Abstract
Epidemiology of bacteria isolated from pyogenic liver abscesses change, and an increase in enterococci has been reported in European hospitals. The aim of this study was to investigate the clinical characteristics and outcome of enterococcal PLA. We performed a retrospective analysis of patients with microbiologically confirmed PLA at three German university centers. Indicators of enterococcal PLA were determined using binary logistic regression, and survival analysis was performed using Kaplan–Meier statistics and Cox regression analysis. Enterococci were isolated in 51/133 (38%) patients with PLA. Patients with enterococcal PLA had smaller abscess diameter (4.8 vs. 6.7 cm, p = 0.03) than patients with non-enterococcal PLA, but had more frequent polymicrobial culture results. In univariate logistic regression analysis, alcohol abuse (OR 3.94, 95% CI 1.24–12.49, p = 0.02), hepatobiliary malignancies (OR 3.90, 95% CI 1.86–8.18, p < 0.001) and cirrhosis (OR 6.36, 95% CI 1.27–31.96, p = 0.02) were associated with enterococcal PLA. Patients with enterococcal PLA had a higher mortality than patients with non-enterococcal PLA (hazard ratio 2.92; 95% confidence interval 1.09–7.80; p = 0.03), which remained elevated even after excluding patients with hepatobiliary malignancies, cirrhosis, and transplant recipients in a sensitivity analysis. The increased mortality was associated with non-fecal enterococci but not in patients with Enterococcus faecalis. In this retrospective, multicenter study, enterococcal PLA was common and indicated an increased risk of mortality, underscoring the need for close clinical monitoring and appropriate treatment protocols in these patients.
Subject terms: Liver diseases, Bacterial infection
Introduction
Pyogenic liver abscess (PLA) is an important diagnosis in patients with fever of unknown cause. In Western countries, the incidence ranges from 1.0 to 3.6 per 100,0001, but in Asia it can reach up to 17 per 100,0002 and the mortality rate is up to 15%3. Treatment of a PLA is based on empiric antibiotic therapy in combination with percutaneous drainage, either ultrasound- or CT-guided, or surgical resection for multilocular abscesses. Antibiotic therapy is based on third-generation cephalosporins (TGC) or Piperacillin/Tazobactam (PTZ)4. However, knowledge of the microbial spectrum and resistance profiles is critical for choosing the most effective empirical treatment.
The vast majority of PLA are caused by bacterial pathogens, but there are notable differences between different regions. Studies from Southeast Asia report a high proportion of PLA caused by Klebsiella pneumoniae, whereas in Western countries Gram-negative Enterobacterales such as Escherichia coli as well as Gram-positive cocci are the most common pathogens1,2,5–8 with increasing prevalence of enterococci and multidrug-resistant bacteria9–11.
Among Gram-positive isolates, enterococci are of great clinical importance because they are naturally resistant to TGC and, in the case of Enterococcus faecium, often exhibit beta-lactam resistance. Enterococci are frequently detected in intra-abdominal infections such as cholangitis, spontaneous bacterial peritonitis, or infected pancreatic necrosis12–15. A recent study from a German tertiary center9 reported that enterococci were the most common pathogen in PLA (29%), with one in three enterococci identified being resistant to vancomycin. However, the impact of enterococcal infections on mortality is controversial16,17, and enterococci have also been associated with mortality in intra-abdominal infections in some studies12.
Therefore, the aim of this retrospective cross-sectional study was to investigate the bacterial spectrum and antibacterial resistance profile in patients with PLA from three German centers and to analyze the association of enterococcal PLA with survival.
Patients and methods
Study design
To characterize the pathogen spectrum and resistance patterns in patients with PLA, data from patients who underwent microbiological testing between 2009 and January 2020 were retrospectively collected at three German university centers (Jena University Hospital, Aachen University Hospital, and Technical University of Munich). Microbiological sampling from the PLA was performed either by percutaneous puncture guided by ultrasound or computed tomography (CT), or during surgical procedures. Microbial cultures were processed and analyzed according to standard local procedures. Patients were on antibiotics prior to microbiological samplings (n = 47, 35%) or received antibiotics directly after puncture (n = 86, 65%). Medical records, including patient records, electronic health records, imaging data, laboratory data, and nursing documentation were used to retrospectively determine clinical and laboratory data. The following variables were recorded in the medical records: Age, sex, comorbidities, medication, including antibiotics and changes in antibiotic therapy, intensive care unit (ICU) treatment, endoscopic diagnostic procedures, and interventions, length of hospital stay, and mortality. Patients without microbiological growth or without calculated antimicrobial therapy were excluded from the analysis. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the internal review boards (University Hospital Jena 3783-05/13 University of Munich 614/19 S-KH and University Hospital Aachen EK125-20) and written informed consent was waived by the ethics committees of the Jena University Hospital, the ethics committee of Aachen University Hospital and the ethics committee of Technical University Munich as only routine data was used.
Gram-negative bacteria were defined as multidrug-resistant if they were non-susceptible to at least one out of three antimicrobial categories according to the interim definition of the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC)18. In addition, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus spp. (VRE) were defined as multidrug-resistant bacteria.
Statistical analysis
Categorical variables were expressed as absolute and relative frequencies and compared using Fisher's exact test. Continuous variables were expressed as medians with interquartiles and compared using the Mann–Whitney U test as variables were not normally distributed. Indicators of enterococcal liver abscess were determined by univariate and multivariable binary logistic regression analysis. Survival analysis was performed using Kaplan–Meier statistics and Cox regression analysis. Survival data were right-censored at loss to follow-up or at 365 days, whichever occurred earlier. Multivariable Cox proportional hazards models were used to evaluate the effects of covariates on short-term survival. A significance level of 0.05 was selected for all statistical tests. Analyses were performed using IBM SPSS Statistics 27 (IBM Corp., Armonk, NY, USA), and data were plotted using Prism 8 (GraphPad, La Jolla, CA, USA).
Results
Baseline characteristics
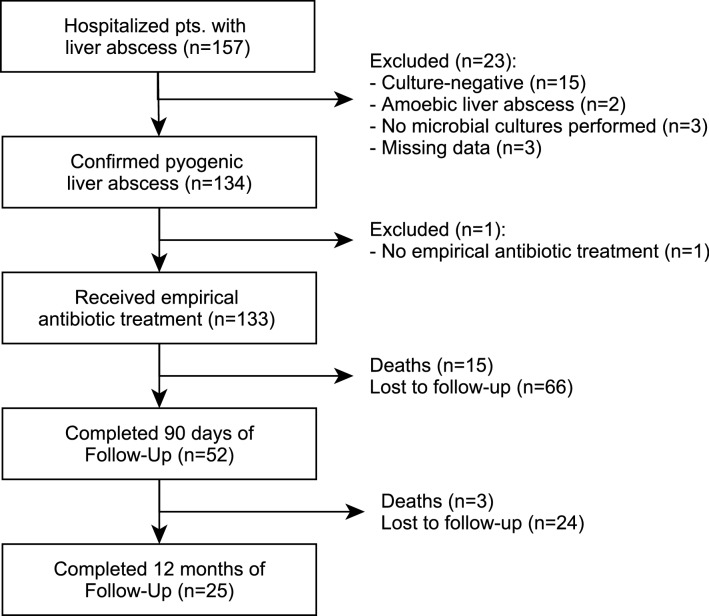
A total of 157 patients with liver abscess were identified at the three study centers. Twenty-four patients were excluded from the analysis because of negative cultures (n = 15; 9.5%), amebic abscess (n = 2; 1.2%), no microbiological sampling (n = 3; 1.9%), no empirical treatment (n = 1; 0.6%), or other missing data (n = 3; 1.9%), and 133 patients empirically treated for PLA were included in the analysis (Fig. 1).
Figure 1.
Patients included into the analysis.
The median age of the patients was 65 years and 79 patients (59%) were male. Fifty-two patients (39%) had a history of hepatobiliary carcinoma, and 60 patients (48%) had previously undergone biliary stenting. The median abscess diameter was 5.6 cm. Abscess drainage was performed in 87% of patients. For empiric therapy, the most commonly used antibiotic was piperacillin/tazobactam in 42 patients (32%), followed by ampicillin/sulbactam (23 patients; 17%), fluoroquinolones (22 patients; 16%), carpabenems (20 patients; 15%), and ceftriaxone (19 patients; 14%). Twenty-nine patients (22%) received combination therapy with metronidazole, vancomycin was added in 6 patients (5%), and linezolid was added in 2 patients (2%). Empirical therapy did not correlate with microbial spectrum or patient outcome (data not shown).
The majority of culture results were polymicrobial (72 cultures; 54%) with a median number of two bacterial species. The most frequently isolated pathogens were from the order Enterobacterales in 92 patients (69%), followed by enterococci (51 patients, 38%) and streptococcal species (25 patients, 19%) (Table 1). Most frequent Enterobacterales were E. coli (60 patients; 45%), Klebsiella spp. (30 patients,;23%) and Citrobacter spp. (13 patients; 10%).
Table 1.
Baseline characteristics, microbial spectrum and treatment in patients with pyogenic liver abscess stratified by outcome.
| Missing data | Patients with PLA (N = 133) | Survivors (N = 115) | Non-survivors (N = 18) | P value | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age (years) | 0 | 65 (56–74) | 64 (55–74) | 65 (58–74) | 0.49 |
| Male sex | 0 | 79 (59) | 47 (41) | 7 (39) | 1.00 |
| Smoker | 19 | 32 (28) | 27 (28) | 5 (31) | 0.77 |
| Alcohol use disorder | 23 | 15 (14) | 12 (13) | 3 (19) | 0.46 |
| Hepatobiliary cancer (incl. liver metastasis and pancreatic cancer) | 0 | 52 (39) | 48 (42) | 4 (22) | 0.76 |
| Previous bile duct stent | 8 | 60 (48) | 49 (45) | 11 (65) | 0.19 |
| Cirrhosis | 0 | 9 (7) | 7 (6) | 2 (11) | 0.35 |
| Liver transplant recipient | 0 | 10 (8) | 6 (5) | 4 (22) | 0.03* |
| COPD | 1 | 16 (12) | 15 (13) | 1 (6) | 0.70 |
| Long-term dialysis | 1 | 11 (8) | 7 (6) | 4 (22) | 0.04* |
| Previous surgery | 1 | 56 (42) | 49 (43) | 7 (39) | 0.80 |
| Antibiotic exposure | 1 | 27 (20) | 22 (19) | 5 (29) | 0.34 |
| Abscess criteria | |||||
| Abscess diameter (cm) | 23 | 5.6 (3.7–8.1) | 5.7 (3.8–8.5) | 4.9 (3.1–6.8) | 0.16 |
| Polymicrobial culture results | 0 | 72 (54) | 63 (55) | 9 (50) | 0.80 |
| Number of bacterial species | 0 | 2 (1–2) | 2 (1–2) | 1.5 (1–3) | 1.00 |
| Anaerobes | 0 | 20 (15) | 19 (17) | 1 (6) | 0.31 |
| Enterobacterales | 0 | 92 (69) | 80 (70) | 12 (67) | 0.79 |
| Enterococci | 0 | 51 (38) | 39 (34) | 12 (67) | 0.02* |
| Streptococci | 0 | 25 (19) | 25 (22) | 0 (0) | 0.02* |
| Non-fermenters | 0 | 7 (5) | 6 (5) | 1 (6) | 1.00 |
| ESBL-Enterobacterales | 0 | 20 (15) | 17 (15) | 3 (17) | 0.73 |
| CR-Enterobacterales | 0 | 2 (2) | 2 (2) | 0 (0) | 1.00 |
| VRE | 0 | 9 (7) | 5 (5) | 3 (17) | 0.10 |
| MRSA/MRSE | 0 | 3 (2) | 2 (2) | 1 (6) | 0.36 |
| Management | |||||
| Intensive care | 0 | 39 (29) | 28 (24) | 11 (61) | 0.004* |
| Abscess drainage | 1 | 115 (87) | 98 (86) | 17 (94) | 0.47 |
Medians with interquartile ranges and frequencies with percentages are shown. P values from Mann–Whitney U test or Fisher’s exact test. *p < 0.05.
PLA pyogenic liver abscess, MRSA methicillin-resistant Staphylococcus aureus, MRSE methicillin-resistant Staphylococcus epidermidis, VRE Vancomycin-resistant enterococci, ESBL extended-spectrum beta-lactamase, CR Carbapenem-resistant.
Isolated enterococci included 29 isolates of E. faecium, 16 isolates of Enterococcus faecalis and 6 other enterococci. The isolation of enterococci did not differ between the three study centers (Aachen 38.6%, Munich 40.9% and Jena 34.3% of the patients).
Characteristics of patients with enterococcal pyogenic liver abscess
Patients with enterococcal PLA had a smaller abscess diameter (4.8 vs. 6.7 cm, p = 0.03) than patients with non-enterococcal PLA but were more likely to have polymicrobial culture results (75% vs. 41%, p < 0.001) (Table 2).
Table 2.
Baseline characteristics, microbial spectrum and treatment in patients with pyogenic liver abscess stratified by the isolation of Enterococcus spp.
| Non-enterococcal PLA (n = 82) | Enterococcal PLA (n = 51) | P value | |
|---|---|---|---|
| Age (years) | 65 (53–75) | 64 (59–70) | 0.71 |
| Male sex | 46 (56) | 33 (65) | 0.37 |
| Smoker | 15 (21) | 17 (39) | 0.06 |
| Alcohol use disorder | 5 (7) | 10 (24) | 0.02 |
| Hepatobiliary cancer (incl. liver metastasis and pancreatic cancer) | 22 (27) | 30 (59) | < 0.001 |
| Previous bile duct stent | 34 (45) | 26 (53) | 0.46 |
| Cirrhosis | 2 (2) | 7 (14) | 0.03 |
| Liver transplant recipient | 6 (7) | 4 (8) | 1.00 |
| Chronic obstructive pulmonary disease | 5 (6) | 11 (22) | 0.01 |
| Long-term dialysis | 2 (2) | 9 (18) | 0.003 |
| Previous surgery | 29 (35) | 27 (54) | 0.046 |
| Antibiotic exposure | 16 (20) | 11 (22) | 0.83 |
| Polymicrobial abscess culture | 34 (41) | 38 (75) | < 0.001 |
| Abscess diameter (cm) | 6.7 (4–8.8) | 4.8 (3.1–7.5) | 0.03 |
| Intensive care | 17 (21) | 22 (42) | 0.02 |
| Death within 365 days | 6 (7) | 12 (24) | 0.02 |
Medians with interquartile ranges and frequencies with percentages are shown. P values from Mann–Whitney U test or Fisher’s exact test.
PLA Pyogenic liver abscess.
There were no differences in age (64 vs. 65 years, p = 0.71) and gender (male 65% vs. 56%, p = 0.37) between the two groups. Patients with enterococcal liver abscess were significantly more likely to have cirrhosis (14% vs. 2%, p = 0.03) or hepatobiliary malignancies (59% vs. 27%, p < 0.001). The number of patients who had previously undergone biliary stenting (53% vs. 45%, p = 0.46) or liver transplantation (8% vs. 7%, p = 1.00) did not differ between the two groups. Concomitant diseases other than liver were also more common in patients with enterococcal PLA, such as long-term dialysis (18% vs. 2%; p = 0.003), chronic obstructive pulmonary disease (COPD) (22% vs. 6%, p = 0.01), and previous intra-abdominal surgery (54% vs. 35%, p = 0.046). Notably, prior antibiotic exposure within 14 days to sampling was not associated with enterococcal isolation (22% vs. 20%, p = 0.83).
In univariate logistic regression analysis, alcohol use disorder (OR 3.94, 95%-CI 1.24–12.49, p = 0.02), hepatobiliary malignancies (OR 3.90, 95%-CI 1.86–8.18, p < 0.001) and cirrhosis (OR 6.36, 95%-CI 1.27–31.96, p = 0.02) were associated with enterococcal PLA. In multivariate analysis with stepwise backward elimination, alcohol abuse (OR 6.16, 95%-CI 1.75–21.68, p = 0.005) and hepatobiliary malignancies (OR 5.29, 95%-CI 2.18–12.82, p < 0.001) were found to be independent indicators PLA caused by Enterococcus spp. (Table 3).
Table 3.
Indicators of enterococcal pyogenic liver abscess.
| Univariate logistic regression | Multivariable logistic regression* | |||||
|---|---|---|---|---|---|---|
| Univariate Odds ratio | 95% CI | P value | Adjusted Odds ratio | 95% CI | P value | |
| Alcohol abuse documented | 3.94 | 1.24–12.49 | 0.02 | 6.16 | 1.75–21.68 | 0.005 |
| Hepatobiliary cancer (incl. liver metastasis and pancreatic cancer) | 3.90 | 1.86–8.18 | < 0.001 | 5.29 | 2.18–12.82 | < 0.001 |
| Cirrhosis | 6.36 | 1.27–31.96 | 0.02 | Excluded from model | ||
*Backward stepwise conditional exclusion model. CI Confidence interval.
Survival analysis
Within 12 months, 18 of the 133 patients died, 15 of them within the first 90 days. (Fig. 1). Demographic characteristics did not differ between survivors and non-survivors. Liver transplant recipients (22% vs. 5%; p = 0.03) with PLA and patients receiving long-term hemodialysis (22% vs. 6%, p = 0.04) were at significantly higher risk for fatal outcome. In terms of underlying pathogen spectrum, non-survivors had a lower prevalence of streptococci (0% vs. 22%; p = 0.02) and a higher prevalence of enterococci (67% vs. 34%, p = 0.02). There were no significant differences in ESBL-producing Enterobacterales (17% vs. 15%; p = 0.79), carbapenem-resistant Enterobacterales (0% vs. 2%; p = 1.00), or MRSA/MRSE (6% vs. 2%; p = 0.36) between non-survivors and survivors (Table 1).
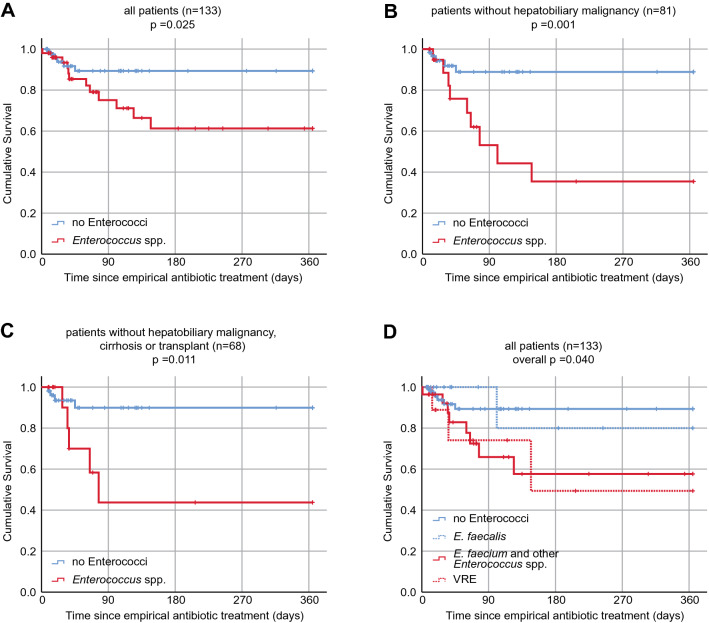
Within 365 days, 12 of the patients with enterococcal PLA died compared to 6 patients with non-enterococcal PLA (p = 0.025 by log-rank test) (Fig. 2A). This difference persisted in the sensitivity analysis when only patients without hepatopancreatic malignancy (Fig. 2B) or only patients without hepatopancreatic malignancy, cirrhosis and transplantation (Fig. 2C) were analyzed. The univariate hazard ratio for death was 2.92 (95% CI 1.09–7.80; p = 0.03) in patients with enterococcal PLA (Table 4).
Figure 2.
Survival in patients with enterococcal pyogenic liver abscess.
Table 4.
Association of enterococcal pyogenic liver abscess with mortality.
| Univariate Cox regression | Adjusted Cox regression* | |||||
|---|---|---|---|---|---|---|
| Univariate hazard ratio | 95% CI | P value | Adjusted hazard ratio | 95% CI | P value | |
| Enterococcal pyogenic liver abscess | 2.92 | 1.09–7.80 | 0.03 | 2.84 | 1.06–7.62 | 0.04 |
| No enterococci | 1.00 (ref.) | 1.00 (ref.) | ||||
| E. faecalis | 0.93 | 0.11–7.74 | 0.95 | 0.88 | 0.10–7.44 | 0.91 |
| E. faecium and other enterococci incl. VRE | 3.63 | 1.34–9.83 | 0.01 | 3.52 | 1.29–9.58 | 0.01 |
*Adjusted for age and sex.
CI Confidence interval, VRE Vancomycin-resistant enterococci.
The presence of E. faecalis was associated with mortality comparable to that of patients with non-enterococcal PLA (HR 0.93; 95%-CI 0.11–7.72; p = 0.95), whereas the presence of Enterococcus faecium or non-faecalis non-faecium Enterococcus species was associated with significantly higher mortality (HR 3.63, 95%-CI 1.34–9.83, p = 0.01) (Fig. 2D, Table 4). The associations of PLA by Enterococcus spp. and E. faecium with survival remained significant after adjustment for age and sex (Table 4).
Discussion
In this retrospective analysis from three German tertiary centers, we demonstrate that enterococci can be isolated in more than one third of patients with PLA and indicate an increased risk of mortality. The high proportion of enterococci in PLA is consistent with another recent study from Central Europe, which shows Enterococcus spp. as the most common pathogen in PLA9, but significantly higher than in Asian cohorts2,7,8.
At present, the most common route of PLA is ascending cholangitis and invasion of liver parenchyma on the ground of obstruction by gallstone disease, strictures or malignancy19. Cohorts from our and other European University centers9 are often enriched for patients with underlying hepatobiliary strictures who underwent prior biliary tract interventions. In patients with malignant biliary obstruction, antibiotic exposure predisposes to enterococcal bactibilia20, presumably predisposing to cholangiogenic liver abscess by enterococci. In our study, 48% of patients had a bile duct stent previously or currently implanted, which can often be colonized with enterococci in addition to Gram-negative bacteria or fungi14,21,22. However, in contrast to our previous analysis of acute ascending cholangitis14,21, neither the presence of biliary stents nor previous antibiotic exposure was significantly associated with enterococcal PLA in our analysis. In the present study, only alcohol use disorders, cirrhosis, and hepatobiliary cancer were linked to isolation of Enterococcus spp. from PLA in univariate analysis. Considering that gut flora reach the biliary system either often by ascent from the intestine, fecal enterococcal overgrowth as seen after TGC use23 or changes in the microbiota composition may contribute. The gut microbiota of patients with alcohol use disorders and alcoholic liver disease is often enriched in Enterococcus spp, especially when other risk factors such as proton pump inhibitors are present24,25.
Enterococci are part of the physiological flora of the gastro-intestinal tract. It remains controversial whether enterococci have pathologic significance, as they are usually described as having low virulence17. In our analysis, isolation of enterococci was associated with higher mortality, even after exclusion of patients with concomitant hepatobiliary disease, suggesting a possible influence of enterococci on mortality. In contrast to other intraabdominal infections in vulnerable patients12, we did not observe a correlation between coverage of enterococci by empirical therapy and outcome. This could be due to the fact that PLA requires several weeks of antibiotic therapy and the duration of empiric therapy is rather short in relation to definitive therapy.
Over the last years, vancomycin-resistant Enterococcus spp. increased, accounting for 19% of all Enterococcus isolates in blood stream infections26 and up to 33% of enterococcal isolates from PLA9. In our analysis, patients with PLA due to E. faecalis, which is usually susceptible to beta-lactams and vancomycin27, had a prognosis comparable to that of non-enterococcal PLA, whereas more resistant enterococcal species such as E. faecium with and without vancomycin resistance were associated with higher mortality. The main cause of the increasing proportion of VRE is antibiotic exposure. Therefore, from an antibiotic stewardship perspective, our results may justify deferring enterococcal-specific therapy in non-critically ill patients with PLA until culture results are available. However, this needs to be clarified in a larger, prospective, randomized cohort.
This study has some limitations. First, it is a retrospective analysis of complex and often lengthy multimodal therapeutic approaches. In particular, a structured follow-up including data on the resolution of PLA were missing in some cases. Although the phenotypic characteristics of enterococcal PLA are quite robust because only patients with microbiologically confirmed PLA were included, there is a risk of bias in the mortality analysis because approximately 50% of patients were lost to follow-up within 90 days. Second, because of the nature of a noninterventional observational study, we could not distinguish whether enterococci were causative for the increased mortality or merely a surrogate marker for sicker patients. Third, all study sites were university hospitals, so patients with biliary structures, repeated antibiotic exposure, and in an immunocompromised state were likely overrepresented. However, the association between enterococci and increased mortality remained stable even after exclusion of patients with concomitant hepatobiliary disease in a sensitivity analysis.
Despite these limitations, our data show that patients with alcohol consumption and malignant biliary strictures are at risk for enterococcal PLA. Enterococcal PLA, particularly PLA by E. faecium and other non-faecalis enterococci, indicates a higher risk of mortality, underscoring the need for close clinical monitoring and individualized treatment protocols in these patients.
Abbreviations
- PLA
Pyogenic liver abscess
- MRSA
Methicillin-resistant Staphylococcus aureus
- MRSE
Methicillin-resistant Staphylococcus epidermidis
- VRE
Vancomycin-resistant enterococci
- ESBL
Extended-spectrum beta-lactamase
- CR
Carbapenem-resistant
Author contributions
K.G. (Data curation: Equal; Formal analysis: Supporting; Investigation: Equal; Writing—original draft: Supporting; Writing—review & editing: Supporting). Ohm D (Data curation: Equal; Supporting; Writing—review & editing: Supporting). S.W. (Data curation: Equal; Supporting; Writing—review & editing: Supporting). Brozat JF (Data curation: Equal; Supporting; Writing—review & editing: Supporting). R.M.S., C.T. and A.S. (Resources: Supporting; Writing—review & editing: Supporting). T.B. and P.A.R. (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Software: Lead; Supervision: Lead; Visualization: Lead; Writing—original draft: Lead; Writing—review & editing: Lead).
Funding
Open Access funding enabled and organized by Projekt DEAL. T.B. was supported by the German Research Foundation (DFG) (SFB1382 Project ID 403114013/B07).
Competing interests
Outside the submitted work, T.B. has received honoraria from Intercept Pharmaceuticals, Falk Foundation, AbbVie, CSL Behring, Merck, and Norgine, and travel expenses from Gilead. A.S. obtained consulting fees from Abbvie, Amgen, Astellas, Biogen, Celltrion, Consal, CSL Behring, Galapagos, Gilead, Institut Allergosan, Janssen, MSD, Norgine, Pfizer Pharma, Roche, Shire and Takeda, lecture fees and travel support from Abbvie, Astellas, Celltrion, Falk Foundation, Ferring, Janssen, MSD, Recordati Pharma, and Takeda, and research support from Abbvie and Celltrion. P.A.R. received lecture and consulting fees from CSL Behring, Boston Scientific and Dr. Wilmar Schwabe and travel grants from Bayer and Merz Pharma. The other authors report no potential conflicts of interest relevant to the manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: T. Bruns and Philipp A. Reuken.
References
- 1.Meddings L, Myers RP, Hubbard J, et al. A population-based study of pyogenic liver abscesses in the United States: Incidence, mortality, and temporal trends. Am. J. Gastroenterol. 2010;105:117–124. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 2.Tsai F-C, Huang Y-T, Chang L-Y, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg. Infect. Dis. 2008;14:1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo S-H, Lee Y-T, Li C-R, et al. Mortality in Emergency Department Sepsis score as a prognostic indicator in patients with pyogenic liver abscess. Am. J. Emerg. Med. 2013;31:916–921. doi: 10.1016/j.ajem.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 4.Lübbert C, Wiegand J, Karlas T. Therapy of liver abscesses. Viszeralmedizin. 2014;30:334–341. doi: 10.1159/000366579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerwenka H. Pyogenic liver abscess: Differences in etiology and treatment in Southeast Asia and Central Europe. World J. Gastroenterol. 2010;16:2458–2462. doi: 10.3748/wjg.v16.i20.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo JZW, Leow JJJ, Ng PLF, et al. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J. Hepato-Biliary-Pancreat Sci. 2015;22:156–165. doi: 10.1002/jhbp.174. [DOI] [PubMed] [Google Scholar]
- 7.Siu LK, Yeh K-M, Lin J-C, et al. Klebsiella pneumoniae liver abscess: A new invasive syndrome. Lancet Infect. Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 8.Chung DR, Lee SS, Lee HR, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J. Infect. 2007;54:578–583. doi: 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Mücke MM, Kessel J, Mücke VT, et al. The role of Enterococcus spp. and multidrug-resistant bacteria causing pyogenic liver abscesses. BMC Infect. Dis. 2017 doi: 10.1186/s12879-017-2543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar P, Ghosh S, Rath D, et al. Multidrug resistant citrobacter: An unusual cause of liver abscess. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Carlo P, Pantuso G, Cusimano A, et al. Two cases of monomicrobial intraabdominal abscesses due to KPC–3 Klebsiella pneumoniae ST258 clone. BMC Gastroenterol. 2011;11:103. doi: 10.1186/1471-230X-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuken PA, Pletz MW, Baier M, et al. Emergence of spontaneous bacterial peritonitis due to enterococci: Risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol. Ther. 2012;35:1199–1208. doi: 10.1111/j.1365-2036.2012.05076.x. [DOI] [PubMed] [Google Scholar]
- 13.Lutz P, Nischalke HD, Krämer B, et al. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur. J. Clin. Invest. 2017;47:44–52. doi: 10.1111/eci.12701. [DOI] [PubMed] [Google Scholar]
- 14.Reuken PA, Torres D, Baier M, et al. Risk factors for multi-drug resistant pathogens and failure of empiric first-line therapy in acute cholangitis. PLoS ONE. 2017;12:e0169900. doi: 10.1371/journal.pone.0169900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuken PA, Kruis W, Maaser C, et al. Microbial spectrum of intra-abdominal abscesses in perforating Crohn’s disease: Results from a prospective German registry. J. Crohns. Colitis. 2018;12:695–701. doi: 10.1093/ecco-jcc/jjy017. [DOI] [PubMed] [Google Scholar]
- 16.Harbarth S, Uckay I. Are there patients with peritonitis who require empiric therapy for enterococcus? Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2004;23:73–77. doi: 10.1007/s10096-003-1078-0. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee I, Iredell JR, Woods M, et al. The implications of enterococci for the intensive care unit. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2007;9:69–75. [PubMed] [Google Scholar]
- 18.Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Mavilia MG, Molina M, Wu GY. The evolving nature of hepatic abscess: A review. J. Clin. Transl. Hepatol. 2016;4:158–168. doi: 10.14218/JCTH.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura T. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig. Dis. Sci. 2000;45:2183–2186. doi: 10.1023/A:1026640603312. [DOI] [PubMed] [Google Scholar]
- 21.Lübbert C, Wendt K, Feisthammel J, et al. Epidemiology and resistance patterns of bacterial and fungal colonization of biliary plastic stents: A prospective cohort study. PLoS ONE. 2016;11:e0155479. doi: 10.1371/journal.pone.0155479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rerknimitr R, Fogel EL, Kalayci C, et al. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest. Endosc. 2002;56:885–889. doi: 10.1067/mge.2002.129604. [DOI] [PubMed] [Google Scholar]
- 23.Suppola JP, Volin L, Valtonen VV, et al. Overgrowth of enterococcus faecium in the feces of patients with hematologic malignancies. Clin. Infect. Dis. 1996;23:694–697. doi: 10.1093/clinids/23.4.694. [DOI] [PubMed] [Google Scholar]
- 24.Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llorente C, Jepsen P, Inamine T, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat. Commun. 2017;8:837. doi: 10.1038/s41467-017-00796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayobami O, Willrich N, Reuss A, et al. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020;9:1180–1193. doi: 10.1080/22221751.2020.1769500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuch A, Willems RJL, Werner G, et al. Insight into antimicrobial susceptibility and population structure of contemporary human Enterococcus faecalis isolates from Europe. J. Antimicrob. Chemother. 2012;67:551–558. doi: 10.1093/jac/dkr544. [DOI] [PubMed] [Google Scholar]