Abstract
Transcranial magnetic stimulation (TMS) reveals decreased efficacy of long-term potentiation-like (LTP-like) plasticity in Alzheimer’s disease (AD). However, it is not yet known whether LTP-like plasticity is also impaired in prodromal AD, or how abnormal TMS measures are related to established AD biomarkers. Here, we investigated the LTP-like response to intermittent theta-burst stimulation in 17 amyloid-positive participants with amnestic mild cognitive impairment (MCI) and 10 cognitively unimpaired controls. Our results showed a lack of LTP-like effect in MCI compared with controls that was unrelated to quantitative amyloid-beta burden on positron emission tomography. Surprisingly, greater LTP-like response was related to worse memory function in the MCI group, highlighting the complex role of neuroplasticity in the prodromal stages of AD. Overall, our results demonstrate abnormal LTP-like plasticity using intermittent theta-burst stimulation assessment in amyloid-positive participants with MCI. These findings support the potential for development of TMS measures as prognostic markers or therapeutic targets in early-stage symptomatic AD.
Keywords: Transcranial magnetic stimulation, Mild cognitive impairment, Alzheimer’s disease, Plasticity, Amyloid
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia, is commonly preceded by a prodromal stage of amnestic mild cognitive impairment (MCI; “2017 Alzheimer’s Disease Facts and Figures,” 2017; Vos et al., 2015). The progressive accumulation of amyloid-beta (Aβ), tau, and neurodegeneration and can now be measured in vivo and used for clinical diagnosis in the early clinical and preclinical stages of AD (Jack et al., 2018). Alterations in cortical activity are associated with this neurodegenerative process, yet we lack an understanding of when neurophysiologic changes first occur and whether they are adaptive or maladaptive. Declines in long-term potentiation (LTP), enhancement of long-term depression (LTD), and synaptic dysfunction have been strongly implicated in synaptic pathology in AD (Mota et al., 2014; Selkoe, 2002). Animal models of AD suggest increases in oligomeric Aβ, which predates fibrillary Aβ deposition, alters synaptic plasticity years before cell death occurs (Shankar et al., 2007), and that toxic species of tau may also inhibit LTP and memory formation (Tracy et al., 2016). Behavioral and imaging studies in humans may document the consequences of altered plasticity (Maass et al., 2015; Sperling, 2007), but cannot directly characterize the efficacy of neuroplastic mechanisms.
Transcranial magnetic stimulation (TMS) has emerged as a promising physiologic tool to assess mechanisms of plasticity in humans in vivo. When TMS is applied over the primary motor cortex (M1), the resultant hand muscle activation can be measured using electromyography (EMG) and provides an index of cortico-motor excitability in the form of the average amplitude of motor evoked potentials (MEPs). Repetitive TMS (rTMS) can induce changes in cortical activity and metabolism that outlast the stimulation, in a manner that resembles the mechanisms of neural plasticity (Hoogendam et al., 2010). Intermittent theta-burst simulation (iTBS) is a protocol of ultra-high frequency patterned rTMS which may capture the efficacy of NMDA-receptor–dependent plasticity (Huang et al., 2007). In healthy adults, a 3-minute application of iTBS to M1 has been shown to increase MEP amplitude for up to 40 minutes, which is thought to represent an LTP-like response (Wischnewski and Schutter, 2015). Using this TMS-iTBS approach in M1, a number of prior studies have shown reduced LTP-like plasticity in patients with AD compared with healthy older adults (Di Lorenzo et al., 2016; Koch et al., 2012). Altered LTP-like plasticity also shows prognostic value for AD (Motta et al., 2018) and is associated with faster clinical progression (Koch et al., 2015). However, it has not yet been shown whether the efficacy of neuroplastic mechanisms is impaired in the earlier clinical stage of MCI, or whether LTP-like plasticity may be related to other AD disease markers in MCI, such as Aβ burden or cognitive dysfunction.
To address these knowledge gaps, the present study assessed TMS-iTBS measures of LTP-like plasticity in participants with amnestic MCI who were amyloid-positive and demographically similar to cognitively unimpaired healthy controls (HCs). We predicted that, compared with HCs, the participants with MCI would show reduced modulation of cortico-motor excitability after iTBS. In addition, we compared LTP-like plasticity measures with Aβ burden assessed with [18F]florbetapir positron emission tomography (PET) in the MCI group and with measures of memory and executive function in both groups. We predicted that reduced cortico-motor plasticity would be related to higher Aβ burden and worse performance on memory tests in the MCI group.
2. Materials and methods
2.1. Participants
This prospective cross-sectional study recruited adult study participants aged 50–90 with a diagnosis of amnestic MCI and a demographically similar cohort of HCs. All experimental procedures took place between 2016 and 2019 at the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center (BIDMC). Patients with MCI were recruited either by direct referral from a physician in the Cognitive Neurology Unit at BIDMC or through community self-referrals. Clinical records of potential participants were first prescreened by a study coordinator for eligibility before entering the study for initial screening.
Exclusion criteria common to both groups consisted of unstable medical conditions, history of neuropsychiatric illness, contraindications to magnetic resonance imaging (MRI) or TMS, or premorbid intelligence quotient below 80 as measured by the age-adjusted Wechsler Test of Adult Reading (Bright and van der Linde, 2020).
Thirty-two participants with a presumptive diagnosis of amnestic MCI were enrolled. Twenty-six patients met the clinical criteria of a diagnosis of amnestic MCI (per DSM-V and Key Symposium criteria; Sachs-Ericsson and Blazer, 2015; Winblad et al., 2004), a Clinical Dementia Rating global score of 0.5, and Mini-Mental Status Examination score ≥21. Two patients were excluded because of difficulty tolerating iTBS, and three withdrew for personal reasons. Four participants who completed all measures were subsequently excluded from the present analysis for negative amyloid status. Of the 17 Aβ+ MCI participants included in the present study, 16 had their amyloid status confirmed on [18F]-florbetapir PET and one on lumbar puncture-based assessment of cerebrospinal fluid (CSF).
Fourteen HCs with normal cognition (Mini-Mental Status Examination ≥27) were enrolled. A history of diabetes was considered an exclusion criterion for the HC group since prior work from our group has shown abnormal iTBS aftereffects in participants with type-2 diabetes (Fried et al., 2017a). Three participants were excluded for not meeting general study criteria, and one withdrew for personal reasons.
The final cohort consisted of 10 HCs and 17 participants with MCI. Assuming an α = 0.05, this sample provided 80% power to observe at least a large effect size (Cohen’s d ≥ 1.16) for the between-groups measurements of LTP-like response. In terms of within-group correlations between LTP-like response and other measures, there was 80% power to observe at least a large effect (|ρ| ≥ 0.59) in the MCI group and at least a very-large effect (|ρ| ≥ 0.71) in the HC group. All participants underwent equivalent measures including a standardized neurological examination, medical history review, neuropsychological screening, structural MRI scan, and a TMS-iTBS visit.
2.2. Neuropsychological testing
A comprehensive cognitive testing battery was performed by a psychometrist under the supervision of a senior level neuropsychologist. Neuropsychological tests and inventories were drawn from the National Alzheimer’s Coordination Center’s Uniform Data Set version 3.0 (Weintraub et al., 2018) including the Geriatric Depression Scale (GDS, 15-item), Functional Activities Questionnaire (FAQ, 30-item), Trail Making Test Part A (TMT-A, time in seconds), TMT Part B (TMT-B, time in seconds), Craft 21 Story Recall: Immediate verbatim (Story Recall Immediate, 44-item), Craft 21 Story Recall: Delayed verbatim (Story Recall Delayed, 44-item), Benson Complex Figure Recall (Figure Recall, 17-item), Number Span Test Forwards (NST-F, longest span), Number Span Test Backwards (NST-B, longest span), Semantic Fluency (# animals named in 1 minute), and Phonemic Fluency (# L-words named in 1 minute). The Alzheimer’s Disease Assessment Scale–cognitive subscale was also administered (ADAS-Cog Total, 70-item); subscores were obtained for the word list immediate recall test (ADAS-Cog Recall, 10-item) and the delayed recognition test (ADAS-Cog Recognition, 12-item; Graham et al., 2004). Additional assessments included the Digit Symbol Substitution Test (DSST, # correct in 1.5 minutes; Jaeger, 2018) and the Rey Auditory Verbal Learning Test (RAVLT). RAVLT subscores were obtained at 20-minute delayed recall (RAVLT Recall, % correct) and 20-minute delayed recognition (RAVLT Recognition, % correct; Gale et al., 2007). To equalize the scale across measures and facilitate statistical analysis, raw scores for each neuropsychological measure were transformed into z-scores using published normative values (Amariglio et al., 2012; Gale et al., 2007; Graham et al., 2004; Weintraub et al., 2018, 2009). Scores on the TMT-A, TMT-B, ADAS-Cog Total, ADAS-Cog Recall, and ADAS-Cog Recognition were inverted so that higher scores reflected better performance across all measures.
To provide domain-specific measures of cognitive functioning to relate to our TMS-iTBS measures, z-scores from individual tests were averaged together to create composite scores within cognitive domains of learning and memory (Story Recall Immediate, Story Recall Delayed, RAVLT Recall, RAVLT Recognition, Figure Recall, ADAS-Cog Recall, ADAS-Cog Recognition) and executive function (TMT-A, TMT-B, NST-F, NST-B, DSST, Semantic Fluency, and Phonemic Fluency). This approach, modeled after one from the Alzheimer’s disease neuroimaging initiative (Crane et al., 2012; Gibbons et al., 2012) and used in previous studies by our group relating cognition to cortical atrophy (Buss et al., 2018) and restingstate electroencephalography (EEG) oscillatory power (Benwell et al., 2020) in participants with early-AD, was adopted to allow iTBS aftereffects to be related to broad categories of cognitive processing rather than specific tests.
2.3. Saliva-based genotyping
Saliva was obtained and used to obtain genotyping of apolipoprotein (APOE) and brain-derived neurotrophic factor (BDNF) in participants who consented to genetic testing. APOE was included since previous literature has described differences in iTBS aftereffects in patients with AD with an APOE4 allele (Koch et al., 2017). BDNF was included because it is known to alter the aftereffects of iTBS (Cheeran et al., 2008). The APOE genotype was determined as the presence of at least one E4 allele (APOE4 status; dichotomous). The BDNF genotype was determined as the presence of at least one Met allele (BDNF-Met status; dichotomous). Genotyping results were available from 16 participants with MCI and 8 HC participants.
2.4. Amyloid biomarker determination
All participants with MCI included in the present analysis were categorized as Aβ+ using either CSF or PET amyloid biomarkers (Palmqvist et al., 2015). CSF was obtained from one participant by lumbar puncture and used to determine evidence of cortical Aβ deposition based on a clinical CSF cutoff level of Aβ42 < 600 (Niemantsverdriet et al., 2017). Amyloid PET scans were obtained from the remaining 16 participants with MCI on BIDMC’s Siemens Biograph 64mct multidetector helical PET-CT scanner (Siemens Healthcare). A 10-minute emission scan, acquired with a 128 × 128 matrix (zoom × 2), was obtained 50 minutes after intravenous injection of 10 mCi (370MBq) of [18F]florbetapir (Doraiswamy et al., 2012). A qualitative read for the presence of cortical brain amyloid was performed by a board-certified nuclear medicine specialist to determine Aβ status. The nuclear medicine specialist was blinded to TMS results.
A continuous quantitative measure representing total Aβ burden was assessed from the 16 participants with available amyloid PET scans using MIMneuro software, version 6.8.2 (MIM Software Inc., Cleveland, OH). Default affine registration of images was accepted. Using cerebellar uptake as the standard, amyloid z-scores for the precuneus, lateral temporal lobe, inferior medial frontal gyrus, anterior cingulate gyrus, superior parietal lobule, and posterior cingulate gyrus were calculated based on comparison to a database of normative values (Clark et al., 2012). Z-scores from each individual brain region were averaged to create a global Aβ burden score.
2.5. Structural MRI
Structural MRI was acquired for neuronavigation during TMS. All participants underwent a T1-weighted anatomical MRI scan on a 3T scanner (GE Healthcare, Ltd., UK) using a 3D inversion recovery spoiled gradient echo sequence: 162 axial-oriented slices for whole-brain coverage; 240-mm field-of-view; 0.937-mm × 0.937-mm × 1-mm native resolution; flip angle = 12°; TE/TR ≥ 3.2/8.2 ms; TI = 450; duration ≥256 s (4 m 16 s).
2.6. TMS-EMG
All TMS procedures conformed to consensus guidelines for the safe application of TMS endorsed by the International Federation of Clinical Neurophysiology (Rossini et al., 2015). A Navigated Brain Stimulation system (Nexstim Plc, Finland) used each participant’s T1-weighted anatomical MRI for TMS targeting. Single-pulse TMS was administered using a figure-of-eight coil (Nexstim), inducing a monophasic (posterior-anterior) current in the brain. The motor cortex stimulation site was determined as the location of maximal activation of the right first dorsal interosseous in response to stimulation. MEPs were recorded from the first dorsal interosseous using surface EMG; resting motor threshold (RMT) was measured as the minimum stimulation intensity required to evoke an MEP on at least 5 out of 10 single-pulse TMS trials. A MagPro figure-of-eight coil (MagVenture), inducing a biphasic (anterior-posterior–posterior-anterior) current in the brain, was used to assess the active motor threshold and administer iTBS.
Cortico-motor excitability and mechanisms of plasticity were assessed using an established TMS-iTBS protocol (Fried et al., 2017a). Cortico-motor excitability was assessed before and after iTBS in blocks of 35 pulses at an intensity of 120% RMT. Three blocks were collected before iTBS, and all 105 MEPs were averaged together to derive a pre-iTBS measure cortico-motor excitability (Pre-iTBS). Additional blocks were collected at 5 minutes (T5), 10 minutes (T10), 20 minutes (T20), and 30 minutes (T30) after iTBS. To minimize the effect of outliers, individual MEPs with peak-to-peak amplitudes >2.5 SD from the mean of each block were excluded from analysis (Fried et al., 2017a). MEP peak-to-peak amplitudes (μV) were measured to assess the effect of iTBS on cortico-motor excitability within each group.
2.7. Statistical methods
Statistical analyses were performed using JMP Pro 13.0 (SAS Institute Inc., Cary, NC) and Stata 14.2 (StataCorp, College Station, TX). Significance was determined using a two-tailed 95% confidence interval (α < 0.05). Nonparametric tests and/or log10 transformations were applied when the assumptions of normality were not met. All p-values shown are uncorrected unless otherwise declared. When appropriate, individual p-values were subjected to a 5% false discovery rate (FDR) threshold using the Benjamini-Hochberg method.
To test group differences in demographic characteristics, neurocognitive status, and baseline physiologic measures, data were compared using independent-samples t-tests or Mann-Whitney U tests for continuous variables and Fisher’s exact tests for categorical variables.
To test the effect of iTBS on cortico-motor excitability within each group, MEP amplitudes (μV) were log10-transformed and entered as dependent variables into separate random-effects linear models for each group. Each model included the main fixed effect of time (Pre-iTBS, T5, T10, T20, T30) and accounted for interindividual variance in repeated measures using crossed-random effects for subject and subject*time. Post-test diagnostics revealed the residuals for the MCI group were still not normally distributed; thus, the analysis was rerun using a nonparametric Skilling–Mack test. Post hoc comparisons of each post-iTBS time point to Pre-iTBS MEP amplitudes were performed with paired-samples t-tests.
To compare the iTBS-induced modulation of MEP amplitudes between groups, MEP amplitudes at each post-iTBS time point were expressed as the ratio of Pre-iTBS MEP amplitude, log10-transformed, and entered as the dependent variable into a mixed-effects linear model. The model included the between-groups factor of diagnosis (HC, MCI) and the within-group factor of time (T5, T10, T20, T30) in a full-factorial design. Planned post hoc between-groups comparisons of each time point were performed using independent samples t-tests. Levene tests demonstrated variances were equivalent between groups (p-values ≥ 0.143), so pooled variance tests were used. For the time point log10 (T5/Pre-iTBS), which showed the biggest group difference (see Section Primary Analysis), an exploratory post hoc nested regression approach was used to investigate how adding demographic and clinical covariates to the model changed the regression coefficient of diagnosis.
Secondary analyses were performed to investigate whether the difference between groups in LTP-like plasticity was related to global Aβ burden and cognitive function. The T5 time point was chosen a posterior because it represented the greatest difference between groups (see Section Primary Analysis). This is further supported by the prior literature showing above-average test-retest reproducibility of iTBS aftereffects at T5 in mild AD (Fried et al., 2017b). Simple linear regression (Pearson correlation) analyses were used to test the relationships of global Aβ burden with log10 (T5/Pre-iTBS) in the MCI group and between log10 (T5/Pre-iTBS) and the composite learning and memory and executive function scores within both groups.
2.8. Protocol approvals and patient consents
The present study was performed on human participants. All study participants provided written informed consent on enrollment consistent with the Declaration of Helsinki. All forms and procedures were approved by the BIDMC Institutional Review Board.
3. Results
TMT-A data were not available in one participant with MCI; TMT-B data were not available in 3 participants with MCI. Data at the T30 time point were missing in one participant with MCI because of time constraints; that participant’s partial data were included in the primary mixed-effects models (see Section Statistical Methods).
3.1. Primary analysis
Table 1 shows demographic information and clinical characteristics of the MCI and HC groups. The MCI group had higher scores on ADAS-Cog Total (p < 0.001) and GDS (p = 0.024) than HCs. Otherwise, the 2 groups did not differ in any of the demographic characteristics (p-values ≥ 0.101). There were no between-group differences in RMT, active motor threshold, or Pre-iTBS MEP amplitude (p-values ≥ 0.152), indicating equivalent stimulation parameters between HC and MCI.
Table 1:
Demographics and Clinical Characteristics
| HC (n=10) | MCI (n=17) | 2-tailed p-value | |
|---|---|---|---|
| Age (mean ± SD) b | 66.7 ± 7.3 | 69.6 ± 9.1 | 0.327 |
| Female (%) c | 60.0 % | 47.1 % | 0.695 |
| Right Handedness (%) c | 90% | 88.2% | 1.000 |
| Years of Education (mean ± SD) a | 16.2 ± 2.5 | 16.9± 2.2 | 0.447 |
| IQ† (mean ± SD)b | 117.1 ± 12.4 | 118.6 ± 6.3 | 0.578 |
| ADAS-Cog Total (mean ± SD) b | 3.3 ± 1.8 | 11.9 ± 3.4 | <0.001 |
| GDS (mean ± SD) b | 1.3 ± 2.9 | 2.3 ± 2.1 | 0.024 |
| Diabetes (%) c | 0% | 11.8% | 0.516 |
| Hypertension (%) c | 20.0% | 35.3% | 0.666 |
| Hyperlipidemia (%) c | 40.0% | 76.5% | 0.101 |
| Amyloid z-score (mean ± SD) | n/a | 8.1 ± 3.2 | n/a |
| APOE4 allele ≥ 1 (%) c | 62.5% | 81.2% | 0.362 |
| BDNF-Met allele ≥ 1 (%) c | 25% | 25% | 1.000 |
| Monophasic RMT (mean ± SD) a | 57.2 ± 10.2 | 55.2 ± 9.2 | 0.623 |
| Biphasic AMT (mean ± SD) a | 40.5 ± 7.7 | 38.5 ± 6.5 | 0.493 |
| Pre-iTBS MEP (μV) (mean ± SD) b | 899.4 ± 676.6 | 1426.3 ± 1007.2 | 0.152 |
Demographic characteristics of HC and MCI participants are shown. MCI participants had impaired global cognition (ADAS-Cog) compared with controls.
Based on age-adjusted WTAR
t-test
Mann-Whitney U test
Fisher’s exact test
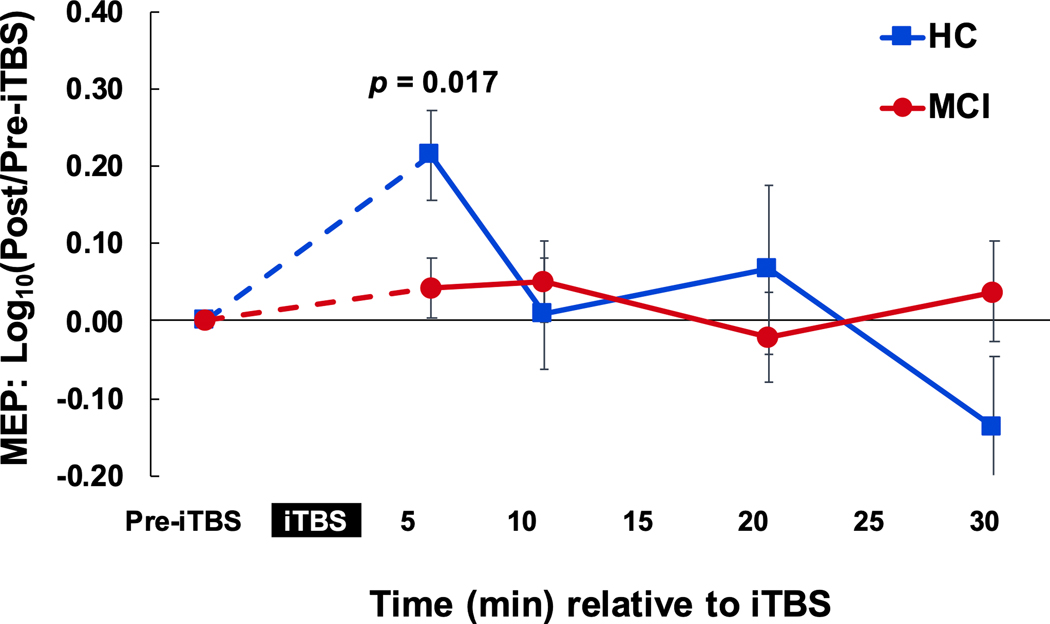
The random-effects linear model showed a significant main effect of time on MEP amplitudes in the HC group (F4,36 = 4.13, p = 0.007), but not in the MCI group (X2r = 2.52, p = 0.641). Post hoc comparisons of each post-iTBS time point to Pre-iTBS revealed that for HCs, T5 was different from Pre-iTBS (p = 0.005; Fig. 1) and remained so after FDR correction. These results indicate that iTBS induced facilitation of cortico-spinal excitability in HCs, but not MCI.
Fig. 1.
iTBS aftereffects on cortico-motor excitability. Lack of LTP-like plasticity response after iTBS in MCI, compared with demographically similar HCs. There was a significant effect of iTBS on MEP amplitudes in the HC group which was not present in the MCI group. Pairwise comparisons show that this difference was driven by a significant increase in cortico-motor excitability between at 5 minutes after iTBS in the HC group. Abbreviations: HC, healthy control; iTBS, intermittent theta-burst simulation; LTP, long-term potentiation; MCI, mild cognitive impairment; MEP, motor evoked potential.
For the between-group comparison of iTBS aftereffects, the mixed-effects linear model revealed a significant effect of time (F3,74 = 3.50, p = 0.020) and diagnosis*time interaction (F3,74 = 3.49, p = 0.020), but no main effect of diagnosis (F3,25 = 0.03, p-value = 0.854), indicating the group differences in iTBS aftereffects varied across time. Post hoc t-tests revealed the diagnosis*time interaction was driven by iTBS aftereffects at T5 that were higher in HCs than MCI (p = 0.017). This effect did not remain significant after FDR correction (p = 0.068).
3.1.1. Nested regression of covariates
Table 2 shows the results of the nested regression of covariates. Pre-iTBS MEP amplitude was the only factor that significantly predicted log10 (T5/Pre-iTBS) when added to the model (p = 0.029). Furthermore, adding Pre-iTBS MEP decreased the β-coefficient of diagnosis by >20%, suggesting that group variation in cortical excitability (i.e., nonsignificantly higher excitability in the MCI group) may account for some of the observed between-group difference in iTBS aftereffects. With respect to the genotyping results, neither APOE-ε4 status nor BDNF-Met status modified the effect of diagnosis (%Δ in β-coefficient <3%). Although baseline differences in depression were present between groups, GDS was not found to be a significant predictor of log10 (T5/Pre-iTBS), and the p-value of diagnosis remained significant after inclusion of GDS in the model.
Table 2:
Effect of covariates on the relationship between Diagnosis and Log10(T5/Pre-iTBS)
| %Δβdiagnosis | ΔPdiagnosis | ΔR2model | P covariate | |
|---|---|---|---|---|
| Diagnosis plus Pre-iTBS MEP | −24.62 | 0.039 | 0.146 | 0.029 |
| Diagnosis plus Age | −11.56 | 0.012 | 0.094 | 0.084 |
| Diagnosis plus Diabetes | −8.85 | 0.015 | 0.033 | 0.319 |
| Diagnosis plus Hyperlipidemia | −8.75 | 0.026 | 0.010 | 0.579 |
| Diagnosis plus Biphasic AMT | −7.31 | 0.009 | 0.051 | 0.210 |
| Diagnosis plus IQ† | −3.68 | 0.004 | 0.041 | 0.266 |
| Diagnosis plus Monophasic RMT | −2.86 | 0.005 | 0.016 | 0.489 |
| Diagnosis plus Years of Education | −2.18 | 0.006 | 0.004 | 0.736 |
| Diagnosis plus BDNF-Met status | 0.00 | 0.005 | 0.004 | 0.765 |
| Diagnosis plus GDS | 0.14 | 0.005 | 0.000 | 0.987 |
| Diagnosis plus Right Handedness | 1.51 | −0.003 | 0.064 | 0.159 |
| Diagnosis plus APOE4 status | 2.75 | 2.75 | 0.003 | 0.790 |
| Diagnosis plus Female | 5.03 | −0.003 | 0.033 | 0.318 |
| Diagnosis plus Hypertension | 9.71 | −0.008 | 0.073 | 0.133 |
A nested regression was used to test the effects of clinical and demographic variables on the between-group difference in LTP-like plasticity. Covariates are listed in order of effect size. Negative %Δβ values represent a weakening of the association between Diagnosis and Log10(T5/Pre-iTBS), while positive values represent a strengthening of the association.
Based on age-adjusted Wechsler Test of Adult Reading; GDS = Geriatric Depression Scale; AMT = active motor threshold; RMT = resting motor threshold; MEP = motor evoked potential
3.2. Amyloid imaging results
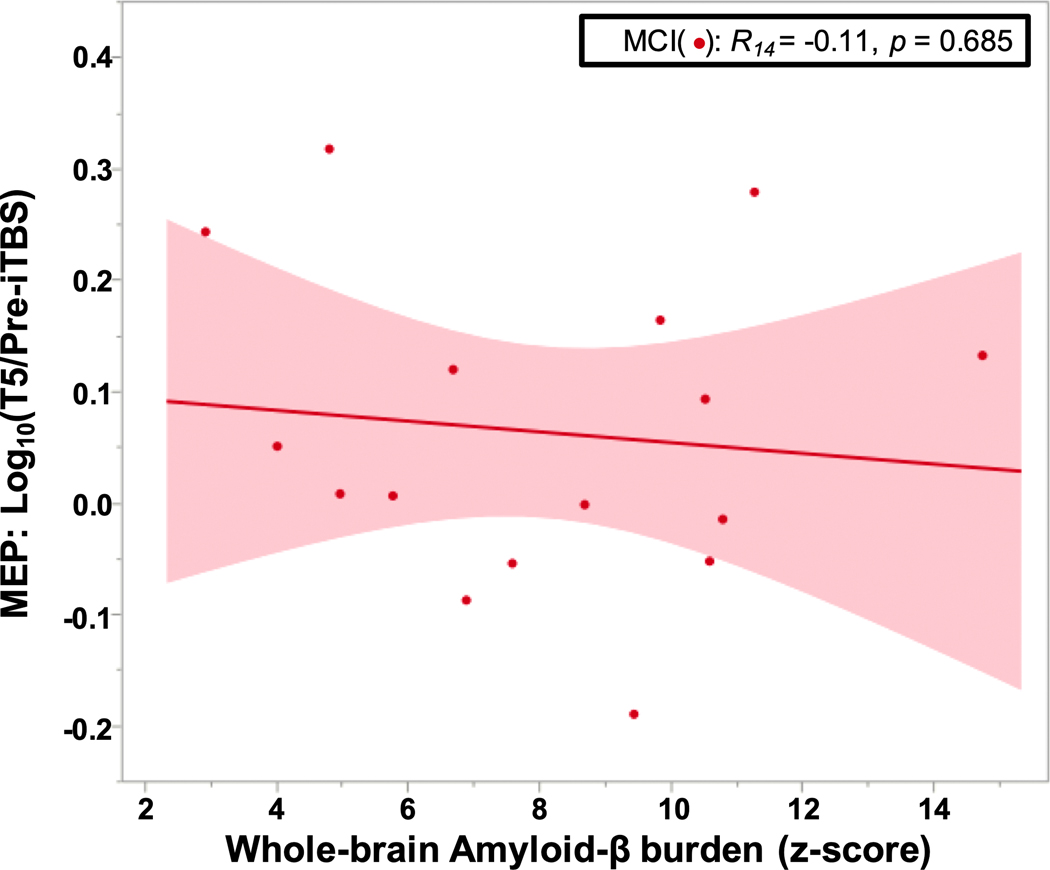
Within the MCI group, the linear regression showed no significant relationship between the global Aβ burden z-score and log10 (T5/Pre-iTBS) (p = 0.685; Fig. 2).
Fig. 2.
No association of amyloid burden with LTP-like plasticity in amyloid-positive MCI. No relationship between the global PET amyloid z-score and log ratio of MEP amplitudes pre- and post-iTBS in MCI. Abbreviations: iTBS, intermittent theta-burst simulation; LTP, long-term potentiation; MCI, mild cognitive impairment; MEP, motor evoked potential; PET, positron emission tomography.
3.3. Neuropsychological testing results
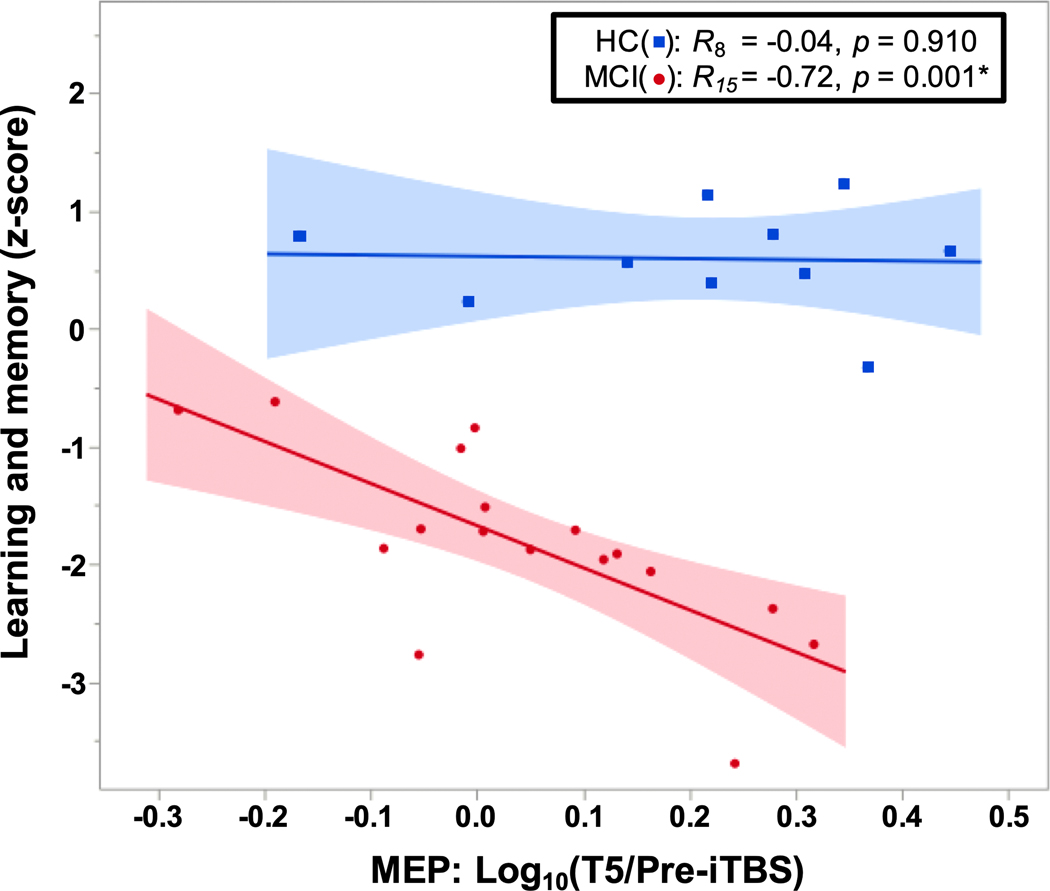
In MCI, linear regression analyses showed a relationship between log10 (T5/Pre-iTBS) and the learning and memory score (p = 0.001; Fig. 3), but not the executive function score (p = 0.233). By comparison, log10 (T5/Pre-iTBS) was not significantly associated with either composite score in the HC group (p-values ≥ 0.763). After FDR correction, the association with learning and memory remained significant in the MCI group. These results indicate that the severity of memory impairments in MCI is inversely related to the degree of LTP-like facilitation.
Fig. 3.
Relationship between LTP-like plasticity and memory. Relationship between memory and LTP-like response at T5. There was a significant association between greater LTP-like response and lower learning and memory composite score in the MCI group, but not in the HC group. Abbreviations: HC, healthy control; LTP, long-term potentiation; MCI, mild cognitive impairment.
To directly compare our results with the prior literature (Di Lorenzo et al., 2019), we also tested the relationships between log10 (T5/Pre-iTBS) and the RAVLT recall scores. Our results (see Supplementary Material and Figure S1) showed a significant negative correlation similar to what was observed between log10 (T5/Pre-iTBS) and the learning and memory composite score.
4. Discussion
Our results demonstrate abnormal LTP-like plasticity using iTBS assessment in participants with MCI. The findings extend results from prior studies, which have shown a decrease in LTP-like plasticity in AD (Di Lorenzo et al., 2016), to the earlier clinical stage of amyloid-positive amnestic MCI. We found no relationship between LTP-like plasticity and Aβ burden on a PET scan in MCI. We also demonstrated an unexpected association of greater LTP-like plasticity with lower memory performance on cognitive tests in MCI. Longitudinal studies are needed to understand the extent to which neurophysiologic changes may serve as prognostic markers or future therapeutic targets in MCI and in earlier, presymptomatic, stages of AD.
TMS assessments have shown reduced efficacy of neuroplastic mechanism plasticity in older healthy individuals compared with younger adults (Freitas et al., 2011), with more severe abnormalities in LTP-like plasticity in dementia due to AD (Brem et al., 2013; Koch et al., 2012). Our results add to this literature by showing that LTP-like plasticity is similarly disrupted in MCI. Other potential TMS markers of plasticity include 5-Hz repetitive TMS, which demonstrates impaired facilitation in MCI compared with controls, and has been reported to predict clinical progression (Trebbastoni et al., 2016). Both techniques may capture common neuroplastic mechanisms, particularly because iTBS uses theta-frequency bursts of pulses delivered at 5-Hz intervals. However, because 5-Hz rTMS-induced facilitation relies heavily on the timing of breaks during the protocol (Rothkegel et al., 2010), iTBS is likely a more robust marker of the efficacy of mechanisms supporting LTP-like plasticity (or lack thereof). Taken together, these findings deepen our understanding of the electrophysiologic changes occurring in MCI, a common clinical manifestation of prodromal AD which has been the target of multiple recent therapeutic drug trials seeking to slow or prevent cognitive decline (Selkoe, 2013).
Contrary to our initial prediction, we found no association between LTP-like response and Aβ burden on [F18]florbetapir PET, suggesting that global levels of fibrillary Aβ deposition do not directly alter LTP-like plasticity. This finding fits with the prior literature, which shows a relationship between LTP-like plasticity and elevated CSF total-tau and phospho-tau (Koch et al., 2015), but not with decreased CSF Aβ−42 (Koch et al., 2017). Because tau accumulation tracks closely with cortical atrophy and cognitive decline (Xia et al., 2017), a decrease in LTP-like plasticity may reflect underlying synaptic toxicity or serve as a physiologic correlate of global cognition. This is in line with findings of reduced LTP-like plasticity in older adults with type-2 diabetes (Fried et al., 2017a), which is associated with accelerated cognitive aging and increased risk of dementia (Allen et al., 2004). In the future, a systematic study comparing synaptic plasticity across Aβ− and Aβ+ MCI compared with Aβ− and Aβ+ HCs would help to elucidate the effect of Aβ positivity on LTP-like plasticity. Likewise, including tau PET would also clarify whether the lack of LTP-like plasticity in early-AD is more closely associated with global tau burden. Furthermore, this would allow for investigation of the relationship between LTP-like plasticity and a variety of factors influencing healthy versus pathological cognitive aging including tau deposition, cortical atrophy, cerebrovascular disease, cognitive reserve, and physical activity.
Soluble Aβ concentrations, which are not detected by [F18]florbetapir PET, could also drive impairments in LTP-like plasticity in AD and could alternatively explain our findings. The soluble oligomeric form of Aβ is thought to cause most of the synaptic neurotoxic effects attributable to Aβ by altering synaptic function and structure (Mucke and Selkoe, 2012). Aβ isolated from human AD brains has been shown to impair brain plasticity in rodent models, interrupting in vivo memory for a passive avoidance task, decreasing hippocampal dendritic spine density, inhibiting LTP, and facilitating LTD (Shankar et al., 2008). Toxic forms of Aβ partially inhibit NMDA receptors, causing an enhancement of LTD over LTP and subsequent synaptic loss (Shankar et al., 2007). In addition, impaired plasticity in AD occurs in the context of mounting neuronal injury. Along with its effects on neuroplasticity, oligomeric amyloid also causes neurons to exhibit toxic overstimulation and excitotoxicity (Palop et al., 2007). Similarly, our results support multiple concurrent synaptic-toxic effects, with Pre-iTBS MEP amplitude showing nonsignificantly greater values in MCI compared with HCs. In the future, assessment of LTP-like plasticity could be further standardized by adjusting stimulation intensity to elicit a set amplitude (e.g., 1 mV MEP amplitude), which may better adjust for differences in cortical excitability than stimulating at 120% RMT in patients with neurodegenerative disorders. Assessment of LTP-like plasticity should also be integrated with other measures reflecting neuroplasticity and synaptic function such as EEG, fMRI, and FDG-PET to understand how different neurophysiologic abnormalities interact in AD.
The modulation of MEPs by iTBS—which we take as an indication of LTP-like plasticity—was inversely related to learning and memory function in our participants with MCI. This finding was contrary to our initial hypothesis and opposite to the effect seen in other studies looking at a more advanced stage of dementia due to AD, where increased LTP-like plasticity was associated with better verbal memory (Di Lorenzo et al., 2019; Motta et al., 2018). Intriguingly, this observation is consistent with autopsy data from the Religious Order Study, which showed that presynaptic glutamatergic bouton density correlated with better cognition in AD dementia, but with decreased cognitive function in Aβ+ MCI (Bell et al., 2007). Although the explanation for this finding is not yet certain, it could be related to a broader loss of homeostatic mechanisms in the prodromal stages of AD (Styr and Slutsky, 2018). For example, cortical hyperexcitability, epileptiform discharges, and seizures are known to occur in the early stages of AD (Vossel et al., 2016). A reduced LTP-like response to iTBS in MCI could reflect an adaptive mechanism offering neuroprotection against toxic hyperexcitability (Styr and Slutsky, 2018). In this model, the ability to downregulate LTP in MCI could indicate greater adaptive neuronal responses, lead to less global neurotoxicity, and therefore be associated with improved memory function. Indeed, modulation of NMDA-r strength is the primary mechanism of action of memantine, which has evidence of clinical efficacy in the moderate to severe stages of dementia due to AD (Parsons et al., 2007). Alternatively, our ability to measure LTP-like plasticity using iTBS may itself be impaired by increases in cortical excitability. In a hyperactive cortex, AMPA receptors may near saturation, limiting our ability to induce an LTP-like response using iTBS (Styr and Slutsky, 2018). Our finding that Pre-iTBS MEP amplitude accounts for a significant covariate in the relationship between diagnosis and LTP-like plasticity could support this possibility. Taken together, an important conclusion is that the interpretation of TMS measures of LTP-like plasticity may vary in different stages of disease progression. In AD dementia, decreased LTP-like plasticity is associated with faster clinical progression (Koch et al., 2015; Motta et al., 2018), but it is not clear if decreased LTP-like plasticity is similarly a negative predictor in MCI. A critical next step is to assess alterations in neuroplasticity and cortical excitability in individuals with presymptomatic AD, before onset of cognitive decline, and to track individuals over time. This may suggest causal relationships between TMS measures in AD, determine the extent to which neurophysiologic alterations carry prognostic value, and point toward new therapies aimed at modulation LTP or cortical excitability in presymptomatic and prodromal AD.
Strengths of this study include thorough characterization of our participants with clinical and cognitive testing, neuroimaging, and TMS measures. Our study design included assessment of amyloid biomarker status in participants with MCI, which increases the generalizability of our findings to other cohorts with biomarker-defined prodromal AD. Limitations of this study include our relatively small number of participants and unknown Aβ biomarker status in the HC group. Because the presence of Aβ+ participants in the HC group would be expected to decrease the difference in TMS measures between our groups, this should not affect the validity of our findings. Second, because our assessment of Aβ used a global assessment of brain amyloid burden on PET, we are unable to draw conclusions about the relationship of regional amyloid (i.e., within the motor cortex) and LTP-like plasticity. Third, because we did not assess tau-biomarker status in either group, we were not able to test whether the previously observed relationship between tau and LTP-like plasticity in AD is also present in MCI. This is not expected to change the accuracy or generalizability of our results. Further biomarker-driven studies are needed to study differences in LTP-like plasticity among different neurodegenerative disorders and would help elucidate the relationship between iTBS measures and proteins involved in neurodegeneration. Finally, because the present analysis used TMS-EMG assessments in M1, we were unable to measure LTP-like plasticity outside of the primary motor cortex. In the future, combining TMS with EEG could be used to assess cortical excitability and LTP-like plasticity in areas with the highest burden of AD pathology in early disease stages, such as the parietal or frontal cortices, which may be more useful prognostically as assessments of synaptic function in the preclinical and prodromal AD.
5. Conclusions
Our results show decreased LTP-like plasticity (as measured by the iTBS-induced modulation of MEP amplitude) in MCI, extending prior work in AD to the earlier clinical stage of MCI. Decreased LTP-like plasticity was not correlated with Aβ burden on a PET scan. Unexpectedly, greater LTP-like plasticity was related to lower memory scores in MCI. Additional longitudinal studies are needed to determine how LTP-like plasticity and other measures of synaptic function evolve over the clinical course of AD progression and the extent to which TMS measures show promise as future prognostic measures or therapeutic targets in the earliest stages of AD.
Supplementary Material
Acknowledgments
Study funding: This study was primarily supported by grants from the National Institutes of Health (NIH; R21 NS082870, R21 AG051846). S.S.B. was further supported by the Sidney R. Baer Jr. Foundation (01028951), American Academy of Neurology (2016–0229), and the Alzheimer’s Association (2019-AACSF-643094). M.M.S. is supported by the CURE (Citizens United for Research in Epilepsy) foundation, the Football Players Health Study at Harvard University, and the NIH (R01 MH115949, R01AG060987, R01 NS073601, P01 AG031720–06A1). A.P.L. was also supported by the Sidney R. Baer, Jr. Foundation, Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758), the Football Players Health Study at Harvard University, and by the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, the American Academy of Neurology, the Alzheimer’s Association, the Sidney R. Baer Jr. Foundation, The Football Players Health Study, or DARPA.
Role of the funding sources: The funding sources did not play a role in study design, data collection, analysis, or interpretation, or in the writing of the report or the decision to submit the article for publication.
Footnotes
Disclosure statement
S.S.B. serves as a consultant for Kinto Care. A.P.L. serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Cognito, Linus Health, Magstim, and Nexstim and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging, and on various methods of transcranial current stimulation. D.Z.P., K.D., E.K., M.O., K.D., M.S., and P.J.F. report no disclosures.
Data statement: Anonymized data will be shared on request from any qualified investigator.
CRediT authorship contribution statement
Stephanie S. Buss: Formal analysis, Investigation, Data curation, Project administration, Writing - original draft. Daniel Z. Press: Conceptualization, Supervision, Writing - review & editing. Katherine McDonald: Investigation, Data curation, Writing - review & editing. Erin Kitchener: Investigation, Writing - review & editing. Margaret O’Connor: Conceptualization, Writing - review & editing. Kevin Donohoe: Data curation, Formal analysis, Writing - review & editing. Mouhsin M. Shafi: Supervision, Writing - review & editing. Alvaro Pascual-Leone: Conceptualization, Investigation, Methodology, Resources, Supervision, Funding acquisition, Writing - review & editing. Peter J. Fried: Conceptualization, Formal analysis, Investigation, Methodology, Data curation, Project administration, Writing - review & editing.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.neurobiolaging.2020.08.021.
Publisher's Disclaimer: Please use this PDF proof to check the layout of your article. If you would like any changes to be made to the layout, you can leave instructions in the online proofing interface. Making your changes directly in the online proofing interface is the quickest, easiest way to correct and submit your proof. Please note that changes made to the article in the online proofing interface will be added to the article before publication, but are not reflected in this PDF proof.
References
- 2017 Alzheimer’s Disease Facts and Figures, 2017. Alzheimer’s & dementia. J. Alzheimers Assoc 13, 325–373. [Google Scholar]
- Allen KV, Frier BM, Strachan MWJ, 2004. The relationship between type 2 diabetes and cognitive dysfunction: longitudinal studies and their methodological limitations. Eur. J. Pharmacol 490, 169–175. [DOI] [PubMed] [Google Scholar]
- Amariglio RE, Frishe K, Olson LE, Wadsworth LP, Lorius N, Sperling RA, Rentz DM, 2012. Validation of the face name associative memory exam in cognitively normal older individuals. J. Clin. Exp. Neuropsychol 34, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KFS, Bennett DA, Cuello AC, 2007. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J. Neurosci 27, 10810–10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell CSY, Davila-Pérez P, Fried PJ, Jones RN, Travison TG, Santarnecchi E, Pascual-Leone A, Shafi MM, 2020. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol. Aging 85, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem A-K, Atkinson NJ, Seligson EE, Pascual-Leone A, 2013. Differential pharmacological effects on brain reactivity and plasticity in Alzheimer’s disease. Front. Psychiatry 4, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright P, van der Linde I, 2020. Comparison of methods for estimating premorbid intelligence. Neuropsychol. Rehabil 30, 1–14. [DOI] [PubMed] [Google Scholar]
- Buss SS, Padmanabhan J, Saxena S, Pascual-Leone A, Fried PJ, 2018. Atrophy in distributed networks predicts cognition in Alzheimer’s disease and type 2 diabetes. J. Alzheimers Dis 65, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC, 2008. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. (Lond.) 586, 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Arora A, Carpenter AP, Flitter ML, Joshi AD, Krautkramer MJ, Lu M, Mintun MA, Skovronsky DM, AV-45-A16 Study Group, 2012. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678. [DOI] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, Alzheimer’s Disease Neuroimaging Initiative, 2012. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 6, 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F, Motta C, Bonnì S, Mercuri NB, Caltagirone C, Martorana A, Koch G, 2019. LTP-like cortical plasticity is associated with verbal memory impairment in alzheimer’s disease patients. Brain Stimul. 12, 148–151. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo F, Ponzo V, Bonnì S, Motta C, Negrão Serra PC, Bozzali M, Caltagirone C, Martorana A, Koch G, 2016. LTP-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann. Neurol 80, 202–210. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, Grundman M, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Clark CM, Joshi AD, Mintun MA, Skovronsky DM, Pontecorvo MJ, AV45-A11 Study Group, 2012. Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology 79, 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, Bashir S, Vernet M, Peña-Gómez C, Pascual-Leone A, 2011. Changes in cortical plasticity across the lifespan. Front. Aging Neurosci 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Jannati A, Davila-Pérez P, Pascual-Leone A, 2017. Reproducibility of single-pulse, paired-pulse, and intermittent theta-burst TMS measures in healthy aging, type-2 diabetes, and Alzheimer’s disease. Front. Aging Neurosci 9, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Schilberg L, Brem A-K, Saxena S, Wong B, Cypess AM, Horton ES, Pascual-Leone A, 2017. Humans with type-2 diabetes show abnormal long-term potentiation-like cortical plasticity associated with verbal learning deficits. J. Alzheimers Dis 55, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Baxter L, Connor DJ, Herring A, Comer J, 2007. Sex differences on the Rey auditory verbal learning test and the brief visuospatial memory test-revised in the elderly: normative data in 172 participants. J. Clin. Exp. Neuropsychol 29, 561–567. [DOI] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging Initiative, 2012. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 6, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DP, Cully JA, Snow AL, Massman P, Doody R, 2004. The Alzheimer’s Disease Assessment Scale-Cognitive subscale: normative data for older adult controls. Alzheimer Dis. Assoc. Disord 18, 236–240. [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GMJ, Di Lazzaro V, 2010. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y, 2007. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol 118, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, 2018. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, 2018. Digit Symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol 38, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Bonnì S, Ponzo V, Caltagirone C, Martorana A, 2012. Impaired LTP- but not LTD-like cortical plasticity in Alzheimer’s disease patients. J. Alzheimers Dis 31, 593–599. [DOI] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Del Olmo MF, Bonní S, Ponzo V, Caltagirone C, Bozzali M, Martorana A, 2015. Reversal of LTP-like cortical plasticity in Alzheimer’s disease patients with tau-related faster clinical progression. J. Alzheimers Dis 50, 605–616. [DOI] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Loizzo S, Motta C, Travaglione S, Baiula M, Rimondini R, Ponzo V, Bonnì S, Toniolo S, Sallustio F, Bozzali M, Caltagirone C, Campana G, Martorana A, 2017. CSF tau is associated with impaired cortical plasticity, cognitive decline and astrocyte survival only in APOE4-positive Alzheimer’s disease. Sci. Rep 7, 13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lövden M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze H-J, Müller NG, Düzel E, 2015. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry 20, 585–595. [DOI] [PubMed] [Google Scholar]
- Mota SI, Ferreira IL, Rego AC, 2014. Dysfunctional synapse in Alzheimer’s disease - a focus on NMDA receptors. Neuropharmacology 76 Pt A, 16–26. [DOI] [PubMed] [Google Scholar]
- Motta C, Di Lorenzo F, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, Mercuri NB, Caltagirone C, Martorana A, Koch G, 2018. Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 89, 1237–1242. [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ, 2012. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect. Med 2, a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemantsverdriet E, Ottoy J, Somers C, De Roeck E, Struyfs H, Soetewey F, Verhaeghe J, Van den Bossche T, Van Mossevelde S, Goeman J, De Deyn PP, Mariën P, Versijpt J, Sleegers K, Van Broeckhoven C, Wyffels L, Albert A, Ceyssens S, Stroobants S, Staelens S, Bjerke M, Engelborghs S, 2017. The cerebrospinal fluid aβ1e42/aβ1e40 ratio improves concordance with amyloid-PET for diagnosing Alzheimer’s disease in a clinical setting. J. Alzheimers Dis 60, 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Alzheimer’s Disease Neuroimaging Initiative, Minthon L, Blennow K, Olsson M, Hansson O, Swedish BioFINDER Study Group, 2015. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 85, 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-Q, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L, 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Stöffler A, Danysz W, 2007. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology 53, 699–723. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U, 2015. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 126, 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel H, Sommer M, Paulus W, 2010. Breaks during 5Hz rTMS are essential for facilitatory after effects. Clin. Neurophysiol 121, 426–430. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Blazer DG, 2015. The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Ment. Health 19, 2–12. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, 2013. The therapeutics of Alzheimer’s disease: where we stand and where we are heading. Ann. Neurol 74, 328–336. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, 2002. Alzheimer’s disease is a synaptic failure. Science 298, 789–791. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL, 2007. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci 27, 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ, 2008. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med 14, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, 2007. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann. N. Y. Acad. Sci 1097, 146–155. [DOI] [PubMed] [Google Scholar]
- Styr B, Slutsky I, 2018. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci 21, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy TE, Sohn PD, Minami SS, Wang C, Min S-W, Li Y, Zhou Y, Le D, Lo I, Ponnusamy R, Cong X, Schilling B, Ellerby LM, Huganir RL, Gan L, 2016. Acetylated tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes Tauopathy-related memory loss. Neuron 90, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebbastoni A, Pichiorri F, D’Antonio F, Campanelli A, Onesti E, Ceccanti M, de Lena C, Inghilleri M, 2016. Altered cortical synaptic plasticity in response to 5-Hz repetitive transcranial magnetic stimulation as a new electrophysiological finding in amnestic mild cognitive impairment converting to Alzheimer’s disease: results from a 4-year prospective cohort study. Front. Aging Neurosci 7, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SJB, Verhey F, Frölich L, Kornhuber J, Wiltfang J, Maier W, Peters O, Rüther E, Nobili F, Morbelli S, Frisoni GB, Drzezga A, Didic M, van Berckel BNM, Simmons A, Soininen H, K1oszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Muscio C, Herukka S-K, Salmon E, Bastin C, Wallin A, Nordlund A, de Mendonça A, Silva D, Santana I, Lemos R, Engelborghs S, Van der Mussele S, Alzheimer’s Disease Neuroimaging Initiative, Freund-Levi Y, Wallin ÅK, Hampel H, van der Flier W, Scheltens P, Visser PJ, 2015. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138, 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF, Darwish SM, Van Berlo V, Barnes DE, Mantle M, Karydas AM, Coppola G, Roberson ED, Miller BL, Garcia PA, Kirsch HE, Mucke L, Nagarajan SS, 2016. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann. Neurol 80, 858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, Giordani B, Kramer J, Loewenstein D, Marson D, Mungas D, Salmon D, Welsh-Bohmer K, Zhou X-H, Shirk SD, Atri A, Kukull WA, Phelps C, Morris JC, 2018. Version 3 of the alzheimer disease centers’ neuropsychological test battery in the Uniform data set (UDS). Alzheimer Dis. Assoc. Disord 32, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC, 2009. The Alzheimer’s disease centers’ Uniform data set (UDS): the neuropsychological test battery. Alzheimer Dis. Assoc. Disord 23, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC, 2004. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J. Intern. Med 256, 240–246. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJLG, 2015. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 8, 685–692. [DOI] [PubMed] [Google Scholar]
- Xia C, Makaretz SJ, Caso C, McGinnis S, Gomperts SN, Sepulcre J, Gomez-Isla T, Hyman BT, Schultz A, Vasdev N, Johnson KA, Dickerson BC, 2017. Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in Typical and atypical alzheimer disease. JAMA Neurol. 74, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.