ABSTRACT
The ESKAPE pathogens (Enterococcus spp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) are identified to be multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan drug-resistant (PDR); thereby, imposing severe challenges in the treatment of associated infections. ESKAPE pathogens colonize on various biotic and abiotic surfaces; biofilms formed by these pathogens are a potential source for food contamination. Moreover, biofilms play a pivotal role in the development of antimicrobial-resistant (AMR) strains. Hence, the frequent isolation of antimicrobial-resistant ESKAPE pathogens from food products across the globe imposes a threat to public health. A comprehensive understanding of the adhesion signaling involved in the polymicrobial and single-species biofilm will assist in developing alternative preservation techniques and novel therapeutic strategies to combat ESKAPE pathogens. The review provides a comprehensive overview of the signaling mechanisms that prevail in the ESKAPE pathogens for adhesion to abiotic and biotic surfaces and molecular mechanisms associated with poly-microbial biofilm-assisted AMR in ESKAPE.
KEYWORDS: ESKAPE, polymicrobial, biofilms, antimicrobial resistance, signaling
Introduction
The presence of antimicrobial-resistant ESKAPE pathogens in food products is a matter of global concern. Most of the foodborne diseases caused by ESKAPE pathogens are mainly linked to animal or plant origin foods such as milk, meat, poultry, vegetables or instigated from the food-related environment such as agricultural land food industry, hospital kitchen [1]. Besides, food processing and storage conditions also increase the risk of disseminating ESKAPE pathogens in food items [1]. Earlier, there were hardly reports of antibiotic-resistant ESKAPE pathogens in food. However, nowadays, several incidences of the prevalence of antibiotic-resistant ESKAPE pathogens in food have been observed. Multidrug-resistant Enterococcus spp. have been found in chicken meat samples (Assam-India) [2], ready to eat dairy products, poultry sector, and animal food (Poland, Kwazulu-Natal-South Africa, and the U.S., respectively) [3–5]. A food poisoning outbreak due to S. aureus (possessing three antibiotic resistance genes; mecA, mecC, and mupA) occurred in Italy (in 2015) and affected 24 customers of a local restaurant [6]. Moreover, chicken, turkey, and pork products from grocery stores in Arizona and Flagstaff (The United States of America) were contaminated with ampicillin, tetracycline, cefazolin-resistant K. pneumoniae [7]. However, A. baumannii strains causing enterogenic sepsis were isolated from the hospital kitchens of Portugal and Brazil [8]. Furthermore, multidrug-resistant P. aeruginosa was found as a potential contaminant in vegetables (Italy), fish farms (Egypt) [9], and water (United States) [10]. Likewise, Enterobacter spp. were found in the milk samples collected from various regions of West Bengal, India [11] and milk powder production units in the United States [12].
Furthermore, ESKAPE biofilms impose severe complexities in the food industry. Recently, the correlation of food safety and biofilms has been described for S. aureus and P. aeruginosa [13]. Biofilms play a pivotal role in antimicrobial resistance. In the last few decades, numerous bacterial strains have developed resistance to various commonly used antibiotics due to genetic, social, and environmental factors. AMR is one of the global issues, requiring extensive coordinated effort to diminish its consequences [14,15]. Despite new antibiotic discoveries, the treatment of multi-drug-resistant (MDR), extensively drug-resistant (XDR), and pan drug-resistant (PDR) bacterial infections remain challenging, leading to severe morbidity and mortality. The resistance to antimicrobials can be attributed to one of the mechanisms adopted by bacteria, i.e. the ability to form a biofilm.
Biofilm is a unique multicellular structure where bacteria are embedded in the self-secreted extracellular polysaccharide matrix and proteinaceous material, which protects them from environmental stresses, antimicrobial agents, and the host immune system. Inside biofilm, bacteria are 1000 times more resistant to antibiotics as compared to their planktonic counterparts [16,17]. The National Institute of Health (NIH) estimates that 65 and 80% of all microbial and chronic infections are associated with biofilm formation [18].
Based on the current need, the World Health Organization (WHO) has prepared a list of few priority pathogens for which new antimicrobials need to be developed. The list consists of ‘ESKAPE’ pathogens viz. Enterococcus spp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp [19]. These pathogens possess an elevated level of antibiotic resistance, mainly due to the ability to form biofilm [20]. The extended-spectrum β-lactamase (ESBL) or carbapenem-resistant K. pneumoniae (CRKP), Enterobacter spp. and Carbapenem-resistant P. aeruginosa (CRPA), A. baumannii (CRAB) belong to critical priority pathogens. However, vancomycin-resistant Enterococcus spp. (VRE) and vancomycin or methicillin-resistant S. aureus (VRSA/MRSA) belong to high priority pathogens and cause a preponderance of mortality and economic burden worldwide [21]. The colonized VRE infections’ treatment cost was 1167 USD higher than that of planktonic VRE infection [22]. The total hospital charge and ICU cost of MRSA infections were found to be 2.07 and 6.71 times higher than methicillin-sensitive S. aureus MSSA infections. However, MRSA infections involving colonization increase total hospital charge by 2193 USD compared to colonization and 56,900 USD compared to MSSA colonization [22]. On average, the total hospital cost of drug-resistant K. pneumoniae infections was observed to be higher than drug-sensitive infections by 1574. USD Specifically, CRKP incurs 1.95 times higher total hospital cost than Carbapenem-sensitive K. pneumoniae infections.
Furthermore, infections involving colonization of K. pneumoniae showed an additional increase in the total hospital cost. In biofilm-forming ESBL positive and non-biofilm forming ESBL positive K. pneumoniae infections, the total hospital cost difference was found to be 1808 USD [22]. CRAB, CRPA, and Carbapenem-resistant and ESBL positive Enterobacter spp. express unique adhesion proteins to colonize on biotic and abiotic surfaces and cause bloodstream, urinary, respiratory tract infections. The infections of CRPA cause a 1.23–1.68 times increase in the hospital cost compared to Carbapenem-sensitive P. aeruginosa (CSPA). Few studies showed that the total hospital cost required to treat CRAB infections is 1.21–1.68 times higher than Carbapenem-sensitive Acinetobacter baumannii (CSAB) infections and infections involving colonization of CRPA and CRAB further elevate the total hospital cost required for treatment of such infections [22–24]. Recent available data states that ESKAPE pathogens represent the lion’s share (42.2%) in bloodstream infection and increase hospital stay by 3.3 days, cost by 5500, USD and mortality by 2.1% [25]. Thus, antibiotic resistance and the ability to form single species and polymicrobial biofilms reduce the efficacy of current treatments available for ESKAPE infections and thereby incur an economic burden to the healthcare system [25].
Adhesion is a preliminary step of biofilm formation, hence, a thorough understanding of adhesion signaling in the development of single species or polymicrobial biofilms of ESKAPE is highly desired to develop novel treatment strategies against ESKAPE infections. This effort offers an additional advantage in regulating other (Non-ESKPAE) bacterial infections as drug resistance and pathogenesis mechanisms in ESKAPE are representative for other pathogenic bacteria, and the regulation strategy of ESKAPE infections can be implemented to other bacterial infections [26]. Hence, in this review, we first describe signaling involved in the adhesion of ESKAPE pathogens to biotic or abiotic surfaces and the contribution of adhesion signaling in polymicrobial biofilms initiation. Subsequently, we focus on the molecular mechanisms of AMR mediated by single and multispecies biofilm in ESKAPE pathogens. Finally, we comment on the complexity of designing the treatment strategy for ESKAPE pathogens and highlight novel molecular targets for efficient treatment.
The ESKAPE signaling for adhesion
Bacterial adhesion to biotic or abiotic surfaces is a complex interplay between bacteria, adhering surface, and the surrounding medium, and it is considered a prime step in bacterial pathogenesis, hence, act as a potential therapeutic target. ESKAPE pathogens produce several families of proteins, which facilitate bacterial adhesion to the surface [27,28]. With the advancement in Omics, it has been discovered that quorum sensing plays an essential role in the initial attachment and biofilm formation in pathogenic bacteria. ESKAPE pathogens form single species or polymicrobial biofilms. In the majority of biofilm-associated human infections, microbes hardly exist as single species biofilm and form polymicrobial biofilms. Polymicrobial biofilms may consist of multiple bacteria from different genera or species from a different kingdom. Recent observations state that infections involving polymicrobial biofilm are relatively more challenging to treat than monomicrobial biofilm-associated infections and have a high mortality rate [29,30].
Nevertheless, we do not have a complete understanding of the factors involved in ESKAPE biofilms initiation. Therefore, in this section of the review, we describe signaling involved in ESKAPE pathogens’ adhesion to biotic or abiotic surfaces in monomicrobial or polymicrobial biofilms.
Enterococcal signaling and adhesion
Enterococci are Gram-positive pathogenic bacteria commonly found in water, raw and processed food (such as meat, vegetables, milk, fermented foods), soil, plant, and humans’ gastrointestinal tracts animals. Enterococci produce biogenic amines and can cause several food-associated infections [31,32]. Enterococcus spp. colonizing food items possess antibiotic resistance genes, and through the food chain, these isolates colonize the intestine or transfer antibiotic-resistant genes to other intestinal enterococci. However, Enterococcus faecalis and Enterococcus faecium are predominantly associated with several hospital-acquired and urinary tract infections [33], and their pathogenesis is mainly mediated through adhesion and biofilm formation ability of the strain [34]. Enterococcus spp. employ aggregation substances (agg, asa1), cytolysin (cyl), gelatinase (gelE), extracellular surface protein (Esp), collagen-binding proteins (ace, acm), and adhesion-like endocarditis antigens (efaAfs and efaAfm) to colonize food and biotic surfaces (Table 1) [35,36].
Table 1.
Adhesins expressed by Enterococcus spp., Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp. (ESKAPE) pathogens
| Pathogen | Type of adhesins | Adhesins | Function | Reference |
|---|---|---|---|---|
| Enterococcus spp. | Aggregation substances | agg, asa1 | Promote aggregation and involved in binding to epithelial cell and abiotic surface | [35,45] |
| Extracellular surface protein | Esp | Involved in cell- cell adhesion, abiotic surface attachment, binding to host tissue, and immune evasion | [35,45] | |
| - | Ace, Acm | Involved in binding to type I and type IV collagen and ECM proteins. | [36] | |
| E. faecalis endocarditis antigen A | EfaAfs | Hydrolyses elastin, gelatin, hemoglobin, collagen, and attachment to biotic and abiotic surface | [36] | |
| LPXTG surface proteins | SgrA | Adhesion to polystyrene abiotic surface and to the extracellular matrix molecules nidogen 1 and nidogen 2 | [42] | |
| LPXTG surface proteins -MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules) | EcbA | Attach to components of the extracellular matrix, protein, host tissue-collagen, fibronectin, fibrinogen, laminin. | [42] | |
| Acm | Binds to collagen type I | [42] | ||
| Scm | Binds to collagen type V | [42] | ||
| ScbA | Binds to fibrinogen | [42] | ||
| PilA, PilB | Host tissue adhesion and biofilm | [34] | ||
| S. aureus | Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMMs) | Fibronectin binding protein FnBPA, | Involved in intercellular adhesion and binding to fibronectin | [50] |
| Fibronectin binding protein FnBPB | Mediate intercellular adhesion and binding to fibronectin | [50] | ||
| Clumping factor ClfA | Binds to platelets which are attached to damaged heart valves | [50] | ||
| Clumping factor ClfB | Binding to epithelial cells | [50] | ||
| SdrD | Interacts with protein Desmoglein-1 (Dsg1) or DSG2 that are present on the epithelial cells. | [50] | ||
| SdrE | Binding to plasma protein, epithelial cells and platelet aggregation | [50] | ||
| IsdA | Binding to Fetuin, Fibrinogen, Transferrin, Fibronectin, Hemoglobin, Hemin |
[50] | ||
| IsdB | Binding to hemin, hemoglobin | [50] | ||
| Surface protein SasC | Mediate intercellular adhesion | [120] | ||
| Surface protein SasG | Intercellular adhesion, nasal epithelial cell binding | [120] | ||
| Collagen binding Cna | Binding to collagenous tissues | [50,120] | ||
| Bbp | Attachment to bone sialoprotein | [120] | ||
| Plasma sensitive (Pls) | Binding to nasal epithelial cells and intercellular adhesion | [50] | ||
| Non-covalently linked surface-associated protein | Autolysin Atl | Involved in the binding to polystyrene, fibrinogen, fibronectin, endothelial cells | [50] | |
| Aaa | Binding to fibrinogen, fibronectin, vitronectin | [50] | ||
| Bap | Intercellular adhesion and attachment to polystyrene | [50] | ||
| Spa | Bacterial cell aggregation and binding to the platelet receptor | [50] | ||
| SERAMs (Eap/Emp/Map/P70/Amp) | Binding to ECM components- fibrinogen, fibronectin, vitronectin | [120] | ||
| Membrane spanning protein (Ebh and Ebps) | Attachment to ECM components fibrinogen, elastin | [50] | ||
| Non-proteinaceous surface adhesins | Polysaccharide intercellular adhesion PIA produced by gene products encoded by icaA, icaD, icaB, icaC | Involved in intercellular adhesion, binding to epithelial cells and abiotic surfaces such as polystyrene, orthodontic surface | [113] | |
| wall teichoic acid (WTA) | Binding to human epithelial cell, polystyrene, glass | [50] | ||
| lipoteichoic acid (LTA) | Involved in binding to medical devices | [50] | ||
| K. pneumoniae | Chaperone/Usher-Type 1 Fimbriae | FimA, FimC, FimD, FimF, FimG, FimH | Adhesion to mannose-containing structures on host cells and extracellular matrix | [61,114] |
| Chaperone/Usher- Type 3 Fimbriae |
Mannose-resistant Klebsiella-like (MR/K) hemagglutinins MrkA, MrkB, MrkC, MrkD |
Involved in binding to biotic (human extracellular matrix such as type V collagen) and abiotic surface |
[61,115] | |
| A. baumannii | Usher-chaperone pili | CsuC-CsuE | Colonize medical devices, and biotic surfaces | [116] |
| Autotransporter adhesin | The Acinetobacter trimeric Autotransporter adhesin (Ata) |
Adhesion to human endothelial and epithelial cells | [117] | |
| P. aeruginosa | Type IV pili | PilA#, PilE, PilD, PilV, PilW, PilX | Binding to stainless steel, polyvinylchloride and polystyrene, human epithelial cell | [61,118] |
| Tad type pili | Flp#, TadA, TadB, TadC, TadD, TadF, TadG, FppA, RcpA, RcpC, TadZ | Attachment to epithelial cells, cellular aggregation, binding to abiotic surface | [61] | |
| Nucleation/precipitation type pili | FapC#, FapA, FapB, FapD, FapE, FapF | Attachment to plastic and glass surface | [118] | |
| Enterobacter spp. | Chaperone/Usher | FimA, (Type I)#, FimC, FimD, FimF, FimG, FimH | *Adhesion to glass surface, plastic polymers and receptors (mannose) of host cells | [61] |
| Nucleation/Precipitation CooA | CsgA(curli)#, CsgB, CsgG, CsgE, CsgE, CsgF, CsgD | *Adhesion to epithelial cells, glass, polystyrene surface | [61,119] |
*The functions of the adhesins mentioned in the table are estimated based on the other organisms of the Enterobacteriaceae family; however, it is not explicitly studied in the Enterobacter spp.
#Major adhesins
Zinc metalloprotease enzyme gelatinase and secreted serine protease play a prime role in the adhesion and biofilm formation in Enterococcus spp [37,38]. Moreover, its expression is dependent on the fsr locus (size ~2.8 kb) consisting four genes fsrA, fsrB, fsrD, and fsrC. The fsr locus encoding a two-component regulatory system is similar to the S. aureus Accessory gene regulator (Agr) system. It senses the cell population in the surrounding and regulates the virulence mechanism. As cell density increases, Gelatinase Biosynthesis Activating pheromone (GBAP) accumulates in the surrounding and is sensed by the FsrC sensor. Further, it gets phosphorylated and activates FsrA, the response regulator. Phosphorylated response regulator (FsrA) controls the transcription of fsrBCD, ef1097 locus (located upstream of the fsr operon) and gelE (encoding gelatinase)-sprE (encoding serine protease) operon. Mutation in the fsrA, fsrB, fsrC affects the expression of the downstream genes, gelE, and sprE, indicating the importance of fsr operon in the expression of surface adhesion genes gelE and sprE [39]. It has also been reported that the fsr system is also directly or indirectly regulated by a glucose-dependent transcriptional regulator [40]. In addition to Fsr quorum sensing, Enterococcus spp. communicate using peptide pheromones, e.g. Cpd, Cob, and Ccf16, and stimulate the transfer of pheromone responsive plasmid carrying agg/asa1 genes encoding aggregation substances through conjugation apparatus [41].
Enterococcus spp. encode the pilA and pilB pilus-like structures, and three LPXTG surface proteins, namely Orf903, Orf2351 (SgrA), and Orf2430 (EcbA), anchored to the cell wall. LPXTG comprises conserved Leu-Pro-X-Thr-Gly sequence, where X represents any amino acid, substrate motif sortase, and a hydrophobic domain. The LPXTG motif is cleaved by sortase, which further anchors the surface protein to the peptidoglycan of the cell wall. In E. faecium, SgrA and EcbA attach to the ECM components and are highly conserved in antibiotic-resistant strains and used as a marker for clinical isolates. SgrA does not assist E. faecium binding to biotic surfaces, such as human bladder cells, kidney cells, human intestinal epithelial cells. Instead, they mediate the adhesion of E. faecium cells to abiotic surfaces [42]. LPXTG surface proteins also include MSCRAMMs (Microbial Surface Components Recognizing Adhesive Matrix Molecules) and are involved in adhesion to extracellular matrix proteins and host tissue (Table 1). These proteins bind to collagen, fibronectin, fibrinogen, laminin. E. faecium expresses Acm, Scm (Figure 1). Acm mainly binds to collagen type I, and Scm binds to collagen type V and fibrinogen [42]. However, E. faecalis expresses Ace and binds to collagen [27,28,43]. Thus, E. faecium possesses three fibrinogen-binding adhesins SgrA, ScbA, and Scm (Table 1).
Figure 1.

Enterococcus spp. signaling involved in adhesion
At high cell density, the extracellular concentration of the Gelatinase Biosynthesis Activating pheromone (GBAP) rises in the surrounding and recognized by by FsrC, sensor kinase. Further, it gets phosphorylate and activate FsrA, the response regulator. Phosphorylated response regulator (FsrA) controls the transcription of fsrB, fsrD, fsrC, ef1097, gelE, sprE and other adhesion genes which encode FsrB, FsrD, FsrC, Enterocin O-16, gelatinase, Serine protease and other adhesion proteins (Esp, Scm, Acm/Ace, Ebp pilus, EcbA, piliA/piliB, SgrA).
Esp, a protein containing 1873 amino acids, is also involved in biofilm formation in Enterococcus spp [37]. It has been demonstrated that Esp protein encoded by the esp gene is predominantly associated with the initial attachment of the E. faecalis to the abiotic surfaces, e.g. food, polystyrene, and polyvinyl chloride plastic surfaces [44,45]. It has been demonstrated that the expression of Esp is growth-dependent and is controlled by environmental signals [40]. Enterococcal biofilm regulator B (EbrB), located upstream of esp, responds to environmental signals and plays a key role in Enterococcal Esp expression and intestinal colonization [34,46]. However, the specific role of Esp in Enterococcus spp. biofilm formation is not yet completely understood. The expression of E. faecalis endocarditis antigen A (efaAfs) and E. faecium endocarditis antigen A (efaAfm) adhesins are also involved in enterococcal attachment to abiotic and biotic surfaces [34]. Moreover, genes ebpABC locus, srt, and pil are responsible for pili production on the cell surface and contribute to cell adhesion and biofilm formation (Table 1) [34]. E. faecalis also possesses the LuxS homologous gene, which is involved in biofilm formation [47].
Several other factors like Enterococcal polysaccharide antigen (Epa), autolysin (atn), Secretory antigen like genes (sal A, salB), D-alanine lipoteichoic acid (dltA), the sugar-binding transcriptional regulator, Biofilm on plastic (bopD), The endocarditis and biofilm-associated pili (ebp) operon, its downstream gene, sortase (srtC), Transcriptional regulator of ebp operon (ebpR) are found to be involved in adhesion and biofilm formation in E. faecalis (Figure 1) (Table 1) [40].
Thus, Enterococcus employs complex and multistage signaling and adhesion process. The influence of food molecules and microflora on the expression of adhesins and aggregation factors is yet to be investigated.
Staphylococcus aureus signaling and adhesion
S. aureus is a foremost etiological agent of severe diseases in humans and animals, e.g. sepsis, pneumonia, and bacteremia. S. aureus colonization to biotic and abiotic surfaces contributes to foodborne diseases and imposes huge economic loss [48,49].
Adherence to the surface is a prerequisite step for S. aureus infections [50,51]. S. aureus possesses Agr and LuxS quorum-sensing systems that involve the production of autoinducer peptide (AIP) and AI-2, respectively, and regulate expression of the biofilms and virulence factors [52,53]. The Agr QS system is a two-component QS system and consists of 4 genes. As AIP concentration in the surrounding increases, it attaches to the membrane-bound AgrC histidine kinase. The AIP-AgrC complex formation triggers autophosphorylation of AgrA, which consequently induces the Agr operon. The activation of the P2 and P3 promoters of agr operon synthesizes RNAII and RNAIII transcripts, respectively. RNAII induces the Agr system by transcribing the Agr operon (AgrBDCA). The agrD of AgrBDCA operon encodes the pro-autoinducing peptide (Pro-AIP), which is further processed into the cyclic AIP (~7–9 peptide long) by the AgrB transmembrane protein. However, RNAIII induces the production of δ-toxin or δ-PSM encoding the toxins and concurrently represses the expression of fibronectin-binding proteins A and B, rot, coagulase, other surface proteins, and protein A. The down-regulation of Rot causes the induction of genes that encode the production of additional toxins, proteases, enterotoxins, superantigens, ureases, and lipases. Thus, during the initial stage of the biofilm formation, i.e., in the exponential phase of the growth, adhesins and other surface proteins are upregulated, and during the biofilm maturation phase, i.e., the stationary phase of bacterial growth, α-hemolysin, δ hemolysins, nucleases, proteases are upregulated (Figure 2)[51].
Figure 2.
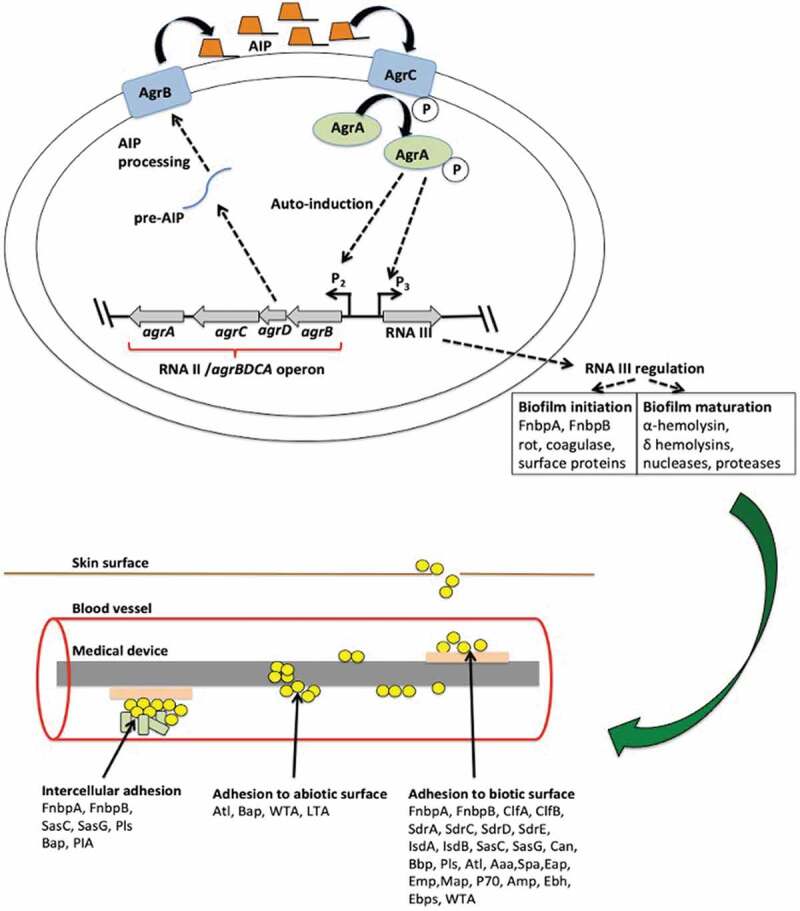
Staphylococcus aureus signaling involved in adhesion
S. aureus possesses Agr QS system. An increased extracellular AIP concentration is sensed by a membrane-bound AgrC histidine kinase and triggers autophosphorylation of AgrC, which further phosphorylates AgrA. Activated AgrA induces the Agr operon and activates the P2 and P3 promoter of agr operon leading to the synthesis of the RNAII and RNAIII transcripts respectively. RNAII encodes the pro- autoinducing peptide AgrD (Pro-AIP), which is further processed into the cyclic AIP by the AgrB transmembrane protein and secreted out of the cell. Whereas, RNAIII encodes proteins for the initial attachment of the biofilm. S. aureus expresses a unique set of proteins for adhesion to the biotic surface that is mentioned in this figure.
S. aureus also possesses a sarA two-component QS system. At higher cell density, expression of the sarA increases, and it further up-regulates expression of surface proteins, adhesins, PIA, EPS, and down-regulates nuclease and protease expression. Thus, sarA facilitates the preliminary attachment of the bacteria to the surface. However, after initial adhesion, Agr is activated to produce nucleases, proteases, phenol soluble modulins (PSMs), causing biofilm dispersal.
Surface adhesins involved in the initial adhesion
Adhesion of S. aureus to the abiotic surface, e.g., food and medical devices, relies on physicochemical properties of the abiotic surface and non-proteinaceous surface adhesins, e.g. lipoteichoic acid (LTA), wall teichoic acid (WTA), non-covalently linked surface-associated proteins autolysins (AtlA, AtlE), and Accumulation associated proteins (Aap). However, S. aureus adherence to biotic surfaces is mainly mediated by MSCRAMMs (Fibronectin Binding protein FnBPA, FnBPB, ClfA, ClfB, SdrA, SdrC, SdrD, SdrE, IsdA, IsdB, Surface Protein C (Sas C), Surface protein G (SasG), Collagen binding protein Cna, Bpb, Pls). Similar to Enterococcus spp., S. aureus possesses LPXTG motifs consisting of MSCRAMMs involved in cell adhesion [50]. The SdrD protein from the MSCRAMMs category interacts with the protein Desmoglein-1 (Dsg1) or DSG2 present on the epithelial cells (Table 1) [54].
Surface adhesins involved in the accumulation/maturation phase
After initial attachment to the surface polysaccharide, intercellular adhesion (PIA), i.e., N-acetylglucosamine (PNAG), is synthesized by Ica operon. The extracellular matrix is formed by PNAG, extracellular DNA (eDNA), cell wall anchored proteins (CWA), and followed by biofilm maturation. Along with PIA proteins, few adhesion proteins like SasG, Bap, FnBps play a key role during the biofilm accumulation/maturation phase (Table 1) [52].
S. aureus forms PIA dependent and PIA independent biofilms. The ica locus consists of 4 genes, icaA, icaB, icaC, and icaD. Their synthesis is controlled by environmental factors or stress conditions like the presence of ethanol or antibiotics, anaerobic conditions, extreme temperature, and solute concentrations (Table 1) [52]. Among these genes, icaA and icaD are involved in synthesizing N-acetylglucosaminyl transferase and is responsible for exopolysaccharide synthesis. The gene icaD assists the transport of N-acetyl glucosamine (NAG) to the cell surface. The product of the gene icaB fixes NAG to the surface through deacylation. However, the icaADBC operon has two regulators; the IcaR encoded by icaR, located upstream of the operon, and TcaR (transcriptional regulator of the teicoplanin-associated locus). The deletion of icaR increases PIA expression, but the deletion of tcaR does not significantly upregulate PIA synthesis. The expression of the IcaR is repressed by Rbf (regulator of the biofilm formation), and it is upregulated by Spx (global stress response gene). In the presence of anaerobic conditions, SrrAB (Staphylococcal respiratory response regulator) binds to the upstream region of the icaADBC, and upregulates PIA synthesis. It was observed that the deletion of the icsADBC operon does not inhibit biofilm formation and virulence, which indicates that S. aureus possesses ica independent pathway for biofilm formation [52]. This pathway involves accumulation-associated protein (Aap), biofilm-associated proteins (Bap), Fibronectin-binding proteins (FnBPA, FnBPB), Surface proteins (SasG, SasC), Autolysins (AtlA, AtlE), wall teichoic acid (WTA). The signal AI-2 encoded by luxS decreases biofilm formation using the ica independent pathway. The sigB operon present in the S. aureus synthesizes σB and induces expression of ica operon, and facilitates initial attachment of the bacteria to surfaces (Table 1) (Figure 2) [52].
K. pneumoniae signaling and adhesion
Due to increased carbapenem resistance, K. pneumoniae infections have limited treatment options and stand second in the most severe Gram-negative infections. K. pneumoniae is an opportunistic pathogen and causative of hospital-acquired infections with an estimated 27–37% mortality rate. K. pneumoniae predominantly causes extraintestinal infections such as urinary tract and bloodstream infection. However, it has also been found to be involved in foodborne infections [55]. Several multidrug-resistant K. pneumoniae strains occur on raw vegetables, chicken or pork liver, poultry dish, bean sprout [56]. Another study demonstrated that ESBL-producing K. pneumoniae colonized on the hospital kitchen surfaces and foodstuffs was a prime reason for the foodborne nosocomial outbreak in March 2008 [57].
As biofilms offer myriad advantages to the bacteria (e.g. escape from immune response, enhanced protection from antimicrobial drugs), biofilm-associated K. pneumoniae infections are relatively more difficult to cure than infections with planktonic K. pneumoniae [58].
K. pneumoniae possesses an AI-2 quorum sensing system, and it regulates the expression of biofilm-associated genes [59]. The luxS gene encoding LuxS protein synthesizes AI-2 precursor 4,5-dihydroxyl-2,3-pentanedione from S-ribosylhomocysteine. Moreover, the AI-2 quorum sensing system regulates the synthesis of usher-chaperone pili and other adherence factors involved in K. pneumoniae biofilm formation to biotic or abiotic surfaces [59,60]
K. pneumoniae express Type 1 FimA, FimC, FimD, FimF, FimG, FimH pili, and Type 3 MrkA, MrkB, MrkC, MrkD pili from the class Chaperone/Usher [61]. However, type 3 fimbriae strongly enhance biofilm formation in K. pneumoniae C3091 as compared to type 1 fimbriae (Table 1) [62].
Among them, MrkA plays a key role in binding to abiotic surfaces. However, MrkD is involved in the adhesion to the human extracellular matrix (Table 1) [63]. The potential role of MrkA in adhesion to the abiotic surface has been demonstrated; albeit, the molecular mechanisms are yet to unrevealed. Type 3 fimbriae are generally expressed by both clinical and environmental isolates of K. pneumoniae and facilitate adhesion to a wide range of cell types, including tracheal cells, urinary bladder, respiratory tissue, trypsinized buccal cells [64]. Biofilm formation was enhanced in K. pneumoniae isolates with upregulated magA, aero, remA, rmpA2, allS, wcaG, and iutA genes [58]. Furthermore, exogenous glucose level also modulates the expression of adhesins and virulence genes in K. pneumoniae through the interplay of the CRP-cAMP and c-di-GMP signaling pathways (Figure 3) [65].
Figure 3.

K. pneumoniae signaling involved in adhesion
In the presence of glucose, intracellular concentration of cAMP and cAMP receptor protein (CRP) complex decreases and concentration of c-di GMP increases. MrkH protein binds to c-di-GMP and upregulates transcription of mrkABCDF operon encoding type 3 fimbriae. A locus mrkHIJ senses the intracellular concentration of c-di-GMP and regulates the expression of type 3 fimbriae. The MrkH possesses autoregulatory activity and activates expression of type 3 fimbriae. However, the MrkJ represses the expression of the mrkABCDF by hydrolyzing c-di-GMP. Concurrently, increased concentration of glucose upregulates LuxS expression, which in turn increases extracellular concentration of autoinducer-2 (AI-2) signal molecule. AI-2 is recognized and imported by ABC transporter LsrACDB. Further, the AI-2 is phosphorylated by the LsrK and binds to LsrR. The binding of AI-2 to LsrR, upregulate lsr operon. However, a direct link between AI-2 mediated quorum sensing and expression of type 1 and 3 fimbriae is not yet established.
Acinetobacter baumannii signaling and adhesion
A. baumannii is ubiquitous, aerobic gram-negative pathogenic bacilli. The increase in the rate of antibiotic resistance is imposing several challenges in the treatment of A. baumannii infections [66]. A. baumannii cause severe foodborne infections. It is generally found in the raw milk and may also colonize the utensils. The infections caused by A. baumannii primarily affect immunocompromised patients in the healthcare setting, like premature infants in the hospital. A. baumannii colonizes in the gastrointestinal tract during infection; however, its presence gets masked by other commonly associated foodborne bacteria. Hence, detection of A. baumannii infection is difficult [67].
Expression of adherence factor in A. baumannii is a multistage process and involves complex signaling mechanisms. The adherence of A. baumannii is regulated by cell density, nutrient availability, the concentration of free cations, and physicochemical properties of the adhering surface. A. baumannii employs a two-component regulatory system BfmRS, which induces the expression of the pili belonging to the class usher-chaperone required for the initiation of the biofilm on biotic or abiotic surfaces [66]. The BfmRS system consists of a sensor kinase and response regulator encoded by bfmS and bfmR genes. An increase in cell density induces the BfmRS system and activates the expression of pili encoded by polycistronic, 6 ORF containing csuA/BABCDE operon. Prior studies showed that these pili play a crucial role in the initial adhesion of the A. baumannii to the abiotic surface (Table 1) (Figure 4).
Figure 4.

Acinetobacter baumannii signaling involved in adhesion
A. baumannii possesses BfmRS and AHL based adhesion signaling pathway. In BfmRS, BfmS represents a sensor kinase, and BfmR is a response regulator which upon phosphorylation activates expression of the csuA/BABCDE operon for the synthesis of the Usher fimbriae. However, the AHL molecule is synthesized by abaI gene, which upon binding to the cognate receptor regulates expression of the adhesion-associated genes.
Though the inactivation of BfmR completely inhibits biofilm formation, the deletion of BfmS does not entirely inhibit biofilm in A. baumannii. This indicates the presence of another interaction of BfmR and the probability of another biofilm-producing pathway.
Metal cations and few additional proteins also control A. baumannii biofilm; however, the regulation’s basic mechanism remains to be explored. Synthesis of Bap encoded by the bap gene is crucial for biofilm maturation (Figure 4) [66]. However, molecular factors and environmental conditions involved in the synthesis of Bap need further investigation.
Alternative signaling regulating biofilm formation in A. baumannii involves N-acyl homoserine lactone (AHL) signal synthesized using AI synthase (abaI).
It has been demonstrated that adhesion of A. baumannii to biotic surfaces such as human alveolar epithelial cells can occur without the involvement of CsuA/BABCDE operon. The two-component system, BfmRS, is not just confined for binding to abiotic surfaces; instead, it also plays a vital role in adhesion to biotic surfaces. In conclusion, A. baumannii uses different regulatory systems for adhesion to biotic and abiotic surfaces. The role of food-associated polymicrobial communities in the secretion of adhesins, virulence factors, biofilm formation, and dispersal needs further investigation.
Pseudomonas aeruginosa signaling and adhesion
P. aeruginosa is frequently associated with numerous human diseases. P. aeruginosa causes foodborne diseases due to its ability to colonize a wide range of surfaces. Pseudomonas spp. can form biofilms on foods such as meat, fish, sprouts, vegetables, or food containing environments such as food processing units, floors, pipes, drainage systems, and walls. Certain isolates of P. aeruginosa can resist pasteurization and often contribute to FBD [68,69]. Immunosuppressed patients, particularly transplant recipients, patients with burns, and HIV, are at increased risk for P. aeruginosa infection [70]. P. aeruginosa is a principal etiological agent in nosocomial infections such as pneumonia, community-acquired pneumonia, urinary tract infections, bacterial keratitis (contact lens associated eye infections), chronic lung infections in patients with cystic fibrosis, infections due to severe burns, and implant-associated infections [71]. The mortality rate of this infection is very high due to the amalgamation of weak host defenses and bacterial resistance to antibiotics [72]. P. aeruginosa gets embedded into the self-secreted extracellular polymeric matrix called as biofilm and becomes resistant to antibiotics. QS systems in P. aeruginosa direct synthesis of biofilm and play a vital role in the expression of virulence factors, including proteases, elastase, pyocyanin, pyoverdine, lectins, rhamnolipids, and toxins. Such virulence factors indirectly affect the development and maintenance of biofilms [73]. P. aeruginosa has four major quorum-sensing systems, first mediated through 3-oxo- C12 HSL, second mediated through C4-HSL, third mediated through 2-heptyl-3- hydroxy-4-quinolone also known as the Pseudomonas quinolone signal (PQS) system, and the fourth recently discovered integrated QS system (IQS) (Figure 4). LasI/LasR and RhlI/RhlR are two major quorum-sensing systems based on AHL signal molecules. LasI produces AHL signal molecule, N-(3-oxo-dodecanoyl)- L-homoserine lactone (OdDHL), which diffuses out of the cell and when it reaches critical threshold concentration, it is recognized by transcriptional regulator LasR. The dimer of OdDHL-LasR then binds to the target promoter and induces expression of lasI as well as rhlR/rhlI and PQS systems. The mechanism of the rhlI/R system is similar to lasI/R but consists of N-butanoyl-L-homoserine lactone (C4-HSL, BHL), AHL synthase RhlI and the cognate receptor RhlR to which BHL binds [74]. The rhlI gene, which is homologous to luxI and lasI encodes the biosynthesis of BHL and is found downstream of the rhlABR cluster [75]. The third QS system is based on PQS. PQS is chemically distinct from the AHL signals of the las and rhl systems. The PQS synthesis cluster consists of pqsABCD, phnAB, and pqsH. PqsR is a transcriptional regulator, which binds to the promoter region of pqsABCD and controls the expression of PQS. PqsR is the cognate receptor of PQS and together induces expression of PqsABCD. The fourth inter-cellular communication signal was recently discovered as 2- (2-hydroxyphenyl)-thiazole-4-carbaldehyde (IQS). This system integrates environmental stress cues with the quorum sensing network, e.g. phosphate depletion condition. The ambBCDE gene cluster encodes proteins required for IQS signal synthesis. Though the signal molecule is known for the IQS system, the receptor is not yet discovered (Figure 5) [70,75].
Figure 5.

Signaling involved in Pseudomonas aeruginosa adhesion
P. aeruginosa possesses four quorum sensing systems namely, LasI/R, RhlI/R, IQS (Integrated quorum sensing system), and PQS (Pseudomonas quinolone signal mediated systems). LasI produces 3-oxo- C12 HSL, and it is secreted out of the cell. The signal attains critical concentration and is recognized by LasR. The signal and LasR complex regulates expression adhesion and virulence genes (LasA protease, LasB elastase, Rhl, IQS, PQS, and other adhesion factors). Similarly, RhlI produces C4-HSL, which is recognized by RhlR. The RhlR and C4-HSL complex regulates expression of LasB elastase, RhlAB rhamnolipid, Phz pyocyanin, PelB, Psl. The PQS signal is synthesized by pqsABCD, phnAB, and pqsH cluster and binds to the PqsR receptor. The signal receptor complex further activates PQS and RhlI/R system. However, the IQS system responds to environmental stress cues. IQS receptor and signal complex stimulate the expression of other target genes (LecA, LecB, Rhl system). Arrows represent the stimulatory effect.
The AHL based quorum-sensing system LasI/R is at the top of all four P. aeruginosa QS system hierarchy. The critical threshold concentration of OdDHL signal leads to the formation of dimer OdDHL/LasR. The complex further triggers the expression of rhlR, rhlI, lasI. LasR-OdDHL also induces PqsR, the transcriptional regulator of the PQS biosynthesis operon pqsABCD. PQS system enhances the transcription of rhlI, thus inducing BHL production and the overall expression of the rhl QS system and also regulates rhl-dependent phenotypes. Recently, it was found that the IQS system is also controlled by LasI/R under rich medium conditions. Inhibition of either lasR or lasI expression completely abrogates the expression of ambBCDE, which is a synthesis cluster for the production of IQS (Figure 5) [72].
P. aeruginosa expresses several different categories of pili such as Type IV pili, Tad type pili (encoded by Tight adherence locus), and Nucleation/precipitation type pili. Type IV pili have PilA major proteins and PiliB, PilE, PilD, PilV, PilW, PilX minor proteins. Tad type pili consist of Flp major protein and their minor proteins are TadA, TadB, TadC, TadD, TadF, TadG, TadZ, FppA, RcpA, RcpC. Type IV piliB. Precipitation/nucleation type pili are composed of major protein-FapC and FapA, FapB, FapD, FapE, FapF as the minor assembly of proteins (Table 1) [61].
In P. aeruginosa, the phenomenon of QS and the process of adhesion to biotic or abiotic surfaces are interlinked. Association of the P. aeruginosa to the biotic or abiotic surface increases expression of LasR receptor and induces binding of the cell to 3-oxo-C12-HSL complex. The signal-receptor complex further activates all other QS systems of P. aeruginosa and mediates expression of surface adhesins, pili binding to ECM components collagen, fibrinogen of the host tissue [76,77]
P. aeruginosa also possesses an alternate adhesion mechanism expressed upon increased c-di-GMP level within the cell and involves CdrA (C-di GMP regulated A adhesin) and CdrB (C-di GMP regulated B adhesin). This mechanism is independent of expression of the fimbriae and binds Polysaccharide synthesis locus (Psl) to endorse biofilm maturation [61]. The RhlI/R quorum-sensing system in P. aeruginosa regulates the expression of Polysaccharide encoding locus (Pel) and Polysaccharide synthesis locus (Psl), which are involved in adherence, aggregation, and maturation of the biofilm. However, the PQS quorum-sensing system regulates expression of the Lectin binding protein LecA, LecB involved in the cell recognition and cell adhesion (Figure 4) [75,78,79].
In addition to the above-mentioned adhesion factors, P. aeruginosa also expresses alginate to facilitate adhesion and also responds to DksA, Ppk1, epinephrine/nor-epinephrine to enhance adhesion to the surface [61,80,81]
Enterobacter signaling and adhesion
Enterobacter spp. are one of the major contaminants in dairy products. Enterobacter spp. enters into the food products through manufacturing, storage, and other processing procedures. Biofilms produced by Enterobacter spp. facilitate its survival despite the control measures followed in the food industry [82].
Among all Enterobacter spp., E. cloacae are often isolated from severe multidrug-resistant infections. The E. cloacae complex (ECC) has been included in the WHOs priority list pathogens for drug development due to increased resistance to broad-spectrum antibiotics and last resort drug carbapenem. Enterobacter spp. express several different types of pili FimA, FimC, FimD, FimF, FimG, FimH belonging to Chaperone/Usher class and Curli-CsgA, CsgB, CsgD, CsgE, CsgF, CsgG from the class Precipitation and Nucleation (Table 1) [61].
ECC does not produce AHL signal molecule; however, it responds to the AHL signal produced by another organism through signal receptor SdiA. Activation of the SdiA-mediated quorum sensing system negatively regulates the expression of the Curli genes [83]. Moreover, the external addition of AHL also inhibits biofilm formation in ECC. Thus, SdiA inactivation increases expression of the genes encoding fimbrial structures and promotes adhesion of ECC to biotic and abiotic surfaces (Figure 6) [83]
Figure 6.

Signaling involved in Enterobacter spp. adhesion
Enterobacter spp. respond to N-acyl homoserine lactone (AHL) produced by surrounding bacteria. The AHL signal molecule binds to SdiA (Orphan LuxR). Further, the SdiA-AHL complex regulates the expression of Curli adhesins. However, the detailed mechanism of inhibition is not known in Enterobacter spp. Moreover, Enterobacter spp. also possess LuxS and produce autoinducer-2 (AI-2) signal molecule. AI-2 is recognized and imported by ABC transporter LsrACDB. Further, the AI-2 which is phosphorylated by the LsrK molecule binds to Lsr R and upregulate lsr operon. However, the role of the AI-2 signaling pathway in the expression of adhesins in Enterobacter spp. is not yet clear.
ESKAPE signaling in polymicrobial biofilm
The ESKAPE adhesion signaling involved in the initiation of polymicrobial biofilm is complex due to diversity in the biofilm-forming organisms [84]. Adhesion factor and other metabolites of one organism facilitate the recruitment of another organism and initiate polymicrobial biofilm formation.
The Polysaccharide encoding locus (Pel) and Polysaccharide synthesis locus (Psl) synthesized by P. aeruginosa help in the adhesion of E. faecalis to form a polymicrobial biofilm [85]. Another ESKAPE pathogen, S. aureus, is often isolated from the polymicrobial infections and uses either symbiotic or competitive interaction with other organisms to form a polymicrobial biofilm. S. aureus employs symbiotic interaction with E. faecalis, Candida albicans, Haemophilus influenzae, or competitive with P. aeruginosa, Lactobacillus spp., and Streptococcus pneumoniae [86].
In the host, S. aureus activates the lysis of red blood cells that release hemin and NAD, which supports adhesion and growth of H. influenzae. Moreover, serine proteases secreted by S. aureus during adhesion signaling facilitate attachment of another bacteria viz. Enterococcus spp. which otherwise synthesizes the same factor through its Fsr adhesion signaling pathway [86]. However, P. aeruginosa synthesizes virulence factors such as phenazine (PZ), quinolone oxidase (QQ), pyocyanin (PY), and hydrogen cyanide (HCN) through its QS pathway, and these factors impose stress to S. aureus survival and thereby enhance the development of small colony variant (SCV) by S. aureus. SCV offer increased antibiotic resistance to S. aureus and leads to failure of the traditional treatment. Besides, S. aureus synthesizes eDNA and enhances co-colonization of P. aeruginosa [86]. In addition, alginate produced by P. aeruginosa and host immune protein calprotectin induce co-colonization of S. aureus and P. aeruginosa in the respiratory tract. It was also observed that S. aureus and P. aeruginosa co-colonize in the early stage of infection and later get dispersed and form distinct biofilms. This suggests that P. aeruginosa and S. aureus signaling involved in the adhesion are mutual and competitive [87].
The Als3 is expressed by C. albicans hyphae and plays a prime role in the colonization of S. aureus with C. albicans [88,89]. The adhesion protein Eap expressed by S. aureus facilitates the adherence of other bacteria to form polymicrobial biofilms [90].
E. faecalis frequently colonizes with E. coli in urinary tract infections. E. faecalis strain under iron limiting conditions secretes L-ornithine, which induces synthesis of siderophore in E. coli, and enhances the growth of E. coli in polymicrobial biofilm. Furthermore, E. coli gets attracted toward the AI-2 signal molecule produced by E. faecalis and triggers polymicrobial biofilms. E. faecalis, P. aeruginosa, and E. coli form polymicrobial biofilms and cause diabetic foot ulcers where every strain synergistically develops a biofilm matrix [90]
The adhesion factor MrkD expressed by K. pneumoniae strain triggers co- colonization of P. aeruginosa and protects the strain from biofilm dispersal [87]. P. aeruginosa produces various factors including phenazines, rhamnolipid, elastase, alkaline protease through the QS system and enhances co-existence with S. epidermidis, S. aureus, and Burkholderia cepacia on abiotic surfaces and wound infections [91]. A. baumannii shares adhesion factors and forms dual-species biofilm with P. aeruginosa in several infections [92]. Also, alginate produced by P. aeruginosa is utilized by S. parasanguinis. Moreover, other streptococcal adhesins, BapA1, and Fap1 trigger co-colonization in the CF lung [93]. E. cloacae forms polymicrobial biofilms with S. aureus and E. faecalis in surgical polypropylene mesh (PPM) associated infections [94]. Polymicrobial biofilm composed of A. baumannii, E. faecalis, Corynebacterium callunae Stenotrophomonas maltophilia, and Acinetobacter junii was observed on dairy-processing unit in Tianjin, China [95].
The signaling involved in the initiation of polymicrobial biofilms of ESKAPE pathogens is not much explored and needs further investigation to develop potent antimicrobials.
Molecular mechanism of AMR mediated by monomicrobial and polymicrobial biofilm in ESKAPE
Though it is well established through several studies that monomicrobial and polymicrobial biofilms enhance antimicrobial resistance, the underlying molecular mechanism for these observations remains inconclusive in the ESKAPE pathogen. ESKAPE Biofilms aggravate AMR problem through several molecular mechanisms [96,97].
Slow or incomplete penetration of Antimicrobials
The antibiotics oxacillin, cefotaxime and vancomycin hardly diffuse through biofilms of S. aureus and contribute to antibiotic resistance. However, some observations suggest that biofilm-associated antibiotic resistance also depends on the structure of the biofilm, bacterial growth conditions, bacterial strain and antimicrobials [97]. Moreover, P. aeruginosa biofilm when treated with fluorescently labeled, positively charged tobramycin antibiotic showed that biofilm does not only act as physical diffusion barrier but slows penetration of antibiotic and give enough time to bacteria to adapt genetically to that antibiotic concentration and contribute AMR [96]. A recent study revealed that E. faecalis tends to locate at the bottom of the biofilm, and P. aeruginosa forms a more structured biofilm on the top of the E. faecalis biofilm. The dual-species biofilm had much thicker ECM than its monospecies, potentially contributing to the increase in virulence of polymicrobial biofilm infections and protecting the bacteria from antimicrobial reagents and other environmental stresses [85].
Components of the biofilm matrix-Polysaccharides, eDNA
P. aeruginosa synthesizes Psl and Pel, major components of the biofilm matrix. Psl found to be involved in developing resistance to antibiotics such as tobramycin, colistin, polymixinB, and ciprofloxacin during early stage of the biofilm through mainly electrostatic interaction. However, role of the Pel contributing antimicrobial resistance is still controversial [97]. Furthermore, the extracellular DNA is responsible for the antibiotic resistance. Molecular mechanism of eDNA in contributing antibiotic resistance is relatively better studied in P. aeruginosa. It was observed that subinhibitory concentration of methicillin triggers synthesis of eDNA in S. aureus and P. aeruginosa biofilm and increase tolerance to tobramycin, gentamicin, polymyxin B and colistin by 3 fold, 2 fold, 4 fold and 8 fold respectively [97]. Besides, eDNA is negatively charged molecule, it chelates cations like Mg2+, and in Mg2+ limiting conditions bacteria like P. aeruginosa promote PhoPQ and PmrAB two-component signaling pathways responsible for antibiotic resistance. The expression of PmrAB is responsible for spermidine synthesis in P. aeruginosa that spermidine stays in the outer membrane and reduces membrane permeability of the antimicrobials thereby increasing AMR. In addition, acidic environment created by eDNA in P. aeruginosa biofilm also triggers activation of PhoPQ and PmrAB pathways and contributes antibiotic resistance [97].
Enzymes modifying antibiotics
K. pneumoniae secretes β-lactamase, which is accumulated in the biofilm matrix and can inactivate ampicillin and thus all bacteria within biofilms become tolerant to ampicillin [97]. Similarly, P. aeruginosa biofilm matrix also contains β-lactamase encoded by chromosomal ampC and contributes AMR in monomicrobial and polymicrobial biofilms [97]
Heterogeneity within biofilm
Heterogeneity within mono-microbial or polymicrobial biofilm can be in terms of gene expression, nutrient availability, hypoxic conditions. The hypoxic condition within P. aeruginosa biofilm up-regulates expression of the mexEF-oprN and mexCD-oprJ efflux pump and contributes to antibiotic resistance [96,97]. Extracellular release of Class-D-lactamases from A. baumannii within biofilm, protect other carbapenem-sensitive pathogens residing within same biofilm [98,99]. The PA1875-1877 efflux pump operon identified in P. aeruginosa consists of 4 genes- PA1875 or opmL which encode the outer membrane, PA1876 encoding transporter and PA1877 responsible for the membrane fusion proteins of ATP-binding cassette transporter. It was observed through several studies that this operon is 10 times more expressed in the P. aeruginosa within biofilm as compared to its planktonic counterpart and elevates biofilm resistance to multiple drugs like tobramycin, gentamicin, and ciprofloxacin. Moreover, this efflux pump also helps in increasing antibiotic resistance to other bacteria within biofilm [100].
Intra and Interspecies signaling
The biofilm-assisted AMR characteristic is mainly enhanced synergistically when bacteria communicate in a polymicrobial community [101]. The LasI/R and RhlI/R systmes of P. aeruginosa are responsible for the resistance to tobramycin and colistin. P. aeruginosa QS system regulates synthesis of HAQ which further disturbs electron transport chain by binding to the complex cytochrome bc1 at Qi. The HAQ regulated inhibition of respiratory chain leads to reduction in membrane potential and an increase in ROS which later causes autolysis and thereby generation of eDNA contributing to AMR. It was also observed that QS in bacteria helps other bacteria within biofilm to gain resistance to antibiotics without participation in the QS process. Similarly, the AgrD involved in the Agr two-component QS system of S. aureus found to be one of the key molecules in acquiring resistance to rifampin. Moreover, in E. faecalis, GelE protease-gelatinase synthesized via Fsr quorum-sensing system induces resistance to gentamicin, daptomycin and linezolid exclusively within biofilm by releasing eDNA [96]. Also, a study depicted that IcaABCD operon involved in adhesion signaling is responsible for biofilm formation in S. aureus, thus conferring resistance to erythromycin, gentamicin, tetracycline, cefoxitin, chloramphenicol and teicoplanin [102].
In the polymicrobial biofilms, diffusible signal factor (DSF) synthesized by Stenotrophomonas maltophilia is recognized by sensor kinase BptS of two-component system in P. aeruginosa and causes upregulation of PmrA and thereby leads to colistin and polymixin B. Polymicrobial biofilms comprising P. aeruginosa and C. albicans possess increased antibiotic resistance due to ethanol synthesized by C. albicans. S. aureus isolated from polymicrobial biofilm-associated HAIs found to contain vancomycin resistance gene which was originally present in E. faceium. This suggests the transfer of resistance gene within biofilm [97].
Horizontal gene transfer and Secretion systems
Inside biofilm, horizontal gene transfer through plasmid is relatively efficient than planktonic stage due to close proximity, sessile conditions, high cell density, increased genetic competence and accumulation of genetic elements for the uptake of resistance genes. In case of S. aureus, rate of transfer was found to be 10,000 times higher in biofilm than planktonic cells. However, within E. faecalis biofilm, plasmid copy number was found to be 1.6–2-fold greater compared to planktonic state [97]. Horizontal transfer of the blaNDM-1 gene has been studied in P. aeruginosa and A. baumannii in biofilms [103]. Type VI secretion system (T6SS) viz. Hcp secretion islands (HIS) I, II, III which are involved in the transfer of various proteins also contribute for antibiotic resistance in P. aeruginosa [97].
Dispersion of cells from Biofilms
Dispersal phenomenon of the biofilm allows bacteria with different inherent and acquired properties to release and colonize in new niche [104]. The dispersion may involve release of single type of cells, cell aggregates or movement of entire biofilm from one location to other [105]. Hence, the dispersal of the ESKAPE biofilm may cause other type of infection with different combinations of species within polymicrobial biofilm and may become extremely difficult to treat than that of earlier infection due to enhanced antibiotic resistance. A. baumannii cells dispersed from biofilm showed enhanced resistance to ciprofloxacin (4-fold higher MIC) and tetracycline (2-fold increased MIC) and also showed cross resistance to erythromycin. Furthermore, it also showed enhanced (10 times greater) efficiency of biofilm formation [106]. However, when a similar study was carried out for P. aeruginosa cells dispersed from the biofilm, it was observed that antibiotic susceptibility property of dispersed cells was influenced by dispersion mechanism. The glutamate induced dispersion caused increased resistance to colistin. However, biofilm dispersed through NO did not affect colistin susceptibility of dispersed cells [107]. Studies in P. aeruginosa revealed that the dispersed bacteria forms small colony variants, contributing to increased virulence in host [108]. However, studies in S. aureus also showed increased tolerance to antibiotics such as oxacillin in cell aggregates dispersed from biofilm [109]. Study on K. pneumoniae biofilms revealed that dispersed cells show enhanced adhesion on both biotic and abiotic surfaces [110], which may be a result of elevated metabolic activity in biofilm dispersed cells. Moreover, other studies on K. pneumoniae provide evidence that planktonic bacteria are phagocytosed at lower level as that of dispersed cells. In contrast, cells dispersed from biofilm of P. aeruginosa can resist engulfment by macrophages more than the planktonic cells [111,112].
Thus, research findings elaborated in this section suggest that mono-microbial and polymicrobial biofilm plays crucial role in the antimicrobial resistance. The biofilm-mediated molecular mechanisms in P. aeruginosa and S. aureus are relatively well established. However, it is not much explored in other ESKAPE pathogens and needs further research.
Summary and Perspective
The frequent isolation of antibiotic-resistant ESKAPE from food is a matter of public health concern. The situation is alarming as one of the notable characteristics of ESKAPE pathogens is their inherent ability to adapt to a range of environmental stress and acquisition of genetic information by horizontal gene transfer. Biofilms play a significant role in antimicrobial resistance and transmission. The mechanisms of regulation of biofilm formation have been explored in clinical isolates; however, the fundamental mechanism of biofilm mediated antibiotic resistance is not much explored in the food isolates. Therefore, the information gained from the clinical isolates could be potentially applied to the food isolates to assess the role of stress encountered during food processing and storage on biofilm formation and antimicrobial resistance.
Adhesion to the abiotic and biotic surfaces is considered to be a key step in the initiation of the biofilm. In the food processing environments ESKAPE encounter a range of microbial species; therefore further investigation on polymicrobial biofilms will help to generate knowledge on role of interspecies signaling in ESKAPE biofilms. The role of MrkA in the adhesion of K. pneumoniae and interspecies signaling involved in the polymicrobial biofilm consisting E. cloacae complex (ECC) and other ESKAPE pathogens, e.g., E. faecalis and S. aureus, are not yet explored. Moreover, further studies involving the elucidation of an alternative pathway to BfmR in A. baumannii are needed to explain adhesion mechanisms.
Future studies along this line will improve our knowledge of the complex interspecies signaling in adhesion and poly-microbial biofilm-mediated antibiotic resistance mechanisms with respect to food-associated infections.
Acknowledgments
AP is supported by the ERASMUS+ grant. RB and PK are supported by the junior research fellowship program of the Symbiosis International (Deemed University).
Funding Statement
The work was supported by the Ramalingaswami fellowship program of Department of Biotechnology, India under grant BT/RLF/Re-entry/41/2015; Major research project grant of Symbiosis International (Deemed University) under grant SIU/SCRI/MJRP-Approval/2019/1556; ERASMUS+ under grant 598515-EPP-1-2018-1-IN-EPPKA2-CBHE-JPDepartment of Biotechnology, Ministry of Science and Technology [BT/RLF/Re-entry/41/2015]; Major research project grant of Symbiosis International (Deemed University) [SIU/SCRI/MJRP-Approval/2019/1556].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
AP searched the data, wrote the article, and reviewed and edited the manuscript before submission. RB and PK searched data and wrote the article. SD prepared an outline of the review and critically reviewed and edited the manuscript before submission. All authors made substantial contributions in the preparation of the manuscript and have read and approved the final submitted manuscript.
References
- [1].Pérez-Rodríguez F, Mercanoglu Taban B.. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: risk factors and mitigation strategies. Front Microbiol. 2019;10:2091. Available from: https://pubmed.ncbi.nlm.nih.gov/31555256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Borah D, Singh V, Gogoi B, et al. Prevalence of multidrug resistant (MDR) novel Enterococcus faecium strain VDR03 in broiler chicken meat samples collected from Dibrugarh Town, Assam (India). Res J Microbiol. 2016;11(4):126–132. . [Google Scholar]
- [3].Molechan C, Amoako DG, Abia ALK, et al. Molecular epidemiology of antibiotic-resistant Enterococcus spp. from the farm-to-fork continuum in intensive poultry production in KwaZulu-Natal, South Africa. Sci Total Environ. 2019;692:868–878. [DOI] [PubMed] [Google Scholar]
- [4].Ge B, Domesle KJ, Gaines SA, et al. Prevalence and antimicrobial susceptibility of indicator organisms Escherichia coli and Enterococcus spp. Isolated from U.S. animal food, 2005–2011. Microorganisms. 2020;8(7):1–14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chajęcka-Wierzchowska W, Zadernowska A, García-Solache M, Ready-to-eat dairy products as a source of multidrug-resistant Enterococcus strains: phenotypic and genotypic characteristics. J Dairy Sci. 2020;103:4068–4077. (5):. [DOI] [PubMed] [Google Scholar]
- [6].Ercoli L, Gallina S, Nia Y, et al. Investigation of a Staphylococcal food poisoning outbreak from a Chantilly Cream Dessert, in Umbria (Italy). Foodborne Pathog Dis. 2017;14(7):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Davis GS, Waits K, Nordstrom L, et al. Intermingled Klebsiella pneumoniae populations between retail meats and human urinary tract infections. Clin Infect Dis. 2015;61:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Malta RCR, Ramos GL, Nascimento Dos Santos J, From food to hospital: we need to talk about Acinetobacter spp. Germs. 2020;10(3):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Algammal AM, Mabrok M, Sivaramasamy E, et al. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and blaTEM, blaCTX-M, and tetA antibiotic-resistance genes. Sci Rep. 2020;10(1):15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McClung RP, Roth DM, Vigar M, et al. Waterborne disease outbreaks associated with environmental and undetermined exposures to water - United States, 2013–2014. MMWR Morb Mortal Wkly Rep. 2017;66(44):1222–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Das A, Guha C, Biswas U, et al. Detection of emerging antibiotic resistance in bacteria isolated from subclinical mastitis in cattle in West Bengal. Vet World. 2017;10(5):517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hayman MM, Edelson-Mammel SG, Carter PJ, et al. Prevalence of Cronobacter spp. and Salmonella in milk powder manufacturing facilities in the United States. J Food Prot. 2020;83(10):1685–1692. . [DOI] [PubMed] [Google Scholar]
- [13].Xu Z, Xie J, Soteyome T, et al. Polymicrobial interaction and biofilms between Staphylococcus aureus and Pseudomonas aeruginosa: an underestimated concern in food safety. Curr Opin Food Sci. 2019;26:57–64. [Google Scholar]
- [14].Bhardwaj AK, Vinothkumar K, Rajpara N.. Bacterial quorum sensing inhibitors : attractive alternatives for control of infectious pathogens showing multiple drug resistance. Recent Pat Antiinfect Drug Discov. 2013;8(1):68–83. [DOI] [PubMed] [Google Scholar]
- [15].Reardon S, Antibiotic resistance sweeping developing world. Nat News. 2014;509:141–142. [DOI] [PubMed] [Google Scholar]
- [16].Mukherji R, Patil A, Prabhune A. Role of extracellular proteases in biofilm disruption of gram positive bacteria with special emphasis on Staphylococcus aureus biofilms. Enzym Eng. 2015;4:1–9. [Google Scholar]
- [17].Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jamal M, Ahmad W, Andleeb S, et al. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81(1):7–11. [DOI] [PubMed] [Google Scholar]
- [19].WHO . WHO publishes list of bacteria for which new antibiotics are urgently needed. GENEVA; 2017:1–4. Available from: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. [Google Scholar]
- [20].Ma YX, Wang CY, Li YY, et al. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv Sci. 2020;7(8):202000779. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mulani MS, Kamble EE, Kumkar SN, et al. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. 2019;10. 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhen X, Lundborg CS, Sun X, et al. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control. 2019;8(1). 10.1186/s13756-019-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11(3):297–308. [DOI] [PubMed] [Google Scholar]
- [24].Wang Z, Qin RR, Huang L, et al. Risk Factors for Carbapenem-resistant Klebsiella pneumoniae infection and mortality of Klebsiella pneumoniae infection. Chin Med J (Engl). 2018;131(1):56–62. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marturano JE, Lowery TJ. ESKAPE pathogens in bloodstream infections are associated with higher cost and mortality but can be predicted using diagnoses upon admission. Open Forum Infect Dis. 2019;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081. [DOI] [PubMed] [Google Scholar]
- [27].Ross CL, Liang X, Liu Q, et al. Targeted protein engineering provides insights into binding mechanism and affinities of bacterial collagen adhesins. J Biol Chem. 2012;287(41):34856–34865. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sattari-Maraji A, Jabalameli F, Node Farahani N, et al. Antimicrobial resistance pattern, virulence determinants and molecular analysis of Enterococcus faecium isolated from children infections in Iran. BMC Microbiol. 2019;19(1):1–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qu Y, Locock K, Verma-Gaur J, et al. Searching for new strategies against polymicrobial biofilm infections: guanylated polymethacrylates kill mixed fungal/bacterial biofilms. J Antimicrob Chemother. 2016;71(2):413–421. . [DOI] [PubMed] [Google Scholar]
- [30].Nabb DL, Song S, Kluthe KE, et al. Polymicrobial interactions induce multidrug tolerance in Staphylococcus aureus through energy depletion. Front Microbiol. 2019;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Giraffa G. Enterococci from foods. FEMS Microbiol Rev. 2002;26(2):163–171. [DOI] [PubMed] [Google Scholar]
- [32].Oprea SF, Zervos MJ. Enterococcus and its association with foodborne illness. Foodborne Dis. 2007;157–174. [Google Scholar]
- [33].Arntzen MØ, Karlskås IL, Skaugen M, et al. Proteomic investigation of the response of Enterococcus faecalis V583 when cultivated in urine. PLoS One. 2015;10(4):1–17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stępień-Pyśniak D, Hauschild T, Kosikowska U, et al. Biofilm formation capacity and presence of virulence factors among commensal Enterococcus spp from wild birds . Sci Rep 2019;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ben Braïek O, Smaoui S. Enterococci: between emerging pathogens and potential probiotics. Biomed Res Int. 2019;2019:5938210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chajecka-Wierzchowska W, Zadernowska A, Łaniewska-Trokenheim Ł. Virulence factors of Enterococcus spp. presented in food. LWT - Food Sci Technol. 2017;75:670–676. [Google Scholar]
- [37].Carniol K, Gilmore MS. Extracellular protease activity in signal transduction, quorum-sensing, and extracellular protease. J Bacteriol. 2004;186(24):8161–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hancock LE, Perego M. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J Bacteriol. 2004;186(17):5629–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ali L, Goraya MU, Arafat Y, et al. Molecular mechanism of quorum-sensing in Enterococcus faecalis: its role in virulence and therapeutic approaches. Int J Mol Sci. 2017;18(5):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mohamed JA, Huang DB. Biofilm formation by enterococci. J Med Microbiol. 2007;56(12):1581–1588. [DOI] [PubMed] [Google Scholar]
- [41].Hashem YA, Amin HM, Essam TM, et al. Biofilm formation in enterococci: genotype-phenotype correlations and inhibition by vancomycin. Sci Rep. 2017;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hendrickx APA, Van Luit-Asbroek M, Schapendonk CME, et al. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect Immun. 2009;77(11):5097–5106. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ali SA, Bin-Asif H, Hasan KA, et al. Molecular assessment of virulence determinants, hospital associated marker (IS16gene) and prevalence of antibiotic resistance in soil borne Enterococcus species. Microb Pathog. 2017. [cited 2020 Mar 23];105:298–306. [DOI] [PubMed] [Google Scholar]
- [44].Toledo-arana A, Valle J, Solano C, et al. The enterococcal surface protein, esp, is involved in Enterococcus faecalis biofilm formation. Society. 2001;67:4538–4545. Available from: https://aem.asm.org/content/67/10/4538.short%0A [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Strateva T, Atanasova D, Savov E, et al. Incidence of virulence determinants in clinical Enterococcus faecalis and Enterococcus faecium isolates collected in Bulgaria. Brazilian J Infect Dis. 2016;20(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Top J, Paganelli FL, Zhang X, et al. The Enterococcus faecium enterococcal biofilm regulator, ebrb, regulates the esp operon and is implicated in biofilm formation and intestinal colonization. PLoS One. 2013;8(5):1–13. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang Y, Li W, Hou B, et al. Quorum sensing LuxS/autoinducer-2 inhibits Enterococcus faecalis biofilm formation ability. J Appl Oral Sci. 2018;26:e20170566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Grace D, Fetsch A. Staphylococcus aureus —A foodborne pathogen. Staphylococcus Aureus. 2018. Elsevier Inc. 10.1016/B978-0-12-809671-0.00001-2. [DOI] [Google Scholar]
- [49].Jhalka K, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Environ Res. 2016;150:528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Clarke SR, Foster SJ, Clarke SR, et al. Surface adhesins of Staphylococcus aureus. Adv Microb Physiol. 2006. [DOI] [PubMed] [Google Scholar]
- [51].Kalia VC. Quorum sensing vs quorum quenching: a battle with no end in sight. Kalia VC, editor. Springer: 2015. [Google Scholar]
- [52].Kirmusaoglu S. Staphylococcal biofilms: pathogenicity, mechanism and regulation of biofilm formation by quorum-sensing system and antibiotic resistance mechanisms of biofilm-embedded microorganisms. In: Dharumadurai D, Nooruddin T, editors. Microbial Biofilms - Importance and Applications. IntechOpen. DOI: 10.5772/62943. [DOI] [Google Scholar]
- [53].Yu D, Zhao L, Xue T, et al. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012;12(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Askarian F, Ajayi C, Hanssen AM, et al. The interaction between Staphylococcus aureus SdrD and desmoglein 1 is important for adhesion to host cells. Sci Rep. 2016;6(1):1–11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Riley LW. Extraintestinal foodborne pathogens. Annu Rev Food Sci Technol. 2020;11(1):275–294. [DOI] [PubMed] [Google Scholar]
- [56].Hartantyo SHP, Chau ML, Koh TH, et al. Foodborne Klebsiella Pneumoniae: virulence potential, antibiotic resistance, and risks to food safety. J Food Prot. 2020;83(7):1096–1103. . [DOI] [PubMed] [Google Scholar]
- [57].Calbo E, Freixas N, Xercavins M, et al. Foodborne nosocomial outbreak of SHV1 and CTX-M-15-producing Klebsiella pneumoniae: epidemiology and control. Clin Infect Dis. 2011;52(6):743–749. . [DOI] [PubMed] [Google Scholar]
- [58].Zheng JX, Lin ZW, Chen C, et al. Biofilm formation in Klebsiella pneumoniae bacteremia strains was found to be associated with CC23 and the presence of wcaG. Front Cell Infect Microbiol. 2018;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ce´cilia A, Balestrino D, Lucile R, et al. Quorum sensing affects biofilm formation through lipopolysaccharide synthesis in Klebsiella pneumoniae. Res Microbiol. 2010;161(7):595–603. . [DOI] [PubMed] [Google Scholar]
- [60].Balestrino D, Haagensen JAJ, Rich C, et al. Characterization of type 2 quorum sensing in Klebsiella pneumoniae and relationship with biofilm formation. J Bacteriol. 2005;187(8):2870–2880. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Berne C, Ducret A, Gail H, et al. Adhesins involved in attachment to abiotic surfaces by Gram- negative bacteria. Microbiol Spectr. 2015;3(4):1–45. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schroll C, Barken KB, Krogfelt KA, et al. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010;10(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lavender HF, Jagnow JR, Clegg S. Biofilm formation in vitro and virulence in vivo of mutants of Klebsiella pneumoniae. Infect Immun. 2004;72(8):4888–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Di Martino P, Cafferini N, Joly B, et al. Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res Microbiol. 2003;154(1):9–16. . [DOI] [PubMed] [Google Scholar]
- [65].Lin CT, Lin TH, Wu CC, et al. CRP-Cyclic AMP regulates the expression of type 3 fimbriae via cyclic di-GMP in Klebsiella pneumoniae. PLoS One. 2016;11:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gaddy J, Actis L. Regulation of Acinetobacter baumannii biofilm formation Jennifer. Future Microbiol. 2009;23:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].De Amorim AMB, Nascimento JDS. Acinetobacter: an underrated foodborne pathogen? J Infect Dev Ctries. 2017;11(2):111–114. [DOI] [PubMed] [Google Scholar]
- [68].Meliani A, Bensoltane A. Review of Pseudomonas attachment and biofilm formation in food industry. Poultry, Fish Wildl Sci. 2015;03:1–7. [Google Scholar]
- [69].Raposo A, Pérez E, De Faria CT, et al. Food spoilage by Pseudomonas spp.—An overview [Internet]. Foodborne Pathog Antibiot Resist. 2016;41–71. 10.1002/9781119139188.ch3 [DOI] [Google Scholar]
- [70].Papaioannou E, Utari PD, Quax WJ. Choosing an appropriate infection model to study quorum sensing inhibition in Pseudomonas infections. Int J Mol Sci. 2013;14:19309–19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].El-shaer S, Shaaban M, Barwa R, et al. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl b -naphthylamide. J Med Microbiol. 2016;65(10):1194–1204. [DOI] [PubMed] [Google Scholar]
- [72].Ganesh PS, Rai VR. Alternative strategies to regulate quorum sensing and biofilm formation of pathogenic Pseudomonas by quorum sensing Inhibitors of Diverse Origins. In: Kalia VC, editor. Biotechnol Appl Quor Sens Inhib. Springer Nature; 2018. p. 33–61. [Google Scholar]
- [73].Philip D, David W. Alginate oligosaccharide-induced modification of the lasI-lasR and rhlI-rhlR quorum sensing systems in Pseudomonas aeruginosa. 2018. [DOI] [PMC free article] [PubMed]
- [74].Yang Y, Xu Z, Zhang Y, et al. A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa. J Microbiol. 2012;50(6):987–993. . [DOI] [PubMed] [Google Scholar]
- [75].Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2014;6(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chuang SK, Vrla GD, Fröhlich KS, et al. Surface association sensitizes Pseudomonas aeruginosa to quorum sensing. Nat Commun. 2019;10(1). 10.1038/s41467-019-12153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Turkina MV, Vikström E. Bacteria-host crosstalk: sensing of the quorum in the context of Pseudomonas aeruginosa Infections. J Innate Immun. 2019;11(3):263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chanda W, Joseph TP, Padhiar AA, et al. Combined effect of linolenic acid and tobramycin on Pseudomonas aeruginosa biofilm formation and quorum sensing. Exp Ther Med. 2017;14(5):4328–4338. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chemani C, Imberty A, De Bentzmann S, et al. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect Immun. 2009;77(5):2065–2075. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Crouzet M, Claverol S, Lomenech AM, et al. Pseudomonas aeruginosa cells attached to a surface display a typical proteome early as 20 minutes of incubation. PLoS One. 2017;12(7):1–24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cambronel M, Tortuel D, Biaggini K, et al. Epinephrine affects motility, and increases adhesion, biofilm and virulence of Pseudomonas aeruginosa H103. Sci Rep. 2019;9(1):1–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Amorim AMB, Nascimento Dos S J. A highlight for non-Escherichia coli and Non-Salmonella sp. Enterobacteriaceae in dairy foods contamination. Front Microbiol. 2017;8:2011–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Culler HF, Couto SCF, Higa JS, et al. Role of SdiA on biofilm formation by atypical enteropathogenic Escherichia coli. Genes (Basel). 2018;9(5):253. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rodrigues ME, Gomes F, Celia R. Candida spp./bacteria mixed biofilms. J Fungi. 2020;6:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Keehoon L, Kang-Mu L, Donggeun Kim SSY. Molecular determinants of the thickened matrix in a dual-species. Appl Environ Microbiol. 2017;83:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nair N, Biswas R, Götz F, et al. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect Immun. 2014;82(6):2162–2169. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Alves PM, Al-Badi E, Withycombe C, et al. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathog Dis. 2018;76(1):76. [DOI] [PubMed] [Google Scholar]
- [88].Peters BM, Ovchinnikova ES, Krom BP, et al. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology. 2012;158:2975–2986. (12):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Demuyser L, Jabra-Rizk MA, Van Dijck P. Microbial cell surface proteins and secreted metabolites involved in multispecies biofilms. Pathog Dis. 2014;70(3):219–230. [DOI] [PubMed] [Google Scholar]
- [90].Ch’ng J-H, Chong KKL, Lam LN, et al. Biofilm-associated infection by enterococci. Nat Rev Microbiol. 2019;17(2):82–94. [DOI] [PubMed] [Google Scholar]
- [91].Childers BM, Van Laar TA, You T, et al. MrkD 1P from Klebsiella pneumoniae strain IA565 allows for coexistence with pseudomonas aeruginosa and protection from protease-mediated biofilm detachment. Infect Immun. 2013;81(11):4112–4120. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang Y-C, Huang T-W, Yang Y-S, et al. Biofilm formation is not associated with worse outcome in Acinetobacter baumannii bacteraemic pneumonia. Sci Rep. 2018;8(1):7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Scoffield JA, Duan D, Zhu F, et al. A commensal Streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS Pathog. 2017;13(4):e1006300–e1006300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Stoodley P, Sidhu S, Nistico L, et al. Kinetics and morphology of polymicrobial biofilm formation on polypropylene mesh. FEMS Immunol Med Microbiol. 2012;65(2):283–290. [DOI] [PubMed] [Google Scholar]
- [95].Wang B, Tan X, Du R, et al. Bacterial composition of biofilms formed on dairy-processing equipment. Prep Biochem Biotechnol. 2019;49(5):477–484. [DOI] [PubMed] [Google Scholar]
- [96].Ciofu O, Tolker-Nielsen T. Tolerance and resistance of pseudomonas aeruginosabiofilms to antimicrobial agents-How P. aeruginosa can escape antibiotics. Front Microbiol. 2019;10. 10.3389/fmicb.2019.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41(3):276–301. [DOI] [PubMed] [Google Scholar]
- [98].Liao YT, Kuo SC, Lee YT, et al. Sheltering effect and indirect pathogenesis of carbapenem-resistant Acinetobacter baumannii in polymicrobial infection. Antimicrob Agents Chemother. 2014;58(7):3983–3990. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44(7):1818–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Poudyal B, Sauer K. The ABC of biofilm drug tolerance: the MerR-like regulator brlr is an activator of ABC transport systems, with PA1874-77 contributing to the tolerance of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob Agents Chemother. 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gabrilska RA, Rumbaugh KP. Biofilm models of polymicrobial infection. Future Microbiol. 2015;10(12):1997–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Neopane P, Nepal HP, Shrestha R, et al. In vitro biofilm formation by Staphylococcus aureus isolated from wounds of hospital-admitted patients and their association with antimicrobial resistance. Int J Gen Med. 2018;11:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tanner W, Atkinson-Dunn R, Goel R, et al. Horizontal transfer of the blaNDM-1 gene to Pseudomonas aeruginosa and Acinetobacter baumannii in biofilms. FEMS Microbiol Lett. 2017;364(8):364. [DOI] [PubMed] [Google Scholar]
- [104].Guilhen C, Forestier C, Balestrino D. Biofilm dispersal: multiple elaborate strategies for dissemination of bacteria with unique properties. Mol Microbiol. 2017;105(2):188–210. [DOI] [PubMed] [Google Scholar]
- [105].Rafii F. Antimicrobial resistance in clinically important biofilms. World J Pharmacol. 2015;4(1):31. [Google Scholar]
- [106].Penesyan A, Nagy SS, Kjelleberg S, et al. Rapid microevolution of biofilm cells in response to antibiotics. Npj Biofilms Microbiomes. 2019;5(1). Available from: 10.1038/s41522-019-0108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chambers JR, Cherny KE, Sauer K. Susceptibility of Pseudomonas aeruginosa dispersed cells to antimicrobial agents is dependent on the dispersion cue and class of the antimicrobial agent used. Antimicrob Agents Chemother. 2017;61(12):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Gernot LM, Tanja Z, Simone K. Analysis and characterization of Staphylococcus aureus small colony variants isolated from cystic fibrosis patients in Austria. Curr Microbiol. 2016;72(5):606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Fux C, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;186(14):4486–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Guilhen C, Miquel S, Charbonnel N, et al. Colonization and immune modulation properties of Klebsiella pneumoniae biofilm-dispersed cells. Npj Biofilms Microbiomes. 2019;5(1). 10.1038/s41522-019-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chua SL, Liu Y, Kuok J, et al. lifestyles. Nat Commun. 2014;5:1–12. [Google Scholar]
- [112].Pettigrew MM, Marks LR, Kong Y, et al. Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection. Infect Immun. 2014;82(11):4607–4619. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Merghni A, Ben Nejma M, Dallel I, et al. High potential of adhesion to biotic and abiotic surfaces by opportunistic Staphylococcus aureus strains isolated from orthodontic appliances. Microb Pathog. 2016;91:61–67. [DOI] [PubMed] [Google Scholar]
- [114].Struve C, Bojer M, Krogfelt KA. Characterization of Klebsiella pneumoniae type 1 fimbriae by detection of phase variation during colonization and infection and impact on virulence. Infect Immun. 2008;76:4055–4065. Available from: https://pubmed.ncbi.nlm.nih.gov/18559432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wilksch JJ, Yang J, Clements A, et al. MrkH, a Novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating Type 3 fimbriae expression. PLOS Pathog. 2011;7(8):e1002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pakharukova N, Tuittila M, Paavilainen S, et al. Structural basis for Acinetobacter baumannii biofilm formation. Proc Natl Acad Sci U S A. 2018;115(21):5558–5563. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Weidensdorfer M, Ishikawa M, Hori K, et al. The Acinetobacter trimeric autotransporter adhesin Ata controls key virulence traits of Acinetobacter baumannii. Virulence. 2019;10(1):68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Dueholm MS, Søndergaard MT, Nilsson M, et al. Expression of Fap amyloids in Pseudomonas aeruginosa, P . fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiologyopen. 2013;2(3):365–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Silva VO, Soares LO, Silva Júnior A, et al. Biofilm formation on biotic and abiotic surfaces in the presence of antimicrobials by Escherichia coli Isolates from cases of bovine mastitis. Appl Environ Microbiol. 2014;80:6136–6145. (19). [DOI] [PMC free article] [PubMed] [Google Scholar]


