Abstract
Most large carnivore populations are declining due to anthropogenic activities including direct persecution, prey depletion, habitat loss and degradation. protected areas (PAs) can help maintain viable large carnivore populations; however, anthropogenic activities occurring near and within PA borders or edges can reduce their effectiveness. We investigated the influence of edge effects on abundance of lions (Panthera leo) and spotted hyenas (Crocuta crocuta) in Maswa Game Reserve (MGR), a part of the Serengeti ecosystem in northern Tanzania. We conducted repeated call-ins to attract and enumerate lions and hyenas at 20 stations in MGR during June–July 2017. We used N-mixture models to estimate hyena and lion abundance in relation to land cover and distance from the south-western MGR borders which are adjacent to villages. We found lowest lion and hyena abundances by the south-western border, with abundance of both species increasing toward the eastern border adjacent to Serengeti National Park. Lions were uniformly distributed among land covers whereas hyenas were more abundant in woodlands. We suggest that reduced lion and hyena abundance near human settlements was in response to depleted prey, due to human actions. We recommend ecologically compatible land uses and effective border patrols to mitigate these adverse effects.
Subject terms: Ecology, Conservation biology
Introduction
Over 60% of large carnivore species are threatened with extinction and nearly 80% are experiencing population declines1, with remaining populations occupying reduced portions of their historic ranges. For example, lions (Panthera leo), are limited to 8% of their historic distributions2 and spotted hyena (Crocuta crocuta) populations, previously considered stable, are also declining1. Although little recognized, large carnivores provide important ecosystem functions, as their trophic position can influence ecological cascades and carbon sequestration1,3,4.Through interference interactions, large carnivores may regulate each other5, as well as prey and mesopredator populations5. Additionally, large carnivores may in part regulate diseases6,7 and provide scavenging opportunities for mesopredators and avian predators8.
As large carnivores are prone to conflict with humans9, viable populations often are maintained within protected areas (PAs)10. These PAs are increasingly effective when surrounded by land use compatible with wildlife11,12. Wildlife landscapes beyond PAs borders can serve as buffers and are critical to large carnivores due to their extensive space use and likelihood of ranging beyond core PAs1. Most PAs in Africa however, harbor large carnivore populations below their carrying capacity13,14. Several factors account for reduced populations including overexploitation (e.g., traditional medicines, illegal and poorly regulated hunting), depletion of prey species9,14,15, human encroachment resulting in habitat loss and degradation, and human-carnivore conflicts14,16. Overall, these and other human actions negatively influence not only large carnivore populations, but also the ecological integrity of PA and buffers9.
Human activities including illegal wildlife harvests, may adversely affect PAs and buffer areas4,11,17,18. For example, wildebeest (Connochaetes taurinus) biomass in Serengeti National Park (SNP) was reduced by at least 75% within 15 km of its border4. Wide-ranging movements of large carnivores result in their greater likelihood of crossing PAs borders, into unprotected zones with greater risk from human conflicts9,19–21.
Our objective was to assess whether lion and spotted hyena abundance varied as a function of distance from the border of Maswa Game Reserve (MGR). We conducted call-in surveys and used N-mixture models to estimate lion and spotted hyena abundance and distribution within MGR, a PA and buffer area within the Serengeti ecosystem of Tanzania. We predicted that lion and hyena abundance would increase as distance from PA edge increased, in particular on the south-western border of MGR where there is greater human habitation. Further, we expected this relationship to vary based on distribution of land covers, with greater hyena abundance in grasslands and lion abundance in forested areas21–23. Finally, we anticipated less tolerance by lions to human disturbances than by hyenas, as felids are typically more sensitive to anthropogenic disturbances than hyaenids and canids17,24.
Results
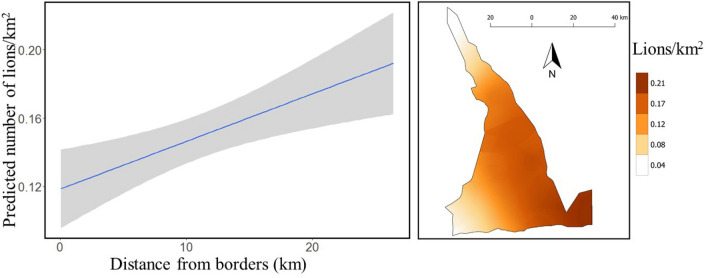
We detected lions at 12 of 20 sites (60%) with maximum counts at these sites ranging from one (1) to 10 individuals. Model goodness-of-fit (GOF) was good, with a Bayesian p-value of 0.48. Detection probability of lions across sites varied from 0.10 to 0.50. Lion density was greater closer to SNP (mean = 0.146 lions/km2, SD = 0.062, 95% CI = 0.046–0.282) than near the south-western border of MGR (mean = 0.039 lions/km2, SD = 0.041, 95% CI = 0.001–0.148) (Fig. 1). Overall, we estimated 303 (SD = 40; 95% CI = 237–392) lions in MGR (0.138 lions/ km2). Land cover did not influence lion abundance and probability of detecting lions did not vary across weeks (Table 1).
Figure 1.
Estimated density of lions (left panel) in relation to distance from south and west borders and their spatial distribution (right panel), Maswa Game Reserve, Tanzania, 2017. Gray shading in left panel represents 95% credible interval.
Table 1.
Covariates influencing abundance of lions and spotted hyenas, Maswa Game Reserve, Tanzania, June–July 2017.
| Species | Covariate | Mean | SD | Rhat | Credible interval | |
|---|---|---|---|---|---|---|
| 2.5% | 97.5% | |||||
| Lion | Distance to border (km) | 0.005 | 0.003 | 1.000 | 0.000 | 0.010 |
| Shrub | − 0.011 | 1.370 | 1.077 | − 0.661 | 0.689 | |
| Sparse grassland | − 1.123 | 1.123 | 1.000 | − 3.766 | 1.661 | |
| Dense grassland | 0.155 | 0.448 | 1.009 | − 0.702 | 1.056 | |
| Woodland | 0.285 | 0.337 | 1.010 | − 0.351 | 0.952 | |
| Closed grassland | 0.170 | 4.112 | 1.008 | − 7.440 | 8.683 | |
| Shrub-grassland | − 0.031 | 0.478 | 1.099 | − 0.938 | 0.927 | |
| Hyena | Distance to border (km) | 0.005 | 0.003 | 1.000 | 0.000 | 0.010 |
| Shrub | − 0.165 | 0.595 | 1.001 | − 1.329 | 1.016 | |
| Sparse grassland | 8.818 | 4.787 | 1.000 | − 0.008 | 18.747 | |
| Dense grassland | 0.286 | 0.860 | 1.001 | − 1.317 | 2.051 | |
| Woodland | 1.632 | 0.411 | 1.002 | 0.833 | 2.447 | |
| Closed grassland | 11.393 | 8.073 | 1.000 | − 4.212 | 27.334 | |
| Shrub-grassland | − 0.610 | 0.799 | 1.002 | − 2.050 | 1.082 | |
Significant covariates are indicated in bold font. Mean = estimate mean, SD = Standard Deviation, Rhat = Gelman–Rubin convergence diagnostic, convergence occurs at Rhat = 1.
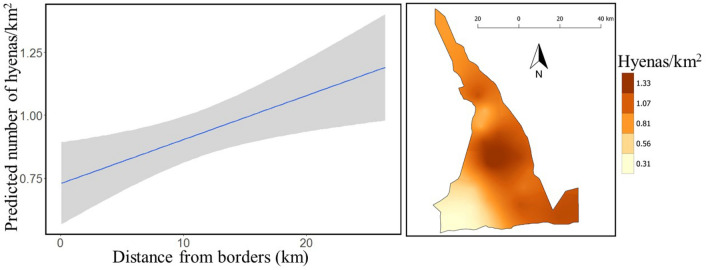
We detected hyenas at all 20 sites (100%) with the maximum count ranging from one (1) to 27 individuals. Model GOF was good25, with a Bayesian p-value of 0.60. Hyena detection probability ranged from 0.47 to 0.66. Hyena abundance was greater further from the south and western borders (mean = 1.264 hyenas/ km2, SD = 0.183; 95% CI = 0.956–1.612) than at the border to human settlements; (mean = 0.300 hyenas/km2, SD = 0.167; 95% CI = 0.067–0.765) (Fig. 2). Overall, we estimated 2 008 (SD = 146; 95% CI = 1 747–2 319) hyenas in MGR (0.940 hyenas/ km2). We found significantly greater hyena abundance within woodlands and probability of detecting hyenas did not vary across weeks (Table 1).
Figure 2.
Estimated density of hyenas (left panel) in relation to distance from south and west borders and their spatial distribution (right panel), Maswa Game Reserve, Tanzania, 2017. Gray shading in left panel represents 95% credible interval.
Discussion
We demonstrated that lion and hyena distributions varied across MGR with both species less abundant near the south-western border, increasing eastward toward the SNP border. Hyena abundance near the SNP border was more than four times higher (1.264 hyenas/km2) than at the south-western border (0.300 hyenas/km2). Although trophy hunting is allowed in MGR, it is unlikely that hunting caused the observed spatial variation in hyena and lion distributions. Four lions were legally harvested in MGR in 2013 and none since that year (MGR, unpublished data). Hyenas are undesirable as trophy animals and therefore rarely hunted in MGR (MGR, unpublished data).
Human disturbances are the probable cause of the observed gradual increase in numbers of lion and hyena from south-western to eastern borders of MGR. South-western areas of MGR receive limited patrols and therefore, daily incursions of poachers, agro-pastoralists, and livestock occur4. Veldhuis et al.4 reported high human encroachment in PAs within the Greater Serengeti Ecosystem (GSE) seven (7) km from the border. These areas within the GSE appear too degraded to support adequate prey biomass4,26 Depleted prey is likely due to poaching which is generally intense within the GSE; over 160,000 animals of various species are killed annually10, but poaching is greater in MGR because bushmeat from poaching is commercialized (i.e., 93% of poachers sell bushmeat27). Poaching and livestock incursions can also indirectly deplete the prey base of large carnivores14,28,29. Large carnivores are killed to reduce livestock depredations and in retaliation of livestock loss4,11,30. Finally, wire snares used for poaching are non-selective and can cause substantial carnivore mortalities (e.g., > 7.5% of adult hyena mortality in SNP22). Low prey density and poaching are therefore likely causes of lower hyena and lion abundance in areas of MGR near human settlements.
We provide the first abundance estimate for hyenas using N-mixture models which are robust as they can account for individual and group responses31,32. Our average density estimates of 0.940 hyenas/km2 is slightly greater than reported for SNP (0.600–0.800 hyenas/km2; Hofer and East, 199325, 0.33–0.74 hyenas/km2; Durant et al.33). To our knowledge, there are no previous estimates for spotted hyenas in our study area. Less interference competition between lions and hyenas due to low lion abundance in MGR could explain greater hyena abundance in MGR than in SNP. Similarly, wild dogs (Lycaon pictus) have not reestablished in SNP, apparently in part due to interference competition with lions34,35. To our knowledge this is the first lion estimate for MGR. Our estimate of 0.138 lions/ km2 is slightly lower than estimates in grasslands of nearby southeastern SNP (0.144 lions/km2), using a similar technique31. Although woodland vegetation and year-around water availability would suggest greater lion abundance in this area36, human activities near the south-western borders of MGR appear to have reduced overall lion density in MGR. Because of low responses, our survey may have excluded most young individuals. But we believe that this has a minimal effect in potential management decisions as young animals are reproductively inactive, and especially for the lions, cubs are prone to higher mortality (up to 50%37).
Contrary to our second prediction we observed more hyenas in the woodlands of MGR, which differed from observed distributions in SNP and Maasai Mara National Reserve (MMNR), Kenya, where hyena distributions were greater in open habitats than woodlands22,23,38. The observed difference in hyena habitat use may be a consequence of adaptability and behavioral plasticity toward anthropogenic disturbances. Increasing human disturbances in MGR4 may have caused hyena to use woodlands and forests more often, which have fewer prey, but denser vegetation that may confer protection from humans23,39. This observation is congruent with our third prediction that hyenas are more adaptable to anthropogenic disturbances than lions. This finding is supported by previous works40, where livestock grazing in the core of hyena territories in MMNR caused hyenas to shift their activities to the peripheries of their territories40.
We found no evidence that land cover influenced lion distributions in MGR, similar to lions in SNP31. Although most carnivore distributions are positively associated with prey density and foraging opportunities41, lion distribution is influenced by prey availability and vulnerability36,42. We therefore suggest that lions are either more strongly influenced by other factors such as prey distribution or avoid encountering humans in more suitable habitats. Including more refined metrics of anthropogenic disturbances could further elucidate drivers of lion distributions.
Our study suggests that large carnivores are spatially depleted in south-western MGR. Similarly, Veldhuis et al.4 reported that wildebeest spend less time in these border areas. Only 11% of the historical Maswa region is currently protected11,26; poaching and overgrazing continue and are likely to further increase with increasing human population (4% annual population increase43). However, MGR acts as a migratory-wildebeest refuge during the short dry season (February–March) which extends through April in drought years11. As demonstrated in other buffer areas, MGR absorbs enormous anthropogenic pressure which could otherwise adversely affect more core PAs such as SNP11,44. Our findings agree with previous studies which suggest that Maswa is ecologically eroded11, which if not rapidly addressed may dramatically and further damage the integrity of the GSE.
Many large carnivore species are increasingly threatened1,31, and limitations to their effective conservation includes a paucity of information on population size and dynamics2. With a few notable examples, knowledge of Tanzania’s large carnivore populations, especially in hunted areas, is limited. Crosmary et al.45 estimated lion populations in Selous Game Reserve, but used a track-count method, which is widely criticized31,46,47. Call-in surveys are generally recommended47–50 and we further demonstrate that call-in surveys appear applicable for enumerating large carnivores in hunted populations, where poorly-regulated harvests may accelerate local declines51. When properly conducted, repeating estimates using our study design may influence management decisions, including setting appropriate harvest quotas52 and assessing the performance various harvest practices (e.g., age limit53).
A long-term solution is needed to address the loss of large carnivore habitat suitability in MGR. Human encroachment inside the reserve needs to be reduced and more compatible land-use practices should be encouraged among local people. These activities could include conservation compatible projects like bee keeping10. Effective patrols and additional ranger posts along the south-western border could reduce poaching and illegal livestock incursions. Importantly, management authorities in MGR can work toward further improving the efficiency of detections, reporting, and responding to illegal activities within the reserve.
Material and methods
Study area
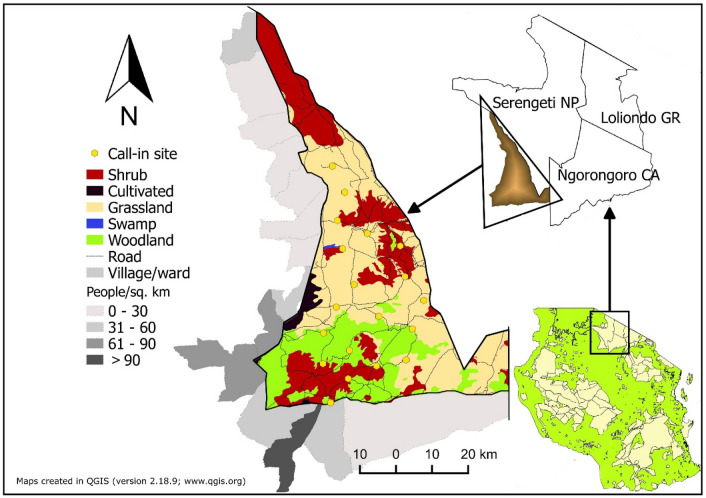
We conducted this study in the dry season during June–July 2017 in the 2200 km2 MGR, Tanzania (Fig. 3). MGR is the south-western portion of the Greater Serengeti Ecosystem (GSE)4. GSE covers 25,000 km2 between Tanzania and Kenya. Comprising of SNP and MMNR as core areas, surrounded by buffers including MGR4. In the east, MGR borders and buffers SNP from several villages found on the western GSE11.Thirteen wards/villages borders MGR to the south and west, some of these villages are more densely populated than a national average (51 people/km2; Fig. 354). Apart from SNP eastern MGR borders Makao Wildlife Management Area (WMA), and Ngorongoro Conservation Area (NCA). Annual rainfall in MGR increases from south (~ 550 mm) to north (~ 850 mm;17), with most precipitation occurring during November–May, with a short dry season in January–March11. Southern MGR is predominantly woodland whereas grasslands are interspersed with dense forest patches in the north44,55. MGR supports over two million migratory ungulates during February–March, but migration can occur through April during dry years7,11. Wildlife is subjected to legal harvests in MGR during July–December.
Figure 3.
Locations of call-in sites used to estimate lion and spotted hyena abundance, Maswa Game Reserve, Tanzania, 2017. Beyond southern and western borders are the neighboring villages/wards. Light gray are less human populated villages/wards than darker grey.
Methods
We established 20 call-in sites throughout MGR, with sites separated by at least 8 km to ensure spatial independence and reduce double counting (Fig. 3;34,48,50). Because of limited nighttime accessibility, our call-in sites were on roads and we randomly selected the first site and systematically placed the remaining sites after each 8-km along the same or nearby road within MGR. We surveyed the 20 sites over five consecutive nights (4 sites each night) in 5 sessions for 5 consecutive weeks during June–July 2017. Typically, we broadcasted vocalizations during 1900–0300 h for 70-min per site following (Belant et al.31,49). We followed previously described procedures for broadcasting vocalizations [see Ref.49] using a lion roar, prey in distress, and spotted hyena whoop call. Prey in distress included warthog (Phacochoerus africanus) for sessions 1 and 4, wildebeest (Connochaetes taurinus) for sessions 2 and 5, and zebra (Equus quagga) for session 3 (see Belant et al.49). We broadcasted calls at up to 116 dB using a commercial game calling system (Foxpro Inc., Lewistown, Pennsylvania, USA) and 4 speakers mounted at 90-degree intervals on the roof of a vehicle about 2.4 m above ground.
We created a 3-km radius (28.27 km2) buffer around each call-in site and used GIS to estimate the percentage of land cover in each. We obtained GIS layers from the Serengeti-Mara database, managed by Tanzania National Parks and Frankfurt Zoological Society (https://serengetidata.weebly.com/). We categorized existing land covers into six classes including sparse grassland, closed grassland, dense grassland, shrub-grassland, shrubland, and woodland31,56 and incorporated these attributes in models as they are known to influence distributions of lions and hyenas32,42. We used a 3-km2 effective radius50,57 to convert abundance into density estimates.
We estimated hyena and lion abundance at call-in sites using N-mixture models58–60 in a Bayesian framework and compared estimated detection probabilities across sites. N-mixture models commonly assume closure in the studied population. We considered lion and hyena population size to be stable during the survey based on dry season stability of residence prey species22. The ‘true’ ecological state Ni describing abundance (i.e. number of individuals in the area of influence of our call-in sites) in site i was defined as a Poisson random variable, with an expected value λi58. A site corresponded to the area of assumed influence of a call-in. We modeled the expected value of the Poisson distribution as a linear combination of an intercept a, and a random site effect εi on the log-scale as:
Because lion and hyena responded to the call site as a group, individual detection was not completely independent. Therefore, we modeled the influence of this detection heterogeneity25,54, while accounting for imperfect detection. We modeled the count process yit in site i during session t conditionally on the true abundance as:
where ρ is a correlation parameter44, such that:
where pit is the individual detection probability in site i during week t. We allowed detection probability pit to vary among sites and sessions, following a non-informative uniform prior:
We next generated the mean session detection probability for 20 call-in sites. We then estimated the population size of each site by first accounting for potential sampling biases. N-mixture models typically rely on several assumptions including population closure, absence of false positives, and independence and homogeneity of detection60. Our sampling approaches mitigated any potential departures from these assumptions as we conducted this survey over a short duration (i.e., 5 weeks) with long distances (> 8 km) between call-in sites which reduced the potential of double counting. Further, we also noted the direction of individual approach and departure during call-ins, recording of individual age classes.
We developed Bayesian models for call-in counts using package “jagsUI”61 in program R version 4.0.362, with non-informative priors for each parameter. We ran three chains of 100,000 iterations after a 10,000 burn-in with a thinning of 10 and monitored convergence of the MCMC chains using Gelman–Rubin convergence diagnostic (R-hat, at convergence R-hat = 163). We assessed GOF of our model based on its derived Bayesian p-value (bpv), with values close to 0.5 suggesting good model fit25. We used a variable selection process for the regression model as our model selection criteria64,65. We present average estimated abundance at call-in sites, as well as corresponding detection probabilities with 95% credible intervals.
As the south-western boundary of MGR borders human settlements and agro-pastoralists. We used distance from call-in site to the nearest border (south or west), to model the influence of this edge effect on abundance of lions and hyenas. Human settlements and agro-pastoralism can influence animal distributions, including survival and increased conflicts with humans3,9,11,18. We simultaneously modeled potential effects of landcovers.
Acknowledgements
We thank the Tanzania Wildlife Research Institute (TAWIRI), Tanzania Wildlife Authority (TAWA), and Commission for Science and Technology (COSTECH) for permission to conduct this research (permit 2016-187-ER-2016-125). P. Kessey, D. Minja, B. Ngereja, M. Shilereyo, B. Lubengo, E. Simmonds and numerous MGR rangers provided valuable assistance.
Author contributions
Conceived and designed the experiments: J.L.B. Performed the experiments: J.L.B., S.B.M., C.M.W., E.R., and I.J.M. Analyzed the data: S.B.M. Contributed analysis tools: J.L.B., P.S.R., C.M.W., I.J.M. and L.M.M. Wrote the paper: S.B.M., J.L.B., R.F., and E.R. Edited the paper: C.M.W., P.S.R., and L.M.M.
Funding
Financial support was provided by Safari Club International Foundation and Camp Fire Conservation Fund.
Data availability
Data available on the request from the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343:1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 2.Riggio J, et al. The size of savannah Africa: A lion’s (Panthera leo) view. Biodivers. Conserv. 2013;22:17–35. [Google Scholar]
- 3.Carbone C, Gittleman JL. A common rule for the scaling of carnivore density. Science. 2002;295:2273–2276. doi: 10.1126/science.1067994. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuis MP, et al. Cross-boundary human impacts compromise the Serengeti-Mara ecosystem. Science. 2019;363:1424–1428. doi: 10.1126/science.aav0564. [DOI] [PubMed] [Google Scholar]
- 5.Palomares F, Caro TM. Interspecific killing among mammalian carnivores. Am. Nat. 1999;153:492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- 6.Tanner E, et al. Wolves contribute to disease control in a multi-host system. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-44148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Bryan CJ, et al. The contribution of predators and scavengers to human well-being. Nat. Ecol. Evol. 2018;2:229–236. doi: 10.1038/s41559-017-0421-2. [DOI] [PubMed] [Google Scholar]
- 8.Prugh LR, Sivy KJ. Enemies with benefits: integrating positive and negative interactions among terrestrial carnivores. Ecol. Lett. 2020;23:902–918. doi: 10.1111/ele.13489. [DOI] [PubMed] [Google Scholar]
- 9.Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- 10.Wilfred P. Towards sustainable wildlife management areas in Tanzania. Trop. Conserv. Sci. 2010;3:103–116. [Google Scholar]
- 11.Sinclair AR, Metzger KL, Mduma SA, Fryxell JM. Serengeti IV: Sustaining Biodiversity in a Coupled Human-Natural System. University of Chicago Press; 2015. [Google Scholar]
- 12.Crooks KR, Sanjayan M. Connectivity Conservation. Cambridge University Press; 2006. [Google Scholar]
- 13.Balme GA, Slotow R, Hunter LT. Edge effects and the impact of non-protected areas in carnivore conservation: Leopards in the Phinda-Mkhuze Complex, South Africa. Anim. Conserv. 2010;13:315–323. [Google Scholar]
- 14.Lindsey P, et al. The performance of African protected areas for lions and their prey. Biol. Conserv. 2017;209:137–149. [Google Scholar]
- 15.Elliot NB, Gopalaswamy AM. Toward accurate and precise estimates of lion density. Conserv. Biol. 2017;31:934–943. doi: 10.1111/cobi.12878. [DOI] [PubMed] [Google Scholar]
- 16.Masenga E, et al. Strychnine poisoning in African wild dogs (Lycaon pictus) in the Loliondo game controlled area, Tanzania. Int. J. Biodivers. Conserv. 2013;5:367–370. [Google Scholar]
- 17.Metzger K, Sinclair A, Hilborn R, Hopcraft JGC, Mduma SA. Evaluating the protection of wildlife in parks: The case of African buffalo in Serengeti. Biodivers. Conserv. 2010;19:3431–3444. [Google Scholar]
- 18.Mogensen NL, Ogutu JO, Dabelsteen T. The effects of pastoralism and protection on lion behaviour, demography and space use in the Mara Region of Kenya. Afr. Zool. 2011;46:78–87. [Google Scholar]
- 19.Kiffner C, Meyer B, Mühlenberg M, Waltert M. Plenty of prey, few predators: what limits lions Panthera leo in Katavi National Park, western Tanzania? Oryx. 2009;43:52–59. [Google Scholar]
- 20.Kiffner C, Stoner C, Caro T. Edge effects and large mammal distributions in a national park. Anim. Conserv. 2013;16:97–107. [Google Scholar]
- 21.Newmark WD. Isolation of African protected areas. Front. Ecol. Environ. 2008;6:321–328. [Google Scholar]
- 22.Hofer H, East M. Population dynamics, population size, and the commuting system of Serengeti spotted hyenas. Serengeti II Dyn. Manag. Conserv. Ecosyst. 1995;2:332. [Google Scholar]
- 23.Holekamp KE, Dloniak SM. Intraspecific variation in the behavioral ecology of a tropical carnivore, the spotted hyena. Adv. Study Behav. 2010;42:189–229. [Google Scholar]
- 24.Crooks KR. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv. biol. 2002;16:488–502. [Google Scholar]
- 25.Martin J, et al. Accounting for non-independent detection when estimating abundance of organisms with a Bayesian approach. Methods Ecol. Evol. 2011;2:595–601. [Google Scholar]
- 26.Prins HH, Grootenhuis JG, Dolan TT. Wildlife Conservation by Sustainable Use. Springer Science & Business Media; 2012. [Google Scholar]
- 27.Knapp EJ. Why poaching pays: a summary of risks and benefits illegal hunters face in Western Serengeti, Tanzania. Trop. Conserv. Sci. 2012;5:434–445. [Google Scholar]
- 28.Revilla E, Palomares F, Delibes M. Edge-core effects and the effectiveness of traditional reserves in conservation: Eurasian badgers in Doñana National Park. Conserv. Biol. 2001;15:148–158. [Google Scholar]
- 29.Lindsey PA, et al. The bushmeat trade in African savannas: Impacts, drivers, and possible solutions. Biol. Conserv. 2013;160:80–96. [Google Scholar]
- 30.Ikanda D, Packer C. Ritual vs. retaliatory killing of African lions in the Ngorongoro Conservation Area, Tanzania. Endanger. Species Res. 2008;6:67–74. [Google Scholar]
- 31.Belant JL, et al. Estimating lion abundance using N-mixture models for social species. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep35920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofer H, East ML. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey I. Social organization. Anim. Behav. 1993;46:547–557. [Google Scholar]
- 33.Durant SM, et al. Long-term trends in carnivore abundance using distance sampling in Serengeti National Park, Tanzania. J. Appl. Ecol. 2011;48:1490–1500. [Google Scholar]
- 34.Swanson AB. Living with Lions: Spatiotemporal Aspects of Coexistence in Savanna Carnivores. University of Minnesota; 2014. [Google Scholar]
- 35.Masenga EH, Lyamuya RD, Mjingo EE, Fyumagwa RD, Røskaft E. Communal knowledge and perceptions of African wild dog (Lycaon pictus) reintroduction in the western part of Serengeti National Park, Tanzania. Int. J. Biodivers. Conserv. 2017;9:122–129. [Google Scholar]
- 36.Hopcraft JGC, Sinclair A, Packer C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005;74:559–566. [Google Scholar]
- 37.Packer C, Pusey AE. Adaptations of female lions to infanticide by incoming males. Am. Nat. 1983;121:716–728. [Google Scholar]
- 38.Kruuk H, Turner M. Comparative notes on predation by lion, leopard, cheetah and wild dog in the Serengeti area, East Africa. Mammalia. 1967;31:1–27. [Google Scholar]
- 39.Green DS, Johnson-Ulrich L, Couraud HE, Holekamp KE. Anthropogenic disturbance induces opposing population trends in spotted hyenas and African lions. Biodiver. Conserv. 2018;27:871–889. doi: 10.1007/s10531-017-1469-7. [DOI] [Google Scholar]
- 40.Kolowski JM, Katan D, Theis KR, Holekamp KE. Daily patterns of activity in the spotted hyena. J. Mamm. 2007;88:1017–1028. [Google Scholar]
- 41.Šálek M, Kreisinger J, Sedláček F, Albrecht T. Do prey densities determine preferences of mammalian predators for habitat edges in an agricultural landscape? Landsc. Urban Plan. 2010;98:86–91. [Google Scholar]
- 42.Mosser A, Fryxell JM, Eberly L, Packer C. Serengeti real estate: density vs. fitness-based indicators of lion habitat quality. Ecol. Lett. 2009;12:1050–1060. doi: 10.1111/j.1461-0248.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt JA. Improving Conservation Efforts in the Serengeti Ecosystem, Tanzania: An Examination of Knowledge, Benefits, Costs, and Attitudes. University of Minnesota; 2010. [Google Scholar]
- 44.Makacha S, Msingwa MJ, Frame GW. Threats to the Serengeti herds. Oryx. 1982;16:437–444. [Google Scholar]
- 45.Crosmary W-G, et al. Lion densities in selous game reserve, Tanzania. Afr. J. Wildl. Res. 2018;48:1–6. [Google Scholar]
- 46.Belant JL, et al. Track surveys do not provide accurate or precise lion density estimates in serengeti. Glob. Ecol. 2019;19:e00651. [Google Scholar]
- 47.Midlane N, O'Riain MJ, Balme GA, Hunter LTB. To track or to call: comparing methods for estimating population abundance of African lions Panthera leo in Kafue National Park. Biodiver. Conserv. 2015;24:1311–1327. doi: 10.1007/s10531-015-0858-z. [DOI] [Google Scholar]
- 48.Ogutu JO, Dublin HT. The response of lions and spotted hyaenas to sound playbacks as a technique for estimating population size. Afr. J. Ecol. 1998;36:83–95. doi: 10.1046/j.1365-2028.1998.113-89113.x. [DOI] [Google Scholar]
- 49.Belant, J. L. et al. Temporal and spatial variation of broadcasted vocalizations does not reduce lion Panthera leo habituation. Wildl. Biol. wlb. 00287 (2017).
- 50.Cozzi G, Broekhuis F, McNutt J, Schmid B. Density and habitat use of lions and spotted hyenas in northern Botswana and the influence of survey and ecological variables on call-in survey estimation. Biodiver. Conserv. 2013;22:2937–2956. [Google Scholar]
- 51.M'soka J, Creel S, Becker MS, Droge E. Spotted hyaena survival and density in a lion depleted ecosystem: The effects of prey availability, humans and competition between large carnivores in African savannahs. Biol. Conserv. 2016;201:348–355. [Google Scholar]
- 52.Croes B, et al. The impact of trophy hunting on lions (Panthera leo) and other large carnivores in the Bénoué Complex, northern Cameroon. Biol. Conserv. 2011;144:3064–3072. [Google Scholar]
- 53.Whitman K, Starfield AM, Quadling HS, Packer C. Sustainable trophy hunting of African lions. Nature. 2004;428:175–178. doi: 10.1038/nature02395. [DOI] [PubMed] [Google Scholar]
- 54.National Bureau of Statistics. Tanzania in Figures 2012 (The United Republic of Tanzania, 2013).
- 55.McNaughton S. Serengeti grassland ecology: The role of composite environmental factors and contingency in community organization. Ecol. Monograph. 1983;53:291–320. [Google Scholar]
- 56.Reed D, Anderson T, Dempewolf J, Metzger K, Serneels S. The spatial distribution of vegetation types in the Serengeti ecosystem: the influence of rainfall and topographic relief on vegetation patch characteristics. J. Biogeogr. 2009;36:770–782. [Google Scholar]
- 57.Sollmann, R., Gardner, B., Belant, J. L., Wilton, C. M. & Beringer, J. Habitat associations in a recolonizing, low‐density black bear population. Ecosphere7 (2016).
- 58.Royle JA, Dorazio RM. Hierarchical Modeling and Inference in Ecology: The Analysis of Data from Populations, Metapopulations and Communities. Elsevier; 2008. [Google Scholar]
- 59.Chandler RB, Royle JA, King DI. Inference about density and temporary emigration in unmarked populations. Ecology. 2011;92:1429–1435. doi: 10.1890/10-2433.1. [DOI] [PubMed] [Google Scholar]
- 60.Royle JA. N-mixture models for estimating population size from spatially replicated counts. Biometrics. 2004;60:108–115. doi: 10.1111/j.0006-341X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 61.Kellner, K. & Meredith, M. Package ‘jagsUI’. (2021).
- 62.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2021).
- 63.Gelman A, Hwang J, Vehtari A. Understanding predictive information criteria for Bayesian models. Stat. Comput. 2014;24:997–1016. [Google Scholar]
- 64.Kuo, L. & Mallick, B. Variable selection for regression models. Indian J. Stat. 65–81 (1998).
- 65.Congdon P. Bayesian Models for Categorical Data. John Wiley and Sons; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on the request from the authors.