ABSTRACT
In response to growing concerns regarding mosquito-borne diseases, scientists are developing novel systems of vector control. Early examples include Oxitec’s OX513A genetically-engineered mosquito and MosquitoMate’s Wolbachia-infected mosquito, and systems using ‘gene-drive’ are in development. Systems based on genetic engineering are controversial and institutions around the world are grappling with the question of who should have a say in how such technologies are field-tested and used. Based on media coverage and public records, we created comparative timelines of the efforts of Oxitec and MosquitoMate to navigate federal and local governance and bring their products to market in the United States. We analyze these timelines with particular attention to the role of public input in technology governance. These cases illustrate how governance of technology in the US is diverse, complex, and opaque. Further, the public response to proposed field trials of the Oxitec product highlights inconsistencies between public expectations for governance and actual practice. As gene-drive mosquito control products develop, both federal and local agencies will find their legitimacy tested without a better procedure for transparently integrating public input.
KEYWORDS: gene drive, mosquito control, United States, technology governance, community and stakeholder engagement
Introduction
In response to growing global concerns regarding mosquito-borne diseases, scientists are developing novel systems of vector control using a new strategy of genetic engineering known as gene-drive (GD) that takes advantage of newfound precision in synthetic biology. These systems feature genetically-engineered (GE) mosquitoes to be released to mate with wild mosquito populations to either modify or reduce disease transmitting mosquito populations. However, the release of GE insects into the environment is controversial, and experimental releases will be needed to accurately model possible outcomes. Governments and institutions around the world, including the United Nations and the World Health Organization, are grappling with the question of who should have a say in how such technologies are tested in the field. In the decade before GD mosquitoes became possible, scientists developed mosquito control systems sharing some key features with GD systems. Two of these systems, Oxitec’s OX513A GE mosquito and MosquitoMate’s Wolbachia-infected mosquito, were received quite differently in the United States (US) and serve as instructive case studies for those concerned about the future governance of GD mosquito systems.
Here we review the timeline and compare efforts to bring to market the MosquitoMate and Oxitec products in the US according to what has been documented in publicly available sources with particular attention to the role of public input and community engagement. Similar to proposed GD systems, both Oxitec and MosquitoMate systems involve the release of laboratory-bred mosquitoes and could not be fully understood before field release. However, where the MosquitoMate system was shepherded down an established path for regulating and testing pesticides, Oxitec encountered misstarts, delays, and controversy as they worked to get their product approved for testing in the US. The comparative timelines, based on publicly available information, suggest that established procedures for the democratic governance of technology are diverse, complex, and opaque. Procedures are not consistently coordinated, often are hard to understand, and lack adequate methods for honoring public input. Furthermore, outcomes are difficult to anticipate. As such, these systems of governance are ill-equipped to manage controversy. We argue that when engaging the public, developers, regulators, and public health agencies should communicate about and address expectations regarding the processes of decision-making as well as the technologies themselves. Engagement should include purposeful and transparent plans for incorporating public input. Such engagement strategies could serve as models for new federal and local procedures.
Background
Mosquito-borne diseases are major public health concerns worldwide. Pathogens responsible for a collection of severe diseases, including malaria, dengue, and Zika, are transmitted to humans by mosquito vectors. While historically associated with tropical climates, the vectors for many of these diseases have recently been identified in new locales. Specifically, populations of Aedes mosquitoes, vectors of dengue, Zika, yellow fever, and chikungunya viruses, have been found in parts of California since 2013 [1]. This is part of a growing area of the US that has become home to Aedes mosquitoes [2]. Although the pathogens that make these mosquitoes dangerous are not yet prevalent in the US, the presence of the vectors signals that this longstanding global public health threat is expanding to new geographical regions. Should infected travelers arrive in these expanded regions and be bitten, these viruses may spread.
The most dangerous species of Aedes mosquitoes (Ae. aegypti and Ae. albopictus) to human health are particularly difficult to control. They prefer to bite humans and therefore adapt well to urban and peri-urban areas. They are drought tolerant, lay their eggs in small amounts of water, such as in dishes under houseplants, and the eggs can dry out (estivate) and stay viable for over a year. These characteristics hinder the application of traditional control techniques such as spraying pesticides or larvicides and mosquito-eating fish to control Aedes populations. Thus, public health officials are particularly concerned, and over the past decade have sought new control methods for these vectors.
Sterile Insect Technique (SIT) is a long-used method for pest control that relies on the wide release of insects that have been rendered impotent, usually through radiation. SIT has been remarkably effective for other types of pests. For example, the screwworm (Cochliomyia hominivorax) has been effectively controlled in North America for decades using SIT [3]. In mosquitoes, SIT systems focus on creating sterile males because male mosquitoes do not feed on blood and therefore can be released. However, SIT has limited effectiveness in mosquitoes because irradiated males often struggle to mate, thus imposing a high fitness cost. Therefore, scientists have sought new ways to create sterile males.
The MosquitoMate product takes advantage of an observed incompatibility between mosquitoes infected and uninfected with Wolbachia bacteria [4,5]. This approach releases infected male mosquitoes into the environment to mate with wild (uninfected) females. Eggs produced by these pairings often do not hatch, thus reducing the total population of mosquitoes. Such a system of mosquito control relies on the ability to reliably sort infected males from infected females before release because infected males and females can successfully reproduce Wolbachia-infected offspring. If too much of the wild population of mosquitoes is infected with Wolbachia the technique will no longer be effective. Therefore, the major technical challenge of Wolbachia systems is reliably sorting lab-raised mosquitoes at scale. Such a system has been developed in partnership by MosquitoMate and Verily [1].
Oxitec’s approach to creating a viable SIT system has been to genetically engineer male mosquitoes that cannot produce viable offspring. Prior to the wide-spread use of CRISPR-Cas9 (CRISPR) for precision genetic engineering, Oxitec developed a dominant lethal system (RIDL – Release of Insects carrying a Dominant Lethal) that uses tetracycline to repress an introduced gene encoding a lethal factor and allows mosquitos to undergo development. Male mosquitoes reared in a tetracycline-rich lab environment are released to mate with wild mosquitoes and produce tetracycline-dependent larvae who would be unable to survive to adulthood, thus decreasing the wild population of mosquitoes. Like the Wolbachia system, RIDL relies on sorting and releasing lab-raised mosquitoes. In the case of this system, preventing the release of engineered females has been particularly important due to public discomfort with the release of GE insects that bite.
Since the introduction of CRISPR, scientists have been working to develop similar systems that would use GE to create mosquitoes with gene-drive (GD) [6,7]. GD is a method of biasing inheritance of certain genes. Where normal inheritance would pass along a trait to half of all offspring, GD would ‘drive’ it into all or nearly all offspring. For example, if a GD mosquito were designed to only produce male progeny, the population of mosquitoes would eventually decline. Another application could be the release of mosquitoes engineered such that they could no longer host infectious agents and would eventually modify the wild population by driving this change into subsequent generations. Such systems offer the possibility of making mosquito populations less likely to carry disease and, perhaps, more effective mosquito control compared to Wolbachia and RIDL systems [8].
Wolbachia, RIDL, and GD systems share many features. They rely on releasing thousands, if not millions, of mosquitoes. They also affect the wild population by disrupting reproduction on a molecular level. Though Wolbachia bacteria are naturally occurring in other species of insects, how they create reproductive incompatibility is not fully understood [9,10]. In addition, the effectiveness and environmental impact of these systems cannot be ascertained fully before open release. Lab and cage trials can offer some insight into dynamics such as mating rates in the presence of competition, characteristics of offspring, and fitness costs of lab-bred mosquitoes, but the conditions of containment, including artificial feeding and a limited mating pool, are known to impact these measurements [11–13]. Additionally, without present infections and a way to trace transmission, the impact of these systems on public health cannot be fully evaluated.
There are also unknown outcomes related to genetic engineering that some segments of the public are particularly anxious about. In our study of California residents’ attitudes toward the possibility of GD mosquitoes, we heard a wide range of concerns, including some that most experts would dismiss as based on misunderstandings of genetics [14]. In addition to concerns about the environmental impact of eliminating an entire species of mosquito, members of the public have expressed concerns about the potential harm to animals that might feed on a GE insect, the possibility of horizontal gene transfer to humans upon being bitten by a GE insect, and unintended mutations leading to super-mosquitoes or pathogens more harmful than the original targets [15].
When weighing these unknowns and difficult-to-predict outcomes with the threat of widespread outbreaks of dengue and Zika, many mosquito control professionals are open to exploring the possibilities of GE and GD mosquito control systems. The question raised again and again by groups of scientists and international organizations is, ‘How should public input be incorporated into plans to test and deploy these systems?’ [16–21] As public health interventions, these systems will impact everyone, although not necessarily equally. When testing novel medical interventions with unknown outcomes, individual informed consent is the model [22]. But individual consent from all community members for field trials of mosquito control systems is not possible. Aside from the practical impossibility of obtaining unanimous consent before going forward, it is also difficult to ensure that the appropriate stakeholders are identified and engaged. Furthermore, to be effective mosquito control requires on-going intervention and surveillance in shared environments and therefore must be decided on and supported collectively over time.
Given this landscape, governance of MosquitoMate’s Wolbachia-infected ‘ZAP male’ mosquito and Oxitec’s GE ‘OX513A’ mosquito serve as important precedents for the next generation of GE and GD mosquito control systems currently in development. These products were perceived and reviewed differently by regulators, local authorities, and members of the public in the US and these differences highlight the complexity and unpredictability of current governance of emerging technologies.
Current governance of emerging vector control technologies in the US
Governance of technologies in the US takes many forms, including both ‘soft’ law, for example, through professional ethical standards, and formalized gatekeeping mechanisms, such as regulation and authorization for testing, sale, or use [23]. In the case of novel mosquito control products, formal forms of governance are first required when planning field trials. Authorization for field trials emerges from an interplay between local and federal government institutions. While the literature on GD suggests the importance of direct public and community engagement, the US has a robust representative democracy intended to ‘engage’ citizens at the polls and delegate much of the decision-making to elected officials. With respect to decisions about the testing and use of novel vector control technologies, the key institutions are federal regulatory agencies and local mosquito control districts (MCDs; also known as Vector Control Districts or Mosquito Abatement Districts). Both regulatory agencies and MCDs are run by experts but controlled by political appointees beholden to elected officials who are in turn beholden to voters. Both types of agencies also have their own customs for directly engaging with citizens.
Regulatory approval is the first form of authorization needed in the US. The federal government has a number of regulatory agencies including the United States Department of Agriculture (USDA), the Food and Drug Administration (FDA), and the Environmental Protection Agency (EPA). It is important to note that these agencies are constrained by their enabling statutes and administrative procedures. Each agency has its own history, including the congressional legislation that originally granted the executive authority to regulate and the history of interpretation and implementation of that legislation. In the 1986 Coordinated Framework for Regulation of Biotechnology, the US Office of Science and Technology Policy declared that new legislation was not needed to regulate biotechnology based on genetic engineering, and that instead this technology required existing regulatory bodies to work together to regulate such products [24]. Each agency has distinct rules and procedures for reviewing new products and often relies on a staff of trained scientists to review new technologies and the safety data provided by applicants.
For many agencies, including the USDA, FDA, and EPA, the primary mechanism for gathering public input on the products they review is a system of public notification and comment. Per the 1946 Administrative Procedure Act, a notice will be posted in the Federal Register and a comment period will typically remain open for 30 days [25]. While notification and comment allows for public input, these systems are designed to collect input from ‘interested persons’ [25] versus a representative sample of the public. Further, agencies have different customs and rules for incorporating these comments. In many cases, they are not required to respond to nor allowed to integrate ‘values-based’ concerns in their decisions [26]. Agencies may decide to regulate a product or they may decide not to regulate a product on the grounds that it is outside of their jurisdiction or does not meet the necessary level of risk to consumers, to the public, or to the environment. USDA and EPA grant experimental use permits (EUP) for field testing, while FDA offers environmental assessment and labeling oversight, but does not grant EUPs [27].
To conduct a field trial, a developer needs more than the oversight of a federal agency; they must work with a host site. Decision-making about field trials and implementation of new vector control techniques is a localized process that takes place within MCDs. The presence and structure of MCDs varies amongst states, but many share a similar general structure. Often, MCDs are controlled by a board of trustees who are either appointed by city governments or are themselves elected city officials. Field trials of new vector control technologies are negotiated with and run by local MCDs. Therefore, in a formal sense, field trials are typically negotiated and approved by a body of representatives empowered by a local democratic process. Historically, and under most circumstances, boards of trustees have been able to work with professional MCD managers to decide on field testing and use of mosquito control products on behalf of their communities. This has often included some degree of community outreach and education but generally has not required a popular vote to approve and move forward with field trials or new techniques.
To trace how these processes worked in the cases of MosquitoMate’s ZAP males and Oxitec’s OX513A mosquitoes, we created comparative timelines of Oxitec’s and MosquitoMate’s efforts to gain regulatory approval and test their products based on publicly available sources.
Methods
In preparation for our own research on public attitudes toward GD mosquitoes, seventeen key informant interviews were conducted with managers of vector control agencies, scientists, and regulatory experts familiar with the regulation of novel mosquito control systems. Many of the key informants discussed Oxitec’s and MosquitoMate’s products as important precursors to new systems based on GE and GD. These cases have set important precedents for the regulation and testing of new products and also illuminate the relevant systems of technology governance currently in place in the US. The interviews gave rise to many questions, as key informants offered speculative and sometimes contradictory accounts of these cases.
To better understand these cases and their official narrative, we gathered press coverage, academic writing, and publicly-available documents related to efforts by Oxitec and MosquitoMate to gain approval for testing in the US. We organized the events represented in these sources into a comparative timeline that traces the movement of regulatory applications and the public rollout of field-testing plans. We initially searched news coverage in LexisNexis (now Nexis Uni) in late 2017 for mentions of ‘Oxitec’ and in early 2018 conducted a complementary search for ‘MosquitoMate’ with no date parameters. We also developed alternative search terms, including ‘Mosquito Mate’ ‘Wolbachia’ and ‘Kentucky’ to find more obscure coverage of MosquitoMate. Follow-up searches have been conducted periodically since our initial searches.
The searches of news coverage were not meant to be exhaustive and we did not systematically review all news coverage. Instead, we looked for clusters of news coverage to identify important events related to these companies and their technologies. Here we reference the most informative articles from established news sources for details about the events. Many events were covered by the Associated Press and therefore such coverage was often repeated by local news outlets. We also included events documented only in the Federal Register [28] and Regulations.gov, publicly-available repositories for procedural documents of the US government, because regulation is not covered evenly by the media.
Importantly, many of our questions could not be answered by the public record. There are clear gaps in the coverage of MosquitoMate due to obvious publication bias – MosquitoMate’s trials met with little or no public opposition and therefore were less newsworthy than the controversies over Oxitec’s trials. No doubt, there are many missing details here and we highlight some of the unanswered questions in the discussion. We present a list of key events, an illustrative comparative timeline, and a narrative of the history of Oxitec and MosquitoMate in the US.
Results
Table 1 lists identified events in chronological order. Figure 1 summarizes the regulatory history of the two products in a comparative timeline. Field tests outside of the US are included for context, as well as key moments in the Oxitec controversy.
Table 1.
Identified events in the development and regulation of Oxitec and MosquitoMate products
| MosquitoMate | Oxitec | ||
|---|---|---|---|
| -- | Prior to 2010 | Jan 2003 Oxitec Company founded | |
| Jan 2009 Draft Guidance #187 – FDA to have jurisdiction over GE animals |
|||
| Nov 2009 Grand Cayman Trial | |||
| |
|
|
Mar 2010 USDA Application: Assessment of OX513A field trials |
| Jan |
Patent filed for “Transfected Mosquito Vectors” |
2011 |
Nov FDA Application: Assessment of OX513A field trials |
| May | EPA Application: EUP for ZAP males trial in American Samoa (4 comments) | 2012 | Jan Publication Critical of Regulatory oversight in Cayman Trial (Reeves et al) |
| Jun | EPA Application Amendment: EUP, add CA, FL, and NY | Mar FKMCD hosts 1st Town Hall Meeting | |
| Jul | EPA EUP granted for American Samoa trial | Apr News coverage of controversy in FL | |
| Sep | EPA EUP granted for CA, FL, and NY | Nov FDA receives petition to deny permission for field trial in Key West |
|
| Nov |
EPA Application Amendment: addition of San Gabriel Valley, CA to EUP |
|
|
| May |
EPA Application: EUP for ZAP males trial in Lexington, KY; Public Comment Period (3 comments) |
2013 |
-- |
| Apr | EPA Request: Date extensions for EUPs | 2014 | Dec FKMCD hosts 2nd Town Hall Meeting |
| Aug | “MosquitoMate” trademarked | ||
| Aug |
EPA grants extensions |
|
|
| -- |
|
2015 |
Aug Oxitec Acquired by Intrexon |
| Mar | Field trials in Clovis CA | 2016 | Jan Brazil Trial Reported as Success |
| Apr | EPA Application: Federal Pesticide Product Registration for ZAP Males; Public Comment Period (10 comments) | Feb Zika in Brazil; Concerns for US | |
| Sep | EPA EUP extended to more CA counties and Monroe County, FL | Mar FDA Posts Draft Environmental Assessment and FONSI; opens comment period (extended through May 13; 2640 comments) |
|
| Apr Referendum in FL announced | |||
| Aug Final FDA FONSI | |||
| Nov Election and Referendum: Split, No GE Mosquitoes in Key Haven |
|||
| |
|
|
Nov FKMCD Board postpones, moves trial |
| Apr | Trial begins in West Keys, FL | 2017 | Jan New Draft Guidance (#236), “Regulation of Mosquito-Related Products” moves jurisdiction to EPA |
| Jul | Begin Debug Fresno Trial in Fresno, CA; partner with Verily | ||
| Sep | EPA Registration Decision: Public Comment Period (10 comments) | ||
| Nov |
EPA Final Registration: ZAP males approved for use in 20 states and Washington D.C. |
|
|
| -- | 2018 | Mar EPA Application: OX513A EUP, Public Comment (564 comments) |
|
| |
|
|
Nov Oxitec announces phase out of OX513A mosquito to be replaced with OX5034 mosquito; withdrawal of EPA application for OX513A |
| -- | 2019 | Sep Publication demonstrating gene transfer from OX513A to wild population in Brazil (Evans et al) |
|
| |
|
|
Sep EPA Application: EUP for OX5034 (343 comments) |
| Apr | Debug Fresno results published | 2020 | Jun EPA Grants EUP for OX5034 |
| Aug Field test of OX5034 approved in FL for summer 2021 |
|||
Figure 1.
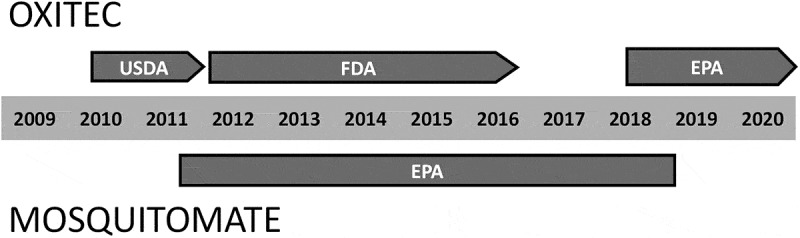
Timeline summarizing .Table 1
MosquitoMate
The experience of MosquitoMate in gaining approval, field testing, and bringing their product to market serves as an example of business-as-usual technology governance in the space of vector control. The process began in the spring of 2012 when MosquitoMate applied to the EPA for an EUP to conduct a trial in American Samoa. The EPA reviewed MosquitoMate’s Wolbachia infected mosquito as a pesticide. Conceptually, this was possible by treating the Wolbachia bacteria itself as a pesticide, reflected in the final labeling of the product that lists Wolbachia as the active ingredient (0.001%) and the male mosquitoes as ‘other ingredients’ (99.999%) [29].
Between 2012 and 2014, the Federal Register recorded the requesting and granting of EUPs for increasingly larger field trials in at least six different locales [30]. The granted EUPs specified the location, scale, and requirements – such as local permissions – for each specific trial. Documents submitted to the EPA suggest that these trials were carried out, but there is little mention of these trials in the media. Documentation of any local outreach or community engagement is not readily or publicly accessible. However, each of the requests for EUPs was accompanied by a public notification and comment period, in which no more than 10 comments were submitted at each juncture. These comments were submitted by scientists, nonprofit groups, other companies, and anonymous members of the public, some of whom appeared to confuse the ZAP male with Oxitec’s more controversial product [30].
In 2017, MosquitoMate conducted larger trials in Fresno, CA and West Keys, FL. Both the timing and the scale of these trials attracted media attention [31–33]. These trials occurred after the appearance of Zika in 2016 raised global interest in controlling the threat of Aedes mosquitoes and shortly after the height of the controversy over GE mosquitoes in Florida (described below). The Fresno trial also impacted a much larger community, involving the release of millions of Wolbachia-infected mosquitoes [31,32] There, MosquitoMate teamed up with Verily, Alphabet Inc.’s life science research arm, and the local MCD to test a scaled-up application of the ZAP males in a project branded ‘Debug Fresno.’ That trial featured an extensive public outreach effort aimed at engaging local residents to secure access to sampling sites as well as raising awareness of the importance of mosquito control and the trial itself [34,35].
In November 2017, the EPA granted MosquitoMate a pesticide product registration for their ZAP male mosquitoes, which allowed MosquitoMate to sell the ZAP male system in 20 states and Washington, D.C. where the climate was deemed similar to testing sites [36]. The product registration also required MosquitoMate to monitor and report on the gender ratio of released mosquitoes and the population of mosquitoes after release.
The ZAP male system is currently available for home and institutional use through the MosquitoMate website [37]. In the April 2020, the results of the Debug Fresno trial were published in Nature Biotechnology [1]. The report describes the release of millions of sorted Wolbachia-infected mosquitoes and a 95.5% decrease in Ae. aegypti compared with control sites.
Oxitec
In their effort to bring the OX513A mosquito to market, Oxitec encountered major regulatory delays, controversy, and community resistance. Compared to the business-as-usual MosquitoMate case, Oxitec’s decade-long struggle to field test OX513A demonstrates the complexity, unpredictability, and opacity of current technology governance.
In 2009, after a local outbreak of dengue raised concerns about the district’s ability to control Aedes mosquitoes, the Florida Keys Mosquito Control District (FKMCD) approached Oxitec about hosting a trial of the OX513A mosquitoes [38]. In planning the trial, the FKMCD drew upon experience with other trials of new vector control strategies and worked to educate and prepare the residents of the proposed site, beginning with public notifications and press releases and followed by educational public meetings [39,40]. However, FKMCD would not initiate the trial without federal regulatory review. Compared to other mosquito control products FKMCD had field-tested in the past, the Oxitec mosquito faced formidable regulatory hurdles that drew out the customary timeframe for such trials.
By 2009, engineering insects for pest control was not a new concept, but until that time the technique had been limited to agricultural applications regulated by the USDA. According to a Nature Biotechnology interview with Oxitec head of regulatory affairs, Camilla Beech, the company submitted a dossier to the USDA in March of 2010 and waited until October 2011 before they were notified that their product was outside the jurisdiction of USDA [41]. This account has been difficult to corroborate through publicly available government documents. Beech’s account raises questions about the communication between agencies required by the Coordinated Framework [24] and the transparency of regulatory processes. In January 2009, a draft guidance for industry document (#187) had claimed FDA jurisdiction over products based on genetic engineering of animals by classifying inserted DNA as a drug [42], so it is unclear why USDA should have taken over a year to respond accordingly. Presumably, draft guidance #187 was the basis for Oxitec’s next application to FDA in late 2011 [39].
Oxitec’s application remained with the FDA for nearly 5 years [43]. While environmental assessment requires creation and collection of much documentation [38,43], similar documents would have been assembled for the MosquitoMate dossier, and the EPA processed their initial application within four months. Even accounting for procedural differences between agencies, the 5-year processing time seems excessive and has been criticized as ‘unconscionable’ [44].
Reasons for this delay was the subject of speculation and disagreement among our key informants. Although US agencies are meant to regulate a product itself, rather than the process by which it is made [24,45], the delay seems likely to be related to the novelty of the Oxitec product within the field of GE animals. One theory is that the delay reflects the challenge of applying rules written for animal drugs to mosquitoes genetically engineered to produce inviable offspring. Oye et al remarked on the ambiguity of these regulatory rules in the case of GE insects, pointing to the difficulty of applying standards for veterinary drugs to products based on the engineering of insects [46]. Cohrssen and Miller opined that regulating a mosquito as a drug created intractable legal problems [44]. Another factor may have been the controversy surrounding this technology and the political implications thereof, of which regulators and agency leaders were well aware. A critical article published in 2012 in PLOS Neglected Tropical Diseases made waves within the scientific community for questioning oversight of Oxitec’s first release in Grand Cayman [47].
As FKMCD awaited regulatory approval, local residents, concerned citizens, and international NGOs began to resist the planned field trials through local protests, petitions, and international awareness campaigns [48–51]. FKMCD organized ‘town hall meetings’ held in 2012 and 2014. While presenters gestured to decisions to be made at the federal and state levels, the focus of the meetings was informing the public about the proposed trials. Presiders framed these events as informational and referred to ‘answering questions’ rather than hearing comments [52,53]. However, at least some participants intended to make their opinions known rather than receive technical information. Taylor and Dewsbury’s analysis of comments highlights how town hall participants expressed their expectations for more robust public engagement from Oxitec and FKMCD beyond media campaigns and public informational events. Participants also voiced a desire for more independent and stringent risk assessment from the FDA [40].
In November of 2012, Florida resident Milagros Demier submitted a petition to the FDA requesting ‘Denial of Genetically Modified Experiment in Key West,’ with over 165,000 signatures. The agency responded with a procedural form letter delaying response until a final decision could be made. Growing national attention to the issue prompted surveys of county residents [54,55] and national polls [56,57] demonstrating broad interest in the topic of GE mosquitoes with competing public opinions about their acceptability.
By spring of 2016, as reports of Zika emerging in Brazil created concerns for the US, hearings held by the House Energy and Commerce Committee drew attention to the FDA’s slow movement in reviewing Oxitec’s application [38]. In March of 2016, the FDA posted an environmental assessment of OX513A for public comment and received 2649 comments – a majority opposing the trial – after news outlets and activist organizations mobilized members of the public [58]. Despite this heated debate in the comments submitted to the FDA and assurances that the agency must review these comments [59], the agency’s procedures apparently do not require specific acknowledgment or direct response to public comments [25]. According to an analysis by Bloss et al, the most prevalent themes raised in these comments were ecological safety (51.2%), human health implications (67.3%), genetically modified organisms generally (65.1%), and mistrust of government (23.6%)” [58]. While the environmental assessment was certainly concerned with ecological safety and human health implications, opinions about the moral acceptability of ‘GMOs’ and government trustworthiness would have been outside of the scope of the FDA’s assessment.
In August 2016, the FDA finalized their finding of no significant impact (FONSI) [60]. For those uninitiated in the field of administrative law, the FONSI issued by the FDA is difficult to interpret. It is not a permit for field testing nor a formal approval of the technology. Rather a FONSI certifies that the agency finds ‘that the proposed field trial would not individually or cumulatively have a significant effect on the quality of the human environment’ and that the agency ‘will not prepare an environmental impact statement’ [60]. Meghani and Kuzma have argued that this decision reflects a questionable reading of safety claims made by Oxitec and criticized it as an example of neoliberal policies that prioritize corporate interests over public safety [61]. As the FDA prepared the final FONSI, they also prepared a formal denial of the 2012 Demier Petition. This 4-page, single-spaced denial memorandum details all of the reasons the agency disagreed with scientific claims of the petition, as well as outlining why other concerns, such as those about liability and economic impacts, were outside the FDA’s jurisdiction [62].
However, the long-awaited FONSI did not allow for the Florida trial to move forward. In April 2016, Monroe County (FL) had announced a voter referendum to be added to the county ballot during the November election, thereby postponing any field testing until after the election, regardless of the FDA finding. Ultimately, the vote showed minority support within the local area selected for the trial, but majority support across the county as a whole [50]. Based on these results, FKMCD chose to postpone and relocate the trial [49]. Further mooting the FONSI, just two months later, the FDA published new draft guidance for industry recommending that products like OX513A that are intended for pest control (rather than disease control) be regulated by the EPA as a biopesticide [63].
Thus, Oxitec’s application arrived at yet a third agency for review. In March of 2018, the EPA published a notice in the Federal Register acknowledging its receipt of Oxitec’s application for an EUP. During the comment period, the EPA received over 500 comments [64]. Later that year, Oxitec announced plans to phase out use of OX513A in favor of the next generation of this system, OX5034, and withdrew the application at the EPA [65].
In late 2019, a team of researchers in the US and Brazil published evidence that some OX513A mosquitoes released in Brazil had persisted in the environment and successfully mated [66]. This report became an occasion for a flurry of anxious headlines and drew fire from the scientific community for erroneously suggesting that the identified mosquitoes were especially robust, and by implication, dangerous [67]. Around the same time, Oxitec applied for an EUP from the EPA to test the new OX5034 system, attracting 343 public comments within the comment period [68]. In 2020, the EPA granted an EUP for trials in Florida and Texas [69] and Florida authorities approved of field tests of the OX5034 mosquito for summer 2021 [70].Summary
Discussion
These comparative timelines illustrate how the MosquitoMate product was reviewed, tested, and approved for market by the EPA in approximately six years while the Oxitec product was passed from USDA to FDA to EPA for nearly ten years before Oxitec withdrew the application in preparation for the next generation of the technology. The idiosyncratic US regulatory rules and procedures create uncertainty about how novel products will be handled and are not designed to manage controversy. Despite the intentions of the 1986 Coordinated Framework [24], work across or between agencies is notoriously difficult. This is illustrated by the failure of communication between the FDA and USDA in 2009 that allowed Oxitec to submit an application to USDA at the same time that FDA claimed jurisdiction over GE animal products. Complexity and diversity also are introduced in the relationships between federal and local agencies. Agencies at both levels were responsible for making intertwined decisions. For example, Florida MCD started planning trials of both Oxitec and MosquitoMate products while awaiting regulatory approval but would not begin trials without it. Likewise, decisions made by the FDA for environmental assessment and EPA for EUPs rely on the identification of specific locations for trials before regulatory consideration.
Because of the diversity and complexity of governance, outcomes are difficult to anticipate and therefore impede planning. Case-by-case interpretation of agency rules is left to regulators and final interpretations are ultimately at the discretion of agency leadership who are typically newly appointed at least with each new presidential administration. The fact that these two similar products could be handled so differently points to an uncertain and contingent process for adjudicating the responsible use of these technologies.
Finally, the complexity and uncertainty contribute to the opacity of the process. In our discussions with key informants, we encountered different, and sometimes contradictory accounts. Indeed, our narrative here, based on published records, contains many unanswered questions. Though many insiders have convictions about what happened in these cases, there is a dearth of primary sources accessible to the public with which to make sense of these technology governance processes. The missing pieces in the public record invite disagreement and speculation in both personal accounts and the secondary literature on these cases. What was the cause of the long FDA deliberation on OX513A? How did agencies process thousands of public comments when they were accustomed to receiving fewer than 100? And what sort of community engagement accompanied MosquitoMate’s early field trials?
The difficulty of understanding the cases of Oxitec and MosquitoMate from publicly available sources reflects the difficulty of keeping these forms of governance transparent. The transparency of technology governance is particularly crucial under conditions of controversy. When there is disagreement, decision-making depends on agreement about how legitimate decisions can be made. Such agreement is not possible without transparent communication and shared expectations about the procedures of governance.
Public input in response to Oxitec’s proposed trial reflected a lack of shared expectations about both the substance of the debate and the process of governance. There was confusion about what form FDA regulation would take, how the federal and local agencies would (or would not) protect communities from harms related to the trials, how public input would be solicited and honored, and, ultimately, how the final decision would be made. The forms of public engagement may have been particularly confusing: the practice of notification and comment and the acceptance of petitions would seem to suggest that comments would be both reviewed and accounted for in agency decision. However, procedures for how to do so are unwritten and the FDA, for example, has strict rules about what type of evidence or concern may be part of a decision. At the local level, informational meetings organized by an MCD could be misinterpreted as a means to access decision-makers or take part in decision-making. Transcripts of the FKMCD town hall meetings suggest that organizers intended to answer questions and address concerns while community members attended to give their opinions to decision-makers. MCDs are accustomed to notifying residents and answering questions, but they are not typically called on to run a deliberative decision-making process. As well as educating citizens about mosquito control, these town hall meetings could have been an opportunity to communicate about the established representative democratic process for MCD decision-making. Given the confusion among experts regarding these governance processes, citizens and experts alike need opportunities to learn about how controversial decisions are made.
Federal and local agencies may not yet have sophisticated procedures for collecting and integrating public input, but these agencies do have opportunities to communicate and inform the public about their current processes. For example, when acknowledging receipt of the Demier petition, FDA representatives could have offered general and accessible information about how petitions would be considered and the types of information that regulators are allowed to consider. When they did respond substantively to the petition, nearly four years after submission, the letter was lawyerly and might easily have been received by lay readers as dismissive and condescending. Before a point-by-point denial of the petition’s claims, the agency representative wrote, ‘To the extent that your petition requests that FDA not agree to the conduct of the trial specifically in Key West, this issue is moot’ because the trial was proposed for Key Haven [62]. The timing and tone make clear that the letter was executed as a legal formality, but it might also have been an opportunity to communicate and engage in a meaningful way to the 165,000 members of the public who signed the petition. While a different style of correspondence would not have quelled the Oxitec controversy, it might have served to reorient some citizens to the processes that have been painstakingly built over the history of our democracy. Our analysis demonstrates that these processes are not obvious; comprehension should not be taken for granted.
We suggest that future attempts at community and public engagement include a concerted effort to attend to expectations and communicate about the governance process as well as listen to and address public concerns about the technology itself. One way to begin is to articulate how public or community input will be collected and incorporated into substantive decisions and then follow through with documentation and dissemination. For example, federal agencies could begin by creating public facing and accessible materials that explain their review processes and how public input may or may not be incorporated. Future petitioners and comment-writers could then be directed to these materials as a way of acknowledging their participation in the process. At the local level, an MCD could commission surveys of a representative sample in their region and pledge to honor the results in their planning of a field trail. Communication about the process contributes to transparency and is an important first step in developing new ways to better negotiate the governance of emerging technologies in the future.
Conclusion
This analysis of the Oxitec and MosquitoMate cases in the US illustrates the imperfect and complex systems of governance that are at play when bringing novel mosquito control products to market. The comparison illuminates the historical structure and idiosyncratic relationships between US regulatory bodies; the ambiguous jurisdictions of novel products embodying emerging technologies; the improvisational interplay between federal and local agencies; and the ways that public input has been honored or disregarded.
On both federal and local fronts, existing modes of community engagement proved insufficient to assuage anxiety or address public concerns about the release of synthetic DNA into the environment. Similarly, the FDA’s inability to meaningfully respond to public comment did not inspire confidence or demonstrate that existing concerns would be taken seriously. Where increased public engagement should have led to more thoughtful and inclusive governance, the surge of public interest in the trial of OX513A mosquitoes instead slowed governance of this technology.
This translates to great uncertainty about how the next generation of novel vector control systems will be handled. In giving the EPA jurisdiction over Oxitec’s product, Draft Guidance for Industry #236 may suggest that GD mosquitoes intended for pest control will be handled as a novel form of pesticide. No doubt developers of these technologies are watching closely to see how Oxitec fairs with field trials sanctioned by the EPA. However, draft guidance documents changed the jurisdiction over GE animals twice since Oxitec’s first application. Further, executive interpretation of the regulatory framework can shift between administrations. For example, the Obama administration ordered an update to the Coordinated Framework which was finalized in January 2017 [27]. In April of that year, the Trump administration formed a new committee to develop recommendations specifically for agricultural biotechnology products, reflecting that administration’s priorities [71].
Regardless of the agency regulating them, when it comes time to test GD mosquitoes, we can expect that activists will oppose release and will use all available channels for public engagement to bring the debate to wide attention. Public debate is meant to be a strength of our democratic system, but without adequate procedures to incorporate and process conflicting points of view, the US regulatory system is not able to properly acknowledge, let alone make use of public input. Our comparative timelines suggest that our institutions of technology governance are not prepared to manage, much less integrate, diverse and conflicting public input in a way that ensures that concerns are addressed at the same time that decisions are made.
Without a better procedure for transparently integrating public input, federal and local agencies will find their legitimacy tested when developers begin to arrange field tests of GD mosquito control products. As a citizenry, we need to incorporate better ways of involving the public into our governance processes that will make use of citizens’ insights and articulation of public interest. While resolving conflicting viewpoints will ultimately be a political decision, there is room to improve the collection and acknowledgment of public opinion. In the case of US regulatory agencies, this will require new legislation or strong leadership from within the executive branch. New systems for public input will require collecting, analyzing, integrating, and acknowledging large amounts of competing public input in a timely and proactive way. With widespread information technology and modern social science methods, such procedures are possible. Deliberative polling [72] and citizen juries [73] are two well-developed examples of methods that could be formalized and integrated into our systems of technology governance.
As a starting point, engagement efforts should not only invite public input but clarify and communicate how input will be honored. Deeper and lasting changes will require leadership and exemplars from stakeholders outside of government agencies as well. Industry leaders, academics, and nonprofit organizations have an opportunity to contribute to technology governance by developing and piloting models of clear and transparent practices for collecting and incorporating public input. Stakeholders also can advocate for or support legislation to address how public input should be honored by regulatory agencies. Building a culture of awareness and shared expectations for how decisions are made can contribute to new forms of governance and institutional resilience in the face of controversy.
Acknowledgments
The authors acknowledge Robert Friedman and Brinda Dass for helpful feedback on early versions of this work, and Cynthia Triplett for assistance in preparing the manuscript. AAJ is a Donald Bren Professor. Author contributions: CES: conceptualization, data curation, formal analysis, investigation, writing-original draft; JN: investigation, writing–review & editing; AAJ: writing–review & editing; OSA: funding acquisition, writing–review & editing; CSB: funding acquisition, conceptualization, supervision, writing–review & editing.
Funding Statement
This work was supported by funding from the Defense Advanced Research Projects Agency (DARPA) Safe Genes program (Contract No. HR0011-17-2-0047; PI: Akbari). The views, opinions, and/or findings expressed are those of the author(s) and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Crawford JE, Clarke DW, Criswell V, et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nature Biotechnology. 2020;38(4):1–11. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention . Zika Virus: potential Range in US. 2017. [cited 2020 Jan 29]; Available from: https://www.cdc.gov/zika/vector/range.html.
- [3].Vargas-Terán M, Hofmann HC, Tweddle NE. Impact of Screwworm Eradication Programmes Using the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson A, editors. Sterile Insect Technique. Springer: Dordrecht; 2005: 629–650.
- [4].Slatko BE, Luck, AN, Dobson, SL, et al. Wolbachia endosymbionts and human disease control. Mol Biochem Parasitol. 2014;195(2):88–95. [DOI] [PubMed] [Google Scholar]
- [5].O’Neill SL. (2018) The use of Wolbachia by the World Mosquito Program to interrupt transmission of Aedes aegypti transmitted viruses. In: Hilgenfeld R and Vasudevan SG (eds) Dengue and Zika: Control and Antiviral Treatment Strategies. Springer: Singapore, 355–360. [DOI] [PubMed] [Google Scholar]
- [6].James S, Collins, FH, Welkhoff, PA, et al. Pathway to Deployment of Gene Drive Mosquitoes as a Potential Biocontrol Tool for Elimination of Malaria in Sub-Saharan Africa: recommendations of a Scientific Working Group. Am J Trop Med Hyg. 2018;98(6_Suppl):1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Raban R, Akbari OS.. Gene drives may be the next step towards sustainable control of malaria. Pathog Glob Health. 2017;111(8):399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alphey L, McKemey, A, Nimmo, D, et al. Genetic control of Aedes mosquitoes. Pathog Glob Health. 2013;107(4):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jiggins FM. Open questions: how does Wolbachia do what it does? BMC Biol. 2016;14(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beckmann JF, Bonneau M, Chen H, et al. The toxin–antidote model of cytoplasmic incompatibility: genetics and evolutionary implications. Trends in Genetics. 2019;35(3):175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Facchinelli L, Valerio, L, Ramsey, JM. Field cage studies and progressive evaluation of genetically-engineered mosquitoes. PLoS Negl Trop Dis. 2013;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wise De Valdez MR, Nimmo, D, Betz, J, et al. Genetic elimination of dengue vector mosquitoes. Proceedings of the National Academy of Sciences, 2011. 108(12): 4772–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pham TB, Phong, CH, Bennett, JB et al. Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet. 2019;15(12):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bloss CS, Triplett C, Schairer C, Buchman A, Akbari O. Perceptions of genetic engineering to control vector-borne disease among California Residents. American Society for Bioethics and Humanities Annual Conference, October, 2019; Pittsburgh, PA. [Google Scholar]
- [15].Kaebnick GE, Heitman, Elizabeth E, Collins, James P, et al. Precaution and governance of emerging technologies. Science. 2016;354(6313):710–711. [DOI] [PubMed] [Google Scholar]
- [16].Schairer CE, Taitingfong, R, Akbari, OS, et al. A typology of community and stakeholder engagement based on documented examples in the field of novel vector control. PLoS Negl Trop Dis. 2019;13(11):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].World Health Organization . Guidance framework for testing of genetically modified mosquitoes. Geneva: World Health Organisation; 2014. [Google Scholar]
- [18].Adelman Z, Akbari O, Bauer J, et al. Rules of the road for insect gene drive research and testing. Nat Biotechnol. 2017;35(8):716–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benedict M, Akbari, O, Bauer, J, et al. Guidance for contained field trials of vector mosquitoes engineered to contain a gene drive system: recommendations of a scientific working group. Vector-borne Zoonotic Dis. 2008;8(2):127–166. [DOI] [PubMed] [Google Scholar]
- [20].National Academies of Sciences Engineering and Medicine . Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. Washington, D.C: National Academies Press; 2016. [PubMed] [Google Scholar]
- [21].Hartley S, Thizy, D, Ledingham, K, et al. Knowledge engagement in gene drive research for malaria control. PLoS Negl Trop Dis. 2019;13(4):e0007233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research , The Belmont Report: ethical Principles and Guidelines for the Protection of Human Subjects of Research. 1979, Washington, D.C.: https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf. [PubMed] [Google Scholar]
- [23].Marchant GE, Allenby B. Soft law: new tools for governing emerging technologies. Bulletin of the Atomic Scientists. 2017;73(2):108–114. [Google Scholar]
- [24].U.S. Office of Science and Technology Policy , Coordinated Framework for Regulation of Biotechnology. 1986, Federal Register:
- [25].United States of America National Archives . Administrative Procedure Act (5 U.S.C. Subchapter II, Section 553). 2017. [cited 2020 Dec 4]; Available from: https://www.archives.gov/federal-register/laws/administrative-procedure/553.html.
- [26].Rudenko L, Palmer MJ, Oye K. Considerations for the governance of gene drive organisms. Pathog Glob Health. 2018;112(4):162–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Biotechnology Working Group , Modernizing the Regulatory System for Biotechnology Products: final Version of the 2017 Update to the Coordinated Framework for the Regulation of Biotechnology. 2017, The Unified Website for Biotechnology Regulation: https://usbiotechnologyregulation.mrp.usda.gov/2017_coordinated_framework_update.pdf.
- [28].United States of America National Archives . Federal Register. 2020. [cited 2020 Apr 15]; Available from: https://www.federalregister.gov/.
- [29].U.S. Environmental Protection Agency , Notice of Unconditional Pesticide Registration for EPA Registration No. 89668–4, ZAP Males and Final Product Label. 2017, Regulations.gov: https://www.regulations.gov/document?D=EPA-HQ-OPP-2016-0205–0033.
- [30].Experimental Use Permits Applications, Amedments, and Issuances in “Mosquito Mate” Dockets 2012–2014. 2020. [cited 2020 Dec 3]; Available from: https://www.regulations.gov/searchResults?rpp=25&so=ASC&sb=postedDate&po=0&s=%22mosquito%2Bmate%22&fp=true&dct=FR%2BPR%2BN%2BO.
- [31].Goldschmidt D. Florida releases experimental mosquitoes to fight Zika. . CNNcom. 2017. [Google Scholar]
- [32].Loria K, 20 million bacteria-infected mosquitoes are getting released into a California city by a division of Google’s parent company. 2017, [cited 2018 Jan 12]. http://www.businessinsider.com/wolbachia-mosquitoes-california-google-alphabet–2017–7.
- [33].Doubek J, To Shrink Mosquito Populaiton, Scientists are Releasing 20 Million Mosquitoes. 2017, [cited 2020 Dec 4]. https://www.npr.org/sections/thetwo-way/2017/07/21/538470321/to-shrink-the-mosquito-population-scientists-are-releasing-20-million-of-them.
- [34].Consolodated Mosquito Abatement District . Debug Fresno. 2019. [cited 2020 Dec 3]; Available from: https://cmad.maps.arcgis.com/apps/MapJournal/index.html?appid=f90115bcf15943928fc82a79af89d71e.
- [35].Verily. Introducing Debug Fresno. 2017. [cited 2018 Feb 20]; Available from: https://www.youtube.com/watch?v=vVozrgEwi_Q.
- [36].U.S. Environmental Protection Agency , Final Registration Decision of the New Active Ingredient Wolbachia pipentis ZAP(wPip) strain in Aedes albopictus. ID: EPA-HQ-OPP-2016-0205-0033. 2017, Regulations.gov: [cited 2020 Dec 7]. https://www.regulations.gov/document?D=EPA-HQ-OPP-2016-0205–0033.
- [37].MosquitoMate.com. 2020. [cited 2020 Dec 3]; Available from: https://mosquitomate.com/?v=3.0.
- [38].Parry H, Testimony before the U.S . House of Representatives Scinece, Space and Technology Committee: science of Zika: the DNA of an Epidemic. 2016
- [39].Maxmen A. Florida abuzz over mosquito plan. Nature; 2012;487(7407):286. [DOI] [PubMed] [Google Scholar]
- [40].Taylor C, Dewsbury B. Barriers to inclusive deliberation and democratic governance of genetic technologies at the science-policy interface. Journal of Science Communication. 2019;18(3):Y02. [Google Scholar]
- [41].Waltz E. A face-lift for biotech rules begins. Nat Biotechnol. 2015;23(12):1221–1222. [DOI] [PubMed] [Google Scholar]
- [42].U.S. Food and Drug Administration , Guidance for Industry on Regulation of Genetically Engineered Animals Containing Heritable recombinant DNA Constructs, Guidance for Industry #187. Federal Register, 2009. 74(11). [Google Scholar]
- [43].Beech C. Regulatory experience and challenges for the release of GM insects. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2014;9(1):71–76. [Google Scholar]
- [44].Cohrssen JJ, Miller HI. 2017. November. Current FDA approach to genetically engineered animals is flawed, The Hill, pp. 6. [cited 2017 Dec 19] http://thehill.com/opinion/healthcare/358893-current-fda-approach-to-genetically-engineered-animals-is-flawed. [Google Scholar]
- [45].Kuzma J. Policy: reboot the debate on genetic engineering. Nature. 2016;531(7593):165–167. [DOI] [PubMed] [Google Scholar]
- [46].Oye KA, et al. Regulating gene drives. Science. 2014;345(6197):626–628. [DOI] [PubMed] [Google Scholar]
- [47].Reeves RG, Denton, JA, Santucci, F, et al. Scientific standards and the regulation of genetically modified insects. PLoS Negl Trop Dis. 2012;6(1):e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kay J. 2016. May. Tell the FDA what you fear more: zika, or GMO mosquitoes? Assoicated Press, pp. 12. [cited 2017 Nov 9]. https://apnews.com/article/bb12a3bc40d249f791507fa7ac89b763. [Google Scholar]
- [49].Glenza J. 2016. November. Genetically modified mosquitoes could be released in Florida Keys by spring; In fight against Zika, British company Oxitec must seek approval from FDA for insects’ release into the wild following Monroe County referendum, The Guardian, pp. 26. [cited 2019 April 22]. https://www.theguardian.com/us-news/2016/nov/26/zika-virus-genetically-modified-mosquitoes-florida. [Google Scholar]
- [50].Joseph A Florida Keys voters split on genetically modified mosquito trial. STAT 2016 Nov 8; Available from: https://www.statnews.com/2016/11/08/florida-keys-voters-split-on-genetically-modified-mosquitoes/.
- [51].McCauley L Florida Keys Residents Resist Controversial GMO Mosquito Trial. 2016. [cited 2019 May 24]; Available from: https://www.organicconsumers.org/news/florida-keys-residents-resist-controversial-gmo-mosquito-trial.
- [52].Florida Keys Mosquito Control District . Town Hall Meeting on Genetically Modified Mosquitoes. 2012. [cited 2021 Jan 13]; Available from: https://www.youtube.com/watch?v=5WQkM-yc7QI.
- [53].Florida Keys Mosquito Control District . Genetically Modified Mosquitoes Town Hall Meeting. 2014. [cited 2021 Jan 13]; Available from: https://www.youtube.com/watch?v=fO31wLQoCJ0.
- [54].Ernst KC, Haenchen, S, Dickinson, K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21(2):320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Adalja A, Sell, TK, McGinty, M, et al. Genetically modified (GM) mosquito use to reduce mosquito-transmitted disease in the US: a community opinion survey. PLoS Curr. 2016;8. DOI: 10.1371/currents.outbreaks.1c39ec05a743d41ee39391ed0f2ed8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Funk C, Hefferon M. Most Americans Accept Genetic Engineering of Animals that Benefits Human Health, but Many Oppose Other Uses. 2018. [cited 2018 oct 2]. https://www.pewresearch.org/science/2018/08/16/most-americans-accept-genetic-engineering-of-animals-that-benefits-human-health-but-many-oppose-other-uses/. [Google Scholar]
- [57].Lull RB, Akin, H, Hallman, WK, et al. Modeling risk perceptions, benefit perceptions, and approval of releasing genetically engineered mosquitoes as a response to Zika virus. Environmental Communication. 2020;14(7):933–953. [Google Scholar]
- [58].Bloss CS, Stoler, J, Brouwer, KC, et al. Public response to a proposed field trial of genetically engineered mosquitoes in the United States. Jama. 2017;318(7):662–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Borio L. Testimony, House Energy and Commerce Subcommitttee on Oversight and Investigations Hearing, “Examining the U.S. Public Health Response to the Zika Virus”. Washington, D.C: Congressional Documents and Publications; 2016. [Google Scholar]
- [60].Food US, Administration D. Finding of No Significant Impact. 2016. [cited 2019 Apr 22]; Available from: https://www.fda.gov/downloads/AnimalVeterinary/DevelopmentApprovalProcess/GeneticEngineering/GeneticallyEngineeredAnimals/UCM514699.pdf.
- [61].Meghani Z, Kuzma J. Regulating animals with gene drive systems: lessons from the regulatory assessment of a genetically engineered mosquito. J Responsible Innovation. 2018;5(sup1):S203–S222. [Google Scholar]
- [62].Forfa T, Petition Denial 2016 Aug 5, ID # FDA-2015-P-1433-0081. 2016, Regulations.gov: https://www.regulations.gov/document?D=FDA-2015-P–1433–0081.
- [63].U.S. Food and Drug Administration , Clarification of FDA and EPA Jurisdiction Over Mosquito-Related Products: guidance for Industry #236. Federal Register, 2017. 82(12). [Google Scholar]
- [64].Comments on Pesticide Experimental Use Permits: applications: oxitec Ltd. ID # EPA-HQ-OPP-2017-0756-0001. 2018, Regulations.gov: [cited 2020 Dec 5]. https://www.regulations.gov/docket?D=EPA-HQ-OPP–2019–0274.
- [65].Klingener N GMO Mosquito Application Withdrawn - But Another Is On The Way. WLRN National Public Radio 2018. [cited 2018 Nov 30]; Available from: http://www.wlrn.org/post/gmo-mosquito-application-withdrawn-another-way.
- [66].Evans BR, Stoler, J, Brouwer, KC, et al. Transgenic Aedes aegypti mosquitoes transfer genes into a natural population. Sci Rep. 2019;9(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Servick K. GM mosquito study draws fire. Science. 2019;365(6459):1234. [DOI] [PubMed] [Google Scholar]
- [68].Comments on Pesticide Experimental Use Permits: applications: oxitec Ltd. ID # EPA-HQ-OPP-2019-0274. 2019, Regulations.gov: [cited 2020 Dec 7]. https://www.regulations.gov/docket?D=EPA-HQ-OPP–2019–0274.
- [69].U.S. Environmental Protection Agency, Notice of Experimental Use Permit,ID: EPA-HQ-OPP-2019-0274-0360. 2020, Regulations.gov: https://www.regulations.gov/document?D=EPA-HQ-OPP-2019-0274–0001. [PubMed]
- [70].LaMotte S, 750 million genetically engineered mosquitoes approved for release in Florida Keys. 2020, [cited 2020 Aug 20]. https://www.cnn.com/2020/08/19/health/gmo-mosquitoes-approved-florida-scn-wellness/index.html.
- [71].Trump DJ, Executive Order . 13874 of June 11, 2019,“Modernizing the Regulatory Framework for Agricultural Biotechnology Products,”. 2019, [cited 2020 Dec 20]. https://www.whitehouse.gov/presidential-actions/executive-order-modernizing-regulatory-framework-agricultural-biotechnology-products/.
- [72].Fishkin JS, Deliberative polling. Consulting the people is not as easy as it sounds, 2018.
- [73].Jefferson Center . How We Work: citizen Juries. 2020. [cited 2020 Apr 17]; Available from: https://jefferson-center.org/about-us/how-we-work/.


