Abstract
Goal
The goal is to present key principles of point-of-care testing (POCT) in educational curricula that meet critical needs for rapid decision-making in disasters, outbreaks of highly infectious diseases, emergency management, and complex crises.
Observations
The coronavirus disease 19 (COVID-19) pandemic unequivocally proved the value of POC strategies. Striking needs identified by COVID-19 challenges have yet to be entirely fulfilled. A comprehensive national survey showed absence of POCT training in public health colleges, schools, and programs. Fundamental improvements in national structuring of POC knowledge, skills, experience, training, dissemination, accreditation, and licensing are necessary, so that multidisciplinary public health teams can respond effectively and efficiently by geospatially optimizing the control and mitigation of highly infectious diseases and other critical challenges.
Conclusions
Four sets of POCT learning objectives were developed for public health and other educational institutions. Global implementation of POC diagnostics in the hands of trained personnel will help avoid untimely worldwide crises, huge economic losses, uncounted excess mortality, and sudden disruptive surges of dangerous infectious threats to personal security and cultural stability.
Key words: antibody test, antigen assay, coronavirus disease 2019 (COVID-19), curriculum, learning objectives, lessons learned, molecular diagnostics, point-of-care specialists, point-of-care testing (POCT), point of need, public health education, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
GOAL, BACKGROUND AND METHODS
Goal
To create a long-term public health paradigm of rapid response diagnosis at points of need, this article identifies necessary skills in point-of-care testing (POCT) for new teaching curricula in colleges, schools, and programs of public health (1,2), in order that faculty can educate public health students and practitioners in POC strategies for highly infectious diseases, emergency management, and crisis preparedness.
Public health disconnect
The disconnect between current public health practice with testing performed in distant slow reference laboratories versus the demonstrated value of rapid COVID-19 testing in communities directly at points of need may continue to threaten family lives and societal values, strong motivators for change in preparation for future outbreaks and epidemics virtually certain to occur. The world goal is to prevent geospatially limited episodes from developing into the next global pandemic.
Mobile testing
Enhancing fundamental knowledge and practical expertise of those working in mobile testing modes, such as vans equipped with portable instruments, will help meet urgent demands for geospatially optimizing containment of COVID-19 and other highly infectious diseases.
Methods
Principles and strategies for pandemic response were derived from lessons learned following the COVID-19 outbreak in Wuhan, China in 2019 and its early spread to America and Southeast Asia (3-5), and before that, from the Ebola epidemic that devastated Western Africa in 2014 and still alarms the continent with recurrences in the Democratic Republic of the Congo and elsewhere (6-10).
Learning objectives
Essential learning objectives were extracted from an original lecture (~500 slides) and workshop course for POC operators and coordinators created for limited-resource settings by Kost et al. (11) This practicum has been taught worldwide for several years, typically in the format of two morning lectures followed by an afternoon hands-on workshop (wet lab) where students using POC devices generate real-time results.
Mathematical and geospatial optimization
Designs for public health POCT curricula were aided by quantitative mathematical and pattern recognition analyses of COVID-19 diagnostics and POC geospatial approaches applied to the current pandemic, which were published open access in the Archives of Pathology and Laboratory Medicine (12-14) and Frontiers of Public Health (15), respectively.
POCT IN PUBLIC HEALTH EDUCATION
A national survey of public health institutions by Kost et al. (1) identified future directions for POCT curricula in public health. The survey showed absence of instruction, hands-on training, and accredited courses in POCT in American colleges, schools, and programs of public health education. Public health certification requirements and textbooks generally do not include POCT instruction.
In the national survey (1), the topic, “POC HIV/HCV ED” testing, appeared in only one course, and “POC diagnostics in local clinics,” in one other. Only one book, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience (16), and one online course on public health preparedness (17) address POCT for disaster and public health crisis intervention.
A 2021 review of PubMed searches and worldwide web searches revealed public health educational institutions have not yet incorporated POCT in curricula. Public health curricula do not address POCT in isolation units during quarantine or societal implementation of POCT broadly in communities, despite unequivocally proven need for POC strategies that enhance standards of care during the current COVID-19 pandemic (3).
PUBLIC HEALTH CURRICULA
Tables 1 through 4 present integrated modular sets of POCT learning objectives customized for public health students. Table 1 learning objectives focus on the mission and the basic principles of POCT (1). Sections II. A. and B. build technical skills. Collaboration with clinical chemists, laboratory scientists, clinical pathologists, inventors, and entrepreneurs will ensure high quality instruction in POC technologies and their design. A recent paper in this journal provided collaborative guidance for training and competency in POCT (18).
Table 1.
Curriculum and learning objectives—mission, principles, and practice
| Section & topics | Learning objectives |
|---|---|
| Part I. | Getting started—the mission |
| Goals, objectives, and overview of uses in public health |
|
| Needs |
|
| Companion Tests |
|
| Part II. | Fundamental principles and practice of POC testing |
| A. Technical | |
| Needs assessment |
|
| Instrument formats, selection, and validation |
|
| Non-invasive monitoring versus in vitro diagnostic testing |
|
| Specimen processing |
|
| Quality assurance (QA), quality control (QC), and proficiency testing (PT) |
|
| Environmental stresses |
|
| Multiplex molecular diagnostics |
|
| B. Design & build | |
| Design criteria |
|
| Commercialization |
|
| Regulatory oversight |
|
| FDA and WHO emergency use declarations |
|
| Accreditation options |
|
| Part III. | Practicum |
| Device hands-on experience |
|
| Results interpretation |
|
| Performance evaluation |
|
| Trouble shooting |
|
| Establishing a POC program |
|
Table 2.
Curriculum and learning objectives—public health sciences
| Section & topics | Learning objectives |
|---|---|
| Part I. | Integration of POC and public health expertise |
| Roles of public health personnel and POC Coordinators |
|
| Training, credentialing, and assuring competency |
|
| Part II. | Health maintenance and noncommunicable diseases (examples) |
| Pregnancy |
|
| Prediabetes and diabetes |
|
| Acute coronary syndromes and acute myocardial infarction |
|
| Part III. | Communicable diseases (examples) |
| HIV |
|
| Influenza A and B |
|
| Malaria |
|
| Strep throat screening |
|
| Tuberculosis and resistance testing |
|
| Part IV. | Geospatial science & geographic information systems (GISs) |
| Small-world networks (SWNs) |
|
| GIS applications to health systems |
|
Table 3.
Curriculum and learning objectives—public health preparedness, emergency management, and the COVID-19 crisis
| Section & topics | Learning objectives |
|---|---|
| Part I. | Preparedness for outbreaks, epidemics, and isolation |
| A. Test metrics | |
| Dynamic evidence-based medicine |
|
| B. Past perspective | |
| Ebola virus and other highly infectious diseases |
|
| B. COVID-19 pandemic | Special section |
| Metrics of testing |
|
| FDA Emergency Use Authorizations (EUAs) |
|
| Management of EUA tests |
|
| Safe spacing & contagion |
|
| Maximize the effectiveness of rapid antigen tests (RAgTs) and empower without intimidating |
|
| C. Workshops | Highly infectious diseases |
| Personal protective equipment (PPE) |
|
| Isolation laboratory and quarantine |
|
| Spatial care pathsTM |
|
| IQCP, its five key components, and plan design |
|
| Global pandemic preparedness |
|
| Part II. | Disasters, emergencies, complex crises, and rapid response |
| Disaster caches and complex crises |
|
| Performance standards |
|
| Telehealth |
|
| Alternate care facilities |
|
| Bioterrorism |
|
Table 4.
Curriculum and learning objectives—public health preparedness, emergency management, and the COVID-19 crisis
| Section & topics | Learning objectives |
|---|---|
| Part I. | Standards, policy, and guidelines |
| A. Lectures | |
| International Organization for Standardization (ISO) |
|
| CDC, FDA, and WHO |
|
| General Accountability Office (GAO) |
|
| Global status |
|
| B. Workshops | |
| Procedures |
|
| Policy and guidelines workshop |
|
| Part II. | Project management and POC value propositions |
| Project management and the POC committee |
|
| How to write a business case and develop value propositions |
|
| Part III. | Global and future vision |
| Course summary |
|
| Learner presentations |
|
| Future vision |
|
Sections II and III in Table 2 cover the general use of POCT in health maintenance, noncommunicable disease, and communicable threats (1). Section IV emphasizes how to position POCT in small-world networks (19) and the use of geographic information systems (20). The reader can refer to the papers and chapters cited for details that will aid instruction.
Table 3, titled Public Health Preparedness and Emergency Management, includes a special Section I.B. of learning objectives addressing POC strategies for COVID-19. It also references the Clinical and Laboratory Standards Institute guidance for Emergency Use Authorization (EUA) tests (21), because the FDA EUA process has been used extensively for COVID-19 diagnostics implemented in the United States. The learning objectives include hands-on workshops in Section I.C. Several of the entries could be assigned as timely Master of Public Health theses.
Table 4 enhances opportunities to embed POC principles and practice in standards, policy, guidelines, project management, and value propositions, so as to form infrastructure for sustainable funding and perpetual improvement in rapid response (1). Please also see Kost (14) for a summary of standards of care guidelines specifically for the use of COVID-19 rapid antigen tests, which have become available worldwide in the fight against COVID-19.
DISCUSSION AND CONCLUSIONS
Point-of-care testing is inherently fast, intrinsically spatial, and immediately actionable. It adds value by decreasing therapeutic turnaround time, speeding decision-making, and quickly enabling correct treatment. The mobility of POCT reduces risk by gatewaying testing before air travel, for facilitated immigration, at drive-ins/-ups/-throughs, in walk-bys and pharmacies, and for other optimal spatially isolated testing sites crucial to economic opening, safe spacing (social distancing), and successful tackling of risky COVID-19 exposure.
Public health policies and guidelines should sustain POC rapid response, as recommended by the logic map of Figure 1. Support can come from the Centers for Disease Prevention and Control, Food and Drug Administration, General Accountability Office, National Institutes of Health, the United Nations, United States Agency for International Development, World Health Organization, and non-government organizations (NGOs). Public health graduates populate these entities, so their awareness of POC strategies is vital to assimilation of POC strategies and their future planning.
Figure 1.
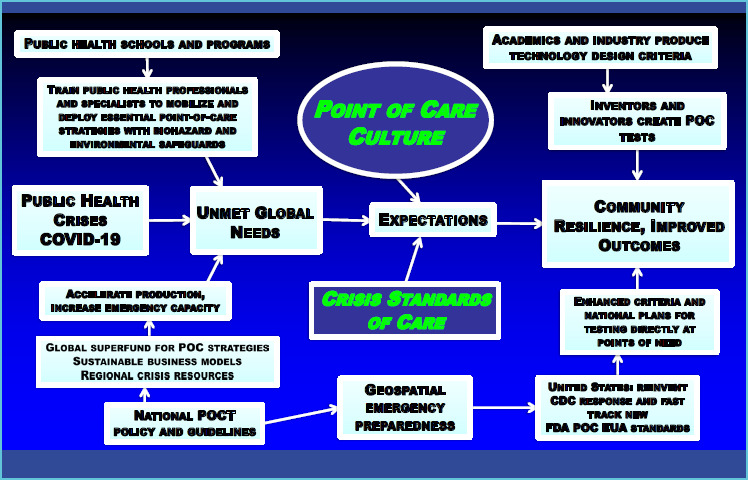
The incorporation of POC knowledge, skills, and culture in public health will lead to community resilience and improved outcomes — an integrative roadmap
The long-term challenge is to train adequate numbers of public health officials who can deliver diagnostic testing in communities worldwide. It makes sense to “train the trainers,” that is, develop new cadres of public health graduates who will enthusiastically reach out to the community and train those at the bedside or on-site in the field in the principles and practice of POCT. Tables 1-4 provide the educational tools to do that.
Educators should modify accreditation standards, so that POCT knowledge will be validated in public health certification exams and sustained long-term to recraft the profession for this type of point-of-need response. Experts from several disciplines can participate productively extending their didactic efforts, methods, and inventions (22-27) in support of public health educators and their curricula.
The COVID-19 pandemic showed us that community contagion inevitably leads to outbreaks in convalescent care homes and deaths among the elderly and highly vulnerable. Therefore, high performance POCT (12-14) must be deployed for screening, triage, and contact tracing of both patients and staff with results immediately available on a daily basis. This represents just one example. Public health leadership can customize course content to meet local community needs for diagnostics by selecting topics from Tables 1-4.
Multiple crises tend to occur simultaneously, and when they do, fixed resources become disabled by electrical shutdowns, physical isolation, and supply failures (16). Communities designate or construct alternate care facilities and plan near-patient critical care testing in support of critically ill and quarantined patients. They implement action plans for patient isolation units close to or just outside emergency rooms to avoid contagion inside and install adjacent isolators and isolation laboratories with POC instrumentation.
Training of community health providers must include the use of PPE and, importantly, practice in operating POC devices while wearing PPE. Mobile vans can be equipped with molecular diagnostics that detect highly infectious threats with high sensitivity and specificity, as well as rapid antigen, antibody, and multiplex SARS-CoV-2 + Influenza A/B tests for COVID-19(5). This will help avoid crowding emergency rooms with potentially contagious patients, because testing will minimize both false negatives (high sensitivity) who spread disease, and false positives (high specificity) who may be committed to units housing infected patients and become infected themselves (12-14).
Government agencies should provide adequate resources and funding (see Figure 1) to sustain community resilience and improve medical and economic outcomes. Grass roots knowledge and skills in POCT, such as awareness of how to improve emergency diagnostics on ambulances (28,29), will help public health leadership create regional safety, while coordinating point-of-impact testing and important countermeasures, such as vaccination and drive-through testing sites (30-33). In fact, POC coordinators, a new and emerging subspeciality professional group in the POC field, can share responsibility for problem-solving strategies at points of need in America and abroad (34).
The COVID-19 pandemic called us all to action, action that can be sustained through creative public health education in POCT. Knowledge learned, taught, and shared worldwide will help fill resilience gaps as the Delta variant becomes a global endemic disease and ubiquitous POCT to deal with it, the new normal.
Acknowledgements
This work was supported in part by the Point-of-Care Testing Center for Teaching and Research (POCT•CTR) and by Dr. Kost, Director.
I thank the creative students, fellows, and international scholars who design and study point-of-care strategies in the United States and worldwide.
I am grateful to have a Fulbright Scholar Award, which supports geospatial analysis of highly infectious diseases and cardiac rescue, strategic POCT field research in the ASEAN Member States, and community and university lectures.
I thank the University of Puthisastra and the National Public Health Laboratory, Phnom Penh, for sponsoring Fulbright research in Cambodia, and the College of Public Health Sciences at Chulalongkorn University, Bangkok, Thailand, for inviting me to be a visiting Fulbright Specialist, 2021-2022.
I am indebted to Doug Kirk, MD, Chief Medical Officer, and to Kimberly Bleichner-Jones, MBA, Executive Director-Administration, UC Davis Health, for their support of this Fulbright program.
The figure and tables are provided courtesy and permission of Knowledge Optimization, Davis, California.
REFERENCES
- 1.Kost GJ, Zadran A, Zadran L, Ventura I. Point-of-care testing curriculum and accreditation for public health — Enabling preparedness, response, and higher standards of care at points of need. Front Public Health. 2019;8(385): 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kost G, Zadran A. Schools of public health should be accredited for, and teach the principles and practice of point-of-care testing. J Appl Lab Med. 2019;4:278-283. [DOI] [PubMed] [Google Scholar]
- 3.Kost GJ. Geospatial hotspots need point-of-care strategies to stop highly infectious outbreaks: Ebola and Coronavirus. Arch Pathol Lab Med. 2020;144:1166-1190. [DOI] [PubMed] [Google Scholar]
- 4.Kost GJ. Geospatial spread of antimicrobial resistance, bacterial and fungal threats to COVID-19 survival, and point-of-care solutions. Arch Pathol Lab Med. 2021;145: 145-167. [DOI] [PubMed] [Google Scholar]
- 5.Eng M, Zadran A, Kost GJ. Covid-19 risk avoidance and management in limited-resource countries — Point-of-care strategies for Cambodia. Omnia Digital Health. 2021. June-July; 112-115. Available from: https://insights.omnia-health.com/laboratorv/covid-19-risk-avoidance-and-management-limited-resource-countries Accessed September 9, 2021.
- 6.Kost GJ, Ferguson WJ, Hoe J, Truong A-T, Banpavichit A, Kongpila S. The Ebola Spatial Care Path™: Accelerating point-of-care diagnosis, decision making, and community resilience in outbreaks. Am J Disaster Med. 2015;10(2): 121-143. [DOI] [PubMed] [Google Scholar]
- 7.Kost GJ, Ferguson W, Truong A-T, et al. Molecular detection and point-of-care testing in Ebola virus disease and other threats: a new global public health framework to stop outbreaks. Expert Rev Mol Diagnostics. 2015; 15(10): 1245-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kost GJ, Ferguson WJ, Truong A-T, et al. The Ebola Spatial Care PathTM: Point-of-care lessons learned for stopping outbreaks. Clin Lab Intnl. 2015;39:6-14. [Google Scholar]
- 9.Kost GJ. Point-of-care testing for Ebola and other highly infectious threats: Principles, practice, and strategies for stopping outbreaks. In: Shephard M, Editor. A Practical Guide to Global Point-of-care Testing. Australia: CSIRO (Commonwealth Scientific and Industrial Research Organization);. 2016, Chapter 24, p. 291-305. [Google Scholar]
- 10.Kost GJ. Molecular and point-of-care diagnostics for Ebola and new threats: National POCT policy and guidelines will stop epidemics. Expert Rev Mol Diagn. 2018;18: 657-673. [DOI] [PubMed] [Google Scholar]
- 11.Kost GJ, Delany C, Gentile NL, Longley A. Point-of-care course for limited-resource countries. A global resource: lecture, combined, interactive, and spatial modules and workshops. Davis, CA: Knowledge Optimization and the POCT•CTR. 2011-2021. [Google Scholar]
- 12.Kost GJ. Designing and interpreting COVID-19 diagnostics: Mathematics, visual logistics, and low prevalence. Arch Pathol Lab Med. 2020;145(3):291-307. [DOI] [PubMed] [Google Scholar]
- 13.Kost GJ. The impact of increasing prevalence, false omissions, and diagnostic uncertainty on Coronavirus Disease 2019 (COVID-19) test performance. Arch Pathol Lab Med. 2021;145(7):797-813. [DOI] [PubMed] [Google Scholar]
- 14.Kost GJ. Diagnostic strategies for endemic coronavirus disease 2019 (COVID-19): Rapid antigen tests, repeat testing, and prevalence boundaries. Arch Pathol Lab Med. 2021; in press. [DOI] [PubMed] [Google Scholar]
- 15.Kost GJ. Geospatial science and point-of-care testing: Creating solution for population access emergencies, outbreaks, and disasters. Front Public Health. 2019;7:1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kost GJ, Ed., Curtis CM.Assoc. Ed. Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Preparedness. Washington DC: AACC Press-Elsevier; 2015, 701pp. [Google Scholar]
- 17.Kost GJ. Point-of-care strategies for disaster preparedness. Institute for Public Health website. https://iph.sdsu.edu/courses/online.php Accessed September 7, 2021. [Google Scholar]
- 18.Yenice S. Training and competency strategies for point-of-care testing. eJIFCC. 2021;32(2):167-178. [PMC free article] [PubMed] [Google Scholar]
- 19.Kost GJ. Using small-world networks to optimize preparedness, response, and resilience. In: Kost GJ, Curtis CM, eds. Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Preparedness. Washington DC: AACC Press-Elsevier; 2015:539-568. [Google Scholar]
- 20.Ferguson W, Kemp K, Kost GJ. Using a geographic information system to enhance patient access to point-of-care diagnostics in a limited-resource setting. Int J Health Geogr. 2016. Mar 1;15:10. doi: 10.1186/sl2942-016-0037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Implementing a Laboratory Test Under Emergency Use Conditions. 1st ed. CLSI white paper EP43-Edl. Allentown, PA: Clinical and Laboratory Standards Institute; 2021. [Google Scholar]
- 22.Ariel E, Owens L. Action research to improve the learning space for diagnostic techniques. J Micro Biol Ed. 2015; 16(2):167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garibaldi BT, Niessen T, Gelber AC, et al. A novel bedside cardiopulmonary physical diagnosis curriculum for internal medicine postgraduate training. BMC Med Ed. 2017;17(182):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouzi F, Hedon C, Blevaque L, et al. Interactive whiteboard use in clinical reasoning sessions to teach diagnostic testing ordering and interpretation to undergraduate medical students. BMC Med Ed. 2019;19(4):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeirnan K, Czapinski J, Bertsch T, Buchman C, Akers J. Training student pharmacists to perform point-of-care testing. Am J Pharm Ed. 2019;83(7):1611-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth IG, Meindl AG, Eckman SL, Franklin AL. Eliciting the student perspective on point-of-care diagnostic testing in association with primary care rotation. J Vet Med Educ. 2019;46(2):225-234. [DOI] [PubMed] [Google Scholar]
- 27.McCool IE, Muir JM, Knollmann-Ritschel EC. Educational care: Point-of-care testing. Acad Path 2020;7:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuzery A, Kost GJ. Point-of-care testing practices, failure modes, and risk-mitigation strategies in emergency medical services programs in the Canadian province of Alberta. Arch Pathol Lab Med. 2020;144:1352-1371. [DOI] [PubMed] [Google Scholar]
- 29.Fuzery AK, Kost GJ. Point-of-care testing by ambulance teams: An opportunity for a new standard. Clin Lab News. 2020;47(4):1, 8-13. [Google Scholar]
- 30.Amoo OS, Ohihoin AG, Musa AZ, et al. Implementation of a modified drive-through sampling strategy for SARS-CoV-2: The Nigerian experience. Pan Afr Med J. 2020; 35(Suppl 2):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang MC, Baek JH, Park D. Lessons from South Korea regarding the early stage of the COVID-19 outbreak. Healthcare (Basel). 2020;8(3):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim E. Comparing the operational strategies of South Korea and Israel’s coronavirus drive through testing centers and the implications on testing capacity. Risk Manag Healthcare Policy. 2020;13:821-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dippel KS, Kelly EK. Implementation of a nurse practitioner-led drive-through COVID-19 testing site. J Nurse Pract. 2021;17(2):185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TLC, Kost GJ. The status of point-of-care testing and coordinators in Vietnam: Needs assessment, technologies, education, exchange, and future mission. Point of Care. 2020;19(1):19-24. [Google Scholar]


