Abstract
Purpose
Racial disparities in coronavirus disease 2019 (COVID-19) outcomes have been described. We sought to determine whether differences in inflammatory markers, use of COVID-19 therapies, enrollment in clinical trials, and in-hospital outcomes contribute to racial disparities between Black and non-Black patients hospitalized for COVID-19.
Methods
We leveraged a prospective cohort study that enrolled 1325 consecutive patients hospitalized for COVID-19, of whom 341 (25.7%) were Black. We measured biomarkers of inflammation and collected data on the use COVID-19-directed therapies, enrollment in COVID-19 clinical trials, mortality, need for renal replacement therapy, and need for mechanical ventilation.
Results
Compared to non-Black patients, Black patients had a higher prevalence of COVID-19 risk factors including obesity, hypertension, and diabetes mellitus and were more likely to require renal replacement therapy (15.8% vs 7.1%, P < .001) and mechanical ventilation (37.2% vs 26.6%, P < .001) during their hospitalization. Mortality was similar between both groups (15.5% for Blacks vs 14.0% for non-Blacks, P = .49). Black patients were less likely to receive corticosteroids (44.9% vs 63.8%, P< .001) or remdesivir (23.8% vs 57.8%, P < .001) and were less likely to be enrolled in COVID-19 clinical trials (15.3% vs 28.2%, P < .001). In adjusted analyses, Black race was associated with lower levels of C-reactive protein and soluble urokinase receptor and higher odds of death, mechanical ventilation, and renal replacement therapy. Differences in outcomes were not significant after adjusting for use of remdesivir and corticosteroids.
Conclusions
Racial differences in outcomes of patients with COVID-19 may be related to differences in inflammatory response and differential use of therapies.
Keywords: African Americans, Convalescent serum, Coronavirus, Corticosteroids, Disparities, M2C2, Remdesivir, SARS-COV-2
Clinical Significance.
-
•
Compared to non-Blacks, Blacks had lower inflammatory burden after adjusting for co-morbidities, suggesting the existence of racial differences in the acute inflammatory response to COVID-19.
-
•
Blacks hospitalized for COVID-19 were less likely to receive corticosteroids and remdesivir prior to the FDA's emergency use authorization for remdesvir, and less likely to be enrolled in clinical trials.
-
•
Differences in outcomes between Blacks and non-Blacks were abrogated after adjusting for differences in therapy.
Alt-text: Unlabelled box
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has disproportionately affected minorities in the United States.1 , 2 The prevalence of COVID-19 is higher in Black patients relative to other racial groups,3 and Black patients have a higher prevalence of risk factors for severe COVID-19, including obesity, hypertension, diabetes mellitus, and kidney disease compared with non-Black patients.4, 5, 6, 7 However, reports on the association between Black race and worse COVID-19 outcomes have been discrepant. Nationwide data suggest Black patients account for a higher percentage of deaths relative to their representation in the general population.8 However, a recent cohort study of more than 11,000 patients presenting to hospitals across the country found that there was no difference in in-hospital mortality between Black and white patients, even after accounting for age, gender, comorbidities, and markers of socioeconomic status.9 Another recent study found that although Black and Hispanic patients were more likely to test positive for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), rates of hospitalization were similar compared with white patients, and Black patients were less likely to die or be discharged on hospice care after adjusting for comorbidities and neighborhood characteristics.10
Whether differences in COVID-19-related hospitalization outcomes between Black and non-Black patients relate to variations in levels of inflammatory markers or use of COVID-19-directed therapies is unclear. Most studies examining racial differences related to COVID-19 are derived from administrative databases limited by the lack of clinical detail in characterizing patients hospitalized for COVID-19. Black patients have been previously reported to receive suboptimal treatment as compared with white patients in a variety of settings, including following admission for pneumonia11 and in-hospital cardiac arrest.12 Minority groups have also been historically underrepresented in clinical trials,13, 14, 15 an especially important consideration given the implications for the generalizability of COVID-19-directed therapeutics.16
The main objective of this study is to understand the reasons underlying apparent racial disparities related to COVID-19, through detailed characterization of the clinical course of Black and non-Black patients hospitalized for COVID-19, including differences in comorbidities, levels of inflammatory markers, use of COVID-19-directed therapies, and participation in clinical trials. We leveraged the Michigan Medicine COVID-19 Cohort (M2C2), a large prospective cohort in which patient hospitalized specifically for COVID-19 were identified and systematically enrolled and characterized.
Methods
Study Design
M2C2 is an ongoing prospective cohort study initiated in February of 2020, which enrolls consecutive adults (≥18 years) with confirmed SARS-CoV-2 infection who were hospitalized specifically for COVID-19 at the University of Michigan. Patients with a positive test for SARS-CoV-2 hospitalized primarily for non-COVID-19 reasons were excluded. Patient eligibility for inclusion into M2C2 was reviewed by 2 independent physicians at the time of admission. Manual chart review and data mining tools were used to gather details of the presentation, demographics, past medical history, home medications, laboratory studies, inpatient medical therapy, hospitalization course, and outcomes. Residual biological samples were collected for biomarker testing. All patients were followed until hospital discharge or in-hospital death. The study was approved by the University of Michigan institutional review board. The data set for this study included patients enrolled in M2C2 from February 1, 2020 to March 26, 2021.
Exposures and Outcomes
The primary exposure was race as documented in the medical record; categorized as non-Hispanic Black versus non-Black patients. We collapsed non-Hispanic white (61.8%), Asian (4.7%), Hispanic (2.5%), and other races (5.3%) into a single “non-Black” category due to their limited numbers.
The outcomes examined were in-hospital death or discharge to hospice, need for mechanical ventilation, and need for renal replacement therapy during hospitalization.
Treatment for COVID-19 was defined as the receipt of any doses of the following therapies: corticosteroids, remdesivir, azithromycin, hydroxychloroquine, tocilizumab, therapeutic anticoagulation (specifically excluding anticoagulation for venous thromboembolism prophylaxis only), and convalescent serum. We also collected data on participation in COVID-19-related clinical trials.
Data on the following inflammatory markers measured within 48 hours of admission were collected: soluble urokinase plasminogen activator receptor (suPAR), C-reactive protein (CRP), interleukin-6 (IL-6), D-dimer, ferritin, lactate dehydrogenase (LDH), and procalcitonin.
Laboratory Measures
Data on levels of CRP, D-dimer, ferritin, LDH, and procalcitonin measured within 48-hours of presentation were extracted from the medical record. We measured suPAR in plasma using a commercially available enzyme-linked immunosorbent assay (SuPARnostic assay by ViroGates, Birkerød) in residual samples obtained from the clinical laboratory. The lower detection limit of the assay is 100 pg/mL with minimal intra- and interassay variation (2.75% and 9.17%, respectively) per the manufacturer. We measured IL-6 with an enzyme-linked immunosorbent assay (Quantikine QuicKitTM ELISA, Human IL-6 Immunoassay). The IL-6 assays have a lower detection limit of 1.70 pg/mL and intra- and interassay variation of 2.6% and 4.5%, respectively, as reported by the assay manufacturer.
Statistical Analysis
We first compared clinical characteristics between Black and non-Black patients. Categorical variables were presented as frequencies with percentages, whereas continuous variables were presented as means with standard deviations if normally distributed or medians with interquartile ranges if non-normally distributed. χ2 tests were used to compare categorical variables and student t-tests and Mann-Whitney U tests were used for normally and non-normally distributed continuous variables, respectively. We also report the use of COVID-19 therapies stratified by period (before and after May 1, 2020, which is date of the Emergency Use Approval (EUA) of remdesivir by the US Food and Drug Administration).
To determine whether racial differences between biomarker levels were independent of clinical characteristics, we used multivariable linear regression models where each biomarker was evaluated separately as the dependent variable and included race as an independent variable. Clinical characteristics included as covariates in each model were: age, gender, body mass index (BMI), diabetes mellitus, hypertension, coronary artery disease, heart failure, and creatinine-derived estimated glomerular filtration rate (eGFR) on admission.
To assess whether Black race was independently associated with outcomes—notably in-hospital death, need for mechanical ventilation, and need for dialysis—and assess the impact of select confounders on the association, we used binary logistic regression with stepwise modeling and each outcome as the dependent variable. Model 0 included race alone; model 1 (clinical characteristics) included race, age, gender, BMI, diabetes mellitus, hypertension, coronary artery disease, heart failure, chronic kidney disease, and eGFR on admission. Model 2 (inflammation) added CRP to model 1, and model 3 (COVID-19 therapies) added use of remdesivir and corticosteroids to the variables in Model 1.
Similarly, we used logistic regression to examine the association between race (independent variable) and the use of COVID-19 therapies; notably remdesivir and corticosteroids (dependent variables in separate models). We included the following covariates in each model: age, sex, BMI, diabetes mellitus, hypertension, coronary artery disease, heart failure, and eGFR on admission. All analyses were performed in SPSS Statistics 27 (IBM).
Results
Differences in Clinical Characteristics Between Black and Non-Black Patients
At the time of database lock for the purpose of this study (March 26, 2021), a total of 1325 patients (mean age 61 years, 57.1% male) had been hospitalized for COVID-19 at the University of Michigan Health System, comprising 341 (25.7%) Black patients and 984 (74.2%) non-Black patients. White individuals represented the majority of non-Black patients (n = 819, 83.2%), followed by Asian (n = 62, 6.3%), other/unknown race (n = 70, 7.1%), and Hispanic (n = 33, 3.4%).
Black patients were on average younger than non-Black patients and had higher average BMI (Table 1 ). The prevalence of cardiovascular and COVID-19 risk factor rates was higher in Black patients compared with non-Black patients: hypertension (75.7% vs 59.0%, P < .001), diabetes mellitus (48.1% vs 35.6%, P < .001), and chronic kidney disease (22.6% vs 16.8%, P = .017) (Table 1).
Table 1.
Demographics and Clinical Characteristics by Self-Reported Race
| Variables | Overall Cohort (n = 1325) | Non-Black Race* (n = 984) | Black Race (n = 341) | P Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 61 (16) | 62 (16) | 58 (15) | .001 |
| Male, n (%) | 757 (57.1%) | 578 (58.7%) | 179 (52.5%) | .045 |
| BMI, kg/m2, mean (SD) | 32 (9) | 31 (8) | 34 (9) | <.001 |
| History of tobacco use, n (%) | 571 (43.1%) | 433 (44.0%) | 138 (40.5%) | .26 |
| Diabetes mellitus, n (%) | 514 (38.8%) | 350 (35.6%) | 164 (48.1%) | <.001 |
| Hypertension, n (%) | 839 (63.3%) | 581 (59.0%) | 258 (75.7%) | <.001 |
| Coronary artery disease, n (%) | 219 (16.5%) | 174 (17.7%) | 45 (13.2%) | .06 |
| Congestive heart failure, n (%) | 167 (12.6%) | 117 (11.9%) | 50 (14.7%) | .18 |
| Chronic kidney disease, n (%) | 242 (18.3%) | 165 (16.8%) | 77 (22.6%) | .017 |
| End-stage renal disease on dialysis, n (%) | 44 (3.3%) | 27 (2.7%) | 17 (5.0%) | .047 |
| Admission eGFR, mean (SD) | 67 (32) | 69 (30) | 64 (37) | .020 |
| Presenting Symptoms, n (%) | ||||
| Fever | 790 (59.6%) | 577 (58.6%) | 213 (62.5%) | .22 |
| Shortness of breath | 990 (74.7%) | 733 (74.5%) | 257 (75.4%) | .75 |
| Diarrhea | 383 (28.9%) | 267 (27.1%) | 116 (34.0%) | .016 |
| Altered mental status | 146 (11.0%) | 115 (11.7%) | 31 (9.1%) | .19 |
| Laboratory Data,† mean (SD) | ||||
| Hemoglobin, g/dL | 13.1 (2.2) | 13.1 (2.3) | 12.9 (2.0) | .11 |
| White blood cell count, k/uL | 7.7 (5) | 7.6 (5.3) | 8 (3.9) | .23 |
| Absolute neutrophil, count, k/uL | 5.9 (4.2) | 5.8 (4.5) | 6.1 (3.4) | .29 |
| Absolute lymphocyte count, k/uL | 1.2 (3.0) | 1.2 (3.4) | 1.1 (1.0) | .50 |
| Aspartate aminotransferase, IU/L | 59 (57) | 57 (55) | 66 (64) | .025 |
| Alanine aminotransferase, IU/L | 46 (52) | 47 (53) | 44 (50) | .37 |
| Total bilirubin, mg/dL | 0.73 (1.11) | 0.72 (1.25) | 0.73 (0.56) | .98 |
| Creatinine, mg/dL | 1.8 (4.6) | 1.6 (4.9) | 2.4 (3.3) | .007 |
| Inflammatory Markers, median (IQR) | ||||
| SuPAR, ng/mL | 7.77 (5.46-12.17) | 7.89 (5.48-12.28) | 7.54 (5.39-12.12) | .45 |
| C-reactive protein, mg/dL | 8.70 (4.70-17.27) | 8.30 (4.10-16.30) | 10.20 (5.80-18.80) | <.001 |
| Lactate dehydrogenase, IU/L | 376 (277-516) | 356 (267-487) | 430 (325-588) | <.001 |
| Interleukin-6, pg/mL | 12.50 (12.50-95.00) | 12.50 (12.50-87.93) | 20.00 (12.50-105.89) | .19 |
| Procalcitonin, ng/mL | 0.18 (0.10-0.48) | 0.17 (0.09-0.41) | 0.24 (0.11-0.70) | <.001 |
| Ferritin, ng/mL | 684 (299-1368) | 624 (270-1256) | 844 (385-1557) | <.001 |
| D-dimer, FEU mg/L | 1.00 (0.57-1.98) | 0.90 (0.54-1.73) | 1.34 (0.73-2.93) | <.001 |
| Outcomes, n (%) | ||||
| Need for mechanical ventilation | 389 (29.4%) | 262 (26.6%) | 127 (37.2%) | <.001 |
| Need for renal replacement therapy | 124 (9.4%) | 70 (7.1%) | 54 (15.8%) | <.001 |
| In-hospital death | 191 (14.4%) | 138 (14.0%) | 53 (15.5%) | .49 |
BMI = body mass index; eGFR = estimated glomerular filtration rate; IQR = interquartile range; SD = standard deviation; suPAR = soluble urokinase plasminogen activator receptor.
Includes 819 white patients, 33 Hispanics, 62 Asian patients, and 70 of unknown race or other.
First value within 48 hours of presentation.
Clinical Presentation
Frequency of respiratory symptoms on presentation of COVID-19 was similar between Black and non-Black patients, with no significant differences in cough, shortness of breath, fever, or altered mental status. Black patients were, however, more likely to have diarrhea on presentation (34.0% vs 27.1%, P = .016) (Table 1).
Black patients had a higher mean CRP (2.4 mg/dL vs 1.6 mg/dL, P = .007) and aspartate aminotransferase (64 IU/L vs 57 IU/L, P = .025) on admission than non-Black patients. There were no significant differences in hemoglobin, white blood cell count, absolute neutrophil/lymphocyte count, aspartate aminotransferase, or total bilirubin (Table 1).
Inflammatory Markers
In univariable analysis, Black patients had higher median CRP (10.2 mg/dL vs 8.3 mg/dL, P < .001), LDH (430 IU/L vs 356 IU/L, P < .001), procalcitonin (20.0 ng/mL vs 12.5 ng/mL, P < .001), ferritin (844 ng/mL vs 624 ng/mL, P < .001), and D-dimer (1.34 FEU mg/L vs 0.90 FEU mg/L, P < .001). There were no differences between Black patients and non-Black patients in suPAR or IL-6 levels (Table 1). After adjusting for clinical characteristics and COVID-19 risk factors, Black race was associated with lower suPAR and CRP levels (Table 2 ).
Table 2.
Biomarkers of Thrombo-Inflammation and Black Race
| Biomarker, β (95%CI) |
|||||||
|---|---|---|---|---|---|---|---|
| SuPAR | C-reactive protein | Lactate dehydrogenase | Interleukin-6 | Procalcitonin | Ferritin | D-dimer | |
| Black race | −1.00 (−1.93 to −0.07) | −7.74 (−13.86 to −1.61) | 73 (31-114) | 19.93(−81.70 to 121.56) | 0.75 (−4.69 to 6.18) | 439 (187-691) | −0.17 (−5.87 to 5.54) |
| Age, per 1 year | −0.03 (−0.06 to 0.00) | −0.20 (−0.41 to 0.02) | −1 (−3 to 0) | −2.01 (−5.93 to 1.91) | 0.03 (−0.17 to 0.22) | −4 (−13 to 5) | 0.09 (−0.11 to 0.29) |
| Male gender | −0.67 (−1.52 to 0.19) | −5.09 (−10.54 to 0.35) | −42 (−80 to −5) | −78.68 (−178.31 to 20.95) | −0.77 (−5.69 to 4.14) | −522 (−747 to −297) | −3.68 (−8.74 to 1.38) |
| BMI, per 1 kg/m2 | 0.07 (0.02-0.12) | 0.05 (−0.29 to 0.39) | 2 (0-5) | 0.30 (−6.17 to 6.76) | −0.16 (−0.46 to 0.15) | 0 (−14 to 14) | 0.39 (0.08-0.71) |
| Diabetes mellitus | 0.54 (−0.39 to 1.48) | 6.20 (0.19-12.21) | −12 (−53 to 30) | 81.59 (−26.96 to 190.15) | −1.62 (−7.03 to 3.80) | 121 (−127 to 367) | −1.47 (−7.04 to 4.09) |
| Hypertension | −0.68 (−1.69 to 0.33) | −0.79 (−7.19 to 5.61) | −54 (−98 to −9) | −46.49 (−165.83 to 72.86) | −3.51 (−9.30 to 2.27) | −204 (−470 to 62) | −3.53 (−9.51 to 2.45) |
| Coronary artery disease | 1.03 (−0.20 to 2.26) | −8.49 (−16.49 to −0.50) | −37 (−92 to 18) | −33.86 (−174.29 to 106.57) | −0.79 (−7.85 to 6.26) | −113 (−439 to 213) | −0.42 (−7.84 to 7.01) |
| Heart failure | 0.62 (−0.71 to 1.94) | 0.46 (−8.23 to 9.15) | 16 (−43 to 74) | 34.63 (−113.93 to 183.19) | −2.34 (−10.10 to 5.41) | −242 (−592 to 109) | −0.68 (−8.58 to 7.22) |
| Admission eGFR | −0.06 (−0.08 to −0.05) | −0.10 (−0.20 to 0.01) | −1 (−2 to 0) | −1.00 (−2.79 to 0.79) | −0.06 (−0.15 to 0.03) | −8 (−12 to −3.6) | 0.00 (−0.09 to 0.10) |
BMI = body mass index; CI = confidence interval; eGFR = estimated glomerular filtration rate.
COVID-19 Therapies
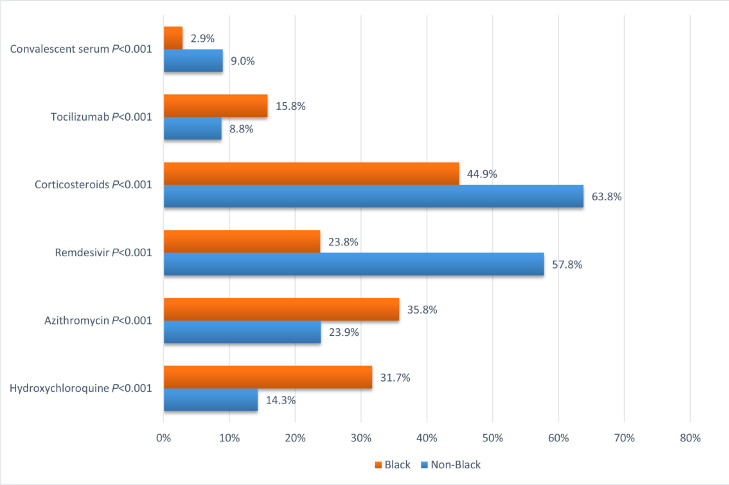
Black patients were more likely to receive hydroxychloroquine (31.7% vs 14.3%, P < .001), azithromycin (35.8% vs 23.9%, P < .001), and tocilizumab (15.8% vs 8.8%, P < .001) and less likely to receive remdesivir (23.8% vs 57.8%, P < .001), corticosteroids (44.9% vs 63.8%, P < .001), and convalescent serum (2.9% vs 9.0%, P < .001) than non-Black patients (Figure 1 ). Black patients were also less likely to be enrolled in any COVID-19-related clinical trial than non-Black patients (15.3% vs 28.2%, P < .001) (Figure 1).
Figure 1.
Use of COVID-19-related therapies according to race. Horizontal bar graph demonstrating the proportion of Black and non-Black patients receiving each therapy with associated P value from univariable analysis. COVID-19 = coronavirus disease 2019.
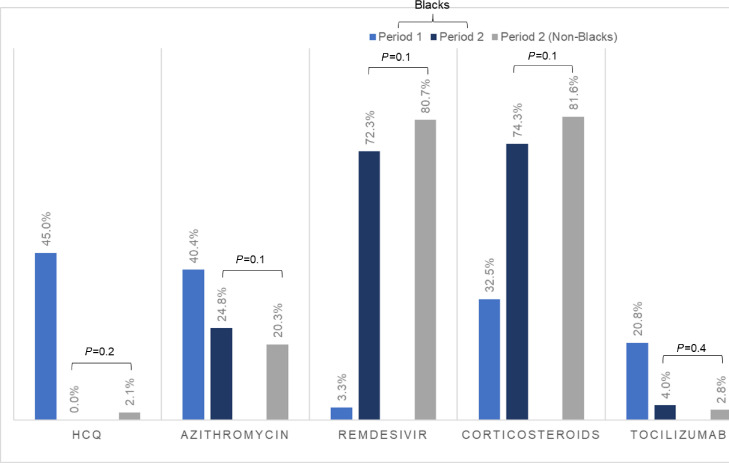
In multivariable analysis adjusting for age, gender, comorbidities, and eGFR at admission, Black race was independently associated with a lower odds of receiving remdesivir (adjusted odds ratio [aOR] 0.24 [0.18-0.32], P < .001), receiving corticosteroids (aOR 0.45 [0.35-0.58], P < .001), or being enrolled in a COVID-19 clinical trial (aOR 0.47, [0.32-0.68], P < .001). (Table 3 ). When examining the use of COVID-19 therapies stratified by time period (prior to or after May 1, 2020, the date of EUA of remdesivir), we noted a significant increase in the use of remdesivir and corticosteroids in Black patients (Figure 2 ), with differences in their use between Black patients and non-Black patients no longer being significant post-EUA.
Table 3.
Multivariable Analysis of Select COVID-19-Directed Interventions
| Intervention, β (95%CI) |
|||
|---|---|---|---|
| Remdesivir | Corticosteroids | Clinical Trial Enrollment | |
| Black race | 0.24 (0.18-0.32) | 0.45 (0.35-0.58) | 0.47 (0.32-0.68) |
| Age, per 1 year | 1.02 (1.01-1.03) | 1.00 (0.99-1.01) | 1.00 (0.99-1.02) |
| Female sex | 0.88 (0.70-1.11) | 0.89 (0.71-1.12) | 0.72 (0.54-0.97) |
| BMI, per 1 kg/m2 | 1.01 (0.995-1.03) | 1.00 (0.99-1.02) | 1.02 (1.00-1.04) |
| Diabetes mellitus | 0.91 (0.70-1.18) | 0.94 (0.73-1.21) | 0.88 (0.64-1.21) |
| Hypertension | 0.93 (0.71-1.23) | 1.03 (0.81-1.39) | 0.97 (0.69-1.35) |
| Coronary artery disease | 1.05 (0.75-1.47) | 0.98 (0.70-1.36) | 0.94 (0.61-1.45) |
| Heart failure | 0.81 (0.56-1.19) | 0.91 (0.63-1.31) | 0.43 (0.24-0.77) |
| eGFR at admission, per 1 mL/min | 1.00 (1.00-1.01) | 1.00 (0.99-1.00) | 1.00 (1.00-1.01) |
BMI = body mass index; CI = confidence interval; COVID-19 = coronavirus disease 2019; eGFR = estimated glomerular filtration rate.
Figure 2.
COVID-19-directed interventions in black patients prior to and after EUA approval of remdesivir. Clustered bar graph showing proportions of Black patients pre- and post-EAU of remdesivir (periods 1 and 2, respectively), in additional to the proportion of non-Black patients in period 2 receiving COVID-19 therapies. P value is derived from the comparison between Black patients and non-Black patients in their receipt of COVID-19 therapies in period 2. COVID-19 = coronavirus disease 2019; EAU = emergency use authorization.
Outcomes
Overall mortality in the cohort was 14.4%. Although there was no statistically significant difference in mortality between Black patients and non-Black patients (15.5 vs 14.0%, P = .49), Black patients were more likely to require mechanical ventilation (37.2% vs 26.6%, P < .001) and renal replacement therapy (15.8% vs 7.1%, P < .001) (Table 1).
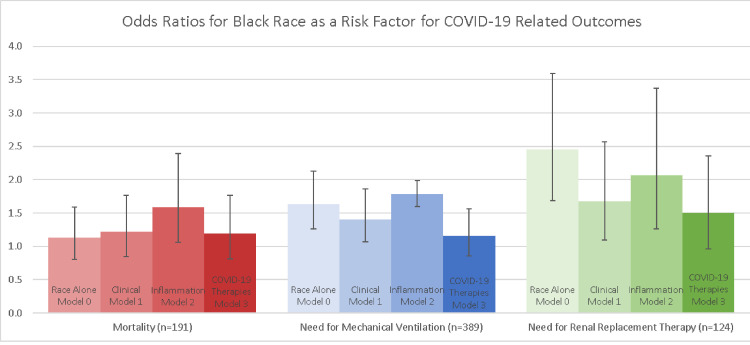
In multivariable analysis, Black race was only associated with increased risk of death after adjustment for inflammation as measured by CRP levels, and the associations with mechanical ventilation and renal replacement therapy were strengthened (Figure 3 ). Adjustment for the use of remdesivir and corticosteroids eliminated these associations, suggesting that the higher odds of mechanical ventilation and renal replacement therapy in Black patients may have been due to the lower rate of use of these therapies in Black patients prior to May 1, 2020.
Figure 3.
Odds ratios for Black race as a risk factor for COVID-19-related outcomes. Bar graphs showing the odds ratios for Black race for mortality, need for mechanical ventilation, and need for renal replacement therapy during hospitalization for COVID-19. Model 0 includes Black versus non-Black race only; model 1 includes race, sex, age, BMI, diabetes mellitus, hypertension, coronary artery disease, heart failure, chronic kidney disease, and eGFR on admission; model 2 adds CRP to model 1; and lastly, model 3 includes the use of remdesivir and corticosteroids. Error bars denote 95% confidence intervals. Dashed line denotes odds ratio of 1, with non-Black race being the reference. BMI = body mass index; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate.
Discussion
There has been much debate on the reasons that underlie racial disparities in COVID-19-related outcomes. We shed light on potential causes in this prospective cohort study of patients hospitalized specifically for COVID-19 who were extensively characterized and had measures of inflammation. We found that Black compared with non-Black patients had similar in-hospital death rates despite a higher prevalence of comorbidities and COVID-19 risk factors, which may be explained by the lower burden of inflammation as measured by CRP and suPAR levels. Black patients were less likely to receive remdesivir and corticosteroids or enroll in clinical trials, which our data suggest may have impacted their outcomes because the associations between Black race and the need for mechanical ventilation or renal replacement therapy were obviated after adjustment for the differential use of these therapies. To our knowledge, this is the first study analyzing the interplay among inflammation, treatment, and outcomes as it relates to racial differences in COVID-19.
Throughout the COVID-19 pandemic, racial disparities in infection rates,17 hospitalization rates,18 , 19 and overall mortality17 , 20 have been described. These differences are likely closely tied to socioeconomic status because they are somewhat attenuated after adjustment for risk factors, including age, sex, comorbidities, insurance, neighborhood, and access to care. Several studies have shown no differences in in-hospital mortality.9 , 20 , 21 A complex interplay between social determinants of health and associated comorbidity burden has been posited as the basis for the observed disparities in COVID-19 infection rate.22, 23, 24 This study builds on the current body of knowledge by providing data on the impact of underlying inflammation and differing treatments to COVID-19-related outcomes.
A recent review posited that observed racial and ethnic differences in COVID-19 outcomes may arise from a differential inflammatory response between groups, driven by underlying differences in chronic inflammation from different burdens of comorbidity, socioeconomic factors, genetic factors, and psychologic stressors.25 Differences in chronic inflammation as measured by CRP levels have been previously described, with several studies finding that Black patients have higher median CRP levels than white patients, though these differences were heavily attenuated after accounting for differences in comorbidities and psychosocial factors.26 , 27 Similarly, after adjusting for cardiovascular and COVID-19 risk factors, Black race compared with non-Black was associated with relatively lower levels of CRP and suPAR, reflecting perhaps an attenuated inflammatory response to SARS-CoV-2 infection and leading to similar survival rates compared to non-Black patients despite their significantly higher burden of comorbidities. Studies on racial differences in acute inflammation are few. In 1 study, Black participants exhibited an attenuated inflammatory response compared to non-Blacks when exposed to endotoxin.28 Nevertheless, these findings warrant further investigation into racial differences in the immune response and their mechanisms.
Our study found substantial treatment differences between Black and non-Black patients that persisted even after multivariable adjustment. Racial disparities in treatment in medicine are well-described in diseases as diverse as pneumonia,29 cardiac arrest,30 appendicitis,31 and end-stage renal disease.32 At the time of writing, no study has evaluated racial differences in the in-hospital management of COVID-19. These differences in the use of COVID-19-directed therapies such as remdesivir are at least partially related to the lower enrollment of Black patients in clinical trials, as we noted its rate of use dramatically increased to levels that were similar by race after its EUA by the U.S. Food and Drug Administration. Disproportionately low enrollment of Black patients in clinical trials has been demonstrated time and again, including in heart failure,33 multiple myeloma,13 cancer phase III trials,34 HIV,35 and others. Many theories have been proposed for why this disparity has been found, and much of the discussion focuses on reluctance among Black patients, a legacy of the unethical, abhorrent Tuskegee Syphilis study,36 and investigator bias.37 , 38 The reasons for reduced enrollment of Black patients in clinical trials in our study unfortunately could not be ascertained. However, we show that differences in management may have major consequences because differences in the incidence of mechanical ventilation and renal replacement therapy were abrogated after adjustment for the use of COVID-19-directed therapies.
Our study has major strengths, including the prospective and systematic inclusion of patients hospitalized specifically for COVID-19, rather than patients hospitalized with a positive SARS-CoV-2 test. We have complemented our assessment of conventional biomarkers of inflammation such as CRP, ferritin, D-dimer, LDH, and procalcitonin with measurements of nonconventional measurements of inflammation such as suPAR and IL-6. We used admission biomarkers levels as opposed to serial measurements to avoid confounding by therapies administered. Serial biomarker measurements may shed light on racial differences to response to therapy. The major limitations are in the observational nature of the study and the inability to capture reasons for not enrolling in clinical trials, precluding making strong conclusions on the impact of racial differences in management on COVID-19-related outcomes.
Conclusion
In conclusion, our study demonstrates significant differences in inflammation, use of COVID-19-directed therapies, and outcomes, notably the need both for mechanical ventilation and renal replacement therapy and death between Black and non-Black patients hospitalized for COVID-19. These findings should prompt additional studies on racial differences in acute inflammation, as well as scrutiny of institutional treatment protocols to reduce racial differences in treatment and clinical trial enrollment.
Acknowledgments
The authors acknowledge the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication. The authors are grateful to the services of the Microbiome Core supported by U2CDK110768, especially Chris Blair; the Michigan Clinical Research Unit including Wrenn Woodard and Dexter Hobdy, and the University of Michigan Medical School Central Biorepository for providing biospecimen storage, management, and distribution services in support of the research reported in the publication.
Footnotes
Funding: RP-B is supported by NIH/NIDDK-1-R01-DK-107956-01, NIH U01 DK119083, and Juvenile Diabetes Research Foundation. SSH is funded by NIH 1R01HL153384, U01-DK119083, R01-DK109720. and the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor (U-M G024231) award.
Conflicts of Interest: The wife of CW works at Regeneron Pharmaceuticals. SSH is a member of the scientific advisory board of Walden Biosciences. TUA, HB, EA, MP, HRS, KP, PO'H, CM, RF, EM, CL, PB, AB, RP-B, JMC report none.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 2.Thebault R, Tran AB, Williams V. The coronavirus is infecting and killing black Americans at an alarmingly high rate. Washington Post. Available at: https://www.washingtonpost.com/nation/2020/04/07/coronavirus-is-infecting-killing-black-americans-an-alarmingly-high-rate-post-analysis-shows/. Accessed April 28, 2020.
- 3.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mensah George A, Mokdad Ali H, Ford Earl S, Greenlund Kurt J, Croft Janet B. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). African American Health. Available at: https://www.cdc.gov/vitalsigns/aahealth/index.html. Accessed August 6, 2020.
- 6.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33(5):409–415. doi: 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carethers JM. Insights into disparities observed with COVID-19. J Intern Med. 2021;289(4):463–473. doi: 10.1111/joim.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19) in the U.S. Available at: https://covid.cdc.gov/covid-data-tracker. Accessed September 17, 2020.
- 9.Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausmann LRM, Ibrahim SA, Mehrotra A, et al. Racial and ethnic disparities in pneumonia treatment and mortality. Med Care. 2009;47(9):1009–1017. doi: 10.1097/MLR.0b013e3181a80fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA J Am Med Assoc. 2009;302(11):1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duma N, Azam T, Riaz IB, Gonzalez-Velez M, Ailawadhi S, Go R. Representation of minorities and elderly patients in multiple myeloma clinical trials. Oncologist. 2018;23(9):1076–1078. doi: 10.1634/theoncologist.2017-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zullig LL, Fortune-Britt AG, Rao S, Tyree SD, Godley PA, Carpenter WR. Enrollment and racial disparities in National Cancer Institute Cancer Treatment Clinical Trials in North Carolina. N C Med J. 2016;77(1):52–58. doi: 10.18043/ncm.77.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: have we improved? Cancer. 2013;119(16):2956–2963. doi: 10.1002/cncr.28168. [DOI] [PubMed] [Google Scholar]
- 16.Carethers JM. Rectifying COVID-19 disparities with treatment and vaccination. JCI Insight. 2021;6(4) doi: 10.1172/jci.insight.147800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths. Ann Intern Med. 2020;174(3):362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu T, Mack JA, Salvatore M, et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an Academic Health Care System. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamoorthy G, Arsene C, Jena N, et al. Racial disparities in COVID-19 hospitalizations do not lead to disparities in outcomes. Public Health. 2021;190:93–98. doi: 10.1016/j.puhe.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabarriti R, Brodin NP, Maron MI, et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an Urban Medical Center in New York. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulware LE. Race disparities in the COVID-19 pandemic-solutions lie in policy, not biology. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.18696. [DOI] [PubMed] [Google Scholar]
- 23.Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowkwanyun M, Reed AL. Racial health disparities and Covid-19 — caution and context. N Engl J Med. 2020;383(3):201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 25.Vepa A, Bae JP, Ahmed F, Pareek M, Khunti K. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab Syndr. 2020;14(5):1043–1051. doi: 10.1016/j.dsx.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 27.Farmer HR, Wray LA, Xian Y, et al. Racial differences in elevated C-reactive protein among US older adults. J Am Geriatr Soc. 2020;68(2):362–369. doi: 10.1111/jgs.16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Race and gender variation in response to evoked inflammationJ Transl Med. Available at: https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-11-63. Accessed September 1, 2021. [DOI] [PMC free article] [PubMed]
- 29.Hausmann LRM, Ibrahim SA, Mehrotra A, et al. Racial and ethnic disparities in pneumonia treatment and mortality. Med Care. 2009;47(9):1009–1017. doi: 10.1097/MLR.0b013e3181a80fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA J Am Med Assoc. 2009;302(11):1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal MK, Kuppermann N, Cleary SD, Teach SJ, Chamberlain JM. Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatr. 2015;169(11):996. doi: 10.1001/jamapediatrics.2015.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ku E, Lee BK, McCulloch CE, et al. Racial and ethnic disparities in kidney transplant access within a theoretical context of medical eligibility. Transplantation. 2020;104(7):1437–1444. doi: 10.1097/TP.0000000000002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3(10):1011–1019. doi: 10.1001/jamacardio.2018.2559. [DOI] [PubMed] [Google Scholar]
- 34.Khandwala P, Desai D, Das DG, Desai A. Racial disparities in cancer clinical trials. J Clin Oncol. 2020;38(29suppl):97. doi: 10.1200/JCO.2020.38.29_suppl.97. [DOI] [Google Scholar]
- 35.Gifford AL, Cunningham WE, Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346(18):1373–1382. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 36.Katz RV, Kegeles SS, Kressin NR, et al. The Tuskegee Legacy Project: willingness of minorities to participate in biomedical research. J Health Care Poor Underserved. 2006;17(4):698–715. doi: 10.1353/hpu.2006.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Getz K, Faden L. Racial disparities among clinical research investigators. Am J Ther. 2008;15(1):3–11. doi: 10.1097/MJT.0b013e31815fa75a. [DOI] [PubMed] [Google Scholar]
- 38.Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126(9):1958–1968. doi: 10.1002/cncr.32755. [DOI] [PubMed] [Google Scholar]