Abstract
Background
Several SARS-CoV-2 variants of concern have been identified that partly escape serum neutralisation elicited by current vaccines. Studies have also shown that vaccines demonstrate reduced protection against symptomatic infection with SARS-CoV-2 variants. We explored whether in-vitro neutralisation titres remain predictive of vaccine protection from infection with SARS-CoV-2 variants.
Methods
In this meta-analysis, we analysed published data from 24 identified studies on in-vitro neutralisation and clinical protection to understand the loss of neutralisation to existing SARS-CoV-2 variants of concern. We integrated the results of this analysis into our existing statistical model relating in-vitro neutralisation to protection (parameterised on data from ancestral virus infection) to estimate vaccine efficacy against SARS-CoV-2 variants. We also analysed data on boosting of vaccine responses and use the model to predict the impact of booster vaccination on protection against SARS-CoV-2 variants.
Findings
The neutralising activity against the ancestral SARS-CoV-2 was highly predictive of neutralisation of variants of concern. Decreases in neutralisation titre to the alpha (1·6-fold), beta (8·8-fold), gamma (3·5-fold), and delta (3·9-fold) variants (compared to the ancestral virus) were not significantly different between different vaccines. Neutralisation remained strongly correlated with protection from symptomatic infection with SARS-CoV-2 variants of concern (rS=0·81, p=0·0005) and the existing model remained predictive of vaccine efficacy against variants of concern once decreases in neutralisation to the variants of concern were incorporated. Modelling of predicted vaccine efficacy against variants over time suggested that protection against symptomatic infection might decrease below 50% within the first year after vaccination for some vaccines. Boosting of previously infected individuals with existing vaccines (which target ancestral virus) is predicted to provide a higher degree of protection from infection with variants of concern than primary vaccination schedules alone.
Interpretation
In-vitro neutralisation titres remain a correlate of protection from SARS-CoV-2 variants and modelling of the effects of waning immunity predicts a loss of protection to the variants after vaccination. However, booster vaccination with current vaccines should enable higher neutralisation to SARS-CoV-2 variants than is achieved with primary vaccination, which is predicted to provide robust protection from severe infection outcomes with the current SARS-CoV-2 variants of concern, at least in the medium term.
Funding
The National Health and Medical Research Council (Australia), the Medical Research Future Fund (Australia), and the Victorian Government.
Introduction
The global spread of SARS-CoV-2 has resulted in substantial morbidity, mortality, and social disruption. Several vaccines have been deployed that protect against symptomatic SARS-CoV-2 infection. Vaccines in use incorporate the ancestral (Wuhan-like) virus or viral spike protein as an immunogen, and both vaccination and previous infection have been shown to provide a degree of protection against symptomatic and severe infection with essentially homologous virus.1 Several SARS-CoV-2 variants of concern have emerged that display increased transmissibility or reduced in-vitro neutralisation by sera from people who are infected with the ancestral strain or immunised.2, 3, 4 Initial reports from clinical trials or from breakthrough community infections suggest that current vaccines might be less protective against symptomatic infection with some SARS-CoV-2 variants.5, 6 Additionally, studies also show that waning antibody tires correlate with reduced protection over time.7, 8 Thus, a major question is the extent to which existing vaccines are likely to protect against variants of concern and how existing vaccines might be used to boost responses to variants.
Titres of neutralising antibodies elicited by current vaccines have been shown to vary by as much as 25-fold.8 Studies analysing vaccine-induced neutralising antibody responses have reported varying reductions in neutralisation titre against variants of concern. Several randomised controlled trials and case-control studies have reported vaccine efficacy against symptomatic and severe infection with SARS-CoV-2 variants of concern.5, 9 Our previous work has shown a correlation between titres of neutralising antibodies (normalised for differences in the assays used) and protection from SARS-CoV-2 infection and has derived a model for predicting vaccine efficacy from mean neutralisation titres.8 In this study, we analysed studies of neutralisation against SARS-CoV-2 variants, with the aim of predicting the efficacy of existing vaccines against variants of concern, new vaccines, and vaccines after waning and boosting.
Research in context.
Evidence before this study
Vaccine-elicited neutralising antibodies responses have reduced neutralising activity against SARS-CoV-2 variants of concern, and reduced vaccine protection from infection with variants of concern has also been observed. The association between neutralisation titres and vaccine efficacy against variants of concern is unclear. Neutralising antibodies wane over time after infection or vaccination, and there is evidence that vaccine efficacy against variants of concern also wanes over time. Understanding how waning immunity and viral variation will affect future vaccine protection and the need for booster vaccines is a public health priority. We searched PubMed between inception and June 30, 2021, for studies (key search terms: “(SARS-CoV-2) AND (variant) AND (neutralising antibodies) OR (efficacy) AND (vaccine)”) and monitored other public sources of information, such as Twitter. We identified 17 studies using live virus assays to examine neutralisation titres against ancestral virus and variants of concern in convalescent and vaccinated individuals from which data were either readily extractable from the original publications or were sent by authors upon request. Additionally, we identified seven studies reporting the efficacy or effectiveness of SARS-CoV-2 vaccines against variants of concern (consisting of a mix of randomised controlled trials and test-negative case-controls) and eight studies reporting neutralising antibody titres following boosting of convalescent or vaccinated individuals from which data were available.
Added value of this study
Previous work has identified that neutralising antibodies to ancestral SARS-CoV-2 virus are a strong predictor of protection from symptomatic infection with a similar virus. Here we extend that work to show that neutralising antibodies are also highly predictive of protection from SARS-CoV-2 variants of concern. Combining data on the decrease in neutralisation titre to variants of concern and the waning of neutralising antibodies with time, we use a statistical model to predict vaccine efficacy to variants of concern over the first year after vaccination. Our analysis suggests that waning immunity and the decrease in neutralisation to the variants of concern is expected to lead to less than 50% efficacy against symptomatic infection with the delta variant within 1 year of vaccination for current vaccines. Analysis of data on the impact of vaccination (boosting) of previously infected individuals with mRNA vaccines shows that boosting leads to high tires of neutralising antibodies, which are predicted to provide protection from symptomatic infection with variants of concern, at least over the first year.
Implications of all the available evidence
The rapid global spread of SARS-CoV-2 variants of concern raises several immunological, public health, and ethical questions about the duration and effectiveness of vaccine-induced protection and the need for vaccine boosting. Our work shows that neutralisation titres can be used to predict vaccine efficacy against variants of concern. This work will be of use to vaccine developers in using immunobridging to predict the efficacy of novel vaccines. Additionally, this work provides useful guidance in predicting vaccine efficacy against novel SARS-CoV-2 variants as they are observed, informing the optimal deployment of current vaccines and the development of the next generation of vaccines. Our work predicts a major loss of protection from symptomatic infection with current variants of concern during the first year after vaccination, which might be reversed by a third booster vaccination. Further work will be needed to confirm these predictions and to assess vaccine protection from severe disease over the longer term.
Methods
Comparison of cross reactivity of different vaccines
For this meta-analysis, we identified 17 published studies2, 3, 4, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 that directly compared neutralisation titres against ancestral virus and the variants of concern, and for which data were either provided in, or were readily extractable from, the original publications or sent by authors (appendix pp 15–16), amounting to 1305 individual neutralisation titres against variants. We focused primarily on assays using live SARS-CoV-2 virus (to reduce the potential variability that might arise from different pseudoviral constructs23), with the exception of the NVX-CoV2373 vaccine, for which only data from neutralisation assays using a spike-expressing pseudovirus were available. This work was approved under the UNSW Sydney Human Research Ethics Committee (approval HC200242).
We calculated the geometric mean decrease in neutralisation titre across different vaccines and variants (comparing with ancestral virus). To assess the impact of vaccine type and laboratory on cross-reactivity in the aggregated data, we performed a multiple linear regression accounting for variant-specific, vaccine-specific, and laboratory-specific effects. We used censoring to account for neutralisation titres below the limits of detection of the assay (appendix pp 7–14).
Estimating neutralisation after boosting
To estimate the impact of boosting on neutralisation, data were taken from studies of boosting of previously infected or vaccinated individuals and were included if the boosting data could be compared with data from naive vaccinated individuals from the same laboratory using the same assay (appendix pp 1–14). To estimate geometric mean neutralisation titre in boosted individuals normalised relative to the mean of convalescent individuals (as defined previously8), we multiplied the fold increase in neutralisation titre conferred with boosting by the normalised neutralisation titre reported in naive individuals in the phase 1/2 trials for each vaccine (appendix p 18).
Predicting the efficacy for variants on the basis of the previously developed model
Previously, we developed and fitted a model of vaccine efficacy to data on the immunogenicity of seven vaccines in phase 1/2 trials and protective efficacy in phase 3 trials (against symptomatic and severe SARS-CoV-2 infection).8 We used this model, as originally published and parameterised, to predict the efficacy of vaccines against each variant, using the fold-change in neutralisation titre estimated against each variant in this study.
Estimating decay in vaccine efficacy
To model the decay in vaccine efficacy we first estimated the normalised neutralisation titres expected to give an initial target efficacy (ie, 95%, 90%, 80%, or 70%) against infection with the ancestral virus (using inverse of supplementary equation 8; appendix p 10). We then allowed these initial normalised neutralisation titres to decay over the first 360 days with a half-life of 108 days (as estimated in Khoury et al8, using data from Dan et al24) and assumed that neutralisation of variants was reduced by the fold changes estimated in this study, and estimated the predicted decrease in efficacy from the model (supplementary equation 8; appendix p 10). Boosting was assumed to increase the normalised geometric mean neutralisation titre to the mean reported in the boosting studies (appendix p 18). The decay rate of neutralisation before and after boosting was assumed to be the same.25 The lower bound on efficacy estimates from the model was determined using a bootstrapping approach (appendix pp 7–14).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
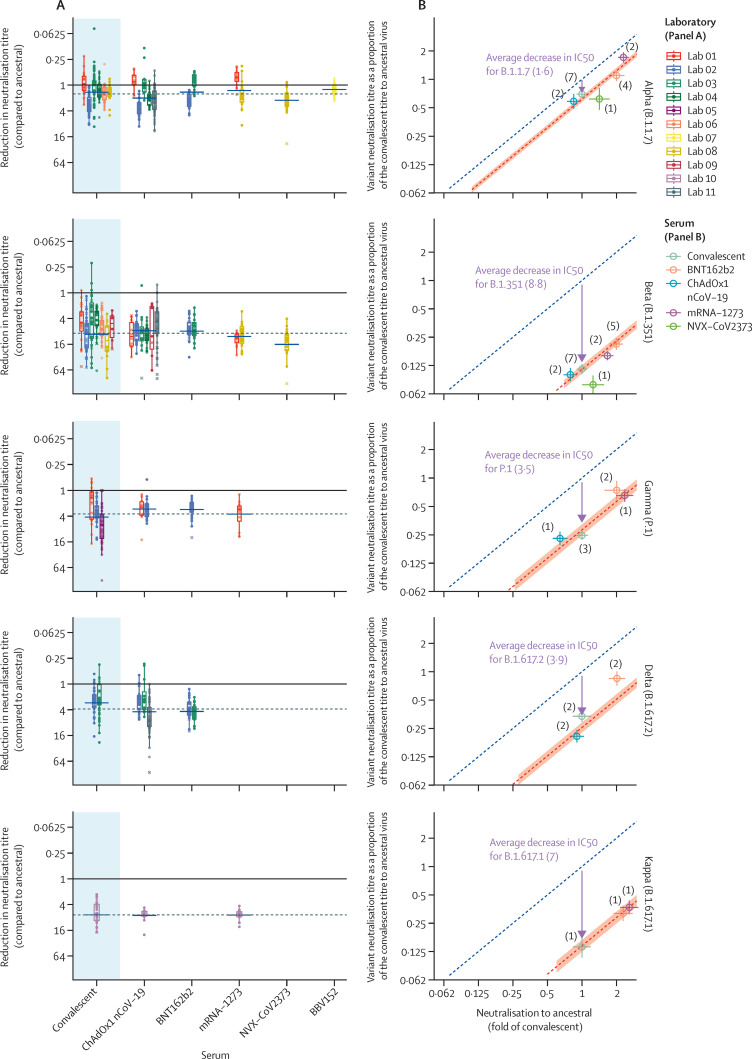
We first aimed to understand whether different vaccines elicit variable cross-reactive neutralisation of SARS-CoV-2 variants. When comparing decreases in neutralisation titre for a given variant between the 17 identified studies, we observed that there was frequently more variability in titres between different laboratories studying the same vaccine than between different vaccines (figure 1A ). Different laboratories used distinct in-vitro assays to measure neutralisation of SARS-CoV-2 (appendix pp 15–16).23 However, within a given study the observed decrease in neutralisation titre for a given variant was very similar for both convalescent and vaccinee serum, suggesting a strong study-specific (or assay-specific) effect (appendix p 21). Our multiple regression model showed that neutralisation against a particular variant was strongly associated with neutralisation against the ancestral virus and that, after adjusting for variant and laboratory effects, whether immunity was acquired through infection or vaccination (and which vaccine was used) was not significantly associated with the loss of neutralisation (p=0·26, likelihood ratio test; figure 1B; appendix p 22). This finding does not imply that all vaccinee serum neutralised variants equally well, rather that the loss of neutralisation of a variant (ie, the average fold decrease in neutralisation against a given variant compared with ancestral virus) was similar between all vaccines studied. Because all vaccines have a similar loss of variant recognition, neutralisation against the ancestral virus can be used to predict neutralisation against the current variants of concern (figure 1B; appendix p 22). The geometric mean of the decrease in neutralisation titres was found to be 1·6-fold for alpha (95% CI 1·5–1·7; 9 studies), 8·8-fold for beta (8·0–9·7; 9 studies), 3·5-fold for gamma (3·1–4·0; 3 studies), and 3·9-fold for delta (3·5–4·4; 3 studies) compared with the ancestral virus (appendix p 23).
Figure 1.
In-vitro neutralisation of SARS-CoV-2 variants of concern
(A) The change in neutralisation titre between the ancestral virus and different SARS-CoV-2 variants for either convalescent individuals (left) or those immunised with different vaccines is shown. Individual colours reflect different studies or laboratories (appendix pp 15–16). Solid dots indicate where titres were measurable for both ancestral and variant neutralisation. Crosses indicate where one titre fell below the limit of detection for that assay. Different studies estimate different changes in neutralisation titre even for the same vaccine or variant combination. The dashed horizonal line indicates the censored mean decrease in titre for a given variant (across all vaccine and convalescent samples), and blue horizontal bars indicate the censored mean titre for a given vaccine or variant combination. The boxes extend between the first and third quartiles, and the whiskers extend to 1·5 times the IQR. (B) The correlation between the mean neutralisation titre against the ancestral virus (x-axis) and mean neutralisation titre against the variants of concern (y-axis) is shown. The predicted line for a 1:1 association is indicated (dashed blue line). The observed mean decrease in neutralisation titre across all vaccines and convalescent individuals is indicated by an arrow (with the length of the arrow representing the decrease in neutralisation titre) and the predicted variant neutralisation is indicated by a dashed red line (shading indicates the 95% CI). Tints indicate the mean neutralisation for a given vaccine or variant combination, averaging across available studies (number of studies indicated).
Several studies have now shown reduced efficacy of vaccination against infection with SARS-CoV-2 variants (appendix p 17). These studies incorporated a variety of study designs, including both randomised controlled trials and observational case-control studies. In addition to these differences in study design, other factors, such as the mechanisms for identifying variant infection and the definitions of mild, moderate, and severe infection, also differed by study (appendix p 17). Despite the variability in study design, we found that predicted serological neutralisation activity against each variant elicited by vaccines was significantly correlated with protection from symptomatic SARS-CoV-2 infection (r S=0·81, p=0·0005; appendix p 24).
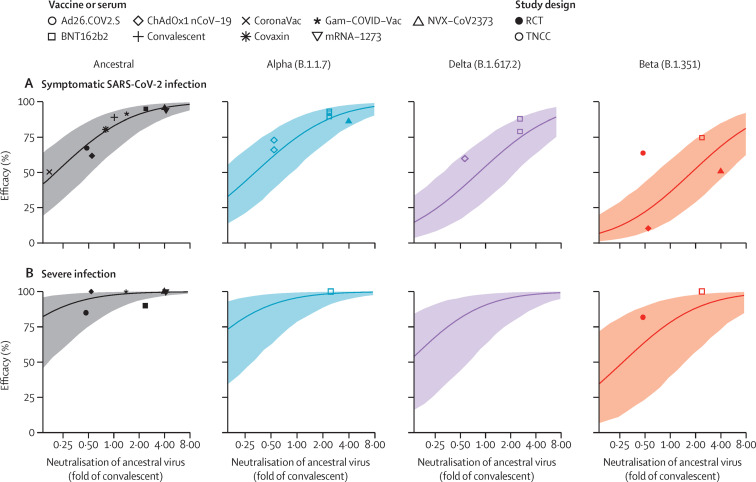
To test whether our previously developed predictive model could also be used to predict efficacy against variants of concern, we took the same model (parameterised from ancestral virus) and applied the geometric mean decrease in neutralisation titres (appendix p 23) to effectively shift the curve for each variant (appendix pp 7–14). These efficacy (effectiveness) studies showed very good agreement with the model predictions for each vaccine, with 13 (93%) of 14 studies falling within the 95% CI of our model (figure 2A ; appendix p 17). These CIs reflect the known sources of uncertainty, including uncertainty in estimates of neutralisation against ancestral virus, uncertainty in estimates of the decrease in neutralisation for each variant, and model-associated uncertainty (appendix p 18). We note that the four randomised controlled trials are placed relatively symmetrically around the predictive line (one above and three below the mean prediction line; figure 2A). However, all 10 observational test-negative case-control studies are placed above the line, indicating a potential role for the design of study in the observed efficacy. Figure 2A shows that our model can use the neutralisation of a new candidate vaccine against ancestral virus to predict efficacy (with a lower bound) against all current variants of concern. Furthermore, our model can predict the efficacy of all current vaccines against a new variant once the fold decrease in neutralisation to that variant has been determined.
Figure 2.
Predicting vaccine efficacy against SARS-CoV-2 variants
The association between mean neutralisation of ancestral SARS-CoV-2 and protection against symptomatic (A) and severe infection (B) with different variants is shown. The line indicates the model prediction of efficacy for a given neutralisation against ancestral virus. Shading indicates 95% CI based on uncertainties in measuring mean neutralisation titre against ancestral virus, the loss of neutralisation against each variant and in model parameters. Individual points shown represent results of different studies of vaccine efficacy against ancestral virus (black) or SARS-CoV-2 variants. Details of studies of ancestral virus are outlined in Khoury et al8 (all of which are randomised controlled trials), and studies of variants of concern are outlined in the appendix (p 17).
Predicting vaccine efficacy against severe SARS-CoV-2 infection is substantially more challenging, owing to the small number of severe infections captured in most efficacy studies (fewer than 100 severe infections were reported across all the studies combined in the original development of the model8). However, even with this limitation, vaccination has been shown to provide significantly better protection against severe disease than against symptomatic SARS-CoV-2 infection.8 Thus, we next considered our model's prediction of efficacy against severe outcomes with variants of concern (figure 2B). The 95% CIs were substantially broader for severe than for symptomatic SARS-CoV-2 infection, indicating the greater uncertainty in the data on protection from severe disease. Furthermore, the point estimates of efficacy from the clinical studies contain considerable uncertainty (appendix p 19). Even so, all but one of the efficacy studies falls within or above the predicted efficacy confidence limits. It should be noted that our model assumes neutralisation alone drives protection against severe disease, but it is possible that non-neutralising antibody functions and recall of other cellular responses might have a crucial role in modulating disease severity, and thus the model might underestimate efficacy against severe SARS-CoV-2 infection.
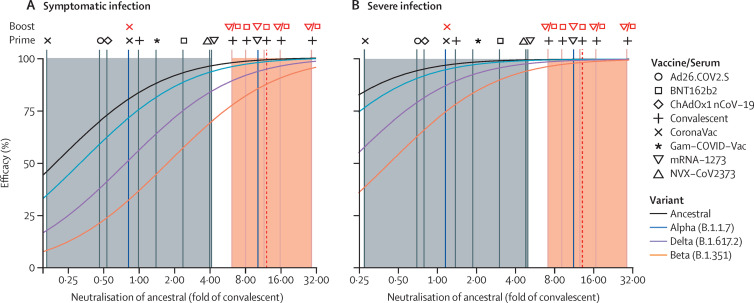
A major question is whether boosting with existing vaccines (that all incorporate only the ancestral spike) will be effective in providing protection against the variants, particularly in the context of waning immunity. Several studies have compared neutralising antibody responses after vaccination of previously infected individuals with those of naive individuals.26, 27, 28, 29, 30 These studies have found that vaccination with a single dose of mRNA vaccine is sufficient to boost responses in previously infected individuals (and indeed a second dose has minimal effect).28, 29 Aggregating these studies, we found that vaccination of convalescent individuals led to 2–10-fold higher neutralisation titres than that seen in naive individuals after primary vaccination (figure 3 ; appendix p 19)26, 27, 28, 29, 30, 31 and improved cross-reactivity to variants.27, 28, 29, 30 Although increases varied between studies, vaccination of convalescent individuals with mRNA vaccines led to boosting of normalised neutralisation titres to 12-fold (range 6–29) higher than that seen in early convalescence (and higher than that seen in any current vaccination regimen; figure 3). This boost in neutralisation titre is expected to increase efficacy against the variants of concern. For example, considering naive or previously infected individuals vaccinated with BNT162b2, the predicted efficacy against the delta variant is expected to increase from 75% (95% CI 53–89) for a naive individual to 95% (85–98) for a previously infected individual (using the previously published model8).
Figure 3.
Predicted effect of a booster dose on neutralising antibody responses
The normalised neutralisation titres (ie, neutralisation titres divided by the geometric mean of a convalescent cohort) against ancestral virus observed following initial vaccination (vertical grey lines) and the effects of vaccination of previously infected individuals (vertical orange lines) are shown. Results for individual studies are indicated as vertical lines, and symbols above the lines indicate the vaccine(s) used and infection history. The geometric mean of boosting seen in previously infected individuals (from all studies) is shown as a dashed red line. Shaded areas indicate the range of mean neutralisation observed following vaccination of naive (grey) or previously infected (orange) individuals. Two studies of a third booster dose in previously vaccinated individuals are shown as vertical blue lines (and the vaccines used are indicated with symbols above the lines). The modelled association between neutralisation and protection from ancestral (black) or variants of concern are shown as coloured curves for either any symptomatic SARS-CoV-2 infection (A) or severe infection (B).
Data on the effects of boosting in vaccinated individuals were available from only two studies (figure 3). Wu and colleagues32 showed that a third dose of mRNA-1273 delivered 6 months after the initial vaccination boosted neutralisation titres by 23-fold compared with pre-boost titres, or 2·5-fold higher than vaccination of naive individuals.31 Pan and colleagues33 studied the effects of a third dose of CoronaVac delivered either 1 month or 6 months after the second dose. They found that a third vaccination at 6 months boosted responses 4·9-fold higher than seen after the initial two-dose regimen. Boosting at 1 month after the initial two-dose regimen led to only a 1·3–2·1-fold increase, suggesting that delayed boosting might be required.
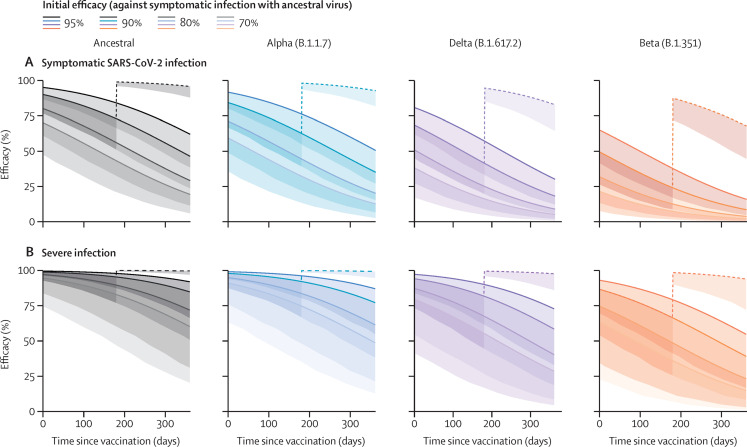
A previous study24 showed a decrease in neutralising antibody titres over the first 8 months of infection, with a half-life of around 3–4 months, and another7 showed a decrease in vaccine protection over this period. We modelled the decrease in immune protection over the first year after vaccination due to the decrease in neutralisation titre against variants of concern and the observed waning in neutralising antibody titres (decaying with a half-life of 108 days;8 figure 4 ). For example, previous infection is thought to provide about 90% protection against the ancestral virus early after infection, but, by 6 months, protection is predicted to decrease to 41% against the delta variant and 24% against the beta variant. However, boosting at 6 months with an mRNA vaccine is expected to increase normalised neutralising antibody titres to 12-fold that of early convalescent titres, leading to protection of 95% for the delta variant and 87% for the beta variant soon after boosting. Although the decay of neutralisation titres after boosting is similar to that after primary infection,25 boosting is still predicted to provide 68% protection from symptomatic infection (lower bound of 95% CI 45) and 94% protection from severe infection (lower bound of 95% CI 72) even against the beta variant of concern 6 months after boosting (figure 4). Together these data and modelling suggest that vaccination of previously infected individuals, even with existing vaccines targeting ancestral virus, will provide robust protection against the current variants of concern, considerably prolonging the duration of efficacy of existing vaccines against these variants.
Figure 4.
Predicted protection from SARS-CoV-2 infection and the effect of an additional booster dose at 6 months
The predicted protection over time is shown for four hypothetical vaccines that initially provide 95%, 90%, 80%, or 70% protection against symptomatic infection with the ancestral virus. It is assumed that neutralisation decays with a half-life of 108 days and variant neutralisation decreases as estimated (appendix p 23). Solid lines are mean model predictions, and shading indicates the lower bound of the 95% CI (indicating the minimal predicted efficacy). The dashed line indicates the predicted effect of boosting previously infected individuals with BNT162b2 or mRNA-1273 6 months after their primary infection (geometric mean of all boosting studies; appendix p 24) and assumes decay after boosting is the same as after initial infection or primary vaccination.
Discussion
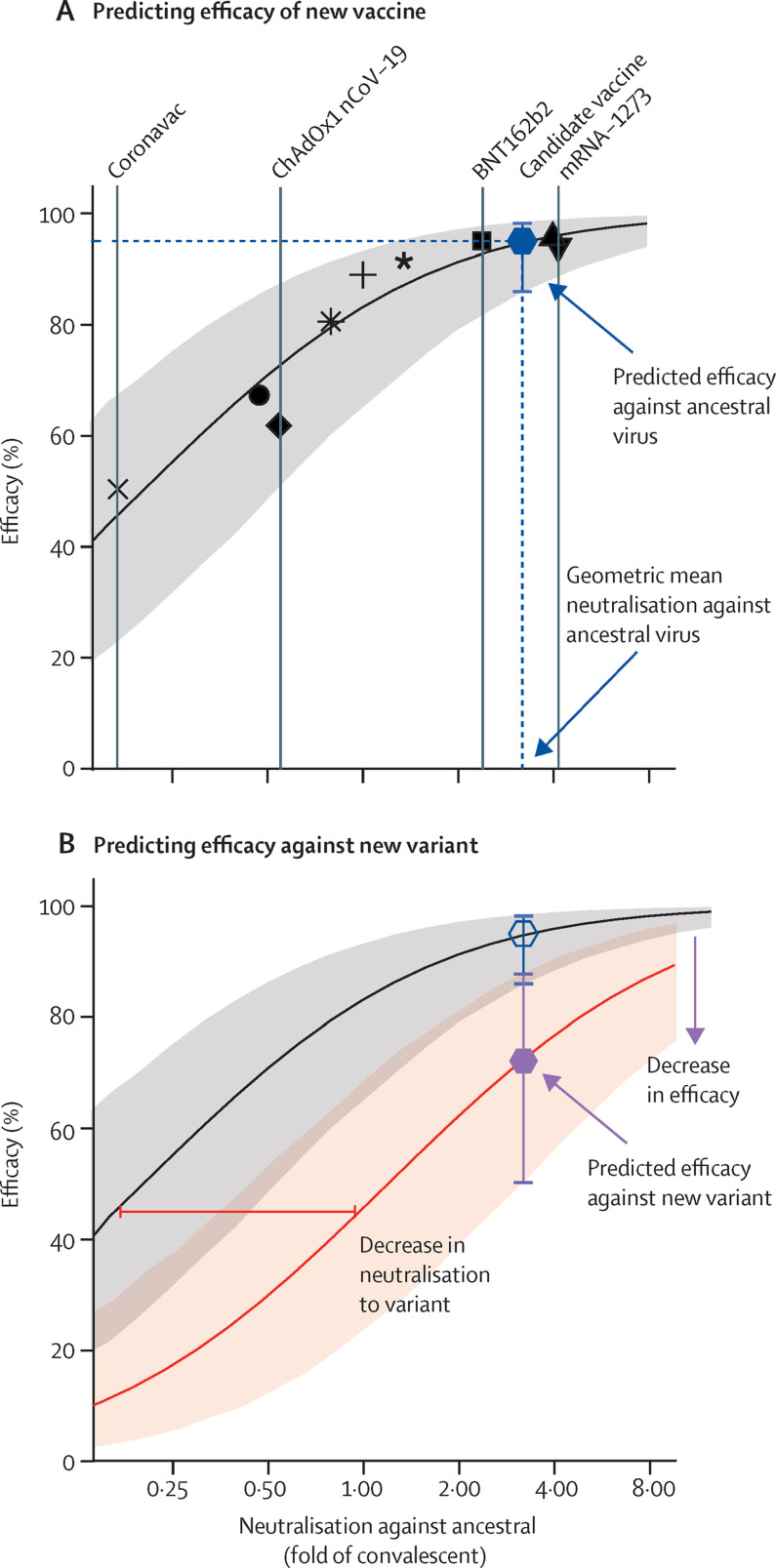
In this study, we integrated data from in-vitro neutralisation assays and efficacy studies incorporating several vaccines in widespread use and used a previously derived model8 to predict vaccine efficacy from in-vitro neutralisation titre against SARS-CoV-2 variants. We found that normalised neutralisation titres remain strongly correlated with protection against SARS-CoV-2 variants and the observed protection is consistent with the predictions of the previous model. A feature of this model that is likely to be useful for future vaccine development, is that it provides not just a predicted efficacy, but also a lower confidence bound on the predicted efficacy against each variant for vaccines of a given potency (measured as neutralisation titre to ancestral virus). It should be noted that all reported vaccine efficacies were above this lower bound. The consistency of the model suggests that it will be useful in predicting the efficacy of new vaccines or the efficacy of current vaccines against new variants (figure 5 ). For new vaccines, the mean antibody titre against the ancestral virus should be determined in 20–30 individuals relative to a panel of existing vaccines and our model can predict its efficacy against both ancestral virus and variants of concern. Similarly, our model can predict the efficacy of all existing vaccines against a new variant once the mean decrease in neutralisation titre for the variant compared with ancestral virus has been determined (preferably across multiple assays, because of the observed assay variation).
Figure 5.
Predicting protective efficacy for new vaccines or new variants
(A) The association between neutralisation and protection can be used for immunobridging to predict vaccine efficacy from in-vitro neutralisation titres. The geometric mean neutralisation titre from 20–30 individuals around 2 weeks after vaccination needs to be measured. These neutralisation titres can then be normalised against sera from convalescent individuals (1–3 months after infection with ancestral SARS-CoV-2 strain), serum from individuals recently vaccinated with common vaccines, and the international standards. This neutralisation (vertical dashed blue line) can then be used to predict efficacy against the ancestral virus (black curve and grey 95% CI). (B) To predict efficacy against a new variant, we need to first estimate the difference in neutralisation titre between the ancestral and new variant SARS-CoV-2 (ideally averaging the decrease measured in different assays across four or more laboratories). This difference in titre can then be used to shift the curve of efficacy against the variant (red arrow, red curve, and pink shaded 95% CI) to the right. The predicted efficacy and 95% CI of the new vaccine against the new variant (purple hexagon and whiskers) can then be ascertained.
However, although the model predictions are consistent with observed vaccine efficacy against symptomatic SARS-CoV2 infection, we have relatively little data on vaccine efficacy against severe SARS-CoV-2 infection and mortality. In part, this is because of limitations in using the test-negative case-control design to estimate efficacy against severe outcomes, given potential difficulties of matching severe SARS-CoV-2 outcomes in PCR-negative controls. Thus, although vaccine protection against severe infection is explored in our study, it is also the result for which we have the lowest confidence based on existing data. The results also hint that the design of study might have a role in the observed efficacy, with all 10 observational test-negative case-control studies placed above the line. It is known that test-negative study designs have several potential confounders,34 and our analyses suggest future studies should investigate whether test-negative case-control studies tend to over-estimate vaccine efficacy.
The combined effects of waning immunity and reduced recognition of the SARS-CoV-2 variants of concern suggest that vaccine boosters might be needed to maintain protection of more than 50% against symptomatic SARS-CoV-2 infection. Our analysis suggests that maximising neutralising antibody responses to the ancestral virus, through booster vaccination of previously infected individuals (with ancestral immunogens), should be an effective strategy to broadly increase neutralisation titres against SARS-CoV-2 variants. The benefits of boosting noted here raise several questions. First, the optimal timing of boosting is unclear. Most boosting studies in convalescent or previously vaccinated individuals occurred around 6 months after infection or vaccination. One study33 comparing early with late boosting of vaccinees would appear to suggest a benefit in delaying to 6 months. Better responses with a larger interval before boosting are consistent with an observed increase in the number of memory B cells within the first months after infection.35 Second, it is unclear whether all vaccines will boost immunity to a similar extent, or whether homologous or heterologous boosting might be preferred or made necessary by anti-vector immunity. However, three doses of a potent mRNA vaccine32 appear to produce greater neutralisation compared with three doses of lower potency vaccine,33, 36 suggesting that high vaccine potency is necessary for maximal boosting. Third, the effects of boosting on efficacy are not clear, although studies37, 38 suggest that vaccination of previously infected individuals leads to increased protection compared with vaccination of naive individuals. Finally, separate from the effects of boosting on immune protection, the ethical challenges of providing a third vaccine dose to selected populations, while others have not received any vaccination, are considerable.39
Our study focused on the effects of boosting with the ancestral SARS-CoV-2 spike immunogen in individuals initially infected with ancestral virus. However, a likely future approach is to develop variant-specific booster vaccines. One study32 compared variant-specific (booster) immunisation with a beta (B.1.351) variant SARS-CoV-2 spike mRNA construct with immunisation with the existing vaccine carrying the ancestral strain. Immunisation with a beta spike immunogen led to greater boosting of the beta-specific response (by a further 10% relative to boosting with the ancestral strain),32 but at the cost of reduced boosting of responses to the ancestral and gamma strains (neutralisation titres against ancestral and gamma strains after boosting with the variant were only 50–60% of the titre achieved using the ancestral spike-based vaccine). Hence, immune imprinting or cross-reactivity to previous immunogens might need to be considered in strategies aimed at boosting neutralising responses to new variants.4, 40
The main limitations of this study centre around difficulties in aggregating neutralisation data from multiple laboratories, most of which use different assays. Additionally, few studies report data on vaccine efficacy or effectiveness against variants of concern, especially for severe outcomes. Our model's predictions of vaccine efficacy against variants of concern, and efficacy over time, therefore have wide CIs. The largest contributing factor to these wide CIs is the uncertainity in our estimate of the mean neutralisation titre for each vaccine (once normalised to convalescent individuals). A standardised assay for assessing neutralisation would greatly improve comparability of results across different laboratories. A consistent definition for severe disease outcomes in clinical trials and observational studies would also assist with the aggregation and comparison of vaccine efficacy from different vaccines against current variants.
In conclusion, our analysis suggests that good protection against the current variants of concern can be achieved by vaccination with existing vaccines (using ancestral spike targets) and that boosting with these vaccines is probably an effective strategy to combat waning of immunity and the current variants of concern. In the future, vaccines targeting novel variants of concern might be required if highly escaped variants arise, but existing vaccines provide an effective method for boosting immunity against the current variants of concern.
Data sharing
All data and code are available on GitHub.
Declaration of interests
DSK is elected executive committee member of the New South Wales branch of the Australian and New Zealand Industrial and Applied Mathematics society. MPD is senior editor for eLife, for which he receives an annual retainer. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by an Australian government Medical Research Future Fund awards GNT2002073 (to MPD, SJK, and AKW), MRF2005544 (to SJK, AKW, JAJ, and MPD), MRF2005760 (to MPD), MRF2007221 (to JAT and MS), an NHMRC programme grant GNT1149990 (SJK and MPD), and the Victorian Government (SJK, AKW, and JAJ). JAJ, DSK, and SJK are supported by NHMRC fellowships. AKW, DC, and MPD are supported by NHMRC Investigator grants. This work would not be possible without the many scientists who generously provided the published data analysed in this study, either through making the data directly available through the original publication or through providing it upon request. The authors thank these scientists for their contribution and the individual sources of data are indicated in the references and appendix.
Contributors
DC, MS, JAT, DSK, and MPD contributed to conceptualisation, supervision, and resources. DC, MS, AR, TES, DSK, and MPD contributed to data curation, methods, formal analysis, and visualisation. All authors contributed to the data collection and writing and reviewed and approved the final report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54:1636–1651. doi: 10.1016/j.immuni.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 3.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220. doi: 10.1016/j.cell.2021.06.020. 36.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. medRxiv. 2021 doi: 10.1056/NEJMoa2110345. published online Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 9.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27:717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939. doi: 10.1016/j.cell.2021.03.055. 54.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulkner N, Ng KW, Wu MY, et al. Reduced antibody cross-reactivity following infection with B.1.1.7 than with parental SARS-CoV-2 strains. eLife. 2021;10 doi: 10.7554/eLife.69317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supasa P, Zhou D, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1·1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201. doi: 10.1016/j.cell.2021.02.033. 11.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Casner RG, Nair MS, et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747. doi: 10.1016/j.chom.2021.04.007. 51.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen X, Tang H, McDanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29:529. doi: 10.1016/j.chom.2021.03.002. 39.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X, Tang H, Pajon R, et al. Neutralization of SARS-CoV-2 variants B.1.429 and B.1.351. N Engl J Med. 2021;384:2352–2354. doi: 10.1056/NEJMc2103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker M, Dulovic A, Junker D, et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edara VV, Pinsky BA, Suthar MS, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wall EC, Wu M, Harvey R, et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet. 2021;398:207–209. doi: 10.1016/S0140-6736(21)01462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury DS, Wheatley AK, Ramuta MD, et al. Measuring immunity to SARS-CoV-2 infection: comparing assays and animal models. Nat Rev Immunol. 2020;20:727–738. doi: 10.1038/s41577-020-00471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccination induces durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021 doi: 10.1126/science.abm0829. published online Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anichini G, Terrosi C, Gandolfo C, et al. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med. 2021;385:90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leier HC, Bates TA, Lyski ZL, et al. Previously infected vaccinees broadly neutralize SARS-CoV-2 variants. medRxiv. 2021 doi: 10.1101/2021.04.25.21256049. published online April 29. (preprint). [DOI] [Google Scholar]
- 28.Stamatatos L, Czartoski J, Wan YH, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372:1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas C, Vogels CBF, Yildirim I, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021 doi: 10.1038/s41586-021-04085-y. published online Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi A, Koch M, Wu K, et al. Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. bioRxiv. 2021 doi: 10.1101/2021.06.28.449914. published online June 28. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu K, Choi A, Koch M, et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv. 2021 doi: 10.1101/2021.05.05.21256716. published online May 6. (preprint). [DOI] [Google Scholar]
- 33.Pan H, Wu Q, Zeng G, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18–59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv. 2021 doi: 10.1101/2021.07.23.21261026. published online July 25. (preprint). [DOI] [Google Scholar]
- 34.Evans SJW, Jewell NP. Vaccine effectiveness studies in the field. N Engl J Med. 2021;385:650–651. doi: 10.1056/NEJMe2110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12 doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Yang J, Wang L, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv. 2021 doi: 10.1101/2021.08.03.21261544. published online Aug 8.. (preprint). [DOI] [Google Scholar]
- 37.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Protection afforded by the BNT162b2 and mRNA-1273 COVID-19 vaccines in fully vaccinated cohorts with and without prior infection. medRxiv. 2021 doi: 10.1101/2021.07.25.21261093. published online July 26. (preprint). [DOI] [Google Scholar]
- 38.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maxmen A. COVID boosters for wealthy nations spark outrage. Nature. 2021 doi: 10.1038/d41586-021-02109-1. https://www.nature.com/articles/d41586-021-02109-1 published online July 30. [DOI] [PubMed] [Google Scholar]
- 40.Wheatley AK, Fox A, Tan H-X, et al. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021;42:956–959. doi: 10.1016/j.it.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code are available on GitHub.