Abstract
Objective:
Sarcoidosis is often treated with glucocorticoids, although the use of biologics is growing. Prescribing patterns for biologics for sarcoidosis patients in U.S. rheumatology practices have never been examined. Given that there are no steroid-sparing FDA-approved therapies for sarcoidosis, we sought to characterize the real-world treatment of sarcoidosis and to assess practice-level variation in prescribing patterns.
Methods:
We conducted an observational study of sarcoidosis patients using data from the Rheumatology Informatics System for Effectiveness (RISE) registry (2014–2018). The RISE registry represents an estimated 32% of the U.S. clinical rheumatology workforce. Adult patients with ≥ 2 codes for sarcoidosis ≥ 30 days apart were included. We examined sarcoidosis-specific medication use at any time during the study period. Data was analyzed at the practice level.
Results:
A total of 3276 patients with sarcoidosis from 184 practices were included. 75.1% were women; with mean age 59.0 ± 12.5; 48.3% were White and 27.6% were Black. Overall, 59.3% of patients were prescribed glucocorticoids; 24.7% received prolonged glucocorticoid therapy (≥10 mg/day for 90 days). 12.1% received a biologic or targeted synthetic disease modifying drug (tsDMARD), most commonly TNF inhibitors. There was wide practice-level variation among 31 practices with ≥ 30 sarcoidosis patients: biologic use ranged from 15.6% to 69.2%; infliximab represented the most common biologic prescribed.
Conclusion:
In a large sample of U.S. rheumatology practices, 12.1% of patients with sarcoidosis received biologics or tsDMARD. We found high variability in biologic use across practices. The significant use of long-term glucocorticoids suggests unmet therapeutic needs in this patient population.
Keywords: Sarcoidosis, practice patterns, anti-TNF drugs, glucocorticoids, health Care
Sarcoidosis is a rare multisystem disease of unknown etiology with adult-onset typically before the fourth decade. The prevalence of sarcoidosis in the United States (U.S.) is estimated to be 35.5 per 100,000 for African Americans and 10.9 per 100,000 for Caucasians (1). The natural history and prognosis of sarcoidosis can vary greatly, from mild and self-limited to severe disease that leads to significant organ impairment and death in 5% of patients (2,3).
The highly variable clinical features and disease course, together with lack of steroid-sparing pharmacologic treatments approved by the U.S. Food and Drug Administration (FDA), explain why treatment for sarcoidosis is not standardized. Current mainstays of treatment include glucocorticoids, disease modifying anti-rheumatic drugs (DMARDs), and tumor necrosis factor inhibitors (TNFi) for some manifestations (4). Glucocorticoids are considered first line treatment for most forms of sarcoidosis but can result in significant cumulative toxicity, even at relatively low doses (5). At present, there is significant variation in medication use for sarcoidosis patients and, as a result, in medical costs (6,7).
Literature on the real-world treatment of sarcoidosis is limited. One study reported that, among 1774 patients with sarcoidosis followed in a large university medical center, treatment was prescribed in 61% of patients, with 55.3% and 5.0% of the cohort receiving glucocorticoids and TNFi, respectively (8). This contrasts with a more recent study which included all patients with sarcoidosis from a large U.S. insurance claims database in which only 22.8% received any treatment (6).
In this study, we used data from the Rheumatology Informatics System for Effectiveness (RISE) registry, which represents an estimated 32% of the U.S. clinical rheumatology workforce, to examine treatment patterns for sarcoidosis. Understanding real-world medication use can identify areas of unmet therapeutic need and inform future clinical trials.
Patients and Methods
Study design and data source:
We performed an observational study using data derived from the RISE registry, a national electronic health record (EHR)-enabled rheumatology registry. RISE is a U.S. registry that passively collects data on all patients seen by participating practices, thereby reducing selection bias present in single insurer claims databases (9). Available data is collected through the EHR mainly from group and private practices across the U.S, and includes individual demographics, diagnoses, procedures, medications, laboratory test results, and vital signs. Rheumatology practices started contributing data to RISE as early as January 2014, but for some practices, many years of historical data is also available. As of 2018, RISE held validated data from 1113 providers in 226 practices with a total of 1,623,504 patients.
Study population and study period:
Patients included in this study were 18 years of age or older and had ≥2 diagnosis codes for sarcoidosis ≥ 30 days apart (N=4888; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 135 or (ICD-10-CM) D86.9) (10). We then excluded subjects who met administrative definitions for other autoimmune conditions (≥2 ICD codes over ≥ 30 days apart for rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, ankylosing spondylitis, inflammatory bowel disease, or psoriatic arthritis, N=1612) to increase the specificity of our case definition and because among patients with more than one condition, it was difficult to infer if drugs were prescribed for sarcoidosis or for the other rheumatic condition. This left 3276 patients with sarcoidosis in the study sample. The study period included all observation time available in the RISE registry.
Demographic / Covariates / Clinical information:
We extracted demographic data on participant age, sex, race/ethnicity, and geographic region (East North Central, West North Central, Mid-Atlantic, Mountain, New England, Pacific, South Atlantic, East South Central, and West South Central). Patients were classified as White, Black or African American, or other (which included Hispanic or Latino, Asian, Native Hawaiian or other Pacific Island, American Indian or Alaska Native, multi-racial, and patients with no race classification). Individual comorbidities examined included a diagnosis of diabetes, asthma, hypertension and cancer based on diagnosis codes at any time, as well as Charlson comorbidity index score based on codes from any time during the study period, calculated according to the Deyo modification (11).
We enumerated the frequencies of ICD-9-CM/ICD-10-CM codes used to specify sarcoidosis specific organ involvement for each patient where possible (Supplemental Table 1).
Medications:
Sarcoidosis-specific therapies used during the study period were examined. Systemic glucocorticoids included prednisone and other oral and intravenous steroids. Prolonged glucocorticoid therapy was defined as ≥ 10 mg of prednisone daily (or its equivalent) for at least three months (12). Conventional disease modifying anti-rheumatic drugs (cDMARDs) included: methotrexate, azathioprine, hydroxychloroquine, leflunomide, sulfasalazine, mycophenolate, cyclosporine, minocycline, and tacrolimus. Biologic DMARDs included TNFi (etanercept, adalimumab, infliximab, golimumab, certolizumab) and non-TNFi biologics (abatacept, rituximab, secukinumab, ustekinumab, omalizumab, anakinra, tocilizumab, sarilumab). Targeted synthetic DMARDs (tsDMARDs) included tofacitinib, baricitinib and apremilast.
Statistical analyses:
Data were presented as the mean ± standard deviation (± SD) or medians with interquartile ranges (IQR) for numerical variables and frequency (percents) for categorical variables. We compared sociodemographic characteristics of sarcoidosis patients to the overall RISE population. We also compared sarcoidosis patients with < 2 years vs. ≥ 2 years of follow-up using t-tests and chi-squared tests. The proportion of patients with sarcoidosis by practice was reported out of the total number of patients in the practice. Among practices with ≥ 30 sarcoidosis patients (“high-volume” practices), we calculated the proportion of patients receiving particular medications, out of the total number of sarcoidosis patients in the practice (practices with fewer than 30 sarcoidosis patients were excluded in order to reduce the random variation in medication use that would result from small practice sample sizes). Statistical significance was defined as P-value <0.05. Data analysis was performed using Stata statistical software version 15 (StataCorp). For privacy protections, we reported no cell sizes < 10. This study was approved by the UCSF and Western IRBs.
Results
Subject characteristics:
A total of 3276 unique sarcoidosis patients with a mean age of 59.1 years (SD=12.5) were included. 75.1% were female. Most patients (48.3%) were White, and 27.6% were Black. Sarcoidosis patients represented between 0.2%−1.8% of patients in the 184 practices included (median 0.2%). The median (IQR) follow-up time during the study period was 1.9 (0.6 – 4.2) years. The mean modified Charlson score was 1.3 (SD=0.9). Other characteristics are summarized in Table 1. Age, sex, and geographic distributions of sarcoidosis patients reflected the underlying population of patients in the RISE registry (Supplemental Table 2). As expected, the proportion of African American patients with sarcoid was significantly higher compared to the proportion in RISE overall (27.6% vs. 7.2%, p<0.001).
Table 1:
Characteristics of patients with sarcoidosis in the RISE registry.
| Characteristics mean±SD / N (%) |
Total patients (N=3276) |
||
|---|---|---|---|
| Age | 59.1 ± 12.5 | ||
| Sex | Female | 2461 | 75.1% |
| Race | White | 1582 | 48.3% |
| Black or African American | 905 | 27.6% | |
| Other* | 789 | 24.1% | |
| Insurance | Medicare | 803 | 24.5% |
| Medicaid | 115 | 3.5% | |
| Private | 1205 | 36.7% | |
| Other§ | 151 | 4.6% | |
| Missing | 1002 | 30.6% | |
| U.S. Geographic Division | East North Central | 107 | 3.3% |
| West North Central | 510 | 15.6% | |
| Mid-Atlantic | 384 | 11.7% | |
| Mountain | 299 | 9.1% | |
| New England | 874 | 26.7% | |
| Pacific | 396 | 12.1% | |
| South Atlantic | 321 | 9.8% | |
| East South Central | 69 | 2.1% | |
| West South Central | 316 | 9.6% | |
| Practice type | Single specialty group | 2205 | 67.3% |
| Multi-specialty group | 555 | 16.9% | |
| Solo practitioner | 289 | 8.8% | |
| Other clinical setting | 186 | 5.7% | |
| Health system | 41 | 1.2% | |
| Comorbidities | Charlson Score; mean±SD | 1.3 ± 0.9 | |
| Hypertension (y/n) | 545 | 16.6% | |
| Asthma (y/n) | 189 | 5.8% | |
| Diabetes (y/n) | 321 | 9.8% | |
| Cancer (y/n) | 177 | 5.4% | |
| Number of visits in RISE; median (IQR) | 3.5 (2–6.5) | ||
| Duration of follow up time (years); median (IQR) | 1.9 (0.6–4.2) | ||
Abbreviations: SD: standard deviation, IQR: interquartile range,
Other race included Hispanic or Latino, Asian, Native Hawaiian or other Pacific Island, American Indian or Alaska Native, and multi-racial,
Other insurance included veterans and other.
Most patients were identified using codes for “sarcoidosis-unspecified” (51.0%; Table 2). Among patients with codes for specific clinical manifestations, musculoskeletal involvement (joints, muscles, and bones) was most commonly coded (22.1%), followed by pulmonary (15.1%), ocular (6.3%), renal (6.3%), neurologic (5.3%), and cardiac manifestations (1.6%). Patients with more than 2 years of follow up were more likely to have received codes for specific disease manifestations (Supplemental Table 3). Rheumatoid factor and the anti-cyclic citrullinated peptide antibody were detected in 8.9% and 5.0%, respectively, among patients with results available as structured data.
Table 2:
Frequency of ICD codes for specific clinical manifestations among patients with sarcoidosis in the RISE registry.
| Parameters mean±SD / N (%) |
Total patients (N=3276) |
||
|---|---|---|---|
| Sarcoidosis clinical manifestations | Sarcoid, unspecified | 1671 | 51.0% |
| Musculoskeletal | 725 | 22.1% | |
| Pulmonary | 532 | 15.1% | |
| Ocular | 208 | 6.3% | |
| Renal | 207 | 6.3% | |
| Neurologic | 175 | 5.3% | |
| Skin | 131 | 4.0% | |
| Cardiac | 54 | 1.6% | |
| Lymph | 23 | 0.7% | |
| Positive RF* (y/n) | 66 | 8.9% | |
| Positive anti-CCP§ (y/n) | 40 | 5.0% | |
Abbreviations: RF: rheumatoid factor, and anti-CCP: anti-cyclic citrullinated peptide antibody. ICD codes for sarcoidosis clinical manifestations are provided in supplementary table.
Number of patients tested for RF =745.
Number of patients tested for anti-CCP = 795.
Patients were categorized as positive for RF or anti-CCP at a level of ≥ 20 units/mL.
Medication use:
Medications prescribed during the study period are shown in Table 3. The majority of patients (59.3%) were prescribed glucocorticoids (at any dose) at some point during the study period; 24.7% of patients received prolonged glucocorticoid therapy (≥ 10 mg/d for ≥ 90 days) and 18.2% of patients received glucocorticoid monotherapy (without any DMARDs). Methotrexate and hydroxychloroquine were the most commonly used csDMARDs. 12.1% received one or more biologic or targeted synthetic DMARD, the most common of which were TNFi (10.9%; top drugs were infliximab (6.7%), and adalimumab (4.4%)), followed by rituximab (0.5%), omalizumab (<10), and abatacept (<10). Biologics were usually used in combination with other csDMARDs; only 29/387 patients received biologic monotherapy. Among high-volume practices (practices with ≥ 30 sarcoidosis patients, N=31), we found large variations in biologic use, ranging from 15.6% to 69.2% of sarcoidosis patients (Figure 1). Among these practices, infliximab was used for between 0–40% of patients. Similar variability was seen with non-TNF biologics, which were used for between 0 and 50% of patients in each practice.
Table 3:
Medications prescribed by U.S. rheumatologists for patients with sarcoidosis
| Medications N (%) |
Total patients (N=3276) |
||
|---|---|---|---|
| None recorded | 731 | 22.3% | |
| Glucocorticoids | Any prednisone or equivalent§ | 1943 | 59.3% |
| Glucocorticoid prolonged* | 811 | 24.7% | |
| Glucocorticoid monotherapy | 598 | 18.2% | |
| Conventional synthetic DMARDs | Methotrexate | 986 | 30.1% |
| Hydroxychloroquine | 941 | 28.7% | |
| Azathioprine | 297 | 9.1% | |
| Mycophenolate | 187 | 5.7% | |
| Leflunomide | 146 | 4.5% | |
| Sulfasalazine | 52 | 1.6% | |
| Cyclosporine | <10 | ||
| Minocycline | 36 | 1.1% | |
| Tacrolimus | 24 | 0.7% | |
| Biologics-TNFi | Infliximab or infliximab biosimilars | 219 | 6.7% |
| Adalimumab | 145 | 4.4% | |
| Etanercept | 16 | 0.5% | |
| Certolizumab | <10 | ||
| Golimumab | <10 | ||
| Biologics-non-TNFi | Rituximab | 17 | 0.5% |
| Omalizumab | <10 | ||
| Abatacept | <10 | ||
| Other biologies¶ | <10 | ||
| Targeted small | Tofacitinib | <10 | |
| molecules | Apremilast | <10 | |
Abbreviations: GC: glucocorticoid, csDMARDs: conventional disease modifying anti-rheumatic drugs, TNFi: tumor necrosis factor inhibitors.
Prednisone or equivalent included prednisone and other oral and intravenous steroids.
Prolonged glucocorticoid therapy was defined as ≥ 10 mg of prednisone daily (or its equivalent) for ≥90 days.
Other biologics include (Belimumab, Tocilizumab, Anakinra, Secukinumab and Ustekinumab).
Figure 1: Proportion of sarcoidosis patients prescribed a biologic or targeted synthetic DMARD by practice (n=31) in the RISE registry.
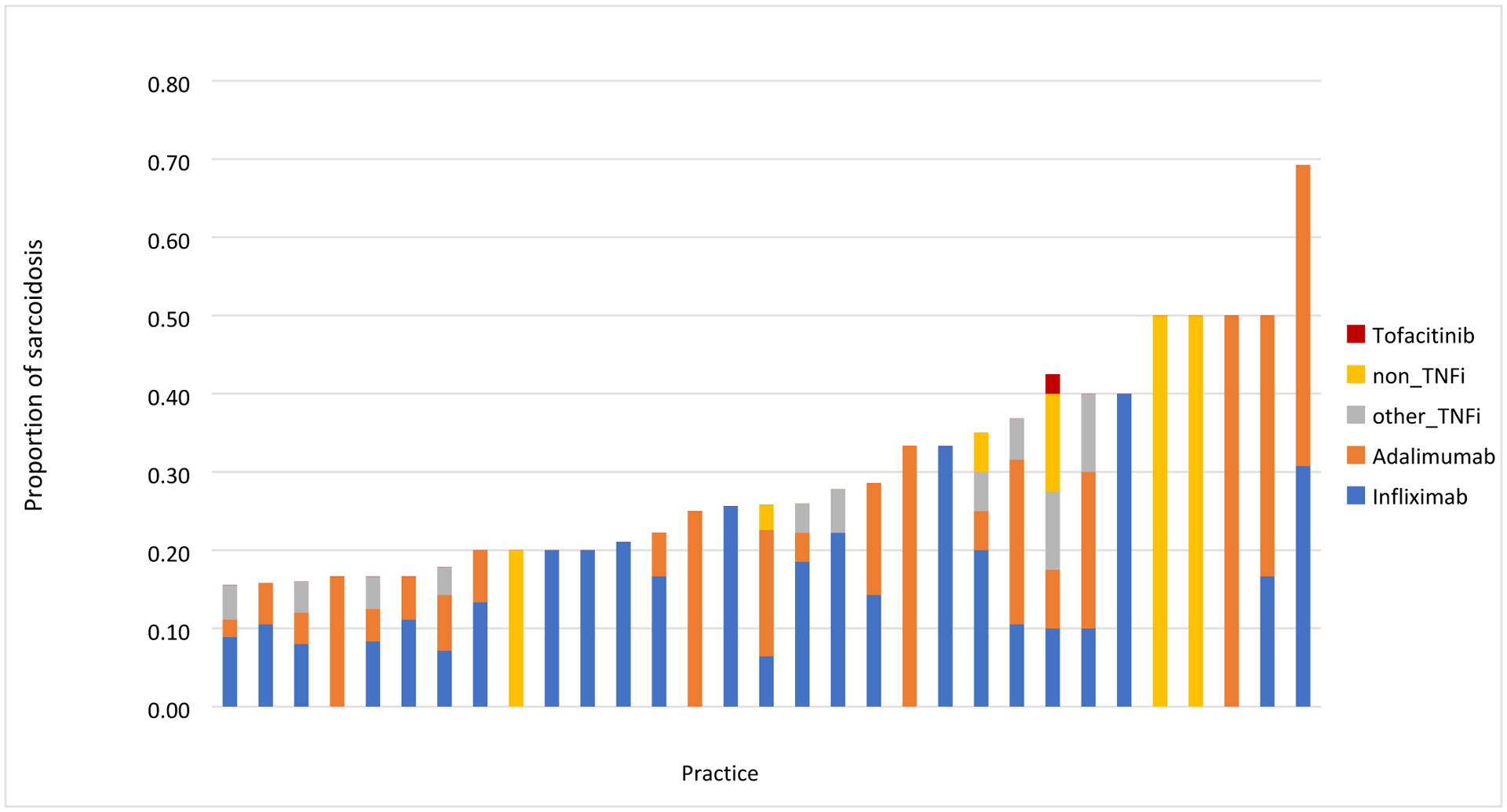
Each bar represents a different practice with at least 30 patients with sarcoidosis. The figure shows that biologics use among these practices ranged from 15.6% to 69.2% of sarcoidosis patients.
Discussion
Using data from the RISE registry, we identified 3276 patients with sarcoidosis, the largest sample for this condition that has been published using real-world EHR data to date. Of these patients, almost a quarter were receiving moderate-dose steroids for a prolonged period, and about 12% received biologic or targeted synthetic DMARDs. We found wide variation in the patterns of biologic use among rheumatology practices. These findings likely reflect the lack of standardized treatment recommendations for this disease.
Rare diseases such as sarcoidosis present unique challenges for researchers working to develop and study treatments or to quantify patient outcomes. It is difficult for a single center to collect enough patients to make important inferences about effective treatments for the disease. Disease-specific registries are often collaborations across academic centers, and do not provide information about treatment patterns in the community. The RISE registry is unique in its inclusion of U.S. clinical rheumatologists: participating practices account for an estimated 32% of the clinical rheumatology workforce, and the registry brings the added benefit that patients are not selected based on severity of disease or having been seen at a tertiary care center, where many of the most severe cases may receive care. We found between 0.2% – 1.8% of patients seen by non-academic rheumatologists carried a diagnosis of sarcoidosis and only 31 out of 184 practices cared for more than 30 patients with the condition. Sarcoidosis is relatively rare, and collecting data is also difficult due to the fact that patients may seek care from clinicians across many different specialties, including neurology, dermatology, pulmonology and rheumatology. This study provides a deeper look into the heterogeneity of this disease among patients seeking care from rheumatologists.
Specific manifestations of sarcoidosis appear to be significantly under-coded in rheumatology EHRs. Not surprisingly for patients seen in rheumatology practices, we found that the most commonly coded organ manifestations were musculoskeletal (22.1%), followed by pulmonary (15.1%). On the contrary, a study of sarcoidosis patients at a disease-specific clinic at a large university medical center showed that the lung was involved in 89%, followed by the skin (26%) and eyes (23%) (8). Some of this difference could be accounted for by studying a sample of patients who see rheumatologists, but in addition, these differences may be a result of the methods used to extract information about disease manifestations-in the current study, we relied only on ICD codes to identify manifestations, whereas prior cohort studies identified manifestations using radiographic or pathologic reports (13,14). The validation of specific manifestations is not possible when data is extracted from the EHR via problem lists as opposed to detailed case abstraction forms. In the future, using natural language processing (NLP) approaches to extract information from clinical notes, may allow for more detailed identification of specific disease manifestations at scale (15,16).
Glucocorticoids are first-line therapy for sarcoidosis (17), and accordingly, we found that more than half of the patients in this study (59.3%) were treated with glucocorticoids. This is similar to previous studies in which prednisone use was reported in 55–65% of sarcoidosis patients (6,18). These proportions were significantly higher when compared to a recent study using Marketscan data, which found that only 25.5% of sarcoidosis patients were prescribed prednisone during a single calendar year, although the difference in follow up time (1 year vs. a median of 2 years) may account for theses discrepancies (19). We also found that 24.7% received prolonged glucocorticoids (≥ 10 mg/daily for ≥ 90 days). This is important because a recent expert consensus guidelines suggested that a maintenance dose of greater than 10 mg of daily prednisone equivalent was suboptimal and associated with significant side effects (20). The finding that a quarter of patients exceed this dose for a prolonged period highlights the need for additional and more effective therapies to be developed in this disease.
12% of patients were prescribed at least one biologic or targeted synthetic DMARD at some point during the study period, presumably to treat signs and symptoms related to their sarcoidosis, since patients with other autoimmune conditions were excluded from the study sample. Infliximab and adalimumab were the most common biologic agents used, which is consistent with prior studies (5), although their overall use was slightly more common than previously reported in the U.S claims-based data analysis (6), perhaps because patients had more musculoskeletal complaints or because they were all seen by rheumatologists, who were comfortable prescribing these drugs.
We observed meaningful variation in the use of biologics and tsDMARDs across practices. For example, 19% of high-volume practices prescribed infliximab as their only biologic for the treatment of sarcoidosis, while nearly 10% of practices prescribed exclusively non-TNFi biologics such as abatacept. Tofacitinib was only prescribed in two practices. Interestingly, trials have shown limited benefit of non-TNFi biologics for sarcoidosis (21–23), and only a few case reports have examined targeted synthetic DMARDs like tofacitinib in patients with cutaneous sarcoidosis, with some improvement in clinical and histological remission in the skin disease (24–26). The variation in medication use might partly be explained by patient factors such as race, insurance status, specific manifestations, or disease severity (27) but likely also reflects prescribing preferences by clinicians (28). It is clear that large studies are needed to evaluate the efficacy and safety of these drugs in patients with sarcoidosis (29).
Using the RISE registry provides a representative sample of non-academic rheumatology practices across the U.S. and reports on the largest sample of sarcoidosis patients to date. Despite the strengths of the current study, its limitations should be addressed. Patients included in this study sought consultation by rheumatologists, thus, the resulting sample still may not be entirely reflective of the general population of patients with sarcoidosis. Our research examined mainly non-academic rheumatology practices, so results may not apply to academic healthcare settings. Although we attempted to be conservative in defining a diagnosis of sarcoidosis by excluding patients with other autoimmune conditions, future work should focus on the validation of codes used to diagnose sarcoidosis and identify its manifestations.
In summary, using data from the RISE registry, we performed the largest study of sarcoidosis patients to date. We found a significant number of patients were receiving chronic glucocorticoids and a clinically important fraction were receiving biologics. With no FDA-approved drugs available for extrapulmonary sarcoidosis, our findings highlight the need for a greater focus on developing standardized treatments for patients with this disease.
Supplementary Material
SIGNIFICANCE and INNOVATIONS.
This study provides the first detailed description of national, real-world treatments provided by rheumatologists to sarcoidosis patients during routine office visits.
About one-fourth of patients received prolonged, moderate doses of glucocorticoids, and 12.1% of patients with sarcoidosis used biologic or targeted synthetic disease modifying drugs (DMARDs), despite absence of FDA approval.
We found wide variations in the patterns of biologic therapies used by U.S. rheumatologists to treat sarcoidosis, likely reflecting the lack of standardized treatment recommendations for this disease.
Funding sources:
The authors report no conflicts of interest. Drs. Yazdany and Schmajuk are supported by the Russell/Engleman Medical Research Center for Arthritis. Dr. Yazdany is supported by NIAMS P30 AR070155 and K24AR074534. Dr. Anastasiou was supported by NIAMS under award number 5T32 AR007304-40. The data presented here was supported by the American College of Rheumatology’s RISE Registry. However, the views expressed represent those of the author(s) and do not necessary represent the views of the American College of Rheumatology. This study protocol was prepared by and the data analysis was executed by the RISE data analytic center at UCSF.
References
- 1.Cozier YC. Assessing the worldwide epidemiology of sarcoidosis: challenges and future directions. Eur Respir J. 2016. Dec;48(6):1545–8. [DOI] [PubMed] [Google Scholar]
- 2.Drent M, Strookappe B, Hoitsma E, De Vries J. Consequences of sarcoidosis. Clin Chest Med. 2015;36(4):727–37. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011. Jan 26;305(4):391–9. [DOI] [PubMed] [Google Scholar]
- 4.James WE, Baughman R. Treatment of sarcoidosis: grading the evidence. Expert Rev Clin Pharmacol. 2018. Jul;11(7):677–87. [DOI] [PubMed] [Google Scholar]
- 5.Beegle SH, Barba K, Gobunsuy R, Judson MA. Current and emerging pharmacological treatments for sarcoidosis: a review. Drug Des Devel Ther. 2013;7:325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc. 2016;13(8):1244–52. [DOI] [PubMed] [Google Scholar]
- 7.Rice JB, White A, Lopez A, Nelson WW. High-Cost Sarcoidosis Patients in the United States: Patient Characteristics and Patterns of Health Care Resource Utilization. J Manag Care Spec Pharm. 2017. Dec;23(12):1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2012. Oct;29(2):119–27. [PubMed] [Google Scholar]
- 9.Yazdany J, Bansback N, Clowse M, Collier D, Law K, Liao KP, et al. Rheumatology Informatics System for Effectiveness: A National Informatics-Enabled Registry for Quality Improvement. Arthritis Care Res. 2016;68(12):1866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungprasert P, Matteson EL, Crowson CS. Accuracy of Diagnostic Coding for Sarcoidosis in Electronic Databases: A Population-Based Study. Lung. 2017. Dec;195(6):713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992. Jun;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 12.Korsten P, Strohmayer K, Baughman RP, Sweiss NJ. Refractory pulmonary sarcoidosis - proposal of a definition and recommendations for the diagnostic and therapeutic approach. Clin Pulm Med. 2016. Mar;23(2):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of Sarcoidosis 1946–2013: A Population Based Study. Mayo Clin Proc. 2016. Feb;91(2):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gribbin J, Hubbard RB, Jeune IL, Smith CJP, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006. Nov;61(11):980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ananthakrishnan AN, Cai T, Savova G, Cheng S-C, Chen P, Perez RG, et al. Improving Case Definition of Crohn’s Disease and Ulcerative Colitis in Electronic Medical Records Using Natural Language Processing: A Novel Informatics Approach. Inflamm Bowel Dis. 2013. Jun;19(7):1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamian L, Wheless L, Crofford LJ, Barnado A. Rule-based and machine learning algorithms identify patients with systemic sclerosis accurately in the electronic health record. Arthritis Res Ther. 2019. Dec 30;21(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Kofahi K, Korsten P, Ascoli C, Virupannavar S, Mirsaeidi M, Chang I, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008. Jun 1;31(6):1189–96. [DOI] [PubMed] [Google Scholar]
- 19.Low D, Simpson KN, Rissmiller R, James E. A NEW SIDE OF SARCOIDOSIS: MEDICATION AND HOSPITALIZATION USE IN A PRIVATELY INSURED PATIENT POPULATION. SARCOIDOSIS Vasc DIFFUSE LUNG Dis. 2019;36(2):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010. May;104(5):717–23. [DOI] [PubMed] [Google Scholar]
- 21.Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014. Nov 1;44(5):1296–307. [DOI] [PubMed] [Google Scholar]
- 22.Bomprezzi R, Pati S, Chansakul C, Vollmer T. A case of neurosarcoidosis successfully treated with rituximab. Neurology. 2010. Aug 10;75(6):568–70. [DOI] [PubMed] [Google Scholar]
- 23.Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol Auckl NZ. 2012;6:1613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damsky W, Thakral D, Emeagwali N, Galan A, King B. Tofacitinib Treatment and Molecular Analysis of Cutaneous Sarcoidosis. N Engl J Med [Internet]. 2018. Dec 26 [cited 2020 Mar 30]; Available from: https://www.nejm.org/doi/10.1056/NEJMoa1805958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damsky W, Young BD, Sloan B, Miller EJ, Obando JA, King B. Treatment of Multiorgan Sarcoidosis With Tofacitinib. ACR Open Rheumatol. 2020. Feb;2(2):106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damsky W, Thakral D, McGeary MK, Leventhal J, Galan A, King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020. Mar;82(3):612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucholc M, O’Kane M, Ashe S, Wong-Lin K. Prescriptive variability of drugs by general practitioners. PLoS ONE [Internet]. 2018. Feb 20 [cited 2020 Feb 23];13(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5819764/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Practice-Level Variation in Quality of Care in the Acr’s Rheumatology Informatics System for Effectiveness (RISE) Registry [Internet]. ACR Meeting Abstracts. [cited 2020 Mar 5]. Available from: https://acrabstracts.org/abstract/practice-level-variation-in-quality-of-care-in-the-acrs-rheumatology-informatics-system-for-effectiveness-rise-registry/ [Google Scholar]
- 29.Shah P, Bechman K, Galloway JL. The evidence for biologic immunotherapy in Sarcoidosis: A systematic review. In 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


