Abstract
Background
Multiple sclerosis (MS) is a chronic immune‐mediated disease of the central nervous system, with an unpredictable course. Current MS therapies such as disease‐modifying therapies focus on treating exacerbations, preventing new exacerbations and avoiding the progression of disability. Siponimod (BAF312) is an oral treatment, a selective sphingosine‐1‐phosphate (S1P) receptor modulator, for the treatment of adults with relapsing forms of MS including active, secondary progressive MS with relapses.
Objectives
To assess the benefits and adverse effects of siponimod as monotherapy or combination therapy versus placebo or any active comparator for people diagnosed with MS.
Search methods
We searched the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Trials Register, which contains studies from CENTRAL, MEDLINE and Embase, and the trials registry databases ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP) on 10 September 2021. We also handsearched relevant journals and screened the reference lists of published reviews and retrieved articles and searched reports (2004 to September 2021) from the MS societies in Europe and America.
Selection criteria
We included randomised parallel controlled clinical trials (RCTs) that evaluated siponimod, as monotherapy or combination therapy, versus placebo or any active comparator in people with MS. There were no restrictions on dose or administration frequency.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We discussed disagreements and resolved them by consensus among the review authors. Our primary outcomes wereworsening disability , relapse and adverse events, and secondary outcomes were annualised relapse rate, gadolinium‐enhancing lesions, new lesions or enlarged pre‐existing lesions and mean change of brain volume. We independently evaluated the certainty of evidence using the GRADE approach. We contacted principal investigators of included studies for additional data or confirmation of data.
Main results
Two studies (1948 participants) met our selection criteria, 608 controls and 1334 treated with siponimod. The included studies compared siponimod with placebo. Overall, all studies had a high risk of bias due to selective reporting and attrition bias.
Comparing siponimod administered at a dose of 2 mg to placebo, we found that siponimod may reduce the number of participants with disability progression at six months (56 fewer people per 1000; risk ratio (RR) 0.78, 95% confidence interval (CI) 0.65 to 0.94; 1 study, 1641 participants; low‐certainty evidence) and annualised relapse rate (RR 0.43, 95% CI 0.34 to 0.56; 2 studies, 1739 participants; low‐certainty evidence). But it might lead to little reduction in the number of participants with new relapse (166 fewer people per 1000; RR 0.38, 95% CI 0.15 to 1.00; 1 study, 94 participants; very low‐certainty evidence). We observed no evidence of a difference due to adverse events for siponimod at 2 mg compared to placebo (14 more people per 1000; RR 1.52, 95% CI 0.85 to 2.71; 2 studies, 1739 participants, low‐certainty evidence). In addition, due to the high risk of inaccurate magnetic resonance imaging (MRI) data in the two included studies, we could not combine data for active lesions on MRI scans. Both studies had high attrition bias resulting from the unbalanced reasons for dropouts among groups and high risk of bias due to conflicts of interest. Siponimod may reduce the number of gadolinium‐enhancing T1‐weighted lesions at two years of follow‐up (RR 0.14, 95% CI 0.10 to 0.19; P < 0.0001; 1 study, 1641 participants; very low‐certainty evidence). There may be no evidence of a difference between groups in the number of participants with at least one serious adverse event excluding relapses (113 more people per 1000; RR 1.80, 95% CI 0.37 to 8.77; 2 studies, 1739 participants; low‐certainty evidence) at six months. No data were available regarding cardiac adverse events.
In terms of safety profile, the most common adverse events associated with siponimod were headache, back pain, bradycardia, dizziness, fatigue, influenza, urinary tract infection, lymphopenia, nausea, alanine amino transferase increase and upper respiratory tract infection. These adverse events have dose‐related effects and rarely led to discontinuation of treatment.
Authors' conclusions
Based on the findings of the RCTs included in this review, we are uncertain whether siponimod interventions are beneficial for people with MS. There was low‐certainty evidence to support that siponimod at a dose of 2 mg orally once daily as monotherapy compared with placebo may reduce the annualised relapse rate and the number of participants who experienced disability worsening, at 6 months. However, the certainty of the evidence to support the benefit in reducing the number of people with a relapse is very low.
The risk of withdrawals due to adverse events requires careful monitoring of participants over time. The duration of all studies was less than 24 months, so the efficacy and safety of siponimod over 24 months are still uncertain, and further exploration is needed in the future. There is no high‐certainty data available to evaluate the benefit on MRI outcomes. We assessed the certainty of the body of evidence for all outcomes was low to very low, downgraded due to serious study limitations, imprecision and indirectness. We are uncertain whether siponimod is beneficial for people with MS.
More new studies with robust methodology and longer follow‐up are needed to evaluate the benefit of siponimod for the management of MS and to observe long‐term adverse effects. Also, in addition to comparing with placebo, more new studies are needed to evaluate siponimod versus other therapeutic options.
Plain language summary
Is siponimod an effective treatment for multiple sclerosis (MS) and does it cause unwanted effects?
Key messages
· We don’t know whether siponimod is an effective treatment for people with multiple sclerosis (MS). At a dose of 2 mg once a day, siponimod may reduce recurrence of symptoms (relapse, calculated as an annual rate) after six months of treatment and may reduce the number of participants whose disability worsens after three months of treatment.
· We don’t know if siponimod causes unwanted effects because studies did not last long enough to fully assess them.
· Future studies should last longer in order to monitor unwanted and beneficial effects of siponimod better, and should use more robust methods. They should compare siponimod with other medicines.
What is multiple sclerosis?
Multiple sclerosis (MS) is a condition caused when the body’s immune system – which defends the body against disease and infection – mistakenly attacks parts of the central nervous system (the brain and spinal cord). Symptoms include problems with balance and walking, and blurred vision. MS is a lifelong condition that can cause serious disability. Some people’s symptoms may develop gradually. However, most people experience ‘attacks’ when new symptoms develop or existing symptoms worsen (called ‘relapse’), followed by periods with no changes to their symptoms (called ‘remittance’). This type of MS is called ‘relapsing remitting’ MS. Eventually, the course of their MS may change. These periods when there are no symptoms, or no worsening of symptoms, may stop and then symptoms may worsen continually. This is called ‘secondary progressive MS’.
How does siponimod work?
Siponimod is a medicine that attaches to the white blood cells (lymphocytes) that attack the central nervous system. This causes the lymphocytes to stay in the lymph glands instead of circulating in the blood to the brain. Fewer lymphocytes reach the brain so the attack by the immune system is reduced. Siponimod is a tablet taken once a day.
What did we want to find out?
We wanted to know if siponimod is an effective treatment for MS and whether it causes any unwanted effects.
We were interested in the number of people:
· who experienced relapses;
· whose disability worsened;
· who left the studies because of unwanted effects of siponimod;
· who developed new or larger brain lesions (damage to the brain); and
· who experienced serious unwanted effects, and which unwanted effects they experienced.
What did we do? We searched for studies that investigated siponimod compared with placebo (a sham medicine that looks and tastes the same as siponimod but with no active ingredients) or another medicine to treat MS. Studies could investigate siponimod alone or combined with another treatment, at any dose over any length of time. Participants had to be aged over 18 years, with confirmed MS.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found two studies with 1948 participants. Both studies compared siponimod with placebo. One study included 1651 people with secondary progressive MS, who were given 2 mg siponimod for up to 3 years. The other study included 297 participants with relapsing remitting MS, who were given siponimod at doses of 10 mg, 2 mg or 0.5 mg for 6 months, or 1.25 mg or 0.25 mg for 3 months. We report the results for 2 mg daily because both studies looked at this dose.
At 2 mg a day, compared to placebo:
· siponimod may lead to a small reduction (166 fewer people per 1000) in the number of people with a new relapse up to six months after starting treatment, and may also reduce relapses when calculated at an annual rate;
· siponimod may reduce the number of people whose disability worsened during the six months after starting treatment (by 56 people per 1000);
· there may be no difference to the number of people (14 more per 1000) who left the studies in the six months after starting treatment due to unwanted effects;
· siponimod may reduce the number of brain lesions of different types after six months and after two years of follow‐up.
· siponimod may make no difference to the number of people with at least one serious unwanted effect in the six months after starting treatment. The most common unwanted effects associated with siponimod were headache, back pain, dizziness, tiredness, influenza, urinary tract infection, reduced white blood cells (lymphopenia), feeling sick, possible liver damage, and infections in the mouth and nose. No information was available about heart‐related unwanted effects.
What are the limitations of the evidence?
Our confidence in the evidence is limited because we found only 2 studies. They did not provide information about everything we were interested and they included people with different types of MS. Also, they did not last long enough to judge the impact of unwanted effects. Both studies were funded by the company that makes siponimod.
Summary of findings
Summary of findings 1. Siponimod compared with placebo for multiple sclerosis.
| Siponimod compared to placebo for multiple sclerosis | ||||||
| Patient or population: people with multiple sclerosis Setting: hospital clinics and specialised multiple sclerosis centres Intervention: siponimod (2 mg) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with siponimod | |||||
| Number of participants with relapses at 6 months | 267 per 1000 |
101 per 1000 (40 to 267) |
RR 0.38 (0.15 to 1.00) |
94 (1 RCT) | ⨁⊝⊝⊝ Very lowa,b,c |
|
| Participants with disability worsening at 6 months | 255 per 1000 | 199 per 1000 (160 to 240) | RR 0.78 (0.65 to 0.94) | 1641 (1 RCT) | ⨁⨁⊝⊝ Lowa,c |
|
| Number of participants who withdrew due to AEs at 6 months | 25 per 1000 |
39 per 1000 (22 to 69) |
RR 1.52 (0.85 to 2.71) |
1739 (2 RCTs) | ⨁⨁⊝⊝ Lowa,c |
|
| Annualised relapse rate | ‐ | ‐ |
RR 0.43 (0.34 to 0.56) |
1739 (2 RCTs) | ⨁⨁⊝⊝ Lowa,c |
|
| Mean number of gadolinium‐enhancing lesions on T1‐weighted brain MRI image at 6 months | ‐ | ‐ |
RR 0.14 (0.10 to 0.19) |
1641 (1 RCT) | ⨁⊝⊝⊝ Very lowa,b,c |
|
| Mean number of new lesions or enlarged pre‐existing lesions on T2‐weighted brain MRI images at 6 months | 1 study reported a small reduction in new lesions (RR 0.19, 95% CI 0.16 to 0.24, P < 0.0001). A second study reported 80% reduction in lesions. | 1739 (2 RCTs) | ⨁⨁⊝⊝ Lowa,c |
We did not perform meta‐analysis because of the incompleteness of data and different time point of evaluation | ||
| Number of participants with at least one SAE at 6 months | 140 per 1000 |
253 per 1000 (52 to 12.28) |
RR 1.80 (0.37 to 8.77) |
1739 (2 RCTs) | ⨁⨁⊝⊝ Lowa,c |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; MRI: magnetic resonance imaging; RR: Risk ratio; SAE: serious adverse event | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to high risk of bias (attrition bias; reporting bias; other bias). bDowngraded one level due to imprecision (low number of participants and wide confidence interval crossing the null). cDowngraded one level due to Indirectness (outcome time frame insufficient to produce benefits attributed to siponimod).
Background
Description of the condition
Multiple sclerosis (MS) is a chronic immune‐mediated disease of the central nervous system that usually begins in young adulthood and causes substantial disability (Mahad 2015). Furthermore, MS has a serious impact on people's quality of life and it is associated with high economic costs for patients, their families and society (Kobelt 2017). With a prevalence of 50 to 300 per 100,000 people, more than two million people are affected worldwide (Thompson 2018a). The female to male sex ratio has increased markedly because of increased incidence of multiple sclerosis in women (Orton 2006).
The aetiology of MS is unclear. Demyelination and significant damage to axons occur in the brain and spinal cord (Thompson 2018a). The course of MS varies greatly and clinical symptoms differ according to the site of lesions in the central nervous system (Newsome 2017). Symptoms include fatigue, weakness, spasticity, visual impairment, tremor, pain, motor paralysis, sexual dysfunction, cognitive impairment, and bladder and bowel problems (Reich 2018; Rommer 2019).
The diagnosis of MS and classification into subtypes are based on the modified 'McDonald criteria' (Thompson 2018b). Approximately 85% of people with MS experience a relapsing remitting course, characterised by acute attacks or relapses with the appearance of neurological deficits followed by a partial or complete remission. More than 50% of people with relapsing remitting MS later change into a more severe course, secondary progressive MS (Scalfari 2014). About 10% to 15% have a primary progressive course from the start or a progressive relapsing course, which is characterised by progression from the start with superimposed relapses (Lublin 1996). Clinically isolated syndrome has been added to the most recent classification (Lublin 2014). Clinically isolated syndrome(CIS) may convert to the relapsing remitting MS subtype.
At present, immune‐modulating, disease‐modifying therapies are indicated for people with relapsing remitting MS or active secondary progressive MS, as evidenced by relapses. A network meta‐analysis of RCTs compared the efficacy and safety of 11 treatments for MS and reached the conclusion that none of the included treatments are effective in decreasing the rate that disability worsens in people with progressive MS (Filippini 2013). Effective treatment options that reduce the frequency of relapses and prevent disability from worsening can have an impact on quality of life of people with MS and help to alleviate the social burden of the disease.
Description of the intervention
In March 2019, the US Food and Drug Administration (FDA) approved siponimod for the treatment of adults with clinically isolated syndrome and relapsing forms of MS including active secondary progressive MS with relapses. In January 2020, siponimod was also approved in the European Union for the treatment of adults with active secondary progressive MS or imaging features of inflammatory activity.
Siponimod is an oral treatment. A titration period is required to reach the maintenance dose of 1 mg or 2 mg. The drug has an average half‐life of about 30 hours and does not need to be phosphorylated in vivo, allowing recovery of peripheral lymphocyte count to a normal range within one week after treatment cessation (Gergely 2012; Shakeri‐Nejad 2015).
The safety profile of siponimod was largely similar to that of fingolimod, with the exception of apparently milder, but not absent, cardiac effects. Like fingolimod, siponimod can cause bradycardia and atrioventricular conduction delays. People treated with siponimod are also at risk for infections, macular oedema, liver injury, hypertension, and respiratory effects consistent with a restrictive airway disease. Progressive multifocal leucoencephalopathy, severe exacerbations after discontinuation, and posterior reversible encephalopathy syndrome are assumed risks associated with this class of therapies, even though they were not observed in siponimod clinical studies (FDA 2019).
How the intervention might work
Siponimod is a sphingosine‐1‐phosphate (S1P) receptor modulator that binds selectively to the S1P1 and S1P5 receptors on lymphocytes and other cell types. Functional antagonism of S1P1 reduces lymphocyte egress from lymph nodes and prevents recirculation of potentially auto‐aggressive lymphocytes and infiltration into the central nervous system (Brinkmann 2009; Chun 2010). Siponimod easily crosses the blood‐brain barrier (Aslanis 2012).
Preclinical data have shown reduction of central nervous system inflammation and indicated effects on repair mechanisms via modulation of S1P1 on astrocytes and S1P5 on oligodendrocytes (Brana 2014; Nuesslein‐Hildesheim 2009). Preclinical studies have also shown that the drug may prevent synaptic neurodegeneration (Gentile 2016), and promote remyelination of the central nervous system (Jackson 2011). Recent experiments on human astrocytes suggest that during neuroinflammation, targeting of S1P1 via siponimod may modulate key astrocyte functions and thereby attain neuroprotection indirectly (Colombo 2020). Clinical studies have suggested that siponimod may be effective in the treatment of people with MS (Kappos 2016; Kappos 2018). In addition, findings from clinical studies indicate that the drug has significant effects in reducing active brain lesions and in improving the disease course (Selmaj 2013).
Another S1P receptor modulator not selective for specific S1P receptor subtypes, fingolimod, was approved for the treatment of relapsing forms of MS. Fingolimod has a known risk for bradyarrhythmia and atrioventricular conduction block. While the S1P1 receptor appears to mediate lymphocyte sequestration, some of the cardiac and vascular effects of S1P modulators are attributed to the S1P receptor subtype 3. A more selective modulator that binds to receptor subtype 1, but not subtype 3, such as siponimod, would be expected to have a lower incidence of cardiovascular effects, without impacting effects on lymphocyte sequestration (FDA 2019).
Why it is important to do this review
Despite the research and the development of many interventions aimed at reducing disease activity or slowing the progression of MS, this is a debilitating condition, a life‐threatening and life‐limiting disease. Therefore, there is an urgent need for effective treatments for people with progressive forms of MS. The increased receptor selectivity and optimised pharmacokinetic characteristics of siponimod prompt an alternative treatment option for people with MS. It is therefore important to conduct a systematic review to evaluate whether these enhancements translate into improved efficacy and safety .
Objectives
To assess the benefits and adverse effects of siponimod as monotherapy or combination therapy versus placebo or any active comparator for people diagnosed with MS.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised parallel controlled clinical trials (RCTs) of at least one year's duration (treatment and follow‐up) that compared siponimod with placebo or any active comparator in people with MS. To date, no cluster or cross‐over trials have been carried out for evaluating siponimod in people with MS.
Types of participants
We included adults (18 years or older) diagnosed with MS, according to the Poser 1983 or McDonald criteria and its revisions (McDonald 2001; Polman 2011; Thompson 2018b). We considered participants with any form of MS (relapsing remitting, secondary progressive, primary progressive and progressive relapsing), regardless of gender, degree of functionality and disease duration.
Types of interventions
Siponimod, as monotherapy or combination therapy, without restrictions regarding dose, administration frequency or duration of use. We included as a comparison intervention placebo or any active comparator. We also allowed concomitant interventions if studies used them in all the comparison groups.
Types of outcome measures
This review focuses on benefit and safety outcomes.
Primary outcomes
The number of participants who experienced new relapses during the treatment and follow‐up periods. A relapse is defined as the appearance of one or more new symptoms due to MS or the deterioration of pre‐existing symptoms, persisting more than 24 hours in the absence of fever and preceded by a period of stability of at least one month (McDonald 2001).
The number of participants who experienced disability worsening measured by Expanded Disability Status Scale (EDSS; Kurtzke 1983). The EDSS is a common measure of MS disability (0 = normal, 3 = mild disability, 6 = care requirement, 7 = wheelchair use, 10 = death from MS) and is used to measure disability worsening in clinical studies. Disability worsening should be confirmed after at least six months of follow‐up, because worsening confirmed after only three months of follow‐up is considered a surrogate marker for unremitting disability. Disability worsening is defined as at least a 1‐point EDSS increase or a 0.5‐point increase if the baseline EDSS was 5.5 or higher, confirmed during two subsequent neurological examinations separated by at least a six‐month interval free of attacks (Kurtzke 1983).
The number of participants who withdrew due to any adverse event out of the total number of participants randomly assigned to each treatment arm.
We assessed primary outcomes after randomisation or at the end of the follow‐up period, and prespecified the following time‐point intervals: short‐term outcomes (less than six months post‐intervention), interim outcomes (six months to less than12 months post‐intervention) and long‐term outcomes (longer than 12 months post‐intervention). We prioritised long‐term outcomes if they are available, otherwise we included short‐term or interim outcomes.
Secondary outcomes
The annualised relapse rate, defined as the mean number of confirmed relapses per participant, adjusted for the duration of follow‐up to annualise it
The mean number of gadolinium‐enhancing lesions on T1‐weighted brain magnetic resonance imaging (MRI) images
The mean number of new lesions or enlarged pre‐existing lesions on T2‐weighted brain MRI images
The mean change of brain volume measured on MRI
The number of participants with at least one serious adverse event as defined in the study, at the end of the treatment period or follow‐up period
Number of participants reporting specific adverse events, including cardiac effects (bradycardia, atrioventricular conduction delays), infections, macular oedema, liver injury, hypertension, respiratory adverse effects, progressive multifocal leukoencephalopathy and encephalopathy syndromes
We assessed primary outcomes after randomisation or at the end of the follow‐up period. We prioritised long‐term outcomes if they were available, otherwise we included short‐term or interim outcomes.
Search methods for identification of studies
We conducted a systematic search without language restrictions on 18 June 2020.
Electronic searches
We searched the following databases.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 9) in the Cochrane Library, (searched 10 September 2021)
MEDLINE (PubMed; 1966 to 10 September 2021)
Embase (Embase.com; 1974 to 10 September 2021)
We also searched the following clinical trials registries.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; who.int/clinical-trials-registry-platform; searched 10 September 2021)
US National Institutes of Health Clinical Trials Register (www.clinicaltrials.gov/; searched 10 September 2021)
Details of search strategies that we used to identify studies can be found in the Specialist Register section on the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System's website (msrdcns.cochrane.org/).
We have listed the keywords that we used for the electronic search in Appendix 1.
Searching other resources
We reviewed the references of included studies, review articles, and conference abstracts of the main MS meetings (European Committee for Treatment and Research in MS (ECTRIMS); European Federation of Neurological Societies (EFNS); European Neurological Society (ENS); American Academy of Neurology (AAN); American Neurological Association (ANA)) from 1990 to 2021. We contacted study authors to request missing data.
Data collection and analysis
Selection of studies
We used the search strategy described in the Search methods for identification of studies section to obtain titles and abstracts of studies. Two review authors (CLJ and LYF) independently screened the titles and abstracts and discarded studies that were not applicable; however, they initially retained studies and reviews that might include relevant data or information on studies. Two review authors (CLJ and LYF) independently assessed the retrieved abstracts and, when necessary, the full‐text articles to determine which studies satisfied the inclusion criteria. The two review authors compared multiple reports of the same study and used the most comprehensive report. They linked together multiple publications as companion reports, but excluded true duplicates. CLJ and LYF resolved discrepancies in judgement by discussion with a third review author (YL), and reported the excluded studies and their reasons for exclusion in the Characteristics of excluded studies table. We recorded the selection process in sufficient detail to complete a PRISMA flow chart (Moher 2009).
Data extraction and management
Two review authors (CLJ and YPJ) independently extracted data from the selected studies using a predefined data extraction form in an Excel spreadsheet. They resolved any disagreements by discussion with a third review author (YL) and entered the data into Review Manager 5 (RevMan 5; Review Manager 2020). When necessary data were unavailable from the study report, we tried to obtain them through correspondence with the study authors.
We extracted from each included study the following information.
Participants: inclusion and exclusion criteria, number of randomised participants, age, gender, clinical baseline characteristics, source of recruitment, type of MS, follow‐up length, and other relevant setting and demographic data
Intervention: dosage, frequency, administration route, treatment duration, co‐interventions, rescue medication and compliance
Comparison: placebo or active control
Outcomes: measures and results of the primary and secondary outcomes that were reported in the included studies (Types of outcome measures).
For dichotomous outcomes we extracted the number of participants who experienced the event on each treatment. For continuous outcomes we extracted mean and standard deviation of the comparison groups. We extracted study arm‐level data when possible. When arm‐level data were not available we extracted effect sizes. We extracted data at the study authors' defined timing points.
Other data
From each included study we extracted data on the following.
Methods: study design, randomisation method; allocation concealment; blinding of participants, personnel and outcome assessors; number of participants withdrawn or lost to follow‐up
Publication details: first author or acronym; number of centres; year of publication; years that the study was conducted (recruitment and follow‐up); publication (full‐text publication, abstract publication, unpublished data); early termination of study
Conflict of interests of study authors and funding source.
Assessment of risk of bias in included studies
Two review authors (YPJ, LYF) independently assessed the risks of bias of each included study according to the Cochrane risk of bias tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We assessed the risk of bias for seven domains, including sequence generation, allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting and other potential sources of bias. We judged each domain as being at low, high or unclear risk of bias. We reached an overall risk of bias judgement for each included study according to the following criteria:
low risk of bias: low risk of bias for all domains;
unclear risk of bias: unclear risk of bias in at least one domain, but not at high risk of bias for any domain;
high risk of bias: high risk of bias in at least one domain, or unclear risk of bias for multiple domains in a way that substantially lowers confidence in the result.
We resolved any disagreement by discussion to reach consensus and, if needed, by discussion with a third review author (YL).
Measures of treatment effect
We calculated dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcomes, we calculated mean difference (MD), or standardised mean difference (SMD) for the same continuous outcome measured with different metrics, and the 95% CIs.
Unit of analysis issues
We included studies with a parallel‐group design, with participants randomised to either intervention or comparison, with subsequent analysis at individual allocation level. We treated multi‐arm studies as multiple, independent, two‐arm studies in pairwise meta‐analysis. We did not find any cluster‐randomised trials or studies with multiple treatment groups that assessed siponimod interventions for MS.
Dealing with missing data
We contacted the study authors to retrieve missing data. We recorded the answers obtained and the dates of our contacts. In order to assess the effect of missing outcome data where not reported or provided, we assumed that both treated and control group participants who were missing had an unfavourable outcome (likely scenario).
Assessment of heterogeneity
We assessed the clinical heterogeneity of the included studies by comparing participants' characteristics (MS type, age, gender, and disease duration), interventions (administration method, dosage and duration, control intervention), using information reported in the Characteristics of included studies. We assessed statistical heterogeneity among the included studies using the Chi2 test and the I2 statistic (Higgins 2003). We planned to perform subgroup and sensitivity analyses to consider possible reasons for heterogeneity when the I2 statistic value was greater than 50% (substantial heterogeneity; Deeks 2021) .
Assessment of reporting biases
We did not perform a funnel plot analysis because we included fewer than 10 studies in the review (Egger 1997). If we include 10 or more RCTs in meta‐analysis in future updates, we will examine potential publication bias using a funnel plot (Page 2021).
Data synthesis
We used Review Manager 2020 to synthesise the available data. We planned to pool results from clinically similar studies. For dichotomous outcomes, we combined RRs with 95% CIs from included studies. We reported adverse event outcomes narratively if a quantitative analysis was not possible. For continuous outcomes, we calculated MD, or SMD if studies measured the outcome on different assessment scales, with 95% CIs. We combined data using a random‐effects model, because we assumed that the studies were not all estimating the same intervention effect, and were estimating intervention effects that follow a distribution across studies (DerSimonian 1986).
If there is an acceptable level of heterogeneity, we planned to perform a meta‐analysis with appropriate data using a random‐effects model in Review Manager 2020. If meta‐analysis was not appropriate because of unacceptable heterogeneity, we presented the results of individual studies in a narrative synthesis (qualitative analysis).
Subgroup analysis and investigation of heterogeneity
For the primary outcomes,we planned to perform the following subgroup analysis to explore potential sources of heterogeneity.
Type of MS (e.g. relapsing remitting, secondary progressive, primary progressive, progressive relapsing)
Dose of siponimod (0.5 mg/day, 2 mg/day, or 10 mg/day)
Duration of follow‐up (12 months, 24 months, 36 months)
Baseline disability score (EDSS score ≤ 3.5 or ≥ 4)
We compared subgroups using the formal statistical test for subgroup differences in Review Manager 2020. We interpreted the results with caution. However, we did not carry out subgroup analyses to consider disease type, duration of follow‐up and disability at baseline due to lack of available data. We only performed subgroup analysis for the different doses of siponimod. If enough data are available in future updates, we intend to continue the subgroup analysis.
Sensitivity analysis
If a sufficient number of studies had been included, we would have undertaken sensitivity analyses to assess the robustness of our review results. Where possible, in future updates, we will assess the impact of studies whose results for important outcomes (included in the summary of findings table) we judge to be at high risk of bias or unclear risk of bias, by removing them from the analysis. We will use the sensitivity analyses to inform the downgrading decisions relating to risk of bias. We also conducted a sensitivity analysis using a likely scenario to assess the impact of missing outcome data.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review as summary of findings tables, according to Cochrane guidance (Schünemann 2021). We provided estimates based on the methodology developed from the GRADE Working Group (Atkins 2004). In the summary of findings tables we included comparison of siponimod with placebo, or with active comparator treatment, and an overall assessment of the evidence for important outcomes.
Number of participants who experienced relapses
Number of participants who experienced disability worsening
Number of participants withdrawn due to adverse events
Annualised relapse rate
Mean number of gadolinium‐enhancing lesions on T1‐weighted brain MRI images
Mean number of new lesions or enlarged pre‐existing lesions on T2‐weighted brain MRI images
Number of participants with at least one serious adverse event
In the summary of findings table, we prioritised long‐term outcomes (longer than 24 months) if they were available, otherwise we included short‐term or interim outcomes.
Two review authors (CLJ and WXQ) independently assessed the certainty of the evidence for each outcome considering risk of bias, indirectness, inconsistency, imprecision of effect estimates, and risk of publication bias using GRADEpro GDT software. We assigned one of four levels of certainty of evidence: high, moderate, low, or very low. We justified our decisions to downgrade the certainty of evidence using footnotes in the summary of findings table and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
We summarised the characteristics of participants, interventions, and outcomes of the included studies in the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
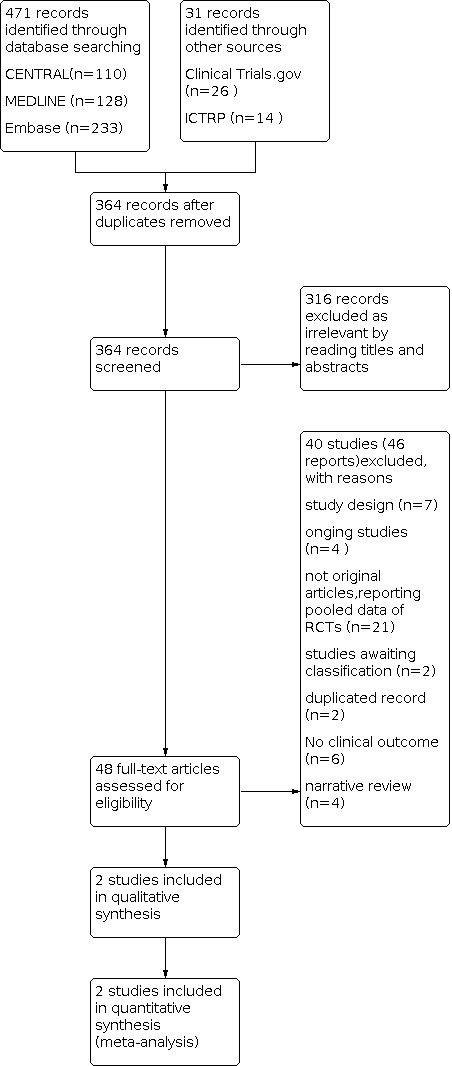
The search strategy identified 471 records (up to 10 September 2021 ): 110 in CENTRAL, 128 in MEDLINE, 233 in Embase, 26 in ClinicalTrials.gov, and 14 in the WHO International Clinical Trials Registry Platform (ICTRP). After removing duplicates (107 references), two review authors (CLJ and LYF) independently screened the titles and abstracts of 364records, excluded 316 records and considered a total of 48 references to be potentially eligible. After reading the full text, 46 reports were ancillary to these primary studies. Finally, we included a total of two RCTs. We found six ongoing studies; four are still ongoing (Characteristics of ongoing studies), and two have been completed and are awaiting classification ( NCT01185821; EUCTR2008‐008719‐25 HU Characteristics of studies awaiting classification). The flow diagram of the process of study identification and selection is presented in Figure 1 (Moher 2009).
1.
Included studies
The review included two studies involving 1948 participants (Kappos 2018; Selmaj 2013). Kappos 2018 compared the efficacy and safety of siponimod 2 mg/day versus placebo for 1651 adults wth secondary progressive MS. While Selmaj 2013 evaluated the dose‐response relation, safety and tolerability of the five doses of siponimod compared with placebo in 297 participants with relapsing remitting MS over three months of treatment. Siponimod was administered orally in both studies (Selmaj 2013; Kappos 2018). Details descriptions of these RCTs are available in the Characteristics of included studies table.
Date of publication
The included studies were published in 2013 (Selmaj 2013), and 2018 (Kappos 2018).
Characteristics of the study design and setting
Both studies were multi‐centre, randomised, double‐blind, controlled, parallel‐group studies: one study was conducted in 292 hospital clinics and specialised MS centres in 31 countries (Kappos 2018), and the other was conducted in 73 medical centres in Europe and North America (Selmaj 2013).
Characteristics of the participants
All participants had a diagnosis of definite MS according to McDonald's diagnostic criteria (McDonald 2001), and were aged from 18 to 60 years. The participants in Selmaj 2013 had had at least one relapse during the previous year, at least two clinical relapses in the previous two years, or one or more gadolinium‐enhancing lesions on MRI at screening, and an EDSS score of 0 to 5. The participants in Kappos 2018 had a history of relapsing remitting MS, EDSS progression in the two years, and no relapse in the three months before randomisation. Baseline demographic and clinical characteristics were well balanced among the groups in the two studies.
Characteristics of the interventions and controls
Both studies assessed the benefits and adverse effects of siponimod on people with MS. Participants in Kappos 2018 received oral siponimod 2 mg once daily or a matching placebo for up to three years or until the occurrence of a prespecified number of confirmed disability progression events. Participants in Selmaj 2013 received once‐daily siponimod 10 mg, 2 mg, or 0.5 mg, or placebo for 6 months in cohort 1, and received siponimod 1.25 mg, siponimod 0.25 mg, or placebo once‐daily for three months in cohort 2.
Characteristics of the outcome measures
The studies reported most outcome measures in the current review. One study reported the number of confirmed disability progression events and the mean change of brain volume, confirmed disability progression was defined as a 1‐point increase in EDSS if the baseline score was 3 to 5, or a 0.5‐point increase if the baseline score was 5.5 to 6.5, confirmed at a scheduled visit at least three months later (Kappos 2018). Both studies reported the number of new or enlarging T2 lesions and the number of new T1 gadolinium‐enhancing lesions. Both studies reported the annualised relapse rate, the number of participants with adverse events, number of participants with serious adverse events and number of participants who withdrew or dropped out from the study because of adverse events.
Excluded studies
Detailed descriptions of excluded studies with reason for exclusion is provided in the Characteristics of excluded studies. Overall, we excluded 40 studies. Reasons for exclusion included:
seven studies for ineligible study design (not RCTs; Hartung 2013; Kappos 2014a; Kappos 2016; Kappos 2017b;Arnold 2020;Shah 2020;Weber 2020),
six studies had no clinical MS‐related outcomes (Kappos 2013; Kappos 2014b; Kappos 2015;Hobart 2021;Penner 2020;Wu 2020),
21 studies were not original articles that reported pooled data of studies (Cree 2019; Fox 2017; Fox 2017a; Giovannoni 2017; Gold 2019; Kappos 2016a; Kappos 2016b; Kappos 2017a; Li 2012a; Li 2012b; Selmaj 2011; Stuve 2012; Stuve 2013; Vermersch 2017; Vermersch 2019;Bar‐Or 2020; Benedict RHB 2021; Giovannoni 2017; Gold 2020; Kappos 2020; Vermersch 2020), and
four studies were narrative reviews (Diener 2018; Gajofatto 2020;Krasnov 2021; Synnott 2020).
two studies were duplicates records (NCT00879658; NCT01665144)
Risk of bias in included studies
Further details of this assessment are available in the Characteristics of included studies and are also presented in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
In Kappos 2018 and Selmaj 2013, sequence generation and allocation concealment were adequate (low risk). Random assignments in both studies were via centralised interactive voice‐response system. Selmaj 2013 generated the allocation sequence by a randomisation number list, Kappos 2018 by blocked randomisation with a block size of six. Both studies reported their methods and we judged them to be appropriate.
Blinding
Both included studies are described as double‐blinded, and reported adequate blinding of participants and personnel. All participants, investigator staff, assessing doctor, and the trained and certified assessor were masked to treatment assignments by use of a blinded treatment code. The drug in the treatment group was reported as identical in appearance to the placebo in the control group. We judged Kappos 2018 and Selmaj 2013 to be at low risk of bias for blinding.
Incomplete outcome data
Both studies provided enough details about the number of and the reasons for drop out. Both studies had a high risk of attrition bias due to a high dropout rate. In Kappos 2018 the dropout rate was 19.6% (18.3% and 22.3% in the siponimod and placebo group, respectively), and in Selmaj 2013 the dropout rate was 11.4%. The reasons and the number for dropping out were carefully recorded but to a large degree they were not balanced between the groups. The risk of attrition bias was high.
Selective reporting
Neither included study provided enough information to assess reporting bias. No full protocol or statistical analysis plan was available in the public domain. We judged Kappos 2018 and Selmaj 2013 at high risk of selective reporting.
Other potential sources of bias
Both studies were sponsored by Novartis Pharma AG. In Kappos 2018, the funder participated in the study design and conduct, data collection, management, analysis and interpretation, and the writing of the study report. In Selmaj 2013, Novartis Pharma AG was involved in the study design, and some study authors are employed by Novartis and contributed to its preparation. We assessed both studies as unclear risk because they received funding but it was unclear if it had an impact on results. Details of other potential sources of bias are reported in the risk of bias tables.
Effects of interventions
See: Table 1
See: Table 1.
We planed to analyse the results at the time points 12, 24 and 36 months but it was not possible because of the small number of included studies. We therefore analysed the available time point results as reported in the studies.
Primary outcomes
Benefit
1. The number of participants who experienced at least one relapse at six months
Only one study assessed this outcome at six months (Selmaj 2013). Overall, the risk of new relapse in participants receiving 10 mg, 2 mg and 0.5 mg siponimod was 18%, 10.2% and 23.3% respectively. To investigate the effect of different doses (0.5 mg, 2 mg, and 10 mg) of siponimod, we performed a subgroup analysis. Subgroup analysis suggested no clear difference between 0.5 mg and 10 mg of siponimod (RR 0.87, 95% CI 0.42 to 1.81; RR 0.68, 95% CI 0.31 to 1.45, respectively), there was a slight reduction in relapse with 2 mg of siponimod administration at six months (RR 0.38, 95% CI 0.15 to 1.00; Analysis 1.4) .
1.4. Analysis.

Comparison 1: Comparison 1: number of participants with at least one relapse at 6 months, Outcome 4: Number of participants with relapses of different doses of siponimod, at 6 months
2. The number of participants who experienced disability worsening measured by Expanded Disability Status Scale (EDSS) at three and six months
Only one study contributed to the analysis of disability worsening at three and six months (Kappos 2018). Based on such data, the risk of disability worsening at three months in participants receiving siponimod treatment was 26.3%, significantly lower than that in participants receiving placebo (31.7%). Compared to placebo, results indicated fewer participants in the siponimod‐treated group experienced disability progression than in the placebo‐treated group at three months' follow‐up (RR 0.83, 95% CI 0.71 to 0.97; 1 study, 1641 participants; Analysis 2.1). In addition, Kappos 2018 reported that the proportions of participants with progression of disability at six months of follow‐up were 19.9% in the siponimod‐treated group and 25.5% in the placebo‐treated group. The results showed that, compared with placebo, 2 mg of siponimod as monotherapy may reduce the proportion of participants with disability progression at six months of follow‐up (RR 0.78, 95% CI 0.65 to 0.94; 1 study, 1641 participants; Analysis 2.2).
2.1. Analysis.

Comparison 2: Comparison 2: number of participants with disability worsening, Outcome 1: Participants with disability worsening at 3 months
2.2. Analysis.

Comparison 2: Comparison 2: number of participants with disability worsening, Outcome 2: Participants with disability worsening at 6 months
No studies provided data for the 12‐month analysis.
3. The number of participants who withdrew from the study because of any adverse events
Both included studies provided data to calculate the number of participants treated with siponimod compared to placebo who withdrew from the study due to any adverse events (Kappos 2018; Selmaj 2013). The results indicated no clear difference with siponimod at 0.5 mg for participants who withdrew due to adverse events at six months' follow‐up (RR 2.62, 95% CI 0.54 to 12.77; Analysis 3.1). Similar results were found when siponimod was used at 2 mg (RR 1.52, 95% CI 0.85 to 2.71; Analysis 3.1). The risk of discontinuing 10 mg of siponimod due to adverse events compared to placebo significantly increased (RR 4.50, 95% CI 1.04 to 19.45; Analysis 3.1).
3.1. Analysis.

Comparison 3: Comparison 3: number of participants who withdrew due to any adverse event, Outcome 1: Number of participants who withdrew due to adverse events (different dose of siponimod)
Secondary outcomes
Benefit
1. The annualised relapse rate
Both studies reported the annualised relapse rate and provided enough information for synthesis of data (Kappos 2018; Selmaj 2013). The results showed that 2 mg of siponimod as monotherapy reduced the annualised relapse rate during the follow‐up period of six months (RR 0.43, 95% CI 0.34 to 0.56; 2 studies, 1739 participants; Analysis 4.1).
4.1. Analysis.

Comparison 4: Comparison 4: annualised relapse rate, Outcome 1: Siponimod 2 mg versus placebo, annualised relapse rate
2. The mean number of gadolinium‐enhancing T1‐weighted lesions
Only one study reported the number of gadolinium‐enhancing T1‐weighted lesions (Kappos 2018). Compared to placebo, the results of Kappos 2018 showed that dosages of 2 mg siponimod reduced the number of gadolinium‐enhancing T1‐weighted lesions at two years of follow‐up (RR 0.14, 95% CI 0.10 to 0.19; P < 0.0001).
3. The mean number of new lesions or enlarged pre‐existing lesions on T2‐weighted brain MRI images
We did not perform meta‐analysis because of the incompleteness of data and different time points of evaluation. Kappos 2018 reported the mean number of new or enlarging pre‐existing lesions on T2‐weighted images. Compared to placebo, there was a clear difference in the reduction of the number of new or enlarging T2‐weighted hyper intense lesions for dosages of 2 mg siponimod at six months (RR 0.19, 95% CI 0.16 to 0.24; P < 0.0001). In Selmaj 2013, after six months of treatment, reductions in the number of new or newly enlarged T2 lesions versus placebo were significant for all siponimod doses apart from for siponimod 0.5 mg (84% for siponimod 10 mg; 80% for siponimod 2 mg; and 58% for siponimod 0.5 mg).
4. The mean change of brain volume measured on MRI
We were unable to undertake a meta‐analysis because of lack of data and different time points of evaluation. Thus we reported only descriptive data on MRI measures of brain volume. Only one study contributed to the analysis of mean change of brain volume (Kappos 2018). The results showed that the brain volume decreased at a lower rate with siponimod than with placebo (adjusted mean percentage brain volume change over months 12 and 24 (−0.50% versus −0.65%; between‐group difference 0.15%, 95% CI 0.07 to 0.23; P = 0.0002).
Safety
5. The number of participants with at least one serious adverse event (serious adverse event)
Data were available from both included studies for this outcome (Kappos 2018; Selmaj 2013). There was no obvious difference in the number of participants with at least one serious adverse event, excluding relapses, between participants receiving 2 mg siponimod and participants receiving placebo at six months (RR 1.80, 95% CI 0.37 to 8.77; P = 0.47; 2 studies, 1739 participants; Analysis 5.1). In addition, the pooled risk of serious adverse events, excluding relapses, in participants receiving 1.25 mg or 10 mg of siponimod was not higher than that in participants receiving placebo at six months (RR 1.98, 95% CI 0.10 to 39.07; P = 0.65; 1 study, 58 participants; and RR 6.31, 95% CI 0.34 to 118.98; P = 0.22; 1 study, 95 participants; Analysis 5.1, respectively). however, compared with the placebo group, the pooled results showed that the incidence of serious adverse events excluding relapses was slightly increased by 0.5 mg of siponimod administration at six months (RR 17.77, 95% CI 1.06 to 298.79; P = 0.05; 1 study, 88 participants; Analysis 5.1).
5.1. Analysis.

Comparison 5: Comparison 5: number of participants with at least one serious adverse event, Outcome 1: Any serious adverse event
6. The number of participants reporting specific adverse events
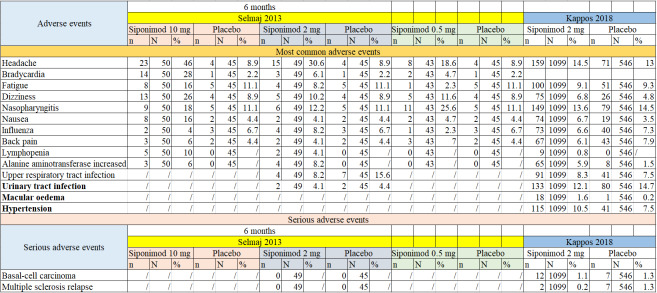
Both included studies reported adverse effects; detailed descriptive data on the type of adverse events, as reported in the included studies, are provided in Figure 4. The most common adverse events included headache (RR 1.75, 95% CI 0.59 to 5.18; P = 0.31; 2 studies, 1739 participants; Analysis 6.1); fatigue (RR 0.96, 95% CI 0.70 to 1.31; P = 0.78; 2 studies, 1739 participants; Analysis 6.2); dizziness (RR 1.40, 95% CI 0.93 to 2.11, P = 0.11; 2 studies, 1739 participants; Analysis 6.3); nasopharyngitis (RR 0.94, 95% CI 0.74 to 1.21, P = 0.65; 2 studies, 1739 participants; Analysis 6.4); nausea (RR 1.85, 95% CI 1.15 to 2.98; P = 0.01; 2 studies, 1739 participants; Analysis 6.5); influenza (RR 0.91, 95% CI 0.63 to 1.30; P = 0.61; 2 studies, 1729 participants; Analysis 6.6); back pain (RR 0.78, 95% CI 0.54 to 1.12; P = 0.18; 2 studies, 1739 participants; Analysis 6.7); lymphopenia (RR 6.73, 95% CI 0.85 to 53.14, P = 0.07; 2 studies, 1739 participants; Analysis 6.8); alanine amino transferase increase (RR 4.21, 95% CI 2.08 to 8.53, P < 0.0001; 2 studies, 1739 participants; Analysis 6.9); upper respiratory tract infection (RR 0.94, 95% CI 0.52 to 1.71; P =0.84; 2 studies, 1739 participants; Analysis 6.10); urinary tract infection (RR 0.83, 95% CI 0.64 to 1.07, P = 0.15; 2 studies, 1739 participants; Analysis 6.11) and bradycardia (RR 2.76, 95% CI 0.30 to 25.54, P = 0.37; 1 study, 94 participantsAnalysis 6.12;. Overall, compared with the placebo group, There were no clear differences in the number of participants with headache, fatigue, dizziness, nasopharyngitis, influenza, back pain, lymphopenia, upper respiratory tract infection, urinary tract infection and bradycardia .
4.
Detailed descriptive data on the type of adverse events
6.1. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 1: Siponimod 2 mg versus placebo, number of participants with headache
6.2. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 2: Siponimod 2 mg versus placebo, number of participants with fatigue
6.3. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 3: Siponimod 2 mg versus placebo, number of participants with dizziness
6.4. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 4: Siponimod 2 mg versus placebo, number of participants with nasopharyngitis
6.5. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 5: Siponimod 2 mg versus placebo, number of participants with nausea
6.6. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 6: Siponimod 2 mg versus placebo, number of participants with influenza
6.7. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 7: Siponimod 2 mg versus placebo, number of participants with back pain
6.8. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 8: Siponimod 2 mg versus placebo, number of participants with lymphopenia
6.9. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 9: Siponimod 2 mg versus placebo, number of participants with alanine aminotransferase increase
6.10. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 10: Siponimod 2 mg versus placebo, number of participants with upper respiratory tract infection
6.11. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 11: Siponimod 2 mg versus placebo, number of participants with urinary tract infection
6.12. Analysis.

Comparison 6: Comparison 6: number of participants reporting specific adverse events, Outcome 12: Siponimod versus placebo, number of participants with bradycardia
Discussion
Summary of main results
This systematic review aimed to evaluate the effects (benefits and adverse effects) of siponimod interventions for people with MS. Two RCTs involving 1942 participants with relapsing remitting MS and secondary progressive MS contributed to the final analysis. Participants with relapsing remitting MS had had at least one relapse during the previous year, and an EDSS of 0 to 5. Participants with secondary progressive MS had a score of 3 to 6.5 on the EDSS and a history of relapsing remitting MS. The treatment duration was three months and six months in Selmaj 2013, and for up to three years in Kappos 2018.
Selmaj 2013 reported siponimod, 10 mg/day, 2 mg/day, 0.5 mg/day, 1.25 mg/day or 0.25 mg/day, administered once daily for relapsing remitting MS and Kappos 2018 reported siponimod 2 mg/day, administered once daily for secondary progressive MS. All studies were sponsored by Novartis Pharma AG and we judged them to be at high risk of bias due to selective reporting and attrition bias. We calculated the treatment effects of interventions based on the available data in the original studies.
Compared to placebo, administration of siponimod at a dose of 2 mg orally once daily as monotherapy may reduce the number of participants with relapse and disability worsening by six months. Compared to placebo, there was also a similar treatment effect on annualised relapse rate for 2 mg dosages of siponimod. However, there was no clear difference in reducing the number of participants with relapse for 10 mg and 0.5 mg dosages of siponimod. We did not conduct meta‐analyses for the MRI outcomes because there was a high risk of selection bias for MRI outcomes and imprecision of MRI data in both studies. Thus, we reported only descriptive data on clinical and MRI measures of outcomes. The results indicated that brain volume decreased at a lower rate with 2 mg of siponimod than with placebo.
Overall, in terms of safety, the risks for any adverse events in participants receiving 2 mg and 10 mg of siponimod were slightly higher than in participants receiving placebo. The risks for any adverse events in participants receiving 0.5 mg siponimod were similar to those in participants receiving placebo at six months of follow‐up. The risks for study drug discontinuation due to adverse events were increased by 10 mg doses of siponimod administration. There was no difference in risk of withdrawal due to adverse events for 0.5 mg and 2 mg dosages of siponimod. The most common adverse events included headache, back pain, bradycardia, dizziness, fatigue, influenza, urinary tract infection, lymphopenia, nausea, alanine amino transferase increase and upper respiratory tract infection.
Overall completeness and applicability of evidence
In this review, we included only two RCTs that evaluated the benefit of siponimod as monotherapy versus placebo in people with MS (including relapsing remitting MS and secondary progressive MS). In one included study, participants received three months or six months of treatment. In the other included study, participants were treated for at least 12 months and had a follow‐up of up to 36 months for some outcomes. Considering that MS is a chronic disease, drugs for the treatment of MS require sufficient administration duration and follow‐up to determine efficacy and safety outcomes; this can increase the uncertainty of these findings. We only included one comparison concerning MRI outcomes in this review. We will evaluate ongoing studies of siponimod compared to other approved disease‐modifying drugs in future updates of the review.
We performed meta‐analyses according to different administration doses (10 mg, 2 mg, 1.25 mg, 0.25 mg and 0.5 mg) using the available data from the studies. We must emphasise that we could not perform meta‐analysis, subgroup analysis or sensitivity analysis on all primary or secondary outcomes as planned due to the small number of included studies and this might increase the uncertainty of these findings. Further, the two studies only included people with relapsing remitting MS and secondary progressive MS, and we found no evidence for other forms of the disease.
In summary, the above limitations could impact the applicability of evidence. The available evidence is limited to these specific interventions and participants, and requires us to be cautious in interpreting the results.
Quality of the evidence
This review identified only two studies with 1942 participants. The overall methodological quality of the included studies was affected by attrition biases (see Table 1). Due to the high attrition bias in both studies, we downgraded the certainty of the evidence for all included outcomes. We downgraded the outcome of disability worsening due to indirectness of evidence because disability worsening was confirmed in under six months. The above factors led to low‐certainty evidence for disability progression. We further downgraded the certainty of evidence for relapses and withdrawals due to adverse events because of imprecision (low number of participants and wide confidence interval crossing the null). The quality of MRI data reported in the primary studies was poor. Overall, we gave a GRADE rating of low certainty for relapses, withdrawals due to adverse events, disability progression, and annualised relapse rate. Overall, the certainty of the body of evidence obtained for each outcome was very low to low.
Potential biases in the review process
To avoid the introduction of bias, we strictly followed all of the recommendations on searching, study selection, data collection, and data analysis from the Cochrane Handbook for Systematic Reviews of Interventions in this review (Higgins 2021). The search strategy for the studies was broad and sensitive, which suggests the likelihood that all RCTs were identified. The authors of this review had no conflicts of interest. Limitations of the review include the lack of some outcome data of included studies, and no assessment of publication bias through funnel plot analysis because there were fewer than 10 studies included in the meta‐analysis.
Agreements and disagreements with other studies or reviews
During the conduct of this review a non‐Cochrane review assessing siponimod treatments for secondary progressive MS was published (Scott LJ 2020). Scott LJ 2020 focuses on therapeutic efficacy and tolerability data relevant to the use of oral siponimod in adults with secondary progressive MS and summarises its pharmacological properties. The authors only included one study (Kappos 2018), in qualitative synthesis. Kappos 2018 is included in the current review.
Compared with review by Scott LJ 2020, we employed strict inclusion and exclusion criteria in the current review, including a comprehensive search strategy, which takes into account a wide range of outcome indicators. In addition, the current review focused on the different doses of siponimod and we performed subgroup analysis. Furthermore, we assessed the methodological quality of included studies using Cochrane's risk of bias tool, and we conducted a comprehensive analysis on the certainty of evidence for the outcomes using GRADE. Thus, our results might be of great value for providing references for the management of MS.
Authors' conclusions
Implications for practice.
There was low‐certainty evidence to support that siponimod at a dose of 2 mg orally once daily as monotherapy by direct comparison with placebo reduced the annualised relapse rate. There was low‐certainty evidence that 2 mg siponimod reduced the number of participants who experienced disability worsening at six months. In addition, the certainty of the evidence to support the benefit in reducing the number of participants with new relapse is low. There is no high‐certainty evidence available to evaluate the benefit on magnetic resonance imaging outcomes. The most common adverse events included headache, back pain, bradycardia, dizziness, fatigue, influenza, urinary tract infection, lymphopenia, nausea, alanine amino transferase increase and upper respiratory tract infection. The severity of these adverse effects was mostly mild to moderate, but had a dose‐related effect.
Implications for research.
The two key aspects to be considered in evaluating the superiority of disease‐modifying drugs for the treatment of multiple sclerosis are prevention of disability worsening and improvement of quality of life. More randomised controlled trials with high methodological quality are required. In particular: treatment duration and follow‐up needs to be longer; long‐term adverse effects, including progressive multifocal leucoencephalopathy and encephalopathy syndromes need to be observed ‐ there is a lack of data about this; and, in addition to placebo, other active controls should be considered to evaluate the efficacy and safety of siponimod.
What's new
| Date | Event | Description |
|---|---|---|
| 8 December 2021 | Amended | Typo corrected |
History
Protocol first published: Issue 6, 2020 Review first published: Issue 11, 2021
Acknowledgements
We thank the editorial base of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS group for reviewing the protocol.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 "multiple sclerosis"
#2 MeSH descriptor Multiple Sclerosis explode all trees
#3 "Demyelinating disease*"
#4 MeSH descriptor Demyelinating Diseases, this term only
#5 "transverse myelitis"
#6 MeSH descriptor Myelitis, Transverse, this term only
#7 "neuromyelitis optica"
#8 "optic neuritis"
#9 MeSH descriptor Optic Neuritis explode all trees
#10 "encephalomyelitis acute disseminated"
#11 MeSH descriptor Encephalomyelitis, Acute Disseminated explode all trees
#12 "devic"
#13 "multiple sclerosis":ti,ab,kw
#14 (demyelinating NEXT disease):ti,ab,kw
#15 (transverse NEXT myelitis):ti,ab,kw
#16 "neuromyelitis optica":ti,ab,kw
#17 "optic neuritis":ti,ab,kw
#18 (devic):ti,ab,kw
#19 "acute disseminated encephalomyelitis":ti,ab,kw
#20 "MS":ti,ab,kw
#21 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20)
#22 Siponimod:ti,ab,kw
#23 BAF312:ti,ab,kw
#24 BAF‐312:ti,ab,kw
#25 baf312:ti,ab,kw
#26 baf 312:ti,ab,kw
#27 (#22 OR #23 OR #24 OR #25 OR #26)
#28 #21 AND #27
MEDLINE (PubMed)
#1 Siponimod[Title/Abstract]
#2 BAF312[Title/Abstract])
#3 BAF‐312[Title/Abstract]
#4 baf312[Title/Abstract]
#5 baf 312[Title/Abstract]
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 "Multiple Sclerosis"[mh]
#8 "Myelitis, Transverse"[mh:noexp]
#9 "Demyelinating Diseases"[mh:noexp]
#10 "Encephalomyelitis, Acute Disseminated"[mh:noexp]
#11 "Optic Neuritis"[mh]
#12 "multiple sclerosis"[Title/Abstract]
#13 "neuromyelitis optica"[Title/Abstract]
#14 "transverse myelitis"[Title/Abstract]
#15 "encephalomyelitis"[Title/Abstract]
#16 "devic"[Title/Abstract]
#17 "MS"[Title/Abstract]
#18 "optic neuritis"[Title/Abstract]
#19 "demyelinating disease*"[Title/Abstract]
#20 "acute disseminated encephalomyelitis"[Title/Abstract]
#21 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR#19 OR #20
#22 #6 AND #21
Embase
#1 'Siponimod'/exp
#2 Siponimod:ti,ab
#3 baf312:ti,ab
#4 'baf 312':ti,ab,
#5 #1 OR #2 OR #3 OR #4
#6 'demyelinating disease'/exp
#7 'encephalomyelitis'/exp
#8 'myelooptic neuropathy'/exp
#9 'multiple sclerosis'/exp
#10 'neuromyelitis optica':ab,ti
#11 'multiple sclerosis':ti,ab
#12 encephalomyelitis:ab,ti
#13 devic:ti,ab
#14 'MS':ti,ab
#15 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
#16 #5 AND #15
Appendix 2. Glossary
ARR: annualised relapse rate
CIS: clinically isolated syndrome
CI: confidence interval
EDSS: Expanded Disability Status Scale
FDA: Food and Drug Administration
MRI: magnetic resonance imaging
MD: mean difference
PPMS: primary progressive multiple sclerosis
PRMS: progressive‐relapsing multiple sclerosis
RCT: randomised controlled trial
RRMS: relapsing remitting multiple sclerosis
RR: risk ratio
SPMS: secondary progressive multiple sclerosis
S1P: sphingosine‐1‐phosphate
SMD: standardised mean difference
Data and analyses
Comparison 1. Comparison 1: number of participants with at least one relapse at 6 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Siponimod 0.5 mg versus placebo | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.42, 1.81] |
| 1.2 Siponimod 2 mg versus placebo | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 1.00] |
| 1.3 Siponimod 10 mg versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.45] |
| 1.4 Number of participants with relapses of different doses of siponimod, at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Siponimod 0.5 mg versus placebo | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.42, 1.81] |
| 1.4.2 Siponimod 10 mg versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.31, 1.45] |
| 1.4.3 Siponimod 2 mg versus placebo | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 1.00] |
1.1. Analysis.

Comparison 1: Comparison 1: number of participants with at least one relapse at 6 months, Outcome 1: Siponimod 0.5 mg versus placebo
1.2. Analysis.

Comparison 1: Comparison 1: number of participants with at least one relapse at 6 months, Outcome 2: Siponimod 2 mg versus placebo
1.3. Analysis.

Comparison 1: Comparison 1: number of participants with at least one relapse at 6 months, Outcome 3: Siponimod 10 mg versus placebo
Comparison 2. Comparison 2: number of participants with disability worsening.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Participants with disability worsening at 3 months | 1 | 1641 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.71, 0.97] |
| 2.2 Participants with disability worsening at 6 months | 1 | 1641 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
Comparison 3. Comparison 3: number of participants who withdrew due to any adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number of participants who withdrew due to adverse events (different dose of siponimod) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Siponimod 10 mg versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 4.50 [1.04, 19.45] |
| 3.1.2 Siponimod 0.5 mg versus placebo | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [0.54, 12.77] |
| 3.1.3 Siponimod 2 mg versus placebo | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.85, 2.71] |
Comparison 4. Comparison 4: annualised relapse rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Siponimod 2 mg versus placebo, annualised relapse rate | 2 | 1739 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.34, 0.56] |
Comparison 5. Comparison 5: number of participants with at least one serious adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Any serious adverse event | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Siponimod 2 mg versus placebo | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.37, 8.77] |
| 5.1.2 Siponimod 10 mg versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 6.31 [0.34, 118.98] |
| 5.1.3 Siponimod 1.25 mg versus placebo | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.10, 39.07] |
| 5.1.4 Siponimod 0.5 mg versus placebo | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 17.77 [1.06, 298.79] |
| 5.2 Any adverse event | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Siponimod 2 mg versus placebo | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.01, 1.25] |
| 5.2.2 Siponimod 10 mg versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.03, 1.40] |
| 5.2.3 Siponimod 0.5 mg versus placebo | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.30] |
5.2. Analysis.

Comparison 5: Comparison 5: number of participants with at least one serious adverse event, Outcome 2: Any adverse event
Comparison 6. Comparison 6: number of participants reporting specific adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Siponimod 2 mg versus placebo, number of participants with headache | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.59, 5.18] |
| 6.2 Siponimod 2 mg versus placebo, number of participants with fatigue | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.31] |
| 6.3 Siponimod 2 mg versus placebo, number of participants with dizziness | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.93, 2.11] |
| 6.4 Siponimod 2 mg versus placebo, number of participants with nasopharyngitis | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.21] |
| 6.5 Siponimod 2 mg versus placebo, number of participants with nausea | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.15, 2.98] |
| 6.6 Siponimod 2 mg versus placebo, number of participants with influenza | 2 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.30] |
| 6.7 Siponimod 2 mg versus placebo, number of participants with back pain | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.54, 1.12] |
| 6.8 Siponimod 2 mg versus placebo, number of participants with lymphopenia | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 6.73 [0.85, 53.14] |
| 6.9 Siponimod 2 mg versus placebo, number of participants with alanine aminotransferase increase | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [2.08, 8.53] |
| 6.10 Siponimod 2 mg versus placebo, number of participants with upper respiratory tract infection | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.52, 1.71] |
| 6.11 Siponimod 2 mg versus placebo, number of participants with urinary tract infection | 2 | 1739 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 6.12 Siponimod versus placebo, number of participants with bradycardia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.12.1 Siponimod 2 mg versus placebo | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.30, 25.54] |
| 6.12.2 Siponimod 10 mg versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 12.60 [1.73, 92.03] |
| 6.12.3 Siponimod 0.5 mg versus placebo | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.20, 22.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kappos 2018.
| Study characteristics | ||
| Methods | Double‐blind, randomised, phase 3 study Multicentre study was done at 292 hospital clinics and specialised MS centres in 31 countries Recruitment period: between 5 February 2013‐2 June 2015 Population included in data analyses: all randomly assigned (2:1) participants who received once daily oral siponimod 2 mg or placebo for up to 3 years |
|
| Participants | 1651 participants with SPMS
Inclusion criteria:
Key exclusion criteria:
Baseline characteristics were similar between groups Age (mean ± SD): siponimod = 48.0± 7.8 years, placebo = 48.1 ± 7.9 years Sex (women): siponimod = 669 (61%), placebo = 323 (59%) Time since diagnosis of MS (mean ± SD): siponimod = 12.9 ± 7.9 years, placebo = 12.1 ± 7.5 years Time from first symptoms of MS (mean ± SD): siponimod = 17.1 ± 8.4 years, placebo = 16.2 ± 8.2 years Number of relapses in previous year (mean ± SD): siponimod = 0.2 ± 0.5 years, placebo = 0.3 ± 0.6 years Number of relapses in previous 2 years (mean ± SD): siponimod = 0.7 ± 1.2 years, placebo = 0.7 ± 1.2 years Number of participants with gadolinium‐enhancing T1 lesions: siponimod = 237/1105 (21%), placebo = 114/546 (21%) EDSS total score (mean ± SD): siponimod = 5.4 ± 1.1, placebo = 5.4 ± 1.0 |
|
| Interventions | Participants were randomly assigned (2:1) to 1 of the 2 groups
|
|
| Outcomes | Primary outcome measure
CDP was defined as a 1‐point increase in EDSS if the baseline score was 3.0–5.0, or a 0.5‐point increase if the baseline score was 5.5–6.5, confirmed at a scheduled visit at least 3 months later. Secondary outcome measures
|
|
| Notes | The study was sponsored by Novartis Pharmaceuticals. The funder participated in the study design and conduct, data collection, management, analysis and interpretation, and the writing of the study report. ClinicalTrials.gov number: NCT01665144 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure that the treatment assignment was unbiased and concealed from patients and study staff, the randomisation list was produced by an interactive response technology provider using a validated system automating the random assignment of patient numbers to randomisation numbers". Sequence generation was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drug and placebo were identical in packaging, labelling, schedule of administration, appearance, taste, and odour.
Randomisation numbers were linked to the different treatment groups, which in turn were linked to medication numbers. A separate medication list was produced by Novartis drug supply management using a validated system that automated the random assignment of medication numbers to packs containing the study drugs". Allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and study staff remained masked to treatment assignment for the duration of the core part of the study". Participants and personnel were blinded to the allocated interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "1. All EDSS scores were obtained by trained, certified assessors who were not otherwise involved in patient management 2.A full neurological examination, including an assessment of walking range, Functional Systems and Expanded Disability Status Scale (EDSS) score, was obtained every 3 months by a trained and certified assessor". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1327 (80%) of 1651 participants completed the study (903 (82%) on siponimod vs 424 (78%) on placebo). Overall, 324 (19.6%) participants withdrew from study, 18.3% (202/1105) in siponimod, 22.3% (122/546) in placebo group. |
| Selective reporting (reporting bias) | High risk | Did not provide enough information to assess reporting bias, no full protocol or statistical analysis plan was available in the public domain |
| Other bias | Unclear risk | Quote: "The study was sponsored by Novartis Pharmaceuticals.The funder participated in the study design and conduct, data collection, management, analysis and interpretation, and the writing of the study report". It is not clear whether this would have affected the results. |
Selmaj 2013.
| Study characteristics | ||
| Methods | An adaptive, dose‐ranging, randomised, phase 2 study Multicentre study was done at 73 specialised MS centres in Canada, USA, Russia, and 9 European countries (Finland, Germany, Hungary, Italy, Norway, Poland, Spain, Switzerland, and Turkey) Recruitment period: 30 March 2009‐22 October 2010 Population included in data analyses:
2 participant cohorts were tested sequentially, and an interim analysis was performed at 3 months |
|
| Participants | 297 participants with RRMS
Inclusion criteria:
Key exclusion criteria:
MS disease characteristics at baseline were generally balanced between treatment groups. Age (mean ± SD): siponimod 10 mg = 36.4 ± 8.4 years, siponimod 2 mg = 37.4 ± 8.9 years, siponimod 1.25 mg = 35.4 ± 8.9 years, siponimod 0.5 mg = 36.0 ± 8.8 years, siponimod 0.25 mg = 37.4 ± 8.4 years, placebo = 35.4 ± 8.6 years Sex (women): siponimod 10 mg = 30 (60%), siponimod 2 mg = 34 (69%), siponimod 1.25 mg = 31 (74%), siponimod 0.5 mg = 30 (70%), siponimod 0.25 mg = 42 (82%), placebo = 45 (73%) Time from first symptoms of MS (mean ± SD): siponimod 10 mg = 6.0 ± 6.1 years, siponimod 2 mg = 7.2 ± 6.8 years, siponimod 1.25 mg = 7.2 ± 6.6 years, siponimod 0.5 mg = 8.7 ± 7.3 years, siponimod 0.25 mg = 7.7 ± 5.7 years, placebo = 8.0 ± 6.6 years Number of relapses in previous year (mean ± SD): siponimod 10 mg = 1.4 ± 0.8 years, siponimod 2 mg = 1.3 ± 0.6 years, siponimod 1.25 mg = 1.3 ± 0.6 years, siponimod 0.5 mg = 1.5 ± 0.9 years, siponimod 0.25 mg = 1.4 ± 0.8 years, placebo = 1.3 ± 0.6 years Number of relapses in previous 2 years (mean ± SD): siponimod 10 mg = 2.0 ± 1.0 years, siponimod 2 mg = 2.1 ± 1.0 years, siponimod 1.25 mg = 1.8 ± 0.8 years, siponimod 0.5 mg = 1.8 ± 1.0 years, siponimod 0.25 mg = 2.0 ± 0.9 years, placebo = 1.8 ± 0.7 years Number of participants with gadolinium‐enhancing T1 lesions: siponimod 10 mg = 22/50 (44%), siponimod 2 mg = 26/48 (54%), siponimod 1.25 mg = 22/42 (52%), siponimod 0.5 mg = 23/43 (53%), siponimod 0.25 mg = 23/51 (45%), placebo = 35/61 (57%) EDSS total score (mean ± SD): siponimod 10 mg = 2.3 ± 1.0, siponimod 2 mg = 2.4 ± 1.2, siponimod 1.25 mg = 2.0 ± 1.0, siponimod 0.5 mg = 2.2 ± 1.3, siponimod 0.25 mg = 2.3 ± 1.1, placebo = 2.3 ± 1.1 |
|
| Interventions | Participants were randomly assigned to 1 of the 2 groups:
Cohort 1: Experimental group 1: siponimod 10 mg once daily (n = 50) Experimental group 2: siponimod 2 mg once daily (n = 49) Experimental group 3: siponimod 0.5 mg once daily (n = 43) Control group: matching placebo once daily (n = 45) Cohort 2: Experimental group 4: siponimod 1.25 mg once daily (n = 42) Experimental group 5: siponimod 0.25 mg once daily (n = 51) Control group: matching placebo once daily (n = 16) |
|
| Outcomes | Primary outcome measure
Assessed by percentage reduction in monthly number of combined unique active lesions at 3 months for siponimod versus placebo Secondary outcome measures
|
|
| Notes | The study was sponsored by Novartis Pharmaceuticals. Novartis Pharma AG was involved in the study design, and some authors of this paper are employed by Novartis and contributed to its preparation. The study design was approved by the steering committee (appendix) who, in conjunction with Novartis Pharma AG, collected and analysed the data, and contributed to the interpretation of the results ClinicalTrials.gov number: NCT00879658 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central interactive voice‐response system automated the random assignment of patient numbers to randomisation numbers; the randomisation number was linked to a treatment group and a unique medication number". Sequence generation was adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "A central interactive voice‐response system automated the random assignment of patient numbers to randomisation numbers; the randomisation number was linked to a treatment group and a unique medication number". Allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, investigator staff, the independent assessing physician, the independent first‐dose administrator, and sponsor staff involved in the conduct of the study remained masked to treatment allocation from the time of randomisation until database lock". Participants and personnel were blinded to the allocated interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, investigator staff, the independent assessing physician, the independent first‐dose administrator, and sponsor staff involved in the conduct of the study remained masked to treatment allocation from the time of randomisation until database lock". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "We included 188 patients in cohort 1 and 109 in cohort 2; 148 (79%) of individuals in cohort 1 and 104 (95%) individuals in cohort 2 completed the study on the study. Overall, 45 (15.2%) participants withdrew from study, 18.3% (40/188) in cohort 1, 4.6% (5/109) in cohort 2 group. |
| Selective reporting (reporting bias) | High risk | Did not provide enough information to assess reporting bias, no full protocol or statistical analysis plan was available in the public domain. |
| Other bias | Unclear risk | Quote: "1. Novartis Pharma AG was involved in the study design, and some authors of this paper are employed by Novartis and contributed to its preparation.
2.Novartis Pharma AG provided funding for editorial assistance by Oxford PharmaGenesis (Oxford, UK), handling of data by Quintiles, and central laboratory monitoring by CoreLab Partners". It is not clear whether this would have affected the results. |
AE: adverse event; ARR: annualised relapse rate; CDP: confirmed disability progression; CUAL: combined unique active lesions; EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging; MS: multiple sclerosis; RRMS: relapsing remitting multiple sclerosis; SAE: serious adverse event; SD: standard deviation; SPMS: secondary progressive multiple sclerosis;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2020 | Non‐RCT, this is a prospective, MTR EXPAND substudy |
| Bar‐Or 2020 | Pooled data of RCTs: results from the exchange study |
| Benedict RHB 2021 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Cree 2019 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Diener 2018 | This study is a narrative review |
| Fox 2017 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Fox 2017a | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Gajofatto 2020 | This study is a narrative review |
| Giovannoni 2017 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Giovannoni 2020 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Gold 2019 | Pooled data of RCTs: subgroup analysis on participants with active SPMS participants |
| Gold 2020 | Pooled data of RCTs: results from the EXPAND study |
| Hartung 2013 | Design: not RCT |
| Hobart 2021 | No clinical outcomes that met the inclusion criteria |
| Kappos 2013 | No clinical outcomes that met the inclusion criteria |
| Kappos 2014a | Design: not RCT |
| Kappos 2014b | No clinical outcomes that met the inclusion criteria |
| Kappos 2015 | No clinical outcomes that met the inclusion criteria |
| Kappos 2016 | Randomised study comparing different doses of siponimod (10 mg, 2 mg, 1.25 mg, 0.5 mg, and 0.25 mg) without a control group |
| Kappos 2016a | Pooled data of RCTs: subgroup analysis on participants previously in EXPAND study |
| Kappos 2016b | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Kappos 2017a | Pooled data of RCTs: results of the phase 3 Study |
| Kappos 2017b | Design: not RCT |
| Kappos 2020 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Krasnov 2021 | Non‐RCT, this is a review |
| Li 2012a | Pooled data of RCTs: results from the phase 2 BOLD study |
| Li 2012b | Pooled data of RCTs: results from the phase 2 BOLD study |
| NCT00879658 | duplicated record |
| NCT01665144 | duplicated record |
| Penner 2020 | No clinical outcomes that met the inclusion criteria |
| Selmaj 2011 | Pooled data of RCTs: results from the phase 2 BOLD study |
| Shah 2020 | Non‐RCT, it's a cross‐sectional study |
| Stuve 2012 | Pooled data of RCTs: results from the phase 2 BOLD study |
| Stuve 2013 | Pooled data of RCTs: results from the phase 2 BOLD study |
| Synnott 2020 | Non‐RCT, this is a review |
| Vermersch 2017 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Vermersch 2019 | Pooled data of RCTs: results from the phase 3 EXPAND study |
| Vermersch 2020 | Pooled data of RCTs: results from the EXPAND study |
| Weber 2020 | Non‐RCT |
| Wu 2020 | No clinical outcomes that met the inclusion criteria |
RCT: randomised controlled trial; SPMS: secondary progressive multiple sclerosis
Characteristics of studies awaiting classification [ordered by study ID]
EUCTR2008‐008719‐25 HU.
| Methods | Multicentre, double‐blind, randomised, multi‐center, adaptive dose‐ranging, placebo‐controlled, parallel‐group study |
| Participants | RRMS |
| Interventions | Experimental: BAF312 Active comparator: placebo |
| Outcomes | Primary outcome measures: monthly number of CUAL during 3 months of treatment Secondary outcome measures:
|
| Notes | This study has been completed (04 May 2011). Partial results have been reported in the site ClinicalTrials.gov. |
NCT01185821.
| Methods | Randomised, parallel assignment |
| Participants | RRMS |
| Interventions | Experimental: BAF312 10 mg/2 mg; BAF312 2 mg/2 mg; BAF312 1.25 mg/2 mg; BAF312 .5 mg/2 mg; BAF312.25 mg/2 mg |
| Outcomes | Primary outcome measure:
|
| Notes | The study has been completed (10 October 2016) |
AE: adverse event; ARR: annualised relapse rate; CUAL: combined unique active lesions; IVCD: intraventricular conduction delay; MRI: magnetic resonance imaging; RRMS: relapsing remitting multiple sclerosis
Characteristics of ongoing studies [ordered by study ID]
EUCTR2012‐003056‐36‐SK.
| Study name | Study of efficacy, safety and tolerability data for BAF312 compared to placebo in patients with secondary progressive multiple sclerosis followed by extended treatment with BAF312 |
| Methods | Parallel RCT |
| Participants | SPMS |
| Interventions | Experimental: siponimod tablet Active comparator: placebo |
| Outcomes | Primary outcome measures: delay in time to 3‐month CDP. Confirmed disability is defined as an increase of score of 1 point in participants with baseline score of 3.0‐5.0 and 0.5 point increase with baseline score of 5.5 to 6.5. Secondary outcome measures: efficacy of siponimod (BAF312) relative to placebo in reducing the increase in T2 lesion volume from baseline |
| Starting date | 31 October 2012 |
| Contact information | clinicaltrial.enquiries@novartis.com |
| Notes | This study is ongoing, but not recruiting participants |
NCT04540861.
| Study name | Managed Access Program (MAP) for Patients Diagnosed With Secondary Progressive Multiple Sclerosis With Active Disease |
| Methods | |
| Participants | Multiple Sclerosis |
| Interventions | Siponimod:1 or 2 mg filmcoated tablets. The tablets have to be taken orally once daily and swallowed whole with water, with or without food |
| Outcomes | |
| Starting date | September 7, 2020 |
| Contact information | novartis.email@novartis.com |
| Notes |
NCT04593927.
| Study name | Long Term Special Drug Use‐results Surveillance for Mayzent in SPMS Patients |
| Methods | |
| Participants | SPMS |
| Interventions | Mayzent |
| Outcomes | Primary Outcome Measures: Incidence of Adverse Events (AE), Serious Adverse Events (SAE) and Adverse Reactions [ Time Frame: 24 months ]A SAE is defined as an AE whose description suggests that it is serious or whose outcome is fatal. |
| Starting date | October 20, 2020 |
| Contact information | novartis.email@novartis.com |
| Notes |
NCT04925557.
| Study name | Study to Assess the Efficacy of Mayzent on Microglia in Secondary Progressive Multiple Sclerosis |
| Methods | |
| Participants | SPMS |
| Interventions | |
| Outcomes | Primary Outcome Measures:Change from baseline in PET Activation at 12 Months [ Time Frame: 12 months ]Change from baseline in PET activation of PBR06 in lesional and non‐lesional NAWM and NAGM in the brain of the patients under treatment with Mayzent when 100% of patients reach 12 months |
| Starting date | June 14, 2021 |
| Contact information | rzivadinov@bnac.net |
| Notes |
CDP: confirmed disability progression; EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging; MSWS‐12: 12‐item multiple sclerosis walking scale; RCT: randomised controlled trial; SPMS: secondary progressive multiple sclerosis
Differences between protocol and review
In the primary and second outcome measures of the protocol, we planned to assess primary outcomes over 12, 24, or 36 months after randomisation or at the end of the follow‐up period. However, we found available data to assess the short‐term outcomes (less than six months post‐intervention) and interim outcomes (six months to less than12 months post‐intervention) only.
We have made minor changes to the main results of the review in the "Summary of Findings" table. We added the outcome annualised relapse rate (ARR) to the summary of findings table because ARR is often included as an outcome measure for clinical trials, and it is easy to quantify these outcomes are the most relevant to clinical practice. At the same time, we removed the number of participants reporting cardiac adverse events mentioned from SoF table.
-
We planned the following subgroup analyses at the protocol stage but did not perform them in this review due to lack of sufficient data.
Duration of follow‐up (12 months, 24 months, 36 months)
Disability at baseline (EDSS scores ≤ 3.5 or ≥ 4).
We added a co‐corresponding author.
Contributions of authors
The initial idea for this review was conceived by CLJ. All review authors were involved in the development of the protocol and responding to feedback, and have agreed the final draft.
Sources of support
Internal sources
-
The Fundamental Research Funds for the Central Universities: No.18LZUJBWZX006;2019jbkyzy002; 2020jbkyzx001, China
The Fundamental Research Funds for the Central Universities
External sources
-
No sources of support provided, Other
No sources of support provided
Declarations of interest
L Cao: none known
Y Lao: none known
L Yao: none known
P Yan: none known
X Wang: none known
Z Yang: none known
M Li: none known
H Li: none known
KH Yang: none known
L
These authors contributed equally to this work.
These authors contributed equally to this work.
Edited (no change to conclusions)
References
References to studies included in this review
Kappos 2018 {published data only}
- Kappos L, Bar-Or A, Cree BA, Fox RJ, Giovannoni G, Gold R, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018;391(10127):1263-73. [DOI] [PubMed] [Google Scholar]
Selmaj 2013 {published data only}
- Selmaj K, Li DK, Hartung HP, Hemmer B, Kappos L, Freedman MS, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurology 2013;12(8):756-67. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnold 2020 {published data only}
- Arnold D, Vermersch P, Cree B, Bar-or A, Giovannoni G, Gold R, et al. Evidence for improved myelination in patients treated with siponimod: Results from the Phase 3 EXPAND MRI Substudy. European Journal of Neurology 2020;27:194-195. [Google Scholar]
Bar‐Or 2020 {published data only}
- Bar-Or A, Weinstock-Guttman B, Mao-Draayer Y, Cox G, Cruz LA, Meng X, et al. Safety and tolerability of conversion to siponimod in patients with relapsing multiple sclerosis: Interim results of the exchange study. Multiple Sclerosis Journal 2020;26:232. [Google Scholar]
Benedict RHB 2021 {published data only}
- Benedict RHB, Tomic D, Cree BA, Fox R, Giovannoni G, Bar-Or A, et al. Siponimod and Cognition in Secondary Progressive Multiple Sclerosis: EXPAND Secondary Analyses. Neurology 2021;96:e376-e386. [DOI] [PubMed] [Google Scholar]
Cree 2019 {published data only}
- Cree B, Fox R, Giovannoni G, Vermersch P, Bar-Or A, Gold R, et al. Siponimod affects disability progression in patients with SPMS independent of relapse activity: results from the phase 3 EXPAND study. PM & R : the Journal of Injury, Function, and Rehabilitation 2019;11:S50. [Google Scholar]
Diener 2018 {published data only}
- Diener HC. Secondary progressive multiple sclerosis: siponimod is more effective than placebo in a double-blind randomized phase III trial. Psychopharmakotherapie 2018;25(5):265-6. [Google Scholar]
Fox 2017 {published data only}
- Fox R, Arnold DL, Bar-Or A, Cree B, Giovannoni G, Gold R, et al. Effects of siponimod on MRI outcomes in patients with secondary progressive multiple sclerosis: results of the phase 3 EXPAND study. Multiple Sclerosis Journal 2017;23(3):34-5. [Google Scholar]
Fox 2017a {published data only}
- Fox R, Kappos L, Bar-Or A, Cree B, Giovannoni G, Gold R, et al. Safety and tolerability of siponimod in patients with secondary progressive multiple sclerosis. Neurology 2017;88 Suppl 16:S33.007. [Google Scholar]
Gajofatto 2020 {published data only}
- Gajofatto A, Turatti M. Siponimod to treat secondary progressive multiple sclerosis. Drugs of Today (Barcelona, Spain : 1998) 2020;56(1):37-46. [DOI] [PubMed] [Google Scholar]
Giovannoni 2017 {published data only}
- Giovannoni G, Baror A, Cree B, Fox R, Gold R, Vermersch P, et al. 2018-The EXPAND study results: safety and tolerability of siponimod in patients with secondary progressive multiple sclerosis. European Journal of Neurology 2017;24:495. [Google Scholar]
Giovannoni 2020 {published data only}
- Giovannoni G, Kappos L, Fox R, Vermersch P, Cree BAC, Benedict R, et al. Sustained reduction of disability and cognitive decline with long-term siponimod treatment in patients with active SPMS: expand data up to 5 years. Multiple Sclerosis Journal 2020;26:235. [Google Scholar]
Gold 2019 {published data only}
- Gold R, Kappos L, Bar-Or A, Vermersch P, Giovannoni G, Fox R, et al. Efficacy of siponimod in secondary progressive multiple sclerosis patients with active disease: the EXPAND study subgroup analysis. Multiple Sclerosis Journal 2019;25:380. [Google Scholar]
Gold 2020 {published data only}
- Gold R, Kappos L, Benedict RHB, Bar-or A, Vermersch P, Giovannoni G, et al. Siponimod slows physical disability progression and decline in cognitive processing speed in SPMS patients with active disease: A Post hoc analysis of the EXPAND study. European Journal of Neurology 2020;27:328-329. [Google Scholar]
Hartung 2013 {published data only}
- Hartung HP, Selmaj K, Li D, Hemmer B, Freedman M, Stuve O, et al. Phase 2 BOLD extension study safety results for siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis. Neurology 2013;80 Suppl 7:P01.176. [Google Scholar]
Hobart 2021 {published data only}
- Hobart J, Piani-Meier D, Cree B, Giovannoni G, Kappos L, Fox RJ, et al. Effect of Siponimod on the MSWS-12 and MSIS-29 in patients with SPMS from the EXPAND study. European Journal of Neurology 2021;28:359. [Google Scholar]
Kappos 2013 {published data only}
- Kappos L, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. P036-siponimod (BAF312) for the treatment of secondary progressive multiple sclerosis: Design of the phase 3 EXPAND trial. Neurology 2014;3(6):752. [Google Scholar]
Kappos 2014a {published data only}
- Kappos L, Stuve O, Hartung H, Freedman M, Li D, Hemmer B, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: results from dose-blinded extension phase of BOLD study. Neurology 2014;82 Suppl 10:P3.151. [DOI] [PubMed] [Google Scholar]
Kappos 2014b {published data only}
- Kappos L, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. Siponimod (BAF312) for the treatment of secondary progressive multiple sclerosis: design of the phase 3 EXPAND trial. Multiple Sclerosis 2014;20(7):927-8. [Google Scholar]
Kappos 2015 {published data only}
- Kappos L, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. Siponimod (BAF312) for the treatment of secondary progressive multiple sclerosis (SPMS): baseline characteristics of the EXPAND study population. Multiple Sclerosis 2015;23(11):317-8. [Google Scholar]
Kappos 2016 {published data only}
- Kappos L, Li D K, Stüve O, Hartung HP, Freedman MS, Hemmer B, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurology 2016;73(9):1089-98. [DOI] [PubMed] [Google Scholar]
Kappos 2016a {published data only}
- Kappos L, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. Baseline subgroup characteristics of EXPAND: a phase 3 study of siponimod (BAF312) for the treatment of secondary progressive multiple sclerosis. Neurology 2016;86 Suppl 16:P3.084. [Google Scholar]
Kappos 2016b {published data only}
- Kappos L, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. Efficacy and safety of siponimod in secondary progressive multiple sclerosis-results of the placebo controlled, double-blind, phase III EXPAND study. Multiple Sclerosis 2016;22:828-9. [Google Scholar]
Kappos 2017a {published data only}
- Kappos L, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. Efficacy of siponimod in secondary progressive multiple sclerosis: results of the phase 3 study. Neurology 2017;88 Suppl 16:CT.002. [Google Scholar]
Kappos 2017b {published data only}
- Kappos L, Vermersch P, Bar-Or A, Gold R, Fox R, Cree B, et al. Efficacy of siponimod on disability progression in SPMS patients with and without on-study relapses. Multiple Sclerosis Journal 2017;23(3):397-8. [Google Scholar]
Kappos 2020 {published data only}
- Kappos L, Giovannoni G, Gold R, Fox RJ, Vermersch P, Benedict RHB, et al. Long-term efficacy of siponimod treatment for up to 5 years in patients with secondary progressive multiple sclerosis: Analysis of the EXPAND extension study. European Journal of Neurology 2020;27:337. [Google Scholar]
Krasnov 2021 {published data only}
- Krasnov VS, Kolontareva YM. Siponimod: a new view at the therapy of secondary progressive multiple sclerosis. ZHURNAL NEVROLOGII I PSIKHIATRII IMENI S S KORSAKOVA 2021;121:124-129. [DOI] [PubMed] [Google Scholar]
Li 2012a {published data only}
- Li D, Selmaj K, Zhao G, Cheng Y, Mercier F, Rochotte E, et al. MRI findings of BOLD: a phase 2 dose-finding study using adaptive design and modeling methods for BAF312 in relapsing-remitting MS. Neurology 2012;78 Suppl 1:S11.005. [Google Scholar]
Li 2012b {published data only}
- Li DK, Hemmer B, Stuve O, Hartung HP, Freedman MS, Rieckmann P, et al. Siponimod (BAF312) treatment leads to early MRI benefits in relapsing-remitting multiple sclerosis patients: results from a phase 2 study. Multiple Sclerosis 2012;18(4):207‐8. [Google Scholar]
NCT00879658 {published data only}
- Safety, Tolerability, Efficacy and Optimal Dose Finding Study of BAF312 in Patients With Relapsing-remitting Multiple Sclerosis. clinicaltrials.gov/show/NCT00879658 (first received 25 April 2020).
NCT01665144 {published data only}
- Exploring the Efficacy and Safety of Siponimod in Patients With Secondary Progressive Multiple Sclerosis (EXPAND). clinicaltrials.gov/show/NCT01665144 (first received 25 April 2020).
Penner 2020 {published data only}
- Penner IK, Giovannoni G, Cree BAC, Fox R, Bar-Or A, Gold R, et al. Effect of siponimod on cognitive processing speed in SPMS patients with active and non-active disease. Multiple sclerosis journal 2020;26:502‐. [Google Scholar]
Selmaj 2011 {published data only}
- Selmaj K, Li D, Stüve O, Kappos L, Hartung H, Hemmer B, et al. BAF312, a selective sphingosine 1-phosphate receptor modulator, effectively suppresses MRI lesion activity in relapsing-remitting multiple sclerosis: findings of an adaptive dose-ranging phase 2 study. Multiple Sclerosis 2011;17(10):S510-1. [Google Scholar]
Shah 2020 {published data only}
- Shah R, Zhang Y, Mavrikis Cox G, Johnson K. The first 12 months of siponimod utilization following approval in the United States. Journal of Managed Care and Specialty Pharmacy 2020;26:S45. [Google Scholar]
Stuve 2012 {published data only}
- Stuve O, Selmaj K, Li D, Hartung H P, Hemmer B, Kappos L, et al. BAF312, a selective sphingosine-1-phosphate receptor modulator improves MRI and clinical outcomes in relapsing-remitting multiple sclerosis (RRMS). Neurology 2012;78 Suppl 1:S30.001. [Google Scholar]
Stuve 2013 {published data only}
- Stuve O, Hartung HP, Freedman M, Li D, Hemmer B, Kappos L, et al. Phase 2 bold extension study efficacy results for siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis. Neurology 2013;80 Suppl 7:P07.110. [DOI] [PubMed] [Google Scholar]
Synnott 2020 {published data only}
- Synnott PG, Bloudek LM, Sharaf R, Carlson JJ, Pearson SD. The Effectiveness and Value of Siponimod for Secondary Progressive Multiple Sclerosis. Journal of Managed Care & Specialty Pharmacy 2020;26:236-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermersch 2017 {published data only}
- Vermersch P, Bar-Or A, Cree B, Fox R, Giovannoni G, Gold R, et al. The EXPAND study results: efficacy of siponimod in secondary progressive multiple sclerosis. European Journal of Neurology 2017;24:44. [Google Scholar]
Vermersch 2019 {published data only}
- Vermersch P, Gold R, Kappos L, Fox R, Bar-Or A, Cree B, et al. Siponimod delays the time to wheelchair in patients with SPMS: results from the EXPAND study. Multiple Sclerosis Journal 2019;25:55-6. [Google Scholar]
Vermersch 2020 {published data only}
- Vermersch P, Fox RJ, Arnold DL, Giovannoni G, Cree BAC, Bar-Or A, et al. Effect of siponimod on grey matter atrophy in patients with secondary progressive multiple sclerosis: Subgroup analyses from the EXPAND study. European Journal of Neurology 2020;27:478. [Google Scholar]
Weber 2020 {published data only}
- Weber M, Klotz L, Schreiber H, Rauser B, ZiemssenT. Baseline characteristics of amasia: First real world data of siponimod treated patients with secondary progressive multiple sclerosis. Multiple Sclerosis Journal 2020;26:518. [Google Scholar]
Wu 2020 {published data only}
- Wu Q, Mills EA, Wang Q, Dowling CA, Fisher C, Kirch B, et al. Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis. JCI Insight 2020;5(3):e134251. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
EUCTR2008‐008719‐25 HU {published data only}
- A phase II, double-blind, randomized, multi-center, adaptive dose-ranging, placebo-controlled, parallel-group study evaluating safety, tolerability and efficacy on MRI lesion parameters and determining the dose response curve of BAF312 given orally once daily in patients with relapsing-remitting multiple sclerosis. http://www.who.int/trialsearch/Trial2.aspx?id=EUCTR2008-008719-25-HU(first received 25 August 2021).
NCT01185821 {published data only}
- Long-term Safety, Tolerability and Efficacy of BAF312 Given Orally in Patients With Relapsing-remitting Multiple Sclerosis. clinicaltrials.gov/show/NCT01185821 (first received 25 August 2021).
References to ongoing studies
EUCTR2012‐003056‐36‐SK {published data only}
- Study of efficacy, safety and tolerability data for BAF312 compared to placebo in patients with secondary progressive multiple sclerosis followed by extended treatment with BAF312. ClinicalTrials.gov/show/EUCTR2012-003056-36-SK (first received 25 August 2020).
NCT04540861 {published data only}
- Managed Access Program (MAP) for Patients Diagnosed With Secondary Progressive Multiple Sclerosis With Active Disease. https://clinicaltrials.gov/ct2/show/NCT04540861.
NCT04593927 {published data only}
- Long Term Special Drug Use-results Surveillance for Mayzent in SPMS Patients. https://clinicaltrials.gov/ct2/show/NCT04593927.
NCT04925557 {published data only}
- Study to Assess the Efficacy of Mayzent on Microglia in Secondary Progressive Multiple Sclerosis. https://clinicaltrials.gov/ct2/show/NCT04925557.
Additional references
Aslanis 2012
- Aslanis V, Faller T, Van de Kerkhof E, Schubart A, Wallström E, Beyerbach A. Siponimod (BAF312) (and/or its metabolites) penetrates into the CNS and distributes to white matter areas. Multiple Sclerosis 2012;18 Suppl 4:279-508. [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brana 2014
- Brana C, Frossard MJ, Pescini Gobert R, Martinier N, Boschert U, Seabrook TJ. Immunohistochemical detection of sphingosine-1-phosphate receptor1 and 5 in human multiple sclerosis lesions. Neuropathology and Applied Neurobiology 2014;40(5):564-78. [DOI] [PubMed] [Google Scholar]
Brinkmann 2009
- Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. British Journal of Pharmacology 2009;158:1173-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chun 2010
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology 2010;33(2):91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2020
- Colombo E, Bassani C, De Angelis A, Ruffini F, Ottoboni L, Comi G, et al. Siponimod (BAF312) activates Nrf2 while hampering NFκB in human astrocytes, and protects from astrocyte-induced neurodegeneration. Frontiers In Immunology 2020;11:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2019
- Food and Drug Administration. Drug Approval Package: Mayzent (siponimod). www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209884Orig1s000SumR.pdf.
Filippini 2013
- Filippini G, Del Giovane C, Vacchi L, D'Amico R, Di Pietrantonj C, Beecher D, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD008933. [DOI: 10.1002/14651858.CD008933.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentile 2016
- Gentile A, Musella A, Bullitta S, Fresegna D, De Vito F, Fantozzi R, et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. Journal of Neuroinflammation 2016;26(13):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gergely 2012
- Gergely P, Nuesslein-Hildesheim B, Guerini D. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. British Journal of Pharmacology 2012;167(5):1035-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 20 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Jackson 2011
- Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. Journal of Neuroinflammation 2011;8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kappos 2016
- Kappos L, Li DK, Stuve O. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD Study. JAMA Neurology 2016;73(9):1089-98. [DOI] [PubMed] [Google Scholar]
Kobelt 2017
- Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J, MSCOI Study Group, European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe. Multiple Sclerosis 2017;23:1123-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurtzke 1983
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444-52. [DOI] [PubMed] [Google Scholar]
Lublin 1996
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46(4):907-11. [DOI] [PubMed] [Google Scholar]
Lublin 2014
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83(3):278-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahad 2015
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurology 2015;14(2):183–93. [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121-7. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. [DOI] [PubMed] [Google Scholar]
Newsome 2017
- Newsome SD, Aliotta PJ, Bainbridge J, Bennett SE, Cutter G, Fenton K, et al. A framework of care in multiple sclerosis, part 2: symptomatic care and beyond. International Journal of MS Care 2017;19(1):42-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuesslein‐Hildesheim 2009
- Nuesslein-Hildesheim B, Gergely P, Wallström E, Luttringer O, Groenewegen A, Howard L, et al. The S1P1/S1P5 receptor modulator BAF312 reverses neurological deficits in ongoing EAE, reduces specific lymphocyte subsets in healthy volunteers and is a potential new multiple sclerosis treatment. Multiple Sclerosis Journal 2009;15(10):438. [Google Scholar]
Orton 2006
- Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurology 2006;5:932-6. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Polman 2011
- Polman CH, Reingold SC, Banwell B. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227-31. [DOI] [PubMed] [Google Scholar]
Reich 2018
- Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. New England Journal of Medicine 2018;378(2):169‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rommer 2019
- Rommer PS, Eichstädt K, Ellenberger D, Flachenecker P, Friede T, Haas J, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Multiple Sclerosis 2019;25(12):1641-52. [DOI] [PubMed] [Google Scholar]
Scalfari 2014
- Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. Journal of Neurology Neurosurgery and Psychiatry 2014;85:67-75. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Scott LJ 2020
- Scott LJ. Siponimod: a review in secondary progressive multiple sclerosis. CNS Drugs 2020;35(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakeri‐Nejad 2015
- Shakeri-Nejad K, Aslanis V, Veldandi UK, Mooney L, Pezous N, Brendani B, et al. Effects of therapeutic and supra therapeutic doses of siponimod (BAF312) on cardiac repolarization in healthy subjects. Clinical Therapeutics 2015;37:2489–505. [DOI] [PubMed] [Google Scholar]
Thompson 2018a
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet 2018;391(10130):1622–36. [DOI] [PubMed] [Google Scholar]
Thompson 2018b
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology 2018;17(2):162-73. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cao 2020
- Cao L, Lao Y, Yao L, Yan P, Wang X, Yang Z, et al. Siponimod for multiple sclerosis. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013647. [DOI: 10.1002/14651858.CD013647] [DOI] [PMC free article] [PubMed] [Google Scholar]