Abstract
BACKGROUND
Post-pericardiectomy right ventricular (RV) failure has been reported but it remains not well-studied. To investigate imaging parameters that could predict RV function and the outcome of patients post-pericardiectomy.
METHODS
We analysed data from a total of 53 CP patients undergoing pericardiectomy. Preoperative, early and at 6 months postoperative echocardiographic (echo) imaging datasets were analysed and correlated with preoperative cardiac magnetic resonance (CMR), cardiac computed tomography scans and histology. The primary endpoint of the study was RV functional status early postoperatively and at 6 months. Secondary endpoint was the need for prolonged inotropic support.
RESULTS
A cause of CP was identified in 26 patients (49%). Inotropic support ≥ 48 hours was required in n = 28 (53%) of patients and was correlated with lower preoperative RV areas by echo or RV volumes by CMR (p < 0.05 for all). A pericardial score based on pericardial thickness/calcification and epicardial fat thickness had good diagnostic accuracy to identify patients requiring prolonged use of inotropes (area under the curve, 0.825; 95% confidence interval, 0.674–0.976). Pericardiectomy resulted in RV decompression and impaired RV function early postoperatively (fractional area change: 40.5% ± 8.8% preoperatively vs. 31.4% ± 10.4% early postoperatively vs. 42.5% ± 10.2% at 6 months, p < 0.001).
CONCLUSIONS
We show that a smaller RV cavity size and a pericardial scoring system are associated with prolonged inotropic support in CP patients undergoing pericardiectomy. RV systolic impairment post decompression is present in most patients, but it is only transient.
Keywords: Pericardiectomy, Constrictive pericarditis, Magnetic resonance, Multislice computed tomography, Echocardiography
INTRODUCTION
Constrictive pericarditis (CP) is caused by fibrosis and calcification of the pericardium leading to impaired ventricular filling and heart failure.1) Multimodality imaging may help the diagnostic assessment of CP; further to echocardiographic (echo) signs of constriction, cardiac computed tomography (CT) and cardiac magnetic resonance (CMR) can provide useful diagnostic information on pericardial thickness, calcification, or pericardial inflammation.2),3)
The severity of pericardial constraint is proportional to the degree of ventricular inter-dependence. Patients with a large degree of ventricular discordance during the respiratory cycle, which can be easily detected as septal bounce by echocardiography or CMR, may benefit the most from pericardiectomy. Those with a mild degree of ventricular discordance and a disproportionate severity in diastolic pressure increase may not benefit from intervention and will be left with residual right-sided heart failure.1),4)
Right heart failure remains the Achille's heel following pericardiectomy,5),6) which could be related to myocardial atrophy secondary to prolonged constriction as well as rapid increase in venous return to the right heart after pericardial decompression. Nonetheless, right ventricular (RV) failure post pericardiectomy has not been systematically investigated and it remains unclear which patients are susceptible to an increased risk of postoperative RV failure due to the effects of rapid decompression.4),7),8)
In the present study, we explored which clinical and imaging factors are associated with postoperative prolonged inotropic support and RV dysfunction in patients who underwent pericardiectomy for CP.
METHODS
Study design
We retrospectively analysed our database and identified a total of 53 patients with a diagnosis of CP who underwent pericardiectomy in our Institution (Royal Brompton and Harefield NHS Foundation Trust, London, UK) between January 2013 and November 2019. The study was registered as a Clinical Audit by the Quality and Safety Department of the Royal Brompton. The diagnosis of CP was made by the clinical care team based on clinical features and multimodality imaging. Patients' medical records were searched for demographics, potential identified causes of CP, details on the in-hospital clinical course, and clinical outcomes within 6 months post-discharge. Medical imaging datasets of preoperative CMR and cardiac CT as well as transthoracic echocardiography (preoperatively, early postoperatively and at 6 months post hospital discharge) were retrieved for independent reviewing and analysis. Representative examples are shown in Figure 1. The primary objective of the study was to evaluate the changes in morphological and functional RV parameters pre- and post-pericardiectomy early (in-hospital) and at 6 months, as assessed by transthoracic echocardiography (RV end-diastolic area [RVEDA], RV end-systolic area [RVESA], RV fractional area change [RVFAC] and secondarily RV end-diastolic diameter [RVEDD] and tricuspid annular plane systolic excursion [TAPSE]). The secondary objective of the study was to explore preoperative imaging features associated with the need for postoperative prolonged inotropic support (defined as ≥ 48 hours, since most patients needed inotropic support in the early postoperative hours).
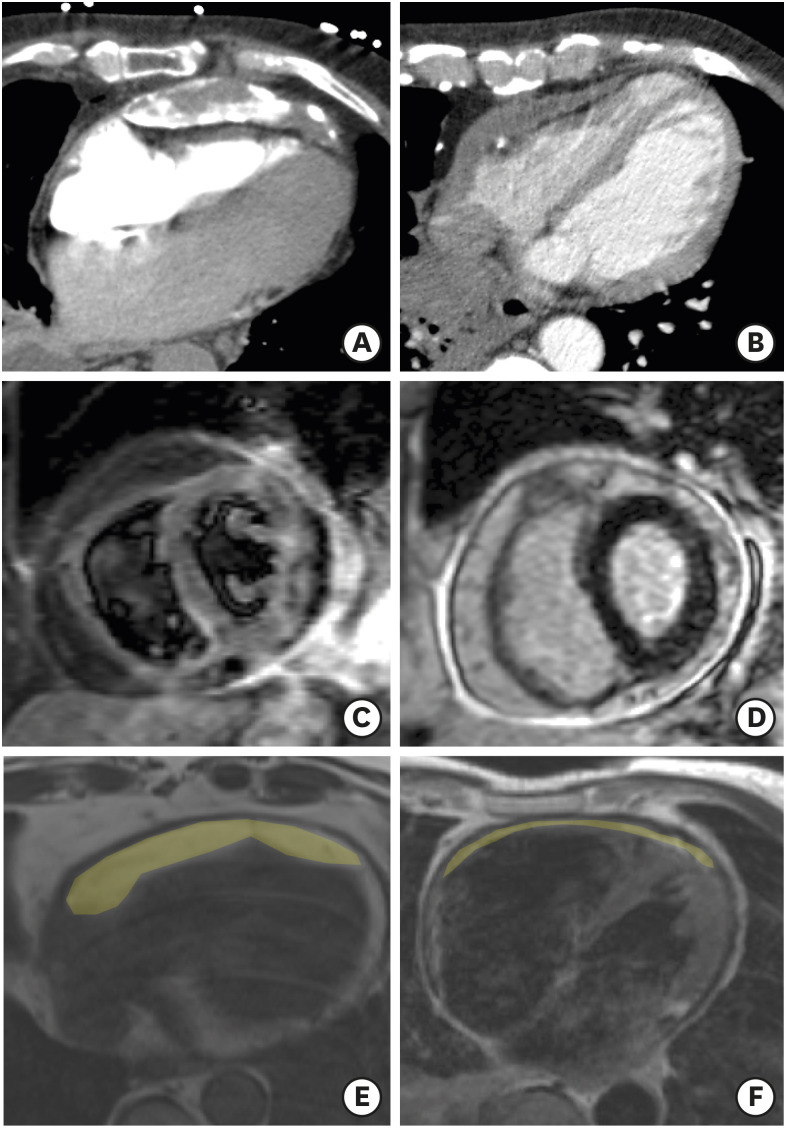
Figure 1. Representative examples of cardiac computed tomography images with (A) thickened and calcified pericardium; (B) thickened but not calcified pericardium. Representative examples of CMR images showing positive (C) pericardial T2-STIR images and (D) pericardial late-gadolinium enhancement. Trans-axial slices from turbo spin echo CMR showing thick (E) compared to thin (F) epicardial fat pad (shaded in yellow).
CMR: cardiac magnetic resonance, echo: echocardiographic, T2-STIR: T2 weighted short tau inversion recovery.
Echo data
Baseline echocardiography was performed according to the international guidelines.9) Specific emphasis was placed on suggestive signs of constriction such as: the inter-ventricular septal shift with the respiratory pattern (septal bounce); the difference in septal and lateral e′ on Tissue Doppler imaging (TDI), inferior vena cava size and collapsibility and hepatic vein expiratory diastolic flow reversal. All measurements were obtained from a mean of 3 beats for patients with sinus rhythm, and 5 beats for those with atrial fibrillation. A comprehensive examination of the right ventricle was performed with a specific focus on linear measurements (basal RV linear dimension), 2D RV areas for the estimation of fractional area change (FAC), and the TAPSE and, where available, with TDI-derived RV S′ wave velocity. A TAPSE < 17 mm was defined as abnormal, and although we report TAPSE values, we acknowledge the limitations of TAPSE in this setting. Therefore, RV dysfunction by echocardiography was defined as RVFAC < 35%.
A preoperative echocardiography was available in 48 patients, an early postoperative echo assessment (median 4 days) was performed in 41 patients, while 6-month echo follow-up was available in 26 patients.
CMR/cardiac CT imaging
Digitally archived CMR studies were retrospectively reviewed. All the examinations were performed on a 1.5-Tesla scanner (Magnetom Sonata or Avanto; Siemens, Erlangen, Germany). Imaging protocols included steady-state free precession (SSFP) breath-hold cines for the assessment of biventricular volumes and function, turbo spin-echo pulse sequences for pericardial morphologic and thickness assessment, free breathing short and long axis SSFP imaging for identification of changes in septal motion during inspiration (ventricular inter-dependence assessment), T2 weighted short tau inversion recovery (T2-STIR) and late gadolinium enhancement (LGE) sequences for the detection of pericardial oedema/inflammation. All studies were reviewed by a single expert reader using semiautomated software (CMR tools; Cardiovascular Imaging Solutions, London, UK). Continuous and categorical CMR variables included left ventricular (LV) and RV end-diastolic and end-systolic volume, LV and RV ejection fraction (LVEF and RVEF; %), left and right atrial volume and pericardial and epicardial fat layer thickness. The pericardium was considered thickened if the maximum diameter was above 3 mm on turbo spin echo images. For each study, the presence or absence of pericardial LGE, high T2-STIR signal, and interventricular septal bounce were also recorded. RV dysfunction by CMR was defined as RVEF < 45%.
Trans-axial non-contrast enhanced CT images were assessed for the presence of pericardial calcification. Pericardial thickness was recorded as the maximum diameter of pericardium on trans-axial images. The pericardium was considered thickened if the maximum diameter was above 3 mm.10)
Baseline CMR/CT data was available in 36 patients (CMR n = 21; CT n = 28) as these exams are not routinely ordered in all clinical cases.
Histology and pericardial scoring
Histopathology reports of pericardial intraoperative specimen were retrospectively reviewed to identify the presence of acute/chronic inflammation, calcification, fibrosis, and granulomas.
We assessed pericardial anatomy by measuring pericardial thickness by either CMR or CT, epicardial fat thickness by either CMR or CT, and assessing the presence/absence of pericardial calcification by CT. A combined “pericardial score” point system of epicardial fat thickness < 5 mm (1 point), thickened pericardium > 5 mm (1 point), and pericardial calcification by CT (1 point) was used.
Pericardiectomy
The surgical approach was through a standard median sternotomy with the aim to avoid cardiopulmonary bypass unless the dissection proved difficult with haemodynamic compromise or a concomitant procedure was required. After inspection and palpation of the surface of the heart to identify a relatively soft area, the pericardium was incised in the midline starting anterior to the aorta. Pericardial flaps were lifted inferiorly and laterally. Then, the pericardium was stripped from phrenic nerve to phrenic nerve laterally, releasing the diaphragm inferiorly and the atrio-ventricular groove superiorly. Bands between the right ventricle and the pulmonary artery were excised if possible. Islands of unstripped pericardium were left over the coronary arteries if adhesions proved too strong to avoid unnecessary damage and bleeding given the known continuity of the visceral pericardium with the adventitia of the vessels. Unless a clear plane could be identified, the pericardium over the right atrium and the cavo-atrial junction was not stripped completely in view of the significant risk of bleeding requiring additional and unnecessary repair.
Statistical analysis
Normally distributed continuous variables are presented as mean (standard deviation) while non-normally distributed ones as median (interquartile range). Categorical variables are presented as absolute numbers and relevant proportions. Continuous variables between groups were compared using unpaired or paired t-test or as appropriate, while categorical ones using χ2. The analysis of variance test was used for the comparison of variables between 3 or more groups. The diagnostic performance of the pericardial score for inotrope use was assessed by calculating the area under the curve for the relevant time points. All tests were 2-tailed, and a p-value of less than 0.05 was considered statistically significant. Analyses were performed using IBM SPSS Statistics for Windows (version 22.0; IBM Corp., Armonk, NY, USA).
RESULTS
Causes of CP and histological findings
The baseline demographics of the study population as well as the cause-specific frequencies of CP for the study population are shown in Table 1. A relevant cause for CP was identified in 26 out of the 53 patients (49%), while for most patients no specific cause was identifiable (51%). Only a small percentage of patients had preoperative LVEF < 5 0% (n = 3 or 6%). On the contrary, there was evidence of RV systolic dysfunction on preoperative TTE in 28% of patients by FAC and 45% of patients by TAPSE. The prevalence of moderate or severe tricuspid regurgitation was of 3 out of 48 patients in the preoperative echocardiography. Moderate or severe tricuspid regurgitation early postoperatively was found in 5 out of the 41 patients with available echocardiography data.
Table 1. Demographic characteristics.
| Characteristics | Value | ||
|---|---|---|---|
| Study population | 53 | ||
| Age (years) | 61.2 ± 14.8 | ||
| Weight (kg) | 87.8 ± 28.0 | ||
| Height (cm) | 160.6 ± 38.0 | ||
| BSA (m2) | 1.96 ± 0.27 | ||
| BMI (kg/m2) | 28.23 ± 4.70 | ||
| Previous medical history | |||
| Pericarditis | 2 (3.8) | ||
| Cardiothoracic surgery | 6 (11.3) | ||
| Tuberculosis | 7 (13.2) | ||
| Radiation | 1 (1.9) | ||
| Autoimmune disease | 4 (7.5) | ||
| Asbestosis | 5 (9.4) | ||
| Malignancy | 1 (1.9) | ||
| Unknown | 27 (50.9) | ||
| Postoperative period | |||
| Length of hospital stay (days) | 16.3 ± 18.8 | ||
| Inotropic support (hours) | 107.4 ± 190.8 | ||
| Death | 7 (13.2) | ||
| MOF | 3 | ||
| Bowel ischaemia | 2 | ||
| Mech. complications/bleeding | 2 | ||
| Pericardial imaging | |||
| Pericardial thickness (mm) | 7.38 ± 4.41 | ||
| Epicardial fat thickness (mm) | 5.91 ± 2.70 | ||
| CT calcification (%) | 10 ± 35.7 | ||
| CMR (pre-operative) | |||
| LVEDV (mL) | 106.71 ± 43.94 | ||
| LVESV (mL) | 44.86 ± 25.48 | ||
| LVSV (mL) | 62.00 ± 21.22 | ||
| LVEF (%) | 59.67 ± 8.02 | ||
| LV mass (g) | 90.14 ± 30.44 | ||
| RVEDV (mL) | 112.33 ± 46.29 | ||
| RVESV (mL) | 54.90 ± 29.18 | ||
| RVSV (mL) | 57.38 ± 20.04 | ||
| RVEF (%) | 52.67 ± 9.35 | ||
| LAV (mL) | 94.71 ± 43.51 | ||
| RAV (mL) | 85.19 ± 42.13 | ||
| Echocardiography (pre-operative) | |||
| LVEDV (mL) | 71.50 ± 28.20 | ||
| LVEDVI (mL/m2) | 37.12 ± 16.59 | ||
| LVESV (mL) | 29.26 ± 13.89 | ||
| LVESVI (mL/m2) | 15.20 ± 7.53 | ||
| LVEDD (cm) | 4.22 ± 0.61 | ||
| IVS (cm) | 0.91 ± 0.16 | ||
| PW (cm) | 0.86 ± 0.16 | ||
| LVEF (%) | 59.25 ± 6.42 | ||
| LAV (mL) | 65.87 ± 23.74 | ||
| RAV (mL) | 57.58 ± 27.53 | ||
| E/A ratio | 1.91 ± 0.91 | ||
| E′ septal (cm/s) | 0.11 ± 0.04 | ||
| E/E′ septal | 8.04 ± 4.52 | ||
| E′ lateral (cm/s) | 0.11 ± 0.03 | ||
| E/E′ lateral | 8.30 ± 5.49 | ||
| TAPSE (mm) | 16.19 ± 4.08 | ||
| RVEDD (cm) | 3.42 ± 0.71 | ||
| RVEDA (cm2) | 15.84 ± 4.18 | ||
| RVESA (cm2) | 9.46 ± 3.13 | ||
| RVFAC (%) | 40.49 ± 8.83 | ||
Data are expressed as mean ± standard deviation or as number (percentage).
BMI: body mass index, BSA: body surface area, CMR: cardiac magnetic resonance, CT: computed tomography, IVS: interventricular septum, LV: left ventricular, LAV: left atrial volume, LVEDV: left ventricular end-diastolic volume, LVEF: left ventricular ejection fraction, LVEDD: left ventricular end-diastolic diameter, LVEDVI: left ventricular end-diastolic volume index, LVESV: left ventricular end-systolic volume, LVSV: left ventricular stroke volume, MOF: multi-organ failure, PW: posterior wall, RAV: right atrial volume, RVEDA: right ventricular end-diastolic area, RVEDD: right ventricular end-diastolic diameter, RVEDV: right ventricular end-diastolic volume, RVEF: right ventricular ejection fraction, RVESA: right ventricular end-systolic area, RVESV: right ventricular end-systolic volume, RVFAC: right ventricular fractional area change, RVSV: right ventricular stroke volume, TAPSE: tricuspid annular plane systolic excursion.
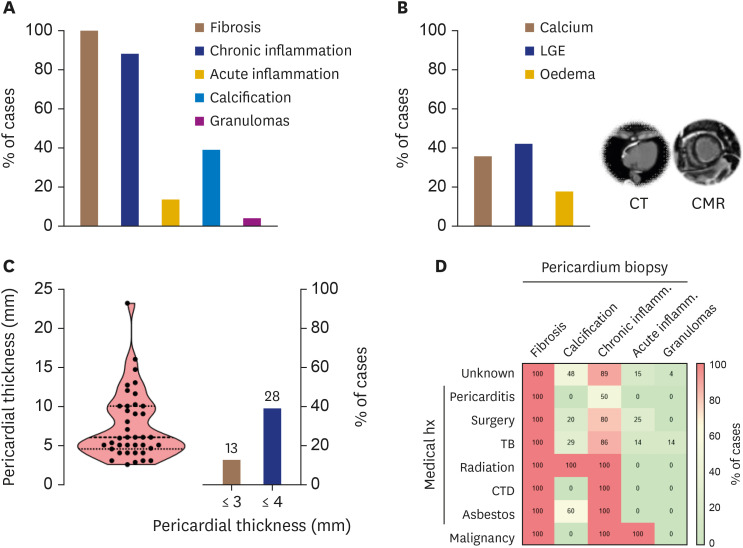
Histological analysis of pericardial biopsy invariably demonstrated evidence of fibrosis and a high prevalence of chronic inflammation (Figure 2A). Evidence of acute inflammation was present in only 13% of cases. Pericardial calcification was prevalent in 39% of cases and absent in cases linked to a history of pericarditis, autoimmune disease, or malignancy. Pericardial calcification by cardiac CT was only moderately correlated with histology (rho = 0.457, p = 0.015). There was evidence of pericardial LGE in 42% of patients, while pericardial oedema, as demonstrated by high T2-STIR signal on CMR, was present in 18% of patients (Figure 2B). There was no correlation between acute or chronic inflammation on histology and pericardial LGE or high T2-STIR signal by CMR (p = not significant [NS] for all). Of note, pericardial thickening (above 3 mm either by CMR or CT) was not present in 13% of patients (Figure 2C). An overview of the relationship between cause-specific CP and the prevalence of histological findings is provided in Figure 2D.
Figure 2. (A) Histological findings of pericardial biopsy and (B) pericardial imaging by CT and CMR in 53 patients with constrictive pericarditis undergoing pericardiectomy. (C) Distribution of pericardial thickness by CT or CMR, and percentage of CP patients with a thickened pericardium. (D) Heatmap for the relationship between cause-specific CP and the prevalence of histological findings.
CMR: cardiac magnetic resonance, CP: constrictive pericarditis, CT: computed tomography, CTD: connective tissue disorder, LGE: late gadolinium enhancement, TB: tubercolosis.
Need for inotropic support post pericardiectomy
Overall, 28 (or 52%) patients undergoing pericardiectomy required inotropic support for ≥ 48 hours. Patients who needed inotropic support for ≥ 48 hours had a significantly smaller RVEDD at baseline by echocardiography, as well as lower RV end-diastolic volume (RVEDV) and RV end-systolic volume (RVESV) preoperatively by CMR (Table 2). There was no correlation between baseline RV function by FAC or RVEF by CMR and prolonged inotropic support (i.e., for ≥ 48 hours). There was no association between LV cavity size or function preoperatively by echocardiography or CMR and the need for inotropic support early postoperatively.
Table 2. Preoperative characteristics and inotropic support.
| Characteristics | Inotropic support | p | ||
|---|---|---|---|---|
| < 48 hours (n = 25) | ≥ 48 hours (n = 28) | |||
| Age (years) | 59.08 ± 17.00 | 63.04 ± 12.62 | 0.337 | |
| BMI (kg/m2) | 28.63 ± 4.35 | 27.85 ± 5.07 | 0.555 | |
| Cardiopulmonary bypass (%) | 5 (21) | 6 (25) | 0.999 | |
| Renal replacement therapy (%) | 0 (0) | 4 (17) | 0.115 | |
| Bleeding (mL) | 864.5 ± 107.1 | 1,242.0 ± 214.1 | 0.146 | |
| CMR (pre-operative) | ||||
| LVEDV (mL) | 115.20 ± 54.23 | 99.00 ± 32.83 | 0.413 | |
| LVESV (mL) | 49.90 ± 31.53 | 40.27 ± 18.85 | 0.401 | |
| LVSV (mL) | 65.30 ± 25.87 | 59.00 ± 16.66 | 0.511 | |
| LVEF (%) | 58.50 ± 8.03 | 60.73 ± 8.25 | 0.539 | |
| LV mass (g) | 97.30 ± 35.09 | 83.64 ± 25.45 | 0.317 | |
| RVEDV (mL) | 132.60 ± 60.84 | 93.91 ± 13.06 | 0.053 | |
| RVESV (mL) | 68.70 ± 36.48 | 42.36 ± 11.93 | 0.035 | |
| RVSV (mL) | 63.70 ± 26.92 | 51.64 ± 8.62 | 0.174 | |
| RVEF (%) | 49.60 ± 8.60 | 55.45 ± 9.50 | 0.157 | |
| LAV (mL) | 73.10 ± 25.81 | 114.36 ± 47.90 | 0.026 | |
| RAV (mL) | 85.70 ± 42.04 | 84.73 ± 44.26 | 0.959 | |
| Echocardiography (pre-operative) | ||||
| LVEDV (mL) | 75.23 ± 31.89 | 68.49 ± 25.07 | 0.421 | |
| LVEDVI (mL/m2) | 38.93 ± 19.40 | 35.59 ± 14.03 | 0.502 | |
| LVESV (mL) | 31.88 ± 16.17 | 27.15 ± 11.64 | 0.251 | |
| LVESVI (mL/m2) | 16.37 ± 8.75 | 14.22 ± 6.34 | 0.341 | |
| LVEDD (cm) | 4.24 ± 0.61 | 4.21 ± 0.63 | 0.868 | |
| LVESD (cm) | 2.88 ± 0.62 | 2.86 ± 0.56 | 0.887 | |
| LVEF (%) | 57.68 ± 6.39 | 60.58 ± 6.27 | 0.121 | |
| LA volume (mL) | 69.45 ± 27.36 | 62.72 ± 20.08 | 0.337 | |
| RA volume (mL) | 59.81 ± 32.73 | 55.62 ± 22.58 | 0.617 | |
| E/A ratio | 1.75 ± 0.70 | 2.04 ± 1.05 | 0.342 | |
| e′ septal (cm/s) | 0.10 ± 0.04 | 0.12 ± 0.04 | 0.302 | |
| E/e′ septal | 7.97 ± 5.61 | 8.09 ± 3.71 | 0.950 | |
| E′ lateral (cm/s) | 0.12 ± 0.03 | 0.11 ± 0.04 | 0.443 | |
| E/e′ lateral | 6.44 ± 2.80 | 9.79 ± 6.64 | 0.068 | |
| TAPSE (mm) | 16.29 ± 4.33 | 16.12 ± 3.96 | 0.889 | |
| RVEDD (cm) | 3.64 ± 0.62 | 3.21 ± 0.74 | 0.047 | |
| RVEDA (cm2) | 16.05 ± 4.69 | 15.66 ± 3.79 | 0.758 | |
| RVESA (cm2) | 9.66 ± 3.31 | 9.29 ± 3.04 | 0.699 | |
| RVFAC (%) | 40.00 ± 7.62 | 40.91 ± 9.88 | 0.733 | |
Data are expressed as mean ± standard deviation or as number (percentage). Statistically significant p-values (< 0.05) are shown in bold fonts.
BMI: body mass index, CMR: cardiac magnetic resonance, LA: left atrium, LAV: left atrial volume, LV: left ventricular, LVEDD: left ventricular end-diastolic diameter, LVEDV: left ventricular end-diastolic volume, LVEDVI: left ventricular end-diastolic volume index, LVEF: left ventricular ejection fraction, LVESD: left ventricular end-systolic diameter, LVESV: left ventricular end-systolic volume, LVESVI: left ventricular end-systolic volume index, LVSV: left ventricular stroke volume, RA: right ventricular, RAV: right atrial volume, RVEDA: right ventricular end-diastolic area, RVEDD: right ventricular end-diastolic diameter, RVEDV: right ventricular end-diastolic volume, RVEF: right ventricular ejection fraction, RVESA: right ventricular end-systolic area, RVESV: right ventricular end-systolic volume, RVFAC: right ventricular fractional area change, RVSV: right ventricular stroke volume, TAPSE: tricuspid annular plane systolic excursion.
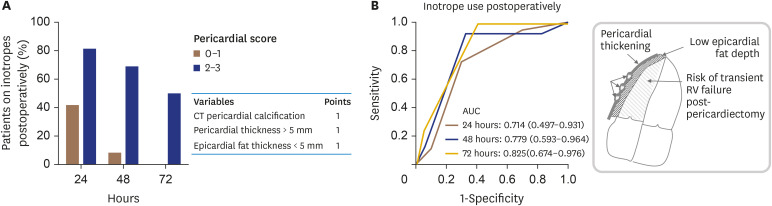
There was a significant negative correlation between epicardial fat thickness and the length of inotropic support (rho = −0.442, p = 0.007). The length of inotropes use was positively correlated with the presence of pericardial calcification by CT (rho = 0.374, p = 0.05) and with a thickened pericardium (i.e., > 5 mm, rho = 0.314, p = 0.05). A combined “pericardial” score point system of epicardial fat thickness < 5 mm (1 point), thickened pericardium > 5 mm (1 point) and pericardial calcification by CT (1 point) was strongly associated with the in-hospital use of inotropes (Figure 3). Patients with a low pericardial score (0–1) were less in need of inotropic support at all time-points post-surgery compared to patients with a high pericardial score (2–3). The prognostic accuracy of the pericardial score for classifying patients at risk for extended inotropic support is shown in Figure 3B.
Figure 3. (A) A pericardial score based on pericardial calcification/thickness and epicardial fat thickness could be used to assess the difficulty of surgical pericardial dissection and to predict the risk for heart failure and prolonged inotropic support postoperatively. (B) Prognostic accuracy of pericardial score to identify patients at risk for prolonged inotropic support (n = 28).
AUC:= area under the curve, CT: computed tomography, RV: right ventricular.
Effects of pericardiectomy on RV anatomy and function
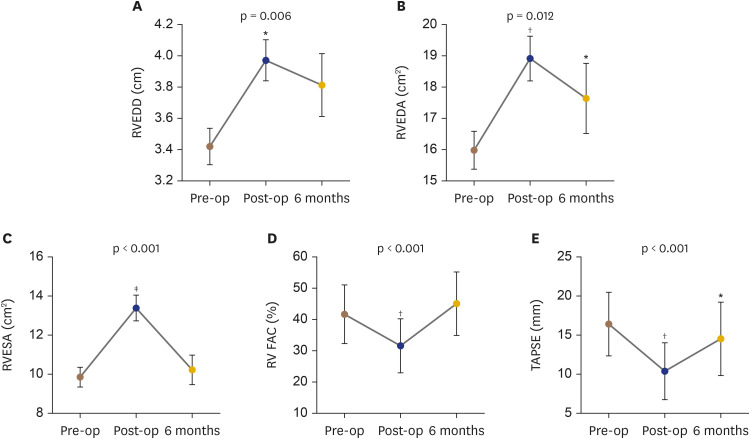
Pericardiectomy led to a rapid decompression of the RV early postoperatively in most patients, as manifested by an increase in RV areas by echocardiography in 85% of patients. In-hospital echocardiography post-pericardiectomy demonstrated a significant change in RVEDD (mean increase by 19.2%, Figure 4A), RVEDA (mean increase by 18%, Figure 4B) and RVESA (mean increase by 35%, Figure 4C). RV systolic function was significantly reduced early post-pericardiectomy as evidenced by a significant reduction in both TAPSE and FAC% by echocardiography (Figure 4D and E). Only 20% of patients did not develop RV dysfunction early postoperatively as defined by echocardiography (i.e., FAC < 35% early postoperatively).
Figure 4. RV cavity size and function preoperatively, postoperatively and at 6 months. Data are shown for changes in (A) RVEDD, (B) RVEDA, (C) RVESA, (D) FAC and (E) TAPSE.
FAC: fractional area change, RV: right ventricular, RVEDA: right ventricular end-diastolic area, RVEDD: right ventricular end-diastolic diameter, RVESA: right ventricular end-systolic area, TAPSE: tricuspid annular plane systolic excursion.
*p < 0.05; †p < 0.001; ‡p < 0.0001 vs. baseline.
At 6 months post-pericardiectomy, RV size (RVEDD and RVEDA) remained significantly increased compared to pre-pericardiectomy status (Figure 4A and B). However, we observed a significant improvement of RV function, as shown by FAC, at 6 months follow-up compared to early postoperative status. There were no significant changes in LV systolic function at 6 months vs. early postoperatively or baseline (p = NS for all).
DISCUSSION
In this study we explore the morphological and functional RV changes that occur early postoperatively and at 6 months post pericardiectomy in a cohort of 53 patients with CP. We demonstrate that: a) RV function is significantly impaired after decompression during the early postoperative period, but its function is improved at 6 months; b) a smaller RV cavity size at baseline as expressed by RVEDD, RVEDA, and RVESA (by either CMR or echocardiography) predicts prolonged inotropic support early postoperatively; and c) also patients with less epicardial fat thickness, and a calcified and thickened pericardium were also at increased risk for prolonged inotropic support. These findings may help to identify those patients at higher risk for RV dysfunction postoperatively.
The clinical diagnosis of CP remains occasionally a challenging one and multimodality imaging can be particularly helpful.11) In our cohort of CP patients pericardial thickening (defined as maximum pericardial thickness > 3 mm) was not present in 13% of patients, which suggests that this may not be a reliable criterion to rule out CP. This confirms previous findings,12) in which up to 18% of patients with surgically proven CP do not present with increased pericardial thickness. On the other hand, septal bouncing has high diagnostic accuracy for CP,13) and in our cohort it was invariably present in free-breathing CMR imaging. Of note, there was only a moderate correlation between histological findings and multimodality imaging, i.e., pericardial calcification by CT and pericardial inflammation by CMR. This could be due to several reasons; a) the patchy involvement of pericardial disease, which may not be detected in the analysed specimen; b) the different timing of CMR imaging compared to the time of surgery and biopsy which may explain why some of these patients did not have evidence of inflammation by CMR; c) another possibility that should be acknowledged may be the low sensitivity of T2-STIR by CMR to detect low levels of chronic inflammation.
RV failure is one of the major complications early post-pericardiectomy,6) but its incidence as well as its predictors have not been systematically explored to date. In a previous risk analysis of a series of CP patients undergoing pericardiectomy, elevated gamma-glutamyl transferase and decreased protein preoperatively could predict RV failure with the need for extracorporeal membrane oxygenation post-surgery.5) Myocardial atrophy secondary to prolonged constriction14) as well as a rapid increase in venous return to right heart after pericardial decompression are presumed causes of RV failure early post-pericardiectomy.6) In our cohort RV was significantly decompressed post-surgery (as evidenced by a significant increase in RV cavity size by echocardiography), and this was paralleled by significant RV systolic impairment. We focused on FAC to assess RV systolic function, since the longitudinal RV function, as measured by TAPSE and RV S′ TDI, is often misleading in the context of cardiac surgery.15) We show that approximately 80% of patients have evidence of RV dysfunction early post-pericardiectomy. Most importantly, we also provide evidence that a higher degree of RV compression preoperatively (as assessed by RV volumes by CMR or RV areas by echocardiography) is associated with the need for prolonged inotropic support postoperatively. To our knowledge, the role of preoperative cardiac imaging in predicting outcomes post-pericardiectomy has never been studied before.
Multimodality imaging has a clear role in the diagnostic assessment of CP,16) but its predictive role in detecting the risk for peri-operative complications in CP patients undergoing pericardiectomy has never been studied to date. Pericardial LGE or high T2-STIR signal were not associated with the in-hospital clinical course of patients. However, we assumed that there is an association between pericardial anatomy (i.e., epicardial fat thickness and pericardial thickness/calcification) and the difficulty of surgical dissection of the pericardium. Our hypothesis is that a thinner epicardial fat layer, as well as a thicker and calcified pericardium with strong adhesions to the underlying RV myocardium, may be associated with a more traumatic surgery causing a higher degree of myocardial damage/injury. This will result in a damaged right ventricle that will require a more prolonged inotropic support postoperatively to recover. We demonstrate that the presence of pericardial calcification by CT, increased pericardial thickness as well as lower epicardial fat thickness were all positively correlated with the length of inotropic support. We suggest that a pericardial scoring system based on these variables may have a role in identifying those patients who need prolonged inotropic support postoperatively. The implication of this would be for the surgical team to adopt more gentle techniques when performing a pericardiectomy in patients with a thin layer of epicardial fat identified by imaging. Certainly, external validation of these findings by independent cohorts would be of value.
Pericardiectomy remains even today a high-risk procedure17),18),19) with high late mortality rates.20) To our knowledge, there is limited literature on the late effects of pericardiectomy on RV anatomy and function. Previous haemodynamic invasive studies suggest an improvement of RV function,14),21) as shown by normalised intra-cardiac pressures on right heart catheterization between 2 and 5 months after the operation. In our cohort we observed an impairment in RV systolic function post-surgery, which however was significantly improved at 6 months. Therefore, it seems that RV functional impairment post pericardiectomy is only transient and limited to the immediate early postoperative period. No significant changes were observed in LV haemodynamics early postoperatively or at 6-month follow-up.
Pericardiectomy has high in-hospital mortality and challenging postoperative management due to the development of RV failure. We show that RV dysfunction develops early postoperatively in most CP patients but is only transient. Multimodality imaging (including CT and/or CMR) to assess pericardial anatomy may be important for surgery planning and/or to identify patients that are at high risk for early postoperative RV dysfunction. CMR can better detect those patients with small RV volumes, which seem to be at risk for prolonged inotropic support.
In conclusion, the present paper shows that in patients undergoing pericardiectomy, RV function is significantly impaired after decompression during the early postoperative period. Predictors of a complicated postoperative course with need for prolonged inotropic support include a smaller preoperative RV cavity size as well as a novel pericardial score based on epicardial fat thickness, pericardial calcification, and pericardial thickness.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Mohiaddin R.
- Data curation: Azzu A, Capoccia M.
- Formal analysis: Antonopoulos AS.
- Investigation: Azzu A.
- Methodology: Mohiaddin R.
- Project administration: Azzu A, Morosin M, Mohiaddin R.
- Supervision: Mohiaddin R.
- Writing - original draft: Antonopoulos AS.
- Writing - review & editing: Capoccia M, Rosendahl U, Mohiaddin R.
References
- 1.Nishimura RA. Constrictive pericarditis in the modern era: a diagnostic dilemma. Heart. 2001;86:619–623. doi: 10.1136/heart.86.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank H, Globits S. Magnetic resonance imaging evaluation of myocardial and pericardial disease. J Magn Reson Imaging. 1999;10:617–626. doi: 10.1002/(sici)1522-2586(199911)10:5<617::aid-jmri5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Young PM, Glockner JF, Williamson EE, et al. MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. Int J Cardiovasc Imaging. 2012;28:1099–1109. doi: 10.1007/s10554-011-9916-0. [DOI] [PubMed] [Google Scholar]
- 4.Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380–1386. doi: 10.1161/01.cir.100.13.1380. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann E, Ismail I, Cebotari S, et al. Right-sided heart failure and extracorporeal life support in patients undergoing pericardiectomy for constrictive pericarditis: a risk factor analysis for adverse outcome. Thorac Cardiovasc Surg. 2017;65:662–670. doi: 10.1055/s-0036-1593817. [DOI] [PubMed] [Google Scholar]
- 6.Yu HT, Ha JW, Lee S, et al. Transient right ventricular dysfunction after pericardiectomy in patients with constrictive pericarditis. Korean Circ J. 2011;41:283–286. doi: 10.4070/kcj.2011.41.5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch C, Penov K, Amorim PA, et al. Risk factors for mortality after pericardiectomy for chronic constrictive pericarditis in a large single-centre cohort. Eur J Cardiothorac Surg. 2015;48:e110–6. doi: 10.1093/ejcts/ezv322. [DOI] [PubMed] [Google Scholar]
- 8.Zhu P, Mai M, Wu R, Lu C, Fan R, Zheng S. Pericardiectomy for constrictive pericarditis: single-center experience in China. J Cardiothorac Surg. 2015;10:34. doi: 10.1186/s13019-015-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Zurick AO, Bolen MA, Kwon DH, et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. JACC Cardiovasc Imaging. 2011;4:1180–1191. doi: 10.1016/j.jcmg.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Syed FF, Schaff HV, Oh JK. Constrictive pericarditis--a curable diastolic heart failure. Nat Rev Cardiol. 2014;11:530–544. doi: 10.1038/nrcardio.2014.100. [DOI] [PubMed] [Google Scholar]
- 12.Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852–1857. doi: 10.1161/01.CIR.0000087606.18453.FD. [DOI] [PubMed] [Google Scholar]
- 13.Bolen MA, Rajiah P, Kusunose K, et al. Cardiac MR imaging in constrictive pericarditis: multiparametric assessment in patients with surgically proven constriction. Int J Cardiovasc Imaging. 2015;31:859–866. doi: 10.1007/s10554-015-0616-z. [DOI] [PubMed] [Google Scholar]
- 14.Viola AR. The influence of pericardiectomy on the hemodynamics of chronic constrictive pericarditis. Circulation. 1973;48:1038–1042. doi: 10.1161/01.cir.48.5.1038. [DOI] [PubMed] [Google Scholar]
- 15.Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 16.Cosyns B, Plein S, Nihoyanopoulos P, et al. European Association of Cardiovascular Imaging (EACVI) position paper: multimodality imaging in pericardial disease. Eur Heart J Cardiovasc Imaging. 2015;16:12–31. doi: 10.1093/ehjci/jeu128. [DOI] [PubMed] [Google Scholar]
- 17.Fang L, Yu G, Huang J, Zhao W, Ye B. Predictors of postoperative complication and prolonged intensive care unit stay after complete pericardiectomy in tuberculous constrictive pericarditis. J Cardiothorac Surg. 2020;15:148. doi: 10.1186/s13019-020-01198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopaldas RR, Dao TK, Caron NR, Markley JG. Predictors of in-hospital complications after pericardiectomy: a nationwide outcomes study. J Thorac Cardiovasc Surg. 2013;145:1227–1233. doi: 10.1016/j.jtcvs.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 19.Kang SH, Song JM, Kim M, et al. Prognostic predictors in pericardiectomy for chronic constrictive pericarditis. J Thorac Cardiovasc Surg. 2014;147:598–605. doi: 10.1016/j.jtcvs.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Biçer M, Özdemir B, Kan İ, Yüksel A, Tok M, Şenkaya I. Long-term outcomes of pericardiectomy for constrictive pericarditis. J Cardiothorac Surg. 2015;10:177. doi: 10.1186/s13019-015-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloster FE, Crislip RL, Bristow JD, Herr RH, Ritzmann LW, Griswold HE. Hemodynamic studies following pericardiectomy for constrictive pericarditis. Circulation. 1965;32:415–424. doi: 10.1161/01.cir.32.3.415. [DOI] [PubMed] [Google Scholar]