Abstract
Apolipoprotein E E4 (APOE4) is a risk factor for cognitive decline. A high blood vitamin C (VC) level reduces APOE4-associated risk of developing cognitive decline in women. In the present study, we aimed to examine the effects of functional variants of VC transporter genes expressed in the brain (SLC2A1, SLC2A3, and SLC23A2) on APOE4-associated risk of developing cognitive decline. This case–control study involved 393 Japanese subjects: 252 cognitively normal and 141 cognitively impaired individuals (87 mild cognitive impairment and 54 dementia). Database searches revealed that rs1279683 of SLC23A2, and rs710218 and rs841851 of SLC2A1 are functional variants that are significantly associated with the altered expression of the respective genes and genotyped as three single nucleotide variants (SNVs). When stratified by SNV genotype, we found a significant association between APOE4 and cognitive decline in minor allele carriers of rs1279683 (odds ratio [OR] 2.02, 95% CI, 1.05–3.87, p = 0.035) but not in the homozygote carriers of the major allele. Significant associations between APOE4 and cognitive decline were also observed in participants with major allele homozygotes of rs710218 (OR 2.35, 95% CI, 1.05–5.23, p = 0.037) and rs841851 (OR 3.2, 95% CI, 1.58–6.46, p = 0.0012), but not in minor allele carriers of the respective SNVs. In contrast, the three functional SNVs showed no significant effect on cognitive decline. Our results imply that functional SNVs of VC transporter genes can affect APOE4-associated risk of developing cognitive decline via altered VC levels in the brain.
Introduction
Cognitive decline in patients with mild cognitive impairment (MCI) and dementia is a complex trait resulting from the interaction between genetic and environmental factors. The E4 variant of apolipoprotein E (APOE4) is a well-known strong genetic risk factor for Alzheimer’s disease (AD) [1] and confers susceptibility to MCI [2]. In addition, genetic association studies performed using genome-wide association analysis and meta-analysis have shown that APOE ε4 (APOε4) confers susceptibility to vascular dementia and dementia with Lewy bodies [3–7]. APOE4 risk of cognitive decline may be modified by interaction with other factors; however, the role of risk modifiers or interacting factors is not well understood. In our previous longitudinal study, we found that high serum vitamin C (VC) levels during normal cognition reduce APOE4-associated risk of cognitive decline, especially in women [8].
VC is a well-known antioxidant in humans and other mammals [9]. VC is also required for diverse biological processes and is involved in several enzymatic reactions, including the synthesis of collagen and neurotransmitters as a cofactor [10]. Studies in animal models have revealed that VC is maintained at relatively high concentrations in the brain during systematic VC deficiency, suggesting the vital role of VC in the brain [11, 12]. Animal studies have showed that chronic VC deficiency causes cognitive dysfunction via mitochondrial dysfunction and increased oxidative stress [13, 14]. Acute administration of VC improved cognitive function in mouse models of AD and aging [15, 16].
As humans cannot biosynthesize VC, its intake through diet is essential for the maintenance of the normal VC levels. The ingested VC is transported from the digestive tract to the body. VC includes ascorbic acid (ASC; reduced form) and dehydroascorbic acid (DHA; oxidized form). ASC absorption occurs in the body via sodium-dependent VC transporter (SVCT), whereas that of DHA occurs via glucose transporters (GLUTs) [17]. A study using gene-knockout mice showed that SVCT1 (encoded by SLC23A1) plays an important role in intestinal absorption and renal reabsorption of ASC [18]. Additionally, the ASC level in neonatal mice with homozygous knockout of SVCT2 (encoded by SLC23A2) was undetectable or considerably reduced in various tissues including the brain, compared with that in heterozygous knockout or wild-type pups. Besides, compared with age-matched wild-type mice, newborn and adult mice with heterozygous knockout of SVCT2 have reduced ASC level in the brain and several other tissues [19, 20]. SVCTs and GLUTs transport VC to various organs. The transport of ASC into the brain is mediated by SVCT2 (encoded by SLC23A2) [21], whereas DHA uptake in the brain occurs via GLUT1 (encoded by SLC2A1) and GLUT3 (encoded by SLC2A3) [22, 23]. Depending on the ASC and DHA levels, the intracellular DHA-to-ASC recycling system is activated, and the amount of VC influx and efflux is regulated by SVCT2 and GLUT1/3 [17]. Thus, VC transporters are important in maintaining VC levels in the body, and in particular, SVCT2 and GLUT1/3 are thought to play important roles in the regulation of VC levels in the brain.
To investigate the relationship between cognitive decline and brain VC level in humans, we need to focus on the roles of brain-expressed VC transporters (SLC23A2, SLC2A1, and SLC2A3) in human cognitive function. We hypothesized that genetic variants of VC transporters (SLC23A2, SLC2A1, and SLC2A3) expressed in the brain could affect the risk of developing APOE4-associated cognitive decline. To test this hypothesis, we first selected functional variants of VC transporter genes from database searches, and then performed case–control association analysis to examine the effects of functional variants on the risk of developing APOE4-associated cognitive decline. In addition, we examined the relationship between functional variants of VC transporter genes and cognitive decline.
Subjects and methods
Study design and grouping by cognitive function
The Nakajima study is a population-based longitudinal cohort study that investigated cognitive decline in aged Japanese individuals. The study started in 2006 in Nakajima, Nanao District, Ishikawa Prefecture, Japan. Nanao City supported our study and provided resident information. The study included questionnaire about personal lifestyle including medical, educational, smoking, and alcohol history. As a part of the Nakajima study, this study involved individuals aged 65 years or older with normal cognitive function during the 2006–2008 baseline survey. The study design has been described in detail previously [8, 24–26]. We assessed participants’ cognitive status using the Mini-Mental State Examination (MMSE) [27] and Clinical Dementia Rating [28–30]. Diagnosis of dementia was based on the guidelines of Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R) [31]. MCI was diagnosed based on the general criteria for MCI by the International Working Group [32]. The diagnosis has been described in detail in a previous report [8]. The blood was collected with the consent of the participants, and serum VC level and APOE phenotype determination, and DNA extraction from blood were performed at SRL, Inc. (Tokyo, Japan).
Participants who cooperated with the baseline survey were followed-up, and their cognitive status was evaluated between 2014 and 2017. In the follow-up survey, we assigned participants with normal cognitive function to the normal group and participants with MCI or dementia to the cognitive decline group.
This study was approved by the medical ethics review board of Kanazawa University (Kanazawa, Japan) (approval numbers 257, 698, 721, 933, 1117, 1188, and 2186). All participants provided written informed consent by signing a form that described the purpose and procedures of the study, the voluntary nature of participation, the right to withdraw from the research without prejudice or penalty, and a guarantee of confidentiality and security of personal data.
Selection and genotyping of the SLC23A2, SLC2A1, and SLC2A3 variants
We hypothesized that genetic variants that alter the function of VC transporters in the brain affect the VC level and APOE4-associated risk of developing cognitive decline. To test this hypothesis, the identification of variants that could affect the function of transporter genes was necessary. Among the three genes, we assumed the following three genetic factors affecting the function of VC transport: gene-overlapping large-scale structural variations (SVs), SNVs and small insertions/deletions (indels) that cause changes in the protein-coding sequences (e.g., frameshift, nonsense, missense, and splice-altering variants), or SNVs and small indels that alter transcript abundance.
Because of the relatively small number of participants in this cohort, we set minor allele frequency (MAF) of 0.15 to detect functional variants with considerable effect on cognitive decline. Using “Genetic Power Calculator” (https://zzz.bwh.harvard.edu/gpc/), this study presented 82.9% power to detect common variants (MAF ≥ 15%) with genotype relative risk of 1.5, assuming 0.05 of type I error rate and 25% of MCI + dementia prevalence [33].
For screening large-scale SVs, we used the Database of Genomic Variants (DGV; http://dgv.tcag.ca/dgv/app/home). In the DGV, we searched for “DGV Gold Standard Variants”, a curated SV, which overlaps with SLC23A2, SLC2A1, and SLC2A3 with at least 15% frequency.
For screening SNVs and small indels that alter respective protein sequences of the three genes, we used the “Genome Variation” database at Japanese Multi Omics Reference Panel (jMorp; https://jmorp.megabank.tohoku.ac.jp/202001/) that provided allele frequencies for SNVs or indels with MAF ≥ 0.01% from the whole-genome sequencing analysis of approximately 8300 Japanese individuals. We selected the following variants with MAF ≥ 15%: frameshift, missense, and splice donor/acceptor variants in SLC23A2, SLC2A1, and SLC2A3, respectively.
For screening SNVs and small indels that alter the amount of transcription, we first obtained all variants with MAFs ≥ 15% in SLC23A2, SLC2A1, and SLC2A3 from the “Genome Variation” database at jMorp. Next, using the Regulome database (http://regulomedb.org/), we searched for variants with score 1 (score 1a – 1f) having evidence of expression quantitative trait loci (eQTLs) and transcriptional factor binding or DNase peak among the obtained variants with MAFs ≥ 15%. We then used the “GTEx” Portal database (https://gtexportal.org/home/) to determine whether the “Regulome DB score 1” variants were eQTLs, which could affect the expression of SLC23A2, SLC2A1, and SLC2A3. If multiple functional variants were found in a single gene from database searches, pairwise linkage disequilibrium values (r2-values) between the variants were evaluated using the LDlink (https://ldlink.nci.nih.gov/) and JPT (Japanese in Tokyo, Japan) databases.
Genotyping of the SLC23A2, SLC2A1, and SLC2A3 variants
Genotyping of functional variants in all the participants was performed by TaqMan SNP Genotyping Assays (Thermo Fisher Scientific, Waltham, MA, USA) using 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Each PCR mixture contained 4 ng of each DNA, 5 μL of TaqMan Genotyping Master Mix (Thermo Fisher Scientific), and 0.5 μL of TaqMan Genotyping Assay Mix (Thermo Fisher Scientific).
Statistical analyses
Chi-square test for categorical variables and Student’s t-test for continuous variables were performed to compare the characteristics of participants at baseline and follow-up survey between the normal cognition and cognitive decline (MCI or dementia) groups. To assess APOE4-associated risk of developing cognitive decline, a multivariate logistic regression analysis was performed using the significant variables (p < 0.1) obtained from the univariate analysis, except for MMSE at the follow-up survey. Because of sex differences in blood VC levels [34], VC values standardized by sex were used in the multivariate analysis. Variables that were statistically significant in the multivariate analysis were used as covariates in the subsequent analyses.
The p values in the Hardy–Weinberg equilibrium test were calculated based on the genotypic distribution of functional variants. To assess the effects of VC transporter genes on APOE4-associated risk of developing cognitive decline, participants were stratified based on the genotype of each variant into two strata: homozygote carriers of the major allele and carriers of the minor allele. In the stratified analysis by variant genotype, a multivariate logistic regression analysis was performed to examine whether there was a significant association between the APOE4 phenotype and cognitive decline (MCI or dementia) with adjustments for significant covariates. The p value, odds ratios (ORs), and 95% confidence interval (CI) of APOE4-associated risk of developing cognitive decline (MCI or dementia) were calculated for each stratum. Assuming a type I error rate of 0.05, statistical power to detect APOE4-associated risk of developing cognitive decline (MCI or dementia) in each stratified analysis was calculated based on the effect size of APOE4 and the frequency of APOE4 phenotype in the follow-up survey of this study. A multivariate logistic regression analysis was also performed to assess genetic association between functional variants of VC transporter genes and cognitive decline (MCI or dementia) in the following two genetic models: dominant model (minor allele) and log-additive model. The p value, OR, and 95% CI of the functional variants for cognitive decline (MCI or dementia) were calculated for each genetic model.
Statistical analyses were performed using R statistical environment (https://www.r-project.org/). The significance level was set at 5%.
Results
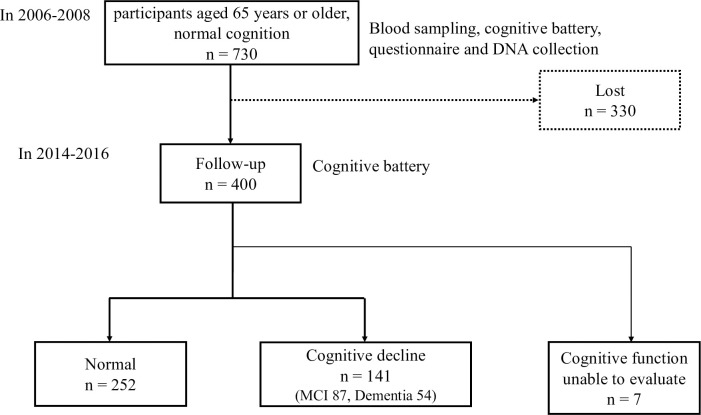
In the baseline survey, 923 individuals aged 65 years or older were included in the study. Among the 923 participants, by applying the previously mentioned diagnostic criteria, 730 were identified with normal cognitive function in the baseline study. In the follow-up survey, 400 participants were included, among which 252 were classified into the normal group and 141 in the cognitive decline group (MCI: 87 and dementia: 54), except for seven participants whose cognitive function could not be determined (Fig 1). The details of the characteristics of participants at baseline and follow-up surveys are summarized in Table 1. The participants in the cognitive decline group in follow-up survey were older than those in the baseline survey, and had a longer follow-up period, shorter education period, and lower MMSE score in both baseline and follow-up surveys, and a higher frequency of APOE4 phenotype than the normal cognition group in the follow-up survey. In female participants of the cognitive decline group, the blood VC level was significantly lower in the baseline survey than that in the follow-up survey. The multivariate logistic regression analysis adjusted for the effects of age, follow-up period, education period, MMSE points at baseline, frequencies of hypertension and hyperlipidemia, standardized VC levels, and sex showed that APOE4 is an independent risk factor for cognitive decline (MCI or dementia) (S1 Table: p = 0.027, OR = 1.91, and 95% CI, 1.10–3.33). In addition, the lost to follow-up participants (n = 330) were older, had lower MMSE scores at baseline, and had a shorter education period than the follow-up participants (n = 400) (S2 Table).
Fig 1. Design of the case–controlled study.
Baseline survey was conducted between 2006 and 2008. The participants aged 65 years or older with normal cognition were selected. In the baseline survey, participants took cognitive battery, underwent blood sampling, and responded to a questionnaire, including medical history. DNA was extracted from the blood. Between 2014 and 2016, follow-up survey was conducted, and the cognitive status was evaluated again. Based on the cognitive status, the participants were classified into normal group (normal cognition) or cognitive decline group (MCI or dementia).
Table 1. Characteristics of the participants with normal cognition (n = 393) at baseline (2006–2008) who presented with normal cognition (n = 252) or MCI/dementia (n = 141) in the follow-up survey (2014–2016).
| Normal cognition at follow-up survey | MCI or dementia at follow-up survey | p value | ||
|---|---|---|---|---|
| N (Male/Female) | 252 (77/175) | 141 (53/88) | 0.18 | |
| Baseline age | 71.4 ± 4.6 | 74.6 ± 5.6 | 5.91 × 10−8 * | |
| Follow-up period (yrs) | 8.12 ± 1.00 | 8.36 ± 1.09 | 0.030 * | |
| Education period (yrs) | 9.97 ± 2.33 | 9.18 ± 2.21 | 5.83 × 10−4 * | |
| Baseline Vitamin C (μg/mL) | ||||
| Male | 5.00 ± 3.03 | 5.39 ± 3.16 | 0.47 | |
| Female | 8.49 ± 3.27 | 7.25 ± 3.28 | 4.01 × 10−3 * | |
| Baseline MMSE (points) | 27.8 ± 1.9 | 26.7 ± 2.2 | 1.41 × 10−6 * | |
| Follow-up MMSE (points) | 28.0 ± 1.8 | 22.8 ± 5.4 | 6.52 × 10−21 * | |
| APOE E4 positive, N(%) | 43 (17.0%) | 40 (29.0%) | 0.014 * | |
| Hypertension, N(%) | 154 (61.1%) | 73 (51.7%) | 0.083 | |
| Hyperlipidemia, N(%) | 94 (37.3%) | 40 (28.4%) | 0.095 | |
| Diabetes mellitus, N(%) | 39 (15.5%) | 23 (16.3%) | 0.89 | |
| Alcohol, N(%) | 96 (38.1%) | 64 (45.3%) | 0.34 | |
| Smoking, N(%) | 56 (22.2%) | 39 (27.6%) | 0.39 | |
Statistical analysis was performed by chi-square test and Student’s t-test between the groups.
* p < 0.05.
In the case of SVs overlapped with SLC23A2, SLC2A1, and SLC2A3 in DGV, we found 1 “DGV Gold Standard” SV in SLC23A2, 0 SV in SLC2A13, and 7 SVs in SLC2A3, all of which did not meet the criterion of at least 15% frequency. In the search with the “Genome Variation” database at jMorp, we found no SNV and small indels altering the protein sequences of SLC23A2, SLC2A1, and SLC2A3, with MAF ≥ 15% in the Japanese population. Therefore, we focused on SNVs and small indels altering the transcript abundance of SLC23A2, SLC2A1, and SLC2A3 in the subsequent analyses.
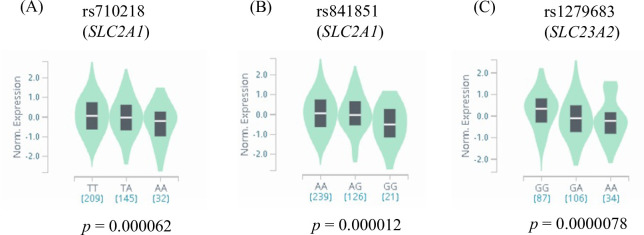
Among 226 SNVs and small indels with MAF ≥ 15% in SLC23A2 from the “Genome Variation” database at jMorp, only one SNV (rs1279683) was classified as a functional variant with the “Regulome DB” score 1d, which had evidence of eQTLs and functional genomic regions such as transcriptional factor binding sites. Using the “GTEx” Portal database, rs1279683 was proven to be an eQTL of SLC23A2 in the spleen tissue, where the most significant association between the genotype of rs1279683 and the SLC23A2 transcription level was observed among 49 human tissues in the “GTEx” Portal database (Fig 2). We also found that the transcription level of SLC23A2 in several brain tissues was significantly different according to the genotype of rs1279683 (data not shown). In SLC2A1, we found two SNVs (rs710218 and rs841851) as functional variants with the “Regulome DB” score 1b among 86 SNVs and small indels with MAF ≥ 15% in the “Genome Variation” database at jMorp. The two SNVs were not in high linkage disequilibrium with each other (pairwise r2 = 0.48) in the JPT database. The “GTEx” Portal database showed that rs710218 and rs841851 were eQTLs of SLC2A1 in the left ventricle tissue, with the lowest p value for the association between the genotype of each SNV and the SLC2A1 transcript level among the 49 human tissues (Fig 2). In the brain tissues, we confirmed that the transcription level of SLC2A1 in the frontal cortex and the nucleus accumbens tended to differ depending on the rs841851 and rs710218 genotypes, respectively, in the “GTEx” Portal database (data not shown). As for SLC2A3, we found six SNVs (rs4883461, rs933552, rs7975829, rs12313154, rs7309332, and rs11610602) as functional variants with the “Regulome DB” score 1f among 99 SNVs and small indels with MAF ≥ 15% in the “Genome Variation” database at jMorp. However, there was no evidence showing that the six SNVs were eQTLs of SLC2A3 in any tissues in the “GTEx” Portal database.
Fig 2. Genotype-specific differences in transcript levels of vitamin C transporter gene.
These data were cited from the GTEx Portal on 11/01/2020 (https://gtexportal.org/). In the “GTEx” Portal database, a significant correlation was observed between the genotype of each SNV and the transcript level of SLC23A2 or SLC2A1 in human tissues. The p value indicates a significant difference between gene expressions by genotype. The genotype-expression association data for tissues with the lowest p values are presented for the 49 human tissues in the “GTEx” Portal database. (A) The transcription level of SLC2A1 in the left ventricle by rs710218 genotype. (B) The transcription level of SLC2A1 in the left ventricle by rs841851 genotype. (C) The transcription level of SLC23A2 in the spleen by rs1279683 genotype.
The database searches revealed rs1279683 (SLC23A2), rs841851 (SLC2A1), and rs710218 (SLC2A1) as functional variants of VC transporters in the brain. These three SNVs were genotyped in 388 participants, excluding five participants in the normal group who had poor DNA quality. The genotype frequencies of the three SNVs are listed in S3 Table. No deviation was found in the genotypic distributions of the three SNVs from the Hardy–Weinberg equilibrium. In the three SNVs, a minor allele of each variant was associated with the reduced expression of SLC23A2 or SLC2A1 (Fig 2). Using the dominant model, we defined homozygote carriers of the major allele as the high expression group, and minor allele carriers as the low expression group in the subsequent analyses.
To examine the impacts of three functional variants on the risk of developing APOE4-associated cognitive decline (MCI or dementia), we assessed the APOE4-associated risk by stratification based on the genotype of each of the three variants (S4 Table), although power calculations showed limited statistical power (ranging from 32% to 55%) in each stratum. In the stratified genetic association analysis by SNV genotype (Table 2), APOE4-associated risk of developing cognitive decline was significant in the low expression group of rs1279683 (AG+AA) (p = 0.035, OR = 2.02, 95% CI, 1.05–3.87), whereas APOE4-associated risk was not significant in the high expression group (GG) (p = 0.71, OR = 1.21, 95% CI, 0.44–3.37). In contrast, both high expression groups of SLC2A1 (rs710218; TT, rs841851; AA) showed a significant APOE4-associated risk of developing cognitive decline (rs710218: p = 0.037, OR = 2.35, 95% CI, 1.05–5.23, rs841851: p = 0.0012, OR = 3.2, 95% CI, 1.58–6.46), whereas none of the low expression groups (rs710218: TA+AA, rs841851: AG+GG) showed a significant APOE4-associated risk of developing cognitive decline (rs710218: p = 0.49, OR = 1.3 and 95% CI, 0.62–2.75, rs841851: p = 0.39, OR = 0.67, 95% CI, 0.27–1.67) (Table 2).
Table 2. Odds ratios of APOE4 for developing MCI and dementia, stratified by genotype of VC transporter genes.
| Gene symbol | SNP ID | Genotype group | APOE4 | ||
|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | |||
| SLC2A1 | rs710218 | TT | 2.35 | 1.05–5.23 | 0.037 * |
| TA+AA | 1.3 | 0.62–2.75 | 0.49 | ||
| rs841851 | AA | 3.2 | 1.58–6.46 | 0.0012 * | |
| AG+GG | 0.67 | 0.27–1.67 | 0.39 | ||
| SLC23A2 | rs1279683 | GG | 1.21 | 0.44–3.37 | 0.71 |
| GA+AA | 2.02 | 1.05–3.87 | 0.035 * | ||
Odds ratio of APOE4 was calculated by multivariate logistic regression analysis, adjusted for effects of baseline age, follow-up period, baseline MMSE points, and sex.
* p < 0.05.
Table 3 shows the results of genetic association analysis of three functional variants of SLC2A1 and SLC23A2 with cognitive decline (MCI or dementia) in the dominant model and log-additive model. In the multivariate logistic regression analysis, none of the SNVs were significantly associated with cognitive decline in either models.
Table 3. Results of association analysis of three functional variants of SLC2A1 and SLC23A2 with cognitive decline (MCI or dementia).
| Gene symbol | SNP ID | Reference | Effect | Odds ratio | 95% CI | p value |
|---|---|---|---|---|---|---|
| Dominant model | ||||||
| SLC2A1 | rs710218 | TT | AA+TA | 1.51 | 0.95–2.38 | 0.081 |
| rs841851 | AA | GG+AG | 1.09 | 0.68–1.76 | 0.72 | |
| SLC23A2 | rs1279683 | GG | AA+GA | 0.93 | 0.58–1.51 | 0.77 |
| Log-additive model | ||||||
| SLC2A1 | rs710218 | T | A | 1.15 | 0.82–1.62 | 0.43 |
| rs841851 | A | G | 1.02 | 0.68–1.51 | 0.94 | |
| SLC23A2 | rs1279683 | G | A | 1.13 | 0.82–1.58 | 0.45 |
Odds ratio of the functional variant was calculated by multivariate logistic regression analysis, adjusted for the effects of APOE4 phenotype, baseline age, follow-up period, baseline MMSE points, and sex. The upper half shows the results of the dominant model and the lower half shows the results of the log-additive model.
Discussion
This case–control study involved the Japanese cohort population to determine the association between VC transporter genes (SLC23A2, SLC2A1, and SLC2A3) and APOE4-associated risk of developing cognitive decline (MCI or dementia). For this purpose, we found three functional SNVs associated with the changes in SLC2A1 and SLC23A2 expression by searching several publicly available databases, and analyzed the effects of these variants on APOE4-associated risk of developing cognitive decline. For the first time, we found subgroups in which APOE4 was not a significant risk factor for cognitive decline, stratified by the SNV genotype, although the statistical power was limited to detect the APOE4-associated risk in each stratified analysis. On the contrary, no association was found between functional SNVs and the risk of developing cognitive decline.
We hypothesized that genetic variants that alter the function of VC transporters in the brain also affect the VC level in the brain and APOE4-associated risk of developing cognitive decline. We applied strict criteria to identify gene variants that could affect gene functions and expression in the available databases. Similar to our study, methods for finding variants associated with the regulation of target gene expression have been reported using various databases [35]. We identified three functional SNVs in SLC23A2 and SLC2A1, with abundant evidence of eQTLs for the target genes. However, we could not find any functional SNVs of SLC2A3 using the present strict criteria. In addition, we found no large-scale SVs, SNVs, and small indels altering protein sequences in the target genes using 15% frequency threshold. The results of this database search do not imply that variants that alter the function and expression of SLC2A3 in humans are absent. Variant filtering based on population frequency was important for our cohort size, and future studies should examine low-frequency functional variants of the brain-expressed VC transporters in larger populations with well-powered replication strategies.
The effects of APOE4 on cognitive decline (MCI or dementia) were clearly different among subgroups stratified by genotypes rs1279683, rs710218, and rs841851. The results showed that the functional variants of SLC2A1 and SLC23A2 may affect or modify the risk of developing APOE4-associated cognitive decline. As for SLC23A2, in the stratified analysis by genotype, APOE4 had a significant risk of cognitive decline in the low expression group of rs1279683, but not in the high expression group. A previous study has shown that in transgenic mice expressing additional copies of SVCT2 (encoded by SLC23A2), the expression of SVCT2 mRNA and the VC levels in organs including the brain were increased accordingly, up to two-fold in the brain depending on the mRNA expression [36]. In addition, heterozygous knockout mice of SVCT2 (encoded by SLC23A2) showed VC deficiency in the brain [37], suggesting that the VC level in the brain changes according to SLC23A2 expression. Cognitive function was reduced in SVCT2 heterozygous knockout mice, and further reduced in APPSWE/PSEN1ΔE9 mice (animal AD model) crossed with SVCT2 heterozygous knockout mice [37]. These findings suggest that the VC level in the brain and cognitive function can be dependent on SLC23A2 expression, and cognitive function can be affected by the brain VC level, especially in animal models with a genetic risk of AD. Whether the rs1279683 genotype in SLC23A2 changes the VC level in the human brain is unclear, and differences in brain SLC23A2 expression might be the reason for susceptibility to cognitive decline in this study.
Contrary to SLC23A2, in the stratified analysis by genotype in SLC2A1, APOE4 was shown to have a significant risk of cognitive decline in the high expression groups of SLC2A1 (rs710218 and rs841851), but not in the low expression groups. SLC2A1 (also called GLUT1) is expressed in the blood–brain barrier (BBB) and transports DHA bidirectionally [38]; however, whether the DHA level in the brain increases or decreases depending on the amount of this transporter, is unclear. The effects of DHA on the brain tissue have not been fully elucidated. In addition, as SLC2A1 is a non-specific DHA transporter and transports not only DHA but also sugars [23], the present results might not be because of the DHA transport function of SLC2A1. To the best of our knowledge, no study has shown that SLC2A1 knockout in animal models alters the in vivo concentration and transport efficiency of DHA or ASC. Therefore, the effect of high expression of SLC2A1 on the amount of ASC or DHA in the brain tissues remains unknown. As the first step in interpreting these results, careful investigation of the relationship among SLC2A1, DHA, and cognitive function using genetically modified animal models is necessary.
Previous studies have reported various mechanisms underlying the effects of APOE4 on AD and other dementing disorders; APOE4 affects (1) Aβ aggregation and clearance, (2) tau phosphorylation and tangle formation, (3) lipid/cholesterol transport and clearance, (4) glucose and mitochondrial metabolism, (5) inflammation, (6) vascular function including BBB, (7) insulin and VEGF signaling, and (8) synaptic and neuronal function [39]. VC has been reported to affect cognitive function in animal models and humans. In a mouse model of AD and VC deficiency in the brain (transgenic APP/PSEN1 mice with heterozygous SVCT2 knockout), VC deficiency exacerbated cognitive performance and Aβ deposition [37]. In addition, higher VC could prevent Aβ deposition, oligomerization, BBB disruption, and mitochondrial oxidant stress in an AD mouse model [13, 14, 40, 41]. These data suggest that VC protects normal cognitive function, particularly in APOE ɛ4-positive individuals with Aβ deposition and oligomerization, BBB disruption, and mitochondrial oxidant stress. In humans, several prospective cohort studies have shown a significant association between higher blood VC level or antioxidant supplements, including VC, and less cognitive decline over time [42–49]. Another study reported that VC supplements in combination with NSAIDs reduced the cognitive decline in APOE4 carriers [49]. However, no randomized controlled studies have revealed the beneficial effects of VC supplementation on cognitive function [50, 51].
This study has some limitations. First, the number of participants was small as we set a high MAF (>15%) for the variants analyzed, which prevented us from testing the interaction effects in the multivariate analysis. Second, the cohort was single without replication. The third limitation is a low follow-up rate. Compared with the follow-up cases, lost cases were older, had lower MMSE scores, and had a shorter education period. The high nonparticipation and low follow-up rate suggest the potential for non-responder bias. Fourth, we did not identify the cause of dementia and MCI such as AD, dementia with Lewy bodies, and vascular dementia. Fifth, we did not include questionnaires on dietary nutrient intake, including VC. Sixth, we could not analyze data stratified by sex because of the small number of participants in this study. Additional large-scale studies in well-powered replication cohorts involving neuroimaging, neuropathology, and questions about dietary nutrients are required to understand the underlying mechanisms of the effects of functional SNVs of VC transporter genes on APOE4-associated risk of developing AD. In addition, as factors controlling the VC level in the body are thought to be complex, future analyses should take into account various factors including other genetic factors such as SLC23A1, which has been reported to be associated with blood VC levels in humans. These further studies will provide a deeper understanding of the relationship between VC and cognitive function in humans.
In conclusion, APOE4 significantly affects cognitive decline (MCI or dementia) in the Japanese population, and the effects are significantly different between subgroups stratified by genotype for each of the three SNVs (rs1279683, rs710218, and rs841851), which were eQTLs for the brain-expressed VC transporters (SLC23A2 or SLC2A1). Our results imply that the functional SNVs of VC transporters can affect APOE4-associated risk of developing cognitive decline via altered VC levels in the brain.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We wish to thank all the residents of Nakajima for their participation in this study. We thank Dr. Hiroaki Yoshikawa, Dr. Minako Hashii, Dr. Akiyoshi Morinaga, Dr. Chiho Ishida, Dr. Kazuya Takahashi, Dr. Akio Akagi, Dr. Taro Ozaki, Dr. Ai Shimizu, Dr. Miwako Asakawa, Dr. Hiroki Yamaguchi, Dr. Yoshihisa Ikeda, Dr. Shutaro Shibata, and Dr. Hidehiro Yokoji for their valuable help throughout this study. We thank the staff of the Department of Neurology of Kanazawa University Hospital, Ioh Hospital National Hospital Organization, Noto General Hospital, and Keiju Medical Center for their clinical and technical support. We also thank Ms. Yoko Iwauchi, a laboratory assistant in the Department of Bioinformatics and Genomics, for supporting the genotyping of the SNVs.
Data Availability
Due to ethical and legal restrictions, the raw data of this study (e.g., individual genotypes and sensitive personal information) are not freely available. The raw data will be made available upon reasonable request and with the permission and approval of the corresponding author (atajima@med.kanazawa-u.ac.jp), and the Medical Ethics Committee of Kanazawa University (rinri@adm.kanazawa-u.ac.jp). All other relevant data are within the paper and its supporting information files.
Funding Statement
This study was partially supported by a grant for the Hokuriku Innovation Cluster for Health Science (to MYa) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan [URL, https://www.mext.go.jp/en/], by a grant for Research and Development Grants for Dementia from the Japan Agency for Medical Research and Development [URL, https://www.amed.go.jp/en/] (JP18dk0207021 (to MYa, to MN-S), JP21dk0207047 (to MYa, to MN-S), JP20dk0207025 (to MYa), and JP21dk0207053 (to MN-S)), and by JSPS KAKEN [URL, https://www.jsps.go.jp/english/index.html] (19K22753) (to AT). There was no additional external funding received for this study. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278: 1349–1356. [PubMed] [Google Scholar]
- 2.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312: 2551–2561. doi: 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelborghs S, Dermaut B, Goeman J, Saerens J, Mariën P, Pickut BA, et al. Prospective Belgian study of neurodegenerative and vascular dementia: APOE genotype effects. J Neurol Neurosurg Psychiatry. 2003;74: 1148–1151. doi: 10.1136/jnnp.74.8.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerreiro R, Gibbons E, Tábuas-Pereira M, Kun-Rodrigues C, Santo GC, Bras J. Genetic architecture of common non-Alzheimer’s disease dementias. Neurobiol Dis. 2020;142: 104946. doi: 10.1016/j.nbd.2020.104946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skrobot OA, McKnight AJ, Passmore PA, Seripa D, Mecocci P, Panza F, et al. Validation study of vascular cognitive impairment genetics meta-analysis findings in an independent collaborative cohort. J Alzheimers Dis. 2016;53: 981–989. doi: 10.3233/JAD-150862 [DOI] [PubMed] [Google Scholar]
- 6.Sun JH, Tan L, Wang HF, Tan MS, Tan L, Li JQ, et al. Genetics of vascular dementia: systematic review and meta-analysis. J Alzheimers Dis. 2015;46: 611–629. doi: 10.3233/JAD-143102 [DOI] [PubMed] [Google Scholar]
- 7.Guerreiro R, Ross OA, K-Rodrigues C, Hernandez DG, Orme T, Eicher JD, et al. Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol. 2018;17: 64–74. doi: 10.1016/S1474-4422(17)30400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noguchi-Shinohara M, Abe C, Yuki-Nozaki S, Dohmoto C, Mori A, Hayashi K, et al. Higher blood vitamin C levels are associated with reduction of Apolipoprotein E E4-related risks of cognitive decline in women: the Nakajima study. J Alzheimers Dis. 2018;63: 1289–1297. doi: 10.3233/JAD-170971 [DOI] [PubMed] [Google Scholar]
- 9.Langlois PL, Lamontagne F. Vitamin C for the critically ill: Is the evidence strong enough? Nutrition. 2019;60: 185–190. doi: 10.1016/j.nut.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 10.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol. Med. 2009;46: 719–730. doi: 10.1016/j.freeradbiomed.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lykkesfeldt J, Trueba GP, Poulsen HE, Christen S. Vitamin C deficiency in weanling guinea pigs: differential expression of oxidative stress and DNA repair in liver and brain. Br J Nutr. 2007;98: 1116–1119. doi: 10.1017/s0007114507787457 [DOI] [PubMed] [Google Scholar]
- 12.Iwama M, Shimokado K, Maruyama N, Ishigami A. Time course of vitamin C distribution and absorption after oral administration in SMP30/GNL knockout mice. Nutrition. 2011;27:471–478. doi: 10.1016/j.nut.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 13.Dixit S, Fessel JP, Harrison FE. Mitochondrial dysfunction in the APP/PSEN1 mouse model of Alzheimer’s disease and a novel protective role for ascorbate. Free Radic Biol Med. 2017;112: 515–523. doi: 10.1016/j.freeradbiomed.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kook SY, Lee KM, Kim Y, Cha MY, Kang S, Baik SH, et al. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014;5: e1083. doi: 10.1038/cddis.2014.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delrobaei F, Fatemi I, Shamsizadeh A, Allahtavakoli. Ascorbic acid attenuates cognitive impairment and brain oxidative stress in ovariectomized mice. Pharmacol Rep. 2019;71:133–138. doi: 10.1016/j.pharep.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 16.Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav. 2009;93: 443–450. doi: 10.1016/j.pbb.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lykkesfeldt J, Tveden-Nyborg P. The Pharmacokinetics of Vitamin C. Nutrients. 2019;11:2412. doi: 10.3390/nu11102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120: 1069–1083. doi: 10.1172/JCI39191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8: 514–517. doi: 10.1038/0502-514 [DOI] [PubMed] [Google Scholar]
- 20.Harrison FE, Dawes SM, Meredith ME, Babaev VR, Li L, May JM. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic Biol Med. 2010;49: 821–829. doi: 10.1016/j.freeradbiomed.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angelow S, Haselbach M, Galla HJ. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003;988: 105–113. doi: 10.1016/s0006-8993(03)03350-x [DOI] [PubMed] [Google Scholar]
- 22.Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, et al. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003;278: 10128–10133. doi: 10.1074/jbc.M210686200 [DOI] [PubMed] [Google Scholar]
- 23.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997(25);272: 18982–18989. doi: 10.1074/jbc.272.30.18982 [DOI] [PubMed] [Google Scholar]
- 24.Noguchi-Shinohara M, Yuki S, Dohmoto C, Ikeda Y, Samuraki M, Iwasa K, et al. Consumption of green tea, but not black tea or coffee is associated with reduced risk of cognitive decline. PLoS ONE. 2014;9: e96013. doi: 10.1371/journal.pone.0096013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugano K, Yokogawa M, Yuki S, Dohmoto C, Yoshita M, Hamaguchi T, et al. Effect of cognitive and aerobic training intervention on older adults with mild or no cognitive impairment: a derivative study of the Nakajima Project. Dement Geriatr Cogn Dis Extra. 2012;2: 69–80. doi: 10.1159/000337224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noguchi-Shinohara M, Yuki S, Dohmoto C, Ikeda Y, Samuraki M, Iwasa K, et al. Differences in the prevalence of dementia and mild cognitive impairment and cognitive functions between early and delayed responders in a community-based study of the elderly. J Alzheimers Dis. 2013;37: 691–698. doi: 10.3233/JAD-130398 [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for clinician. J Psychiatr Res. 1975;12: 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 28.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140: 566–572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43: 2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 30.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58: 397–405. doi: 10.1001/archneur.58.3.397 [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed, revised. Washington DC: Amerian Psychiatric Association;1987. [Google Scholar]
- 32.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment-beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256: 240–246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya T, Nakaji S, Maeda T, Yamada M, Mimura M, Nakashima K, et al. Study design and baseline characteristics of a population-based prospective cohort study of dementia in Japan: the Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD). Environ Health Prev Med. 2020;25: 64. doi: 10.1186/s12199-020-00903-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob RA. Assessment of human vitamin C status. J Nutr. 1990;120 Suppl 11: 1480–1485. doi: 10.1093/jn/120.suppl_11.1480 [DOI] [PubMed] [Google Scholar]
- 35.Yoshihara H, Sugiura-Ogasawara M, Ozawa F, Kitaori T, Ozaki Y, Aoki K, et al. Polo-like kinase 4 and Stromal antigen 3 are not associated with recurrent pregnancy loss caused by embryonic aneuploidy. Hum Genome Var. 2020;7: 18. doi: 10.1038/s41439-020-0106-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison FE, Best JL, Meredith ME, Gamlin CR, Borza DB, May JM. Increased expression of SVCT2 in a new mouse model raises ascorbic acid in tissues and protects against paraquat-induced oxidative damage in lung. PLoS One. 2012;7: e35623. doi: 10.1371/journal.pone.0035623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixit S, Bernardo A, Walker JM, Kennard JA, Kim GY, Kessler ES, et al. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem Neurosci. 2015;6: 570–581. doi: 10.1021/cn500308h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May JM. Vitamin C transport and its role in the central nervous system. Subcell Biochem. 2012;56: 85–103. doi: 10.1007/978-94-007-2199-9_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safieh M, Korczyn AD, Michaelson DM. ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019;17: 64. doi: 10.1186/s12916-019-1299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubio JF, Bielsa FJS, Peinado JR, LaFerla FM, Llort LG, Prado MD, et al. Sex-dependent co-occurrence of hypoxia and β-amyloid plaques in hippocampus and entorhinal cortex is reversed by long-term treatment with ubiquinol and ascorbic acid in the 3 × Tg-AD mouse model of Alzheimer’s disease. Mol Cell Neurosci. 2018;92: 67–81. doi: 10.1016/j.mcn.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 41.Murakami K, Murata N, Ozawa Y, Kinoshita N, Irie K, Shirasawa T, et al. Vitamin C restores behavioral deficits and amyloid-β oligomerization without affecting plaque formation in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;26: 7–18. doi: 10.3233/JAD-2011-101971 [DOI] [PubMed] [Google Scholar]
- 42.Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996;312: 608–611. doi: 10.1136/bmj.312.7031.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45: 718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x [DOI] [PubMed] [Google Scholar]
- 44.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JCM, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287: 3223–3229. doi: 10.1001/jama.287.24.3223 [DOI] [PubMed] [Google Scholar]
- 45.Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am J Clin Nutr. 2003;77: 975–984. doi: 10.1093/ajcn/77.4.975 [DOI] [PubMed] [Google Scholar]
- 46.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61: 82–88. doi: 10.1001/archneur.61.1.82 [DOI] [PubMed] [Google Scholar]
- 47.Devore EE, Grodstein F, van Rooij FJA, Hofman A, Stampfer MJ, Witteman JCM, et al. Dietary antioxidants and long-term risk of dementia. Arch Neurol. 2010;67: 819–825. doi: 10.1001/archneurol.2010.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhari K, Sumien N, Johnson L, D’Agostino D, Edwards M, Paxton RJ, et al. Vitamin C supplementation, APOE4 genotype and cognitive functioning in a rural-dwelling cohort. J Nutr Health Aging. 2016;20: 841–844. doi: 10.1007/s12603-016-0705-2 [DOI] [PubMed] [Google Scholar]
- 49.Fotuhi M, Zandi PP, Hayden KM, Khachaturian AS, Szekely CA, Wengreen H, et al. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti-inflammatory drugs: the Cache County Study. Alzheimers Dement. 2008;4: 223–227. doi: 10.1016/j.jalz.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the women’s antioxidant and cardiovascular study. Circulation. 2009. Jun 2;119(21): 2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002. Jul 6;360(9326): 23–33. doi: 10.1016/S0140-6736(02)09328-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Due to ethical and legal restrictions, the raw data of this study (e.g., individual genotypes and sensitive personal information) are not freely available. The raw data will be made available upon reasonable request and with the permission and approval of the corresponding author (atajima@med.kanazawa-u.ac.jp), and the Medical Ethics Committee of Kanazawa University (rinri@adm.kanazawa-u.ac.jp). All other relevant data are within the paper and its supporting information files.