Abstract
Patients undergoing cardiac surgery develop a marked postoperative systemic inflammatory response. Blood transfusion may contribute to disruption of homeostasis in these patients. We sought to evaluate the impact of blood transfusion on serum interleukin-6 (IL-6), hypoxia induced factor-1 alpha (HIF-1α) levels as well as adverse outcomes in patients undergoing adult cardiac surgery. We prospectively enrolled 282 patients undergoing adult cardiac surgery. Serum IL-6 and HIF-1α levels were measured preoperatively and on the first postoperative day. Packed red blood cells were transfused in 26.3% of patients (mean 2.93 ± 3.05 units) by the time of postoperative sampling. Postoperative IL-6 levels increased over 30-fold and were similar in both groups (p = 0.115), whilst HIF-1α levels (0.377 pg/mL vs. 0.784 pg/mL, p = 0.002) decreased significantly in patients who received red blood cell transfusion. Moreover, greater decrease in HIF-1α levels predicted worse in-hospital and 3mo adverse outcome. Red blood cell transfusion was associated with higher risk of major adverse outcomes (stroke, pneumonia, all-cause mortality) during the index hospitalization. Red blood cell transfusion induces blunting of postoperative HIF-1 α response and is associated with higher risk of adverse thrombotic and pulmonary adverse events after cardiac surgery.
Clinical Trial Registration ClinicalTrials.gov Identifier: NCT03444259.
Subject terms: Interleukins, Predictive markers
Introduction
Transfusion of red blood cells (RBC) increases oxygen delivery in patients with severe perioperative anemia. However, RBC transfusion is associated with higher risk of stroke and all-cause mortality in patients undergoing cardiac surgery1–4. Hypoxia induced factor-1 alpha (HIF-1α) is a key transcription factor that helps the body to adapt to inflammation caused by hypoxia5. In a small pilot study of six patients undergoing surgical repair of the abdominal aorta, we showed that aortic cross-clamping was associated with an increase of HIF-1α unless the patient received RBC transfusion6. In the present study, we sought to evaluate the systemic inflammatory response, HIF-1α levels and the related clinical outcome in patients undergoing adult cardiac surgery.
Results
Patient characteristics
Overall, 282 patients underwent adult cardiac surgery and were included in this analysis (Fig. 1). Most operations were elective (83.0%). Four patients (1.4%) underwent emergency or salvage operation. During the index hospitalization 116/266 (43.6%) patients received RBC transfusion. Within the first 12 postoperative hours and before the second blood sample was obtained, 70/266 (26.3%) patients received RBC transfusion (45 patients received 1–2 RBC units and 25 received more than 2 RBC units). Mean transfusion amount was 2.93 ± 3.05 units among those who received RBC. Patients who received RBC transfusion after the allotted 12-h period (n = 46/266) were excluded from further analyses.
Figure 1.
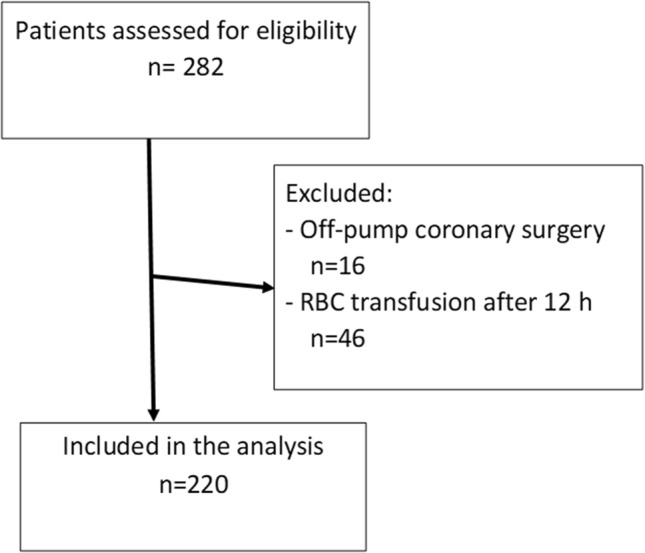
Flow chart of eligibility.
The baseline and perioperative characteristics of patients who received or not RBC transfusion within the first 12 perioperative hours are summarized in Tables 1 and 2. Patients with RBC were more often female, older, had history of congestive heart failure, and treatment for type I diabetes, hypothyroidism and hypertension. Patients who received RBC transfusion during the first 12 h had longer operation time and longer stay at the intensive care unit (ICU) after index operation. To further adjust for baseline differences, a propensity score was calculated by logistic regression. The matching caliper chosen was 0.05. One-to-one propensity score matching provided 43 pairs with similar baseline and operative characteristics except for some difference in the use of treatment for hypertension and BMI.
Table 1.
Baseline characteristics of the study cohorts.
| RBC transfusion | No RBC transfusion | p | RBC transfusion PSM |
No RBC transfusion PSM |
p | |
|---|---|---|---|---|---|---|
| n = 70 | n = 150 | n = 43 | n = 43 | |||
| Age | 70.0 (65.0–76.0) | 65.0 (56.0–71.0) | 0.001 | 69.0 (65.0–76.0) | 69.0 (62.0–76.0) | 0.647 |
| BMI | 26.1 (24.0–30.0) | 28.5 (± 4.5) | 0.011 | 26.5 (± 4.6) | 29.0 (± 4.7) | 0.016 |
| Preoperative eGFR | 64.5 (± 22.2) | 78.1 (67.1–89.4) | < 0.001 | 71.7 (± 21.0) | 70.3 (± 14.6) | 0.713 |
| Female | 28 (40.0%) | 19 (12.7%) | < 0.001 | 12 (27.9%) | 10 (23.3%) | 0.621 |
| Treatment for dyslipidemia | 52 (74.3%) | 97 (64.7%) | 0.155 | 21 (72.1%) | 32 (74.4%) | 0.808 |
| Treatment for diabetes II | 15 (21.4%) | 37 (24.7%) | 0.599 | 8 (18.6%) | 9 (20.9%) | 0.787 |
| Treatment for diabetes I | 7 (10.0%) | 4 (2.7%) | 0.040 | 3 (7.0%) | 1 (2.3%) | 0.616 |
| Treatment for hypertension | 64 (91.3%) | 109 (72.7%) | 0.002 | 41 (95.3%) | 32 (74.4%) | 0.007 |
| Heart failure | 16 (22.9%) | 10 (6.7%) | 0.001 | 4 (9.3%) | 3 (7.0%) | 1.000 |
| Preoperative atrial fibrillation | 16 (22.9%) | 30 (20.0%) | 0.627 | 8 (18.6%) | 6 (14.0%) | 0.559 |
| Coronary artery disease | 49 (70.0%) | 90 (60.0%) | 0.152 | 27 (62.8%) | 29 (67.4%) | 0.651 |
| Previous myocardial infarction | 18 (25.7%) | 33 (22.0%) | 0.543 | 8 (18.6%) | 13 (30.2%) | 0.209 |
| Recent myocardial infarction | 14 (20.0%) | 16 (10.7%) | 0.060 | 5 (11.6%) | 9 (20.9%) | 0.243 |
| Prior PCI | 11 (15.7%) | 19 (12.7%) | 0.540 | 8 (18.6%) | 8 (18.6%) | 1.000 |
| Prior stroke | 8 (11.4%) | 14 (9.3%) | 0.629 | 3 (7.0%) | 1 (2.3%) | 0.616 |
| Carotid artery disease | 3 (4.3%) | 1 (0.7%) | 0.096 | 2 (4.7%) | 1 (2.3%) | 1.000 |
| Peripheral artery disease | 4 (5.7%) | 3 (2.0%) | 0.213 | 1 (2.3%) | 1 (2.3%) | 1.000 |
| Pulmonary artery hypertension | 26 (37.1%) | 41 (27.3%) | 0.141 | 14 (32.6%) | 13 (30.2%) | 0.816 |
| Chronic lung disease | 9 (12.9%) | 12 (8.0%) | 0.253 | 5 (11.6%) | 3 (7.0%) | 0.713 |
| Active smoking | 13 (18.6%) | 25 (16.9%) | 0.760 | 6 (14.0%) | 3 (7.3%) | 0.484 |
| Ex-smoker | 30 (42.9%) | 64 (43.2%) | 0.957 | 22 (51.2%) | 17 (41.5%) | 0.373 |
| Obstructive sleep apnea | 6 (8.6%) | 13 (8.7%) | 0.981 | 3 (7.05) | 5 (11.6%) | 0.713 |
| Any malignancy | 14 (20.0%) | 17 (11.3%) | 0.085 | 8 (18.6%) | 6 (14.0%) | 0.559 |
| Chronic dialysis | 3 (4.3%) | 0 (0.0%) | 0.030 | 1 (2.4%) | 0(0.0%) | 0.494 |
| Hypothyroidism | 14 (20.0%) | 6 (4.0%) | < 0.001 | 6 (14.0%) | 4 (9.3%) | 0.501 |
Continuous variables are reported as median and interquartile range or mean and standard deviation. Categorical variables are reported as counts and percentages.
BMI body mass index, CABG coronary artery bypass grafting, CPAP continuous positive airway pressure, eGFR estimated glomerular filtration rate, LED discoid lupus erythematosus, PCI percutaneous coronary intervention; RBC red blood cell, SLE systemic lupus erythematosus, TAVR transcatheter aortic valve replacement.
Table 2.
Operative characteristics of the study cohorts.
| RBC transfusion | No RBC transfusion | p | RBC transfusion PSM |
No RBC transfusion PSM |
p | |
|---|---|---|---|---|---|---|
| n = 70 | n = 150 | n = 43 | n = 43 | |||
| Elective operation | 55 (78.6%) | 133 (88.7%) | 0.048 | 37 (86.0%) | 39 (90.7%) | 0.501 |
| Urgent operation | 13 (18.6%) | 16 (10.7%) | 0.106 | 4 (9.3%) | 4 (9.3%) | 1.000 |
| Emergency/salvage operation | 2 (2.9%) | 1 (0.7%) | 0.238 | 2 (4.7%) | 0 (0.0%) | 0.494 |
| Type of procedure | ||||||
| AVR biological prosthesis/mechanical prosthesis | 21 (30.0%) | 48 (32.0%) | 0.766 | 12 (27.9%) | 16 (37.2%) | 0.357 |
| CABG | 49 (70.0%) | 87 (58.0%) | 0.088 | 27 (62.8%) | 29 (67.4%) | 0.651 |
| Isolated CABG | 33 (47.1%) | 69 (46.0%) | 0.874 | 19 (44.2%) | 23 (53.5%) | 0.388 |
| MVP | 3 (4.3%) | 15 (10.0%) | 0.150 | 2 (4.7%) | 3 (7.0%) | 1.000 |
| MVR | 7 (10.0%) | 2 (1.3%) | 0.005 | 5 (11.6%) | 0 (0.0%) | 0.055 |
| Surgery on the ascending aorta | 6 (8.6%) | 17 (11.3%) | 0.533 | 4 (9.3%) | 1 (2.3%) | 0.360 |
| David procedure | 0 (0.0%) | 4 (2.7%) | 0.309 | 0 (0.0%) | 0 (0.0%) | – |
| Bentall-DeBono procedure | 1 (1.4%) | 10 (6.7%) | 0.180 | 1 (2.3%) | 1 (2.3%) | 1.000 |
| Maze procedure | 2 (2.9%) | 4 (2.7%) | 1.000 | 1 (2.3%) | 0 (0.0%) | 1.000 |
| Pericardiectomy | 2 (2.9%) | 1 (0.7%) | 0.238 | 1 (2.3%) | 1 (2.3%) | 1.000 |
| LAA closure | 11 (15.7%) | 17 (11.3%) | 0.346 | 6 (14.0%) | 3 (7.0%) | 0.483 |
| Other procedures | 1 (1.4%) | 3 (2.0%) | 1.000 | 1 (2.3%) | 1 (2.3%) | 1.000 |
| Indications for CABG | ||||||
| Stable angina | 22 (31.4%) | 55 (36.7%) | 0.448 | 15 (34.9%) | 18 (41.9%) | 0.506 |
| Unstable angina | 10 (14.3%) | 14 (9.3%) | 0.272 | 5 (11.6%) | 2 (4.7%) | 0.433 |
| NSTEMI | 17 (24.3%) | 16 (10.7%) | 0.008 | 7 (16.3%) | 9 (20.9%) | 0.579 |
| STEMI | 0 (0.0%) | 2 (1.3%) | 1.000 | 0 (0.0%) | 0 (0.0%) | – |
| Operation length (min) | 246 (209–285) | 225 (197–250) | 0.006 | 238 (210–292) | 233 (± 39) | 0.346 |
| Aortic cross-clamping time (min) | 89 (74–112) | 89 (72–106) | 0.363 | 89 (71–102) | 91 (75–111) | 0.678 |
| Cardiopulmonary bypass time (min) | 115 (102–145) | 114 (94–138) | 0.136 | 114 (93–138) | 124 (± 32) | 0.445 |
| Delayed ventilation* | 10 (14.3%) | 0 (0.0%) | < 0.001 | 7 (16.3%) | 0 (0.0%) | 0.012 |
| Duration of mechanical ventilation (hours) | 11.5 (6.4–20.6) | 5.0 (4.0–7.0) | < 0.001 | 15.0 (8.5–21.5) | 5.5 (4.5–8.0) | < 0.001 |
| Blood products administered within 12 h | ||||||
| Packed red blood cells | 70 (100.0%) | 0 (0.0%) | < 0.001 | 43 (100.0%) | 0 (0.0%) | < 0.001 |
| Fresh frozen plasma | 27 (38.6%) | 21 (14.0%) | < 0.001 | 18 (41.9%) | 8 (18.6%) | 0.019 |
| Platelets | 31 (44.3%) | 14 (9.3%) | < 0.001 | 21 (48.8%) | 1 (2.3%) | < 0.001 |
| Intensive care unit variables | ||||||
| Length of ICU stay (hours) | 25 (22–47) | 23 (22–24) | < 0.001 | 24 (22–47) | 23 (22–24) | 0.006 |
| Intravenous fluids (mL, 12 h) | 3878 (2543–5849) | 2752 (2419–3290) | < 0.001 | 4249 (2381–6081) | 3060 (± 1017) | 0.005 |
| Chest drain output (mL, 12 h) | 565 (260–1139) | 368 (290–480) | 0.001 | 600 (320–1195) | 410 (295–530) | 0.009 |
| Diuresis (mL, 12 h) | 1860 (1390–2520) | 1960 (1655–2348) | 0.605 | 1820 (1360–2470) | 2055 (± 749) | 0.678 |
Continuous variables are reported as median and interquartile range or mean and standard deviation. Categorical variables are reported as counts and percentages.
AVR aortic valve replacement, CABG coronary artery bypass grafting, ECMO extracorporeal membrane oxygenation, IABP intra-aortic balloon pump, ICU intensive care unit, LAA left atrial appendix, MVP mitral valve repair, MVR mitral valve replacement; NSTEMI non-ST-elevation myocardial infarction, RBC red blood cell, STEMI ST-elevation myocardial infarction.
*Delayed ventilation > 24 h.
IL-6 and HIF-1α response
As a marker of cytokine derangement, IL-6 levels were increased postoperatively over 30-fold without any difference between the study groups (236.1 pg/mL vs. 211.5 pg/mL, p = 0.130) (Fig. 2). Interestingly, patients who received RBC transfusion had an insufficient adaptive response to hypoxia—measured as significantly decreased HIF-1α levels during the first 12 h postoperatively—compared to those who did not receive any blood (0.377 pg/mL vs. 0.786 pg/mL, p = 0.002) (Fig. 3). Giving more than two units of packed red blood cells dumped HIF-1α response even further (Fig. 4). When adjusted for transfusions over 2 units of packed red blood cells, there was a significant difference in postoperative and delta-HIF-1α levels between the study groups (p < 0.001 and p = 0.047, respectively). In multivariate regression analysis RBC transfusion considerably blunted the HIF-1α response when significant baseline characteristics were included in the model (BMI, preoperative paroxysmal atrial fibrillation, previous myocardial infarction, treatment for dyslipidemia, carotid artery disease, hypothyroidism, operation type coronary artery bypass (CABG), operation indication non-ST-elevation myocardial infarction (NSTEMI) for CABG, RBC transfusion). Results show a significant effect on HIF-1α response (F(9.205) = 3.039), p = 0.002, with R2 = 0.118, suggesting prediction value of 11.8% by the listed factors. Blood transfusion was found to be the highest predictor for HIF-1α response (p = 0.037) (see Supplemental Table S1). Platelet and fresh frozen plasma administration were not included in the model since they are administrated with red blood cells.
Figure 2.
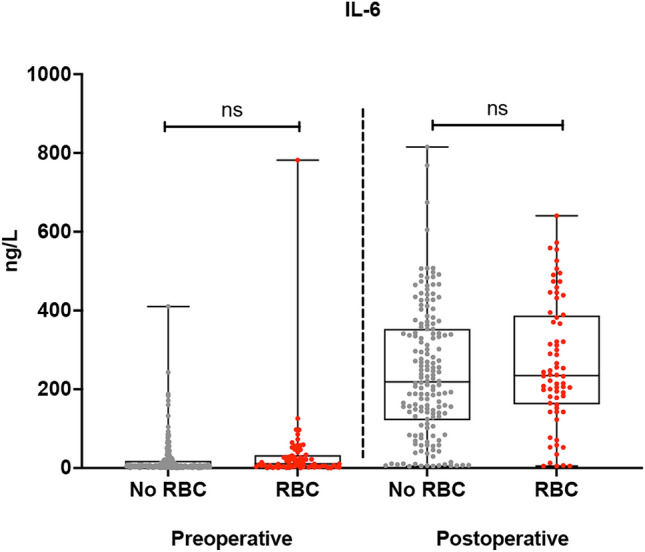
Pre- and postoperative interleukine-6 (IL-6) cytokine levels (ng/L) in with and without red blood cell transfusion (RBC) after adult cardiac surgery. The vertical axis represents IL-6 levels (ng/L). The horizontal axis represents patients who received or not RBC transfusion preoperatively and postoperatively.
Figure 3.
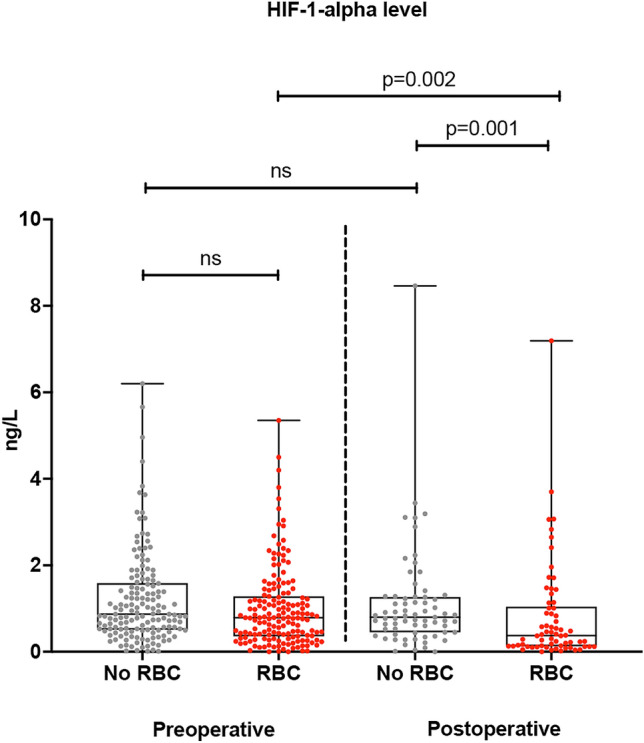
Pre- and postoperative hypoxia-inducible factor 1 alpha (HIF-1-α) levels (ng/L) in patients with or without red blood cell transfusion (RBC) after adult cardiac surgery. The vertical axis represents HIF-1α cytokine levels (ng/L). The horizontal axis represents patients who received or not RBC transfusion preoperatively and postoperatively.
Figure 4.
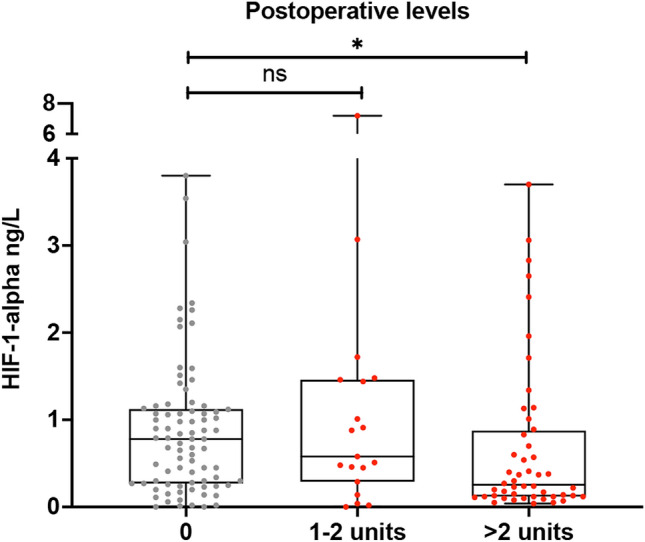
Postoperative hypoxia-inducible factor 1 alpha (HIF-1-α) levels according to the number of transfused red blood cell units. The vertical axis represents HIF-1α cytokine levels (ng/L). The horizontal axis represents red blood cell units in three groups.
Clinical outcomes
Clinical outcome events during hospitalization and at 90 days are summarized in Table 3. Patients who received RBC transfusion during the first 12 h perioperatively had substantially higher rates of strokes, postoperative pneumonia, de novo dialysis, and mortality during the index hospitalization as well as longer intensive care unit and hospital stay. In the PSM cohort, there were more adverse events in the RBC transfusion group and this was mainly driven by higher postoperative pneumonia and stroke rates.
Table 3.
Clinical outcomes.
| RBC transfusion | No RBC transfusion | p | RBC transfusion PSM |
No RBC transfusion PSM |
p | |
|---|---|---|---|---|---|---|
| n = 70 | n = 150 | n = 43 | n = 43 | |||
| Index hospitalization | ||||||
| Death | 6 (8.6%) | 0 (0.0%) | 0.001 | 4 (9.3%) | 0 (0.0%) | 0.116 |
| New-onset atrial fibrillation* | 25 (46.3%) | 43 (35.8%) | 0.191 | 14 (40.0%) | 20 (54.1%) | 0.233 |
| Stroke | 7 (10.0%) | 1 (0.7%) | 0.002 | 5 (11.6%) | 0 (0.0%) | 0.055 |
| TIA | 0 (0.0%) | 0 (0.0%) | – | 0 (0.0%) | 0 (0.0%) | – |
| Postoperative pneumonia | 8 (11.4%) | 2 (1.3%) | 0.002 | 6 (14.0%) | 0 (0.0%) | 0.026 |
| Deep sternal wound infection/mediastinitis | 2 (2.9%) | 2 (1.3%) | 0.594 | 1 (2.3%) | 1 (2.3%) | 1.000 |
| Reoperation for bleeding | 16 (22.9%) | 1 (0.7%) | < 0.001 | 13 (30.2%) | 1 (2.3%) | < 0.001 |
| De novo dialysis | 3 (4.3%) | 0 (0.0%) | 0.031 | 2 (4.7%) | 0 (0.0%) | 0.494 |
| Major adverse events† | 16 (22.9%) | 3 (2.0%) | < 0.001 | 11 (25.6%) | 0 (0.0%) | < 0.001 |
| Length of hospital stay (days) | 10 (7–16) | 7 (7–9) | < 0.001 | 9 (7–15) | 7 (7–9) | 0.012 |
| Heart rhythm at the time of discharge‡ | ||||||
| Sinus rhythm | 52 (80.0%) | 114 (76.0%) | 0.521 | 34 (85.0%) | 29 (67.4%) | 0.062 |
| Atrial fibrillation | 12 (18.5%) | 32 (21.3%) | 0.632 | 6 (15.0%) | 13 (30.2%) | 0.099 |
| Other | 1 (1.5%) | 4 (2.7%) | 1.000 | 0 (0.0%) | 1 (2.3%) | 1.000 |
| Events within 90 days | ||||||
| NOAF | 26 (49.1%) | 48 (40.3%) | 0.286 | 15 (44.1%) | 20 (54.1%) | 0.403 |
| NOAF after index hospitalization | 1 (3.6%) | 5 (6.6%) | 1.000 | 7 (18.4%) | 10 (23.3%) | 0.594 |
| Permanent anticoagulation | 16 (25.4%) | 32 (21.5%) | 0.533 | 8 (21.1%) | 12 (27.9%) | 0.475 |
| Myocardial infarction | 0 (0.0%) | 1 (0.7%) | 1.000 | 0 (0.0%) | 0 (0.0%) | – |
| Stroke and TIA | 9 (13.8%) | 3 (2.0%) | 0.001 | 6 (15.0%) | 1 (2.3%) | 0.052 |
| Death | 9 (12.9%) | 0 (0.0%) | < 0.001 | 5 (11.6%) | 0 (0.0%) | 0.055 |
Continuous variables are reported as median and interquartile range or mean and standard deviation. Categorical variables are reported as counts and percentages.
NOAF New-onset atrial fibrillation, TIA transient ischemic attack.
*Patients with preoperative atrial fibrillation or atrial flutter (n = 60) were excluded from the variable.
†Major adverse event includes stroke, TIA, postoperative pneumonia and death during index hospitalization.
‡Other: pacemaker or junctional rhythm.
Lower decrease in HIF-1α levels—as an indication for minor blunting—predicted better composite outcome of all-cause mortality, stroke, transient ischemic attack (TIA), pneumonia, mediastinitis, acute dialysis or reoperation for bleeding during index hospitalizaton (OR 0.42, 95% CI 0.21–0.84) and at 3mo follow-up (OR 0.50, 95% CI 0.26–0.98) in a multivariable logistic regression model including age and gender as covariates in the overall series and in the PSM cohort.
Discussion
The main finding of this study was that RBC transfusion may significantly reduce the response to hypoxia by inhibiting HIF-1α elevation in the cytokine storm secondary to surgical stress and the use of cardiopulmonary bypass. RBC transfusion significantly increases the risk of major adverse events after cardiac surgery7–9.
RBC use was associated with high rates of in-hospital adverse events. Risk for stroke/TIA was over 16-fold and every tenth patient with RBC use had a stroke/TIA. The clinical setting and baseline differences in groups need to be taken into account when interpreting the results. Patients who received RBC transfusion were frailer at baseline. In PSM analysis almost all transfusion patients were on medication for hypertension, and we observed no difference in the usage of betablockers, ACE-inhibitors/angiotensin receptor blockers or calcium-channel blockers between transfusion groups. Differences in clinical findings during hospitalization indicate more straining surgeries for RBC transfusion patients. In multivariate logistic regression and propensity score analysis we showed that RBC was substantial predictor for these thrombotic events unlike other baseline and perioperative variables. Larger scale studies with similar baseline and clinical presentation have previously reported even more pronounced role for RBC transfusion with worse outcome and mortality10,11.
Our findings are in line with previous studies on transfusions and postoperative stroke occurrence1,2,4. We also found stroke risk to be higher if patient was transfused more than two units12. Etiology of post-operative strokes is often cardioembolic after open-heart surgery13 and a great share is atrial fibrillation-related and derived from the left atrial appendage. Risk for pneumonia was almost tenfold and only renal impairment in addition to RBC was its predictor. These results highlight the findings of abnormal thrombotic and inflammatory milieu after RBC in postoperative setting.
RBC transfusion effect on worsen outcome has been suggested to be related to RBC quality after longer storage time14,15. We provide observational mechanistic hypothesis for RBC-related increase in adverse outcomes. Cardiopulmonary bypass and open-heart surgery induce hypoxia as well as a drastic cytokine storm16. As a sign of this storm, IL-6 levels rose 30-fold postoperatively in both groups. HIF-1α—found ubiquitously—helps the body to adapt to inflammation caused by hypoxia5. We show that blunted HIF-1α was most common in patients who received RBC. To assess whether this finding was independent of other baseline and perioperative factor, we performed multiple sensitivity analyses where RBC remained as the most influential predictor for the abnormal response. With transfusion of more than two units of RBC, post-operative HIF-1α was more pronounced. Greater decrease of HIF-1α was associated with adverse in-hospital and 3mo follow-up outcomes.
Blunted HIF-1α response likely disrupts body’s adaptation to hypoxic stimuli. HIF-1α is a transcription factor which induces transcription of more than 60 genes in hypoxia17 including the de novo synthesis of CD7318. Extra-cellular CD73 (ecto-5'-nucleotidase/NT5E)-derived adenosine production is one of the key pathways in attenuating hypoxia-induced inflammation19,20 and protecting several central organs in the acute hypoxia21. CD73 is a glycosyl-phosphatidylinositol expressing cell surface enzyme found on most human tissues, such as endothelial cells and immune cells. Recent studies have suggested CD73 has a role in myocardial infarction recovery22, central nervous system protection23, and control of alveolar permeability in hypoxia and vascular leak24. To our knowledge, we provide association of RBC with blunted HIF-1α response as well as increased rates of thrombotic and lung injury endpoints for the first time.
The strength of our study was that it was a prospective cohort study with a consecutive patient enrollment. We included in our final analysis patients who had open-heart surgery under CPB and who received packed red blood perioperatively excluding patients who needed red blood cells later the appointed 12 h during index hospitalization. Patients’ operation types did not differ between groups. There were a few limitations in our study. Baseline characteristics differed between groups since patients who needed more often packed red blood were older, female and had more comorbidities. Female sex has been shown to act as an independent risk factor for red blood cell transfusion25. Obviously, patients received RBC for clinical indications such as anemia and perioperative bleeding and the decision on RBC use was always at the treating anesthesiologist’s discretion. We need to consider that RBC transfusion may be a result not a cause for complications. We tackled this challenge using propensity score matching. We registered 33 postoperative outcome events in PSM group and 48 outcome events in total. Since new-onset atrial fibrillation is very common after cardiac surgery, we did not add this covariate in our regression model. In multivariate logistic regression we could only use few explanatory variables due to statistical constraints. We chose age and gender as the other covariates as they are known predictors of worse postoperative outcome. RBC transfusion was nominated an explanatory covariate for postoperative outcome mirroring previous studies. There are multiple studies on red blood cell transfusion related worse postoperative outcome and transfusion has been shown to act an independent predictor10,11. Relatively small sample size as well as observational (yet, prospective) setting are other limitations of this study, and therefore, these findings should be viewed as hypothesis generating. One limitation was that pre- and postoperative blood samples were taken not more than 24 h apart and packed red blood cells were given 12 h perioperatively which meant measured cytokine response happened in a short window.
This study has clinical implications. Decision on RBC use needs to be balanced between its anticipated benefits and harms. This study supports the view that we may need to consider stricter threshold limit for transfusions26,27. Intraoperative autologous blood donation (IAD) has been recently shown to reduce transfusion need and reduction in postoperative complications28. Moreover, hypoxia-inducible inflammation and impaired hypoxia related repair mechanisms require further research and could serve as a target for operative preconditioning and better patient outcome.
In conclusion, the present findings provide evidence that the use of RBC transfusion disrupts the adaptation to cytokine storm and hypoxia and is associated with adverse thrombotic and inflammatory events after adult cardiac surgery.
Methods
Patient selection
The present analysis includes a prospective cohort of 282 consecutive patients who underwent adult cardiac surgery from February 2016 to September 2017 at the Heart Center of the Turku University Hospital, Turku, Finland. Our Institution is a University and tertiary referral hospital for a population of 876,000 inhabitants of Southwest Finland. These patients are participants to the CAREBANK study (Cardiovascular Research Consortium—a Prospective Project to Identify Biomarkers of Morbidity and Mortality in Cardiovascular Interventional Patients), an on-going Finnish prospective cohort study of patients undergoing adult cardiac surgery at the Turku University Hospital, Turku, Finland (ClinicalTrials.gov Identifier: NCT03444259), first posted on 23/02/2018. The cohort consists of all the recruited patients who have undergone open-heart surgery and have 90-day follow-up data by the time of immunological analysis by December 2017. Blood samples from these patients were prospectively obtained pre- and postoperatively. Follow-up data was collected prospectively both by individual contacts by phone calls using a structured questionnaire, and from hospital records at prespecified time points (3, 12 and 24 months). This study focused on in-hospital outcomes and 90-day outcomes on the assumption perioperative blood transfusion related outcomes were most likely seen at these time periods. Mortality data was obtained from the nationwide registry Statistics Finland. The CAREBANK has received approval from the Ethical Committee of the Hospital District of Southwestern Finland and adheres to the Declaration of Helsinki as revised 2002. CAREBANK data was monitored by an independent third party. Written informed consent was obtained from the study subjects. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient serum samples
Consecutive blood samples were collected preoperatively and on the morning of the first postoperative day. Pre- and postoperative serum was centrifuged from the whole blood and ethylenediaminetetraacetic acid plasma. The first samples were obtained on the morning time after fasting or at the emergency setting. The second blood samples were obtained on the first postoperative morning with patients still fasting. The serum samples were labeled and stored at – 70 °C until analyses. The analyses for all 282 samples were done in three separate days and the three persons assigned to do the cytokine analyses were unaware of patients’ procedures and outcomes. IL-6 and HIF-1α analyses were done with the enzyme-linked immunosorbent assay according to manufacturer’s instructions (Elabscience, Houston, Texas, USA). The optical density values were analyzed using Tecan Infinite M200 and Magellan 7.2. software for Microsoft Windows (Tecan Group, Männedorf, Switzerland).
Outcomes
The primary outcome of this study was the evaluation of cytokine IL-6 and HIF-1α levels in respect with RBC transfusion during surgery and/or within 12 h from surgery. The secondary outcomes were postoperative complications during the index hospitalization after open-heart surgery, new onset atrial fibrillation at 90 days, three-month stroke, and morbidity.
The diagnosis of in-hospital new onset atrial fibrillation (NOAF) was confirmed by a 12-lead ECG recording or telemonitoring indicating an atrial fibrillation episode of 10 min or longer. Ischemic stroke was defined as a permanent focal neurological deficit adjudicated by a neurologist and confirmed via computed tomography (CT) or magnetic resonance imaging (MRI). Only ischemic strokes considered definite by the treating neurologist or physician were included in the present study. Pneumonia was defined as symptoms of infection (fever, malaise), elevation of C-reactive protein (CRP) or leucocyte count (excluding leg wound infection), and/or imaging finding consistent with infection (X-ray, CT, magnetic resonance imaging MRI or ultrasound) and/or evident purulent wound secretion or abscess. Mediastinitis was defined as symptoms of infection (fever, malaise), elevation of CRP or leucocyte count (excluding leg wound infection), and/or imaging finding consistent with infection at the mediastinal area and without signs of alternative diagnoses with similar presentation. Re-operation for bleeding was defined as re-sternotomy because of bleeding.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 25 (SPSS Inc., Chicago, IL, USA). Continuous variables are reported as median and inter-quartile range (IQR) or mean and standard deviation (SD). Categorical variables are reported as counts and percentages. The Shapiro–Wilk test of normality was performed to all baseline characteristics. The Chi-square test and Fischer’s exact test were used to evaluate the difference in categorical variables and outcomes whilst continuous variables were evaluated using independent sample T-test or Mann–Whitney-U test. Logistic regression was performed to identify risk factors associated with decreased postoperative HIF-1α levels. Multivariate logistic regression models were performed by including variables of relevance with p-value < 0.05 in the univariable analyses. HIF-1α levels were log-transformed for logistic regression analysis. Statistical significance was set at p < 0.05 in multivariate analysis. Propensity score matching (PSM) was conducted to better evaluate endpoint events and reduce bias. This study was exploratory in nature and a formal sample size calculation was not carried out. We anticipated that a sample of 300 patients would be reasonable to show consistent change in IL-6 and HIF-1α levels pre and post operation.
Supplementary Information
Author contributions
T.O.K., J.J., M.H. and J.G. conceived the idea of the study and were responsible for the design of the study. E.V. performed experiments. E.V., J.L. and T.O.K. accounted for undertaking the data analysis and produced the tables and graphs. The original draft of the manuscript was prepared by E.V. and T.O.K., and all authors contributed for critical revision. T.O.K. supervised the study and acquired funding. All authors approved the final version of the manuscript. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Finnish Medical Foundation, the Finnish Foundation for Cardiovascular Research, Helsinki, Finland; State Clinical Research Fund (EVO) of Turku University Hospital, Turku, Finland. The funding was provided by Turun Yliopistollisen Keskussairaalan Koulutus- ja Tutkimussäätiö, Turun Yliopisto, Orionin Tutkimussäätiö, Sydäntutkimussäätiö, Emil Aaltosen Säätiö, Suomen Kulttuurirahasto, Suomen Lääketieteen Säätiö, Suomen Kardiologinen Seura and also by Kuistilan Muistosäätiö.
Competing interests
Joonas Lehto received research grants from Orion Research Foundation, the Finnish Foundation for Cardiovascular Research, the Finnish Cultural Foundation, Turku University Foundation, and Emil Aaltonen Foundation. K. E. Juhani Airaksinen received research grants from the Finnish Foundation for Cardiovascular Research, the Clinical Research Fund (VTR) of Turku University Hospital, Turku, Finland and lecture fees from Bayer, and Boehringer Ingelheim. K. E. Juhani Airaksinen is a member in the advisory boards of Bayer, Astra Zeneca, and Bristol-Myers Squibb-Pfizer. Jarmo Gunn received research grants from Turku University Research Foundation, Turku, Finland, the Clinical Research Fund (VTR) of Turku University Hospital, Turku, Finland, and an unrestricted grant from Vifor Pharma. Tuomas O. Kiviniemi received lecture fees from Bayer, Boehringer Ingelheim, MSD, Astra Zeneca, St Jude Medical, and Bristol-Myers-Squibb-Pfizer, and research grants from the Finnish Medical Foundation, the Finnish Foundation for Cardiovascular Research, Clinical Research Fund (EVO) of Turku University Hospital, Turku, Finland, Finnish Cardiac Society, the Emil Aaltonen Foundation, the Maud Kuistila Foundation, and an unrestricted grant from Bristol-Myers Squibb-Pfizer. Tuomas O. Kiviniemi is a member of the advisory board of Boehringer-Ingelheim, and MSD. Juho Jalkanen owns stock and is employed by Faron Pharmaceuticals Ltd. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01695-4.
References
- 1.Mikkola R, et al. Use of blood products and risk of stroke after coronary artery bypass surgery. Blood Transfus. 2012;10(4):490–501. doi: 10.2450/2012.0119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwann T, et al. Effects of blood transfusion on cause-specific late mortality after coronary artery bypass grafting—Less is more. Ann. Thorac. Surg. 2016;02(2):465–473. doi: 10.1016/j.athoracsur.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Johnson D, et al. Morbidity and mortality after high-dose transfusion. Anesthesiology. 2016;12(2):387–395. doi: 10.1097/ALN.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 4.Brascia D, et al. Impact of transfusion on stroke after cardiovascular interventions: Meta-analysis of comparative studies. J. Crit. Care. 2017;38:157–163. doi: 10.1016/j.jcrc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Eltzschig H, Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalkanen J, Maksimow M, Jalkanen S, Hakovirta H. Hypoxia-induced inflammation and purinergic signaling in cross clamping the human aorta. Springerplus. 2016 doi: 10.1186/s40064-015-1651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy G, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Kidane B, Klingel M, Parry N. Risks associated with red blood cell transfusion in the trauma population, a meta-analysis. Injury. 2014;45(10):1522–1533. doi: 10.1016/j.injury.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Tuinman P, et al. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: A case control study. Crit. Care. 2011;15(1):R59. doi: 10.1186/cc10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaetano P, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann. Thorac. Surg. 2014;97(1):87–94. doi: 10.1016/j.athoracsur.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Koch C, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit. Care Med. 2006;34(6):1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 12.Mariscalco G, et al. Red blood cell transfusion is a determinant of neurological complications after cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2015;20(2):166–171. doi: 10.1093/icvts/ivu360. [DOI] [PubMed] [Google Scholar]
- 13.Lehto J, et al. Occurrence and classification of cerebrovascular events after isolated bioprosthetic surgical aortic valve replacement: A competing risk analysis of the CAREAVR study. Struct. Heart J. Heart Team. 2018;2(2):157–163. doi: 10.1080/24748706.2017.1419327. [DOI] [Google Scholar]
- 14.Koch C, et al. Duration of red-cell storage and complications after cardiac surgery. N. Engl. J. Med. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 15.Sanders J, et al. Red blood cell storage is associated with length of stay and renal complications after cardiac surgery. Transfusion. 2011;51(11):2286–2294. doi: 10.1111/j.1537-2995.2011.03170.x. [DOI] [PubMed] [Google Scholar]
- 16.Giacinto O, et al. Inflammatory response and endothelial dysfunction following cardiopulmonary bypass: Pathophysiology and pharmacological targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13(2):158–173. doi: 10.2174/1872213X13666190724112644. [DOI] [PubMed] [Google Scholar]
- 17.Semenza G. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 18.Minor M, Alcedo K, Battaglia R, Snider N. Cell type- and tissue-specific functions of ecto-5'-nucleotidase (CD73) Am. J. Physiol. Cell Physiol. 2019;317(6):C1079–C1092. doi: 10.1152/ajpcell.00285.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resta R, Yamashita Y, Thompson L. Ecto-enzyme and signaling functions of lymphocyte CD 73. Immunol. Rev. 1998;161(1):95–109. doi: 10.1111/j.1600-065X.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 20.Colgan S, Eltzschig H, Eckle T, Thompson L. Physiological roles for ecto-5'-nucleotidase (CD73) Purinergic Signal. 2006;2(2):351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le T-T, et al. Purinergic signaling in pulmonary inflammation. Front. Immunol. 2019;10:1633. doi: 10.3389/fimmu.2019.01633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg N, et al. CD73 on T cells orchestrates cardiac wound healing after myocardial infarction by purinergic metabolic reprogramming. Circulation. 2017;136(3):297–313. doi: 10.1161/CIRCULATIONAHA.116.023365. [DOI] [PubMed] [Google Scholar]
- 23.Petrovic-Djergovic D, et al. Tissue-resident ecto-5' nucleotidase (CD73) regulates leukocyte trafficking in the ischemic brain. J. Immunol. 2012;188(5):2387–2398. doi: 10.4049/jimmunol.1003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemela J, et al. IFN-induced adenosine production on the endothelium: A mechanism mediated by CD73 (Ecto-53-nucleotidase) up-regulation. J. Immunol. 2004;172:1646–1653. doi: 10.4049/jimmunol.172.3.1646. [DOI] [PubMed] [Google Scholar]
- 25.Woorst J, et al. Evolution of perioperative blood transfusion practice after coronary artery bypass grafting in the past two decades. J. Cardiol. Surg. 2020;35(6):1220–1227. doi: 10.1111/jocs.14573. [DOI] [PubMed] [Google Scholar]
- 26.Murphy G, et al. Liberal or restrictive transfusion after cardiac surgery. N. Engl. J. Med. 2015;372(11):997–1008. doi: 10.1056/NEJMoa1403612. [DOI] [PubMed] [Google Scholar]
- 27.Mazer CD, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N. Engl. J. Med. 2017;377(22):2133–2144. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann E, et al. Intraoperative autologous blood donation leads to fewer transfusions in cardiac surgery. Ann. Thorac. Surg. 2019;108(6):1738–1744. doi: 10.1016/j.athoracsur.2019.06.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


