Abstract
Hypoxia-inducible transcription factor-1α (HIF-1α) was originally identified as a master regulator of cellular responses to hypoxia. More recently, HIF-1α has emerged as a critical regulator of immune cell function that couples shifts in cellular metabolism to cell type- specific transcriptional outputs. Activation of macrophages with inflammatory stimuli leads to induction of the metabolic program aerobic glycolysis and to HIF-1α stabilization, which reinforce one another in a positive feedback loop that helps drive macrophage activation. This activation of aerobic glycolysis and HIF-1α is important both for production of inflammatory cytokines, such as IL-1β, and for cell intrinsic control of infection. Here we review the importance of HIF-1α for control of bacterial, fungal, and protozoan intracellular pathogens, highlighting recent findings that reveal mechanisms by which HIF-1α is activated during infection and how HIF-1α coordinates antimicrobial responses of macrophages.
Introduction.
Originally discovered as a master regulator of cellular responses to hypoxia [1], the transcription factor Hypoxia inducible factor-1α (HIF-1α) has recently emerged as a key player in macrophage based immune responses. Over the last five years there has been significant progress in our understanding of HIF-1α’s regulation in macrophages, and an increasing awareness of its central role in defense against intracellular pathogens. Here we review mechanisms of HIF-1α regulation, including key studies that demonstrate links between HIF-1α and the shifts in macrophage metabolism that accompany activation and differentiation. In addition, we review recent studies that reveal a role for HIF-1α in defense against intracellular bacterial, fungal, and protozoan pathogens. Interestingly, although the majority of these studies find that HIF-1α promotes classical inflammatory responses and cell intrinsic control of intracellular pathogens, a few studies point to HIF-1α as a factor that downregulates immune responses and impairs control of infection. We also discuss important unanswered questions related to HIF-1α and immunity to intracellular pathogens, including the need to elucidate context dependent roles for HIF-1α during infection with different pathogens in vivo, and to determine cell type specific responses regulated by HIF-1α in macrophages, dendritic cells, neutrophils, and epithelial cells. Finally, the seeming importance of HIF-1α in defense against a wide variety of pathogens suggests that pharmacological agents that activate HIF-1α represent interesting targets for the development of host targeted therapeutics for fighting drug resistant infections, an area that despite a small number of interesting studies, remains largely unexplored.
HIF-1 transcription factor regulation.
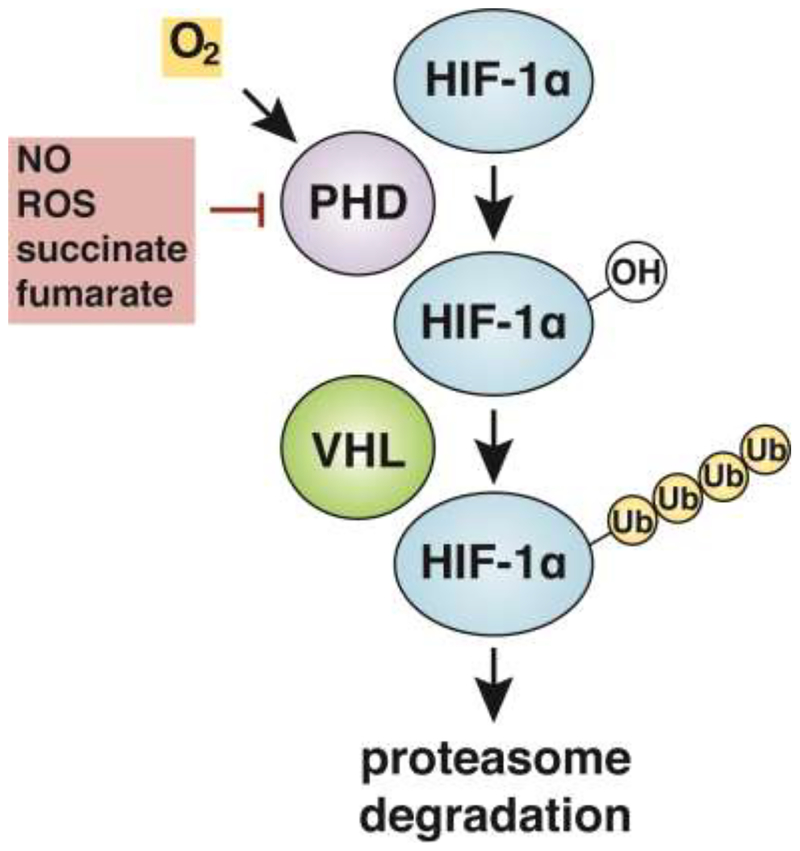
The HIF-1 transcription factor is a heterodimer consisting of two protein subunits, HIF-1α and HIF-1β [2]. Whereas HIF-1β is constitutively expressed in cells, HIF-1α protein levels are highly regulated, both by O2 and by specific metabolites. HIF-1α protein is constitutively transcribed and translated but is rapidly targeted for degradation via hydroxylation of HIF-1α by prolyl hydroxylases (PHDs) [3–5]. O2 dependent hydroxylation facilitates HIF-1α interaction with the Von Hippel-Lindau tumor suppressor protein (VHL), a component of an E3 ubiquitin ligase that ubiquitinates HIF-1α, leading to its degradation by the proteasome [6,7]. Under conditions of low O2, PHD proteins are unable to hydroxylate HIF-1α, leading to a rapid increase in protein levels and transcriptional activation of HIF-1α target genes, a phenomenon known as HIF-1α stabilization (Figure 1). Importantly, HIF- 1α stabilization also occurs under normoxic conditions in response to fluctuating levels of metabolites. For example, increased levels of the TCA cycle intermediates succinate and fumarate stabilize HIF-1α protein by direct inhibition of PHDs, or through promotion of reactive oxygen species production [8–10]. In addition, HIF-1α stabilization can be induced by nitric oxide (NO), which stabilizes HIF-1α directly via S-nitrosylation and indirectly by inhibition of PHDs [11–13]. Although metabolic mechanisms for HIF-1α stabilization were initially discovered in the context of cancer [14], the same mechanisms are operative in activated macrophages, where normoxic HIF-1α stabilization is promoted by succinate, lactate, and NO [15–17].
Figure 1.
In an O2-dependent manner, prolyl hydroxylases (PHDs) hydroxylate HIF-1α, leading to ubiquitination by the Von Hippel-Lindau tumor suppressor protein (VHL) and targeting of the protein for degradation by the proteasome. HIF-1α protein stabilization can occur as a result of low O2 conditions inhibiting hydroxylation by PHDs. In addition, PHDs can be inhibited by ROS, NO, and metabolites including succinate and fumarate.
HIF-1α and metabolic regulation of macrophage function.
In response to activation by TLR ligands or IFN-γ, macrophages alter their metabolism by downregulating oxidative phosphorylation and increasing flux through glycolysis to maintain ATP production [18]. Known as aerobic glycolysis, this metabolic program occurs even under normoxic conditions and is promoted by several factors, including mTOR, AKT, and downregulation of AMPK [19]. Increased glycolytic flux and the accompanying rewiring of central metabolism that accompanies aerobic glycolysis leads to HIF-1α stabilization, a phenomenon observed in macrophages stimulated with TLR ligands as well as in macrophages infected with intracellular pathogens [15,20,21]. Once activated, HIF-1α increases the expression of numerous glycolytic genes, reinforcing glycolytic flux and setting up a positive feedback loop for macrophage activation [18,20].
Why do activated M1 macrophages induce aerobic glycolysis? One possibility is that a switch to aerobic glycolysis enables macrophages to meet the bioenergetic demands of activation [22]. In addition, relying on glycolysis for ATP generation may prepare macrophages to enter hypoxic tissues and areas of inflammation. Indeed, early studies reported that HIF-1α deficient macrophages were defective for ATP production, resulting in profound migration defects to sites of sterile inflammation [23]. However, other studies found no differences in ATP production, or in the numbers of macrophages recruited to sites of inflammation during infection [20,24]. Furthermore, the fact that M2 macrophages, which also have increased biosynthetic demands and migrate into inflamed tissues, do not activate aerobic glycolysis suggests that bioenergetics alone do not explain the switch to aerobic glycolysis in M1 macrophages. Indeed, it has subsequently become clear that the importance of aerobic glycolysis during macrophage activation is in large part due to the activation of HIF-1α, which induces a gene expression program in macrophages that includes expression of inflammatory cytokines and chemokines, as well as inducible nitric oxide synthase (iNOS) and antimicrobial peptides. The specific effectors induced by HIF-1α that contribute to functional control of infection differ depending on the pathogen and cellular context. However, an emerging principle is that HIF-1α couples metabolic cues with immune responses required for control of infection.
HIF-1α and bacterial infections.
The importance of HIF-1α for antibacterial-immunity was initially discovered in the context of infection with Streptococcus spp. Macrophages lacking HIF- 1α were found to be defective for killing both Group B and Group A Streptococci (GAS) [23,24]. Furthermore, mice lacking HIF-1α in macrophages and neutrophils (Hif1afl/flLysMcre) were more susceptible to infection with GAS in a soft tissue infection model. Susceptibility was correlated with a decrease in production of NO and TNF-α by macrophages, and a defect in production of both granule proteases and the antimicrobial peptide CRAMP by neutrophils. These seminal studies established a critical role for HIF-1α in regulating both inflammatory and anti-microbial responses in response to bacterial infection.
Macrophage HIF-1α expression has also been shown to be important for control of intracellular bacterial pathogens that replicate in the macrophage niche. Mice lacking HIF-1α in macrophages are impaired in their ability to control infection with Listeria monocytogenes in the liver, a finding that correlated with decreased production of TNF-α and decreased induction of glycolytic flux by macrophages in infected mice [23]. Interestingly, Francisella tularensis (Ftt) appears to actively impair HIF-1α stabilization in order to facilitate intracellular growth in macrophages. WT Ftt infections do not lead to HIF-1α activation, a result of the immuno- modulatory effect of the Ftt capsule. Capsule deficient mutants induce HIF-1α and are largely attenuated for intracellular growth [25].
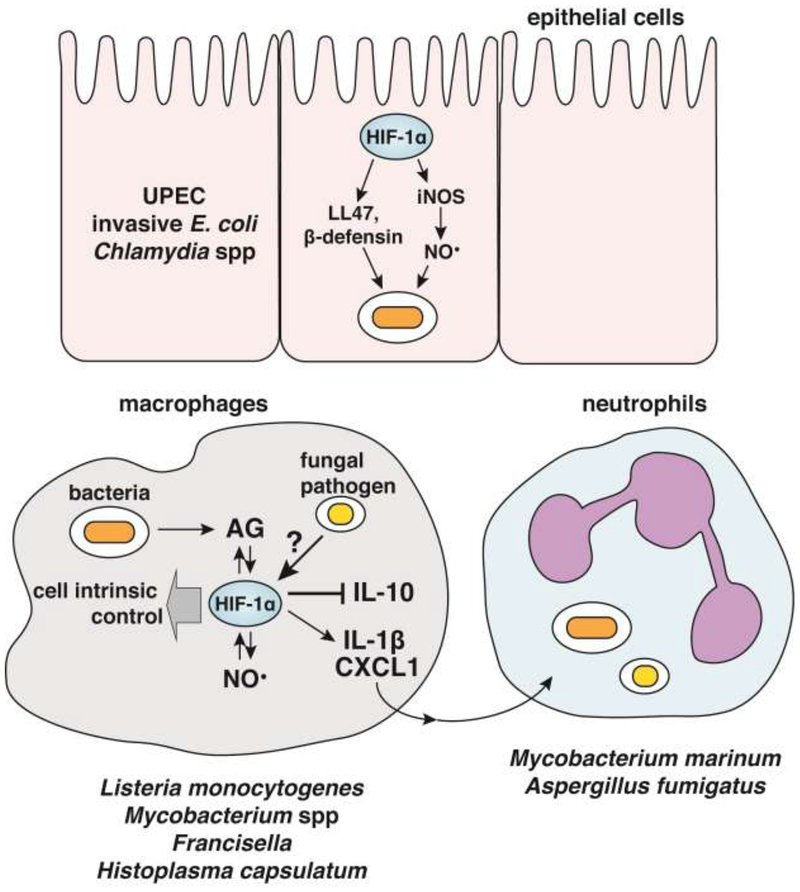
The importance of HIF-1α for host immunity to intracellular bacterial pathogens is best understood in the context of infection with pathogenic mycobacterial species. In a granuloma necrosis model of Mycobacterium avium infection, Hif1afl/flLysMcre mice had increased bacterial burden in livers relative to wild-type, and a more rapid formation of necrotic granulomas [26]. This was originally attributed to HIF-1α deficient macrophages being incapable of adaptation to hypoxia, but more recent studies have implicated a role for HIF-1α during normoxic mycobacterial infections. HIF-1α was found to be important for control of Mycobacterium marinum infections of zebrafish, due to HIF-1α dependent induction of IL-1β in macrophages resulting in enhanced NO production by neutrophils (Figure 2) [27,28]. In this context, HIF-1α stabilizers enhance control of mycobacterial infection, raising the intriguing possibility that exogenous HIF-1α stabilization may enhance immune responses to infection. In the context of infection with Mycobacterium tuberculosis, HIF-1α is a critical mediator of IFN-γ dependent immunity [20]. During Mtb infection, HIF-1α deficient macrophages exhibit decreased IFN-γ dependent expression of iNOS, numerous cytokines and chemokines, genes required for lipid droplet and eicosanoid production, and genes required for induction of aerobic glycolysis [20,29]. As a result, Hif1afl/flLysMcre mice are very susceptible to disease and succumb to infection far earlier than WT [20]. Intriguingly, recent work has found that during Mtb infection HIF-1α stabilization requires NO, establishing a positive feedback loop for macrophage activation [16]. Furthermore, NO has been found to inhibit inflammatory cytokine production both by inhibiting transcription of IL-1β and by inhibiting IL-1β processing by the inflammasome [30,31]. Importantly therefore, HIF-1α simultaneously helps drive NO production to reduce excess inflammation and regulates cell intrinsic antibacterial effectors, many of which remain to be defined [16].
Figure 2.
HIF-1α contributes to cell intrinsic control of epithelial cells infected with invasive UPEC or Chlamydia by inducing expression of iNOS and antimicrobial peptides. In macrophages, HIF-1α, aerobic glycolysis, and NO can promote each other through positive feedback mechanisms that provide host protection to intracellular bacterial pathogens. In the context of infection with the fungal pathogen Histoplasma capsulatum stabilization of HIF-1α results in inhibition of anti-inflammatory cytokine IL-10, while upregulating production of inflammatory cytokines and chemokines like IL-1β and CXCL1 which can promote neutrophil migration to sites of infection.
In addition to its role in myeloid cells, several studies suggest that HIF-1α is also important for defense against infection of epithelial cells with intracellular bacteria. HIF-1α dependent expression of iNOS, β-defensin, and cathelicidins in keratinocytes contributes to defense against uropathogenic E. coli (UPEC) infection in human bladder epithelial cells and in a mouse model of bladder infection (Figure 2) [32]. Indeed, HIF-1α is a key regulator of constitutive β-defensin production in colonic epithelial cells [33]. Importantly, pharmacologic stabilization of HIF-1α was shown to increase control of UPEC infection, suggesting that HIF-1α stabilizers may have therapeutic applications across a range of bacterial infections [32]. HIF-1α was also found to be required for autophagic control of invasive E. coli in intestinal epithelial cells. Furthermore, mice lacking HIF-1α in intestinal epithelial cells were found to be significantly more susceptible to oral challenge with Yersinia enterolitica than wild-type mice [34]. Finally, it was reported that Chlamydia pneumoniae produces a virulence factor that actively degrades HIF-1α during late stages of epithelial cell infection, and that this destabilization of HIF-1α is required for efficient growth of C. pneumoniae under hypoxic conditions [35].
HIF-1α and fungal infections.
Similar to its role in bacterial infections, HIF-1α appears to support immune responses to fungal pathogens. C57BL/6 mice are more susceptible to infection with Coccidioides immitis than DBA/2 mice. This susceptibility correlates with greater HIF-1α dependent gene expression patterns in the resistant DBA/2 mice [36–38]. Furthermore, Hif1afl/flLysMcre mice are highly susceptible to infection with Aspergillus fumigatus. Interestingly, hyphal damage assays and FACS analysis of conidial viability during ex vivo infections indicated that HIF-1α deficient macrophages and neutrophils are not impaired in fungal killing. Instead, Hif1afl/flLysMcre mice displayed a neutrophil recruitment defect, resulting specifically from reduced production of CXCL1 by HIF-deficient macrophages in vivo [39]. Hif1afl/flLysMcre mice are also highly susceptibility to Histoplasma capsulatum infection. In this case, no defect in cell recruitment was observed, however Hif1afl/flLysMcre mice displayed elevated production of the anti-inflammatory cytokine IL-10. Administration of an anti-IL-10 neutralizing antibody reduced fungal burden in Hif1afl/flLysMcre mice and extended survival [40]. Taken together, these data demonstrate that HIF-1α plays an important role in mediating protective inflammatory responses to fungal pathogens by distinct mechanisms (Figure 2).
Although there is limited research on manipulation of HIF-1α for treating fungal disease, it was shown that pharmacological stabilization of HIF-1α during Candida albicans infection of murine and human macrophages resulted in decreased fungal burden. Conversely, treating the media with 2-ME to inhibit HIF-1α increased fungal burden [41]. These results suggest that manipulation of HIF-1α in vitro and in vivo could impact cell intrinsic control of fungal infection, and that HIF-1α stabilization is a promising area of research for the development of novel antifungals.
HIF-1α and protozoan infections
In contrast to the importance of HIF-1α to bacterial and fungal immunity, the role of HIF-1α during protozoan infections is less clear. It was initially reported that Leishmania amazonensis benefits from HIF-1α during infection of murine macrophages, as inhibition of HIF-1α nuclear translocation resulted in decreased pathogen burden in murine macrophages [42]. Similarly, silencing Hif1a using siRNA in the J774 macrophage cell line reduced parasite load whereas over-expressing HIF-1α promoted parasite growth during infection with Leishmania donovani [43]. It was subsequently reported that expression of HIF-1α in dendritic cells (DCs) weakened host immunity to visceral Leishmania donovani infection by suppressing CD8 T cell expansion [44] and by promoting M2 macrophage differentiation during infection [45]. As a consequence, Hif1afl/fl-Cd11c-cre mice, deficient for HIF-1α in DCs, are resistant to visceral Leishmania donovani infection [44]. Conversely, however, Hif1afl/flLysMcre mice suffer a non-resolving cutaneous infection with Leishmania major, whereas WT mice are able to recover [46]. One possible explanation for this apparent discrepancy is the use of different Cre drivers (CD11c-cre vs LysMcre) which create a HIF-1α deficiency in overlapping but distinct populations of myeloid cells [47]. In addition, differences could be attributed to tissue site-specific differences in the immune function of HIF-1α, or differences in immune responses to Leishmania spp. Further research is required to elucidate the role of HIF-1α in cells and tissues of the innate immune system, and how this influences the overall inflammatory milieu during infection.
Conclusions.
HIF-1α is an important regulator of immunity to bacterial, fungal, and protozoan pathogens. Here we have summarized how HIF-1α activation in myeloid and epithelial cells influences immune responses to intracellular pathogen. However, there are still significant gaps in our understanding of HIF-1α mediated immunity. It has been clearly established that HIF-1α controls expression of inflammatory cytokines and chemokines, and induces iNOS and various antimicrobial peptides that clearly contribute to microbial killing both in vitro and in vivo. However, many additional HIF-1α-dependent cell intrinsic antimicrobial effectors, particularly in the context of mycobacterial and fungal infections, remain to be discovered. Interestingly, although most studies focus on the pro-inflammatory activities of HIF-1α, several studies also suggest that HIF-1α can promote downregulation of excessive inflammatory responses, as has been reported for sepsis [48]. HIF-1α appears to influence polarization of some cells towards antimicrobial activation states, and skew others away from microbicidal capabilities. HIF-1α’s complicated and sometimes contradictory roles in these myeloid cell populations requires further investigation. Furthermore, HIF-1α influences the function of lymphocytes, promoting activation and differentiation [49–51], and likely impacts adaptive as well as innate immune responses to intracellular pathogens. Finally, although HIF-1α likely plays a role in immune responses to viruses, this area of HIF-1α biology has been largely unexplored. Nonetheless, the emergent understanding of HIF-1α’s importance to host immunity suggests that HIF-1α manipulation may lead to the development of novel therapies to bolster our increasingly limited arsenal of effective antimicrobial treatments.
Highlights.
The transcription factor HIF-1α has recently gained attention as a master regulator of macrophage-based antimicrobial responses.
HIF-1α couples shifts in metabolism with macrophage activation and differentiation.
HIF-1α contributes to cell intrinsic immunity and helps drive inflammatory responses during a number of bacterial and fungal infections
A small number of pathogens, particularly protozoans, benefit from HIF-1α stabilization, leading to questions about HIF-1α’s varying role in different cell types involved in innate immunity.
HIF-1α manipulation has shown promising results in decreasing microbial burdens both in vitro and in vivo, suggesting that HIF-1α may provide a promising target for host-directed therapeutics.
Acknowledgments.
The authors want to thank Katie Lien, Mariëtta Ravesloot-Chávez, and Erik Van Dis for helpful edits and discussions.
Funding. This work was supported by NIH 1R01AI113270-01A1 to SAS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Majmundar AJ, Wong WJ, Simon MC: Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop- helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92:5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG: HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001, 292:464–468. [DOI] [PubMed] [Google Scholar]
- 4.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. : Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292:468–472. [DOI] [PubMed] [Google Scholar]
- 5.Yu F, White SB, Zhao Q, Lee FS: HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. USA 2001, 98:9630–9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia- inducible factors for oxygen-dependent proteolysis. Nature 1999, 399:271–275. [DOI] [PubMed] [Google Scholar]
- 7.Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, Klausner RD, Pause A: Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 1999, 96:12436–12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J: Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem 2007, 282:4524–4532. [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E: Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol 2007, 27:3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudarshan S, Sourbier C, Kong H-S, Block K, Valera Romero VA, Yang Y, Galindo C, Mollapour M, Scroggins B, Goode N, et al. : Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1α stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol 2009, 29:4080–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, Addeo R, Makuuchi M, Esumi H: Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 2000, 95:189–197. [PubMed] [Google Scholar]
- 12.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li C-Y: Regulation of HIF-1α stability through S-nitrosylation. Mol. Cell 2007, 26:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B: Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol. Biol. Cell 2003, 14:3470–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King A, Selak MA, Gottlieb E: Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene 2006, 25:4675–4682. [DOI] [PubMed] [Google Scholar]
- 15.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. : Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braverman J, Stanley SA: Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB. J. Immunol 2017, 199:1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. : Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L: Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol 2010, 185:605–614. [DOI] [PubMed] [Google Scholar]
- 19.Kelly B, O’Neill LA: Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 2015, 25:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. **.Braverman J, Sogi KM, Benjamin D, Nomura DK, Stanley SA: HIF-1α Is an Essential Mediator of IFN-γ-Dependent Immunity to Mycobacterium tuberculosis. J. Immunol 2016, 197:1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that HIF-1α is required for IFN-γ dependent activation of macrophages and control of the important human pathogen Mycobacterium tuberculosis. RNAseq experiments demonstrate that HIF-1α impacts half of the gene expression changes induced by IFN-γ in Mtb infected cells.
- 21.Gleeson LE, Sheedy FJ, Palsson-McDermott EM, Triglia D, O’Leary SM, O’Sullivan MP, O’Neill LAJ, Keane J: Cutting Edge: Mycobacterium tuberculosis Induces Aerobic Glycolysis in Human Alveolar Macrophages That Is Required for Control of Intracellular Bacillary Replication. J. Immunol 2016, 196:2444–2449. [DOI] [PubMed] [Google Scholar]
- 22.Langston PK, Shibata M, Horng T: Metabolism supports macrophage activation. Front. Immunol 2017, 8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. : HIF-1α is essential for myeloid cell-mediated inflammation. Cell 2003, 112:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS: HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest 2005, 115:1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt EV, Diaz K, Griffin AJ, Rasmussen JA, Crane DD, Jones BD, Bosio CM: Metabolic Reprogramming of Host Cells by Virulent Francisella tularensis for Optimal Replication and Modulation of Inflammation. J. Immunol 2016, 196:4227–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardoso MS, Silva TM, Resende M, Appelberg R, Borges M: Lack of the Transcription Factor Hypoxia-Inducible Factor 1α (HIF-1α) in Macrophages Accelerates the Necrosis of Mycobacterium avium-Induced Granulomas. Infect. Immun 2015, 83:3534–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elks PM, Brizee S, van der Vaart M, Walmsley SR, van Eeden FJ, Renshaw SA, Meijer AH: Hypoxia inducible factor signaling modulates susceptibility to mycobacterial infection via a nitric oxide dependent mechanism. PLoS Pathog 2013, 9:e1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogryzko NV, Lewis A, Wilson HL, Meijer AH, Renshaw SA, Elks PM: Hif-1α-Induced Expression of Il-1β Protects against Mycobacterial Infection in Zebrafish. J. Immunol 2018, doi: 10.4049/jimmunol.1801139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight M, Braverman J, Asfaha K, Gronert K, Stanley S: Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-γ/HIF-1α signaling and supports host defense. PLoS Pathog 2018, 14:e1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra BB, Lovewell RR, Olive AJ, Zhang G, Wang W, Eugenin E, Smith CM, Phuah JY, Long JE, Dubuke ML, et al. : Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat. Microbiol 2017, 2:17072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra BB, Rathinam VAK, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM: Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol 2013, 14:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. **.Lin AE, Beasley FC, Olson J, Keller N, Shalwitz RA, Hannan TJ, Hultgren SJ, Nizet V: Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Escherichia coli Infection. PLoS Pathog 2015, 11:e1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that pharmacological activation of HIF-1α can protect against infection with UPEC, providing important proof of concept for the promise of HIF-1α activators in treating infections with intracellular pathogens.
- 33.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, Bayless AJ, Saeedi BJ, Colgan SP: Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol 2013, 6:1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, et al. : Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology 2008, 134:756–767. [DOI] [PubMed] [Google Scholar]
- 35.Rupp J, Gieffers J, Klinger M, van Zandbergen G, Wrase R, Maass M, Solbach W, Deiwick J, Hellwig-Burgel T: Chlamydia pneumoniae directly interferes with HIF-1α stabilization in human host cells. Cell Microbiol 2007, 9:2181–2191. [DOI] [PubMed] [Google Scholar]
- 36.Kirkland TN, Fierer J: Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect. Immun 1983, 40:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fierer J, Walls L, Eckmann L, Yamamoto T, Kirkland TN: Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. Infect. Immun 1998, 66:4397–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woelk CH, Zhang JX, Walls L, Viriyakosol S, Singhania A, Kirkland TN, Fierer J: Factors regulated by interferon gamma and hypoxia-inducible factor 1A contribute to responses that protect mice from Coccidioides immitis infection. BMC Microbiol 2012, 12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepardson KM, Jhingran A, Caffrey A, Obar JJ, Suratt BT, Berwin BL, Hohl TM, Cramer RA: Myeloid derived hypoxia inducible factor 1-alpha is required for protection against pulmonary Aspergillus fumigatus infection. PLoS Pathog 2014, 10:e1004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. **.Fecher RA, Horwath MC, Friedrich D, Rupp J, Deepe GS: Inverse Correlation between IL-10 and HIF-1α in Macrophages Infected with Histoplasma capsulatum. J. Immunol 2016, 197:565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that HIF-1α promotes host immunity to a fungal pathogen by suppressing the production of IL-10. Mice lacking HIF-1α in macrophages have elevated IL-10 levels during infection, which leads to a suppression of IFN-γ dependent immunity.
- 41.Li C, Wang Y, Li Y, Yu Q, Jin X, Wang X, Jia A, Hu Y, Han L, Wang J, et al. : HIF1α-dependent glycolysis promotes macrophage functional activities in protecting against bacterial and fungal infection. Sci. Rep 2018, 8:3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degrossoli A, Bosetto MC, Lima CBC, Giorgio S: Expression of hypoxia-inducible factor 1α in mononuclear phagocytes infected with Leishmania amazonensis. Immunol. Lett 2007, 114:119–125. [DOI] [PubMed] [Google Scholar]
- 43.Singh AK, Mukhopadhyay C, Biswas S, Singh VK, Mukhopadhyay CK: Intracellular pathogen Leishmania donovani activates hypoxia inducible factor-1 by dual mechanism for survival advantage within macrophage. PLoS One 2012, 7:e38489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. *.Hammami A, Charpentier T, Smans M, Stäger S: IRF-5-Mediated Inflammation Limits CD8+ T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Leishmania Infection. PLoS Pathog 2015, 11:e1004938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. *.Hammami A, Abidin BM, Charpentier T, Fabié A, Duguay A-P, Heinonen KM, Stäger S: HIF-1α is a key regulator in potentiating suppressor activity and limiting the microbicidal capacity of MDSC-like cells during visceral leishmaniasis. PLoS Pathog 2017, 13:e1006616. [DOI] [PMC free article] [PubMed] [Google Scholar]; In contrast to the role of HIF-1α in bacterial and fungal infections, this paper along with [44] demonstrates that HIF-1α activation is detrimental during Leishmania donovani infection, in part because it promotes the emergence of M2 macrophages.
- 46. *.Schatz V, Strüssmann Y, Mahnke A, Schley G, Waldner M, Ritter U, Wild J, Willam C, Dehne N, Brüne B, et al. : Myeloid Cell-Derived HIF-1α Promotes Control of Leishmania major. J. Immunol 2016, 197:4034–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that HIF-1α is required for control of cutaneous leishmaniasis, and that HIF-1α promotes NO production in this context.
- 47.Abram CL, Roberge GL, Hu Y, Lowell CA: Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 2014, 408:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernández-Jiménez E, Toledano V, Cubillos-Zapata C, Rapisarda A, Chen J, et al. : Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 2015, 42:484–498. [DOI] [PubMed] [Google Scholar]
- 49.Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV: Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proc. Natl. Acad. Sci. USA 2002, 99:2170–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phan AT, Goldrath AW: Hypoxia-inducible factors regulate T cell metabolism and function. Mol. Immunol 2015, 68:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng X, Grötsch B, Luo Y, Knaup KX, Wiesener MS, Chen X-X, Jantsch J, Fillatreau S, Schett G, Bozec A: Hypoxia-inducible factor-1α is a critical transcription factor for IL-10- producing B cells in autoimmune disease. Nat. Commun 2018, 9:251. [DOI] [PMC free article] [PubMed] [Google Scholar]