Abstract
Major depressive disorder (MDD) is a primary psychiatric illness worldwide; there is a dearth of new mechanistic models for the development of better therapeutic strategies. Although we continue to discover individual biological factors, a major challenge is the identification of integrated, multidimensional traits underlying the complex heterogeneity of depression and treatment outcomes. Here, we set out to ascertain the emergence of the novel mitochondrial mediator of epigenetic function acetyl-L-carnitine (LAC) in relation to previously described individual predictors of antidepressant responses to the insulin-sensitizing agent pioglitazone. Herein, we report that i) subjects with MDD and shorter leukocyte telomere length (LTL) show decreased levels of LAC, increased BMI, and a history of specific types of childhood trauma; and that ii) these multidimensional factors spanning mitochondrial metabolism, cellular aging, metabolic function, and childhood trauma provide more detailed signatures to predict longitudinal changes in depression severity in response to pioglitazone than individual factors. The findings of multidimensional signatures involved in the pathophysiology of depression and their role in predicting treatment outcomes provide a starting point for the development of a mechanistic framework linking biological networks and environmental factors to clinical outcomes in pursuit of personalized medicine strategies to effectively treat MDD.
Keywords: Acetylcarnitine, Metabolism, Stress
1. Introduction
The large variability in response to antidepressants is a major problem in the treatment of major depressive disorder (MDD) (Akil et al., 2017), which is a leading cause of disability worldwide and a main risk factor for diabetes, cardiovascular diseases, accelerated brain aging and dementia (Friedrich, 2017; Kessler et al., 2003). At present, the most effective therapeutic strategy can only be identified through trial and error and requires multiple trials of different antidepressants and several weeks to test for each given treatment. This situation arises from the complex disease heterogeneity that has made it difficult to identify biomarkers for detecting depressive disorders or predicting individual responses to a particular treatment. Several lines of evidence support the notion that the blanket diagnosis of depression should, in reality, be categorized into distinct clinical endophenotypes. At the same time, our recognition of the effects of early life adversity on the incidence of somatic and neurological illnesses, including MDD, advances (Heim and Nemeroff, 2001; Nemeroff, 2016).
Prior work showed an essential role of the pivotal mitochondrial metabolite acetyl-L-carnitine (LAC) in the epigenetic regulation of neuroplasticity and its role as a promising therapeutic target for depression (Bigio et al., 2016; Cherix et al., 2020; Lau et al., 2017; C. Nasca et al., 2018; C. Nasca et al., 2017; C. Nasca et al., 2013; Wang et al., 2015). In rodent models, decreased central (hippocampus and prefrontal cortex) and systemic levels of LAC have been shown to increase the risk for development of impaired dendritic plasticity of corticohippocampal circuits - implicated in the pathophysiology and treatment of depression. Supplementation of LAC leads to a rapid antidepressant response by regulating the expression of key genes important for dendritic and synaptic plasticity, including the metabotropic glutamate receptor of type-2 (mGlu2 receptors (Nicoletti et al., 2015), insulin signaling pathways and neuroinflammation (NFkB/p65) in discrete hippocampal neurons. In patients with MDD, LAC levels are decreased as compared to age- and sex-matched control subjects, and these changes are accompanied by increased depression severity, early onset of the disease and a treatment-resistant course of the illness (C. Nasca et al., 2018). More recently, we showed a relationship between the epigenetic modulation of glutamatergic function and central insulin resistance (IR) as assessed by measures of the insulin signaling cascade in brain-enriched exosomes (C. Nasca et al., 2020).
Prediction of individuals likely to respond to antidepressant treatments has been largely based on single factors. For example, previous work suggested systemic IR as a biological correlate of antidepressant responses to the insulin-sensitizing agent pioglitazone in subjects with MDD (Lin et al., 2015). As reviewed elsewhere, IR is a metabolic condition important for brain plasticity and reflective of cerebral hypometabolism and aberrant intrinsic connectivity of corticohippocampal circuits in patients with depression (Biessels and Reagan, 2015; Grillo et al., 2019; N. L. Rasgon and McEwen, 2016; Watson et al., 2017; Kenna et al., 2013; Watson et al., 2017; Wroolie et al., 2015). Variability in leukocyte telomere length (LTL) has also been associated with antidepressant responses to pioglitazone (N. Rasgon, Lin, Lin, Epel and Blackburn, 2016). Shortening of telomeres is a primary etiology for brain tissue damage in many neurodegenerative disorders (Federico et al., 2012; Gandhi and Abramov, 2012; Maciejczyk et al., 2019), which are thought to be precipitated by earlier mood disorders, such as depression (N. Rasgon and Jarvik, 2004; N. L. Rasgon and McEwen, 2016). Moreover, multiple lines of evidence showed that childhood trauma is not only a major risk factor for MDD as it is for a deficiency of LAC, metabolic dysfunction and shortening of telomeres (Chen et al., 2014; C. Nasca et al., 2018; Carla Nasca, Kathleen Watson-Lin et al., 2019; Wolkowitz et al., 2011), but is also associated with a poor outcome to treatment with psychopharmacology and/or psychotherapy (Nemeroff, 2016).
Taken together, these prior studies suggest that mitochondrial metabolism, cellular aging, and metabolic function can be intertwined with each other in the pathophysiology and treatment of depression, but these factors have been mostly studied independently in translational research. In the current study, we used as a conceptual platform this evolving framework of epigenetic modulation of brain plasticity to identify multidimensional traits underlying the complex heterogeneity of depression and its treatment. We efficiently used biological samples from a previous randomized, placebo-controlled trial with pioglitazone (Lin et al., 2015; N. Rasgon et al., 2016) to add the new measure of peripheral LAC levels for ascertaining the role of this pivotal mitochondrial metabolite in relation to the previously described individual predictors of antidepressant responses to pioglitazone. Specifically, the purpose of this study was to determine whether multidimensional factors spanning mitochondrial metabolism, cellular aging, metabolic function, and childhood trauma can provide more detailed signatures than individual markers in predicting antidepressant responses.
2. Methods
The Institutional Review Board committees at Rockefeller University and Stanford University approved the current study.
2.1. LAC assessment by ultraperformance liquid chromatography electrospray tandem mass spectrometry
LAC levels were measured as we previously reported (C. Nasca et al., 2018) by ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) with electrospray ionization in positive ion mode on a Xevo-TQD or a TQD tandem mass spectrometer equipped with Acquity UPLC system (Waters Corp). All groups were evenly divided between the experimental plates to account for any interpolate variability and plasma samples were spiked with acetyl–[2H3]-carnitine internal standards. A detailed method for assessment of LAC is described in our previous reports (C. Nasca et al., 2018; C. Nasca et al., 2013). Materials: L-carnitine. HCl and LAC hydrochloride (Sigma Chemical Co.); 2H3-L-carnitine.HCl and acetyl–2H3-L-carnitine.HCl (Cambridge Isotope Laboratories, Inc.); all other reagents, solvents, and solvent additives were purchased from Sigma Chemical Co. or VWR.
2.2. Insulin resistance assessment
A series of laboratory tests were used to measure fasting plasma glucose (FPG), fasting plasma insulin (FPI), as well as to assess the status of insulin resistance versus sensitivity. Insulin resistance status was defined by the presence of at least one of the following criteria: oral glucose tolerance test at 120 min >140 mg/dL, fasting plasma glucose >100 mg/dL; fasting plasma insulin >15 ulU/ml, while insulin sensitivity status was defined by the absence of those criteria.
2.3. Measures of leukocyte telomere length (LTL)
DNA samples were shipped to the laboratory of Professor Elizabeth Blackburn for assessment of LTL. Telomere length was measured by quantitative real-time PCR (qPCR) assays by adapting the previous published original method (Cawthon, 2002). In brief, total genomic DNA was purified using QIAamp DNA blood Mini kit (QIAGEN, Venlo, The Netherlands, Cat#51106) from whole blood stored at −80 °C and quantified by measuring OD260. The telomere thermal cycling profile consists of: cycling for T9 (telomic) PCR: 96 °C for 1 min; denature at 96 °C for 1 s, anneal/extend at 54 °C for 60 s, with fluorescence data collection, 30 cycles. Cycling for S (single-copy gene) PCR: PCR: 96 °C for 1 min; denature at 95 °C for 15 s, anneal at 58 °C for 1 s, extend at 72 °C for 20 s, 8 cycles; followed by denature at 96 °C for 1 s, anneal at 58 °C for 1 s, extend at 72 °C for 20 s, hold at 83 °C for 5 s with data collection, 35 cycles. The primers for the telomere PCR are tel1b (5′-CGGTTT (GTTTGG)5GTT-3′), used at a final concentration of 100 nM, and tel2b (5′-GGCTTG (CCTTAC)5CCT-3′), used at a final concentration of 900 nM. The primers for the single-copy gene (human beta-globin) PCR are hbg1 (5′-GCTTCTGACACAACTGTGTTCACTAGC-3′), used at a final concentration of 300 nM, and hbg2 (5′-CACCAACTTCATCCACGTTCACC-3′) used at a final concentration of 700 nM. The final reaction mix contains 20 mM Tris-HCl, pH 8.4; 50 mM KCl; 200 mM each dNTP; 1% DMSO; 0.4 × Syber Green I; 22 ng Escherichia coli DNA per reaction; 0.4 U of Platinum Taq DNA polymerase (Invitrogen, Waltham, MA, USA). To control for inter-assay variability, eight control DNA samples are included in each run. In each batch, the average telomere to single gene copy ratio T/S ratio of each control DNA is divided by T/S for the same DNA from 10 runs to get a normalizing factor. This is done for all eight samples and the average normalizing factor for all eight samples is used to correct the participant DNA samples to get the final T/S ratio. The T/S ratio for each sample was measured twice. When the duplicate T/S value and the initial value vary by >7%, the sample was run the third time and the two closest values were reported. The coefficient of variation for this study is 2.4%.) per 11 μl reaction; 6 ng of genomic DNA. Tubes containing 26, 8.75, 2.9, 0.97, 0.324 and 0.108 ng of a reference DNA (from the Hela cancer cell line) are included in each PCR run so that the quantity of targeted templates in each research sample can be determined relative to the reference DNA sample by the standard curve method. The same reference DNA was used for all PCR runs.
2.4. Participants
Study participants were recruited at the Department of Psychiatry and Behavioral Sciences at Stanford University. After an initial phone screen, potential participants were evaluated in person to determine study eligibility. All participants determined to be eligible to join the study provided written informed consent before study enrollment. Inclusion criteria included the following: age ranging between 23 and 71 years, a primary diagnosis of MDD, and at least 12 years of education. Study clinicians or trained coordinators conducted the Structured Clinical Interview for DSM-IV (SCID) or Mini International Neuropsychiatric Interview (MINI) to confirm diagnosis of MDD. Exclusion criteria included a history of liver dysfunction, electroconvulsive therapy (ECT) within the 6 months of recruitment, diagnosis of possible or probable dementia or any evidence of cognitive decline, Type I or Type II diabetes, history of significant CVD or myocardial infarction, cerebrovascular, pulmonary disease, cancer, untreated hypothyroidism, unstable or untreated hypertension, osteoporosis or prior history of non-traumatic fracture, and a history of neurological disorder or evidence of neurologic or other physical illness that could produce cognitive deterioration. The baseline cohort included those subjects who completed the Childhood Trauma Questionnaire (CTQ) and joined the randomized controlled trial with pioglitazone (n = 38) or the parallel depression study (n = 9) at the Department of Psychiatry and Behavioral Sciences at Stanford University. Clinical and demographic characteristics were available for all subjects; plasma samples were available for 38 subjects and BMI was not recorded in the database for 2 subjects. Both studies had the same inclusion and exclusion criteria (N. Rasgon et al., 2016; Wroolie et al., 2015). A total of 5 subjects in the active arm and 3 subjects in the placebo arm had psychiatric comorbidities, such as obsessive-compulsive disorder, anxiety and bipolar disorder.
2.5. Randomized controlled trial design and randomization
The randomized controlled trial consisted of a parallel design in which 50% of participants were allocated to 12 weeks of treatment with 30 mg/day pioglitazone and 50% of participants were randomized to 12 weeks treatment with placebo. Random allocation was generated by use of a random number generator that assigned half of the subjects’ identification numbers to each study condition. Randomization and maintenance of the unblinded study list was performed by a staff member of the Stanford University Department of Psychiatry who did not participate in the implementation of the study. Active and placebo medication were bottled and labeled with participant identification numbers by this unblinded staff person according to randomized assignment. Study investigators, coordinators, raters, and clinical laboratory staff remained blinded to subject treatment assignments throughout their participation in the study.
2.6. Clinical and psychiatric assessment
Clinical assessment consisted of a physical examination, including measures of height, weight, and BMI. Other data collected included current medication use and history of failed antidepressant trials. Demographic information, including sex, was also recorded from the participants. The psychiatric examination at screening included SCID or MINI to confirm MDD diagnosis. Trained raters administered the structured depression rating scales: 21-item Hamilton Depression Rating Scale (HDRS-21). Participants completed the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003) to assess childhood adversity in five specific areas: physical, sexual, and emotional abuse, and physical and emotional neglect.
2.7. Statistical analyses
Statistical analyses were conducted using JMP Software from SAS (Statistical Analysis System, Institute, Cary, NC, USA). Two-tailed t tests and χ2 analyses were used to compare, respectively, continuous and categorical demographic and clinical characteristics between treatment groups. Unless otherwise specified, all variables were used as continuous. We used stepwise regression analyses with minimum corrected Akaike information criterion (AIC) to compare fit and hence potential suitability of the independent variables in the predictive models. The choice of predictive variables to be retained in the model is carried out by an iterative procedure in which only the variables that fit specific statistical selection criteria of p-value threshold of 0.05 and minimum AIC are retained in the model. These analyses identified BMI and emotional abuse in the relationship with LAC and leukocyte telomere length. Multifactorial regression analyses were used to determine the relationship between the dependent and independent variables at baseline (Fig. 2A) as well as in predicting treatment outcomes (Fig. 3A). Each test accounts for the probability of a type I error and the p-values are adjusted for the other variables in the model. We used the residual by predicted plots to check for the standard assumptions of linear regression models (e.g.: normal distribution, constant variance). The residuals of both models are randomly scattered around the center line of zero, with no noticeable pattern, confirming the assumptions of linear regression for both models of multidimensional constructs in subjects with MDD and treatment responses (SI Fig. 1A and B). We also used prediction modeling to assess the directionality of the interactions between the multidimensional factors (Fig. 2B and C and Fig. 3B and C). The prediction modelling captures how the relationship between all variables of interest changes as a function of each individual variable. To study the relationship between LAC and IR in treatment outcomes, we used regression analysis and we dichotomized our sample by the presence of insulin resistance (IR) versus insulin sensitivity (IS) status as defined above (Fig. SI2). Statistical significance was set as ⍺ = 0.05. Figures were produced with JMP Software and Adobe Illustrator CC 2019.
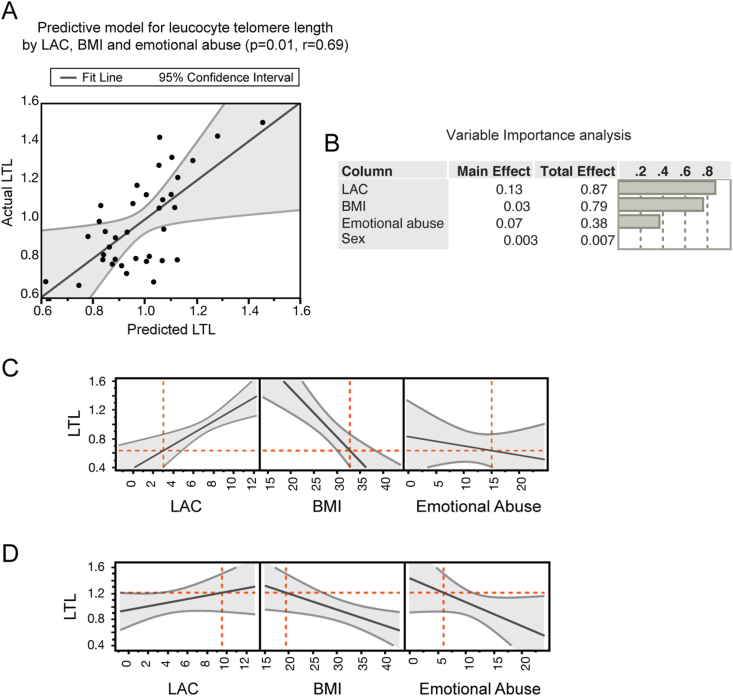
Fig. 2.
Multidimensional biological pathways in potential mitochondrial endophenotypes of depression. A, Multifactorial regression analysis showed that multidimensional factors, including LAC levels, BMI and reported rates of childhood emotional abuse predict leucocytes telomere length (LTL) in subjects with MDD. The model in A depicts LTL predicted values in X and LTL actual values in Y. B Variable importance analysis indicated that the integration of the individual measures results in a superior predictive potential in relation to telomere length as showed by the higher total effects of each variable than the corresponding main effects. C and D, Prediction profilers showed the directionality of the association between the multidimensional measures of mitochondrial metabolism (i.e., LAC levels), metabolic function (i.e., BMI), cellular aging (i.e., LTL), and environmental stress (i.e., childhood trauma). Specifically, we found the lowest LAC levels in those subjects with elevated BMI, high reported rates of childhood emotional abuse and decreased LTL; vice versa subjects with the highest LAC levels are characterized by decreased BMI, low rates of emotional abuse and increased LTL as shown in panel D.
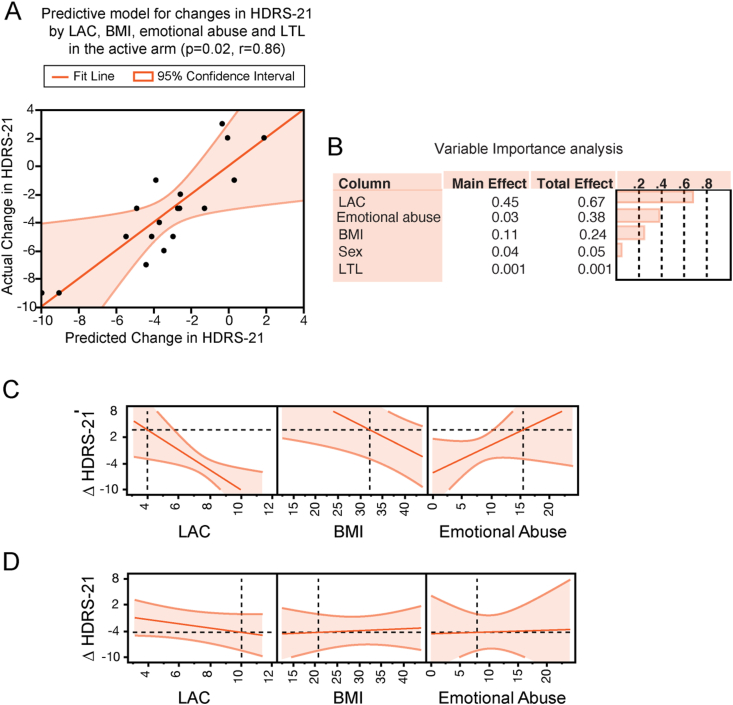
Fig. 3.
Multidimensional predictors of antidepressant responses to pioglitazone in patients with MDD. A, multifactorial regression analysis showed that baseline LAC levels, BMI, LTL and reported rates of childhood emotional abuse predict changes in depression severity in the responses to pioglitazone as assessed using the HDRS-21. A negative change in HDRS-21 indicates improvement in depression severity. The predictive model depicts actual changes in HDRS-21 in Y and predicted changes in HDRS-21 in X as predicted by the interactions between LAC, BMI, emotional abuse, and LTL. B, Variable importance analysis indicated that, of all parameters, LAC levels had the highest variable importance (0.447) in the model predicting changes in depression severity in response to treatment with pioglitazone. Of note, the total effects of each variable were higher than the corresponding main effects. C and D, Prediction profilers show the directionality of changes in depression severity after treatment with pioglitazone as predicted by the interaction of baseline measures of LAC levels, BMI and emotional abuse in a model controlled for LTL. Specifically, decreased baseline LAC levels, elevated BMI and high reported rates of emotional abuse predict lack of changes in depression severity (panel C), vice versa increased baseline LAC levels, decreased BMI and low reported rates of emotional abuse predict decreased depression severity at the HDRS-21 in the responses to pioglitazone (panel D). Both the prediction profilers in B and C are controlled for LTL.
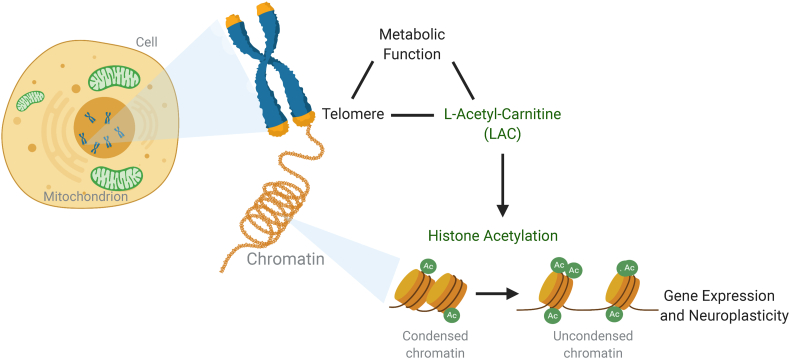
Fig. 1.
Mechanistic model of integrated biological networks for the regulation of brain plasticity. Converging evidence implicate a role for the mitochondrial mediator of epigenetic function acetyl-L-carnitine (LAC) in depression. Recent studies reported decreased levels of LAC in clinical endophenotypes of depression. Preclinical studies showed that supplementation of LAC leads to a rapid antidepressant-like response by promoting epigenetic regulation of glutamatergic function and corresponding changes in specific aspects of neuroplasticity, including dendritic elongation of discrete hippocampal neurons.
3. Results
3.1. Genetic and metabolic correlates of LAC levels in patients with MDD and high reported rates of childhood emotional trauma
The mechanistic model for this study is described in Fig. 1. At baseline, subjects with MDD were evaluated for peripheral levels of the pivotal mitochondrial metabolite LAC (Fig. 1), cellular aging as assessed by LTL, metabolic function as assessed by body mass index (BMI) and specific subscales of childhood trauma as assessed by using the CTQ. The demographic and clinical characteristics are presented in SI Table 1.
Multifactorial regression analysis showed a relationship between LAC levels, LTL, BMI, and childhood emotional abuse, but none of the other types of childhood trauma, in subjects with unremitted depression (Fig. 2A). Specifically, we found that LAC levels, BMI, and reported rates of childhood emotional abuse predict LTL in subjects with MDD (p = 0.01, r = 0.69), and that the interaction of these biological and environmental measures can more accurately predict LTL (p = 0.007) than each individual measure alone (LAC: p = 0.078; BMI: p = 0.014; emotional abuse: p = 0.05). This relationship was controlled for sex, and remained significant after adjusting for age (p = 0.009, r = 0.73) and psychiatric comorbidities (p = 0.03, r = 0.68), corroborating the strength of the relationship between mitochondrial metabolism of LAC, BMI, emotional abuse and LTL in subjects with MDD.
To further ascertain how each individual variable contributes to LTL in subjects with MDD, we assessed the variable importance (Fig. 2B). As expected, the total effects of each variable were higher than individual factors, corroborating the fact that the integration of the individual measures results in a superior predictive potential in relation to telomere length.
Next, we used prediction modeling to assess the directionality of the relationship between LAC levels, LTL, BMI and childhood emotional abuse (Fig. 2C and D). The prediction modelling estimates the interactions between all variables of interest, i.e.: how the relationship between all variables changes when the level of each individual variable is different. We presented two of these conditions in Fig. 2C and D. Specifically, panel C shows the lowest LAC levels in those subjects with elevated BMI, high reported rates of childhood emotional abuse and decreased LTL; vice versa subjects with the highest LAC levels are characterized by decreased BMI, low rates of emotional abuse and increased LTL as shown in panel D.
3.2. Multidimensional predictors of antidepressant responses to pioglitazone in patients with MDD
Driven by the findings of a relationship between mitochondrial metabolism, cellular aging, metabolic function and childhood emotional abuse, we tested the contribution of these interrelated biological pathways in predicting longitudinal changes in depression severity in the responses to pioglitazone in a placebo-controlled study design. Using regression analysis, our data showed that combinatorial measures of baseline LAC levels, LTL, BMI, and emotional abuse predict changes in depression severity in the active arm as assessed by using the HDRS-21 (pioglitazone, p = 0.02, r = 0.86, Fig. 3A). The model was controlled for sex and remained significant after adjusting for age (p = 0.047, r = 0.86) as well as for psychiatric comorbidities (p = 0.03, r = 0.88). The relationship between mitochondrial, metabolic, genetic and environmental factors was specific to the active arm; no relationship was found in the placebo arm (p = 0.2).
We also assessed the variable importance of each factor in predicting treatment responses (Fig. 3B). Of all parameters, LAC levels had the highest variable importance (0.447) in the model predicting changes in depression severity in response to treatment with pioglitazone. Furthermore, the total effects of each variable were higher than individual factors, supporting our hypothesis that a richer set of phenotypical markers can aid in the prediction of antidepressant responses better than the individual variable. These findings are akin to the spirit of precision medicine and support further exploration of mitochondrial metabolism of LAC as a therapeutic target that may help to identify potential mitochondrial endophenotypes of MDD leading to individualized treatment strategies.
Next, using prediction modeling, we assessed the directionality of the relationship between LAC levels, LTL, BMI and childhood trauma in predicting treatment responses to pioglitazone (Fig. 3C and D). Our data showed that decreased baseline LAC levels and the corresponding elevated BMI and high rates of childhood emotional abuse predict lack of changes in depression severity at the HDRS-21 in the responses to pioglitazone (Fig. 3C). Conversely, our data showed that increased baseline LAC levels associated with decreased BMI and low reported rates of childhood emotional abuse in a model controlled for LTL predict greater decline in depression severity at the HDRS-21 in the responses to pioglitazone (Fig. 3D).
We also conducted an exploratory analysis to evaluate the contribution of LAC and IR in predicting longitudinal changes in depression severity. In subjects with IR, but not insulin sensitivity, baseline levels of LAC predicted changes in depression severity at the HDRS-21, independently of the arm (p = 0.02, r = 0.52). Specifically, increased baseline LAC levels predicted greater decline in depression severity at the HDRS-21 (SI Fig. 2A). Conversely, decreased baseline levels of LAC predicted a lack of change in depression severity at the HDRS-21. This relationship was specific to subjects with IR and was not observed in subjects with insulin sensitivity (p = 0.4, SI Fig. 2 B).
4. Discussion
We report that multidimensional signatures integrating mitochondrial metabolism, cellular aging and metabolic function contribute to describe childhood trauma-associated clinical endophenotypes of depression and predict antidepressant responses to pioglitazone. These findings provide a starting point for the development of a mechanistic framework linking specific biological networks and environmental factors to clinical outcomes in pursuit of personalized medicine strategies to effectively treat MDD. These findings also generate new hypotheses for basic neuroscience research to identify the molecular signaling pathways linking specific aspects of mitochondrial metabolism to cellular aging and metabolic function for which rodent models are essential.
A priori multidimensional constructs contribute to describe potential mitochondrial endophenotypes of MDD. We found that shortening of LTL and elevated BMI appear as critical biological pathways involved in a LAC-deficient clinical endophenotype of depression characterized by high rates of childhood emotional abuse. These findings supplement our recent report of a deficiency of the pivotal mitochondrial metabolite LAC in childhood trauma-related endophenotypes of MDD (C. Nasca et al., 2018). The current findings are an outgrowth of a mechanistic framework in rodent models wherein decreased cellular availability of LAC is a signature of excitotoxicity and hippocampal glutamatergic dysfunction (C. Nasca et al., 2018; C. Nasca et al., 2013). In addition to its role in sustaining energy production, LAC is an essential molecule for epigenetic mechanisms of histone acetylation and, in turn, for the regulation of gene expression in brain circuits implicated in depression (C. Nasca et al., 2013; Pettegrew et al., 2000). Because LAC also plays a central role in oxidative stress and energy mobilization, which can affect telomere length and have damaging effects on systemic physiology increasing the risk for elevated BMI (N. L. Rasgon and McEwen, 2016), future studies are needed to further characterize the complex interplay of these integrated biological networks. Taken together the current findings suggest that integrated genetic, metabolic and environmental measures contribute to define potential new mitochondrial endophenotypes of MDD in agreement with the concept of precision medicine (Post, 2018).
In pursuit of modifiable multidimensional predictors of antidepressant responses: a main finding in this study is that multidimensional markers provide more detailed signatures to predict antidepressant responses to pioglitazone. We found that combined measures of mitochondrial metabolism, cellular aging, metabolic function and childhood trauma predict changes in depression severity in response to pioglitazone over time with a stronger ability than each individual measure alone, as shown by the higher total variable importance of the integrated measures than the variable importance of individual components. The specificity of the findings in predicting treatment outcomes in the active arm, but not in the placebo arm, suggest that mitochondrial metabolism of LAC may represent a rate limiting substrate for the regulation of the related biological pathways in the context of antidepressant responses to pioglitazone. This observation is supported by the evidence that utilization of the glutamatergic agent esketamine as an antidepressant leads to dynamic changes in LAC levels and that increased levels of LAC after treatment are linked to decreased severity of depressive symptoms (Rotroff et al., 2016). Collectively, the current work raises the hypothesis for future studies that targeting mitochondrial metabolism of LAC is critical for antidepressant action, possibly via LAC-related regulation of the complex biological pathways of cellular aging and metabolic function.
In agreement with the previously documented link between mitochondrial metabolism and either systemic IR or metabolic profiles of specific brain areas, such as the ventral dentate gyrus and nucleus accumbens (Bigio et al., 2016; Cherix et al., 2020; Larrieu et al., 2017; C. Nasca et al., 2019a,b,c), the current study shows that baseline LAC levels alone predicted changes in depression severity but only in subjects with IR. Within the translational framework of epigenetic regulation of glutamatergic function, supplementation of LAC ameliorates systemic IR (Bigio et al., 2016; Cherix et al., 2020; C. Nasca et al., 2019a,b,c); (McEwen et al., 2015; C. Nasca, Rasgon and McEwen, 2019), and these changes in metabolism are linked to a rapid and enduring antidepressant-like response of LAC at several stress paradigms, including chronic restraint stress and social defeat stress (Cherix et al., 2020; C. Nasca et al., 2019a,b,c). Taken together with our prior report of systemic IR as a biological correlate of the responses to pioglitazone, the current findings support further exploration of the role of mitochondrial metabolism in the pathophysiology of depression and its treatment.
In summary, a main innovation of this study is in its translational approach to identify integrated biological networks and environmental factors for providing more detailed signatures to predict antidepressant responses. Future research is warranted to explore whether these multidimensional predictors of treatment responses generalize to standard antidepressants. Because of the paucity of investigations of mitochondrial metabolism in MDD and antidepressant responses, the current findings of a complex interplay of mitochondrial, metabolic, genetic and environmental factors provide a foundation for future studies to characterize potential new mitochondrial endophenotypes of depression as targets for future personalized medicine strategies.
Funding & disclosure
This work was supported by a NARSAD Young Investigator Grant to CN, a grant from the Robertson Therapeutic Development Foundation to CN, a grant from the Hope for Depression Research Foundation to CN and BMC, and a grant from the Hearst Foundation to NR. The authors have no conflict to declare.
CRediT authors contribution statement
Refer to Carla Nasca for all correspondance regarding L-Acetyl-Carnitine (LAC). Carla Nasca: Conceptualization of the study, interpretation of the data, manuscript writing, design of figures, tables, coordination of research and analyses. Olivia Barnhill, Paolo DeAngelis, Josh Dobbin: contributed to statistical analyses, figures, tables. Jue Lin: performed telomere experiments. James Beasley, Sarah P. Young: performed LAC assessment. Alison Myoraku: contributed to the research. Kathleen Watson: contributed to statistical analyses. Benedetta Bigio: Interpretation of the data, contributed to statistical analyses, figures, tables, and manuscript writing. Bruce McEwen: provided inputs to the research. Natalie Rasgon: interpretation of the data, and inputs on manuscript.
Declaration of competing interest
Authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100407.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Akil H., Gordon J., Hen R., Javitch J., Mayberg H., McEwen B.…Nestler E.J. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2017 doi: 10.1016/j.neubiorev.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T.…Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Biessels G.J., Reagan L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015;16(11):660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- Bigio B., Mathe A.A., Sousa V.C., Zelli D., Svenningsson P., McEwen B.S., Nasca C. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: implications for treatment resistance. Proc. Natl. Acad. Sci. U. S. A. 2016;113(28):7906–7911. doi: 10.1073/pnas.1603111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Epel E.S., Mellon S.H., Lin J., Reus V.I., Rosser R.…Wolkowitz O.M. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J. Affect. Disord. 2014;169:86–90. doi: 10.1016/j.jad.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherix A., Larrieu T., Grosse J., Rodrigues J., McEwen B., Nasca C.…Sandi C. Metabolic signature in nucleus accumbens for anti-depressant-like effects of acetyl-L-carnitine. eLife. 2020;9 doi: 10.7554/eLife.50631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico A., Cardaioli E., Da Pozzo P., Formichi P., Gallus G.N., Radi E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012;322(1–2):254–262. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Friedrich M.J. Depression is the leading cause of disability around the world. Jama. 2017;317(15):1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- Gandhi S., Abramov A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative medicine and cellular longevity. 2012;2012 doi: 10.1155/2012/428010. 428010-428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C.A., Woodruff J.L., Macht V.A., Reagan L.P. Insulin resistance and hippocampal dysfunction: disentangling peripheral and brain causes from consequences. Exp. Neurol. 2019;318:71–77. doi: 10.1016/j.expneurol.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatr. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Kenna H., Hoeft F., Kelley R., Wroolie T., DeMuth B., Reiss A., Rasgon N. Fasting plasma insulin and the default mode network in women at risk for Alzheimer's disease. Neurobiol. Aging. 2013;34(3):641–649. doi: 10.1016/j.neurobiolaging.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R.…Wang P.S. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Larrieu T., Cherix A., Duque A., Rodrigues J., Lei H., Gruetter R., Sandi C. Hierarchical status predicts behavioral vulnerability and nucleus accumbens metabolic profile following chronic social defeat stress. Curr. Biol. 2017;27(14):2202–2210. doi: 10.1016/j.cub.2017.06.027. e2204. [DOI] [PubMed] [Google Scholar]
- Lau T., Bigio B., Zelli D., McEwen B.S., Nasca C. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Mol. Psychiatr. 2017;22(2):227–234. doi: 10.1038/mp.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.W., Wroolie T.E., Robakis T., Rasgon N.L. Adjuvant pioglitazone for unremitted depression: clinical correlates of treatment response. Psychiatr. Res. 2015;230(3):846–852. doi: 10.1016/j.psychres.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejczyk M., Żebrowska E., Chabowski A. Insulin resistance and oxidative stress in the brain: what's new? Int. J. Mol. Sci. 2019;20(4):874. doi: 10.3390/ijms20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Bigio B., Lee F.S., Young S.P., Kautz M.M., Albright A.…Rasgon N. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 2018;115(34):8627–8632. doi: 10.1073/pnas.1801609115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Bigio B., Zelli D., de Angelis P., Lau T., Okamoto M.…McEwen B.S. Role of the astroglial glutamate exchanger xCT in ventral Hippocampus in resilience to stress. Neuron. 2017;96(2):402–413. doi: 10.1016/j.neuron.2017.09.020. e405. [DOI] [PubMed] [Google Scholar]
- Nasca C., Dobbin J., Bigio B., Watson K., de Angelis P., Kautz M.…Rasgon N. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol. Psychiatr. 2020 doi: 10.1038/s41380-020-0804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Menard C., Hodes G., Bigio B., Pena C., Lorsch Z.…Russo S.J. Multidimensional predictors of susceptibility and resilience to social defeat stress. Biol. Psychiatr. 2019;86(6):483–491. doi: 10.1016/j.biopsych.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Rasgon N., McEwen B. An emerging epigenetic framework of systemic and central mechanisms underlying stress-related disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2019;44(1):235–236. doi: 10.1038/s41386-018-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C., Watson-Lin K., Bigio B., Robakis T.K., Myoraku A., Wroolie T.E.…Rasgon N. Childhood trauma and insulin resistance in patients suffering from depressive disorders. Exp. Neurol. 2019;315:15–20. doi: 10.1016/j.expneurol.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Nasca C., Xenos D., Barone Y., Caruso A., Scaccianoce S., Matrisciano F.…Nicoletti F. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl. Acad. Sci. U. S. A. 2013;110(12):4804–4809. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C.B. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89(5):892–909. doi: 10.1016/j.neuron.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Bruno V., Ngomba R.T., Gradini R., Battaglia G. Metabotropic glutamate receptors as drug targets: what's new? Curr. Opin. Pharmacol. 2015;20:89–94. doi: 10.1016/j.coph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Pettegrew J.W., Levine J., McClure R.J. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression. Mol. Psychiatr. 2000;5(6):616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- Post R.M. Myriad of implications of acetyl-l-carnitine deficits in depression. Proc. Natl. Acad. Sci. U. S. A. 2018;115(34):8475–8477. doi: 10.1073/pnas.1811389115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon N., Jarvik L. Insulin resistance, affective disorders, and Alzheimer's disease: review and hypothesis. J Gerontol A Biol Sci Med Sci. 2004;59(2):178–183. doi: 10.1093/gerona/59.2.m178. ; discussion 184-192. [DOI] [PubMed] [Google Scholar]
- Rasgon N., Lin K.W., Lin J., Epel E., Blackburn E. Telomere length as a predictor of response to Pioglitazone in patients with unremitted depression: a preliminary study. Transl. Psychiatry. 2016;6(1) doi: 10.1038/tp.2015.187. e709-e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon N.L., McEwen B.S. Insulin resistance-a missing link no more. Mol. Psychiatr. 2016;21(12):1648–1652. doi: 10.1038/mp.2016.162. [DOI] [PubMed] [Google Scholar]
- Rotroff D.M., Corum D.G., Motsinger-Reif A., Fiehn O., Bottrel N., Drevets W.C.…Kaddurah-Daouk R. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: new mechanistic insights for rapid acting antidepressants. Transl. Psychiatry. 2016;6(9) doi: 10.1038/tp.2016.145. e894-e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Lu Y., Xue Z., Li C., Wang C., Zhao X.…Zhou W. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience. 2015;285:281–291. doi: 10.1016/j.neuroscience.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Watson K., Nasca C., Aasly L., McEwen B., Rasgon N. Insulin resistance, an unmasked culprit in depressive disorders: promises for interventions. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.11.038. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Mellon S.H., Epel E.S., Lin J., Dhabhar F.S., Su Y.…Blackburn E.H. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0017837. e17837-e17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroolie T.E., Kenna H.A., Singh M.K., Rasgon N.L. Association between insulin resistance and cognition in patients with depressive disorders: exploratory analyses into age-specific effects. J. Psychiatr. Res. 2015;60:65–72. doi: 10.1016/j.jpsychires.2014.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.