ABSTRACT
In this study, we performed a year-long in situ incubation experiment on a common ferrous sulfide (Fe-S) mineral, pyrite, at the oxidative deep seafloor in the hydrothermal vent field in the Izu-Bonin arc, Japan, and characterized its microbiological and biogeochemical properties to understand the microbial alteration processes of the pyrite, focusing on Fe(II) oxidation. The microbial community analysis of the incubated pyrite showed that the domain Bacteria heavily dominated over Archaea compared with that of the ambient seawater, and Alphaproteobacteria and Gammaproteobacteria distinctively codominated at the class level. The mineralogical characterization by surface-sensitive Fe X-ray absorption near-edge structure (XANES) analysis revealed that specific Fe(III) hydroxides (schwertmannite and ferrihydrite) were locally formed at the pyrite surface as the pyrite alteration products. Based on the Fe(III) hydroxide species and proportion, we thermodynamically calculated the pH value at the pyrite surface to be pH 4.9 to 5.7, indicating that the acidic condition derived from pyrite alteration was locally formed at the surface against neutral ambient seawater. This acidic microenvironment at the pyrite surface might explain the distinct microbial communities found in our pyrite samples. Also, the acidity at the pyrite surface indicates that the abiotic Fe(II) oxidation rate was much limited at the pyrite surface kinetically, 3.9 × 103- to 1.6 × 105-fold lower than that in the ambient seawater. Moreover, nanoscale characterization of microbial biomolecules using carbon near-edge X-ray absorption fine-structure (NEXAFS) analysis showed that the sessile cells attached to pyrite excreted the acidic polysaccharide-rich extracellular polymeric substances at the pyrite surface, which can lead to the promotion of biogenic Fe(II) oxidation and pyrite alteration.
IMPORTANCE Pyrite is one of the most common Fe-S minerals found in submarine hydrothermal environments. Previous studies demonstrated that the Fe-S mineral can be a suitable host for Fe(II)-oxidizing microbes in hydrothermal environments; however, the details of microbial Fe(II) oxidation processes with Fe-S mineral alteration are not well known. The spectroscopic and thermodynamic examination in the present study suggests that a moderately acidic pH condition was locally formed at the pyrite surface during pyrite alteration at the seafloor due to proton releases with Fe(II) and sulfidic S oxidations. Following previous studies, the abiotic Fe(II) oxidation rate significantly decreases with a decrease in pH, but the biotic (microbial) Fe(II) oxidation rate is not sensitive to the pH decrease. Thus, our findings clearly suggest that the pyrite surface is a unique microenvironment where abiotic Fe(II) oxidation is limited and biotic Fe(II) oxidation is more prominent than that in neutral ambient seawater.
KEYWORDS: biomineralization, microbe-mineral-metal interactions
INTRODUCTION
Submarine hydrothermal environments provide unique and diverse biomes that are significantly based on the primary biomass generated by chemolithoautotrophic microorganisms (1, 2). Considering the potential energy of hydrothermal environments for biomass generation, researchers originally focused on chemolithoautotrophic and/or syntrophic microorganisms that acquire energy from dissolved inorganic compounds [e.g., H2S, H2, CH4, and Fe(II)] in the surrounding seawater (3, 4). However, quantitative estimation of potential energy sources to support microorganisms in the hydrothermal environments indicates that the chemical energy specified for these microorganisms is significantly provided in the form of solid mineral substrates (5). For instance, a quantitative calculation by McCollom (6) indicated that sulfide minerals deposited in the hydrothermal vents represent at least 40% of the total energy available for chemoautotrophic microorganisms hosted in the hydrothermal environments. In the past, researchers have made efforts to understand the microbial community in the sulfide minerals, including inactive chimneys (5, 7, 8); however, at present, the metabolic processes of microorganisms that use the solid sulfide minerals as energy sources are not well known in these environments.
Ferrous sulfide (Fe-S) minerals (e.g., pyrite, marcasite, pyrrhotite, and mackinawite) are the most common and reactive sulfide minerals found in active/inactive submarine hydrothermal environments (9, 10). They are formed by direct precipitation from the hydrothermal fluid and/or plume around the vent (9). Since the Fe-S minerals contain iron (Fe) and sulfur (S) in reduced forms, they undergo oxidative weathering through interaction with ambient seawater at the seafloor. It has been suggested that the release of these electron donors contributes to the metabolic energy of Fe- and S-oxidizing chemolithoautotrophs under aerobic conditions (5, 11, 12). The end product of Fe(II) oxidation, Fe(III) species, has a much lower solubility in the neutral-pH range and is rapidly precipitated as insoluble Fe(III) (hydr)oxides at the Fe-S mineral surface in the ambient seawater, while sulfate as the end product of sulfide oxidation is soluble and flows out in the seawater. Hence, microbial oxidation of Fe(II) in Fe-S minerals includes a unique geobiological process that requires mineralogical and geochemical investigations, in addition to microbiological investigation. In fact, a certain amount of Fe(III) (hydr)oxides is widely observed as an alteration product of the marine Fe-S minerals (see reference 9 and references therein). Edwards et al. (11) directly demonstrated the existence of Fe-oxidizing bacteria that colonize several Fe-S minerals, based on in situ incubation study at the Juan de Fuca Ridge. Recent studies have shown phylogenetic evidence that Fe(II)-oxidizing bacteria host the Fe-S minerals and Fe-S mineral-rich chimneys on the seafloor near hydrothermal and nonhydrothermal environments (13–15). In addition, cultural cultivation and enrichment studies also showed that the Fe-oxidizing bacteria are involved in the Fe-S minerals incubated at the seafloor (5, 12).
It is also known that Fe-S minerals have a unique geochemical property that releases strong acidity during oxidative alteration (16). Because the pH of seawater is kept around neutral in most marine environments, it is expected that a distinctive acidic environment is locally formed at the surface of Fe-S minerals where they interact with the oxidative seawater. This might mean that a unique microbial community, secondary mineral formation, and biogeochemical processes would occur on the Fe-S minerals during seafloor weathering. Considering the biological Fe(II) oxidation reaction in aquatic environments, a detailed understanding of mineralogical and biogeochemical processes could be key information for estimating possible reactions for microbial growth and kinetics of biological Fe(II) oxidation during Fe-S mineral alteration. For instance, previous studies have reported that changes in pH can significantly control the contributions of biotic and abiotic Fe(II) oxidation in aqueous environments (17–19). However, there have been few studies that have performed biogeochemical characterization in addition to microbial community characterization during Fe-S mineral alteration.
In the present study, we performed a year-long in situ incubation/alteration experiment with a common Fe-S mineral, pyrite (FeS2), at the low-temperature deep seafloor around the hydrothermal vent field in the Izu-Bonin arc, Japan. In addition, we aimed to simultaneously characterize the microbial community and mineralogical and biogeochemical properties of the incubated pyrite. For the mineralogical and biogeochemical characterizations, two advanced synchrotron X-ray spectroscopic techniques, X-ray absorption fine-structure analysis (XAFS) and scanning transmission X-ray microscopy (STXM), were applied, which allowed us to quantitatively specify secondary minerals and microbial biomolecules formed in the incubated pyrite with high elemental selectivity and high spatial resolution. In addition, the in situ incubation method applied in the present study has advantages: the observation of microbial activity in situ, which included culturable and nonculturable microbes in the really deep seafloor; and the correlative investigation of the relationship between the entire oxidation history of pyrite and microbial activity. Thus, through this study, we could directly link the microbial activity and mineralogical and biogeochemical information left in the pyrite sample.
RESULTS AND DISCUSSION
Microbial community analysis of the incubated pyrite.
We used high-throughput amplicon sequencing of the microbial 16S rRNA gene from the incubated pyrite samples (at 8 and 12 months) and ambient seawater to investigate microbial community structures. Through data processing and quality filtering, we obtained amplicon sequences with an average length of 411 bp covering the V4-to-V5 region of the 16S rRNA gene for the 8-month pyrite sample (110,565 [74% in raw reads]), 12-month pyrite sample (146,525 [71%]), and ambient seawater sample (83,029 [66%]), respectively. At a 97% identity cutoff, 479, 571, and 316 operational taxonomic units (OTUs) were generated in the 8- and 12-month pyrite and ambient seawater samples, respectively.
As seen in Fig. 1a, at the domain level, pyrite samples were entirely dominated by the domain Bacteria (>99.8%) over Archaea in both incubation periods, while a certain extent of Archaea (22.1%) was contained in the ambient seawater sample (numerical data are shown in Table S3 in supplemental material). This dominance is consistent with previously reported microbial communities of samples containing Fe-S minerals (e.g., inactive chimneys and in situ-incubated Fe-S minerals) (5, 7, 12, 15, 20). In addition, this strong dominance assumes that most microbial cells found in our microscopic analyses (epifluorescence, electron, and X-ray microscopic analyses) of the pyrite samples are bacterial cells. The bacterial community at the class level in Fig. 1b shows that the proportion in both pyrite samples was largely codominated by Alphaproteobacteria (45.8 to 48.5%) and Gammaproteobacteria (22.8 to 27.8%), while this trend was not found in the ambient seawater as a transport medium for microorganisms. This might be in agreement with a previous work showing that geochemistry strongly influences the bacterial community on a given substrate, as well as temperature and location (21). Previous studies also reported a similar dominance of Alphaproteobacteria and Gammaproteobacteria in the Fe-S mineral-included marine samples (7, 12–14, 20, 21), which seems to be a distinct feature of the bacterial community of Fe-S mineral-included samples. Sylvan et al. (13) reported that Alphaproteobacteria (∼18%) and Gammaproteobacteria (∼33%) dominated the Fe-S mineral-rich inactive chimney collected from hydrothermal vent fields. Barco et al. (12) also reported that the bacterial community of incubated pyrrhotite (FeS) was codominated by Alphaproteobacteria (37%) and Gammaproteobacteria (44%). On the other hand, lower detection of Zetaproteobacteria, including important chemolithoautotrophic Fe(II) oxidizer group (Mariprofundus spp.), was found in our pyrite samples (Zetaproteobacteria, <0.01%), which indicates that activity of Zetaproteobacteria was limited on the surface of pyrite. In addition, the substantial number of Zetaproteobacteria (1.2%) were detected in the ambient seawater sample. The seawater sample was collected right above the incubation device placed at the base of active and inactive hydrothermal vents. At the foot of active vents, red-colored Fe(III) hydroxide mats were in situ precipitated (see Fig. 6d below), which indicates that the active vents were associated with Fe(II)-oxidizing environments. Thus, the detection of Zetaproteobacteria in the ambient seawater might result from the Fe(III) hydroxide mats floating in the seawater, though this is a speculation.
FIG 1.
(a) Microbial community structure of incubated pyrite (8- and 12-month samples) and ambient seawater in the level of domain. (b) Bacterial community structure in the level of class. In panels b and c, classes with a relative abundance of <1% were merged and indicated as “Others.” (c) Bacterial community structure in the level of order. Numerical data in panels a to c are shown in Table S3 in the supplemental material.
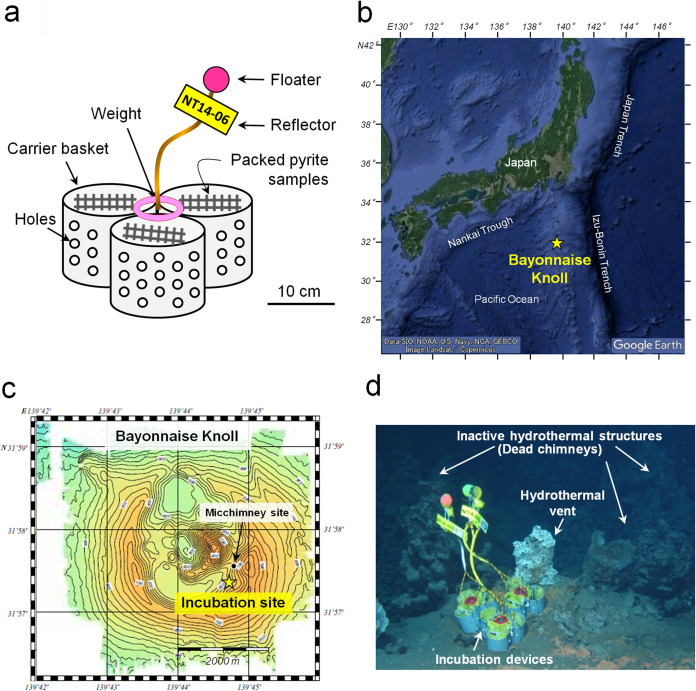
FIG 6.
(a) Schematic image of the incubation device used in this study. (b) Geographic overview of the Bayonnaise Knoll. (c) Bathymetric map of hydrothermal vent field in the Bayonnaise Knoll caldera. A yellow star indicates the in situ incubation site. (d) Dive photograph of the incubation site with incubation devices.
At the order level, Rhodospirillales was the most abundant (25.8 to 34.0%) in both incubation periods, and the orders Ectothiorhodospirales (13.9 to 18.3%), Rhodobacterales (4.9 to 5.0%), Pirellulales (4.4 to 4.5%), Caulobacterales (1.7 to 5.3%), Thiotrichales (1.4 to 1.7%), and Alteromonadales (1.0 to 1.4%) were abundant, as shown in Fig. 1c. These orders were also abundant in previously reported Fe-S mineral-associated marine samples (12–14). Picardal et al. (22) reported the isolation of heterotrophic Fe(II)-oxidizing bacteria of Dechrolospirillum sp. from an Fe-rich creek sediment, which belongs to the order Rhodospirillales, the most abundant bacterial order in our samples. Edwards et al. (11) also reported two isolates of chemolithoautotrophic Fe(II)-oxidizing bacteria from natural Fe-S minerals collected at Juan de Fuca Ridge. These isolates belonged to the orders Caulobacterales and Alteromonadales, which were also abundant in our pyrite samples. Barco et al. (12) isolated a chemolithoautotrophic Fe(II)-oxidizing bacterium (Thiomicrospira sp.) from an enrichment using pyrrhotite incubated at a shallow seafloor in Santa Catalina Island. The isolate belongs to Thiotrichales, which was also abundant in our pyrite samples. These reports and our findings indicate that at the order level, microbes that may be involved in the Fe(II) oxidation reaction were abundantly observed in our pyrite samples. In addition, in the seawater sample, abundant Alphaproteobacteria (22.6%) and Gammaproteobacteria (20.7%) were also observed at the class level. At the order level, groups abundant in the Alphaproteobacteria and Gammaproteobacteria in the pyrite samples, Rhodospirillales, Ectothiorhodospirales, Rhodobacterales, and Caulobacterales were all minor in the seawater samples (Rhodospirillales, 2.4%; Ectothiorhodospirales, 0.2%; Rhodobacterales, 1.0%; Caulobacterales, 0.1%) (Table S3c). Thus, community structures of the Alphaproteobacteria and Gammaproteobacteria in the pyrite were largely different from that in the seawater, while both classes were abundant in both pyrite and seawater samples. A more detailed discussion of the microbial community (e.g., analysis at a level below genus and ecological diversity analysis) will be compiled in a separate paper and is not addressed in this study.
Microscale observation by epifluorescence and electron microscopes.
To obtain information on the morphology of microbe cells (mainly assumed as bacterial cells) and cell density on the pyrite surface, we stained cells on the pyrite with Syto9 (Fig. 2a to d). Images showed that there were various types of cellular morphologies, including short/long-rod, coccus, and short-spiral shapes. The estimated cell densities on the pyrite surface were 1.1 × 105 (±3.8 × 104) cells/mm2 for the 8-month sample and 1.2 × 105 (±4.2 × 104) cells/mm2 for the 12-month sample, showing a slight increase with incubation period. It should be noted that the cell density values are conservative estimates and are predominantly based on the counting of cells observed in a single-layer biofilm. The cell densities of quartz particles (as negative control) in both 8- and 12-month samples were within 1.0 × 103 cells/mm2 (data not shown), which is about 100 times lower than the density of the incubated pyrite. The higher cell density of pyrite clearly suggests that the pyrite composed of reduced-Fe and -S could be a favorable microbial (bacterial) habitat to acquire metabolic energy for growth on the oxidative seafloor (e.g., aerobic Fe and S oxidation). Edwards et al. (11) reported, based on in situ cultivation at the seafloor, that cell densities in several incubated Fe-S minerals, including pyrite, ranged from 0.7 × 105 to 5.0 × 106 cells/mm2 in a 2-month incubation, and the cell density of the pyrite was 1.5 × 105 cells/mm2. Barco et al. (12) also reported cell densities of (5.6 to 8.9) × 103 cells/mm2 in pyrrhotite incubated in a shallow seafloor for approximately 3 months. Compared to terrestrial environments, studies on microbial colonization of pyrite in acid mine drainage (AMD) sites reported cell densities ranging from 2 × 103 cells/mm2 to a maximum value of 8 × 104 cells/mm2 (23, 24). Thus, the cell densities in this study ([1.1 to 1.2] × 105 cells/mm2) were in the range of previously reported values for the Fe-S minerals and were reasonably close to the value of pyrite incubation at the seafloor reported by Edwards et al. (11). The surface morphology of pyrite particles before and after the incubation was studied by scanning electron microscopy (SEM) at a microscale (Fig. 2e to g). Although a plane face was found on the pyrite surface before incubation (Fig. 2e), a large number of altered products with irregular morphology appeared on the incubated pyrite surfaces (Fig. 2f, 8 months, and Fig. 2g, 12 months). Previous studies (5, 11, 12, 25, 26) have reported the occurrence of biological coiled Fe products on the incubated Fe-S minerals as a morphological signature showing the existence of a stalk-forming Zetaprotetobacteria Fe(II) oxidizer (Mariprofundus spp.). However, the appearance on the surface was very limited. In fact, the proportion of Zetaproteobacteria in the pyrite samples was also limited (<0.01%) in the microbial community. Previously reported Zetaproteobacteria Fe(II) oxidizers are typically neutrophiles (87): hence, the pyrite surface, where is expected to be an acidic environment, may not be a suitable environment for them. These findings may indicate a low contribution of the Zetaproteobacteria Fe(II) oxidizer to Fe(II) oxidation in the present pyrite alteration. On the other hand, our findings are based on the limited incubation periods of 8 and 12 months: thus, particularly at the initial incubation stage (∼8 months), the possibility that other lineages might play transitional roles in the pyrite alteration cannot be denied.
FIG 2.
(a to d) Microbe cells (mainly assumed to be bacterial cells) on incubated pyrite particles stained with Syto9. (e to g) SEM images of pyrite surface. (e) Pyrite before incubation. (f and g) Pyrite incubated for 8 months and 12 months, respectively.
Mineralogical characterization and Fe speciation.
The SEM analysis showed the formation of altered products on the pyrite surface, including the microbial cells. Geochemical and mineralogical characterizations of the altered products often provide valuable information to speculate about probable reactions and microbial metabolism processes occurring on the pyrite surface. We first performed bulk X-ray diffraction (XRD) and X-ray absorption near-edge structure (XANES) analyses to gain insight into the mineralogy of the altered products. In the XRD patterns of both 8- and 12-month samples in Fig. S1 in the supplemental material, strong peaks corresponding to pyrite were observed, but no peaks derived from the altered products were observed. This detection failure indicates that the fraction of the altered materials was lower than the detection limit of the XRD analysis. Additionally, it should be noted that if the altered products are composed of poorly crystalline minerals, the bulk XRD analysis is not sensitive to them (27), which is consistent with the lower peak intensities of pyrite compared with that before the in situ incubation (Fig. S1). Figure 3a shows the bulk Fe XANES spectra of incubated pyrite samples and standard materials (siderite, pyrite, jarosite, hematite, goethite, ferrihydrite, and schwertmannite). The Fe XANES analysis can determine Fe chemical (or mineral) species independent on the degree of crystallinity. However, the spectral features of 8- and 12-month pyrite samples were almost identical to those of pyrite standard, as shown in Fig. 3a. The quantitative fitting result also reveals a similar trend—that is, the entire dominance of pyrite fraction. These results also suggest that it is difficult to identify the Fe alteration products in pyrite, even by bulk Fe XANES. The results of bulk XRD and XANES analyses consistently suggest that the amounts of altered materials are much smaller than those of pyrite. The altered minerals may accumulate locally on the pyrite surface, because it could be assumed that the alteration processes mainly proceeded at the pyrite surface in contact with the ambient seawater. From this point of view, we believe that the pyrite surface possesses biogeochemical information during the pyrite alteration process under incubation. Hence, we attempted to apply a surface-sensitive XANES technique, the conversion electron yield (CEY) XANES technique, to selectively identify the altered Fe product at the surface. This technique generally reflects information on the species at the submicrometer scale from the particle surface due to the escape depth of inelastic Auger electrons, depending on the Fe-bearing material species (28). Estimating the probing depth of Auger electrons with the physicochemical properties of pyrite and the universal curve equation proposed by Schroeder (29), it has been shown that the probing depth of CEY Fe XANES for pyrite is <100 nm from the surface (28).
FIG 3.
Fe K-edge XANES spectra of pyrite samples before and after in situ incubation obtained by bulk (a) and CEY (b) measurements. In panels a and b, fitting spectra (dotted lines) were overlapped on the experimental spectra (solid lines) with each component contribution. (c and d) Results of linear combination fittings using bulk Fe XANES (c) and CEY Fe XANES (d).
Figure 3b shows the CEY Fe XANES spectra of the incubation samples and standards. Remarkably, the spectral features of the incubation samples drastically changed from those of the bulk Fe XANES spectra. That is, the intensity of the peak at 7,117 eV specific for pyrite drastically decreased in both 8- and 12-month samples, but a prominent peak around 7,129 eV, which is typical for Fe(III) (hydr)oxides, arose instead (Fig. 3b). This clearly indicates the dominance of Fe(III) (hydr)oxides as Fe-bearing altered products on the pyrite surface (<100 nm). In addition, we should note that the CEY Fe XANES of the pyrite before incubation was almost identical to that of standard pyrite, indicating that there was no alteration product at the surface of the initial pyrite. To quantitatively determine the Fe species in the altered products, a liner-combination fitting was performed. Prior to the fitting, principal-component combination was determined using the spectra of possible components (pyrite, siderite, magnetite, jarosite, hematite, goethite, ferrihydrite, and schwertmannite). The number of components for the analysis was limited to three, according to previous studies (30, 31). As a result, the combination of principal components with the lowest R factor was schwertmannite [FeIII8O8(OH)6(SO4)], ferrihydrite [amorphous FeIII(OH)3], and original pyrite in both 8- and 12-month samples. The quantitative fitting result in Fig. 3d (see Table S4 in the supplemental material) shows 30% schwertmannite and 27% ferrihydrite for the 8-month sample. The 12-month sample contained a larger fraction of schwertmannite (46%) and the same level of ferrihydrite (22%) added to the pyrite. Thus, the dominance of Fe(III) hydroxides (57 to 68%) was observed during both periods. Therefore, these findings strongly suggest that the Fe(III) hydroxides schwertmannite and ferrihydrite were locally formed at the 100-nm surface by the seafloor incubation. These Fe(III) hydroxides were not observed on the quartz glass (control) sample, which also suggests that the Fe(III) hydroxides were the products formed as a result of pyrite alteration. In addition, these findings suggest that the weathering processes involving Fe(II) oxidation proceeded actively on the pyrite surface during incubation. In addition, the fraction of Fe(III) hydroxides increased with the incubation period (from 57% in 8-month samples to 68% in 12-month samples). This is consistent with the pyrite alteration process as an irreversible reaction.
Thermodynamic investigation.
Using the surface-sensitive XANES technique, we found a hidden local Fe(II) oxidation and formation of Fe(III) alteration products at the pyrite surface, schwertmannite and ferrihydrite, which were not detected in the bulk analyses. Previous studies also found similar Fe(III) alteration products at the surface of altered Fe-S minerals (25, 32); however, they have not been characterized and quantified surface sensitively. Our study surface sensitively quantified the specific Fe(III) hydroxides at the surface of altered Fe-S minerals in marine environments by the advanced Fe CEY-XANES technique. The Fe(III) hydroxides are often found in natural aquatic environments through biotic and abiotic Fe(II) oxidation and subsequent precipitation (33). Previous studies have shown that the thermodynamic stability of Fe(III) hydroxide species is largely dependent on physicochemical parameters (e.g., pH and redox conditions and solution components) (34). This implies that Fe(III) hydroxide species and proportions provide information to regulate the thermodynamic and kinetic aspects of the microbial metabolism occurring on the pyrite surface. The ferrihydrite is a ubiquitous Fe(III) hydroxide in widespread neutral aquatic environments, from freshwater to marine systems, aquifers to hydrothermal hot springs, and soils (34). Relatively, the schwertmannite is a “rare” mineral because of its unique formation condition being acidic and sulfate rich (35). It has been reported that the favorable pH of schwertmannite formation ranges between 2.5 and 4.0 in freshwater systems (36).
We show a thermodynamic Fe species diagram parameterized by pH and dissolved O2 in Fig. 4, assuming specific temperature and pressure conditions at the incubation site (5°C and 8,000,000 Pa). The diagram consistently shows that the schwertmannite is the thermodynamically dominant phase in acidic pH between 3.6 and 5.8, and the ferrihydrite is the dominant species at higher pH (>5.8) at the incubation site. Based on our spectroscopic XANES results, the ratios of Fe(III) hydroxides on the pyrite surface (<100 nm) were 30% schwertmannite to 27% ferrihydrite in the 8-month sample and 46% schwertmannite to 22% ferrihydrite in the 12-month sample. The thermodynamic calculation with the ratios estimates that pH values at the pyrite surface were pH 5.7 (8-month sample) and pH 4.9 (12-month sample). Because the seawater pH at the incubation site was 7.5 (Table 1), this suggests that moderately acidic pH conditions were locally formed at the pyrite surface, and that the pyrite surface is a unique acidic microenvironment against neutral ambient seawater.
FIG 4.

Thermodynamic Fe species diagram parameterized by pH and dissolved O2, assuming specific temperature and pressure conditions at the incubation site (5°C and 8,000,000 Pa). According to previous geochemical modeling of Fe species in AMD (85), Fe and sulfur activities were assumed to be 1.0 × 10−3 and 1.0 × 10−2 M, respectively. Gray bars stand for dissolved O2 level and pH at the incubation site studied in this study. The thermodynamic calculation was conducted with the MINTEQ computer program (86). Field data of dissolved major cations and anions were used as model inputs. Ionic strength corrections were made using the Pitzer equation. Carbonate and HCO3− activities were estimated from the alkalinity data of the ambient seawater at the incubation site.
TABLE 1.
Chemical properties of the ambient seawater at the incubation site
| Property | Value |
|---|---|
| Temp, °C | 5.1 |
| Salinity, ‰ | 34 |
| pH | 7.5 |
| Dissolved oxygen, mg/kg | 3.59 |
| Alkalinity, meq/kg | 2.69 |
| Dissolved Fe(II) or sulfidic-S, mg/kg | Undetectable (<0.05) |
| Dissolved Fetotal, Cutotal, or Zntotal, mg/kg | Undetectable (<0.01) |
The reason for the occurrence of the acidic environment would be due to the oxidative alteration of pyrite. When pyrite is exposed to oxidative water, the oxygenation of sulfide in the pyrite releases dissolved Fe(II) and protons (equations 1 to 3), and subsequent oxygenation of dissolved Fe(II) and Fe(III) hydroxide formation also release protons (equations 4 and 5) (16, 24). Thus, it is expected that the protons released by the pyrite oxidation and Fe(III) hydroxide formation result in the low-pH environment on the pyrite surface. In addition, similar releases of protons and acidity occur in the oxidative alteration of the other sulfide and Fe-S minerals (16, 24).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The low-pH condition at the pyrite surface might explain the distinct microbial community found in our pyrite samples, showing the dominance of the domain Bacteria with abundant Alphaproteobacteria and Gammaproteobacteria at the class level (Fig. 1b). A similar trend was consistently found in previously reported microbial communities of marine samples containing Fe-S minerals (7, 12, 15, 20), as mentioned above. However, to the best of our knowledge, no studies have investigated the effect of pH on microbial communities in Fe-S mineral-associated marine samples. The AMD microbial community can be a good analog to discuss the pyrite incubated at the seafloor in this study because the low-pH condition in the AMD site also results from similar biotic and abiotic alteration processes of sulfide minerals (predominantly Fe-S minerals) under oxidative conditions (16, 24). Interestingly, several previous AMD studies reported that the microbial community in AMD is likely to be dominated by the domain Bacteria, with abundant Alphaproteobacteria and Gammaproteobacteria (37–39). Using a high-throughput gene sequencing technique and ecological diversity analysis, Kuang et al. (39) indicated that Alphaproteobacteria, Gammaproteobacteria, and Nitrospira particularly have a strong adaptation to acidic environments in AMD sites. pH is the controlling factor structuring the microbial community across different environments (39–41), as well as the other major factors, such as the solution conductivity and rainfall (42) and oxygen gradient (43) at the local scale. In addition, many studies have repeatedly shown that pH is strong determinant of the bacterial community in normal environments, such as soil (44–47). Notably, even in the extreme AMD environments, the degree of acidity is still the definitive factor structuring the bacterial community (39). Thus, these reports suggest that the lower pH at the pyrite surface observed in the present study could considerably control the bacterial community structure. The acidic environment could be a factor structuring the common trend found in the microbial communities of our and previous Fe-S mineral-associated marine samples.
Kinetic investigation.
pH is an important factor to control the abiotic Fe(II) oxidation kinetics. Previous studies have shown that abiotic Fe(II) oxygenation (oxidation by dissolved O2) rates in solution are extremely sensitive to pH between values of approximately 4 and 8, depending on the change in Fe(II) species. In this pH range, the oxygenation kinetics follows the rate law (48–50)
where brackets indicate activities and k (liters3 mol−3 min−1) represents the rate constant. The equation means that the rate is to be second order with respect to [OH−]. Thus, a 100-fold decrease in the rate of reaction occurs for a unit decrease in pH in the given [Fe(II)] and [O2] (48–50). Previous studies based on field studies have demonstrated that the same rate law can be applied in natural seawater with a salinity of 31 to 36‰ (50, 51), while they reported half-times of Fe(II) nearly 100 times larger than those observed in freshwater at the same pH. In the present study, the thermodynamically estimated pH at the pyrite surface ranged between 4.9 and 5.7 against the ambient seawater of pH 7.5 (Table 1), which was 1.8 to 2.6 units lower. This unit difference indicates that the abiotic Fe(II) oxidation rate at the pyrite surface would be 3.9 × 103- to 1.6 × 105-fold lower than that in the ambient seawater at the incubation site, based on simple calculation with the rate law (49). On the other hand, the rate of biotic (microbial) Fe(II) oxidation does not decrease with a decrease in pH, as reported in previous studies (19). According to the raw rate of microbial Fe(II) oxidation [by an acidophilic Fe(II) oxidizer] developed by Pesic et al. (52), the microbial oxidation rates should rather increase at lower pH. This is also consistent with the biochemical model illustrated by Nordstrom and Southam (53), where ATP synthesis depends on proton diffusion from the surrounding medium into the cell. A lower external pH creates a steeper proton gradient through the cell wall leading to more rapid ATP synthesis. In addition, several terrestrial AMD studies on biotic and abiotic Fe(II) oxidation have clearly shown that the rate of Fe(II) oxidation in the field is much higher than that abiotic Fe(II) oxidation under sterile laboratory conditions around pH 5 (17–19). These studies indicate that biotic (microbial) activity increases the Fe(II) oxidation rates beyond the abiotic rates under low-pH conditions. Clair et al. (54) also reported, based on a microcosm experiment with natural freshwater, that when the pH decreased about 2 units (from pH 7.3 to pH 5.6), the net Fe(II) oxidation rate decreased by only 1 order of magnitude. They estimated that the Fe(II)-oxidizing bacteria were responsible for more than 85% of the net Fe(II) oxidation rate at pH 5.6 (54). Therefore, these reports and our estimates suggest that the pyrite surface is a unique microenvironment where abiotic Fe(II) oxidation is limited and biotic (microbial) Fe(II) oxidation is likely to be more prominent than that in neutral ambient seawater. Of course, the terrestrial field data may only be an analog to discuss the Fe(II) oxidation rate in the deep seafloor field.
Nanoscale observation by X-ray microscopy.
Mineralogical speciation on the pyrite surface and the thermodynamic and kinetic investigations showed the possibility that biogenic Fe(II) oxidation is more dominant at the acidic pyrite surface. To determine how microbes (mainly assumed as bacteria) inhabit the solid Fe(II) substrate or respond to the acidic stress at the pyrite surface, we applied the STXM technique to obtain information on the biogenic Fe(II) oxidation mechanism in the microbe-pyrite interaction. The STXM-based near-edge XAFS (NEXAFS) analysis offers multiple advantages: a high spatial resolution of 50 nm, a nondestructive analytical technique with a high elemental specificity, and X-ray spectroscopic chemical speciation useful for the analysis of hydrated biomolecules (55, 56). Thus, the STXM technique can be a useful tool for investigating the mechanism of biological processes occurring at the microbe-pyrite interface based on carbon (C) and Fe chemical speciation (57).
In this study, we analyzed a total of 35 cells attached to pyrite particles in the 12-month incubation sample using the STXM technique. In some of the cells, Fe(III) hydroxides as end products of Fe(II) oxidation were found in the cell-pyrite interface and/or on the cells, which are likely to be involved in biogenic Fe(II) oxidation (alteration) of solid pyrite. In this study, we present two representative results. The STXM-based Fe and C images of the microbial cells on the pyrite surface are shown in Fig. 5a to f. The Fe speciation in several regions of interest (ROIs) was performed by the STXM-based Fe 2p NEXAFS, divided into the regions of pyrite, pyrite-cell interface, and cell. Previous studies have reported that the peak-top energies of Fe 2p NEXAFS typically shift depending on the Fe(II) species (707.5 eV) and Fe(III) species (709.2 eV) (58), whereas the spectral shape is almost the same among the Fe(III) hydroxide species (59). The peak-top energy in the spectra of the cell-pyrite interface (ROIs 2, 5, and 6) and Fe particles on the cell (ROI 1) overlapped with that of the Fe(III) peak top, while the pyrite region (ROIs 3 and 7) is close to that of the Fe(II) (pyrite) peak-top (Fig. 5g). In addition, the spectral features of the interface regions were identical to those of Fe(III) hydroxides such as ferrihydrite and schwertmannite of model compounds. This finding indicates the existence of Fe(III) hydroxides at the cell-pyrite interface, which is also in agreement with the Fe CEY XANES results showing the dominance of Fe(III) hydroxides at the pyrite surface. The STXM-based C 1s NEXAFS spectra of the cell and cell-pyrite interface regions are shown in Fig. 5h. The spectral features of the model compounds in C NEXAFS indicated clear differences among acidic and neutral polysaccharides, lipid, protein, and nucleic acid. (Functional groups and transitions corresponding to each peak are summarized in Table S5 in the supplemental material.) In particular, the peak-top energies of these molecules also vary depending on the molecular species (55, 60), thus we distinguished the localization of major microbial biomolecules using the fingerprinting C NEXAFS. The spectrum of the cell region (ROIs 4 and 8 in Fig. 5h) includes specific peaks corresponding to aromatic (I), aliphatic (II), amide (III), and O-alkyl (V) C, which suggests that the cell regions mainly contain abundant protein, lipid, and nucleic acid. In contrast, a significant difference was observed in the spectra of the cell-pyrite interface regions. The spectra of the interface regions (ROIs 2 and 6) mainly include carboxyl [peak (IV)], typical for acidic polysaccharides, whereas low contributions of aliphatic (II), amide (III), and O-alkyl (V) peaks were found compared to the cell regions. The peak-top energies of ROIs 2 and 6 slightly but clearly shifted to higher energies and were close to that [peak (IV) at 288.6 eV] of acidic polysaccharide (alginate). These findings suggest that acidic polysaccharide materials are mainly localized at the pyrite-cell interface. A similar localization of the acidic polysaccharides at the cell-pyrite interface was also reported in our previous study on bacterial pyrite leaching experiments (57, 61). In addition, sessile cells attached to the pyrite surface in our 12-month incubation sample were successfully stained with the lectin concanavalin A (ConA), with specificity for polysaccharides (see Fig. S2 in the supplemental material), which supports the spectroscopic results from the C NEXAFS. These findings and reports consistently indicate that the sessile bacteria attached to pyrite excreted polysaccharide-rich extracellular polymeric substances (EPSs) at the pyrite surface. Recent studies have recognized the important role of EPSs at the cell-mineral interface in pyrite bio-oxidation (bioleaching). Mitsunobu et al. (57) reported that organic ligands in acidic exopolysaccharide-rich EPS increase pyrite solubility by complexing Fe(II) in the pyrite and subsequently accelerating the biogenic Fe(II) oxidation. The key function of microbial EPSs is the mediation of the initial attachment of cells to different substrata and/or protection against environmental stress and dehydration. Previous studies on bacterial pyrite leaching suggested that complexation of metals with acidic polysaccharides in the EPSs allows bacterial cells to have a positive charge (62–64), which leads to preferential attachment of bacteria to the negative pyrite surface by electrostatic interactions. In addition, Singh et al. (65) reported that EPSs generally have a functional role to maintain optimal pH conditions and localized solute concentrations. Fowler et al. (66) implied that the polysaccharide-rich EPSs formed during the bacterial pyrite leaching are responsible for pH buffering from the acidity at the pyrite surface. Based on direct atomic force microscopy observation, Becker et al. (67) suggested that the pyrite-bioleaching bacteria respond to the acidic stress at the pyrite surface by excreting EPSs as a pH buffer. Thus, these reports, as well as the present study, suggest that the polysaccharide-rich EPSs observed at the pyrite-cell interface have a possible function to promote biogenic Fe(II) oxidation and pyrite alteration and to protect the microbes from the acidity stress at the pyrite surface, which may play a key role in promoting or maintaining the biotic contribution to Fe(II) oxidation at the pyrite surface, as expected in the kinetic investigation.
FIG 5.
(a to f) STXM-based C, Fe, and merged images of two representative areas selected from sessile cells on the incubated pyrite in the 12-month incubation. The C image was obtained by dividing the two images at 282 eV (C 1s pre-edge energy) and 304 eV (post-edge energy). The Fe image was also obtained by dividing two images at 700 eV (Fe 2p pre-edge energy) and 730 eV (post-edge energy). Yellow boxes in panels c and f indicate the regions of interest (ROIs) where STXM-based C and Fe NEXAFS spectra were measured. (g) STXM-based Fe 2p NEXAFS spectra of model compounds (ferrihydrite, schwertmannite, and pyrite) and ROIs in the incubated pyrite particles. The gray bars in panel g were overlapped to show the specific Fe(II) and Fe(III) peaks at 707.5 and 709.1 eV, respectively. (h) STXM-based C 1s NEXAFS spectra of model compounds and ROIs in the incubated pyrite particles. In the C NEXAFS, sodium alginate (acidic polysaccharide), agarose (neutral polysaccharide), albumin (protein), Escherichia coli DNA (nucleic acid), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (lipid) were selected as model compounds for major microbial biomolecules, as in previous studies (60). The gray bars labeled (I) to (V) in panel h stand for the peak energies of the major organic functional groups present in the model compounds shown in Table S5 in supplemental material.
MATERIALS AND METHODS
Pyrite sample.
The in situ incubation of pyrite was performed using powder sample to facilitate the applications of XAFS, STXM, and DNA-based microbial community analysis. The pyrite was natural and in single crystal form (volume of 1 to 5 cm3) from the Navajún mine in Spain (Hori Mineralogy Co.). The cubic crystals, broken into pieces of several millimeters, were ground in an agate mortar with a pestle and sieved to a particle size of 50 to 100 μm. The powdered pyrite was subsequently cleaned with boiled 6 M HCl, rinsed with deionized water and acetone to remove Fe and S compounds, and dried at 100°C in an oven (57). For the colonization experiment, an aliquot of pyrite powder was packed into the membrane filters. Pyrite powder (0.1 g) was placed between two nylon filter sheets with a pore size of 40 μm (JE-40; Advantec) and sealed on all sides using a thermal sealer. The packed pyrite was put into a coarse nylon net (pore size, 5 mm) to facilitate the setting into a carrier basket. All of the packed pyrite samples were sterilized by soaking in ethanol for 2 h, gently sonicated for 5 min in ethanol, and dried at room temperature (RT) (68). Finally, to minimize air oxidation of pyrite and microbial contamination, all samples were packed into an O2-impermeable polymer bag (AP-1522; As One) with inert Ar gas in a clean bench and stored until the setting into the carrier baskets. In addition, quartz powder (particle size of 50 to 100 μm; Wako) as a negative control for the cell count was also prepared and set into the carrier baskets with the same way.
Sample deployment.
The pyrite colonization experiment was performed in carrier baskets according to a previous similar study (5). The carrier baskets were designed to hold the pyrite packs during placement on the seafloor and not to directly touch the sediment (Fig. 6a). The carrier basket unit was composed of three polyvinyl chloride (PVC) cylinders (diameter, 12 cm; height, 18 cm). The packed pyrite samples were set in each cylinder using Tygon bands. A lead weight with polymer cover was affixed to the center of the unit using a Tygon band to prevent unwanted movement after placement on the seafloor (Fig. 6a). Holes were drilled into the side of the PVC cylinder to allow water circulation over the packed pyrite samples. The polypropylene rope (diameter, 10 mm) tied with a floater and reflector was equipped to facilitate handling of the baskets by the manipulator arm of the deep-sea remotely operated vehicle (ROV).
Study site and sample collection.
The pyrite samples were incubated on the seafloor near the active hydrothermal vent field in the main Bayonnaise Knoll caldera located in the eastern part of the back-arc rift zone of the Izu-Bonin arc (Fig. 6b and c). Geological and geophysical surveys (69, 70) have reported that there are many active/inactive hydrothermal Fe-S deposits (including pyrite, chalcopyrite, and sphalerite) in the Bayonnaise Knoll field.
The carrier baskets with pyrite samples were placed at ambient seafloor conditions (31°57′37″N, 139°44′73″E; depth, 778 m) at the base of active and inactive hydrothermal structures around Micchimney site (Fig. 6c and d). The deployment of incubation devices was performed during ROV Hyper-Dolphin dive no. 1649 on 16 April 2014 in cruise NT14-06 of research vessel (R/V) Natsushima (JAMSTEC). The incubation devices were recovered twice during Hyper-Dolphin dive no. 1757 (12 December 2014) in cruise NT14-21 of the R/V Natsushima and Hyper-Dolphin dive no. 1809 (26 April 2015) in cruise KY15-07 of R/V Kaiyo (JAMSTEC), indicating that the incubation periods were 8 and 12 months, respectively. After the recovery dive, the collected pyrite samples were immediately subdivided into 50-ml Falcon tubes and stored in the refrigerator at −80°C during cruises. After returning to the port, all the samples were transported to our laboratory with dry ice and stored at −80°C in the laboratory until subsequent analysis.
The chemical analysis of the ambient seawater around the incubation device was performed mainly using seawater sample in the deployment cruise. The obtained chemical properties of the ambient seawater are summarized in Table 1 (and see Table S1 in the supplemental material). The temperature and dissolved O2 (DO) concentration were directly measured at the site by remotely manipulating each probe with the ROV arms. According to the data, the incubation site was oxidative (DO, 3.59 mg/kg) and under a low-temperature condition (5.1°C). The pH and concentrations of inorganic components in the ambient seawater were obtained based on the analysis of seawater sample collected at the incubation site. A gas-tight water sampler (71) that can be operated by ROV arms was used to collect the ambient seawater right above the incubation device. After recovery of the collected seawater onboard, pH and alkalinity were immediately measured using a pH electrode (D-51; Horiba) and automatic titration system (702 SM Titrino; Metrohm) with 0.10 M HCl, respectively. At the same time, dissolved Fe(II) and sulfidic-S concentrations were also determined on board after filtration with a 0.20-μm-pore polytetrafluoroethylene (PTFE) membrane filter (28HP-020AN; Advantec) by colorimetry using the phenanthroline method for Fe(II) [for Fe(II)] (72) and the methylene blue method (for sulfidic-S) (73). The total concentrations of dissolved metals (Fe, Cu, and Zn) in the seawater were analyzed onshore; the concentrations were less than the detection limit (<10 μg/kg). For that, the collected seawater was immediately passed through a 0.20-μm-pore PTFE membrane filter, acidified with concentrated HCl (pH of <2), and stored at 4°C during the cruise. The quantification was performed by inductivity-coupled plasma optical emission spectroscopy (ICP-OES) (730-ES; Varian) with a device installed at Ehime University.
DNA extraction and phylogenetic analysis.
Microbial DNA samples of incubated pyrite were extracted from pyrite powder (approximately 80 to 90 mg) using the PowerMax soil DNA isolation kit (Mo-Bio Laboratories). The microbial DNA in the ambient seawater was obtained by filtering the 2 liters of seawater collected at the incubation site (in the deployment cruise) using a sterile membrane filter with pore size of 0.20 μm (28HP-020AN; Advantec). The DNA was eluted with 100 μl of TE (Tris-EDTA) solution and stored at −20°C prior to further analysis. Microbial community structure was estimated using high-throughput amplicon sequencing of the microbial 16S rRNA gene. The V4-to-V5 region of the 16S rRNA gene was amplified from the DNA samples using the primer set U530F and U907R (74) and LA Taq DNA polymerase with GC buffer (TaKaRa). The details of the primer sets are summarized in Table S2 in the supplemental material. For amplification, Illumina adaptor sequence (ACACTCTTTCCCTACACGACGCTCTTCCGATCT) and Illumina Multiplexing PCR primer 2.0 sequence (GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT) were added to the 5′ ends of the primers. The PCR amplification conditions consisted of denaturation at 96°C for 1 min and 25 to 28 amplification cycles of denaturation at 96°C for 25 s, annealing at 52°C for 45 s, and elongation at 72°C for 1 min. The amplicons were mixed with Illumina PhiX control libraries and sequenced using the Illumina MiSeq platform (Illumina) at JAMSTEC. Amplicon sequence data were processed using the following procedure. Raw paired-end reads were merged using PEAR v0.9.10 (75), and primer sequences were removed using Cutadapt v1.10 (76). Low-quality (Q score of <30 in over 3% of sequences) and short (<150-bp) reads were filtered out using a custom Perl script. The resulting sequences were analyzed using the QIIME2 v2019.4.0 pipeline (77). Unique amplicon sequence variants were generated using the DADA2 plugin wrapped in QIIME2, and chimera sequences were removed (78). The sequences were clustered into operational taxonomic units (OTUs) with 97% sequence identity in each library. The taxa were assigned to the OTUs for 16S rRNA genes using the QIIME2 plugin feature-classifier classify-sklearn (79) against the SILVA 132 database (80).
Scanning electron and epifluorescence microscopy analyses.
The surface morphology of the incubated pyrite was investigated using SEM (VE-8800; Keyence). A small portion of frozen pyrite powder samples was transferred to a 2-ml sterile plastic tube, and then an ethanol dehydration series of 50, 70, 80, 90, and 100% (vol/vol) was performed just before the SEM analysis. The sample was mounted on carbon tape (no. 7321; Nissin EM), dried at room temperature (RT), and analyzed by SEM after Pt coating under a vacuum condition at 10 Pa.
Cell counts on the incubated pyrite samples were performed by direct staining with Syto9 (Invitrogen). The pyrite powder stored at −80°C was partially fixed with formalin (final concentration, 3.7%) for 48 h at 4°C and stored in Tris-buffered saline (TBS)-ethanol solution at 4°C. The fixed pyrite suspension was transferred to a 2-ml sterile plastic tube, and 0.1 mM Syto9 solution was added to stain the cells. In addition, staining of extracellular polysaccharides was performed using a lectin technique as previously described by Mangold et al. (81). Briefly, the pyrite suspension was washed with deionized water and stained for 30 min with Cy3-labeled concanavalin A (ConA) (Protein Mods) at a final concentration of 50 μg/ml. The stained sample was mounted on a glass slide and observed by epifluorescence microscope (BX-51; Olympus).
Bulk XRD analysis.
The XRD analysis was performed to identify mineralogical characteristics of the altered products in the pyrite sample. The frozen pyrite sample was directly freeze-dried at −50°C under vacuum condition (<20 Pa) for 48 h with a freeze-dryer (DC400; Yamato Scientific). After the freeze-drying, the powder sample was mounted on a low background quartz holder and analyzed using XRD spectrometer (Ultima IV; Rigaku) with a step size of 0.02° and a time interval of 1s. After the XRD analysis, the freeze-dried samples were immediately packed into O2-impermeable polymer bag with inert Ar gas and stored again at −80°C until subsequent Fe XANES analysis.
Fe K-edge XANES analysis.
The Fe K-edge XANES was measured to identify the Fe species and minerals formed during pyrite incubation. The analysis was performed at beamline BL01B1 at SPring-8 (Hyogo, Japan) with a Si(111) double-crystal monochromator and two mirrors. Two types of Fe XANES, bulk and CEY Fe XANES, were measured. For the bulk XANES analysis, freeze-dried pyrite samples were diluted to approximately 1 wt% in boron nitride and pelleted for transmission detection mode for bulk XANES. The CEY XANES measurement was performed using the CEY detection system installed at BL01B1. A portion of the freeze-dried pyrite sample was placed on electrically conductive carbon tape (no. 7321; Nissin EM) attached to the graphite electrode stage in the CEY detector unit. The detector unit was canted 4° against the incident X ray and covered with a metal box to be filled with helium gas during measurement. The high-voltage power was fixed at 400 V in all the CEY XANES measurements.
The energy calibration in the Fe XANES was performed using a pre-edge peak maximum of hematite at 7,111 eV according to a previous study (82). All XAFS measurements were carried out at RT under ambient air condition. All Fe XANES data were processed using REX2000 (Rigaku). The uncertainty in the XANES analysis was evaluated by the R factor given by the following equation:
STXM analysis.
The STXM analyses for C 1s and Fe 2p NEXAFS were conducted at STXM apparatuses installed in BL-4U at UVSOR (Okazaki, Japan) (83) in Japan. The theoretical spatial and spectral resolutions of both STXM apparatuses were less than 50 nm and ±0.1 eV, respectively. The STXM analyses were performed at RT and at 1/6 atm He. For the sample preparation, a small amount of the frozen pyrite sample was transferred into a sterile plastic tube and thawed at RT, and then 0.1 ml sterile water was added to the tube. The pyrite suspension was dropped onto a silicon nitride (Si3N4) membrane (NT050A; Norcada) and air-dried slowly at RT. All STXM data processing was carried out using the aXis2000 software, an IDL-based analytical package (Hitchcock; http://unicorn.mcmaster.ca/aXis2000.html). A careful examination showed that in the present study, there was no apparent photo alteration of C and Fe during the STXM analyses.
All model compounds for C NEXAFS, albumin, sodium alginate, agarose, Escherichia coli DNA, and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, were obtained from a Japanese reagent provider, Wako Pure Chemicals. For Fe NEXAFS and XANES model compounds, natural siderite and jarosite were obtained from the Japanese mineral provider Hori Mineralogy. The Fe (hydr)oxide minerals, ferrihydrite, goethite, schwertmannite, magnetite, and hematite were synthesized as described by Schwertmann and Cornell (84).
Data availability.
Amplicon sequence data were deposited in the DDBJ Sequence Read Archive under accession no. DRA011908. All data were registered using BioProject ID PRJDB11582.
ACKNOWLEDGMENTS
We thank the captains and crews of R/V Natsushima and Kaiyo, as well as the operation teams of ROV Hyper-Dolphin, for their invaluable help when obtaining in situ incubation and seawater samples. We also thank the scientists M. Shimanaga (Kumamoto University), K. Inoue (The University of Tokyo), and S. Kawagucci (JAMSTEC) for their help in conducting our research cruises.
This work was financially supported by MEXT KAKENHI (grants 15H05330, 25610164, and 19H03087). Synchrotron-based experiments in this study were performed with the approvals of JASRI (proposal no. 2015B1452 and 2017B1556), Photon Factory (proposal no. 2016G575 and 2018G545), and UVSOR (proposal no. S-16-MS-2002 and S-17-MS-2024).
Footnotes
Supplemental material is available online only.
Contributor Information
S. Mitsunobu, Email: mitsunobu.satoshi.dy@ehime-u.ac.jp.
H. Makita, Email: makita@kaiyodai.ac.jp.
Knut Rudi, Norwegian University of Life Sciences.
REFERENCES
- 1.Jannasch HW. 1985. The chemosynthetic support of life and microbial diversity at deep-sea hydrothermal vents. Proc R Soc Lond B 225:277–297. [Google Scholar]
- 2.Karl DM. 1995. Ecology of free-living hydrothermal vent communities, p 35–124. In Karl DM (ed), The microbiology of deep-sea hydrothermal vents. CRC Press, Boca Raton, FL. [Google Scholar]
- 3.McCollom TM, Shock EL. 1997. Geochemical constraints on chemolitoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Acta 61:4375–4391. 10.1016/S0016-7037(97)00241-X. [DOI] [PubMed] [Google Scholar]
- 4.McCollom TM. 1999. Methanogenesis as a potential source of chemical energy for primary biomass production by autotrophic organisms in hydrothermal systems on Europa. J Geophys Res 104:30729–30742. 10.1029/1999JE001126. [DOI] [Google Scholar]
- 5.Edwards KJ, McCollom TM, Konishi H, Buseck PR. 2003. Seafloor bioalteration of sulfide minerals: results from in situ incubation studies. Geochim Cosmochim Acta 67:2843–2856. 10.1016/S0016-7037(03)00089-9. [DOI] [Google Scholar]
- 6.McCollom TM. 2000. Geochemical constraints on primary productivity in submarine hydrothermal vent plumes. Deep Sea Res I 47:85–101. 10.1016/S0967-0637(99)00048-5. [DOI] [Google Scholar]
- 7.Suzuki Y, Inagaki F, Takai K, Nealson KH, Horikoshi K. 2004. Microbial diversity in inactive chimney structures from deep-sea hydrothermal systems. Microb Ecol 47:186–196. 10.1007/s00248-003-1014-y. [DOI] [PubMed] [Google Scholar]
- 8.Takai K, Nunoura T, Horikoshi K, Shibuya T, Nakamura K, Suzuki Y, Matthew S, Gary JM, Christenson BM, deRonde CEJ, Butterfield DA, Ishibashi J, Lupton JE, Evans LJ. 2009. Variability in microbial communities in black smoker chimneys at the NW caldera vent field, Brothers volcano, Kermadec arc. Geomicrobiol J 26:552–569. 10.1080/01490450903304949. [DOI] [Google Scholar]
- 9.Herzig PM, Hannington MD. 1995. Polymetallic massive sulfides at the modern seafloor: a review. Ore Geol Rev 10:95–115. 10.1016/0169-1368(95)00009-7. [DOI] [Google Scholar]
- 10.Hannington MD, Jonasson IR, Herzig PM, Petersen S. 1995. Physical and chemical processes of seafloor mineralization at mid-ocean ridges, p 115–157. In Humphris SE, Zierenberg RA, Mullineaux LS, Thomson RE (ed), Hydrothermal systems: physical, chemical, biological, and geological interactions. Geophysical Monograph Series 91. American Geophysical Union, Washington, DC. [Google Scholar]
- 11.Edwards KJ, Rogers DR, Wirsen CO, McCollom TM. 2003. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic alpha- and gamma-Proteobacteria from the deep sea. Appl Environ Microbiol 69:2906–2913. 10.1128/AEM.69.5.2906-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barco RA, Hoffman CL, Ramírez GA, Toner BM, Edwards KJ, Sylvan JB. 2017. In-situ incubation of iron-sulfur mineral reveals a diverse chemolithoautotrophic community and a new biogeochemical role for Thiomicrospira. Environ Microbiol 19:1322–1337. 10.1111/1462-2920.13666. [DOI] [PubMed] [Google Scholar]
- 13.Sylvan JB, Toner BM, Edwards KJ. 2012. Life and death of deep-sea vents: bacterial diversity and ecosystem succession on inactive hydrothermal sulfides. mBio 3:e00279-11. 10.1128/mBio.00279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylvan JB, Sia TY, Haddad AG, Briscoe LJ, Toner BM, Girguis PR, Edwards KJ. 2013. Low temperature geomicrobiology follows host rock composition along a geochemical gradient in Lau Basin. Front Microbiol 4:61. 10.3389/fmicb.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato S, Ikehata K, Shibuya T, Urabe T, Ohkuma M, Yamagishi A. 2015. Potential for biogeochemical cycling of sulfur, iron and carbon within massive sulfide deposits below the seafloor. Environ Microbiol 17:1817–1835. 10.1111/1462-2920.12648. [DOI] [PubMed] [Google Scholar]
- 16.Konhauser KO. 1998. Diversity of bacterial iron mineralization. Earth-Sci Rev 43:91–121. 10.1016/S0012-8252(97)00036-6. [DOI] [Google Scholar]
- 17.Noike TN, Nakamura K, Matsumoto J. 1983. Oxidation of ferrous iron by acidophilic iron-oxidizing bacteria from a stream receiving acid mine drainage. Water Res 17:21–27. 10.1016/0043-1354(83)90282-8. [DOI] [Google Scholar]
- 18.Kirby CS, Elder Brady JA. 1998. Field determination of Fe2+ oxidation rates in acid mine drainage using a continuously-stirred tank reactor. App Geochem 13:509–520. 10.1016/S0883-2927(97)00077-2. [DOI] [Google Scholar]
- 19.Williamson MA, Kirby CS, Rimstidt JD. 2006. Iron dynamics in acid mine drainage, p 2411–2423. In Barnhisel RI (ed), 7th International Conference Acid Rock Drainage (ICARD), St. Louis, MO. [Google Scholar]
- 20.Kato S, Takano Y, Kakegawa T, Oba H, Inoue K, Kobayashi C, Utsumi M, Marumo K, Kobayashi K, Ito Y, Ishibashi JI, Yamagishi A. 2010. Biogeography and biodiversity in sulfide structures of active and inactive vents at deep-sea hydrothermal fields of the Southern Mariana Trough. Appl Environ Microbiol 76:2968–2979. 10.1128/AEM.00478-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toner BM, Lesniewski RA, Marlow JJ, Briscoe LJ, Santelli CM, Bach W, Orcutt BN, Edwards KJ. 2013. Mineralogy drives bacterial biogeography of hydrothermally inactive seafloor sulfide deposits. Geomicrobiol J 30:313–326. 10.1080/01490451.2012.688925. [DOI] [Google Scholar]
- 22.Picardal FW, Zaybak Z, Chakraborty A, Schieber J, Szewzyk U. 2011. Microaerophilic, Fe(II)-dependent growth and Fe(II) oxidation by a Dechlorospirillum species. FEMS Microbiol Lett 319:51–57. 10.1111/j.1574-6968.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 23.Edwards KJ, Schrenk MO, Hamers R, Banfield JF. 1998. Microbial oxidation of pyrite: experiments using microorganisms from an extreme acidic environment. Am Mineral 83:1444–1453. 10.2138/am-1997-11-1233. [DOI] [Google Scholar]
- 24.Nordstrom DK, Alpers CN. 1999. Geochemistry of acid mine waters, p 133–160. In Plumlee GS, Logsdon MJ (ed), The environmental geochemistry of mineral deposits. Society of Economic Geologists, Littleton, CO. [Google Scholar]
- 25.Orcutt BN, Bach W, Becker K, Fisher AT, Hentscher M, Toner BM, Wheat CG, Edwards KJ. 2011. Colonization of subsurface microbial observatories deployed in young ocean crust. ISME J 5:692–703. 10.1038/ismej.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramírez GA, Hoffman CL, Lee MD, Lesniewski RA, Barco RA, Garber A, Toner BM, Wheat CG, Edwards KJ, Orcutt BN. 2016. Assessing marine microbial induced corrosion at Santa Catalina Island, California. Front Microbiol 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji K, Nakano K, Takahashi Y, Hayashi K, Ro C-U. 2010. X-ray spectrometry. Anal Chem 82:4950–4987. 10.1021/ac101069d. [DOI] [PubMed] [Google Scholar]
- 28.Itai T, Takahashi Y, Uruga T, Tanida H, Iida A. 2008. Selective detection of Fe and Mn species at mineral surfaces in weathered granite by conversion electron yield X-ray absorption fine structure. Appl Geochem 23:2667–2675. 10.1016/j.apgeochem.2008.05.020. [DOI] [Google Scholar]
- 29.Schroeder SLM. 1996. Towards a ‘universal curve’ for total electron-yield XAS. Solid State Comm 98:405–409. 10.1016/0038-1098(96)00035-X. [DOI] [Google Scholar]
- 30.Takahashi Y, Minamikawa R, Hattori HK, Kurishima K, Kihou N, Yuita K. 2004. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38:1038–1044. 10.1021/es034383n. [DOI] [PubMed] [Google Scholar]
- 31.Mitsunobu S, Toda M, Hamamura N, Shiraishi F, Tominaga Y, Sakata M. 2020. Millimeter-scale topsoil layer blocks arsenic migration in flooded paddy soil. Geochim Cosmochim Acta 274:211–227. 10.1016/j.gca.2020.01.038. [DOI] [Google Scholar]
- 32.Toner BM, Santelli CM, Marcus MA, Wirth R, Chan CS, McCollom T, Bach W, Edwards KJ. 2009. Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochim Cosmochim Acta 73:388–403. 10.1016/j.gca.2008.09.035. [DOI] [Google Scholar]
- 33.Fortin D, Langley S. 2005. Formation and occurrence of biogenic iron-rich minerals. Earth-Sci Rev 72:1–19. 10.1016/j.earscirev.2005.03.002. [DOI] [Google Scholar]
- 34.Cornell RM, Schwertmann U. 2003. The iron oxides: structure, properties, reactions, occurrences and uses, 2nd and extended ed. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 35.Scheinost AC. 2005. Metal oxides, p 428–438. In Hillel D (ed), Encyclopedia of soils in the environment. Academic Press, Waltham, MA. [Google Scholar]
- 36.Burgos WD, Borch T, Troyer LD, Luan F, Larson LN, Brown JF, Lambson J, Shimizu M. 2012. Schwertmannite and Fe oxides formed by biological low-pH Fe(II) oxidation versus abiotic neutralization: impact on trace metal sequestration. Geochim Cosmochim Acta 76:29–44. 10.1016/j.gca.2011.10.015. [DOI] [Google Scholar]
- 37.Baker BJ, Banfield JF. 2003. Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152. 10.1016/S0168-6496(03)00028-X. [DOI] [PubMed] [Google Scholar]
- 38.Amaral-Zettler LA, Zettler ER, Theroux SM, Palacios C, Aguilera A, Amils R. 2011. Microbial community structure across the tree of life in the extreme Río Tinto. ISME J 5:42–50. 10.1038/ismej.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuang JL, Huang LN, Chen LX, Hua ZS, Li SJ, Hu M, Li JT, Shu WS. 2013. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J 7:1038–1050. 10.1038/ismej.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lear G, Niyogi D, Harding J, Dong Y, Lewis G. 2009. Biofilm bacterial community structure in streams affected by acid mine drainage. Appl Environ Microbiol 75:3455–3460. 10.1128/AEM.00274-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sul WJ, Cole JR, Jesus EDC, Wang Q, Farris RJ, Fish JA, Tiedje JM. 2011. Bacterial community comparisons by taxonomy-supervised analysis independent of sequence alignment and clustering. Proc Natl Acad Sci USA 108:14637–14642. 10.1073/pnas.1111435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards KJ, Gihring TM, Banfield JF. 1999. Seasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environment. Appl Environ Microbiol 65:3627–3632. 10.1128/AEM.65.8.3627-3632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Toril E, Aguilera A, Souza-Egipsy V, Lopez Pamo E, Sanchez Espana J, Amils R. 2011. Geomicrobiology of La Zarza-Perrunal acid mine effluent (Iberian Pyritic Belt, Spain). Appl Environ Microbiol 77:2685–2694. 10.1128/AEM.02459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fierer N, Jackson RB. 2006. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631. 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauber CL, Hamady M, Knight R, Fierer N. 2009. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120. 10.1128/AEM.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N. 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. 10.1038/ismej.2010.58. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths RI, Thomson BC, James P, Bell T, Bailey M, Whiteley AS. 2011. The bacterial biogeography of British soils. Environ Microbiol 13:1642–1654. 10.1111/j.1462-2920.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- 48.Stumm W, Lee G. 1961. Oxygenation of ferrous iron. Ind Eng Chem 53:143–146. 10.1021/ie50614a030. [DOI] [Google Scholar]
- 49.Singer PC, Stumm W. 1970. Acidic mine drainage: the rate determining step. Science 167:1121–1123. 10.1126/science.167.3921.1121. [DOI] [PubMed] [Google Scholar]
- 50.Millero FJ, Sotolongo S, Izaguirre M. 1987. The oxidation kinetics of Fe(II) in seawater. Geochim Cosmochim Acta 51:793–801. 10.1016/0016-7037(87)90093-7. [DOI] [Google Scholar]
- 51.Kester DR, Byrne RH, Liang YG. 1975. Chapter 3, p 56–79. In Church TM (ed), Marine chemistry in the coastal environment. ACS Symposium Series, vol 18. American Chemical Society, Washington, DC. [Google Scholar]
- 52.Pesic B, Oliver DJ, Wichlacz P. 1989. An electrochemical method of measuring the oxidation rate of ferrous to ferric iron with oxygen in the presence of Thiobacillus ferrooxidans. Biotechnol Bioeng 33:428–439. 10.1002/bit.260330408. [DOI] [PubMed] [Google Scholar]
- 53.Nordstrom DK, Southam G. 1997. Geomicrobiology of sulfide mineral oxidation, p 361–390. In Banfield JF, Nealson KH (ed), Geomicrobiology: interactions between microbes and minerals, vol RIM 35. Mineralogial Society of America, Chantilly, VA. [Google Scholar]
- 54.Clair B, Pottenger J, Debes R, Hanselmann K, Shock E. 2019. Distinguishing biotic and abiotic iron oxidation at low temperatures. ACS Earth Space Chem 3:905–921. 10.1021/acsearthspacechem.9b00016. [DOI] [Google Scholar]
- 55.Chan CS, Fakra SC, Edwards DC, Emerson D, Banfield JF. 2009. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim Cosmochim Acta 73:3807–3818. 10.1016/j.gca.2009.02.036. [DOI] [Google Scholar]
- 56.Miot J, Benzerara K, Obst M, Kappler A, Hegler F, Schädler S, Bouchez C, Guyot F, Morin G. 2009. Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Appl Environ Microbiol 75:5586–5591. 10.1128/AEM.00490-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitsunobu S, Zhu M, Takeichi Y, Ohigashi T, Suga H, Jinno M, Makita H, Sakata M, Ono K, Mase K, Takahashi Y. 2016. Direct detection of Fe(II) in extracellular polymeric substances (EPS) at the mineral-microbe interface in bacterial pyrite leaching. Microbes Environ 31:63–69. 10.1264/jsme2.ME15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeichi Y, Inami N, Suga H, Ono K, Takahashi Y. 2014. Development of a compact scanning transmission X-ray microscope at the Photon Factory. Chem Lett 43:373–375. 10.1246/cl.130948. [DOI] [PubMed] [Google Scholar]
- 59.Toner BM, Fakra SC, Manganini SJ, Santelli CM, Marcus MA, Moffett JW, Rouxel O, German CR, Edwards KJ. 2009. Preservation of iron(II) by carbon-rich matrices in a hydrothermal plume. Nat Geosci 2:197–201. 10.1038/ngeo433. [DOI] [Google Scholar]
- 60.Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ. 2011. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J 5:717–727. 10.1038/ismej.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitsunobu S, Zhu M, Takeichi Y, Ohigashi T, Suga H, Makita H, Sakata M, Ono K, Mase K, Takahashi Y. 2015. Nanoscale identification of extracellular organic substances at the microbe-mineral interface by scanning transmission X-ray microscopy. Chem Lett 44:91–93. 10.1246/cl.140880. [DOI] [Google Scholar]
- 62.Gehrke T, Telegdi J, Thierry D, Sand W. 1998. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl Environ Microbiol 64:2743–2747. 10.1128/AEM.64.7.2743-2747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sand W, Gehrke T. 2006. Interfacial processes involving iron(III) ions and acidophilic bacteria. Res Microbiol 157:49–56. 10.1016/j.resmic.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Noël N, Florian B, Sand W. 2010. AFM & EFM study on attachment of acidophilic leaching organisms. Hydrometallurgy 104:370–375. 10.1016/j.hydromet.2010.02.021. [DOI] [Google Scholar]
- 65.Singh R, Paul D, Jain RK. 2006. Biofilms: implications in bioremediation. Trends Microbiol 14:389–397. 10.1016/j.tim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Fowler TA, Holmes PR, Crundwell FK. 1999. Mechanism of pyrite dissolution in the presence of Thiobacillus ferrooxidans. Appl Environ Microbiol 65:2987–2993. 10.1128/AEM.65.7.2987-2993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becker T, Gorham N, Shiers DW, Watling HR. 2011. In situ imaging of Sulfobacillus thermosulfidooxidans on pyrite under conditions of variable pH using mode atomic force microscopy. Process Biochem 46:966–976. 10.1016/j.procbio.2011.01.014. [DOI] [Google Scholar]
- 68.Edwards KJ, Bond PL, Banfield JF. 2000. Characteristics of attachment and growth of Thiobacillus caldus on sulphide minerals: a chemotactic response to sulphur minerals? Environ Microbiol 2:324–332. 10.1046/j.1462-2920.2000.00111.x. [DOI] [PubMed] [Google Scholar]
- 69.Iizasa K. 1993. Petrographic investigations of seafloor sediments from the Kita-Bayonnaise submarine caldera, Shichito-Iwojima Ridge, Izu-Ogasawara Arc, northwestern Pacific. Mar Geol 112:271–290. 10.1016/0025-3227(93)90173-S. [DOI] [Google Scholar]
- 70.Watanabe S, Hayashi KI. 2014. Mineralogy, sulfur isotope and fluid inclusion studies of hydrothermal ore at the Hakurei deposit, Bayonnaise Knoll, Izu-Bonin arc. Resour Geol 64:77–90. 10.1111/rge.12029. [DOI] [Google Scholar]
- 71.Saegusa S, Tsunogai U, Nakagawa F, Kaneko S. 2006. Development of a multibottle gas-tight fluid sampler WHATS II for Japanese submersibles/ROVs. Geofluids 6:060404065855002–060404065855240. 10.1111/j.1468-8123.2006.00143.x. [DOI] [Google Scholar]
- 72.Saywell LG, Cunningham BB. 1937. Determination of iron. Ind Eng Chem Anal Ed 9:67–69. 10.1021/ac50106a005. [DOI] [Google Scholar]
- 73.Trueper HG, Schlegel HG. 1964. Sulphur metabolism in thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okeni. Antonie Van Leeuwenhoek 30:225–238. 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- 74.Hirai M, Nishi S, Tsuda M, Sunamura M, Takaki Y, Nunoura T. 2017. Library construction from subnanogram DNA for pelagic sea water and deep-sea sediments. Microbes Environ 32:336–343. 10.1264/jsme2.ME17132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Kobert K, Flouri T, Stamatakis A. 2014. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30:614–620. 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 77.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang K, Bin Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Gregory Caporaso J. 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mangold S, Laxander M, Harneit K, Rohwerder T, Claus G, Sand W. 2008. Visualization of Acidithiobacillus ferrooxidans biofilms on pyrite by atomic force and epifluorescence microscopy under various experimental conditions. Hydrometallurgy 94:127–132. 10.1016/j.hydromet.2008.05.044. [DOI] [Google Scholar]
- 82.Mitsunobu S, Harada T, Takahashi Y. 2006. Comparison of antimony behavior with that of arsenic under various soil redox conditions. Environ Sci Technol 40:7270–7276. 10.1021/es060694x. [DOI] [PubMed] [Google Scholar]
- 83.Ohigashi T, Arai H, Araki T, Kondo N, Shigemasa E, Ito A, Kosugi N, Katoh M. 2013. Construction of the scanning transmission X-ray microscope beamline at UVSOR. J Phys Conf Ser 463:e012006. 10.1088/1742-6596/463/1/012006. [DOI] [Google Scholar]
- 84.Schwertmann U, Cornell RM. 2000. Iron oxides in the laboratory: preparation and characterization, 2nd and extended ed. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 85.Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M. 1996. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim Cosmochim Acta 60:2111–2121. 10.1016/0016-7037(96)00091-9. [DOI] [Google Scholar]
- 86.Felmy AR, Girvin DC, Jenne EA. 1983. MINTEQ—a computer program for calculating aqueous geochemical equilibria. Final project report, USEPA contract 68-03-3098. USEPA, Arlington, VA. [Google Scholar]
- 87.McAllister SM, Moore RM, Gartman A, Luther GM, III, Emerson D, Chan CS. 2019. The Fe(II)-oxidizing Zetaproteobacteria: historical, ecological and genomic perspectives. FEMS Microbiol Ecol 95:fiz015. 10.1093/femsec/fiz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S5, Fig. S1 and S2. Download aem.00977-21-s0001.pdf, PDF file, 0.4 MB (431.4KB, pdf)
Data Availability Statement
Amplicon sequence data were deposited in the DDBJ Sequence Read Archive under accession no. DRA011908. All data were registered using BioProject ID PRJDB11582.