Abstract
Background
To improve physical activity (PA) in patients with chronic obstructive pulmonary disease (COPD), providing a target PA value based on the individual patient’s condition may be a useful interventional strategy. However, to determine the target value, a predictive PA value for each patient is required.
Research Question
What is the reference equation consisting of PA-related factors to determine the predictive PA value for each patient with COPD?
Material and Methods
In this prospective cross-sectional observational study, we measured the PA with a triaxial accelerometer and several other factors including demographic factors, pulmonary function, dyspnea, exercise capacity, muscle strength, nutrition, and indicators of several comorbidities in stable Japanese outpatients with COPD aged ≥40 years old and detected PA-related factors by a multiple regression analysis and stepwise method. We created reference equations for four indices of PA using multiple linear regression equations.
Results
Two hundred and twenty-seven patients were registered. The equations of duration at ≥2.0 metabolic equivalents (METs) and step count consisted of 4 factors: 6-minute walk distance, modified Medical Research Council dyspnea scale, anxiety score of the Hospital Anxiety and Depression Scale, and the forced expiratory volume in 1 second % of predicted value. Those of duration at ≥3.0 METs and total activity at ≥3.0 METs consisted of 5 factors: the above 4 factors and age or brain natriuretic peptide. There was no fixed bias or proportional bias between the measured and predictive values in patients with non-high measured PA values.
Conclusion
We determined reference equations for four indicators of PA using PA-related factors in Japanese patients with COPD. The predictive values calculated using the equations could be useful for deciding target PA values for each patient.
Clinical Trial Registration
UMIN-CTR; No.: UMIN000025459; URL: https://www.umin.ac.jp/ctr/index.htm.
Keywords: predictive value, COPD, metabolic equivalents, step count
Plain Language Summary
Study Question: What is the reference equation consisting of physical activity (PA)-related factors to determine the predictive PA value for each patient with COPD?
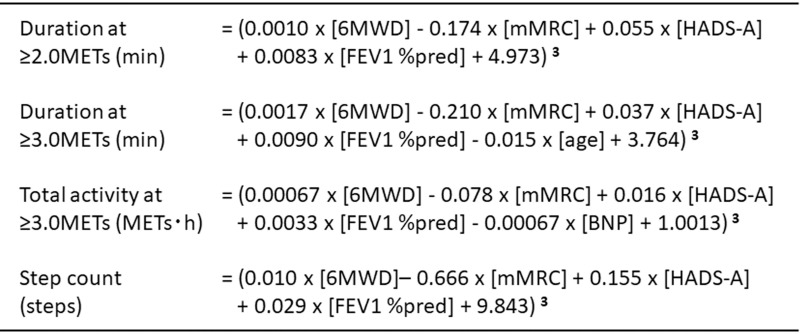
Results: The equations of four indicators of PA were consisted of 6-minute walk distance (6MWD), modified Medical Research Council dyspnea scale (mMRC), anxiety score of the Hospital Anxiety and Depression Scale (HADS-A), forced expiratory volume in 1 second % of predicted value (FEV1 %pred), age, or serum brain natriuretic peptide (BNP). The equations were follows; Duration at ≥2.0 METs (min) = (0.0010 ∙ [6MWD] − 0.174 ∙ [mMRC] + 0.055 ∙ [HADS-A] + 0.0083 ∙ [FEV1 %pred] + 4.973)3; Duration at ≥3.0 METs (min) = (0.0017 ∙ [6MWD] − 0.210 ∙ [mMRC] + 0.037 ∙ [HADS-A] + 0.0090 ∙ [FEV1 %pred] − 0.015 ∙ [age] + 3.764) 3; Total activity at ≥3.0 METs (METs∙h) = (0.00067 ∙ [6MWD] − 0.078 ∙ [mMRC] + 0.016 ∙ [HADS-A] + 0.0033 ∙ [FEV1 %pred] − 0.00067 ∙ [BNP] + 1.0013) 3; Step count (steps) = (0.010 ∙ [6MWD] − 0.666 ∙ [mMRC] + 0.155 ∙ [HADS-A] + 0.029 ∙ [FEV1 %pred] + 9.843) 3.
Interpretation: The predictive values calculated with these equations might be useful for setting the target PA value in each patient.
Introduction
Physical activity (PA) is the strongest predictor of mortality in patients with chronic obstructive pulmonary disease (COPD);1 therefore, improving PA is an important issue in the management of COPD. The addition of activity counseling showed favorable effects on PA,2 so goal setting may be an important factor in counseling. The World Health Organization recommends that older adults engage in at least 150–300 minutes of moderate-intensity aerobic PA or at least 75–150 minutes of vigorous-intensity aerobic PA or an equivalent combination of moderate- and vigorous-intensity activity throughout the week. They should also do muscle-strengthening activities at moderate or greater intensity that involve all major muscle groups on two or more days a week.3
In patients with COPD, a systematic review demonstrated the efficacy of providing target values of step count for improving PA; however, efficacy was not observed in patients with severe COPD or in studies with an intervention duration of six months or longer.4 In most reports, the target values were set at 5%, 20%, 800 steps, or 400 steps more than the current step count.5–8 However, in the report in which 400 steps over the current count were required, approximately 63% of participants did not feel they could comfortably reach their given step count goal, and nearly 40% believed that their goal was too high. Furthermore, both perceptions increased as age increased.9 That is, the requirement of a uniformly increased amount of PA that does not consider the disease state or comorbidities might be too much for patients with COPD to bear, especially those with advanced disease. This might prevent patients from maintaining their motivation to stay physically active for a longer duration. Therefore, it may be important to set the target value in consideration of the predictive PA value according to the individual patient’s condition.
To calculate the predictive PA value of an individual patient, we recently developed a simple reference equation for the step count in Japanese patients with COPD from a retrospective study using demographic factors, pulmonary function, and the dyspnea scale.10 However, COPD was reported to be associated with many other factors aside from the pulmonary function or dyspnea. These factors include the exercise capacity,11,12 nutritional disturbance,13,14 diabetes mellitus,15–17 anemia,18,19 cardiovascular disease,20,21 muscle weakness,22,23 depression,24,25 and anxiety25–27 and may also influence PA in COPD.28,29
In the present study, we extracted the PA-related factors among a pool of factors, including demographic factors, the pulmonary function, exercise capacity, dyspnea, and comorbidities, and created precise reference equations for PA in Japanese patients with COPD that reflect the patient’s condition using extracted PA-related factors.
Materials and Methods
Subjects
Patients were recruited from 21 institutes belonging to the National Hospital Organization of Japan from January 2017 to February 2020. Entry criteria were stable outpatients with COPD diagnosed based on a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7 and aged ≥40 years old. Patients with the following were excluded: 1) clinically relevant bronchial asthma, 2) receiving oxygen therapy, 3) a history of lung resection, 4) a history of exacerbation within three months, 5) PA extremely suppressed by other diseases (including neuromuscular disease, bone and joint disease, active malignant disease, myocardial infarction, etc.), and 6) participation in this study deemed inappropriate by the attending physician. Written informed consent was obtained from all participants. This study was approved by National Hospital Organization Central Review Board (approval number H28-0411001, April 11, 2016) and registered with UMIN-CTR (UMIN000025459, January 10, 2017).
Protocol
This was a prospective cross-sectional observational study. On day 0 (visit 1), demographic information, post-bronchodilator spirometry, grip strength, upper arm circumference, triceps branch subcutaneous fat thickness, modified Medical Research Council (mMRC) dyspnea score, Hospital Anxiety and Depression Scale (HADS), and blood tests were evaluated. Demographic information included the age, gender, height, weight, body mass index (BMI), smoking history, presence or absence of medication for COPD, whether or not pulmonary rehabilitation was conducted, and presence or absence of comorbidities. Blood tests included assessments of red blood cells, hemoglobin, fasting blood sugar, hemoglobin A1c, albumin, and brain natriuretic peptide (BNP). From day 0 to day 14 (up to day 28), PA measurement with a triaxial accelerometer Active Style Pro HJA-750C® (Omron Healthcare, Kyoto, Japan) and diary record were required. A 6-minute walk test and adverse event evaluation were performed at day 14 (or up to day 28) (Visit 2). Four indicators of PA were employed: duration of PA at ≥3.0 metabolic equivalents (METs), duration of PA at ≥2.0 METs, total activity at ≥3.0 METs (METs∙h), and step count. Data were registered via Electronic Data Capture system, and the management and monitoring of the data were carried out by a data center of the National Hospital Organization.
Diary and Mean Temperature
Patients were asked to keep a diary every day. The contents of the diary included information about holidays, weather, and the details of any unusual activities. The mean temperature each day was detected from the records at the meteorological station closest to each institute where the patients were recruited.
Measurement of PA
Participants wore an accelerometer for 24 h, except when bathing or engaged in water-based activities. From the obtained 15 days (up to 29 days), we excluded the first and last days,30,31 rainy days,30–33 holidays, days with unusual activity, and days with a mean temperature of less than 2.5°C31,33,34 based on the diary contents and temperature readings. Of the remaining valid days, the mean value of the first 3 days was used as a representative value of the PA of each patient.30,35 When the number of valid days was less than 3, the patient data were excluded from the analysis.
PA-Related Factors and the Reference Equations
First, we extracted the PA-related factors among a pool of factors, including demographic factors, the pulmonary function, exercise capacity, dyspnea, and comorbidities for four indicators of PA. Next, as the frequencies of these indicators were not normally distributed, we transformed these indicators to normal distributions, and then we created precise reference equations of four transformed indicators using the PA-related factors detected by a stepwise method. Finally, we created each reference equation by reversing the transformations.
Reproducibility of Predictive Values
After the reference equations were created, the relationships between the measured PA values and the predictive values calculated using the equations were evaluated, and the presence of systematic biases between both data sets were evaluated.
Sample Size Determination
In our previous study, the correlation coefficients between the duration of physical activity at ≥3.0 METs and FEV1 % of predicted value (FEV1 %pred) or mMRC score were 0.500 or −0.313, respectively.12 Assuming a 2-sided α-value of 0.05 and β-value of 0.10, the required number of patients was 38 for r=0.5 and 113 for r=0.3; assuming a 20% dropout rate, the numbers should then be 48 and 142, respectively. Furthermore, to perform a multiple regression analysis, the number of samples per independent variable was recommended to be more than 10. Assuming 20 among 28 variables were detected as associated factors by simple regression analysis and were employed for multiple regression analysis, the required number of patients was 200, and assuming a 20% dropout rate, the number should be 250. Therefore, we set the sample size at 250.
Statistical Analyses
Simple and multiple linear regression analyses were used to assess the relationships between PA and several factors. The D’Agostino & Pearson test was used for the evaluation of the normal distribution. Box-Cox transformation was used to normalize the distribution. The stepwise method was used for the detection of PA-related factors. Spearman correlation co-efficient was used for comparisons between the measured and predictive PA values and Bland-Altman Plot was used for evaluating systematic biases. Calculations were performed using the GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics (IBM Japan, Tokyo, Japan) software programs.
Results
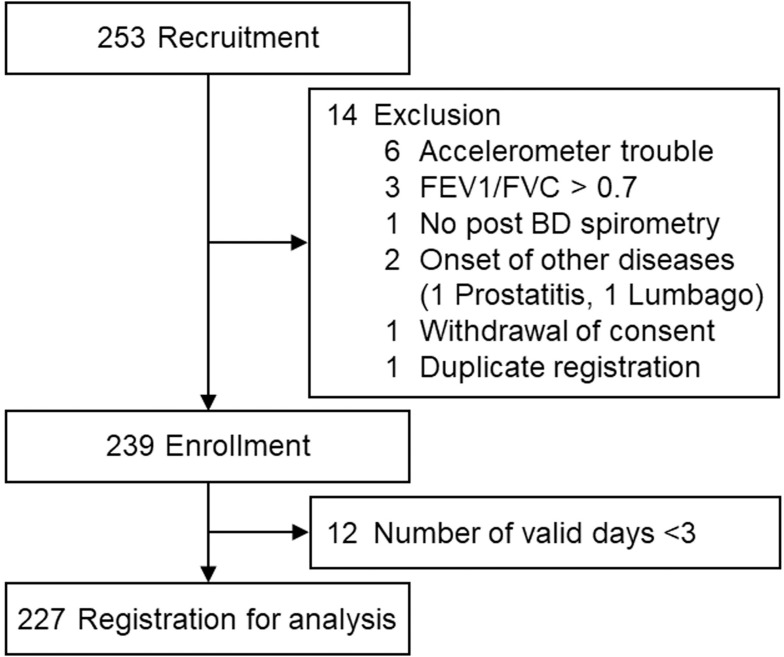
Two hundred and fifty-three patients were recruited, and 14 were excluded (accelerometer trouble: 6, post-bronchodilator FEV1/FVC ≥0.7:3, spirometry performed without a bronchodilator: 1, other diseases occurred: 2, withdrawal of consent: 1, duplicate registration: 1), so 239 were enrolled. Among the enrolled patients, 12 were excluded because the number of valid days was fewer than 3. Ultimately, 227 patients were registered for the analysis (Figure 1).
Figure 1.
Flow diagram.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BD, bronchodilator.
The average age was 73.1 years old, and the study population included 213 male patients (93.8%) and mild to very severe COPD patients with an average FEV1 %pred of 49.7%. The duration at ≥3.0 METs was 60.3 ± 36.4 min, duration at ≥2.0 METs 223.8 ± 93.1 min, total activity at ≥3.0 METs 3.31 ± 2.12 METs∙h, step count 4350.2 ± 2845.8 steps (Table 1).
Table 1.
Characteristics
| Age (years) | 73.1 ± 6.7 |
| Gender [M/F] | 213/14 |
| Height (cm) | 163.6 ± 6.7 |
| Weight (kg) | 60.2 ± 10.2 |
| BMI (kg/m2) | 22.5 ± 3.4 |
| Smoking | |
| [non/ex/curr] | 5/187/35 |
| (Pack·Year) | 64.2 ± 66.5 |
| COPD stage [I/II/III/IV] | 54/110/48/15 |
| Treatment [yes/no] | 207/21 |
| [LAMA/LABA/ICS/Theo/Muco/Other] | 175/154/63/3/22/62 |
| Pulmonary Rehabilitation [yes/no] | 15/212 |
| Comorbidity [yes/no] | 168/59 |
| Pulmonary function | |
| IC (L) | 2.22 ± 0.56 |
| FVC (L) | 3.28 ± 0.78 |
| FVC % pred (%) | 99.6 ± 19.5 |
| FEV1 (L) | 1.64 ± 0.60 |
| FEV1/FVC (%) | 49.7 ± 13.2 |
| FEV1% pred (%) | 62.7 ± 20.9 |
| mMRC | 1.2 ± 0.9 |
| 6-minute walk test | |
| Distance (m) | 390.0 ± 102.6 |
| Lowest SpO2 (%) | 89.2 ± 5.1 |
| Grip strength (kgf) | 31.8 ± 7.4 |
| Blood tests | |
| Fasting plasma glucose (mg/dL) | 109.1 ± 25.9 |
| Hemoglobin A1c (%) | 6.0 ± 0.6 |
| Red blood cells (×104/μL) | 473.1 ± 55.0 |
| Hemoglobin (g/dL) | 14.7 ± 1.5 |
| Brain Natriuretic Peptide (pg/mL) | 36.0 ± 46.1 |
| Albumin (g/dL) | 4.2 ± 0.3 |
| Nutrition | |
| Upper arm circumference (cm) | 26.8 ± 3.2 |
| Subcutaneous fat thickness of triceps brachii (cm) | 1.34 ± 0.76 |
| Anxiety score of HADS | 3.3 ± 2.6 |
| Depression score of HADS | 4.3 ± 3.1 |
| Physical activity | |
| Duration at ≥3.0 METs (min) | 60.3 ± 36.4 |
| Duration at ≥2.0 METs (min) | 223.8 ± 93.1 |
| Total activity at ≥3.0 METs (METs∙h) | 3.31 ± 2.12 |
| Step count (steps) | 4350.2 ± 2845.8 |
Abbreviations: BMI, body mass index; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta 2 adrenergic agonist; ICS, inhaled corticosteroid; Theo, theophylline; Muco, mucolytic agent; IC, inspiratory capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; %pred, % of predicted value; mMRC, modified Medical Research Council; HADS, Hospital Anxiety and Depression Scale, METs, metabolic equivalents.
In the simple regression analysis, the age, FEV1/FVC, FEV1 %pred, 6-minute walk distance (6MWD), lowest SpO2 during 6-minute walk test, anxiety score of HADS (HADS-A), mMRC, and presence of COPD treatment were found to be related to all indicators of PA, and the gender, height, BMI, IC, FVC % of predicted value, upper arm circumference, grip strength, and BNP were shown to be related to some of the 4 indicators of PA (Table 2). In the multiple regression analysis, the 6MWD, mMRC, and HADS-A were related to all 4 indicators, the FEV1 %pred was related to the duration at ≥3.0 METs and ≥2.0 METs, the age was related to the duration at ≥3.0 METs, and the BNP was related to the total activity at ≥3.0 METs (Table 3).
Table 2.
Simple Regression Analysis Results
| Duration at ≥2.0 METs | Duration at ≥3.0 METs | Total Activity at ≥3.0 METs | Step Count | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age (years) | −0.131 | 0.049 | −0.194 | 0.003 | −0.197 | 0.003 | −0.208 | 0.002 |
| Gender [M/F] | −0.157 | 0.018 | −0.073 | 0.275 | −0.064 | 0.336 | −0.002 | 0.976 |
| Height (cm) | −0.149 | 0.025 | −0.039 | 0.561 | −0.031 | 0.639 | 0.086 | 0.195 |
| Weight (kg) | 0.010 | 0.876 | 0.094 | 0.160 | 0.094 | 0.156 | 0.056 | 0.403 |
| BMI (kg/m2) | 0.102 | 0.126 | 0.134 | 0.044 | 0.131 | 0.049 | 0.029 | 0.668 |
| Smoking [non/ex/curr] | 0.002 | 0.980 | 0.022 | 0.739 | 0.029 | 0.662 | −0.039 | 0.559 |
| Smoking (Pack·Year) | −0.085 | 0.208 | −0.006 | 0.927 | −0.004 | 0.956 | 0.069 | 0.308 |
| IC (L) | 0.122 | 0.066 | 0.210 | 0.002 | 0.212 | 0.001 | 0.195 | 0.003 |
| FVC %pred (%) | 0.193 | 0.219 | 0.248 | <0.001 | 0.245 | <0.001 | 0.247 | <0.001 |
| FEV1/FVC | 0.303 | <0.001 | 0.311 | <0.001 | 0.306 | <0.001 | 0.236 | <0.001 |
| FEV1 %pred (%) | 0.335 | <0.001 | 0.370 | <0.001 | 0.365 | <0.001 | 0.315 | <0.001 |
| 6MWD (m) | 0.278 | <0.001 | 0.411 | <0.001 | 0.407 | <0.001 | 0.393 | <0.001 |
| Lowest SpO2 during 6-minute walk test (%) | 0.154 | 0.021 | 0.196 | 0.003 | 0.198 | 0.003 | 0.176 | 0.008 |
| Upper arm circumference (cm) | 0.134 | 0.044 | 0.155 | 0.019 | 0.157 | 0.018 | 0.104 | 0.117 |
| Subcutaneous fat thickness of triceps brachii (cm) | −0.017 | 0.796 | −0.058 | 0.381 | −0.057 | 0.390 | −0.130 | 0.051 |
| Grip strength (kgf) | 0.116 | 0.081 | 0.136 | 0.041 | 0.128 | 0.054 | 0.150 | 0.024 |
| Fasting plasma glucose (mg/dL) | −0.037 | 0.575 | 0.058 | 0.385 | 0.076 | 0.253 | 0.004 | 0.958 |
| Hemoglobin A1c (%) | −0.042 | 0.531 | 0.016 | 0.806 | 0.037 | 0.579 | −0.014 | 0.839 |
| Red blood cells (×104/mL) | −0.048 | 0.474 | −0.059 | 0.377 | −0.054 | 0.420 | −0.059 | 0.379 |
| Hemoglobin (g/dL) | −0.097 | 0.146 | −0.063 | 0.343 | −0.060 | 0.371 | −0.051 | 0.443 |
| Brain Natriuretic Peptide (pg/mL) | −0.065 | 0.330 | −0.148 | 0.026 | −0.152 | 0.022 | −0.049 | 0.467 |
| Albumin (g/dL) | 0.117 | 0.078 | 0.122 | 0.066 | 0.124 | 0.062 | 0.112 | 0.094 |
| Anxiety score of HADS | 0.163 | 0.014 | 0.142 | 0.033 | 0.148 | 0.026 | 0.156 | 0.019 |
| Depression score of HADS | −0.039 | 0.563 | −0.042 | 0.533 | −0.039 | 0.557 | −0.017 | 0.796 |
| mMRC | −0.314 | <0.001 | −0.392 | <0.001 | −0.377 | <0.001 | −0.323 | <0.001 |
| Treatment [yes/no] | −0.164 | 0.014 | −0.212 | 0.001 | −0.205 | 0.002 | −0.216 | 0.001 |
| Rehabilitation [yes/no] | −0.125 | 0.060 | −0.091 | 0.173 | −0.096 | 0.150 | 0.031 | 0.647 |
| Comorbidity [yes/no] | −0.002 | 0.980 | 0.022 | 0.739 | 0.029 | 0.662 | −0.003 | 0.967 |
Abbreviations: BMI, body mass index; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta 2 adrenergic agonist; ICS, inhaled corticosteroid; Theo, theophylline; Muco, mucolytic agent; IC, inspiratory capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; %pred, % of predicted value; 6MWD, 6-minute walk distance; HADS, Hospital Anxiety and Depression Scale; mMRC, modified Medical Research Council, METs, metabolic equivalents.
Table 3.
Multiple Regression Analysis Results
| Duration at ≥2.0 METs | Duration at ≥3.0 METs | Total Activity at ≥3.0 METs | Step Count | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| 6MWD | 0.187 | 0.010 | 0.241 | 0.001 | 0.250 | 0.001 | 0.299 | <0.001 |
| mMRC | −0.210 | 0.005 | −0.239 | 0.001 | −0.211 | 0.005 | −0.153 | 0.043 |
| HADS-A | 0.148 | 0.019 | 0.119 | 0.047 | 0.142 | 0.020 | 0.156 | 0.012 |
| FEV1 %pred | 0.171 | 0.028 | 0.766 | 0.047 | – | – | – | – |
| Age | – | – | −0.190 | 0.008 | – | – | – | – |
| BNP | – | – | – | – | −0.142 | 0.018 | – | – |
Abbreviations: METs, metabolic equivalents; 6MWD, 6-minute walking distance; mMRC, modified Medical Research Council; HADS-A, anxiety score of Hospital Anxiety and Depression Scale; FEV1 %pred, forced expiratory volume in 1 second % of predicted value; BNP, brain natriuretic peptide.
Since the frequencies of the PA indicators were not normally distributed (Figure S1), we transformed these indicators to normal distributions using Box-Cox transformation (λ=0.33) (Figure S2). In the simple regression analysis for the transformed PA values, the IC, FVC % of predicted value, FEV1/FVC, FEV1 %pred, 6MWD, lowest SpO2 during the 6MWD test, mMRC, and presence of COPD treatment were related to all indicators of PA. The age, gender, height, BMI, upper arm circumference, subcutaneous fat thickness of triceps brachii, grip strength, BNP, and HADS-A were related to some of the 4 indicators of PA (Table S1). In the stepwise method, the 6MWD, mMRC, HADS-A, and FEV1 %pred was related to all indicators of PA, the age was related to the duration at ≥3.0 METs, and the BNP was related to the total activity at ≥3.0 METs (Table 4).
Table 4.
Factors Detected by the Stepwise Method
| Duration at ≥2.0 METs | Duration at ≥3.0 METs | Total Activity at ≥3.0 METs | Step Count | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| 6MWD | 0.139 | 0.044 | 0.221 | 0.001 | 0.240 | <0.001 | 0.295 | <0.001 |
| mMRC | −0.199 | 0.007 | −0.256 | <0.001 | −0.240 | <0.001 | −0.180 | 0.011 |
| HADS-A | 0.178 | 0.004 | 0.127 | 0.026 | 0.141 | 0.013 | 0.119 | 0.047 |
| FEV1 %pred | 0.213 | 0.002 | 0.245 | <0.001 | 0.242 | <0.001 | 0.175 | 0.008 |
| Age | – | – | −0.132 | 0.020 | – | – | – | – |
| BNP | – | – | – | – | −0.127 | 0.022 | – | – |
Abbreviations: METs, metabolic equivalents; 6MWD, 6-minute walking distance; mMRC, modified Medical Research Council; HADS-A, anxiety score of Hospital Anxiety and Depression Scale; FEV1 %pred, forced expiratory volume in 1 second % of predicted value; BNP, brain natriuretic peptide.
Finally, the reference equations for four indicators were created by the multiple linear regression formula with PA-related factors (Figure 2).
Figure 2.
Reference equations of physical activity. METs, metabolic equivalents. Units: Duration at ≥2.0 METs (min), Duration at ≥3.0 METs (min), Total activity at ≥3.0 METs (METs∙h), Step count (steps), 6MWD (m), mMRC (point), HADS-A (point), FEV1 %pred (%), age (years old), BNP (pg/mL).
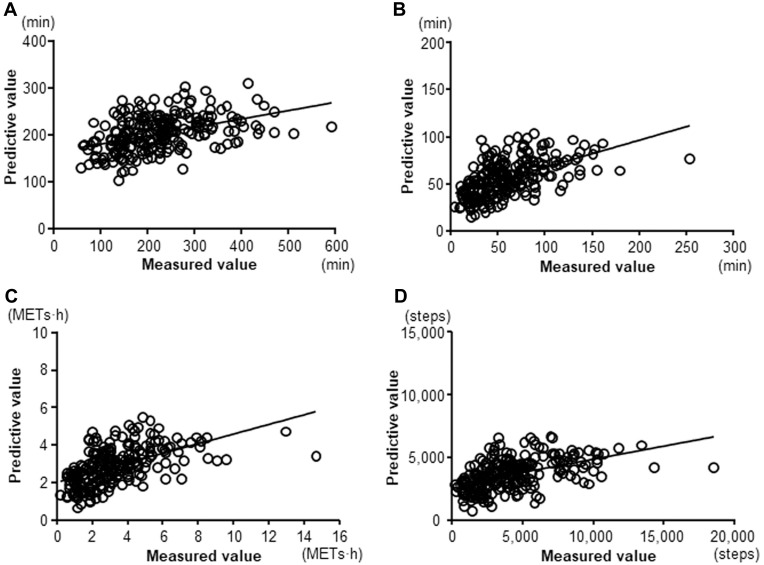
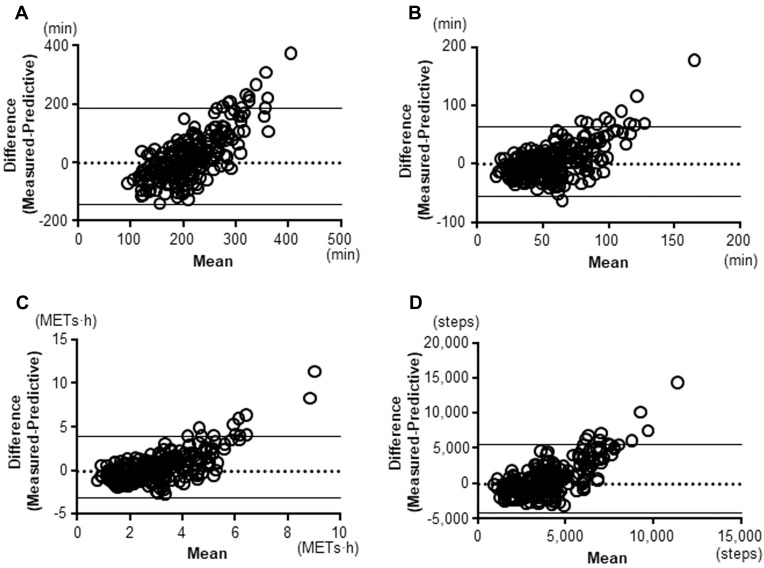
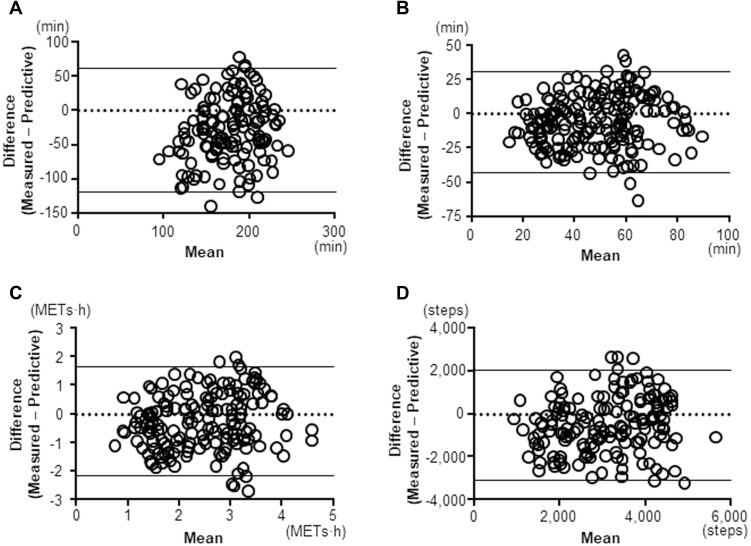
The correlations between the measured and predictive PA value were statistically significant in all indicators (≥2.0 METs: r=0.462, p<0.0001; ≥3.0 METs: r=0.598, p<0.0001; total activity at ≥3.0 METs: r=0.614, p<0.0001; Step: r=0.539, p<0.0001) (Figure 3). The 95% coefficient interval of the mean difference between the measured and predictive value of the duration at ≥2.0 METs was −143.6 to 185.7 min, that of the duration at ≥3.0 METs was −54.8 to 64.4 min, that of the total activity at ≥3.0 METs was −3.08 to 3.94 METs∙h, and that of the step count was −4239.1 to 5545.2 steps, which indicated that there was no fixed bias in any indicators. The correlation coefficients between difference and mean values on Bland-Altman Plots were 0.75 in the duration at ≥2.0 METs (p<0.001), 0.629 in the duration at ≥3.0 METs (p<0.001), 0.700 in the total activity at ≥3.0 METs (p<0.001), and 0.730 in the step count (p<0.001), which indicated that there were proportional biases in all indicators. The measured values were high compared to the predictive values in the patients with high measured PA values (Figure 4). However, these proportional biases disappeared when the patients whose measured values for the duration at ≥2.0 METs, duration at ≥3.0 METs, total activity at ≥3.0 METs, and step count were <231 min, <87 min, <4.4 METs∙h, or <5108 steps, respectively, were selected (Figure 5).
Figure 3.
Relationships between the measured and predictive values. (A) duration at ≥2.0 METs; r=0.462, P<0.0001, (B) duration at ≥3.0 METs; r=0.598, P<0.0001, (C) total activity at ≥3.0 METs; r=0.614, P<0.0001, (D) Step count; r=0.539, P<0.0001.
Abbreviation: METs, metabolic equivalents.
Figure 4.
Bland-Altman Plots. (A) duration at ≥2.0 METs, (B) duration at ≥3.0 METs, (C) total activity at ≥3.0 METs, (D) Step count.
Abbreviation: METs, metabolic equivalents.
Figure 5.
Bland-Altman Plots after excluding highly active patients. (A) duration at ≥2.0 METs (patients with <231 minutes of measured value), (B) duration at ≥3.0 METs (patients with <87 minutes of measured value), (C) total activity at ≥3.0 METs (patients with <4.4 METs∙h of measured value), (D) Step count (patients with <5108 steps of measured value).
Abbreviation: METs, metabolic equivalents.
Discussion
Among demographic factors, the pulmonary function, dyspnea, exercise capacity, muscle strength, and indices of several comorbidities, all indicators of PA were found to be related to the 6MWD, mMRC, HADS-A, and FEV1 %pred and partly related to the age or BNP level. We were able to create reference equations for indicators of PA, including the duration at ≥2.0 METs, duration at ≥3.0 METs, total activity at ≥3.0 METs, and step count, using these PA-related factors in Japanese patients with COPD. No fixed bias between the measured values and the predictive values was not observed in any indicators; however, proportional biases were observed in all indicators of PA.
We previously developed a simple reference equation for step count in Japanese patients with COPD using the age, IC, and mMRC.10 However, PA in COPD can be influenced by many other factors. In the current study, we prospectively evaluated the influences of 28 total factors, as follows: exercise capacity, muscle strength, presence of medication for COPD, presence of pulmonary rehabilitation, presence of comorbidity, and types of comorbidities (eg anemia, diabetes mellitus, cardiac dysfunction, malnutrition, depression, and anxiety) in addition to demographic factors, the pulmonary function, and dyspnea. The stepwise method after Box-Cox transformation selected four to five PA-related factors for each indicator of PA, and more precise reference equations were able to be developed using these related factors. The 6MWD, mMRC, HADS-A, and FEV1 %pred were the PA-related factors for all 4 indicators of PA, and age and BNP were those for some indicators.
The relationship between exercise capacity and PA has been reported in several studies,11,12,22,36–39 so exercise capacity may be an important factor influencing PA. Patients with a low exercise capacity can have low PA; however, patients with a high exercise capacity do not always have high PA. Furthermore, exercise capacity can improve with pulmonary rehabilitation, but PA does not.40–42 In another report, the exercise capacity improved significantly after three months of pulmonary rehabilitation, but PA did not, although it did improve after six months.43 These results suggest that exercise capacity and PA do not always change in tandem.
The relationship between dyspnea and PA has also been reported in several studies.12,36,37,39,44,45 Dyspnea and PA were related as a whole; however, the degree of mMRC in patients was widely distributed, even in patients with similar PA values.44
The relationship between the FEV1 %pred and PA has been reported in several studies,11,22,36,37,46 although it is very modest.22 The correlation coefficient between the FEV1 %pred and PA was lower than that between the exercise capacity and PA in several reports.22,36,37 On the contrary, in a systematic review with a meta-regression analysis, only the FEV1 %pred was a PA-related factor, though the majority of adopted studies had a small number of patients less than 90 and a mean FEV1 %pred below 50%.46 In the current study, both FEV1 %pred and exercise capacity were detected as PA-related factors with larger number of patients (n=227) and relatively higher FEV1% pred (62.7%).
HADS-A was selected as a PA-related factor of PA. Unexpectedly, a higher risk of anxiety was shown to be related to a higher level of PA in the current study. Several studies found no relationship between anxiety and PA in the patient cohort with relatively higher score of HADS-A.47,48 However, Nguyen et al reported that increased anxiety was associated with higher levels of PA in patients with COPD with relatively low score of HADS-A (5.5±4.1),49 which was compatible with our results. In the current study, patients had relatively low level of anxiety with a mean HADS-A of 3.3±2.6, and the results were similar to those of Nguyen’s report. Further study is required to clarify the effect of anxiety on PA in COPD.
In addition to the 6MWD, mMRC, HADS-A, and FEV1 %pred, age was related to the duration of PA at ≥3.0 METs, and BNP was related to the total activity at ≥3.0 METs. Generally, PA is expected to decrease with age, and the relationship between age and PA in patients with COPD has been reported.38,50 In the current study, age was related to the duration of PA at ≥3.0 METs but not with the duration at a lower level of PA or with the step count. The step count as an indicator does not include the factor of intensity and it is counted as the same number of steps regardless of the walking speed. Kawagoshi et al reported that age is correlated with a fast-walking pace (≥2 km/h) but not with a slow-walking pace (<2 km/h).38 Luzak et al reported that the time per day of moderate-to-vigorous PA in patients over 61 years old was significantly shorter than in those under 55 years old, while the time per day of light PA was not markedly different between those age groups.50 These findings were compatible with our own, suggesting that age might influence the indicators of relatively high-intensity PA.
Cardiovascular disease is more common in patients with COPD than in those without it,20,21,51 and the BNP level in heart failure patients is correlated with their PA.52 Watz et al reported that BNP was correlated with the PA level (total daily energy expenditure divided by whole-night sleeping energy expenditure) and step count in patients with COPD.51 BNP was correlated with the total activity at ≥3.0 METs but not with the duration of PA or step count in the current study. BNP might affect indicators of PA, including factors of both the intensity and duration. Further studies will be required to clarify the meanings of indicators of PA reflecting the cardiac function.
In our previous report, which demonstrated a simple reference equation for step count, the age, IC, and mMRC were extracted as PA-related factors of the step count;10 however, the 6MWD, HADS-A, FEV1 %pred, and mMRC were extracted in the current study. IC was not extracted as a PA-related factor, although it was reported to be more closely related to PA than FEV1.53 IC showed multicollinearity with the 6MWD, which was not used in the previous analysis as an independent variable (r=0.270, p<0.001), and with FEV1 %pred (r=0.458, p<0.001), which suggests that using both the 6MWD and FEV1 %pred may help predict the step count more precisely than the IC. Alternatively, the prospective nature of the present study and retrospective nature of the previous study might have resulted in the extraction of different PA-related factors.
There were proportional biases between measured and predictive PA values in all indicators, and the measured values were higher than the predictive values in the patients with high measured values. This implied that there were some patients who could already maintain a relatively high level of PA and did not need further improvement of PA. After excluding the patients whose measured PA values were already high, proportional biases disappeared in all indicators of PA. This suggests that these equations may be particularly useful for patients with a low PA who need to improve their values.
The newly developed reference equations could make it possible to calculate a predictive value of PA that reflects each patient’s condition. While it will be necessary to develop a detailed method of setting target values as the next step, we could determine an executable target value of PA for each patient based on the current and predictive values. In the previous reports in which target values of PA were provided to the patients with COPD, the target values were set based on the current values but not on the individual predictive values,5–8 resulting in that the effect of providing a target on PA could not be maintained for more than 6 months.4 The target values based on current and predictive values of PA could become a realistic, practical, and sustainable one to improve PA for each patient; therefore, we may be able to develop precision medicine based on PA for patients with COPD.
Several limitations associated with the present study warrant mention. First, socio-environmental conditions were not included as independent variables. Having a job,54 living with an active housemate,55 walking a dog, and grandparenting56 are related to increased amounts and intensities of PA. Evaluating the influence of socio-environmental conditions will be required in future studies. Second, the recruited patients were all Japanese, so the newly developed equations can only be reasonably applied to Japanese patients with COPD. Developing such equations of PA in each country might be required. Third, most of the recruited patients were male (93.8%). While gender was not selected as a PA-related factor for PA, this might not be conclusive. Ichinose et al reported that gender was related to the duration at ≥2.0 METs in Japanese patients with COPD, although the coexistence of bronchial asthma was not always excluded in the study.54 A further study with an increased number of female patients is required.
Conclusions
Using the PA-related factors of the 6MWD, mMRC, HADS-A, FEV1 %pred, and partly age or BNP, we were able to create reference equations of four indicators of PA in Japanese patients with COPD. The predictive values calculated with these equations might be useful for setting the target PA value in each patient.
Acknowledgments
Patient recruitment was performed at the following 18 institutes belonging to the National Hospital Organization (NHO): NHO Asahikawa Medical Center, NHO Takasaki General Medical Center, NHO Yokohama Medical Center, NHO Kanazawa Medical Center, NHO Tenryu Hospital, NHO Minami Kyoto Hospital, NHO Kinki-chuo Chest Medical Center, NHO Osaka Toneyama Medical Center, NHO Osaka Minami Medical Center, NHO Himeji Medical Center, NHO Nara Medical Center, NHO Minami Wakayama Medical Center, NHO Wakayama Hospital, NHO Minami-Okayama Medical Center, NHO Yamaguchi-Ube Medical Center, NHO Ehime Medical Center, NHO Kochi National Hospital, NHO Fukuoka Hospital. We thank Dr. Tadatoshi Suruda for assistance in conducting research and Mr. Brian Quinn for proofreading the manuscript.
Funding Statement
This study was supported by a grant from Japanese National Hospital Organization (NHO)-Evidence-based Medicine (EBM) study group.
Abbreviations
6MWD, 6-minute walk distance; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FEV1 %pred, forced expiratory volume in 1 second % of predicted value; FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; HADS-A, anxiety score of Hospital Anxiety and Depression Scale; METs, metabolic equivalents; mMRC, modified Medical Research Council; PA, physical activity.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by National Hospital Organization Central Review Board (approval number H28-0411001, April 11, 2016) and registered with UMIN-CTR (UMIN000025459, January 10, 2017). Written informed consent was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
YM received lecture fee from Nippon Boehringer Ingelheim. Other authors have no conflicts of interest.
References
- 1.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi: 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 2.Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2016;11:3121–3136. doi: 10.2147/COPD.S121263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO guidelines on physical activity and sedentary behaviour; 2020:43. Available from https://www.who.int/publications/i/item/9789240015128. Accessed June 16, 2021. [PubMed]
- 4.Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386. doi: 10.1177/1753466618787386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan CM, Maddocks M, Canavan JL, et al. Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2017;195(10):1344–1352. doi: 10.1164/rccm.201607-1372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorrink SN, Kort HS, Troosters T, Zanen P, Lammers JJ. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J. 2016;48(4):1019–1029. doi: 10.1183/13993003.00083-2016 [DOI] [PubMed] [Google Scholar]
- 7.Wan ES, Kantorowski A, Homsy D, et al. Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use. Respir Med. 2017;130:102–110. doi: 10.1016/j.rmed.2017.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moy ML, Janney AW, Nguyen HQ, et al. Use of pedometer and internet-mediated walking program in patients with chronic obstructive pulmonary disease. J Rehabil Res Dev. 2010;47(5):485–496. doi: 10.1682/JRRD.2009.07.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson SA, Wan ES, Shimada SL, Richardson CR, Moy ML. Age and attitudes towards an internet-mediated, pedometer-based physical activity intervention for chronic obstructive pulmonary disease: secondary analysis. JMIR Aging. 2020;3(2):e19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi M, Minakata Y, Tanaka R, et al. Simple standard equation for daily step count in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1967–1977. doi: 10.2147/COPD.S218705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522–530. doi: 10.1016/j.rmed.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 12.Minakata Y, Sugino A, Kanda M, et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir Investig. 2014;52(1):41–48. doi: 10.1016/j.resinv.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017 [DOI] [PubMed] [Google Scholar]
- 14.Gea J, Sancho-Muñoz A, Chalela R. Nutritional status and muscle dysfunction in chronic respiratory diseases: stable phase versus acute exacerbations. J Thorac Dis. 2018;10(Suppl 12):S1332–S1354. doi: 10.21037/jtd.2018.02.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004;27(10):2478–2484. doi: 10.2337/diacare.27.10.2478 [DOI] [PubMed] [Google Scholar]
- 16.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi: 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 17.Methvin JN, Mannino DM, Casey BR. COPD prevalence in southeastern Kentucky: the burden of lung disease study. Chest. 2009;135(1):102–107. doi: 10.1378/chest.08-1315 [DOI] [PubMed] [Google Scholar]
- 18.John M, Hoernig S, Doehner W, Okonko DD, Witt C, Anker SD. Anemia and inflammation in COPD. Chest. 2005;127(3):825–829. doi: 10.1378/chest.127.3.825 [DOI] [PubMed] [Google Scholar]
- 19.Similowski T, Agusti A, MacNee W, Schonhofer B. The potential impact of anaemia of chronic disease in COPD. Eur Respir J. 2006;27(2):390–396. doi: 10.1183/09031936.06.00143704 [DOI] [PubMed] [Google Scholar]
- 20.Cazzola M, Bettoncelli G, Sessa E, Cricelli C, Biscione G. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(2):112–119. doi: 10.1159/000281880 [DOI] [PubMed] [Google Scholar]
- 21.Hannink JD, van Helvoort HA, Dekhuijzen PN, Heijdra YF. Heart failure and COPD: partners in crime? Respirology. 2010;15(6):895–901. doi: 10.1111/j.1440-1843.2010.01776.x [DOI] [PubMed] [Google Scholar]
- 22.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi: 10.1164/rccm.200407-855OC [DOI] [PubMed] [Google Scholar]
- 23.Butcher SJ, Pikaluk BJ, Chura RL, Walkner MJ, Farthing JP, Marciniuk DD. Associations between isokinetic muscle strength, high-level functional performance, and physiological parameters in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:537–542. doi: 10.2147/COPD.S34170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eagan TM, Ueland T, Wagner PD, et al. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J. 2010;35(3):540–548. doi: 10.1183/09031936.00088209 [DOI] [PubMed] [Google Scholar]
- 25.Cafarella PA, Effing TW, Usmani ZA, Frith PA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627–638. doi: 10.1111/j.1440-1843.2012.02148.x [DOI] [PubMed] [Google Scholar]
- 26.Okubadejo AA, O’Shea L, Jones PW, Wedzicha JA. Home assessment of activities of daily living in patients with severe chronic obstructive pulmonary disease on long-term oxygen therapy. Eur Respir J. 1997;10(7):1572–1575. doi: 10.1183/09031936.97.10071572 [DOI] [PubMed] [Google Scholar]
- 27.Kim HF, Kunik ME, Molinari VA, et al. Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics. 2000;41(6):465–471. doi: 10.1176/appi.psy.41.6.465 [DOI] [PubMed] [Google Scholar]
- 28.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt 2):337–346. doi: 10.1242/jeb.048074 [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Watz H, Wouters EF, Cazzola M. COPD: the patient perspective. Int J Chron Obstruct Pulmon Dis. 2016;11(Spec Iss):13–20. doi: 10.2147/copd.s85977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugino A, Minakata Y, Kanda M, et al. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration. 2012;83(4):300–307. doi: 10.1159/000330046 [DOI] [PubMed] [Google Scholar]
- 31.Minakata Y, Sasaki S. Data reproducibility and effectiveness of bronchodilators for improving physical activity in COPD patients. J Clin Med. 2020;9(11):3497. doi: 10.3390/jcm9113497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alahmari AD, Mackay AJ, Patel AR, et al. Influence of weather and atmospheric pollution on physical activity in patients with COPD. Respir Res. 2015;16:71. doi: 10.1186/s12931-015-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turrisi TB, Bittel KM, West AB, et al. Seasons, weather, and device-measured movement behaviors: a scoping review from 2006 to 2020. Int J Behav Nutr Phys Act. 2021;18(1):24. doi: 10.1186/s12966-021-01091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlanetto KC, Demeyer H, Sant’anna T, et al. Physical activity of patients with COPD from regions with different climatic variations. COPD. 2017;14(3):276–283. doi: 10.1080/15412555.2017.1303039 [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto S, Minakata Y, Azuma Y, et al. Verification of a motion sensor for evaluating physical activity in COPD patients. Can Respir J. 2018;2018:8343705. doi: 10.1155/2018/8343705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117(5):1359–1367. doi: 10.1378/chest.117.5.1359 [DOI] [PubMed] [Google Scholar]
- 37.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi: 10.1183/09031936.00024608 [DOI] [PubMed] [Google Scholar]
- 38.Kawagoshi A, Kiyokawa N, Sugawara K, et al. Quantitative assessment of walking time and postural change in patients with COPD using a new triaxial accelerometer system. Int J Chron Obstruct Pulmon Dis. 2013;8:397–404. doi: 10.2147/COPD.S49491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Remoortel H, Hornikx M, Demeyer H, et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax. 2013;68(10):962–963. doi: 10.1136/thoraxjnl-2013-203534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mador MJ, Patel AN, Nadler J. Effects of pulmonary rehabilitation on activity levels in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2011;31(1):52–59. doi: 10.1097/HCR.0b013e3181ebf2ef [DOI] [PubMed] [Google Scholar]
- 41.Kanao K, Shiraishi M, Higashimoto Y, et al. Factors associated with the effect of pulmonary rehabilitation on physical activity in patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int. 2015;17(1):17–23. doi: 10.1111/ggi.12656 [DOI] [PubMed] [Google Scholar]
- 42.Mesquita R, Meijer K, Pitta F, et al. Changes in physical activity and sedentary behaviour following pulmonary rehabilitation in patients with COPD. Respir Med. 2017;126:122–129. doi: 10.1016/j.rmed.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 43.Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134(2):273–280. doi: 10.1378/chest.07-2655 [DOI] [PubMed] [Google Scholar]
- 44.Hayata A, Minakata Y, Matsunaga K, Nakanishi M, Yamamoto N. Differences in physical activity according to mMRC grade in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2203–2208. doi: 10.2147/COPD.S109694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesquita R, Spina G, Pitta F, et al. Physical activity patterns and clusters in 1001 patients with COPD. Chron Respir Dis. 2017;14(3):256–269. doi: 10.1177/1479972316687207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders T, Campbell N, Jason T, et al. Objectively measured steps/day in patients with chronic obstructive pulmonary disease: a systematic review and meta-Analysis. J Phys Act Health. 2016;13(11):1275–1283. doi: 10.1123/jpah.2016-0087 [DOI] [PubMed] [Google Scholar]
- 47.Miravitlles M, Cantoni J, Naberan K. Factors associated with a low level of physical activity in patients with chronic obstructive pulmonary disease. Lung. 2014;192(2):259–265. doi: 10.1007/s00408-014-9557-x [DOI] [PubMed] [Google Scholar]
- 48.Yu T, Ter Riet G, Puhan MA, Frei A. Physical activity and risk of comorbidities in patients with chronic obstructive pulmonary disease: a cohort study. NPJ Prim Care Respir Med. 2017;27(1):36. doi: 10.1038/s41533-017-0034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen HQ, Fan VS, Herting J, et al. Patients with COPD with higher levels of anxiety are more physically active. Chest. 2013;144(1):145–151. doi: 10.1378/chest.12-1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luzak A, Heier M, Thorand B, et al. Physical activity levels, duration pattern and adherence to WHO recommendations in German adults. PLoS One. 2017;12(2):e0172503. doi: 10.1371/journal.pone.0172503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177(7):743–751. doi: 10.1164/rccm.200707-1011OC [DOI] [PubMed] [Google Scholar]
- 52.Doi S, Tamura A, Minagawa T, Osaka A, Sata M. Classification of physical activity in patients with heart failure categorized as New York Heart Association class I or II. J Med Invest. 2020;67(1.2):124–133. doi: 10.2152/jmi.67.124 [DOI] [PubMed] [Google Scholar]
- 53.Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834–840. doi: 10.1016/j.rmed.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 54.Ichinose M, Minakata Y, Motegi T, et al. A non-interventional, cross-sectional study to evaluate factors relating to daily step counts and physical activity in Japanese patients with chronic obstructive pulmonary disease: STEP COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:3385–3396. doi: 10.2147/COPD.S277782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mesquita R, Nakken N, Janssen DJA, et al. Activity levels and exercise motivation in patients with COPD and their resident loved ones. Chest. 2017;151(5):1028–1038. doi: 10.1016/j.chest.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 56.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. Socio-environmental correlates of physical activity in patients with chronic obstructive pulmonary disease (COPD). Thorax. 2017;72(9):796–802. doi: 10.1136/thoraxjnl-2016-209209 [DOI] [PMC free article] [PubMed] [Google Scholar]