Abstract
We present a case of vertebral osteomyelitis with an adjacent abdominal aortic mycotic aneurysm caused by a highly penicillin-resistant Streptococcus pneumoniae strain. The occurrence of all three phenomena in a single patient has not been previously described. This presentation offers the opportunity to reflect on the increasing incidence of S. pneumoniae as a resistant pathogen, the treatment of highly penicillin-resistant S. pneumoniae, and the etiologic agents of both vertebral osteomyelitis and mycotic aneurysm.
CASE REPORT
A 52-year-old woman with no significant medical history presented to Thomas Jefferson University Hospital in March of 1999. She complained of low, central back pain without radiation. The pain had begun after a fall approximately 2 months before admission. The pain was persistent despite the use of over-the-counter nonsteroidal anti-inflammatory agents and a muscle relaxant. The pain was associated with a poor appetite, loss of 20 pounds, and subjective fevers. The patient denied chills or night sweats. She also denied current or past headache, stiff neck, rhinorrhea, sore throat, otalgia, or cough. There was no chest pain, shortness of breath, or dyspnea on exertion. There was no abdominal pain, nausea, vomiting, or flank pain.
In the week prior to admission, the back pain had intensified and was now associated with lower-extremity weakness, urinary urgency, paresthesias, and constipation. She was admitted for urgent evaluation of symptoms consistent with spinal cord compression.
Physical exam revealed a temperature of 98.5°F, a pulse rate of 100 beats per min, 18 respirations per min, and a blood pressure of 150/80 mm Hg. The heart rate was regular, without a murmur, rub, or gallop. The lungs were clear without rales, rhonchi, wheezes, or crackles. The abdomen was soft and nontender without organomegaly. The upper extremities were neurologically normal. The lower extremities had bilateral weakness in hip flexion, knee extension, and pedal plantar and dorsiflexion that was symmetrical and graded 1 to 2 out of 5. The knees had 1+ deep tendon reflexes. The ankle reflexes were not elicited. The toes were up-going on the left and down-going on the right. The rectal tone was normal.
Laboratory data obtained on admission was as follows: erythrocyte count, 8.6 × 103/ml (87% neutrophils, 1% bands, 11% lymphocytes, 1% monocytes); hemoglobin, 8.5 g/dl, hematocrit, 25.2%; platelets, 406 × 103/ml. The creatinine level was 0.7 g/dl of serum. The erythrocyte sedimentation rate was 130 mm/h. A urine dipstick showed 1+ protein and 3+ blood. Microscopic analysis of the urine showed >100 erythrocytes and 21 to 50 leukocytes per high-power field. The total bilirubin was 1.3 mg/dl with a direct bilirubin fraction of 0.6 mg/dl. The aspartate aminotransferase was 25 IU/liter, the alanine aminotransferase was 17 IU/liter, and the gammaglutyltransferase was 78 IU/liter. The albumin was 2.3 g/dl. Alkaline phosphatase was 139 IU/liter. A chest X-ray showed no active infiltrates and was otherwise normal.
A computed tomographic scan of the lumbar spine showed a destructive process within the disc space at the L3-to-L4 level which extended into the paraspinal space, consistent with discitis and osteomyelitis. There was obliteration of the fat plane surrounding the psoas muscle, as well as the fat plane posterior to the aorta, iliacs, and inferior vena cava. Dilation of the aorta was also demonstrated and was felt to be consistent with a mycotic aneurysm. Severe tricompartment stenosis was shown at the L3-to-L4 and L4-to-L5 levels.
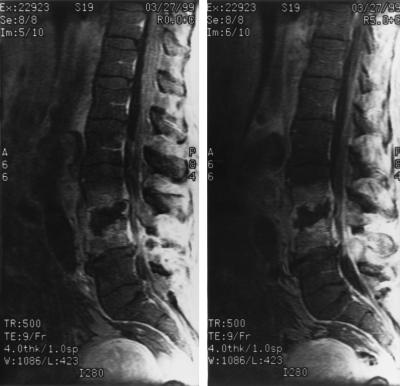
A magnetic resonance image of the spine (Fig. 1) was consistent with discitis and osteomyelitis at L3 to L4, with prevertebral and psoas microabscess formation present. Infection was found to extend into the posterior elements, with significant narrowing of the spinal canal at L4 and evidence of meningeal enhancement. The question of dilation of the abdominal aorta at the L3-to-L4 level raised the concern about a mycotic aneurysm.
FIG. 1.
Magnetic resonance imaging of the lumbar spine demonstrating destruction of the vertebral bodies at L3 and L4 with prevertebral and psoas microabscess formation. Dilation of the abdominal aorta at this level can also be appreciated. The spinal canal shows significant narrowing with meningeal enhancement.
A computed tomographic scan of the abdomen demonstrated a 5-cm juxtarenal abdominal aortic aneurysm located directly superior to an area of extensive bony destruction of the end plates of L3 and L4, with surrounding inflammatory changes of the psoas muscle.
After imaging and evaluation by neurosurgeons, the Infectious Diseases Service was consulted. The differential diagnosis included bacterial and mycobacterial infection, as well as malignancy. The bacteria considered likely were Staphylococcus aureus and Salmonella species. Decadron was initiated for the spinal cord compression. Blood cultures were drawn, and empiric antibiotic therapy included 2 g of nafcillin given intravenously every 4 h with 60 mg of gentamicin given every 12 h. At the time of the Infectious Diseases Service consultation, the patient was noted to have new abdominal pain and urgent vascular surgery consultation was requested. Shortly thereafter, the patient was found to be unresponsive and pulseless. Despite aggressive resuscitative measures, she expired 48 h after admission.
Blood cultures returned posthumously with gram-positive cocci which were identified as Streptococcus pneumoniae. The sensitivities of the organism were as follows: penicillin resistant (MIC, 4.0 μg/ml), cefotaxime resistant (MIC, >2.0 μg/ml), ceftriaxone resistant (MIC, 2.0 μg/ml), vancomycin susceptible (MIC, 0.12 μg/ml), clindamycin susceptible (MIC, ≤0.03 μg/ml), erythromycin susceptible (MIC, ≤0.03 μg/ml), and tetracycline susceptible (MIC, ≤0.06 μg/ml). The MICs were determined by using the Dade MicroScan Microstrep MIC Panel.
Autopsy revealed a ruptured infrarenal abdominal aneurysm measuring 5 by 5 by 4 cm with retroperitoneal extravasation of approximately 2.5 liters of clotted blood. The aneurysm was adherent posteriorly to the L4 vertebral body. The intervertebral disc, vertebral bodies, and paravertebral soft tissues showed nonspecific softening without discrete abscess formation.
Additional autopsy data revealed that the abdominal aorta displayed a mycotic aneurysm with a mixed acute and chronic inflammatory infiltrate and abscess formation in the medial layer, adventitia, and adjacent musculature. Atheromatous plaque formation was identified adjacent to the aneurysm. An elastic tissue stain revealed narrowing and focal destruction of the medial layer adjacent to the aneurysm. Gram staining of the aneurysm revealed multiple gram-positive cocci in the media and adventitia. The L4 and L5 vertebral bodies showed chronic inflammation and associated fibrosis of the bone marrow. Microbiologic studies were not performed on the vertebral tissue.
Discussion.
“Acute infective osteomyelitis of the bones of the vertebral column is a disease rarely met with, and beyond this, when it occurs, it is often only recognized in the post-mortem room” (18). This quote from an 1896 description of 21 cases of vertebral osteomyelitis predates the era of modern bacteriology, microbiologic diagnostic techniques, surgical intervention, and antibiotic therapy. The descriptions of dying patients are both gruesome and informative. The untreated patient develops local extension of the disease, as well as disseminated abscess formation. Mortality in this small series was 71%. There is no mention of any microscopic examination for bacteria.
Over 100 years ago, little beyond elegant case descriptions was known about vertebral osteomyelitis. Subsequent series and reviews have revealed the microbiology behind these infections (2, 7, 25). More recent reviews, by a single author in the postantibiotic era, confirm the bacteriology of this rare infection (26–28). The predominant organism found in vertebral osteomyelitis is S. aureus. Less commonly implicated are coagulase-negative staphylococci (especially in the era of spine surgery and implantable hardware and devices), gram-negative organisms, beta-hemolytic streptococci, and enterococci. Rarer pathogens include fungi (usually in an immunocompromised host) and tubercle bacilli. S. pneumoniae has been occasionally associated with sickle cell disease but is otherwise rarely reported as a cause of vertebral osteomyelitis.
Recent case reports have brought attention to S. pneumoniae as an important pathogen in vertebral osteomyelitis (4, 13, 17, 24). In general, increasing resistance to penicillins and cephalosporins has been well documented (6, 15) although resistance to antibiotics is not clearly associated with any increase in mortality (21). Accordingly, antibiotic resistance has also been recognized in vertebral osteomyelitis (3, 8, 12).
Our patient did not have any antecedent history that would suggest an initial focus of pneumococcal infection. The case presented did involve a mycotic aneurysm which involved the abdominal aorta adjacent to the infected lumbar vertebrae. A rupture of the aneurysm caused her death. Whether the initial process was a vertebral osteomyelitis which eroded the abdominal aorta or a mycotic aneurysm which eroded posteriorly to cause vertebral osteomyelitis is not known.
The term mycotic aneurysm was originated by Osler in the Gulstonian lectures of 1885 to designate the pathophysiology involved in the formation of arterial dilation due to septic degradation of the arterial wall. The mushroom shape of the aneurysm led to the term “mycotic,” which has since been applied primarily to fungal infections but still carries its original designation for intravascular infections. The bacteria were believed to originate from infected heart valves during an episode of infective endocarditis and embolize to peripheral arteries. Most infected abdominal aneurysms arise either from direct extension of an adjacent infectious process or by microbial arteritis, in the absence of known infective endocarditis (23).
Mycotic aortic aneurysm is a recognized complication in patients who have lumbar vertebral osteomyelitis (22). The bacteriology of mycotic aneurysms includes S. aureus and salmonellae, but S. pneumoniae has also been reported, albeit infrequently (5, 11, 14). A penicillin-resistant pneumococcus has been reported in only one case of a mycotic aneurysm (1).
The treatment of infections caused by highly penicillin-resistant S. pneumoniae remains controversial. Serious infections such as bacteremias and meningitis are difficult to treat because of poor drug penetration into the central nervous system and high burdens of organisms. Various regimens have been tested, including high-dose penicillin, chloramphenicol, carbapenems, high-dose cephalosporins, quinolones, vancomycin, and rifampin (10). There is no uniformly recommended antibiotic or antibiotic combination, and research is needed to determine optimal therapy (9, 16).
While unusual, outbreaks of pneumococcal disease have occurred and a recent report highlights an outbreak of multidrug-resistant pneumococcal pneumonia (20). Prevention of pneumococcal infection with multivalent polysaccharide vaccine is felt to be effective, underutilized, and perhaps one of the last lines of defense against seriously invasive disease caused by an organism with rapidly expanding resistance (19).
The case presented highlights many important points about several processes. We believe this is the only reported case to involve highly penicillin-resistant S. pneumoniae and vertebral osteomyelitis with an adjacent mycotic aneurysm. It also provides an opportunity to emphasize the necessity of a coordinated effort between the clinician and the microbiology laboratory to develop a reasonable therapeutic plan.
Acknowledgments
We thank Donald Jungkind for his assistance with the microbiological aspects of this case.
REFERENCES
- 1.Albrecht W E, Papasian C J, Bamberger D M, Fiorella R, Riddell S W. Infected abdominal aortic aneurysm due to penicillin-, ceftriaxone- and cefotaxime-resistant Streptococcus pneumoniae. J Clin Microbiol. 1997;35:985–987. doi: 10.1128/jcm.35.4.985-987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose G B, Alpert M, Neer C S. Vertebral osteomyelitis: a diagnostic problem. JAMA. 1966;197:619–622. [PubMed] [Google Scholar]
- 3.Antony S J. Multidrug-resistant pneumococcus causing vertebral osteomyelitis. J Natl Med Assoc. 1997;89:634–635. [PMC free article] [PubMed] [Google Scholar]
- 4.Babinchak T J, Riley D K, Rotheram E B. Pyogenic vertebral osteomyelitis of the posterior elements. Clin Infect Dis. 1997;25:221–224. doi: 10.1086/514529. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer R E, van Bockel J H, van Dissel J T. Streptococcus pneumonia, an emerging pathogen in mycotic aneurysms? Neth J Med. 1998;52:16–21. doi: 10.1016/s0300-2977(97)00067-3. [DOI] [PubMed] [Google Scholar]
- 6.Caputo G M, Appelbaum P C, Liu H H. Infections due to penicillin-resistant pneumococci. Arch Intern Med. 1993;153:1301–1310. [PubMed] [Google Scholar]
- 7.Carson H W. Acute osteomyelitis of the spine. Br J Surg. 1930;18:400–408. [Google Scholar]
- 8.Chemlal K, Trouillet J L, Carbon C, Yeni P. Vertebral osteomyelitis and meningitis due to a penicillin-resistant pneumococcal strain. Eur J Clin Microbiol Infect Dis. 1996;15:893–895. doi: 10.1007/BF01691227. [DOI] [PubMed] [Google Scholar]
- 9.Friedland I R, Mc Cracken G H. Management of infections caused by antibiotic-resistant S. pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 10.Friedland I R, Paris M, Ehrett S, Hickey S, Olsen K, McCracken G H. Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993;37:1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelabert H A, Quinones-Baldrich W J. Mycotic aneurysm of the suprarenal aorta secondary to Streptococcus pneumonia: an unusual pathogen. Ann Vasc Surg. 1991;5:529–532. doi: 10.1007/BF02015277. [DOI] [PubMed] [Google Scholar]
- 12.Gelfand M S, Cleveland K O. Penicillin-resistant pneumococcal vertebral osteomyelitis. Clin Infect Dis. 1992;15:746–747. doi: 10.1093/clind/15.4.746. [DOI] [PubMed] [Google Scholar]
- 13.Gelfand M S, Miller J H. Pneumococcal vertebral osteomyelitis in an adult. South Med J. 1987;80:534–535. doi: 10.1097/00007611-198704000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Gomes M N, Choyke P L, Wallace R B. Infected aortic aneurysms: a changing entity. Ann Surg. 1992;215:435–442. doi: 10.1097/00000658-199205000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman J, Cetron M C, Farley M M, et al. The prevalence of drug-resistant S. pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumonia. Clin Infect Dis. 1992;15:199–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 17.Kutas L M, Duggan J M, Kauffman C A. Pneumococcal vertebral osteomyelitis. Clin Infect Dis. 1995;20:386–390. doi: 10.1093/clinids/20.2.286. [DOI] [PubMed] [Google Scholar]
- 18.Makins G H, Abbott F C. On acute primary osteomyelitis of the vertebrae. Ann Surg. 1896;23:510–539. doi: 10.1097/00000658-189601000-00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musher D M. Pneumococcal outbreaks in nursing homes. N Engl J Med. 1998;338:1915–1916. doi: 10.1056/NEJM199806253382610. [DOI] [PubMed] [Google Scholar]
- 20.Nuorti J P, Butler J C, Crutcher J M, et al. An outbreak of multi-drug resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med. 1998;338:1861–1868. doi: 10.1056/NEJM199806253382601. [DOI] [PubMed] [Google Scholar]
- 21.Pallares R, Linares J, Vadillo M, et al. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 22.Rubery P T, Smith M D, Cammisa F P, Silane M. Mycotic aortic aneurysm in patients who have lumbar vertebral osteomyelitis: a report of two cases. J Bone Joint Surg. 1995;77:1729–1732. doi: 10.2106/00004623-199511000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Scheld W M, Sande M A. Endocarditis and intravascular infections. In: Mandell G L, Douglas R G, Bennett J E, editors. The principles and practice of infectious diseases. 4th ed. Edinburgh, Scotland: Churchill Livingstone, Ltd.; 1995. p. 772. [Google Scholar]
- 24.Schleiter G, Gantz N M. Vertebral osteomyelitis secondary to Streptococcus pneumonia: a pathophysiologic understanding. Diagn Microbiol Infect Dis. 1986;5:77–80. doi: 10.1016/0732-8893(86)90094-5. [DOI] [PubMed] [Google Scholar]
- 25.Stone D B, Bonfiglio M. Pyogenic vertebral osteomyelitis. Arch Intern Med. 1963;112:491–500. doi: 10.1001/archinte.1963.03860040087007. [DOI] [PubMed] [Google Scholar]
- 26.Waldvogel F A, Lew D P. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 27.Waldvogel F A, Medoff G, Swartz M N. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:316–322. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 28.Waldvogel F A, Vasey H. Osteomyelitis: the past decade. N Engl J Med. 1980;303:360–370. doi: 10.1056/NEJM198008143030703. [DOI] [PubMed] [Google Scholar]