Abstract
Aim: This study investigated whether the small dense low-density lipoprotein cholesterol (sd-LDL-c) level is associated with the rapid progression (RP) of non-culprit coronary artery lesions and cardiovascular events (CE) after acute coronary syndrome (ACS).
Methods: In 142 consecutive patients with ACS who underwent primary percutaneous coronary intervention for the culprit lesion, the sd-LDL-c level was measured using a direct homogeneous assay on admission for ACS and at the 10-month follow-up coronary angiography. RP was defined as a progression of any pre-existing coronary stenosis and/or stenosis development in the initially normal coronary artery. CEs were defined as cardiac death, myocardial infarction, stroke, or coronary revascularization.
Results: Patients were divided into two groups based on the presence ( n =29) or absence ( n =113) of RP after 10 months. The LDL-c and sd-LDL-c levels at baseline were equivalent in both the groups. However, the sd-LDL-c, triglyceride, remnant lipoprotein cholesterol (RL-c), and apoC3 levels at follow-up were significantly higher in the RP group than in the non-RP group. The optimal threshold values of sd-LDL-c, triglyceride, RL-c, and apoC3 for predicting RP according to receiver operating characteristics analysis were 20.9, 113, 5.5, and 9.7 mg/dL, respectively. Only the sd-LDL-c level (≥ 20.9 mg/dL) was significantly associated with incident CEs at 31±17 months (log-rank: 4.123, p =0.043).
Conclusions: The sd-LDL-c level on treatment was significantly associated with RP of non-culprit lesions, resulting in CEs in ACS patients. On-treatment sd-LDL-c is a residual risk and aggressive reduction of sd-LDL-c might be needed to prevent CEs.
Keywords: Small dense low-density lipoprotein cholesterol, Acute coronary syndrome, Rapid progression, Triglyceride, Apolipoprotein C3
See editorial vol. 28: 1130-1132
Introduction
Acute coronary syndrome (ACS) is the most serious manifestation of ischemic heart disease, which is the leading cause of death worldwide. Statins, the low-density lipoprotein-cholesterol (LDL-c) lowering medications, are recommended for reducing the subsequent risk of major cardiovascular events (CEs) in patients with ACS 1) . The suggested target LDL-c level is less than 70 mg/dL for the secondary prevention in ACS 2) . However, some patients present with CEs even after achieving an LDL-c level below the guideline-directed goal. Thus, the potential target of residual risk management remains unresolved to improve the clinical outcomes following ACS events.
Several reports have indicated that in ACS patients, vulnerable plaque exists not only in culprit lesions but also in non-culprit coronary segments 3 , 4) . The PROSPECT study showed that half of the CEs after ACS are adjudicated to be related to the progression of non-culprit lesions 5) . Regarding plaque changes, numerous randomized controlled trials (RCTs) of lipid-lowering therapy using intravascular ultrasound (IVUS) have revealed the efficacy of plaque stabilization with aggressive lipid-lowering therapy 6 - 8) . A recent study investigating changes in non-culprit lesions using IVUS in ACS patients reported that the incidence of CEs was higher in patients with plaque progression and in those with plaque regression who have an on-treatment LDL-c level of ≥ 70 mg/dL than in patients with plaque regression who have an LDL-c level of <70 mg/dL 9) . Thus, inhibition of rapid progression (RP) of non-culprit coronary lesions is important for secondary prevention in ACS; however, the detailed mechanisms and predictors of RP are not fully understood.
A pooled study of nine clinical trials using IVUS demonstrated that coronary atheroma progression was more closely associated with the treatments of non-high-density lipoprotein cholesterol (non-HDL-c) and triglyceride (TG) than with that of LDL-c 10) . A meta-analysis of eight RCTs of statins reported that the on-treatment levels of non-HDL-c were more strongly associated with the risk of CEs than those of LDL-c and apolipoprotein (apo) B in statin-treated patients 11) . Moreover, on a background of statin treatment in patients with ACS and myocardial infarction (MI), fasting TG levels have been associated with the risk of CEs 12 , 13) . Non-HDL-c comprises both LDL-c and TG-rich lipoprotein (TRL) cholesterols such as very low-density lipoprotein and remnant lipoprotein (RL) cholesterols. TRL accumulation has been suggested to pose a residual risk in patients receiving effective statin therapy. Numerous studies, including ours 14) , have demonstrated that TGs are positively associated with small dense (sd)-LDL particles and negatively associated with large buoyant (lb)-LDL particles 14 - 16) . The predominance of sd-LDL particles is strongly associated with an impaired glucose regulation 17 , 18) , postprandial hyperlipidemia 19) , and metabolic syndrome 16 , 20) . Several clinical studies have demonstrated that sd-LDL particles are more atherogenic than lb-LDL particles 21) . Numerous studies, including ours 22 - 25) , have indicated that sd-LDL cholesterol (sd-LDL-c) levels are more strongly associated with the risk of incident coronary artery disease (CAD), severity of coronary atherosclerosis 22 , 23) , and incident CEs in secondary prevention 24 , 25) than the level of LDL-c in Japanese populations 21 - 27) . However, it remains unclear whether the levels of sd-LDL-c and TRL before percutaneous coronary intervention (PCI) and during the treatment period can predict RP and/or CEs after ACS.
Aim
This study aimed to investigate whether the levels of sd-LDL-c and various lipid biomarkers can predict RP and long-term prognosis in patients with ACS under lipid-management after PCI.
Methods
Study Subjects
This single-center observational cohort study enrolled 142 consecutive patients with ACS who underwent primary PCI between January 2014 and December 2017 at the Showa University Hospital. Serum samples were collected just before the emergency coronary angiography (CAG) on admission for ACS and before the 10-month follow-up CAG. ACS comprised ST-segment elevation MI (STEMI) and non-ST-segment elevation ACS (NSTE-ACS). STEMI was defined as the presence of anginal symptoms (>20 min) associated with electrocardiographic ST-segment elevation of at least 0.1 mV in two or more limb leads or at least 0.2 mV in two or more precordial leads and a rise in cardiac-specific troponin I levels. NSTE-ACS included non-STEMI (NSTEMI) and unstable angina pectoris (UAP). NSTEMI was defined as the presence of ischemic symptoms in the absence of ST elevation on electrocardiogram with elevated cardiac-specific troponin I levels. UAP was defined as having newly developed and accelerated chest symptoms on exertion or rest angina without a significant rise in cardiac-specific troponin I levels. A diagnosis of hypertension (HT) was made if patients had a history of HT or had a systolic blood pressure of ≥ 140 mmHg or diastolic blood pressure of ≥ 90 mmHg 21) . Diabetes mellitus (DM) was diagnosed if a patient’s fasting blood glucose level was ≥ 126 mg/dL, 2-hour glucose level during an oral glucose tolerance test was ≥ 200 mg/dL, random serum glucose level was ≥ 200 mg/dL, glycated hemoglobin (HbA1c) value was ≥ 6.5%, and/or if a patient received treatment with any hypoglycemic agents 21) . Dyslipidemia (DL) was defined as the current use of lipid-lowering medications and/or meeting the criteria of the Japan Atherosclerosis Society for fasting serum lipid levels (i.e., LDL-c ≥ 140 mg/dL, HDL-c <40 mg/dl, or TG ≥ 150 mg/dL) 21) . A serum creatinine-based formula was used to calculate estimated glomerular filtration rate (eGFR): eGFR=194×creatinine −1.094 ×age −0.287 (×0.739 for women) 28) . Body mass index was calculated as weight (in kilograms) divided by height (in meters) squared. Patients who smoked at least one cigarette daily in the past or on admission were classified as smokers.
Patients on hemodialysis, taking drugs for thyroid dysfunction, or currently receiving treatment for malignancy or those with nephrotic syndrome, renal dysfunction (serum creatinine ≥ 1.5 mg/dL), severe hepatic disease, infectious disease, or any other serious condition were excluded. The Institutional Review Board of the Showa University approved the study protocol (No. 2855). The study conformed to the ethical principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Measurement of Biomarkers
The levels of total cholesterol, TG, HDL-c, HbA1c, immunoreactive insulin (IRI), and apolipoprotein were assayed by standard laboratory procedures. The RL-cholesterol (RL-c) level was measured by an immunoprecipitation method using an immunoaffinity mixed gel (RLP-C; Japan Immunoresearch Laboratories, Takasaki, Japan) containing anti-apo A-I (apoA) and anti-apo B-100 (apoB) monoclonal antibodies until March 31, 2016 29) . Thereafter, the RL-c level was measured by another homogenous assay (RemL-C; Kyowa Medex, Tokyo, Japan) 30) . Although the RLP-C and RemL-C assays are different, they have been reported to have significant positive correlation with each other 31) . Lipoprotein(a) levels were measured using a latex agglutination immunoassay. The kits used for the LDL-c and sd-LDL-c tests were both provided by Denka Seiken, Tokyo, Japan. The serum LDL-c level was determined by a direct homogenous assay using detergents (LDL-EX; Denka Seiken). The serum samples were frozen at −80℃ until used for the direct homogeneous assay to measure sd-LDL-c levels, as previously described 32) . Non-HDL-c levels were estimated by subtracting the HDL-c concentration from the total cholesterol concentration. The lb-LDL-c concentration was estimated by subtracting the sd-LDL-c concentration from the LDL-c concentration. The high-sensitivity C-reactive protein (hs-CRP) was assayed by the Dade Behring BN method 33) . The plasma N-terminal pro-brain natriuretic peptide (NTproBNP) level was measured using an Elecsys proBNP immunoassay (Roche Diagnostics, Risch, Switzerland). The serum malondialdehyde-modified LDL (MDA-LDL) level was measured using a sandwich enzyme-linked immunosorbent assay system (SRL, Inc. Tokyo, Japan).
CAG Analysis and Definition of RP of CAG
In this study, CAG was performed by experienced cardiologists using standard techniques after the direct intracoronary injection of 2.5 mg isosorbide dinitrate into the coronary arteries to exclude potential effects of coronary spasm. After all coronary arteries were injected, at least 2 views of the right coronary arteries and 6 views of the left coronary arteries were analyzed.
All quantitative CAGs at index PCI and follow-ups were reviewed and analyzed using CAAS 5 software (Pie Medical Imaging BV, Maastricht, the Netherlands) by at least two experienced interventional cardiologists who were blinded to the clinical data. If the QCA data was different between two operators, a subsequent re-analysis of the same frame was performed and the average value was employed. The stem size of a Judkins coronary catheter was used for calibration to determine absolute measurements in millimeters. Coronary arteries were divided into 15 segments according to the classification of the American Heart Association Grading Committee 34) . The measurements for each segment were performed on end-diastolic frames, in which the severity of stenosis appeared maximum. All the coronary arteries, the right coronary artery, left anterior descending coronary artery, and left circumflex artery were analyzed from the base to the tip, with a reference diameter of ≥ 1 mm. A lesion with intermediate stenosis (percent diameter stenosis range: 30%–70%) and without ischemia at either baseline or follow-up was defined as the non-culprit lesion. We excluded lesions located within 10 mm of PCI-treated lesions and those within 10 mm of non-culprit lesions from the analysis. As in previous studies, the RP of non-culprit lesions was determined by the following criteria: 1) ≥ 10% reduction in the diameter of a pre-existing stenosis ≥ 50%; 2) ≥ 30% reduction in the diameter of a pre-existing <50% stenosis; 3) development of a new stenosis with ≥ 30% reduction in the diameter of a segment that was normal on initial CAG; or 4) progression of any lesion to total occlusion on follow-up CAG 35 , 36) .
Definition of Clinical Outcomes
Follow-up data were obtained by reviewing medical records and scripted telephone interviews. A CE was defined as cardiac death, non-fatal MI, hospitalization for ischemic or hemorrhagic stroke, or total coronary revascularization. Total revascularization included target lesion revascularization (TLR), target vessel revascularization (TVR), and non-TVR. TLR was defined as the revascularization of the target lesion due to restenosis or re-occlusion within the stent or 5 mm in and adjacent to the distal or proximal segment. TVR was defined as revascularization of the target vessel or any segment of the coronary artery containing the target lesion. Non-TVR was defined as the revascularization of any segment of the non-target coronary artery.
Statistical Analysis
Statistical analyses were performed using JMP statistical software version 14 (SAS Institute, Cary, NC, USA). All data are presented as mean±standard deviation (SD) or proportion. Normally distributed continuous variables were compared using unpaired Student’s t -test and non-normally distributed continuous variables were compared using the Mann–Whitney test or Wilcoxon’s signed-rank test. Categorical variables were compared using the Fisher’s exact test or chi-square test. Correlation coefficients between sd-LDL-c and other lipid markers were determined by linear regression analyses. Variables (including clinical, laboratory, and angiographic variables) known or suspected to be associated with the presence of RP were assessed using univariate and multivariate logistic regression analyses. Thereafter, receiver operating characteristic (ROC) curves were constructed. The area under the curve (AUC), sensitivity, and specificity for predicting RP were calculated, with an AUC of 0.50 indicating no accuracy and an AUC of 1.00 indicating maximum accuracy. Multivariate logistic regression was used to determine the predictors of RP. Variables that had clinically meaningful lipid profiles for plaque progression based on the current guidelines (LDL-c ≥ 70 mg/dL, non-HDL-c ≥ 100 mg/dL and TG ≥ 150 mg/dL) were included in the multivariate regression analysis. All statistical analyses were two-tailed. A p value of <0.05 was considered statistically significant.
Results
Patient Characteristics
The average 10-month follow-up CAG revealed that 29 (20.4%) of the 142 patients had RP of non-culprit lesions, however, no patient had lesion progression to total occlusion. Among 29 patients with RP of non-culprit lesions, 6 had ≥ 10% reduction in the diameter of a pre-existing stenosis of ≥ 50%, 8 had ≥ 30% reduction in the diameter of a pre-existing stenosis of <50%, and 15 had a new lesion with ≥ 30% stenosis in a previously normal segment. Patients with at least one RP lesion were classified into the RP group ( n =29) and those without RP lesions were classified into the non-RP group ( n =113). No significant differences in the variables were noted between the two groups ( Table 1 ) . Statins were administered to 98.6% of the patients.
Table 1. Characteristics of the patients.
| Variable | RP group n = 29 | Non-RP group n = 113 | p value | ||
|---|---|---|---|---|---|
| Age, yrs | 64±10 | 66±11 | 0.24 | ||
| Gender, female | 2 (6.9%) | 24 (21.2%) | 0.07 | ||
| BMI, kg/m 2 | 24.7±3.3 | 24.4±3.6 | 0.63 | ||
| Coronary risk factors | |||||
| Hypertension | 17 (58.6%) | 73 (64.6%) | 0.53 | ||
| Diabetes mellitus | 8 (27.6%) | 35 (31.0%) | 0.72 | ||
| Dyslipidemia | 11 (37.9%) | 39 (34.5%) | 0.73 | ||
| Smoking | 25 (86.2%) | 79 (69.9%) | 0.08 | ||
| Family history of CVD | 6 (20.7%) | 20 (17.7%) | 0.71 | ||
| Prior MI | 1 (3.5%) | 8 (7.1%) | 0.44 | ||
| Prior PCI | 0 (0.0%) | 8 (7.8%) | 0.14 | ||
| Clinical presentation | 0.96 | ||||
| STEMI | 22 (75.9%) | 83 (73.5%) | |||
| NSTEMI | 6 (20.7%) | 26 (23.0%) | |||
| UAP | 1 (3.5%) | 4 (3.5%) | |||
| Peak CK, U/L | 2295±1937 | 2280±2376 | 0.68 | ||
| Ejection fraction, % | 49.0±11.0 | 52.3±10.8 | 0.09 | ||
| Medications | On admission | At follow-up | On admission | At follow-up | |
| Statin | 8 (27.6%) | 29 (100%) | 29 (25.7%) | 111 (98.2%) | 0.83 |
| Ezetimibe | 0 (0.0%) | 4 (13.8%) | 4 (3.6%) | 9 (8.0%) | 0.3 |
| EPA | 0 (0.0%) | 2 (6.9%) | 5 (4.4%) | 7 (6.2%) | 0.25 |
| Fibrate | 3 (10.4%) | 2 (6.9%) | 7 (6.2%) | 1 (0.9%) | 0.46 |
Data are expressed as mean±standard deviation (SD) or number (%). Abbreviations: BMI, body mass index; CVD, cardiovascular disease; CK, creatine kinase; EPA, eicosapentaenoic acid; PCI, percutaneous coronary intervention; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; RP, rapid progression; STEMI, ST-segment elevation myocardial infarction; UAP, unstable angina pectoris.
Angiographic Characteristics
A follow-up CAG was performed at 10±3 months. The basic angiographic parameters are listed in Table 2 . No significant differences were noted between the groups. Approximately one-third of the patients had multivessel diseases. The majority of culprit lesions were treated with second-generation drug-eluting stent implantation. Binary restenosis occurred in 10 patients (7%).
Table 2. Angiographic characteristics.
| Variable | RP group n = 29 | Non-RP group n = 113 | p value |
|---|---|---|---|
| Culprit vessel | 0.27 | ||
| LMT | 1 (3.5%) | 2 (1.8%) | |
| LAD | 17 (58.6%) | 58 (51.3%) | |
| LCX | 0 (0.0%) | 13 (11.5%) | |
| RCA | 11 (37.1%) | 40 (35.4%) | |
| No. diseased vessel | 0.92 | ||
| 1 | 19 (65.5%) | 72 (63.7%) | |
| 2 | 7 (24.1%) | 31 (27.4%) | |
| 3 | 3 (10.3%) | 10 (8.9%) | |
| Culprit lesion PCI variables | 0.59 | ||
| DES | 29 (100%) | 109 (96.5%) | |
| BMS | 0 (0.0%) | 1 (0.9%) | |
| POBA | 0 (0.0%) | 3 (2.7%) | |
| In stent restenosis in culprit lesion | 1 (3.5%) | 9 (8.0%) | 0.36 |
| CAG follow-up period | 10±3 | 10±3 | 0.75 |
Data are expressed as number or mean±standard deviation (SD). Abbreviations: BMS, bare metal stent; CAG, coronary angiography; DES, drug-eluting stent; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LMT, left main trunk; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; RCA, right coronary artery; RP, rapid progression.
Laboratory Findings
The laboratory findings at baseline and follow-up are compared in Table 3 . There was no significant difference at baseline in both groups. The LDL-c, non-HDL-c, lb-LDL-c, sd-LDL-c, apoB, MDA-LDL, and plasma glucose levels were significantly decreased at follow-up in both the groups, whereas the RL-c, eGFR, NTproBNP, hs-CRP, and IRI levels were significantly decreased only in the non-RP group. The sd-LDL-c, TG, RL-c, and apoC3 levels at follow-up were significantly higher in the RP group than in the non-RP group. Both absolute and percent changes in sd-LDL-C and RL-c were non-significantly modestly greater in the non-RP group, while the changes in TG and apoC3 were markedly different between the groups ( Supplementary Table 1 ) . The achievement rate of an LDL-c level of <70 mg/dL was similar in both the groups (41.4% vs. 43.4%, p =0.84).
Table 3. Laboratory characteristics.
| Variable | RP group n = 29 | Non-RP group n = 113 | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | p value (vs. baseline) | Baseline | Follow-up | p value (vs. baseline) | |
| TG, mg/dL | 114 (76-160.5) | 138 (96-192.5) * | 0.26 | 109 (55.5-162.5) | 99 (71-145) | 0.36 |
| LDL-c, mg/dL | 131.5±37.7 | 80.0±20.5 | <0.0001 | 122.6±39.0 | 75.5±20.0 | <0.0001 |
| HDL-c, mg/dL | 49.6±16.1 | 50.1±15.2 | 0.91 | 46.9±11.4 | 48.8±12.3 | 0.23 |
| Non-HDL-c, mg/dL | 154.9±40.3 | 107.3±22.0 | <0.0001 | 146.6±41.1 | 99.0±21.7 | <0.0001 |
| lb-LDL-c, mg/dL | 93.6±36.0 | 53.1±16.3 | <0.0001 | 85.9±36.0 | 53.1±16.6 | <0.0001 |
| sd-LDL-c, mg/dL | 37.9±13.7 | 26.9±9.6 * | 0.0008 | 36.6±20.5 | 22.4±8.9 | <0.0001 |
| apolipoprotein A1, mg/dL | 127.7±32.0 | 135.7±25.3 | 0.3 | 123.2±22.5 | 130.4±23.2 | 0.018 |
| apolipoprotein B, mg/dL | 106.7±23.2 | 77.2±15.2 | <0.0001 | 99.4±28.5 | 71.5±15.6 | <0.0001 |
| apolipoprotein C3, mg/dL | 9.9 (7.5-12.6) | 10.1 (8.4-13.0) * | 0.06 | 8.9 (6.6-11.1) | 8.7 (7.1-11) | 0.41 |
| RL-c, mg/dL | 5.8 (3.7-8.0) | 4.3 (2.7-6.9) * | 0.12 | 5.0 (3.4-7.5) | 3.1 (2.1-4.9) | <0.0001 |
| MDA-LDL, U/L | 127.9±43.3 | 90.9±30.4 | 0.0004 | 128.1±55.1 | 87.6±29.2 | <0.0001 |
| lipoprotein(a), mg/dL | 14.1 (7.6-26) | 14.4 (8.7-38.4) | 0.44 | 13 (5-23) | 16 (7-31.5) | 0.12 |
| HbA1c(N), % | 6.1±1.0 | 6.2±0.8 | 0.75 | 6.4±1.3 | 6.3±0.8 | 0.8 |
| eGFR, mL/min/1.73 m 2 | 76.7±20.8 | 66.9±19.0 | 0.06 | 75.5±23.0 | 67.8±15.8 | 0.004 |
| NT-pro BNP, pg/mL | 229 (82-816) | 166 (94.5-316) | 0.37 | 255 (64.5-839) | 146 (72-345) | 0.025 |
| hs-CRP, mg/dL | 0.123 (0.033-0.384) | 0.064 (0.038-0.101) | 0.18 | 0.144 (0.057-0.380) | 0.055 (0.029-0.106) | <0.0001 |
| Glucose, mg/dL | 144±58 | 115±29 | 0.009 | 150±58 | 113±29 | <0.0001 |
| IRI, µU/mL | 15 (8-36) | 9.85 (5.6-13.8) | 0.02 | 12.2 (6.9-20.2) | 7.1 (4.8-11.6) | <0.0001 |
Data are expressed as mean±standard deviation (SD), or median (25% and 75% quartiles). * p <0.05, compared with corresponding Non-RP group by unpaired t -test or Wilcoxon’s signed-rank test. Abbreviations: apo A1, apolipoprotein A1; apo B, apolipoprotein B; apo C3, apolipoprotein C3; eGFR, estimated glomerular filtration rate; HbAc1, glycated hemoglobin; HDL-c, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IRI, immunoreactive insulin; LDL-c, low-density lipoprotein cholesterol; lb-LDL-c, large buoyant low-density lipoprotein cholesterol; MDA-LDL, malondialdehyde-modified low-density lipoprotein; non-HDL-c, non-high-density lipoprotein cholesterol; NTproBNP, N-terminal pro-brain natriuretic peptide; RL-c, remnant lipoprotein cholesterol; RP, rapid progression; sd-LDL-c, small dense low-density lipoprotein cholesterol; TG, triglyceride.
Supplementary Table 1. Baseline and follow-up lipid markers.
| Variable | RP group n = 29 | Non-RP group n = 113 | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | p value (vs. baseline) | Baseline | Follow-up | p value (vs. baseline) | |
| TG, mg/dL | 114 (76 - 160.5) | 138 (96 - 192.5) * | 0.26 | 109 (55.5 - 162.5) | 99 (71 - 145) | 0.36 |
| Change in TG , mg/dL | 19 (-15 - 57) * | -9 (-51.5 - 21.5) | ||||
| Percent of change in TG, % | 25.0 (-12.4 - 50.9) * | -9.7 (-31.3 - 33.6) | ||||
| LDL-c, mg/dL | 131.5±37.7 | 80.0±20.5 | <0.0001 | 122.6±39.0 | 75.5±20.0 | <0.0001 |
| Change in LDL-c, mg/dL | -51.5±40.5 | -47.1±41.4 | ||||
| Percent of change in LDL-c, % | -34.8±23.7 | -33.2±24.9 | ||||
| Non-HDL-c, mg/dL | 154.9±40.3 | 107.3±22.0 | <0.0001 | 146.6±41.1 | 99.0±21.7 | <0.0001 |
| Change in non-HDL-c, mg/dL | -47.7±42.7 | -47.5±43.6 | ||||
| Percent of change in non-HDL-c, % | -26.6±22.9 | -28.0±22.7 | ||||
| Sd-LDL-c, mg/dL | 37.9±13.7 | 26.9±9.6 * | 0.0008 | 36.6±20.5 | 22.4±8.9 | <0.0001 |
| Change in sd-LDL-c, mg/dL | -11.0±15.1 | -14.2±19.7 | ||||
| Percent of change in sd-LDL-c, % | -22.3±31.0 | -26.5±35.0 | ||||
| Apo B, mg/dL | 106.7±23.2 | 77.2±15.2 | <0.0001 | 99.4±28.5 | 71.5±15.6 | <0.0001 |
| Change in Apo B, mg/dL | -28.4±26.4 | -28.8±28.6 | ||||
| Percent of change in Apo B, % | -23.9±20.2 | -24.6±22.5 | ||||
| Apo C3, mg/dL | 9.9 (7.5 - 12.6) | 10.1 (8.4 - 13.0) * | 0.06 | 8.8 (7.1 - 11) | 8.7 (7.1 - 11) | 0.41 |
| Change in Apo C3, mg/dL | 1.8 (-0.2 - 3.6) * | -0.2 (-2.4 - 1.4) | ||||
| Percent of change in Apo C3, % | 18.2 (-0.8 - 45.4) * | -2.2 (-21.4 - 16.6) | ||||
| RL-c, mg/dL | 5.8 (3.7 - 8.0) | 4.3 (2.7 - 6.9) * | 0.12 | 5.0 (3.4 - 7.5) | 3.1 (2.1 - 4.9) | <0.0001 |
| Change in RL-c, mg/dL | -1.2 (-3.3 - 0.15) | -1.6 (-3.9 - -0.1) | ||||
| Percent of change in RL-c, % | -26.7 (-49.6 - -3.9) | -33.3 (-58.0 - -1.5) | ||||
Data are expressed as mean±standard deviation (SD), or median (25% and 75% quartiles). * p <0.05, compared with corresponding Non-RP group by unpaired t -test or Wilcoxon’s signed-rank test.
Correlation analyses between the levels of sd-LDL-c and other lipid markers at baseline and follow-up revealed that sd-LDL-c level was significantly related to the levels of LDL-c, non-HDL-c, TG, RL-c, and apoC3 ( Supplementary Table 2 ) . According to the simple regression analysis, LDL-c (mg/dL)=46.17+1.296×sd-LDL-c (mg/dL) and non-HDL-c (mg/dL)=58.26+1.812×sd-LDL-c (mg/dL).
Supplementary Table 2. Correlation coefficients between lipid markers at baseline and follow-up.
| baseline | LDL-c | non-HDL-c | TG | RL-c | apoC3 | hs-CRP |
|---|---|---|---|---|---|---|
| sd-LDL-c | 0.390 | 0.535 | 0.462 | 0.478 | 0.495 | -0.092 |
| LDL-c | ----- | 0.949 | 0.040 | 0.228 | 0.150 | -0.105 |
| non-HDL-c | ----- | ----- | 0.288 | 0.462 | 0.381 | -0.081 |
| TG | ----- | ----- | ----- | 0.867 | 0.738 | -0.009 |
| RL-c | ----- | ----- | ----- | ----- | 0.729 | -0.106 |
| apoC3 | ----- | ----- | ----- | ----- | ----- | -0.027 |
| follow-up | LDL-c | non-HDL-c | TG | RL-c | apoC3 | hs-CRP |
| sd-LDL-c | 0.590 | 0.759 | 0.546 | 0.459 | 0.526 | -0.102 |
| LDL-c | ----- | 0.887 | -0.031 | -0.019 | 0.056 | -0.140 |
| non-HDL-c | ----- | ----- | 0.353 | 0.370 | 0.378 | -0.146 |
| TG | ----- | ----- | ----- | 0.912 | 0.726 | -0.042 |
| RL-c | ----- | ----- | ----- | ----- | 0.667 | -0.060 |
| apoC3 | ----- | ----- | ----- | ----- | ----- | -0.028 |
The sd-LDL-c level is significantly related to the levels of LDL-c, non-HDL-c, TG, RL-c, and apoC3. Furthermore, TG is significantly related to RL-c and apo C3. Abbreviations: apo C3, apolipoprotein C3; LDL-c, low-density lipoprotein cholesterol; non-HDL-c, non-high-density lipoprotein cholesterol; RL-c, remnant lipoprotein cholesterol; sd-LDL-c, small dense low-density lipoprotein cholesterol; TG, triglyceride.
Prediction of RP and CEs
ROC analysis revealed the optimal cutoff values of lipid biomarkers that predicted RP ( Fig.1 ) . The levels of sd-LDL-c, TG, apoC3, and RL-c at follow-up that predicted RP were 20.9 mg/dL (AUC: 0.652, p =0.021; sensitivity: 75.9%; specificity: 51.3%), 113 mg/dL (AUC: 0.652, p =0.06; sensitivity: 69%; specificity: 62.8%), 9.7 mg/dL (AUC: 0.663; p =0.012; sensitivity: 69%; specificity: 61%), and 5.5 mg/dL (AUC: 0.641, p =0.007; sensitivity: 41.4%; specificity: 82.3%), respectively. On multivariate logistic regression model analysis including sd-LDL-c, RL-c, LDL-c, TG, and non-HDL-c showed that only an sd-LDL-c level of ≥ 20.9 mg/dL (odds ratio [OR], 3.50; 95% confidence interval [CI]: 1.07–11.48; p =0.039) at follow-up was a powerful predictor for RP of the culprit lesion independent of higher RL-c and achievement of target levels of LDL-c, TG, and non-HDL-c ( Table 4 ) . However, higher sd-LDL-c levels failed to be an independent predictor for RP when apo C3 was included in the multivariate analysis.
Fig.1. Receiver operating characteristic (ROC) curves for predicting rapid progression (RP).
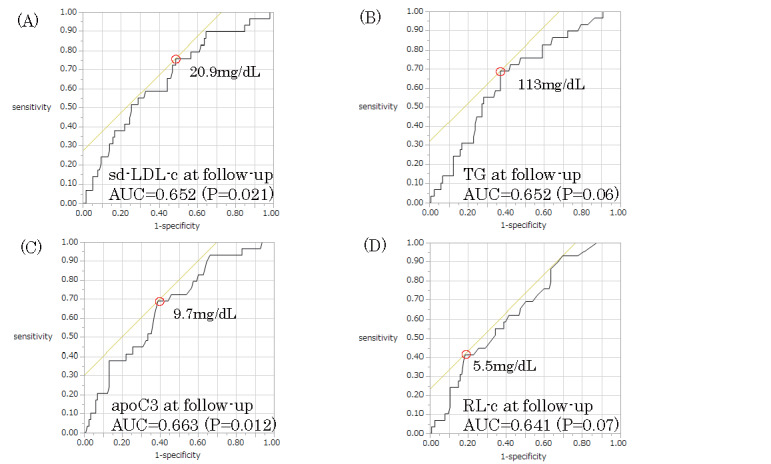
Based on ROC analysis, the optimal thresholds for predicting RP are as follows: follow-up small dense low-density lipoprotein cholesterol (sd-LDL-c) level=20.9 mg/dL (A) follow-up triglyceride (TG) level=113 mg/dL (B), follow-up apolipoprotein C3 (apo C3) level=9.7 mg/dL (C), and remnant lipoprotein cholesterol (RL-c) level=5.5 mg/dL (D).
Table 4. Univariate and Multivariate logistic regression analyses as predictors for the RP.
| Univariate analysis | Mutivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Sd-LDL-c ≥ 20.9 mg/dL | 2.65 | 1.21 - 5.81 * | 3.37 | 1.06 - 10.74 * | 3.50 | 1.07 - 11.48 * | 2.68 | 0.83 - 8.71 |
| Apo C3 ≥ 9.7 mg/dL | 2.71 | 1.32 - 5.53 ** | - | - | - | - | 2.91 | 1.01 - 8.34 * |
| RL-c ≥ 5.5 mg/dL | 2.33 | 1.24 - 4.37 * | - | - | 4.47 | 0.94 - 21.21 | - | - |
| LDL-c ≥ 70 mg/dL | 1.10 | 0.56 - 2.15 | 0.52 | 0.14 - 2.00 | 0.59 | 0.15 - 2.27 | 0.51 | 0.13 - 1.98 |
| TG ≥ 150 mg/dL | 1.67 | 0.87 - 3.21 | 1.02 | 0.37 - 2.77 | 0.33 | 0.07 - 1.67 | 0.61 | 0.20 - 1.87 |
| Non-HDL-c ≥ 100 mg/dL | 1.59 | 0.81 - 3.12 | 1.53 | 0.39 - 6.08 | 1.26 | 0.31 - 5.11 | 1.49 | 0.38 - 5.91 |
Model 1 was adjusted for sd-LDL-c, LDL-c, TG, and non-HDL-c.
Model 2 was adjusted for sd-LDL-c, RL-c, LDL-c, TG, and non-HDL-c. Model 3 was adjusted for sd-LDL-c, apo C3, LDL-c, TG, and non-HDL-c. OR, odds ratio: CI, confidence interval. * p < 0.05. ** p <0.01.
Although CEs occurred in 30 (21.1%) patients (non-fatal MI, 2 patients; stroke, 3 patients; and revascularization, 25 patients [TLR: 9 patients, TVR: 2 patients, and non-TVR: 14 patients]) during the 31±17 months of follow-up, no cardiac death occurred ( Supplementary Table 3 ) . Patients with RP had a significantly increased risk of CEs (log-rank 14.878, p <0.0001; Fig.2 ). The Kaplan–Meier event-free survival curves were analyzed using the cutoff values for predicting RP. The analysis revealed that patients with an sd-LDL-c level of ≥ 20.9 mg/dL at follow-up had a significantly increased risk of CEs (log-rank 4.123, p =0.043; Fig.3 ). Total revascularization, especially non-TVR, was performed more often in the group with high sd-LDL-c during the follow-up period ( Supplementary Table 4 ) .
Supplementary Table 3. Comparison of cardiovascular events between the RP and non-RP groups.
| Variable |
RP group n = 29 |
non-RP group n = 113 |
p value |
|---|---|---|---|
| cardiac death | 0 (0%) | 0 (0%) | not detectable |
| non fatal MI | 1 (3.5%) | 1 (0.9%) | 0.35 |
| stroke | 1 (3.5%) | 2 (1.8%) | 0.6 |
| total revascularization | 11 (37.9%) | 14 (12.4%) | 0.001 |
| TLR | 0 (0%) | 9 (8.0%) | 0.11 |
| TVR | 0 (0%) | 2 (1.8%) | 0.47 |
| non-TVR | 11 (37.9%) | 3 (2.7%) | 0.0001 |
Abbreviations: MI, myocardial infarction; RP, rapid progression; TLR, target lesion revascularization; TVR, target vessel revascularization.
Fig.2. Kaplan–Meier event-free survival curves.
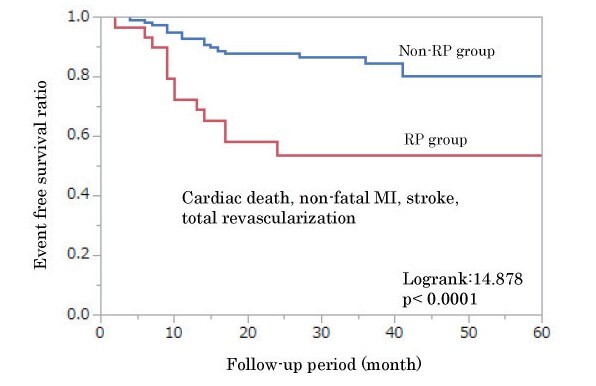
Patients are divided into two groups based on rapid progression (RP). Patients with RP have a significantly increased risk of cardiovascular events (CEs; log-rank 14.878, p <0.0001).
Fig.3. Kaplan–Meier event-free survival curves.
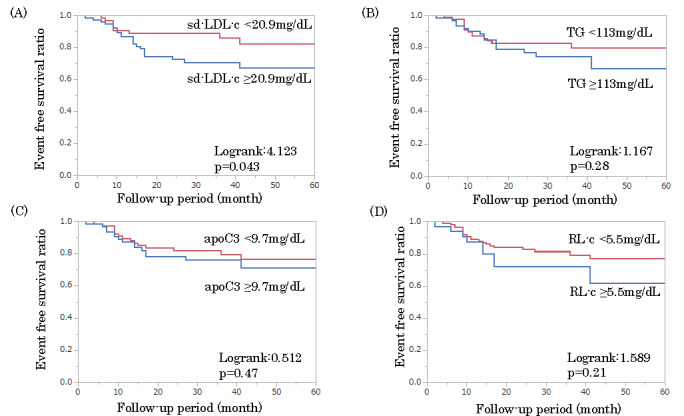
Patients are divided into two groups based on the cutoff value of rapid progression (RP): small dense low-density lipoprotein cholesterol (sd-LDL-c), 20.9 mg/dL; triglyceride (TG), 113 mg/dL; apolipoprotein C3 (apo C3), 9.7 mg/dL; and remnant lipoprotein cholesterol (RL-c), 5.5 mg/dL. Patients with an sd-LDL-c level of ≥ 20.9 mg/dL at follow-up have a significantly increased risk of cardiovascular events (CEs; log-rank 4.123, p =0.043).
Supplementary Table 4. Comparison of cardiovascular events between the high sd-LDL-c and low sd-LDL-c groups.
| Variable |
high sd-LDL-c group n = 77 |
low sd-LDL-s group n = 65 |
p value |
|---|---|---|---|
| cardiac death | 0 (0%) | 0 (0%) | not detectable |
| non fatal MI | 1 (1.3%) | 1 (1.5%) | 0.9 |
| stroke | 2 (2.6%) | 1 (1.5%) | 0.66 |
| total revascularization | 18 (23.4%) | 7 (10.8%) | 0.04 |
| TLR | 4 (5.2%) | 5 (7.7%) | 0.54 |
| TVR | 2 (2.6%) | 0 (0%) | 0.12 |
| non-TVR | 12 (15.6%) | 2 (3.1%) | 0.008 |
Total revascularization, especially non-TVR, was performed more often in the high sd-LDL-c group during the follow-up period. Abbreviations: MI, myocardial infarction; RP, rapid progression; sd-LDL-c, small dense low-density lipoprotein cholesterol; TLR, target lesion revascularization; TVR, target vessel revascularization
Discussion
This study presents four novel findings. First, the levels of sd-LDL-c and three TRL biomarkers (TG, RL-c, and apoC3) at follow-up are effective predictors of the RP of non-culprit lesions. Second, the presence of RP lesions is significantly associated with a higher incidence of 3-year CEs. Third, among the four lipid biomarkers, the only predictive factor for 3-year CEs in ACS patients was an sd-LDL-c level above the cutoff value (≥ 20.9 mg/dL). Fourth, none of the lipid biomarkers measured at the onset of ACS in the present study could predict RP and CEs after ACS.
The significant association between sd-LDL-c level and CEs is consistent with the findings of our two previous cohort studies that included patients with stable CAD and used two different assays for sd-LDL-c level measurement 24 , 25) . In the cohort of elderly male patients with stable CAD, a significant association was observed between sd-LDL-c levels and incident CEs in diabetic and non-diabetic patients, statin users and in patients with hypertriglyceridemia 25) . This study revealed a new mechanism of CEs, especially non-TVR, which is considered under RP due to the sd-LDL-c level. A previous study of 136 patients with stable CAD who underwent CAG at baseline and follow-up demonstrated a positive association between sd-LDL levels, which was determined by four different laboratory methods, and angiographic progression of CAD, after adjusting for the LDL-c, HDL-c, and TG levels 37) . In addition, the study indicated that this association was significantly greater for the follow-up sd-LDL-c levels than the baseline sd-LDL-c levels 37) . A previous retrospective study of 332 patients with stable angina who underwent PCI reported that higher levels of MDA-LDL, an oxidized LDL, before PCI were significantly associated with CEs during the follow-up period (median follow-up: 2.9 years) 38) . On the other hand, the present study failed to demonstrate an association between MDA-LDL and RP. The discrepancies in the findings might be due to differences in the study population and clinical outcomes.
This is the first study to show a positive association between the on-treatment TG, apoC3, and RL-c levels and RP. These three markers are strongly correlated with each other. ApoC3 is closely associated with TG metabolism and is a key regulator of fasting and non-fasting TG levels 39) . Both the CAD risk and TG level were up to 30%–40% lower in the carriers than in the non-carriers of apoC3 gene mutation 40) . A prospective study of 190 patients treated with statins for 3 months after ACS revealed that a high RLP-C level (≥ 5.4 mg/dL) was significantly associated with CEs during the follow-up period (mean follow-up: 30 months) 41) . In addition, our previous cohort study demonstrated that the RLP-C level was significantly higher in patients with CEs than in those without CEs; however, the age-adjusted Cox regression analysis did not support the significant association between the RLP-C level and CE occurrence 25) . In the RP group, both TG and apoC3 marginally increased at follow-up than at baseline. In the non-RP group, the RL-c level was significantly lower at follow-up than at baseline. These results suggest that TRL accumulation plays an important role in RP. The sd-LDL-c level was significantly correlated with the levels of both LDL-c and TRL (TG, apoC3, and RL-c), whereas the TRL level was not correlated with the LDL-c level. These differences suggest that only the sd-LDL-c level, not the TRL level, at follow-up is associated with CEs after ACS. Thus, future studies with a large sample size are required to evaluate the association between the TRL levels and CEs in patients.
The lb-LDL-c levels at follow-up were similar between the two groups. This is in good agreement with the findings of our previous studies 22 - 24) and a general prospective study 42) . Thus, intensive sd-LDL-c-lowering may be necessary for both primary and secondary prevention of CAD. A simple regression analysis revealed that an sd-LDL-c level of 20.8 mg/dL was equivalent to approximately 73 mg/dL of LDL-c and 96 mg/dL of non-HDL-c. These levels are consistent with the target levels of LDL-c (<70 mg/dL) and non-HDL-c (<100 mg/dL) mentioned in the current guidelines 21 , 43) . According to the 2019 European Society of Cardiology (ESC) guidelines 44) , the target levels of LDL-c and non-HDL-c for very high-risk patients are <55 mg/dL and <85 mg/dL, respectively; thus, the target value of sd-LDL-c after the input of these values in the formulas is estimated to be approximately 5–15 mg/dL. A secondary analysis of the FOURIER trial with proprotein convertase subtilisin/kexin type 9 inhibitor evolocumab in patients with a high CAD risk demonstrated a monotonic relationship between the achieved LDL-c level and CEs, down to an LDL-c level of <7.7 mg/dL 45) . This suggests that an intensive sd-LDL-c reduction up to zero level, might prevent the progression of coronary atherosclerosis. Future studies with a large sample size are required to evaluate the association between extremely low levels of sd-LDL-c and CEs in patients.
A high prevalence of sd-LDL is a main feature of pre-diabetic and diabetic dyslipidemia and insulin resistance 18 , 46) . These disorders exhibit postprandial glucose variability. Previous studies have demonstrated that glucose variability is associated with the RP of non-culprit lesions 47) and coronary plaque vulnerability in ACS patients 48 , 49) . The association between the sd-LDL-c level and RP might be due to glucose variability. In addition, the sd-LDL-c level was reported to be associated with thrombosis and inflammation 50) . These factors also affect coronary atherosclerosis. However, the present study failed to demonstrate an association between the sd-LDL-c level or the TRL level and hs-CRP at both baseline and follow-up. Future studies are required to evaluate the association between glucose variability, RP, and the levels of sd-LDL-c, TRL, and thrombotic and inflammatory markers.
This study has a few limitations. First, this was a single-center study with relatively small sample size. Hence, multicenter studies with large sample sizes are required to investigate whether CEs can be reduced with interventions for sd-LDL-c reduction. Second, morphological assessment of non-culprit lesions using intravascular imaging was not performed in all patients. Previous studies have suggested that positive remodeling, vulnerable plaque, and/or micro-vessels might play an important role in lesion progression. Future studies should validate the relationship between the morphological changes in plaque progression and lipid profiles at various time points. Third, the relationship between lipid profiles and inflammation markers was not assessed 50) .
Conclusion
The on-treatment sd-LDL-c, TG, apoC3, and RL-c levels are associated with the RP of non-culprit lesions after ACS. A higher sd-LDL-c level may predict RP and CEs after ACS. Therefore, on-treatment sd-LDL-c is a residual risk and aggressive reduction of the sd-LDL-c level may be essential to improve the long-term clinical outcomes in ACS patients. A large cohort study is required to determine the appropriate target level of sd-LDL-c.
Conflict of Interest
Shinji Koba received research funding from Denka Seiken Co., Ltd. The other authors have no conflicts of interest to declare.
Financial Support
The funding agencies had no role in the preparation of the manuscript.
Acknowledgments
We are grateful for the valuable help of the nursing staff of the catheterization laboratory and all cardiologists at the Department of Cardiology of the Showa University Hospital for this study. We also gratefully acknowledge Motoko Ohta, Yasuki Itoh, Kei Iwadate, and Maki Namatame for their technical assistance in this study. We would like to thank Editage (www.editage.com) for English language editing.
References
- 1).Larsen AI, Tomey MI, Mehran R, Nilsen DW, Kirtane AJ, Witzenbichler B, Guagliumi G, Brener SJ, Genereux P, Kornowski R, Dudek D, Gersh BJ and Stone GW: Comparison of outcomes in patients with ST-segment elevation myocardial infarction discharged on versus not on statin therapy (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol, 2014; 113: 1273-1279 [DOI] [PubMed] [Google Scholar]
- 2).Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT and Group ESCSD: 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J, 2016; 37: 2999-3058 [Google Scholar]
- 3).Asakura M, Ueda Y, Yamaguchi O, Adachi T, Hirayama A, Hori M and Kodama K: Extensive development of vulnerable plaques as a pan-coronary process in patients with myocardial infarction: an angioscopic study. J Am Coll Cardiol, 2001; 37: 1284-1288 [DOI] [PubMed] [Google Scholar]
- 4).Rioufol G, Finet G, Ginon I, Andre-Fouet X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF and Tabib A: Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation, 2002; 106: 804-808 [DOI] [PubMed] [Google Scholar]
- 5).Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW and Investigators P: A prospective natural-history study of coronary atherosclerosis. N Engl J Med, 2011; 364: 226-235 [DOI] [PubMed] [Google Scholar]
- 6).Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H and Daida H: Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation, 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 7).Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M and Investigators J-A: Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302 [DOI] [PubMed] [Google Scholar]
- 8).Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T, Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K, Oshima S, Kaikita K, Hokimoto S, Ogawa H and Investigators P-I: Impact of Dual Lipid-Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: The Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol, 2015; 66: 495-507 [DOI] [PubMed] [Google Scholar]
- 9).Endo H, Dohi T, Miyauchi K, Kuramitsu S, Kato Y, Okai I, Yokoyama M, Yokoyama T, Ando K, Okazaki S, Shimada K, Suwa S and Daida H: Clinical significance of non-culprit plaque regression following acute coronary syndrome: A serial intravascular ultrasound study. J Cardiol, 2019; 74: 102-108 [DOI] [PubMed] [Google Scholar]
- 10).Puri R, Nissen SE, Shao M, Elshazly MB, Kataoka Y, Kapadia SR, Tuzcu EM and Nicholls SJ: Non-HDL Cholesterol and Triglycerides: Implications for Coronary Atheroma Progression and Clinical Events. Arterioscler Thromb Vasc Biol, 2016; 36: 2220-2228 [DOI] [PubMed] [Google Scholar]
- 11).Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM, Jr., Ridker PM and Kastelein JJ: Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA, 2012; 307: 1302-1309 [DOI] [PubMed] [Google Scholar]
- 12).Schwartz GG, Abt M, Bao W, Demicco D, Kallend D, Miller M, Mundl H and Olsson AG: Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. Journal of the American College of Cardiology, 2015; 65: 2267-2275 [DOI] [PubMed] [Google Scholar]
- 13).Suzuki K, Oikawa T, Nochioka K, Miura M, Kasahara S, Sato M, Aoyanagi H, Shiroto T, Takahashi J, Miyata S, Sakata Y and Shimokawa H: Elevated Serum Non-HDL (High-Density Lipoprotein) Cholesterol and Triglyceride Levels as Residual Risks for Myocardial Infarction Recurrence Under Statin Treatment. Arterioscler Thromb Vasc Biol, 2019; 39: 934-944 [DOI] [PubMed] [Google Scholar]
- 14).Hayashi T, Koba S, Ito Y and Hirano T: Method for estimating high sdLDL-C by measuring triglyceride and apolipoprotein B levels. Lipids Health Dis, 2017; 16: 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ and Shepherd J: Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis, 1994; 106: 241-253 [DOI] [PubMed] [Google Scholar]
- 16).Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D’Agostino RB, Vasan RS and Robins SJ: Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation, 2006; 113: 20-29 [DOI] [PubMed] [Google Scholar]
- 17).Ban Y, Koba S, Tsunoda F, Yokota Y, Ezumi H, Kondo T, Suzuki H and Katagiri T: Predominance of small dense low-density lipoproteins and abnormal glucose regulation in patients with acute coronary syndrome. Circ J, 2006; 70: 393-401 [DOI] [PubMed] [Google Scholar]
- 18).Hsu H, Hsu P, Cheng MH, Ito Y, Kanda E, Schaefer EJ and Ai M: Lipoprotein Subfractions and Glucose Homeostasis in Prediabetes and Diabetes in Taiwan. J Atheroscler Thromb, 2019; 26: 890-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Koba S, Tsunoda F, Hirano T, Iso Y, Suzuki H, Geshi E and Katagiri T: Postprandial changes in LDL phenotypes in patients with myocardial infarction. Eur J Clin Invest, 2005; 35: 171-179 [DOI] [PubMed] [Google Scholar]
- 20).Okazaki M, Usui S, Ishigami M, Sakai N, Nakamura T, Matsuzawa Y and Yamashita S: Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol, 2005; 25: 578-584 [DOI] [PubMed] [Google Scholar]
- 21).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for E and Clinical Management of A: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, Iso Y, Suzuki H and Katagiri T: Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis, 2006; 189: 206-214 [DOI] [PubMed] [Google Scholar]
- 23).Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, Kobayashi Y and Katagiri T: Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb, 2008; 15: 250-260 [DOI] [PubMed] [Google Scholar]
- 24).Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T and Kobayashi Y: Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb, 2014; 21: 755-767 [DOI] [PubMed] [Google Scholar]
- 25).Sakai K, Koba S, Nakamura Y, Yokota Y, Tsunoda F, Shoji M, Itoh Y, Hamazaki Y and Kobayashi Y: Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr Gerontol Int, 2018; 18: 965-972 [DOI] [PubMed] [Google Scholar]
- 26).Higashioka M, Sakata S, Honda T, Hata J, Yoshida D, Hirakawa Y, Shibata M, Goto K, Kitazono T, Osawa H and Ninomiya T: Small Dense Low-Density Lipoprotein Cholesterol and the Risk of Coronary Heart Disease in a Japanese Community. J Atheroscler Thromb, 2020; 27: 669-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Higashioka M, Sakata S, Honda T, Hata J, Shibata M, Yoshida D, Goto K, Kitazono T, Osawa H and Ninomiya T: The Association of Small Dense Low-Density Lipoprotein Cholesterol and Coronary Heart Disease in Subjects at High Cardiovascular Risk. Journal of Atherosclerosis and Thrombosis, 2021; 28: 79-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A and Matsuo S: Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol, 2009; 13: 621-630 [DOI] [PubMed] [Google Scholar]
- 29).Nakajima K, Saito T, Tamura A, Suzuki M, Nakano T, Adachi M, Tanaka A, Tada N, Nakamura H, Campos E and et al.: Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta, 1993; 223: 53-71 [DOI] [PubMed] [Google Scholar]
- 30).Miyauchi K, Kayahara N, Ishigami M, Kuwata H, Mori H, Sugiuchi H, Irie T, Tanaka A, Yamashita S and Yamamura T: Development of a homogeneous assay to measure remnant lipoprotein cholesterol. Clin Chem, 2007; 53: 2128-2135 [DOI] [PubMed] [Google Scholar]
- 31).Masuda D and Yamashita S: Postprandial Hyperlipidemia and Remnant Lipoproteins. J Atheroscler Thromb, 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Ito Y, Fujimura M, Ohta M and Hirano T: Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 33).Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J and Rifai N: Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem, 2001; 47: 418-425 [PubMed] [Google Scholar]
- 34).Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML and Roe BB: A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation, 1975; 51: 5-40 [DOI] [PubMed] [Google Scholar]
- 35).Zouridakis EG, Schwartzman R, Garcia-Moll X, Cox ID, Fredericks S, Holt DW and Kaski JC: Increased plasma endothelin levels in angina patients with rapid coronary artery disease progression. Eur Heart J, 2001; 22: 1578-1584 [DOI] [PubMed] [Google Scholar]
- 36).Nakachi T, Kosuge M, Hibi K, Ebina T, Hashiba K, Mitsuhashi T, Endo M, Umemura S and Kimura K: C-reactive protein elevation and rapid angiographic progression of nonculprit lesion in patients with non-ST-segment elevation acute coronary syndrome. Circ J, 2008; 72: 1953-1959 [DOI] [PubMed] [Google Scholar]
- 37).Williams P, Zhao X-Q, Marcovina S, Otvos J, Brown BG and Krauss R: Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis (Amsterdam), 2014; 233: 713-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Ito T, Fujita H, Tani T and Ohte N: Malondialdehyde-modified low-density lipoprotein is a predictor of cardiac events in patients with stable angina on lipid-lowering therapy after percutaneous coronary intervention using drug-eluting stent. Atherosclerosis (Amsterdam), 2015; 239: 311-317 [DOI] [PubMed] [Google Scholar]
- 39).Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM and Witztum JL: Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med, 2014; 371: 2200-2206 [DOI] [PubMed] [Google Scholar]
- 40).Tg, Hdl Working Group of the Exome Sequencing Project NHL, Blood I, Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O’Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E and Kathiresan S: Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med, 2014; 371: 22-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Nguyen SV, Nakamura T and Kugiyama K: High remnant lipoprotein predicts recurrent cardiovascular events on statin treatment after acute coronary syndrome. Circ J, 2014; 78: 2492-2500 [DOI] [PubMed] [Google Scholar]
- 42).Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E and Ballantyne CM: Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS and Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 139: e1082-e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O and Group ESCSD: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J, 2020; 41: 111-188 [DOI] [PubMed] [Google Scholar]
- 45).Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni-Berthold I, Lopez-Miranda J, Schiele F, Mach F, Ott BR, Kanevsky E, Pineda AL, Somaratne R, Wasserman SM, Keech AC, Sever PS, Sabatine MS and Investigators F: Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet, 2017; 390: 1962-1971 [DOI] [PubMed] [Google Scholar]
- 46).Hirano T: Pathophysiology of Diabetic Dyslipidemia. J Atheroscler Thromb, 2018; 25: 771-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Kataoka S, Gohbara M, Iwahashi N, Sakamaki K, Nakachi T, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, Ebina T, Umemura S and Kimura K: Glycemic Variability on Continuous Glucose Monitoring System Predicts Rapid Progression of Non-Culprit Lesions in Patients With Acute Coronary Syndrome. Circ J, 2015; 79: 2246-2254 [DOI] [PubMed] [Google Scholar]
- 48).Kuroda M, Shinke T, Sakaguchi K, Otake H, Takaya T, Hirota Y, Osue T, Kinutani H, Konishi A, Takahashi H, Terashita D, Uzu K and Hirata K: Association between daily glucose fluctuation and coronary plaque properties in patients receiving adequate lipid-lowering therapy assessed by continuous glucose monitoring and optical coherence tomography. Cardiovasc Diabetol, 2015; 14: 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Kuroda M, Shinke T, Sakaguchi K, Otake H, Takaya T, Hirota Y, Sugiyama D, Nakagawa M, Hariki H, Inoue T, Osue T, Taniguchi Y, Iwasaki M, Nishio R, Kinutani H, Konishi A, Hiranuma N, Takahashi H, Terashita D and Hirata KI: Effect of daily glucose fluctuation on coronary plaque vulnerability in patients pre-treated with lipid-lowering therapy: a prospective observational study. JACC Cardiovasc Interv, 2015; 8: 800-811 [DOI] [PubMed] [Google Scholar]
- 50).Hsu S, Jang M-H, Torng P-L and Su T-C: Positive Association Between Small Dense Low-Density Lipoprotein Cholesterol Concentration and Biomarkers of Inflammation, Thrombosis, and Prediabetes in Non-Diabetic Adults. J Atheroscler Thromb, 2019; 26: 624-635 [DOI] [PMC free article] [PubMed] [Google Scholar]


