Abstract
The objective of this study is to identify post SARS-CoV-2 vaccine BNT162b2 (BioNTech & Pfizer) side effects in patients with systemic lupus erythematosus (SLE) at the Cayetano Heredia Hospital, Lima, Peru. A descriptive observational study was designed in patients with SLE at the Immuno-Rheumatology Department of the Cayetano Heredia Hospital, Lima, Peru, immunized with the BNT162b2 vaccine from May 21 to June 30, 2021. Of the total number of patients seen in the service, 100 received the vaccine’s 1st dose, and 90 patients received the 2nd dose; 90% and 92.2% presented symptoms within 10 days after immunization (1st and 2nd doses, respectively), being pain at the inoculation site the most frequent (87%); most of the symptoms presented were of mild intensity. There were 27 episodes of post-immunization flare, 9% and 20% after the 1st and 2nd doses, respectively; the predominant type of flare was articular (85.1%), followed by dermal (18.5%). It was found that a history of renal involvement was associated with the risk of developing flare RR 0.38 (0.15–0.91) and the use of hydroxychloroquine and azathioprine prior to immunization 0.20 (0.06–0.63) and 7.96 (2.70–23.43) respectively. In 100 SLE patients immunized with BNT162b2 vaccine against SARS-CoV-2, 27% of SLE reactivation episodes occurred, two patients were hospitalized for flare severity, and none died.
|
Key Points • Up to 92.2% presented some type of symptom after vaccination, being mostly local and of mild intensity. • Of the population studied, there were 27 episodes of post-vaccination flare, most of which were mild. • In the studied population, taking hydroxychloroquine and having a history of renal disease were associated with a lower risk of presenting post-vaccination flare. |
Keywords: (MeSH NLM): Coronavirus, Autoimmune diseases, COVID-19, Systemic lupus erythematosus, Vaccines
Introduction
To date, the main tools for COVID-19-related mortality are vaccines. The new methods in the development of vaccines (mRNA and viral vectors) initially caused concern in the population given the lack of previous experience with this technology; however, clinical trials showed adequate levels of efficacy and safety in the included population [1–3].
However, these studies did not include patients with autoimmune diseases, which have not made it possible to establish a level of efficacy and/or safety for this group of patients (considering the immunological alteration inherent to these diseases, immunosuppressive therapy, and the possible impact on disease activity) [1, 4–6].
Some studies have documented post-immunization side effects in patients with autoimmune diseases; and in the particular case of SLE, post-immunization episodes of disease reactivation (flare) have been described, as in the case of pneumococcal, influenza, and human papilloma virus vaccines [7, 8]. However, to date there are limited communications studying the manifestations presented in patients with SLE vaccinated against SARS-CoV-2 [9–12].
The main objective of the present study was to evaluate the manifestations and tolerance following immunization with the BNT162b2 vaccine against SARS-CoV-2 in patients with SLE in a public hospital in Peru.
Materials and methods
A descriptive observational study was designed, where the study population were patients with the diagnosis of SLE attended at the Immuno-Rheumatology Service of the Cayetano Heredia Hospital, Lima, Peru, who were immunized with the BNT162b2 vaccine (BioNTech & Pfizer) between May 21 and June 30, 2021.
Patients with a diagnosis of SLE were included, according to the classification criteria of SLICC 2012 [13] and/or ACR/EULAR 2019 [14], all older than 18 years, who had received at least one dose of BNT162b2 vaccine (BioNTech & Pfizer) during the established period. Patients who had been immunized with another type of SARS-CoV-2 vaccine and patients not authorizing telephone follow-up and/or face-to-face post-immunization evaluation were excluded.
The variables recorded were sex (male-female), age, time of illness, comorbidities, history of documented SARS-Cov-2 infection, presence of flare within 6 months prior to immunization, type of systemic involvement by SLE, usual treatment for SLE, clinical manifestations following the 1st and 2nd doses of immunization (local reaction, pain at the site of inoculation, headache, fever, fatigue, joint pain, myalgia, nausea, vomiting, abdominal pain, diarrhea), presence of flare after the 1st and/or 2nd dose of immunization (confirmed by a rheumatologist in a face-to-face evaluation), and type of flare presented. Flare was defined as a measurable increase in disease activity in one or more organ systems involving clinical or laboratory findings (new or worse) [15], using laboratory parameters (ESR, CRP, 24-h proteinuria, DNAds, serum complement) before and after immunization.
In the case of post-immunization manifestations, symptom intensity was graded as follows: mild, if the symptom did not interfere with daily activities; moderate, if the symptom partially interfered with the normal performance of daily activities; severe, if the symptom prevented the performance of daily activities. For the variable, fever was graded 38.0 to 38.5 (mild), 38.6 to 39 (moderate), and greater than 39 (high).
Procedures
As part of routine care, all patients were evaluated face to face prior to SARS-CoV-2 immunization. Subsequently, with their consent, they were followed up within 10 days by telephone. Similarly, in the case of patients with suspected disease reactivation, they were re-evaluated face-to-face to confirm the flare and document the corresponding characteristics.
The present study was approved by the Cayetano Heredia Hospital ethics committee (code 039–2021); the identity of the participants was respected and protected; only the researchers had access to the database, which was encrypted to maintain the confidentiality of the participants.
For the analysis of numerical variables, the mean/standard deviation or median/interquartile range was used for parametric and nonparametric distribution, respectively; for dichotomous variables, absolute and relative frequency was used. For categorical variables, the chi-square or Fisher test was used when any of the assumptions were not met; in both cases, statistical significance was established for a p value <0.05 and a 95% confidence interval (95% CI). Covariate adjustment for possible confounding factors was also performed using Poisson regression.
Microsoft Office Excel 2016® and the statistical program STATA v15® were used as computer support.
Results
A total of 100 patients with SLE immunized with the BNT162b2 vaccine (BioNTech & Pfizer) were studied; the mean age was 38.9 years, 94% were women; the most frequent systemic manifestations of SLE were articular 74%, renal 60%, and dermal 23%. The 81% of patients were taking treatment to control SLE, 28% presented reactivation of SLE within 6 months prior to immunization (Appendices Table 3 and Figure 2).
Table 3.
Clinical-epidemiological characteristics of patients with SLE immunized against SARS-CoV-2
| Variables | Total (N = 100) |
|---|---|
| Age, years | 35 (27.5–49) |
| Sex - n (%) | |
|
Female Male |
94 (94%) 6 (6%) |
| Time of illness (SLE), years | 7.5 (4–13) |
|
Comorbidities* Hypothyroidism Chronic kidney disease APS† Arterial hypertension Rheumatoid arthritis Sjögren’s syndrome Others‡ |
55 (55%) 15 (15%) 13 (13%) 7 (7%) 7 (7%) 6 (6%) 5 (5%) 20 (20%) |
| Associated commitment of SLEǂ | |
|
Articulate Renal Dermal Hematological Mucocutaneous Pulmonary Neurological Cardiovascular |
74 (74%) 60 (60%) 23 (23%) 21 (21%) 17 (17%) 15 (15%) 15 (15%) 4 (4%) |
Usual treatment of SLE
|
|
|
Prednisone Hydroxychloroquine Azathioprine Mycophenolate Methotrexate Cyclophosphamide Leflunomide Cyclosporine No treatment |
71 (71%) 75 (75%) 24 (24%) 23 (23%) 8 (8%) 5 (5%) 2 (2%) 1 (1%) 9 (9%) |
| COVID-19 background | |
|
Yes No |
39 (39%) 61 (61%) |
| Hospitalization for COVID-19 | 3 (3%) |
| SLE reactivation in the last 6 months (documented by rheumatologist physician) | 28 (28%) |
Data are n (%) or median (IQR).
*12 patients presented 2 or more comorbidities
†APS antiphospholipid syndrome
‡Juvenile idiopathic arthritis (1), avascular necrosis (1), primary biliary cirrhosis (1), diabetes (2), transverse myelitis (1), fibromyalgia (4), pulmonary fibrosis (2), glaucoma (2), asthma (1), aortic stenosis (1), hepatitis C (2), celiac disease (1), pregnancy (1)
ǂ70 patients had 2 or more systems affected by SLE
 72 patients were taking 2 or more drugs
72 patients were taking 2 or more drugs
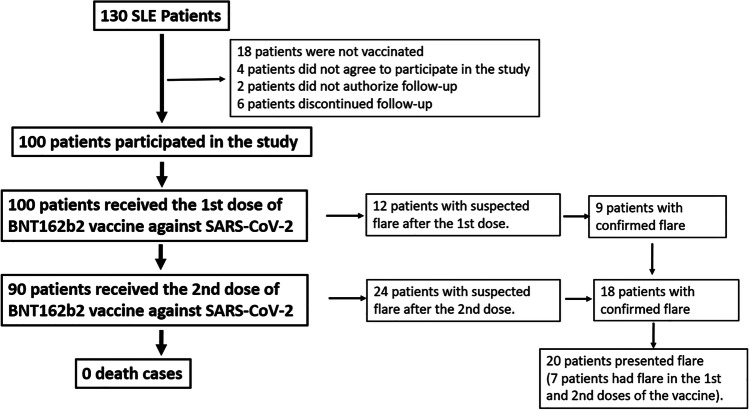
Fig. 2.
Flow chart
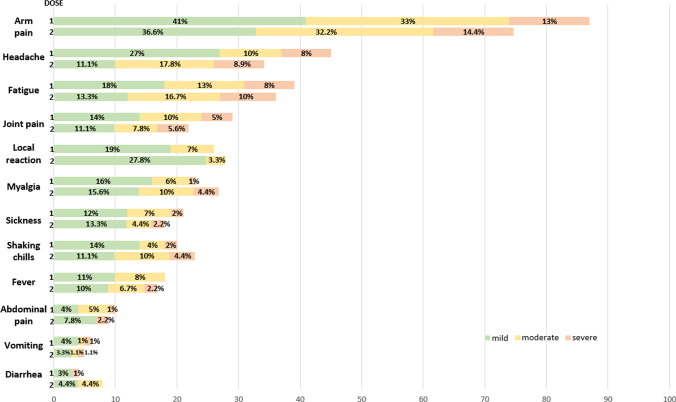
Of the total, 100 patients received the 1st vaccine dose and 90 the 2nd dose; 90% and 92.2% presented symptoms within 10 days after immunization (1st and 2nd doses, respectively), with pain at the inoculation site being the most frequent (87%). Most of the symptoms presented were of mild intensity. On average, these symptoms occurred 1.1 ± 0.5 days after vaccination, and the duration was 3.5 ± 1 days (Fig. 1).
Fig. 1.
Symptoms presented in patients with lupus after the 1st and 2nd doses of immunization against SARS-CoV-2
There were 27 episodes of flare after the immunization process, 9% (9/100) and 20% (18/90) after the 1st and 2nd doses, respectively; seven patients presented reactivation in both immunization processes. The predominant type of flare was arthritis (synovitis, swelling, phlogosis) (85.1%), followed by dermal (18.5%) (Table 1, Appendix Table 4). The time of onset of reactivation was 2.3 ± 0.8 days; the duration was 7.3 ± 3 days; it was found that the history of renal involvement was associated with the risk of developing flare RR 0.38 (0.15–0.91) and the use of hydroxychloroquine and azathioprine prior to immunization 0.20 (0.06–0.63) and 7.96 (2.70–23.43), respectively (Table 2).
Table 1.
Clinical characteristics of flares post SARS-CoV-2 immunization
| Total | 20a | |
|---|---|---|
| Age-years | 38.65 ± 14.13 | |
| Sex-no. (%) | ||
|
Female Male |
18 (90%) 2 (10%) |
|
| Length of illness (SLE)-years | 7.04 ± 4.85 | |
| SLE treatmentb | ||
|
Prednisone Hydroxychloroquine azathioprine Mycophenolate Methotrexate Leflunomide No treatment |
16 (80%) 11 (55%) 10 (50%) 3 (15%) 3 (15%) 1 (5%) 3 (15%) |
|
| SLE outbreak in the past 6 months | 8 (40%) | |
| Confirmed COVID-19 history | 13 (65%) | |
| Flare episodes after immunization | ||
|
1st dose 2nd dose 1st and 2nd doses |
9 (45%) 18 (90%) 7 (35%) |
|
| Type of flare | 1era dosis | 2da dosis |
|
Arthritis Malar erythema Alopecia Lupus pneumonitis leukopenia Myopericarditis |
6 (30%) 1 (5%) - 1 (5%) 1 (5%) 1 (5%) |
17 (85%) 4 (20%) 1 (5%) - - - |
| Hospitalization for flare | 2 | |
a20 patients presented post-immunization flare; however, there were 27 flare episodes (given that 7 patients had flare episodes in the 1st and 2nd doses)
b15 patients use 2 or more drugs
Table 4.
Clinical characteristics of patients who presented flare post-immunization against SARS-CoV-2
| Sex/age (years) | Illness time (SLE) | SLE-associated systemic involvement | Standard treatment | Flare in 6 months prior to immunization | Flare type after the 1st immunization dose | Flare type after the 2nd immunization dose | |
|---|---|---|---|---|---|---|---|
| 1 |
F 21 |
2 years | Renal, cardiovascular (valvulitis, pericarditis), articular | Prednisone Hydroxychloroquine Azathioprine | Yes |
Cardiac flare-up (Myopericarditis) Decrease in LVEF 30% * Pericardial effusion 600 cc Articular flare-up In-hospital management (corticosteroid therapy 1 mg/kg, mycophenolate) |
Did not vaccinate |
| 2 |
F 37 |
5 years | Renal, hematologic (leukopenia), articular | No treatment | No |
Hematological flare-up (leukopenia 2000) †. Outpatient management. |
Musculoskeletal (arthritis
 ) flare-up. Outpatient management
) flare-up. Outpatient management |
| 3 |
F 37 |
6 months | Articular, alopecia | No treatment | No | Musculoskeletal (arthritis) flare-up. Outpatient management | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 4 |
M 80 |
5 years | Hematologic (leukopenia), pulmonary (ILD‡), articular. | Prednisone Mycophenolate | No | Pulmonary flare-up (lupus pneumonitis), in-hospital management (methylprednisolone pulses, cyclophosphamide) | Did not vaccinate |
| 5 |
F 44 |
11 years | Dermal (malar erythema), mucocutaneous (oral ulcers), articular | Prednisone Methotrexate Leflunomide | Yes | Musculoskeletal (arthritis) flare-up. Outpatient management | Dermal flare-up (erythema malar), Musculoskeletal (arthritis) flare-up. Outpatient management |
| 6 |
F 27 |
10 years | Dermal (malar erythema), articular, neurological (convulsions) | No treatment | Yes | Musculoskeletal (arthritis) flare-up. Outpatient management | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 7 |
F 37 |
6 years | Renal, articular | Hydroxychloroquine Mycophenolate | No | Musculoskeletal (arthritis) flare-up. Outpatient management | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 8 |
M 42 |
19 years | Hematologic (plateletopenia), pulmonary (pleural effusion), dermal (malar erythema), articular | Prednisone Hydroxychloroquine Azathioprine Methotrexate | Yes | Musculoskeletal (arthritis) flare-up. Outpatient management | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 9 |
F 43 |
9 years | Dermal (malar erythema), mucocutaneous (oral ulcers), articular, neurological (convulsions) | Prednisone | No | Musculoskeletal (arthritis) flare-up, dermal flare-up (erythema malar), outpatient management | Musculoskeletal (arthritis) flare-up, dermal flare-up (erythema malar), outpatient management |
| 10 |
F 21 |
5 years | Renal, dermal (malar erythema), articular | Prednisone Mycophenolate | No | – | Dermal flare-up (erythema malar, alopecia), outpatient management |
| 11 |
F 34 |
14 years | Renal, mucocutaneous (oral ulcers), articular. | Prednisone Hydroxychloroquine Azathioprine | No | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 12 |
F 42 |
5 years | Articular | Prednisone Methotrexate | No | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 13 |
F 29 |
5 years | Renal, hematologic (plateletopenia), mucocutaneous, articular | Prednisone Hydroxychloroquine Azathioprine | No | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 14 |
F 55 |
3 years | Polyserositis, articular | Prednisone Hydroxychloroquine Azathioprine | Yes | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 15 |
F 38 |
12 years | Renal | Prednisone Hydroxychloroquine Azathioprine | No | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 16 |
F 24 |
4 years | Renal, articular | Prednisone | No | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 17 |
F 46 |
9 years | Dermal, articular | Prednisone Hydroxychloroquine Azathioprine | No | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
| 18 |
F 25 |
3 years | Articular | Prednisone Hydroxychloroquine Azathioprine | Yes | – | Dermal flare-up (erythema malar) Musculoskeletal (arthritis) flare-up. Outpatient management |
| 19 |
F 33 |
4 months | Hematologic (hemolytic anemia, antiphospholipid antibodies) | Prednisone Hydroxychloroquine Azathioprine | Yes | – | Musculoskeletal (arthritis) flare-up. vascular (Raynaud’s) flare-ups. Outpatient management |
| 20 |
F 58 |
12 years | Hematologic (plateletopenia), articular | Prednisone Hydroxychloroquine Azathioprine | Yes | – | Musculoskeletal (arthritis) flare-up. Outpatient management |
*Patient in previous evaluations had LVEF 46%
†In the control prior to immunization, the patient presented 5000 leukocytes
 Arthritis (synovitis, tumefaccion, flogosis, limitacion funcional)
Arthritis (synovitis, tumefaccion, flogosis, limitacion funcional)
‡ILD diffuse interstitial lung disease. LVEF left ventricular ejection fraction
Table 2.
Associated factors to flare after SARS-COV-2 immunization
| Flare | No flare | P valuea | RR-IC | |
|---|---|---|---|---|
|
Age, years Median (IQR) |
37 (28–43.5) |
35 (27.5–51) |
0.945 | – |
| Sex | ||||
| Male | 2 (33.3%) | 4 (66.7%) | 0.597 | – |
| Female | 18 (19.2%) | 76 (80.8%) | ||
|
Time of illness, years Median (IQR) |
5 (3.5–10.5) | 8 (4–14) | 0.218 | – |
| Comorbidities | 13 (23.6%) | 42 (76.4%) | 0.315 | – |
| Systematic commitment to SLE | ||||
| Articular | 15 (20.3%) | 59 (79.7%) | 0.909 | – |
| Renal | 8 (13.3%) | 52 (86.7%) | <0.001b | 0.38 (0.15–0.91) |
| Dermatological | 6 (26.1%) | 17 (73.9%) | 0.391 | – |
| Hematological | 7 (33.3%) | 14 (66.7%) | 0.122 | – |
| Mucocutaneous | 5 (29.4%) | 12 (70.6%) | 0.322 | – |
| Pulmonary | 3(20%) | 12 (80%) | 1 | – |
| Neurological | 3 (20%) | 12 (80%) | 1 | – |
| Cardiovascular | 1 (25%) | 3 (75%) | 1 | – |
| Regular treatment for SLE | ||||
| No treatment | 3 (33.3%) | 6 (66.7%) | 0.378 | – |
| Hydroxychloroquine | 11 (14.7%) | 64(85.3%) | <0.001b | 0.20 (0.06–0.63) |
| Prednisone | 16 (22.5%) | 55 (77.5%) | 0.321 | – |
| Azathioprine | 10 (41.7%) | 14 (58.3%) | <0.001b | 7.96 (2.70–23.43) |
| Mycophenolate | 3 (13%) | 20 (87%) | 0.553 | – |
| Methotrexate | 3 (37.5%) | 5 (62.5%) | 0.196 | – |
| Cyclophosphamide | 0 | 5 (100%) | – | – |
| COVID-19 background | 13 (33.3%) | 26 (66.7%) | <0.001b | 1.68 (0.69–4.09) |
| Flare within 6 months prior to immunization | 8 (28.6%) | 20 (71.4%) | 0.181 | – |
aP value <0·05 indicates statistical significance at 95% confidence interval
bPoisson regression analysis, for a P value adjusted for age, sex, treatment use, history of confirmed COVID-19 and renal involvement, with 95% confidence interval
Discussion
Infections are among the main causes of morbidity and mortality in patients with SLE; therefore, vaccination is advised (especially vaccines originating from non-living microorganisms) [16]. While the benefit of immunization against infections in SLE patients is undeniable, it should also be noted that isolated cases of post-immunization flare have been reported in the literature [7, 8]. In the case of SARS-CoV-2 vaccines, especially mRNA and viral vectors, theoretical mechanisms have been proposed that could lead to a possible SLE reactivation (production of interferon alpha as well as a probable molecular mimicry of the SARS-CoV-2 Spike protein) [2, 3].
In a study of adverse reactions associated with the SARS-CoV-2 vaccine (Pfizer/BioNTech and Moderna) in systemic autoimmune diseases [5], it was found that 89% presented local symptoms and 69% systemic symptoms (mainly fatigue, headache, myalgia). Boekel [11] reported that in patients with systemic autoimmune diseases vaccinated mainly with Pfizer/BioNTech and AstraZeneca, 51% presented mild adverse effects and 21% moderate effects. In our study, 87% presented local symptoms (pain at the inoculation site), and 69% presented systemic symptoms (mainly headache, fatigue, joint pain, and myalgias) in both the 1st and 2nd doses of immunization.
A possible relationship between the SARS-CoV-2 vaccine and autoimmunity has been described in observational studies. Watad et al. [10] reported a series of 27 patients, of whom 17 presented reactivation of their baseline autoimmune disease and 10 presented debut of autoimmunity after SARS-CoV-2 immunization. In our study, we found 27 flare episodes (clinical and/or laboratory worsening) after immunization (9 and 18 after the 1st and 2nd doses, respectively). The most frequent type of reactivation was arthritis (synovitis, phlogosis, functional limitation, accompanied by elevation of acute phase reactants: ESR/CRP) which occurred in 66.6% in the 1st dose and 94.4% in the 2nd dose, followed by skin and scalp involvement (malar erythema and alopecia). Our results are similar to those reported by Izmirly et al. [9] who reported, in a multiethnic study, up to 11.5% of flare episodes in patients with lupus after SARS-Cov-2 immunization (mainly Pfizer).
Felten et al. [12], in a study of 696 patients with SLE (VACOLUP), described 21 (3%) self-report episodes of SLE flare (medically confirmed) being mostly musculoskeletal involvement (90%). Although our study found a higher proportion of flare episodes (27%), this could be due to the fact that in VACOLUP, the population was mostly European/USA, the difference in SLE activity in relation to ethnicity is known [17], and that the online survey was a self-report by patients, which could represent a possible underreporting in the number of flares.
For the cases of articular reactivation, given the functional limitation, short courses of increased prednisone doses (or initiation in patients who did not use steroids) were used, with which clinical improvement was evidenced. Similarly, two patients required in-hospital management due to the severity of the condition (lupus pneumonitis and myopericarditis, respectively) which were managed with high doses of corticosteroids (methylprednisolone pulses) and immunosuppressants. After which they were discharged and due to the possible risk of a new episode of flare after the 2nd vaccine dose, immunization was not recommended.
It should be noted that 10 patients chose not to receive the second dose of the vaccine; among the main reasons were fear of reactivation of SLE, severe reactions after the 1st dose of immunization, and SARS-CoV-2 infection between both doses; one patient had an abortion at 4 weeks of gestation after the 1st vaccine dose.
The present study has limitations; causality cannot be established in relation to immunization and the flare presented. Likewise, SLEDAI-2 k levels were not compared since not all patients had immunological tests. However, the main strength of the present analysis is that the patients were evaluated prior to immunization, and the episode of SLE reactivation was also established by evaluating (clinically and laboratorial) all cases with suspected flare.
Conclusion
Although our series describes episodes of reactivation following SARS-CoV-2 immunization (mainly mild), we advise that the COVID-19 immunization process should continue to be a priority for patients with SLE.
Appendix 1
Appendix 2
Appendix 3
Data availability
Only the researchers had access to the database, which was encrypted to maintain the confidentiality of the participants.
Code availability
Not applicable.
Declarations
Ethics approval
The present study was approved by the Cayetano Heredia Hospital ethics committee (code 039–2021).
Consent to participate
All participants in the present study gave verbal consent.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGill COVID-19 Vaccine Tracker Team. COVID19 vaccine tracker. https://covid19.trackvaccines.org/vaccines/ Date Accessed 15 Aug 2021
- 2.Boekel L, Hooijberg F, van Kempen ZLE, Vogelzang EH, Tas SW, Killestein J, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3(4):241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):243–e245. doi: 10.1016/S2665-9913(21)00039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moutsopoulos HM. A recommended paradigm for vaccination of rheumatic disease patients with the SARS-CoV-2 vaccine. J Autoimmun. 2021;121:102649. doi: 10.1016/j.jaut.2021.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL et al (2021) Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 10.1136/annrheumdis-2021-220231
- 6.Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik-Wang JM et al (2021) Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 10.1136/annrheumdis-2021-220289 [DOI] [PMC free article] [PubMed]
- 7.Murdaca G, Orsi A, Spanò F, Puppo F, Durando P, Icardi G, et al. Influenza and pneumococcal vaccinations of patients with systemic lupus erythematosus: current views upon safety and immunogenicity. Autoimmun Rev. 2014;13(2):75–84. doi: 10.1016/j.autrev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case–control study. Ann Rheum Dis. 2013;72(5):659–664. doi: 10.1136/annrheumdis-2012-201393. [DOI] [PubMed] [Google Scholar]
- 9.Izmirly PM, Kim MY, Samanovic M, Fernandez-Ruiz R, Ohana S, Deonaraine KK (2021) Evaluation of immune response and disease status in SLE patients following SARS-CoV-2 vaccination. Arthritis Rheumatol. 10.1002/art.41937 [DOI] [PMC free article] [PubMed]
- 10.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boekel L, Kummer LY, van Dam KPJ, Hooijberg F, van Kempen Z, Vogelzang EH, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3(8):e542–e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felten R, Kawka L, Dubois M, Ugarte-Gil MF, Fuentes-Silva Y, Piga M (2021) Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 10.1016/S2665-9913(21)00221-6 [DOI] [PMC free article] [PubMed]
- 13.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R. 2019 European league against rheumatism/American College of Rheumatology Classification Criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruperto N, Hanrahan LM, Alarcón GS, Belmont HM, Brey RL, Brunetta P, et al. International consensus for a definition of disease flare in lupus. Lupus. 2011;20(5):453–462. doi: 10.1177/0961203310388445. [DOI] [PubMed] [Google Scholar]
- 16.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases – version 1. Arthritis Rheumatol. 2021;73(7):1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004;83(1):1–17. doi: 10.1097/01.md.0000104742.42401.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Only the researchers had access to the database, which was encrypted to maintain the confidentiality of the participants.
Not applicable.