Abstract
Background
Many individuals take long-term immunosuppressive medications. We evaluated whether these individuals have worse outcomes when hospitalised with COVID-19 compared with non-immunosuppressed individuals.
Methods
We conducted a retrospective cohort study using data from the National COVID Cohort Collaborative (N3C), the largest longitudinal electronic health record repository of patients in hospital with confirmed or suspected COVID-19 in the USA, between Jan 1, 2020, and June 11, 2021, within 42 health systems. We compared adults with immunosuppressive medications used before admission to adults without long-term immunosuppression. We considered immunosuppression overall, as well as by 15 classes of medication and three broad indications for immunosuppressive medicines. We used Fine and Gray's proportional subdistribution hazards models to estimate the hazard ratio (HR) for the risk of invasive mechanical ventilation, with the competing risk of death. We used Cox proportional hazards models to estimate HRs for in-hospital death. Models were adjusted using doubly robust propensity score methodology.
Findings
Among 231 830 potentially eligible adults in the N3C repository who were admitted to hospital with confirmed or suspected COVID-19 during the study period, 222 575 met the inclusion criteria (mean age 59 years [SD 19]; 111 269 [50%] male). The most common comorbidities were diabetes (23%), pulmonary disease (17%), and renal disease (13%). 16 494 (7%) patients had long-term immunosuppression with medications for diverse conditions, including rheumatological disease (33%), solid organ transplant (26%), or cancer (22%). In the propensity score matched cohort (including 12 841 immunosuppressed patients and 29 386 non-immunosuppressed patients), immunosuppression was associated with a reduced risk of invasive ventilation (HR 0·89, 95% CI 0·83–0·96) and there was no overall association between long-term immunosuppression and the risk of in-hospital death. None of the 15 medication classes examined were associated with an increased risk of invasive mechanical ventilation. Although there was no statistically significant association between most drugs and in-hospital death, increases were found with rituximab for rheumatological disease (1·72, 1·10–2·69) and for cancer (2·57, 1·86–3·56). Results were generally consistent across subgroup analyses that considered race and ethnicity or sex, as well as across sensitivity analyses that varied exposure, covariate, and outcome definitions.
Interpretation
Among this cohort, with the exception of rituximab, there was no increased risk of mechanical ventilation or in-hospital death for the rheumatological, antineoplastic, or antimetabolite therapies examined.
Funding
None.
Introduction
As of Oct 1, 2021, SARS-CoV-2 has infected more than 43 million people in the USA and caused more than 698 000 deaths.1 Although increasing vaccination uptake and other public health measures have reduced the burden of the pandemic, substantial morbidity and mortality continue to accrue in the unvaccinated and non-immune population.
Evidence is mixed regarding the impact of immunosuppression and immunosuppressive medicines on COVID-19 outcomes. Immunosuppression increases the incidence and severity of many infectious diseases; case reports from China and Europe, as well as guidelines from WHO and the US Centers for Disease Control and Prevention indicate that conditions requiring pharmacological immunosuppression, such as solid organ transplant2 and cancer, are risk factors for SARS-CoV-2 infection. However, previous studies have found that individuals with autoimmune diseases, such as rheumatoid arthritis or inflammatory bowel disease, have a greater incidence of COVID-19, but not of resultant invasive ventilation or death.3, 4, 5 Case series of patients with solid organ transplant with SARS-CoV-2 infection have compared the risk of severe COVID-19 outcomes to that of the general population and found higher hospitalisation and case fatality rates in the initial months of the pandemic (March to June, 2020).6, 7, 8 Despite the insights from these early studies, some questions remain unanswered, such as whether time trends in COVID-19 management could explain these apparent increased risks.
Research in context.
Evidence before this study
The evidence regarding the impact of immunosuppression and immunosuppressive medicines on COVID-19 outcomes is mixed, with previous studies finding that these individuals might have an increased risk of infection but not resultant invasive ventilation or death. It is unclear whether associations vary by medication class.
Added value of this study
This retrospective cohort study evaluated the risk of severe COVID-19 outcomes for individuals using 15 pharmacological classes that alter immune function, using electronic health information from 42 health systems in the USA. In this cohort, with the exception of rituximab, there was no increased risk of mechanical ventilation or in-hospital death for the rheumatological, antineoplastic, or antimetabolite therapies examined. Our sample size was large enough to consider separately a variety of drug classes with distinct molecular mechanisms of action, including the targeting of B-cell versus T-cell mediated immunity.
Implications of all the available evidence
Our results add to a growing body of evidence on the overall safety of most long-term immunosuppressive medications against the backdrop of continued COVID-19-related morbidity and mortality. These findings are important because of how commonly these medicines are used, and due to ongoing questions regarding the degree to which they increase the risks of poor outcomes among individuals who are hospitalised with COVID-19.
As with many other clinical contexts, for any given immunosuppressive condition, many potential drug combinations can be used. Several single-centre evaluations, including our own,9 have reported no increased risk of severe COVID-19 among those taking long-term immunosuppressive medicines,10, 11, 12 and the theoretical possibility that such medicines might dampen the cytokine storm associated with severe COVID-19 has not been substantiated in the literature. Much of the previous long-term immunosuppressive medication literature has used small samples of patients, precluding the evaluation of specific medicine classes.
To address these research gaps, we performed a retrospective cohort study using data from the National COVID Cohort Collaborative (N3C), the largest US electronic health record repository, which captures COVID-19 care delivered between January, 2020, and June, 2021. In addition to evaluating overall risk, we also evaluated whether the therapeutic class of immunosuppressive medications alters the risk of invasive mechanical ventilation or death.
Methods
Study design and population
The N3C is a national electronic health record repository supported by the National Institutes of Health (NIH) National Center for Advancing Translational Science.13, 14 It contains detailed inpatient and outpatient records, as well as drug exposure information, for a racially, ethnically, and geographically diverse group of individuals. Data are reviewed for completeness and accuracy by a data quality team, and the data are harmonised using the Observational Medical Outcomes Partnership Common Data Model.13 As of June 17, 2021, the N3C had records for more than 2 130 000 COVID-19-positive individuals, the majority of which were from outpatient encounters. We used individual patient data to conduct our analyses. The N3C data transfer to the National Center for Advancing Translational Sciences (NCATS) is performed under a Johns Hopkins University Reliance Protocol (#IRB00249128) or individual site agreements with the NIH.
We defined COVID-19-positive individuals as those with confirmed SARS-CoV-2 infection (at least one positive SARS-CoV-2 test result, more than 99% of which were by RT-PCR) or suspected SARS-CoV-2 infection. Suspected infections required at least one strong positive diagnosis code, or two weak positive codes, as outlined in the GitHub repository.15 We defined a COVID-19-related hospitalisation as the first inpatient visit up to 21 days after the date of confirmed or suspected SARS-CoV-2 infection. To account for delays in test reporting while minimising the possibility of nosocomial infections,16 we also included hospitalised individuals designated as COVID-19-positive up to 5 days after admission. We limited our analyses to individuals with complete hospitalisation episodes, documented by either discharge or death.
We sequentially excluded individuals with missing data on age or sex, those younger than 18 years, those transferred to the N3C data partner already on a ventilator, and individuals with implausible information, such as a COVID-19 diagnosis in 2018 or a date of death predating their date of admission. In addition, we excluded six clinical sites from our analysis that did not meet N3C standards of data quality, leaving 42 sites for analysis.14
Exposure
We defined two mutually exclusive exposure groups: immunosuppressed individuals and non-immunosuppressed individuals up to and including at the time of admission. Individuals were considered immunosuppressed if they had exposure to at least one of the following: rheumatological drugs (interleukin inhibitors, Janus kinase inhibitors, tumour necrosis factor (TNF) inhibitors, or any other drug in the WHO Anatomical Therapeutic Chemical [ATC] L04: selective immunosuppressants), antimetabolite drugs (azathioprine, calcineurin inhibitors, or mycophenolic acid [formulated either as mycophenolate sodium or mycophenolate mofetil]), cancer therapies (anthracyclines, checkpoint inhibitors, cyclophosphamide, protein kinase inhibitors, or any other drug in the WHO ATC class L01: antineoplastic agents), rituximab, targeted cancer therapies, and oral glucocorticoids (dexamethasone, prednisone, prednisolone, or methylprednisolone; appendix pp 2–4). We classified people as having long-term immunosuppression at the time of admission if they used one or more of these medications; in addition, we used the electronic health record fields of prescription record start and stop dates, and we required immunosuppression to be started at least 14 days before the date of admission, and either continued during admission or actively stopped on or after the date of admission. We excluded 57 people whose only immunosuppressant was a glucocorticoid prescribed on or after the date of COVID-19 diagnosis but before admission. For oral glucocorticoids, we further required a diagnosis that was consistent with long-term use of steroids (appendix p 15). People without use of any of the immunosuppressive drugs on the date of admission were considered non-immunosuppressed.
Outcomes
Our primary outcome was the time from admission to invasive mechanical ventilation, using the standard N3C definition, which uses concept codes for condition occurrence, procedures, and observations.14 Our secondary outcome was time from admission to in-hospital death. To reduce their effect in the models, we winsorised the upper 1% of times to event.17 For people whose first ventilation code could not precisely define the date of ventilation, such as “Respiratory support, 24–96 hours” or “Respiratory support, greater than 96 hours”, we used the shortest date of the interval range as the time to event.
We selected covariates a priori for use in a propensity score model based on the availability of data and information from the peer-reviewed literature,18, 19 government and international agency recommendations, and our own clinical, biostatistical, and epidemiological expertise. We used the following covariates: the week of admission, contributing data site, age, sex as recorded in the electronic health record, self-reported race and ethnicity, smoking history, body-mass index, days between COVID-19 diagnosis and hospital admission, medication use for chronic conditions, and relevant comorbidities (appendix pp 5–8).
Statistical analysis
We characterised our study cohort using means with SDs for continuous variables and frequency with percentages for count variables. We then constructed propensity scores,20 using a logistic regression model including each of the aforementioned covariates to predict the probability of being on immunosuppressive medications at the time of admission. For the propensity score estimation, we created missing data indicators for each variable, because this effectively creates a match on both observed and missing data patterns.
We used propensity score matching, given substantial areas of non-overlap for the exposed and unexposed groups, with a 4:1 nearest neighbour matching algorithm without replacement and a calliper of 0·2 pooled SDs of the estimated propensity score.21 We evaluated the absolute value of the standardised mean difference (SMD) in the unmatched and the propensity score matched sample, using the R cobalt package, to assess covariate balance in a sample-size independent manner. We implemented doubly robust adjustment, in which covariates that remained unbalanced (SMD >10%) after matching were included in the regression models described in the following paragraph.22
We assessed for elevated risk of outcomes comparing immunosuppressed individuals and non-immunosuppressed individuals with all 15 immunosuppressive drug classes combined, as well as separately by class of medication and broad indication for use. We used cluster-robust SEs that accounted for the matched nature of the data to calculate unadjusted and adjusted hazard ratios (HRs) and 95% CIs. We used Fine and Gray's proportional subdistribution hazards models to estimate the risk of mechanical ventilation, accounting for the competing risk of death.23 We used Cox proportional hazards models to estimate the risk of death.24 We calculated the E-value to quantify the amount of independent unmeasured confounding that would have to be present in order to qualitatively change the interpretation of results.25
We generated new propensity scores and repeated the propensity score matching process in each subgroup and sensitivity analysis, as well as the set of doubly robust adjustment variables. We stratified models by race and ethnicity groups, which were generally reported by the patient or family member at the time of hospital registration in the local electronic health record.26 We grouped race and ethnicity as done in the US Census and evaluated whether or not the associations of interest differed by patient race and ethnicity. We also disaggregated data by sex, in accordance with Sex and Gender Equity in Research guidelines for reporting of sex information; gender identity was not available.
We did a sensitivity analysis to assess whether the absence of glucocorticoid dose information could create exposure misclassification by excluding individuals who had a record of glucocorticoid use without any dose information. In a second sensitivity analysis, we restricted the cohort to individuals with at least one previous health system encounter before COVID-19 diagnosis, to assess whether the lack of lookback data could have affected covariate ascertainment. Third, we included only people who were hospitalised for at least 2 days, because individuals discharged the same or next day might be clinically distinct from those with longer stays. Fourth, we added vital signs and laboratory values at the time of admission to the model. Given that these variables might be strongly associated with the outcome, we did not include them in our primary analyses but instead considered them in sensitivity analyses. Lastly, for the people whose ventilation procedure code indicated a date range rather than a single date, we varied the choice of date to consider the latest day in the period.
Data extraction and management were performed using Spark SQL (version 3.0.2) and Python, and analyses used SparkR, in the N3C Enclave.
Role of the funding source
There was no funding source for this study.
Results
Of 231 830 potentially eligible adults in the N3C Enclave who were hospitalised for COVID-19 between Jan 1, 2020, and June 11, 2021, we identified 222 575 people who met the inclusion criteria (appendix p 17). The mean age of patients was 59 years (SD 19) and 111 269 (50%) were male. The most common comorbidities were diabetes (50 656 [23%] patients), pulmonary disease (36 760 [17%]), and renal disease (29 772 [13%]; appendix p 10). The mean length of hospital stay was 8·5 days (SD 13·9). Trends in hospital admissions coincided with national trends in infection waves, with peaks in March to April, July, and November to December, 2020 (appendix p 18). Immunosuppressed adults were older, more often female, and less frequently Hispanic or Latinx than non-immunosuppressed adults (table 1 ). Among hospitalised adults, 16 494 (7%) had active medication records for immunosuppressive medications at the time of admission (appendix p 9), including medications commonly used for a rheumatological condition (5366 [33%] patients), antimetabolite drugs (4288 [26%]), or for cancer treatment (3569 [22%]). Comorbidities were more prevalent in the immunosuppressed population (appendix p 10). Vital signs on the first day of admission were similar (appendix p 11); abnormal creatinine and troponin concentrations and abnormal white blood cell counts were more prevalent in the immunosuppressed group.
Table 1.
Characteristics of individuals on date of hospitalisation with confirmed or suspected COVID-19, by immunosuppressed status before COVID-19
| Immunosuppressed (n=16 494) | Non-immunosuppressed (n=206 081) | ||
|---|---|---|---|
| Age, years | 61 (16) | 59 (19) | |
| Sex | |||
| Female | 9231 (56%) | 102 075 (50%) | |
| Male | 7263 (44%) | 104 006 (50%) | |
| Race and ethnicity | |||
| Asian | 335 (2%) | 6612 (3%) | |
| Hispanic or Latinx | 1672 (10%) | 30 759 (15%) | |
| Non-Hispanic Black | 3820 (23%) | 38 461 (19%) | |
| Non-Hispanic White | 7989 (48%) | 92 629 (45%) | |
| Another race | 113 (1%) | 1030 (<1%) | |
| Missing or unknown | 2565 (16%) | 36 590 (18%) | |
| Current or former smoker | 4814 (29%) | 36 544 (18%) | |
| Body-mass index, kg/m2 | |||
| Underweight (<18·5) | 329 (2%) | 1850 (1%) | |
| Not overweight or obese (18·5–24·9) | 2893 (18%) | 18 899 (9%) | |
| Overweight (25·0–29·9) | 3299 (20%) | 26 494 (13%) | |
| Obese (≥30·0) | 5789 (35%) | 40 757 (20%) | |
| Missing | 4184 (25%) | 118 081 (57%) | |
| Days between COVID-19 diagnosis and hospital admission | 1·6 (4·0) | 1·3 (3·7) | |
| Solid organ transplant recipient | 3423 (21%) | 2338 (1%) | |
| Cardiovascular disease | 5922 (36%) | 34 116 (17%) | |
| Chronic hypertension | 12 397 (75%) | 94 658 (46%) | |
Continuous variables are represented as mean (SD), and categorical variables as count (%).
We included 12 841 immunosuppressed and 29 386 non-immunosuppressed individuals in the propensity score matched analyses. In this cohort, some but not all SMDs indicated remaining imbalance between groups (appendix p 19). The overlap of the propensity scores between groups before and after propensity score matching is shown in the appendix (p 20).
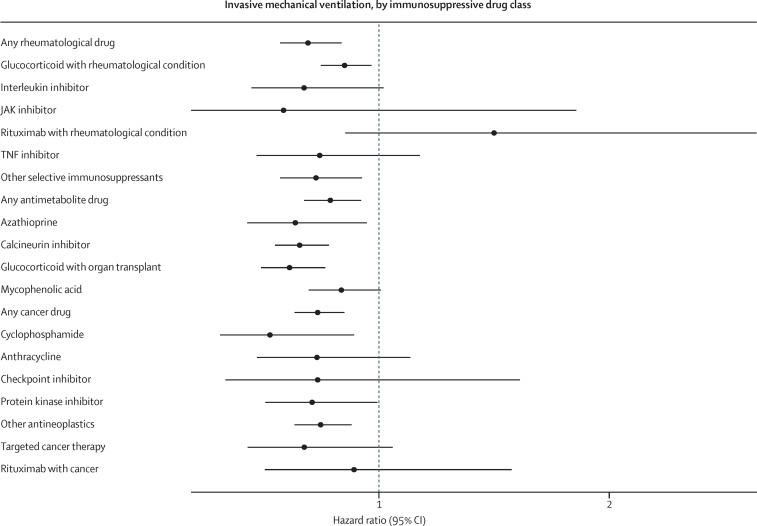
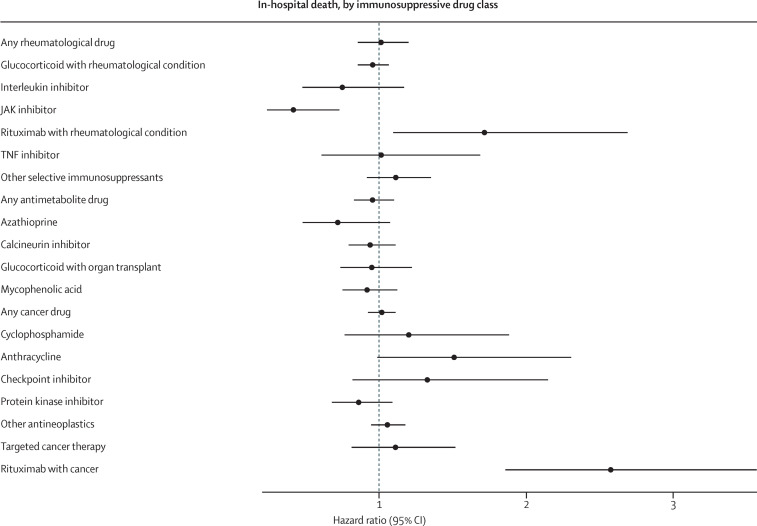
In the entire cohort, 14 740 (7%) of 222 575 people received invasive mechanical ventilation (appendix p 21) and 21 801 (10%) people died (appendix p 22). Invasive mechanical ventilation was an indicator of poor prognosis, because 6878 (47%) of people who required ventilation later died in hospital. In unadjusted analyses, individuals who were immunosuppressed were at greater risk of invasive mechanical ventilation (9% vs 6%; HR 1·36, 95% CI 1·29–1·43) and in-hospital death (14% vs 9%; 1·05, 1·01–1·10; table 2 ). However, in the propensity score matched cohort, immunosuppression was associated with a reduced risk of invasive ventilation (8% vs 9%; 0·89, 0·83–0·96) and there was no overall association between long-term immunosuppression and the risk of in-hospital death (14% vs 12%; 0·97, 0·91–1·02). These analyses had an E-value of 1·50 for invasive mechanical ventilation and 1·21 for death (appendix p 12). The direction of the results when people were grouped by treatment indications (rheumatological, antimetabolite, or cancer therapies) were similar to overall results (figures 1, 2); immunosuppression was associated with a significantly reduced risk of invasive mechanical ventilation (HRs of 0·69–0·79; appendix p 13), and no significant effects were seen on in-hospital death (appendix p 14).
Table 2.
Association between long-term immunosuppression and clinical outcomes in COVID-19
| Invasive mechanical ventilation | In-hospital death | |
|---|---|---|
| Immunosuppressed group, n (%) | ||
| Entire cohort (n=16 494) | 1520 (9%) | 2334 (14%) |
| Matched cohort (n=12 841) | 1089 (8%) | 1743 (14%) |
| Non-immunosuppressed group, n (%) | ||
| Entire cohort (n=206 081) | 13 220 (6%) | 19 467 (9%) |
| Matched cohort (n=29 386) | 2730 (9%) | 3564 (12%) |
| Comparison of immunosuppressed vs non-immunosuppressed adults | ||
| Unadjusted regression in entire cohort, HR (95% CI) | 1·36 (1·29–1·43) | 1·05 (1·01–1·10) |
| Unadjusted regression in matched cohort, HR (95% CI) | 0·88 (0·82–0·94) | 1·01 (0·96–1·07) |
| Propensity score matching with doubly robust adjustment, HR (95% CI) | 0·89 (0·83–0·96) | 0·97 (0·91–1·02) |
| E-value | 1·50 | 1·21 |
| Propensity score matching with doubly robust adjustment among male patients (n=18 798), HR (95% CI) | 0·86 (0·78–0·95) | 0·97 (0·90–1·05) |
| Propensity score matching with doubly robust adjustment among female patients (n=23 199), HR (95% CI) | 0·89 (0·81–0·99) | 0·95 (0·88–1·04) |
HR=hazard ratio.
Results from sensitivity analyses varying the exposure definition for glucocorticoids, ascertaining covariates from previous health system experience, applying a minimum length of stay of 2 days, and adding laboratory and vital sign measures from the day of admission yielded similar findings to the main analyses (appendix p 15). For individuals with a range of dates rather than a single date of invasive mechanical ventilation placement, using the longest date in the range, rather than the shortest, did not change the interpretation of results.
For invasive mechanical ventilation, each of the drug classes was associated with reduced or null effects; no drug class was associated with an increase in invasive mechanical ventilation (figure 1 , appendix p 13). For in-hospital death, we found a significant reduction with JAK inhibitors (HR 0·42, 95% CI 0·24–0·73; figure 2 , appendix p 14). Rituximab in rheumatological conditions (1·72, 1·10–2·69) and as a cancer therapy (2·57, 1·86–3·56) was associated with an increased risk of in-hospital death. All other drugs evaluated did not have statistically significant associations with in-hospital death. Although not statistically significant, the effect size suggests a potentially elevated risk of death for people with anthracycline prescriptions (1·51, 0·99–2·31).
Figure 1.
Association between long-term immunosuppression and invasive mechanical ventilation, by medication class
Analyses were done in the propensity score matched cohort, with doubly robust adjustment for any remaining covariate imbalances after matching.
Figure 2.
Association between long-term immunosuppression and in-hospital death, by medication class
Analyses were done in the propensity score matched cohort, with doubly robust adjustment for any remaining covariate imbalances after matching.
We evaluated potential effect measure modification by racial and ethnic identity. Immunosuppressive drug use was protective against mechanical ventilation for non-Hispanic Black (HR 0·82, 95% CI 0·70–0·95) and for people with missing or unknown racial and ethnic identity (0·68, 0·55–0·84); this finding was consistent albeit not significant in non-Hispanic White individuals (0·90, 0·82–1·00). However, among Asian and Hispanic or Latinx people and individuals of another race, immunosuppression had no significant effect (appendix p 16). Consistent with the overall effect estimate, the risk of in-hospital death in each racial and ethnic group was not significantly different between immunosuppressed and non-immunosuppressed individuals.
In analyses stratified by sex as recorded in the electronic health record, none of the drug classes were associated with an increased risk of invasive mechanical ventilation. Most of the classes had protective effects in male patients (appendix p 23) and non-significant effects in female patients (appendix p 24). For in-hospital death, rituximab was associated with an increased risk of death in female patients with cancer, but not in female patients with a rheumatological condition or male patients for either indication (appendix pp 25–26). The risk of in-hospital death with checkpoint inhibitors was increased for male patients, but not female patients.
Discussion
Although cases, hospitalisations, and deaths were decreasing in the USA in mid-2021, the COVID-19 pandemic is ongoing worldwide and important questions remain. In this analysis of more than 220 000 adults hospitalised with COVID-19, there was no discernible increased risk of invasive mechanical ventilation or in-hospital death with most of the therapies we examined. These findings are important because of how commonly these therapies are used, and because of ongoing questions regarding the degree to which they increase the risks of poor outcomes among individuals who are hospitalised with COVID-19.
Our findings regarding immunosuppressive therapies extend the results of our earlier work and that of others examining the association between use of these medication classes and COVID-19 outcomes. In contrast to some studies that have suggested that people with immunosuppressive conditions are at increased risk of infection,3, 4, 5 our finding of no increased risk of severe disease could be attributable to a combination of factors, including our focus on immunosuppressive medications rather than diagnoses, restriction to hospitalised patients with COVID-19, and analyses with statistical power and methods to address confounding and effect modification. By using a larger and more diverse cohort, our results add to a growing body of evidence suggesting the overall safety of several products in the context of continued COVID-19-related morbidity and mortality. Our sample size also allowed us to examine specific subclasses of therapies that vary considerably in their mechanisms of action, and we found similar safety of these various classes with respect to the outcomes examined among this cohort.
Although in our main analyses, and in the subgroup analyses by rheumatological and antimetabolite drugs, we found that immunosuppression reduced the risk of invasive mechanical ventilation, we did not find this with rituximab. Rituximab is a chimeric monoclonal antibody that binds to the cell surface protein CD20 and induces B-cell apoptosis. This mechanism of action powerfully interferes with antibody response to infection, and it can lead to prolonged viral replication. Therefore, the null effect for ventilation and an increased risk of death are plausible, given the impaired antiviral humoral response.27, 28
Of note, we found a decreased risk of death with chronic JAK inhibitor use. Baricitinib and tofacitinib have each shown to be efficacious in clinical trials as COVID-19 therapies among individuals not using them before SARS-CoV-2 infection.29, 30 An international registry of patients with rheumatoid arthritis and COVID-19 reported an increased odds of death for people on JAK inhibitors, as compared with TNF inhibitors. These results might differ from ours given that their population was not restricted to hospitalised patients.31
Our results generate important scientific and clinical questions for further exploration. For example, studies are needed to assess whether long-term immunosuppressive use, especially with products such as glucocorticoids, could attenuate the mortality benefit attributable to dexamethasone for patients with COVID-19 requiring supplemental oxygen.32 Also, our study was not designed to inform questions regarding whether long-term immunosuppressive medicines, present at hospital admission, should be continued during hospitalisation for COVID-19, and if so, under what treatment protocols. Of course, such protocols, as well as current clinical practice, might vary for different subpopulations of individuals, such as those with rheumatological diseases as compared with those with a history of solid organ transplant. It is also unclear whether the associations we describe could be in part due to differential treatment across our study groups once hospitalised. Immunosuppressed patients could have been hospitalised at earlier stages in disease and treated more aggressively because of the perception of higher risk, both of which could account for the decreased risk of ventilation and absence of an increase in mortality. Of note, we found no significant differences in the proportions of immunosuppressed and non-immunosuppressed people who received remdesivir, in-hospital dexamethasone, or pre-admission monoclonal antibodies for SARS-CoV-2 management. Future studies could account for the time-varying nature of in-patient treatment, as well as treatment indicators, such as laboratory measures of inflammation or ability to mount an inflammatory response.
Our analyses have several limitations. First, the N3C does not contain information on advanced directives, which might lead to misclassification of risk of ventilation and death. Second, the N3C does not contain information on supplemental oxygen. Given that the RECOVERY trial found that dexamethasone reduced the risk of death in people with oxygen requirements,32 but increased the risk of death if they did not require supplemental oxygen, it is possible that the null effect we report is an average of increased and decreased risk by an unmeasured confounder. Third, we used WHO ATC classes for a standardised definition for immunosuppressive medications, which does not include therapies that some might consider to be immunosuppressive, such as hydroxychloroquine, medications for HIV care, or multiple sclerosis, or other immunocompromising conditions. Instead, in this analysis, these people were considered to be non-immunosuppressed. Fourth, individuals who stopped their immunosuppressive medication in the short term before admission, such as at the time of COVID-19 diagnosis, due to concern of immunosuppression leading to worse outcomes, and did not report current immunosuppression at the time of admission would be misclassified as non-immunosuppressed in this analysis. Fifth, care delivered in the N3C might not represent settings outside of academic medical centres or in hospitals outside of the USA. Finally, our analysis was strongly dependent on valid risk adjustment, but we recognise that the Charlson-Deyo instrument might not fully capture the risks associated with underlying comorbidities and indication for immunosuppressive therapy. Residual and unmeasured confounding due to indication, particularly among the subset of cancer patients, could be a source of bias.
The limitations notwithstanding, our analyses also have several strengths. We used a national, diverse cohort of more than 220 000 adults in the USA hospitalised with COVID-19. Our analyses include patients with confirmed SARS-CoV-2 infection, as well as patients with suspected COVID-19. Given that the average sensitivity of SARS-CoV-2 tests is around 80%, restricting analyses to individuals who tested positive could have excluded truly positive individuals. By using a hospitalised cohort, we believe that there should be few, if any, false positives within the suspected COVID-19 designation. In addition, we used doubly robust propensity score methods, and considered the competing risk of death for analyses examining ventilation. Finally, our sample size was large enough to consider separately a variety of drug classes with distinct molecular mechanisms of action, including the targeting of B-cell versus T-cell mediated immunity.
In conclusion, in this cohort, with the exception of rituximab, there was no increased risk of ventilation or death for the rheumatological, antineoplastic or antimetabolite therapies examined. This information could be useful not only to guide future research, but also to clinicians and patients navigating treatment decisions together.
Data sharing
The code for this work can be found at https://github.com/National-COVID-Cohort-Collaborative/CS-ISC. The N3C Data Enclave (covid.cd2h.org/enclave) houses fully reproducible, transparent, and broadly available limited and de-identified datasets (Health Insurance Portability and Accountability definitions are available at https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html). Data are accessible by investigators at institutions that have signed a Data Use Agreement with the NIH, who have taken human subjects and security training, and who attest to the N3C User Code of Conduct. Investigators wishing to access the limited dataset must also supply an institutional review board protocol. All requests for data access are reviewed by the NIH Data Access Committee. A full description of the N3C Enclave governance has been published; information about how to apply for access is available on the NCATS website: https://ncats.nih.gov/n3c/about/applying-for-access. Reviewers and health authorities will be given access permission and guidance to aid reproducibility and outcomes assessment. A frequently asked questions page about the data and access has been created at https://ncats.nih.gov/n3c/about/program-faq.
Declaration of interests
RBM reports grants and other fees from Vitaeris/CSL Behring; other fees from the American Journal of Transplantation; and grants from Mallinckrodt, CSL Behring, Transplant Genomics, and Quark Phamaceuticals, outside the submitted work. RCP reports grants from Trasher Foundation and investigator-sponsored studies from Merck, outside the submitted work. JSi reports consulting fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs, Adept Field Solutions, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM, Trio Health, Medscape, WebMD, and Practice Point Communications, the National Institutes of Health, and the American College of Rheumatology; is on the speaker's bureau of Simply Speaking; receives support from OMERACT to attend their biennial meeting; serves as a member of the US Food and Drug Administration (FDA) Arthritis Advisory Committee; serves as the Chair of the Veterans Affairs Rheumatology Field Advisory Committee, is the editor and director of the UAB Cochrane; owns stock options in TPT Global Tech, Vaxart Pharmaceuticals, and Charlotte's Web Holdings, and previously owned stock options in Amarin, Viking, and Moderna Pharmaceuticals, outside the submitted work. PGA reports consulting fees from EMD Serono; has participated on a data safety monitoring board or advisory board for Humanigen; and has personal stock or stock options with Johnson and Johnson. HBM reports investigator initiated research grants from Bristol Myers Squibb. GCA is a current member and past chair of the FDA Peripheral and Central Nervous System Advisory Committee, is a co-founding principal and equity holder in Monument Analytics, a health care consultancy with clients in the life sciences industry as well as plaintiffs in opioid litigation, and is a past member of OptumRx's National Pharmacy and Therapeutics Committee. BTG is a member of the FDA Pulmonary and Asthma Drug Advisory Committee, has received speaking fees from Gilead Sciences, and consulting fees from Janssen Research and Development. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
KMA received doctoral training support from the National Heart, Lung and Blood Institute Pharmacoepidemiology T32 Training Program (T32HL139426). ALO was supported by the Clinical and Translational Science Award from NCATS (UL1TR002649). JSi was supported by NCATS (UL1TR003096). RCP was supported by the NIH National Institute of Allergy and Infectious Diseases (K23AI120855). HBM is supported by the National Institute on Aging (1K01AG070329). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave (covid.cd2h.org/enclave) and supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organisations (covid.cd2h.org.dtas) and the organisations and scientists (covid.cd2h.org/duas) who have contributed to the ongoing development of this community resource: https://doi.org/10.1093/jamia/ocaa196. The authors acknowledge the following individuals for contributions to this work: Huijun An and Corey Joseph (Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA), Paul T Kocis (Penn State Health, Milton S Hershey Medical Center, Hershey, PA, USA), Saad Ljazouli (Palantir Technologies, Denver, CO, USA), Soko Setoguchi (Rutgers Center for Pharmacoepidemiology and Treatment Science, New Brunswick, NJ, USA), and Hythem Sidky (National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD, USA).
Contributors
KMA, BAB, and ESR did the literature search. KMA prepared the figures. All authors contributed to the study design. KMA, ALO, HBM were responsible for data collection. KMA did the data analysis. All authors contributed to data interpretation. KMA, ESR, and GCA wrote the original draft of the manuscript. All authors contributed to the writing, review, and editing of the manuscript. KMA and HBM directly accessed the data. KMA, ESR, and HBM verified the underlying data reported in the manuscript using reports from the statistical analyses performed in the N3C Enclave. All authors have seen and approved the final text.
N3C core teams
Principal investigators: Melissa A Haendel, Christopher G Chute, Kenneth R Gersing, Anita Walden. Workstream subgroup and administrative leaders: Melissa A Haendel, Tellen D Bennett, Christopher G Chute, David A Eichmann, Justin Guinney, Warren A Kibbe, Hongfang Liu, Philip R O Payne, Emily R Pfaff, Peter N Robinson, Joel H Saltz, Heidi Spratt, Justin Starren, Christine Suver, Adam B Wilcox, Andrew E Williams, Chunlei Wu. Data Ingest and Harmonization Team: Christopher G Chute, Emily R Pfaff, Davera Gabriel, Stephanie S Hong, Kristin Kostka, Harold P Lehmann, Richard A Moffitt, Michele Morris, Matvey B Palchuk, Xiaohan Tanner Zhang, Richard L Zhu. Phenotype Team: Emily R Pfaff, Benjamin Amor, Mark M Bissell, Marshall Clark, Andrew T Girvin, Stephanie S Hong, Kristin Kostka, Adam M Lee, Robert T Miller, Michele Morris, Matvey B Palchuk, Kellie M Walters. Project Management and Operations Team: Anita Walden, Yooree Chae, Connor Cook, Alexandra Dest, Racquel R Dietz, Thomas Dillon, Patricia A Francis, Rafael Fuentes, Alexis Graves, Julie A McMurry, Andrew J Neumann, Shawn T O’Neil, Usman Sheikh, Andréa M Volz, Elizabeth Zampino. Partners from NIH and other federal agencies: Christopher P Austin, Kenneth R Gersing, Samuel Bozzette, Mariam Deacy, Nicole Garbarini, Michael G Kurilla, Sam G Michael, Joni L Rutter, Meredith Temple-O’Connor. Analytics Team: Benjamin Amor, Mark M Bissell, Katie Rebecca Bradwell, Andrew T Girvin, Amin Manna, Nabeel Qureshi. Publication Committee Management Team: Mary Morrison Saltz, Christine Suver, Christopher G Chute, Melissa A Haendel, Julie A McMurry, Andréa M Volz, Anita Walden. Publication Committee Review Team: Carolyn Bramante, Jeremy Richard Harper, Wenndy Hernandez, Farrukh M Koraishy, Federico Mariona, Saidulu Mattapally, Amit Saha, Satyanarayana Vedula. We thank these core teams as well as key liaisons at data partner sites, regulatory staff at data partner sites, and individuals at the sites who are responsible for creating the datasets and submitting data to N3C.
Contributor Information
National COVID Cohort Collaborative Consortium:
Supplementary Material
References
- 1.Johns Hopkins University & Medicine Coronavirus resource center. https://coronavirus.jhu.edu
- 2.Mirjalili M, Shafiekhani M, Vazin A. Coronavirus disease 2019 (COVID-19) and transplantation: pharmacotherapeutic management of immunosuppression regimen. Ther Clin Risk Manag. 2020;16:617–629. doi: 10.2147/TCRM.S256246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2021;80:660–666. doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.England BR, Roul P, Yang Y, et al. Risk of COVID-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021 doi: 10.1002/art.41800. published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Silva KM, Jorge A, Cohen A, et al. COVID-19 Outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2021;73:914–920. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottio T, Bagozzi L, Fiocco A, et al. COVID-19 in heart transplant recipients: a multicenter analysis of the northern Italian outbreak. JACC Heart Fail. 2021;9:52–61. doi: 10.1016/j.jchf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heldman MR, Kates OS, Safa K, et al. COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study. Am J Transplant. 2021;21:2774–2784. doi: 10.1111/ajt.16692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen KM, Mehta HB, Palamuttam N, et al. Association between chronic use of immunosuppressive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US health system. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1488. published online Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:e93–e95. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28:427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett TD, Moffitt RA, Hajagos JG, et al. The National COVID Cohort Collaborative: clinical characterization and early severity prediction. medRxiv. 2021 https://doi.org/2021.01.12.21249511 published online Jan 23. (preprint). [Google Scholar]
- 15.National COVID Cohort Collaborative National COVID Cohort Collaborative phenotype and data acquisition. 2021. https://github.com/National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition
- 16.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwet J-P, Rivest L-P. Outlier resistant alternatives to the ratio estimator. J Am Stat Assoc. 1992;87:1174–1182. [Google Scholar]
- 18.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19. Ann Intern Med. 2021;174:33–41. doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Rubin DB, Thomas N. combining propensity score matching with additional adjustments for prognostic covariates. J Am Stat Assoc. 2000;95:573–585. [Google Scholar]
- 22.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173:761–767. doi: 10.1093/aje/kwq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 25.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 26.Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501–1518. doi: 10.1111/j.1475-6773.2006.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2021;80:e67. doi: 10.1136/annrheumdis-2020-218075. [DOI] [PubMed] [Google Scholar]
- 28.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40:2015–2021. doi: 10.1007/s00296-020-04699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code for this work can be found at https://github.com/National-COVID-Cohort-Collaborative/CS-ISC. The N3C Data Enclave (covid.cd2h.org/enclave) houses fully reproducible, transparent, and broadly available limited and de-identified datasets (Health Insurance Portability and Accountability definitions are available at https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html). Data are accessible by investigators at institutions that have signed a Data Use Agreement with the NIH, who have taken human subjects and security training, and who attest to the N3C User Code of Conduct. Investigators wishing to access the limited dataset must also supply an institutional review board protocol. All requests for data access are reviewed by the NIH Data Access Committee. A full description of the N3C Enclave governance has been published; information about how to apply for access is available on the NCATS website: https://ncats.nih.gov/n3c/about/applying-for-access. Reviewers and health authorities will be given access permission and guidance to aid reproducibility and outcomes assessment. A frequently asked questions page about the data and access has been created at https://ncats.nih.gov/n3c/about/program-faq.