Abstract
T2 ribonuclease family (RNaseT2) proteins are secretory and nonspecific endoribonucleases that have a large conserved biological role. Family members of RNaseT2 are found in every organism and carry out important biological functions. However, little is known about the functions of these proteins in legumes, including potential roles in symbiotic nodulation. This study aimed to characterize and perform bioinformatic analysis of RNaseT2 genes in four legume species that their genome was sequenced. In total, 60 RNaseT2 genes were identified and characterized. By analyzing their phylogeny, we divided these RNaseT2 into five clades. Expression analysis of RNaseT2 genes indicated that these genes are expressed in various tissues, and the most expression level was related to the pod, flower, and root. Moreover, GmaRNS9 expression analysis in soybean was consistent with in silico studies and demonstrated that this gene usually has high root tip expression. GmaRNS9 expression was reduced by Bradyrhizobium japonicum inoculation and nodule formation. Reduced expression of this gene was possibly controlled by the GmNARK gene either directly or pleiotropically through increased phosphorus requirements during increased nodulation. However, the nutrient stress (phosphate and nitrate starvation) led to an increase in the expression level of GmRNS9. In silico and quantitative gene expression analyses showed that RNaseT2 genes could play important roles in the growth and development of legumes as well as nodulation.
Keywords: In silico, RNaseT2, Gene expression, Phylogeny, Biochemical properties, Abiotic stresses, Legume
Introduction
In most organisms, the ribonucleases, including transferase type RNases, are important secretory enzymes. These enzymes are associated with RNA metabolism (Vidalino et al. 2012). These RNases are able to cleave RNA and form mononucleotides, or oligonucleotides with 2′, 3′-cyclic phosphate at the 3′-side, yielding 3′-mononucleotides or oligonucleotides with a 3′-phosphate (Deshpande and Shankar 2002). The 2′, 3′-cyclizing RNases are classified into three separate groups based on their base traits and molecular mass, including RNase A with molecular masses of around 14 kDa (such as bovine pancreatic RNase A), RNaseT1 family with molecular masses of 11–12 kDa (such as RNaseT1 from Aspergillus oryzae), and T2 ribonuclease (RNaseT2) family with molecular masses of 24–36 kDa (such as RNase Rh of Rhizopus niveus and one RNaseT2 of A. oryzae). The primary structure of RNase Rh and RNaseT2 from the third group has been clarified by Horiuchi et al. (1988) and Kawata et al. (1988), respectively.
RNasesT2 are known as transferase type RNases and are classified because of their similarity to RNaseT2 from A. oryzae (Tanaka et al. 2000; MacIntosh et al. 2001; Luhtala and Parker 2010; Gundampati et al. 2011). These enzymes are single-strand-specific endoribonucleases and show activity in a wide range of pH preferences. Members of the RNaseT2 family are also represented in the genome of almost every organism ever examined, including bacteria, viruses, fungi, animals, and plants, except for some bacteria and trypanosomes and carry out important biological functions (Irie 1999; Deshpande and Shankar 2002; MacIntosh et al. 2010; MacIntosh and Castandet 2020). In addition, RNaseT2 enzymes are extremely conserved in most eukaryotic genomes, and their expression patterns and phylogenetic relationships indicate that they may carry out important housekeeping roles and biological functions. Nevertheless, the nature of these functions is still unknown (Hillwig 2009; Hillwig et al. 2011; Itagaki et al. 2013). For example, Rny1 (the budding yeast RNaseT2) degrades and cleaves RNA in vacuole under nutrient starvation, resulting in 3′ phosphorylated nucleotides. Also, a similar role has been observed for RNS2, its orthologue in Arabidopsis (Bassham and MacIntosh 2017). Haud et al. (2011) reported that lack of RNaseT2 in zebrafish causes accumulation of undigested rRNA in brain cell lysosomes. Besides, it has been shown that mutations in RNaseT2 cause familial cystic leukoencephalopathy due to rRNA accumulation in the lysosomes of human neurons (Haud et al. 2011). As previously mentioned, eukaryotic RNaseT2 family members are localized in the vacuole or lysosome and function in rRNA degradation. Deficiency of rRNA degradation in rns2 leads to a decline in cytoplasmic nucleoside and nucleotide concentration (Kazibwe et al. 2020). These RNases are usually glycosylated in eukaryotic cells or typically enter the secretory pathway (Irie 1999; Deshpande and Shankar 2002; Andersen and Collins 2012).
Interestingly, studies have shown that Rny1 secretes out of the cell and plays a role in scavenging nutrients from RNAs in the extracellular space. Hence, the main localization of Rny1 is extracellular or vacuolar space (MacIntosh et al. 2001; Huang et al. 2015). On the other hand, Thompson and Parker (2009) indicated that during oxidative stress in yeast, Rny1 releases to the cytoplasm from the vacuole cleaves cytosolic RNAs and promotes cell death. In fact, the release of Rny1 to cytosol modulates cell survival during stress and has not been observed in yeast cytosol under normal conditions.
The crystal structures of RNaseT2 family members from plants, fungi, and bacteria reveal the presence of a conserved α/β core structure (with seven β strands and six α helices) that is similar to the structure observed in the fungal ribonuclease (RNase Rh from R. niveus) (De Leeuw et al. 2007; Luhtala and Parker 2010). This core includes the conserved active site that is responsible for degrading RNA. RNasesT2 have two Conserved Active Sites, CAS I and II, around two very conserved His residues, which are catalytically important (Itagaki et al. 2013; Kobayashi et al. 2003).
Plant RNases T2 family are divided into two subfamilies, namely S-RNases and S-like RNases (MacIntosh et al. 2010). They are specifically expressed during leaf senescence (Lers et al. 1998, 2006). Moreover, they are involved in nutrition stress such as phosphorus limitation, where they scavenge phosphates from ribonucleotides and during wounding or pathogen invasion, as a protective function (Kock et al. 1998; Bodenhausen and Reymond 2007; Zheng et al. 2014). Also, RNaseT2 enzymes contribute to rRNA and tRNA degradation in plants (Ramanauskas and Igic 2017). Some RNases T2, including S-RNases, are responsible for self-incompatibility in plants by being a genetic barrier that prevents inbreeding in some plant families such as Solanaceae and Scrophulariaceae and Rosaceae (MacIntosh et al. 2001; Melissa Sue Hillwig 2009; Luhtala and Parker 2010). Several RNaseT2 genes have been reported in plant species that are induced under different conditions. RNase LE and Rnase LX, in Lycopersicon esculentum (tomato) and RNS1, RNS2 and RNS3 in Arabidopsis thaliana, are induced during senescence and under phosphate-limiting conditions. RNase NW, in tobacco, and ZRNaseII, in Zinnia elegans, were found in response to wounding. At the same time, NGR3 gene in Nicotiana glutinosa, was identified 48 h after tobacco mosaic virus (TMV) infection, and eight RNaseT2 (OsRNS1-8) in rice were expressed under biotic and abiotic stresses (Taylor et al. 1993; Ye and Droste 1996; Kariu et al. 1998; Hillwig 2009; Luhtala and Parker 2010; MacIntosh et al. 2010).
Legumes are an important family of angiosperms as they can form a symbiosis with bacteria, called rhizobia that fix atmospheric nitrogen gas (Mirzaei et al. 2017; Solis-Miranda et al. 2020). Symbiotic development leads to altered gene expression level and the formation of nitrogen-fixing nodules (Solis-Miranda et al. 2020). Legumes in high soil nitrogen conditions are able to control nodule formation through auto-regulation of nodulation (AON) as a regulatory system (Gresshoff et al. 2015). In the AON system, the emergence of early nodulation generates a signal from roots to shoots and in turn, from shoots to roots, inhibiting further nodulation (Ferguson et al. 2010). This root signal is characterized as a small set of Rhizobium-induced CLE peptides (called RIC1 and RIC2 for soybean similar to Arabidopsis peptide CLAVATA3 (CLV3) (Reid et al. 2011). The peptides are tri-arabinosylated for transport to the shoot where they interact with receptor GmNARK (a leucine-rich repeat receptor-like kinase (LRR-RLK) (Searle et al. 2003; Gresshoff et al. 2015; Corcilius et al. 2017). GmNARK is also involved in inhibiting nodulation by nitrate, as deficient mutants show the ability to nodulate under inhibitory conditions (Ferguson et al. 2019). Many factors, conditions, elements, and genes are effective in soybean nodulation and nitrogen fixation. Phosphorus deficiency decreases photosynthesis, nodule dry weight, nitrogen fixation, and plant growth in legumes (Singh 2010). In addition, a high amount of mineral nitrogen in soil has a negative effect on nodulation (Ferguson et al. 2010; Coskan and Dogan 2011; Reid et al. 2011). As legumes are important in providing food for the world’s population and also able to fix atmospheric nitrogen through a symbiotic association with soil bacteria, further works should be done for characterizing genes related to legume nodulation, growth, and nutrient stress responses (Mirzaei et al. 2014, 2017; Mirzaei and Azizkhani 2019). As mentioned before, RNasesT2 are represent in almost all organisms and are involved in various biological functions. However, there is no genome-wide investigation of these genes and functions of their proteins in legumes, including growth, development, and potential roles in symbiotic nodulation. In the current study, we performed a genome-wide investigation of RNaseT2 genes in four legume species. Moreover, biochemical features, phylogenetic relationship, and gene expression were analyzed using various bioinformatics tools and qRT-PCR.
Materials and methods
Identification of RNaseT2 genes
BLAST searches were used to identify RNaseT2 genes from four legume species. Blastp search was conducted using the protein sequence of all five RNaseT2 from A. thaliana (RNS1-5) as the query in Phytozome database (version (v) 12.1) for RNaseT2 genes of Glycine max (Wm82.a2.v1), Medicago truncatula (Mt4.0v1) and Phaseolus vulgaris (v2.1). RNaseT2 genes of Lotus japonicus were obtained from the Kazusa database (v3). The protein of A. thaliana RNaseT2 was taken from the NCBI database (Goodstein et al. 2012). Accordingly, genes were selected that contained the RNaseT2 genes domain with scores above 32.5, identity of more than 20%, and E-value less than 0.01. Furthermore, to ensure all legume RNaseT2 are found, a reverse blast was performed. As a result, the genes that had the same blast results as the first results of the blast were selected for this study. Also, in addition to the mentioned databases, the Ensembl Plants database (https://plants.ensembl.org/index.html) were used to confirm the genomic features, exons, introns and UTRs of all identified genes. Also, homologous genes with Arabidopsis were found using the pair-wise analysis in the EMBLE-EBI database (https://www.ebi.ac.uk/Tools/psa/emboss_needle/). Identity of more than 35% was considered for determining the very close homologs. Then, the legumes proteins with the highest identity were selected as the closest homologous to the Arabidopsis RNasesT2.
Phylogenetic analysis
Multiple sequence alignments were conducted on amino acid sequences of RNaseT2 protein using MUSCLE with default parameters (Edgar 2004). Phylogenetic analysis was conducted on the conserved region of RNaseT2 protein between amino acid numbers 326 to 438 according to multiple sequence alignments (gaps are considered). In phylogenetic analysis, genes with short protein sequences were ignored. A maximum-likelihood phylogenetic tree was created for edited sequence alignment with the UGENE program, using PhyML substitution model with 1000 bootstraps and default parameters (Soltis and Soltis 2003; Okonechnikov et al. 2012).
Protein domains, cellular localization, and biochemical characterization
SMART EMBL (http://smart.embl-heidelberg.de/) and SIGNALP4.1 (www.cbs.dtu.dk/services/SignalP/) servers were used to identify protein domains and signal peptides of the genes through the default server’s settings (Schultz et al. 1998; Emanuelsson et al. 2007; Letunic et al. 2015). Also, the WOLF PSORT server (an update of PSORT II for fungi/animal/plant sequences) was used to estimate the cellular localization of identified RNaseT2 proteins in legumes (Horton et al. 2007). The isoelectric point (pI) was obtained using the ProtParam tool in EXPASY (http://www.web.expasy.org/compute-pi/) (Artimo et al. 2012). The instability index was evaluated using the Guruprasad et al. (1990) method.
Gene expression analysis
In silico analysis of the RNaseT2 gene family expression in legume
The expression data for RNaseT2 genes in G. max, M. truncatula and A. thaliana were obtained from BAR resource (http://bar.utoronto.ca/) (Schmid et al. 2005; Benedito et al. 2008; Libault et al. 2010a). The expression data for RNaseT2 genes in L. japonicus and P. vulgaris were downloaded from the Lotus Base and PvGEA, respectively (O’Rourke et al. 2014; Mun et al. 2016). Expression data were represented as heat maps for each species through CIMminer-One Matrix server (CIMminer-One Matrixdiscover.nci.nih.gov) (Sahu et al. 2017; Solis-Miranda et al. 2020). The expression profiles were only obtained of the 4, 11, 4, 13, and 8 genes for A. thaliana, G. max, L. japonicus, P. vulgaris and M. truncatula.
Plant growth conditions and qRT-PCR assays
Soybean WT Bragg and its isogenic supernodulating nark mutant line, nts 382, were used for gene expression analysis. The seeds were surface-sterilized in 70% (v/v) ethanol, followed by several rinses with distilled water. Plants were grown in standard conditions (25 °C/18 °C and 16-h-light) in a greenhouse in sterilized perlite and pots where sterile growing conditions were required. In the first analysis, to induce nodulation and to study nodulation effect on GmaRNS9 expression, the plants were inoculated with ~ 100 ml of Bradyrhizobium japonicum (CB1809) grown in yeast-mannitol broth (YMB) and diluted to an OD600 0.01, or only with YMB media as mock. For the first analysis, the plants were irrigated every other day with 50 ml B & D nutrient solution, lacking nitrogen or having a solution of KNO3 (5 mM) (Libault et al. 2010b). In the second analysis, soybean plants were placed under nitrate and phosphate stress (0 or 5 mM of N or P) to evaluate the effect of nutrient stresses on GmaRNS9 expression. In this section, plants were watered with modified B & D nutrient solution lacking nitrogen (N) and phosphorous (P). P and N were added to the nutrient as KH2PO4 and KNO3 at a concentration of 5 mM where it was required. In general, both experiments were conducted with four treatments and each treatment with three replications in a completely randomized design.
Root tip samples (5 mm long) were collected and pooled from 21-day-old plants (at least five plants were used) and were immediately frozen and homogenized in liquid nitrogen using mortar and pestle. According to the manufacturer’s instructions, RNA was extracted from 0.1 g of root tip samples using TRIzol solution (Invitrogen, USA). RNA concenteration was determined using UV spectrophotometer. DNA contamination was removed using DNaseI (Fermentase, Lithuania). Approximately 1 μg of RNA was subjected to 1 unit of DNaseI at 37 °C for 40 min. The reactions were inactivated by adding 1 μl of 25 mM EDTA and incubating at 65 °C for 10 min. RNA was converted to cDNA in a 20 μl reaction mixture containing 0.5 mM deoxynucleoside triphosphates (dNTPs), 1 μl of 50 μm oligo (dT) primers, 40 units of RNase inhibitor, 0.5 μg of DNA-free RNA, 1 × first-strand buffer, 5 mM dithiothreitol (DTT) and 100 units of MMLV reverse transcriptase (Fermentas, Lithuania) at 50 °C for 60 min. Finally, cDNA was confirmed using GmCONS6, primers with PCR (Libault et al. 2008, 2009). Gene-specific primer pairs were designed based on the target gene sequences using the Gene Runner 5.06; (forward primer: AAAGGCTGGGCATCACTC; reverse primer: AGGATTTGAAGGAGGTCTAC) (http://www.generunner.com). The qRT-PCR assays were performed using SYBR Premix EX Taq™ II Master (TAKARA, Japan), in a real-time PCR system. All reactions were performed in three biological replicates and two technical replicates (duplicate for each cDNA sample). The expression level was normalized to the mRNA expression level of GmCons6 (Libault et al. 2008, 2009). The thermal profile of the qRT-PCR reaction was 95 °C for 5 min activation and denaturation, followed by 44 cycles of 95 °C for 15 s and 63 °C for 40 s. Finally, a dissociation curve was generated by increasing temperature starting from 65 to 95 °C to determine the specificity of the reactions. The specificity of primer sets was confirmed by analyzing the dissociation curve profile of each qRT-PCR amplicon, and the efficiency of primers was considered 100%. The data were analyzed using the∆∆ ct method (Livak and Schmittgen 2001). Mean comparisons based on the Duncan test was performed using SPSS software (Kisa et al. 2017).
Results
Identification of RNaseT2 genes
Studies of the RNaseT2 gene family are mainly focused on the model plants, A. thaliana, and Oryza sativa L. (Taylor and Green 1991; Igic and Kohn 2001; MacIntosh et al. 2010). Thus little information was available on RNaseT2 gene identification, characterization, and expression profiling analysis in legume species. This study performed a genome-wide investigation of the RNaseT2 gene family in legume species, including G. max, M. truncatula, P. vulgaris, and L. japonicus. All the RNaseT2 genes from these legume species were acquired by Blastp search. We identified a total of 60 candidate RNaseT2 genes in four legume species. Our results revealed that 13 RNaseT2 genes in G. max are distributed over six chromosomes out of 20 chromosome, 27 RNaseT2 in M. truncatula are situated on seven chromosomes out of eight chromosomes, 13 RNaseT2 in P. vulgaris genes are located on four chromosomes out of 11 chromosomes, seven RNaseT2 genes in L. japonicus are located on three chromosomes out of six chromosomes (Table 1). Although MacIntosh et al. (2010) predicted 12 RNaseT2 genes in G. max, we identified an additional new gene in the soybean genome called GmaRNS13. It should be noted that all genes in this article were named based on Taylor and Green (1991), and MacIntosh et al. (2010).
Table 1.
Genomic characteristic and number of exons of the RNaseT2 genes in the studied legumes species and A. thaliana
| Species | Gene name | Genome locus | Chromosome | Start | End | Gene length (bp) | Transcripts (bp) | Peptide (aa) | Exons | Introns |
|---|---|---|---|---|---|---|---|---|---|---|
| A. thaliana | RNS1 | AT2G02990 | CHR 2 | 783,506 | 874,811 | 1306 | 693 | 230 | 4 | 3 |
| RNS2 | AT2G39780 | CHR 2 | 16,591,222 | 16,593,775 | 2554 | 780 | 259 | 9 | 8 | |
| RNS3 | AT1G26820 | CHR 1 | 9,292,446 | 9,293,784 | 1339 | 669 | 222 | 4 | 3 | |
| RNS4 | AT1G14210 | CHR 1 | 4,851,778 | 4,857,964 | 1187 | 744 | 247 | 4 | 3 | |
| RNS5 | AT1G14220 | CHR 1 | 4,858,642 | 4,859,601 | 959 | 687 | 228 | 4 | 3 | |
| G. max | GmaRNS1 | Glyma02g12010 | CHR 2 | 10,303,196 | 1,035,744 | 2549 | 684 | 227 | 4 | 3 |
| GmaRNS2 | Glyma16g03118 | CHR 16 | 2,733,624 | 2,740,420 | 6797 | 810 | 269 | 8 | 7 | |
| GmaRNS3 | Glyma02g07150 | CHR 2 | 5,766,138 | 5,767,678 | 1541 | 696 | 231 | 3 | 2 | |
| GmaRNS4 | Glyma02g07140 | CHR 2 | 5,757,818 | 5,759,434 | 1617 | 696 | 231 | 3 | 2 | |
| GmaRNS5 | Glyma02g07130 | CHR 2 | 5,741,318 | 5,742,800 | 1483 | 696 | 231 | 3 | 2 | |
| GmaRNS6 | Glyma07g06520 | CHR 7 | 5,293,201 | 5,300,088 | 6809 | 813 | 270 | 9 | 8 | |
| GmaRNS7 | Glyma02g12020 | CHR 2 | 10,311,315 | 10,313,811 | 2497 | 681 | 226 | 4 | 3 | |
| GmaRNS8 | Glyma20g04820 | CHR 20 | 5,179,170 | 5,181,667 | 2498 | 684 | 227 | 4 | 3 | |
| GmaRNS9 | Glyma20g04830 | CHR 20 | 5,185,449 | 5,187,759 | 2302 | 717 | 238 | 4 | 3 | |
| GmaRNS10 | Glyma01g05840 | CHR 1 | 5,615,672 | 5,619,252 | 3581 | 717 | 238 | 5 | 4 | |
| GmaRNS11 | Glyma01g05850 | CHR 1 | 5,627,602 | 5,631,187 | 3586 | 681 | 226 | 4 | 3 | |
| GmaRNS12 | Glyma03g35230 | CHR 3 | 40,508,841 | 40,512,126 | 3286 | 696 | 231 | 3 | 2 | |
| GmaRNS13 | Glyma02g07135 | CHR 2 | 5,745,915 | .5747298 | 1384 | 699 | 232 | 3 | 2 | |
| P. vulgaris | PhvuRNS1 | Phvul.002G084500 | CHR 2 | 13,697,023 | 13,699,157 | 2135 | 681 | 226 | 4 | 3 |
| PhvuRNS2 | Phvul.002G170800 | CHR 2 | 32,802,508 | .32804250 | 1743 | 756 | 251 | 4 | 3 | |
| PhvuRNS3 | Phvul.002G084600 | CHR 2 | 13,220,621 | 1,322,180 | 1560 | 684 | 227 | 4 | 3 | |
| PhvuRNS4 | Phvul.010G110200 | CHR 1 | 37,289,320 | 37,295,432 | 6113 | 807 | 268 | 9 | 8 | |
| PhvuRNS5 | Phvul.006G112700 | CHR 6 | 22,842,035 | 22,842,782 | 748 | 660 | 219 | 2 | 1 | |
| PhvuRNS6 | Phvul.004G064100 | CHR 4 | 8,885,613 | 8,888,113 | 2501 | 654 | 217 | 3 | 2 | |
| PhvuRNS7 | Phvul.006G112900 | CHR 6 | 22,852,423 | 22,853,143 | 721 | 633 | 210 | 2 | 1 | |
| PhvuRNS8 | Phvul.006G112800 | CHR 6 | 22,846,925 | 22,847,773 | 849 | 666 | 221 | 2 | 1 | |
| PhvuRNS9 | Phvul.004G050200 | CHR 4 | 6,009,604 | 6,010,313 | 710 | 648 | 215 | 2 | 1 | |
| PhvuRNS10 | Phvul.004G052000 | CHR 4 | 6,660,899 | 6,661,614 | 716 | 639 | 212 | 2 | 1 | |
| PhvuRNS11 | Phvul.004G056000 | CHR 4 | 7,314,258 | 7,314,829 | 572 | 486 | 161 | 2 | 1 | |
| PhvuRNS12 | Phvul.004G056100 | CHR 4 | 7,332,977 | 7,333,551 | 575 | 489 | 162 | 2 | 1 | |
| PhvuRNS13 | Phvul.004G055900 | CHR 4 | 7,309,017 | 7,310,748 | 1732 | 408 | 135 | 3 | 2 | |
| L. japonicus | LjRNS1 | Lj2g3v1277800 | CHR 2 | 20,463,586 | 20,464,988 | 1402 | 677 | 226 | 4 | 3 |
| LjRNS2 | Lj2g3v1277810 | CHR 2 | 20,467,335 | 20,469,342 | 2007 | 677 | 226 | 4 | 3 | |
| LjRNS3 | Lj3g3v2921110 | CHR 3 | 36,451,904 | 36,452,640 | 736 | 601 | 200 | 2 | 1 | |
| LjRNS4 | Lj0g3v0178959 | CHR 1 | 86,826,543 | 86,827,629 | 1086 | 679 | 225 | 2 | 1 | |
| LjRNS5 | Lj0g3v0178969 | CHR 1 | 86,827,028 | 86,827,679 | 654 | 390 | 129 | 1 | – | |
| LjRNS6 | Lj0g3v0219759 | CHR 1 | 112,350,466 | 112,351,053 | 587 | 375 | 124 | 1 | – | |
| LjRNS7 | Lj0g3v0261499 | CHR 1 | 135,511,382 | 135,511,990 | 608 | 383 | 127 | 1 | – | |
| M. truncatula | MedtrRNS1 | Medtr5g040940 | CHR 5 | 17,998,340 | 18,000,220 | 1881 | 687 | 228 | 4 | 3 |
| MedtrRNS2 | Medtr5g040960 | CHR 5 | 18,005,673 | 18,007,398 | 126 | 693 | 230 | 4 | 3 | |
| MedtrRNS3 | Medtr5g041010 | CHR 5 | 18,028,304 | 18,030,068 | 1765 | 684 | 227 | 4 | 3 | |
| MedtrRNS4 | Medtr5g041040 | CHR 5 | 18,034,232 | 18,035,843 | 1612 | 687 | 228 | 4 | 3 | |
| MedtrRNS5 | Medtr5g041080 | CHR 5 | 18,046,846 | 18,048,292 | 1447 | 708 | 235 | 4 | 3 | |
| MedtrRNS6 | Medtr5g041095 | CHR 5 | 18,051,970 | 18,053,262 | 1293 | 687 | 228 | 4 | 3 | |
| MedtrRNS7 | Medtr3g030660 | CHR 3 | 9,731,677 | 9,732,682 | 1006 | 822 | 273 | 2 | 1 | |
| MedtrRNS8 | Medtr3g030640 | CHR 3 | 9,712,341 | 9,713,262 | 922 | 807 | 268 | 2 | 1 | |
| MedtrRNS9 | Medtr6g071425 | CHR 6 | 26,453,813 | 26,454,740 | 928 | 843 | 280 | 2 | 1 | |
| MedtrRNS10 | Medtr8g028080 | CHR 8 | 10,419,405 | 10,425,244 | 5840 | 861 | 286 | 10 | 9 | |
| MedtrRNS11 | Medtr2g021910 | CHR 2 | 7,476,372 | 7,484,903 | 8532 | 2190 | 729 | 13 | 12 | |
| MedtrRNS12 | Medtr5g086770 | CHR 5 | 37,507,709 | 37,510,819 | 3111 | 666 | 221 | 5 | 4 | |
| MedtrRNS13 | Medtr2g021750 | CHR 2 | 7,422,390 | 7,423,599 | 1210 | 1050 | 349 | 3 | 2 | |
| MedtrRNS14 | Medtr2g021830 | CHR 2 | 7,445,199 | 7,445,926 | 728 | 576 | 191 | 2 | 1 | |
| MedtrRNS15 | Medtr5g086410 | CHR 5 | 37,342,035 | 37,342,780 | 746 | 651 | 216 | 2 | 1 | |
| MedtrRNS16 | Medtr2g104370 | CHR 2 | 44,944,607 | 44,945,639 | 1033 | 606 | 201 | 3 | 2 | |
| MedtrRNS17 | Medtr6g071505 | CHR 6 | 26,504,057 | 26,504,969 | 913 | 810 | 269 | 2 | 1 | |
| MedtrRNS18 | Medtr1g048200 | CHR 1 | 18,244,235 | 18,245,342 | 1108 | 918 | 305 | 2 | 1 | |
| MedtrRNS19 | Medtr4g057825 | CHR 4 | 21,322,416 | 21,323,542 | 1127 | 627 | 208 | 2 | 1 | |
| MedtrRNS20 | Medtr6g090200 | CHR 6 | 34,263,142 | 34,263,902 | 761 | 633 | 210 | 2 | 1 | |
| MedtrRNS21 | Medtr5g022810 | CHR 5 | 9,037,425 | 9,038,278 | 854 | 690 | 229 | 3 | 2 | |
| MedtrRNS22 | Medtr2g104300 | CHR 2 | 44,925,214 | 44,925,807 | 594 | 408 | 135 | 2 | 1 | |
| MedtrRNS23 | Medtr5g459500 | CHR 5 | 24,537,707 | 24,538,132 | 426 | 426 | 141 | 1 | – | |
| MedtrRNS24 | Medtr2g104330 | CHR 2 | 44,934,975 | 44,935,274 | 300 | 300 | 99 | 1 | – | |
| MedtrRNS25 | Medtr5g075480 | CHR 5 | 32,099,626 | 32,102,556 | 2931 | 645 | 214 | 3 | 2 | |
| MedtrRNS26 | Medtr5g097710 | CHR 5 | 42,794,078 | 427,994,473 | 396 | 321 | 106 | 2 | 1 | |
| MedtrRNS27 | Medtr6g090230 | CHR 6 | 34,270,969 | 34,272,712 | 1744 | 348 | 106 | 2 | 1 |
RNaseT2 genes in legumes and Arabidopsis have different lengths (Table 1). This variation was due to the differences in the length of the UTRs, number, and the length of their introns and exons. According to this, among RNases T2 in Arabidopsis, RNS2 is the largest gene (2554 bp) with eight introns in large coding regions. Also, GmaRNS6 with 6809 bp and GmaRNS2 with 6797 bp are the largest genes in soybean. GmaRNS6 has eight introns where the first one is in 5′ UTR upstream from the coding region. GmaRNS2 has seven introns within the coding region. GmaRNS10 has no 5′ and 3′ UTRs but contains five exons with four large introns. GmaRNS12 has two introns within the coding region, with no UTRs. PhvuRNS13 does not have UTRs. PhvuRNS5, PhvuRNS7, PhvuRNS9, PhvuRNS10, PhvuRNS11, and PhvuRNS12 do not have UTRs. They have only one small intron within two large exons. In M. truncatula, MedtrRNS10 and MedtrRNS11, despite the absence of UTRs, are large genes due to the number and length of their exon–intron structures. MedtrRNS10 contains nine introns and ten exons and 5840 bp in length. MedtrRNS11 has 13 exons with a length of 8532 bp. MedtrRNS24, MedtrRNS23, LjRNS5, LjRNS6, and LjRNS7 contain only one exon without any intron and UTRs. In fact, their entire gene is an extended coding region (Table 1).
Active-site residues and phylogeny
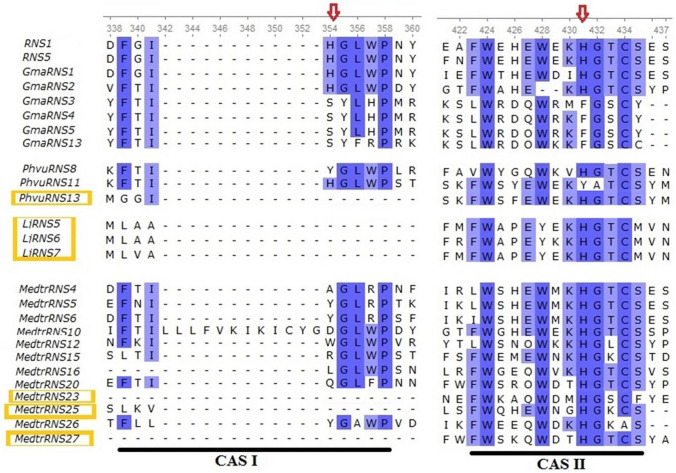
Multiple sequence alignment revealed stretches of the conserved region ranging from the amino acid residues 339–358 (CAS I) and 423–435 (CAS II) as shown in Figs. 1 and 2. Also, some other highly conserved amino acids were seen for many of the sequences (Fig. 1). According to this, in legumes and Arabidopsis, 43 proteins containing two essential catalytic histidines in active site residues (CAS I and CAS II), and 22 proteins (GmaRNS3, GmaRNS4, GmaRNS5, GmaRNS13, MedtrRNS4, MedtrRNS5, MedtrRNS6, MedtrRNS10, MedtrRNS12, MedtrRNS15, MedtrRNS16, MedtrRNS20, MedtrRNS23, MedtrRNS25, MedtrRNS26, MedtrRNS27, PhvuRNS8, PhvuRNS11, PhvuRNS13, LjRNS5, LjRNS6, LjRNS7) were identified without one or two catalytic histidines. These genes could have lost their RNase activity due to a mutation in catalytic histidines at the conserved active sites (Figs. 1, 2). Catalytic histidines in CASI and CASII of GmaRNS3, GmaRNS4, GmaRNS5 and GmaRNS13 were changed to serine (S) and phenylalanine (F), respectively. The conserved histidines in CAS I of PhvuRNS8 and CAS II of PhvuRNS11 were replaced with tyrosine (Y). In CAS I of MedtrRNS4, MedtrRNS10, MedtrRNS12, MedtrRNS15, MedtrRNS16 and MedtrRNS20, the catalytic histidines were to alanine (A), aspartic acid (D), tryptophan (W), arginine (R), leucine (L) and glutamine (Q), respectively. Histidines in CAS I of MedtrRNS5, MedtrRNS6 and MedtrRNS26 were replaced with tyrosine (Y) (Fig. 2). Furthermore, PhvuRNS13, MedtrRNS23, MedtrRNS25, MedtrRNS27, LjRNS5, LjRNS6, and LjRNS7 have entirely lost their CAS I (Fig. 2).
Fig. 1.
Protein sequence alignment of all the RNaseT2 proteins. Residues conserved are shaded. The two conserved active site (CAS) regions and mutations in these sites are indicated, and the two catalytic histidines (No. 354 and 431) are marked with red arrows
Fig. 2.
Mutations in conserved active site residues in plant RNasesT2. The catalytic histidines are demonstrated with red arrows. PhvuRNS13, MedtrRNS23, MedtrRNS25, MedtrRNS27, LjRNS5, LjRNS6 and LjRNS7 proteins have lost their CAS I and showed using a yellow box. Mutation in either one or the two histidines leads to loss of their RNases activity. RNS1, RNS5, GmaRNS1, and GmaRNS2 are active RNases that only are shown for comparison
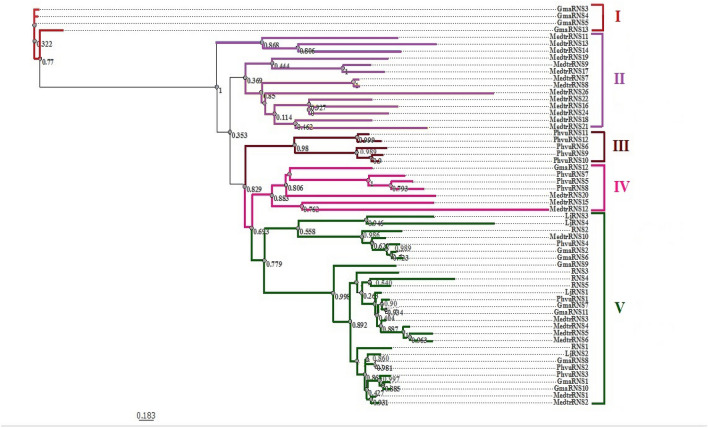
Based on the phylogenetic analysis of the conserved domain of RNaseT2 protein sequences using PhyML maximum-likelihood method, we established that they were distributed into five clades, named I, II, III, IV and V (Soltis and Soltis 2003; Okonechnikov et al. 2012) (Fig. 3). The clade V was the biggest clade, consisting of proteins from all studied species, 5 proteins of A. thaliana, 4 proteins of L. japonicus, 4 proteins of P. vulgaris, 8 proteins of G. max and 7 proteins of M. truncatula. Clades I, II and III included only RNaseT2 proteins of G. max, M. truncatula, and P. vulgaris, respectively (Fig. 3).
Fig. 3.
Phylogenetic tree of the RNaseT2 proteins from G. max, M. truncatula, P. vulgaris, L. japonicus and A. thaliana species based on protein sequences of RNaseT2. Clades I, II, III, IV and V are indicated. Phylogenetic tree made with maximum-likelihood with UGENE program, using PhyML substitution model with 1000 bootstraps and default parameters
Homologs
Conservation analysis and levels of the identity of each sequence to Arabidopsis led to the identification of homologs. According to this, no homology was observed between legumes RNaseT2 with RNS4 in Arabidopsis. However, other RNaseT2 proteins of A. thaliana have homology with some of RNaseT2 proteins in the legumes studied. Among the RNasesT2 of G. max, GmaRNS1, GmaRNS9, and GmaRNS10 have homology to RNS1 (with identity over 47%). GmaRNS2 and GmaRNS6 are homologous to RNS2 (identity of 60%). RNS5 homologs were GmaRNS4, GmaRNS7, GmaRNS8, GmaRNS11, GmaRNS12, and GmaRNS13. GmaRNS12 has homology to both RNS3 and RNS5. In M. truncatula, MedtrRNS1, MedtrRNS2, and MedtrRNS18 have high homology and identity to RNS1, MedtrRNS10 to RNS2, MedtrRNS4 to RNS3, and MedtrRNS3, MedtrRNS5 and MedtrRNS6 to RNS5 of A. thaliana. In L. japonicus, only LjRNS2 had 64% identity with RNS1 and LjRNS1 and 64% identity with RNS5. RNasesT2 of PhvuRNS7 including PhvuRNS2 and PhvuRNS3 have homology to RNS1, PhvuRNS4 to RNS2, PhvuRNS7, PhvuRNS8, PhvuRNS9, PhvuRNS10, PhvuRNS11 and PhvuRNS12 to RNS3 and PhvuRNS1, PhvuRNS5, PhvuRNS6, PhvuRNS8 and PhvuRNS10 to RNS5. Furthermore, PhvuRNS8 and PhvuRNS10 are homologs for both RNS5 and RNS3 (identity > 40%) (Table 2).
Table 2.
The closest homologs to the Arabidopsis RNaseT2 with % identity of homology (homology more than 35%)
| Homologs | % Identity (%) | |
|---|---|---|
| RNS1 | GmaRNS1 | 73 |
| GmaRNS9 | 47 | |
| GmaRNS10 | 69 | |
| PhvuRNS2 | 65 | |
| PhvuRNS3 | 72 | |
| MedtrRNS1 | 67 | |
| MedtrRNS2 | 67 | |
| MedtrRNS18 | 61 | |
| LjRNS2 | 64 | |
| RNS2 | GmaRNS2 | 60 |
| GmaRNS6 | 60 | |
| PhvuRNS4 | 62 | |
| MedtrRNS10 | 58 | |
| RNS3 | GmaRNS12 | 43 |
| MedtrRNS4 | 58 | |
| PhvuRNS7 | 45 | |
| PhvuRNS8 | 42 | |
| PhvuRNS9 | 42 | |
| PhvuRNS10 | 40 | |
| PhvuRNS11 | 38 | |
| PhvuRNS12 | 39 | |
| RNS4 | – | – |
| RNS5 | GmaRNS4 | 37 |
| GmaRNS7 | 74 | |
| GmaRNS8 | 71 | |
| GmaRNS11 | 71 | |
| GmaRNS12 | 43 | |
| GmaRNS13 | 36 | |
| MedtrRNS3 | 67 | |
| MedtrRNS5 | 52 | |
| MedtrRNS6 | 50 | |
| PhvuRNS1 | 74 | |
| PhvuRNS5 | 45 | |
| PhvuRNS6 | 43 | |
| PhvuRNS8 | 42 | |
| PhvuRNS10 | 40 | |
| LjRNS1 | 66 |
The gap line for RNS4, indicates a lack of homology and identity more than 35% with other RNaseT2 proteins
Analysis of RnaseT2 domains, cellular localization, and biochemical features
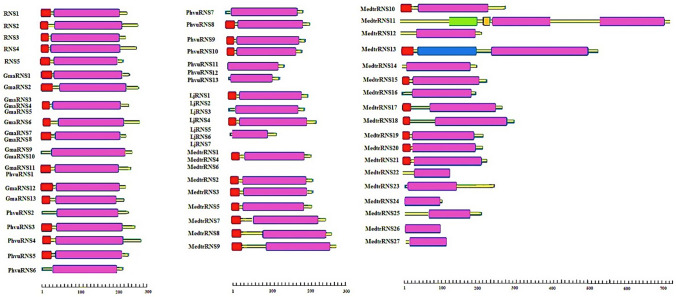
We further investigated protein structure, cellular localization, and biochemical properties of all RNaseT2 amino acid sequences including 60 in legume and 5 in Arabidopsis. As shown in Fig. 4, a clear structural pattern was similar among most RNaseT2 proteins except for a few of them. Most of the RNaseT2 proteins have a signal peptide and RNaseT2 domain. The most prominent difference between these RNaseT2 proteins is the location and length of the domains (Fig. 4). RNaseT2 proteins lacking signal peptide included GmaRNS9 and GmaRNS10 proteins in G. max, MedtrRNS11, MedtrRNS12, MedtrRNS14, MedtrRNS16, MedtrRNS22, MedtrRNS23, MedtrRNS24, MedtrRNS25, MedtrRNS26 and MedtrRNS27 proteins in M. truncatula, PhvuRNS2, PhvuRNS6, PhvuRNS7, PhvuRNS11, PhvuRNS12 and PhvuRNS13 proteins in P. vulgaris, and LjRNS3, LjRNS5, LjRNS6 and LjRNS7 proteins in L. japonicus. Furthermore, among the proteins studied, MedtrRNS13 protein in M. truncatula has an extra domain called thioredoxin. MedtrRNS11, in addition to RNaseT2 domain, encodes two more domains called GTP-binding elongation factor thermal unstable (GTP-EF Tu) and transmembrane helix regions. Moreover, it has two RNaseT2 domains placed after GTP-EF Tu and transmembrane helix region domains (Fig. 4).
Fig. 4.
Depicted protein structure of RNaseT2 proteins. Signal peptide (red box), RNaseT2 domain (purple box), GTP-EFTU domain (green box), transmembrane helix regions (yellow box) and thioredoxin domain (blue box). GmaRNS9, GmaRNS10, MedtrRNS11, MedtrRNS12, MedtrRNS14, MedtrRNS16, MedtrRNS22, MedtrRNS23, MedtrRNS24, MedtrRNS25, MedtrRNS26, MedtrRNS27, PhvuRNS2, PhvuRNS6, PhvuRNS7, PhvuRNS11, PhvuRNS12, PhvuRNS13, LjRNS3, LjRNS5, LjRNS6 and LjRNS7 proteins lack a signal peptide. In addition to, MedtrRNS13 and MedtrRNS11 have unusual domains
The predicted subcellular localization of Arabidopsis and legume RNasesT2 proteins indicated their presence in extracellular and intracellular compartments (Table 3). For example, RNS1, RNS2, and RNS4 were predicted to be mostly present in extracellular and vacuolar space, consistent with the previous report (MacIntosh and Castandet 2020). RNS5 and RNS3 were mostly localised in chloroplasts and cytoplasm, respectively. Besides intracellular portions, GmaRNS7, GmaRNS9, GmaRNS10, GmaRNS11, GmaRNS12, and GmaRNS13 in G. max and PhvuRNS2, PhvuRNS3, PhvuRNS4, PhvuRNS5, PhvuRNS10 and PhvuRNS13 in P. vulgaris were predominantly predicted to be localised in extracellular space. GmaRNS6, PhvuRNS1, PhvuRNS8 and PhvuRNS9 were predicted to be chloroplasts in addition to being extracellular. Most RNases in L. japonicus (LjRNS1, LjRNS2, LjRNS3, LjRNS5, LjRNS6 and LjRNS7), RNS3 in A. thaliana, PhvuRNS6 and PhvuRNS11 in P. vulgaris and GmaRNS3, GmaRNS4 and GmaRNS5 in G. max were located in the cytoplasm. Moreover, some proteins in addition to being extracellular, were predicted to be mostly present in organelles, such as GmaRNS2 in vacuoles, PhvuRNS7 and MedtrRNS4 in the nucleus, MedtrRNS27 in mitochondria. However, some proteins such as RNS5, PhvuRNS11, MedtrRNS6, MedtrRNS11, MedtrRNS12, MedtrRNS14, MedtrRNS22 and MedtrRNS26 were predicted to be only present intracellular space without any extracellular roles (Table 3).
Table 3.
Cellular localization of the RNAseT2 proteins identified in Arabidopsis and legumes
| Proteins | Cellular localization |
|---|---|
| RNS1 | extr: 8, vacu: 4, chlo: 1, plas: 1 |
| RNS2 | extr: 11, vacu: 3 |
| RNS3 | cyto: 6, nucl: 4, chlo: 1, plas: 1, extr: 1, pero: 1 |
| RNS4 | extr: 6, vacu: 6, plas: 1, golg: 1 |
| RNS5 | chlo: 4, E.R.: 3, plas: 2, pero: 2, vacu: 1, golg: 1, cyto_nucl: 1 |
| GmaRNS1 | plas: 3.5, E.R._plas: 3.5, cyto: 3, extr: 3, E.R.: 2.5, nucl: 1, mito: 1 |
| GmaRNS2 | vacu: 7, extr: 6, chlo: 1 |
| GmaRNS3 | cyto: 5, nucl: 3.5, cysk_nucl: 2.5, chlo: 2, mito: 1, extr: 1, golg: 1 |
| GmaRNS4 | cyto: 5, nucl: 3, chlo: 2, extr: 2, cysk: 1, golg: 1 |
| GmaRNS5 | cyto: 4, chlo: 3, nucl: 3, mito: 2, extr: 1, golg: 1 |
| GmaRNS6 | chlo: 6, extr: 5, vacu: 2, nucl: 1 |
| GmaRNS7 | extr: 7, chlo: 6, nucl: 1 |
| GmaRNS8 | E.R.: 3.5, E.R._plas: 3.5, cyto: 3, plas: 2.5, nucl: 2, mito: 1, extr: 1, vacu: 1 |
| GmaRNS9 | extr: 5, nucl: 2, cyto: 2, vacu: 2, chlo: 1, mito: 1, E.R.: 1 |
| GmaRNS10 | extr: 5, E.R.: 2.5, E.R._plas: 2.5, plas: 1.5, nucl: 1, cyto: 1, mito: 1, vacu: 1, golg: 1 |
| GmaRNS11 | extr: 8, vacu: 3, golg: 2, plas: 1 |
| GmaRNS12 | extr: 7, vacu: 5, plas: 1, golg: 1 |
| GmaRNS13 | extr: 12, cyto: 1, plas: 1 |
| PhvuRNS1 | chlo: 6, extr: 6, nucl: 1, cyto: 1 |
| PhvuRNS2 | extr: 3, E.R.: 3, nucl: 2, cyto: 2, plas: 2, mito: 1, golg: 1 |
| PhvuRNS3 | extr: 8, vacu: 2, E.R.: 2, mito: 1, plas: 1 |
| PhvuRNS4 | extr: 7, chlo: 3, cyto: 2, nucl: 1, vacu: 1 |
| PhvuRNS5 | extr: 11, vacu: 2, plas: 1 |
| PhvuRNS6 | cyto: 9.5, cyto_E.R.: 5.5, nucl: 1, mito: 1, extr: 1, golg: 1 |
| PhvuRNS7 | nucl: 4, cyto: 4, extr: 2, pero: 2, chlo: 1, plas: 1 |
| PhvuRNS8 | chlo: 11, extr: 3 |
| PhvuRNS9 | chlo: 4, extr: 4, nucl: 3, cyto: 1, plas: 1, E.R.: 1 |
| PhvuRNS10 | extr: 9, nucl: 2, cyto: 1, vacu: 1, E.R.: 1 |
| PhvuRNS11 | cyto: 8, chlo: 5, nucl: 1 |
| PhvuRNS12 | nucl: 9.5, cyto_nucl: 6, cyto: 1.5, chlo: 1, mito: 1, extr: 1 |
| PhvuRNS13 | extr: 7, chlo: 2, cyto: 2, nucl: 1, cysk: 1, golg: 1 |
| LjRNS1 | chlo: 6, nucl: 3, extr: 3, cyto: 1, plas: 1 |
| LjRNS2 | cyto: 3, extr: 3, E.R._plas: 3, plas: 2.5, E.R.: 2.5, nucl: 2, mito: 1 |
| LjRNS3 | cyto: 5, nucl: 4, chlo: 3, extr: 1, vacu: 1 |
| LjRNS4 | extr: 10, golg: 2, plas: 1, vacu: 1 |
| LjRNS5 | cyto: 5, extr: 5, chlo: 3, nucl: 1 |
| LjRNS6 | chlo: 7, mito: 3, nucl: 1, cyto: 1, extr: 1, cysk: 1 |
| LjRNS7 | cyto: 5, extr: 5, chlo: 3, nucl: 1 |
| MedtrRNS1 | plas: 3.5, E.R._plas: 3.5, cyto: 3, E.R.: 2.5, nucl: 2, extr: 2, mito: 1 |
| MedtrRNS2 | extr: 6, vacu: 3, E.R.: 2, nucl: 1, mito: 1, plas: 1 |
| MedtrRNS3 | extr: 6, chlo: 5, cyto: 2, nucl: 1 |
| MedtrRNS4 | cyto_nucl: 6.5, nucl: 6, chlo: 3, cyto: 3, extr: 2 |
| MedtrRNS5 | cyto_nucl: 6, nucl: 4, cyto: 4, chlo: 3, mito: 1, extr: 1, pero: 1 |
| MedtrRNS6 | chlo: 12, mito: 2 |
| MedtrRNS7 | extr: 6, chlo: 4, vacu: 2, cyto: 1, plas: 1 |
| MedtrRNS8 | chlo: 5, extr: 5, vacu: 2, cyto: 1, plas: 1 |
| MedtrRNS9 | extr: 7, golg: 3, vacu: 2, cyto: 1, plas: 1 |
| MedtrRNS10 | extr: 8, vacu: 4, mito: 1, golg: 1 |
| MedtrRNS11 | E.R.: 4.5, E.R._plas: 3.5, nucl: 3, cyto: 2, plas: 1.5, mito: 1, vacu: 1, pero: 1 |
| MedtrRNS12 | cyto: 9.5, cyto_nucl: 7, mito: 2, plas: 1 |
| MedtrRNS13 | extr: 6, vacu: 2, E.R.: 2, golg: 2, chlo: 1, plas: 1 |
| MedtrRNS14 | chlo: 7, mito: 6.5, cyto: 1 |
| MedtrRNS15 | vacu: 6, extr: 5, chlo: 1, nucl: 1, plas: 1 |
| MedtrRNS16 | cyto: 6, nucl: 4, mito: 1, plas: 1, extr: 1, cysk: 1 |
| MedtrRNS17 | extr: 9, cyto: 2, nucl: 1, vacu: 1, mito 1, plas: 1 |
| MedtrRNS18 | vacu: 6, extr: 4, plas: 2, mito: 1, E.R.: 1 |
| MedtrRNS19 | chlo: 9, cyto: 2, extr: 2, E.R.: 1 |
| MedtrRNS20 | extr: 9, chlo: 1, mito: 1, plas: 1, vacu: 1, E.R.: 1 |
| MedtrRNS21 | extr: 5, vacu: 3, plas: 2, golg: 2, chlo: 1, nucl: 1 |
| MedtrRNS22 | nucl: 6.5, cyto_nucl: 4, mito: 3.5, cyto_mito: 2.5, chlo: 2, plas: 1 |
| MedtrRNS23 | cyto: 5, chlo: 4, extr: 3, nucl: 1, plas: 1 |
| MedtrRNS24 | cyto: 9, nucl: 3, extr: 2 |
| MedtrRNS25 | chlo: 5, cyto: 3, nucl: 2, mito: 1, extr: 1, vacu: 1, E.R.: 1 |
| MedtrRNS26 | chlo: 10, cyto: 3, nucl: 1 |
| MedtrRNS27 | mito: 6, cyto: 5, chlo: 1, nucl: 1, extr: 1 /cyto: 8, extr: 4, plas: 1, cysk: 1 |
Endoplasmic reticulum (ER), mitochondria (mito), chloroplasts (chlo), cytoplasm (cyto), nuclear (nucl), vacuole (vacu), Golgi (golg), proxysome (pro), plastid (plas) and extra cellular (extr)
The biochemical features for these RNasesT2 were presented in Table 4. The pI value is the pH at which a molecule carries no net electrical charge (Skoog and Wichman 2013). The theoretical pI of all Arabidopsis RNaseT2 proteins was acidic (5.08–5.98), while the theoretical pI of legume RNaseT2 varied from acidic to alkaline (4.39–9.28). Twenty-eight proteins were acidic (4.39–6.86), and 32 were alkaline (7.11–9.28). The theoretical pI average of RNaseT2 proteins was almost neutral (6.99). The highest was predicted for MedtrRNS18 with 9.28 and the lowest associated with MedtrRNS23 with 4.39. The instability index (II) indicates a half-life or stability of proteins (Ikai 1980; Gasteiger et al. 2005; Rawlings et al. 2010). Proteins with an instability index less than 40 are stable and larger than 40 are unstable (Gasteiger et al. 2005). The instability index of Arabidopsis proteins was more than 40 (40.23–48.11). In legumes, instability index ranged from 17.28 (MedtrRNS26) to 59.47 (MedtrRNS18). The average instability index of the proteins studied in this study was 40.64 (Table 4).
Table 4.
Biochemical features of RNaseT2 protein sequences from A. thaliana, G. max, M. truncatula, p. vulgaris and L. japonicas
| Species | Gene name | Theoretical isoelectric point (pI) | Instability index |
|---|---|---|---|
| RNS1 | 5.08 | 40.23 | |
| RNS2 | 5.98 | 43.43 | |
| A. thaliana | RNS3 | 5.71 | 48.11 |
| RNS4 | 5.86 | 41.72 | |
| RNS5 | 5.08 | 40.46 | |
| GmaRNS1 | 4.5 | 42.82 | |
| GmaRNS2 | 6.57 | 52.7 | |
| GmaRNS3 | 7.49 | 42.77 | |
| GmaRNS4 | 6.3 | 45.92 | |
| GmaRNS5 | 7.49 | 45.92 | |
| GmaRNS6 | 7.46 | 39.22 | |
| G. max | GmaRNS7 | 5.31 | 43.32 |
| GmaRNS8 | 5.46 | 39.2 | |
| GmaRNS9 | 6.41 | 37.19 | |
| GmaRNS10 | 4.82 | 47.04 | |
| GmaRNS11 | 4.94 | 45.32 | |
| GmaRNS12 | 8.66 | 34.68 | |
| GmaRNS13 | 5.75 | 40.05 | |
| PhvuRNS1 | 5.34 | 42.59 | |
| PhvuRNS2 | 6.57 | 41.05 | |
| PhvuRNS3 | 4.82 | 42.22 | |
| PhvuRNS4 | 5.21 | 53 | |
| PhvuRNS5 | 7.11 | 37.08 | |
| PhvuRNS6 | 6.33 | 39.22 | |
| P. vulgaris | PhvuRNS7 | 8.3 | 29.45 |
| PhvuRNS8 | 7.52 | 34.3 | |
| PhvuRNS9 | 8.89 | 29.71 | |
| PhvuRNS10 | 8.92 | 36.06 | |
| PhvuRNS11 | 7.56 | 54.04 | |
| PhvuRNS12 | 8.99 | 42.14 | |
| PhvuRNS13 | 8.56 | 54.03 | |
| LjRNS1 | 4.74 | 48.91 | |
| LjRNS2 | 4.82 | 38.31 | |
| LjRNS3 | 8.8 | 29.48 | |
| L. japonicas | LjRNS4 | 7.52 | 25.85 |
| LjRNS5 | 4.99 | 24.41 | |
| LjRNS6 | 8.88 | 33.24 | |
| LjRNS7 | 4.81 | 24.92 | |
| MedtrRNS1 | 4.84 | 41.34 | |
| MedtrRNS2 | 5.6 | 38.72 | |
| MedtrRNS3 | 4.79 | 47.26 | |
| MedtrRNS4 | 8.44 | 52.71 | |
| MedtrRNS5 | 5.79 | 42.06 | |
| MedtrRNS6 | 6.86 | 38.79 | |
| MedtrRNS7 | 8.83 | 45.08 | |
| MedtrRNS8 | 8.83 | 43.19 | |
| M. truncatula | MedtrRNS9 | 7.66 | 38.84 |
| MedtrRNS10 | 6.08 | 46.59 | |
| MedtrRNS11 | 7.77 | 46.82 | |
| MedtrRNS12 | 8.14 | 36.75 | |
| MedtrRNS13 | 8.57 | 30.15 | |
| MedtrRNS14 | 7.61 | 40.92 | |
| MedtrRNS15 | 8.34 | 42.83 | |
| MedtrRNS16 | 8.92 | 35.22 | |
| MedtrRNS17 | 8.25 | 44.99 | |
| MedtrRNS18 | 9.28 | 59.47 | |
| MedtrRNS19 | 8.51 | 35.84 | |
| MedtrRNS20 | 6.86 | 30.09 | |
| MedtrRNS21 | 7.57 | 26.56 | |
| MedtrRNS22 | 7.7 | 45.31 | |
| MedtrRNS23 | 4.39 | 42.09 | |
| MedtrRNS24 | 8.60 | 49.85 | |
| MedtrRNS25 | 5.54 | 38.05 | |
| MedtrRNS26 | 5.20 | 17.28 | |
| MedtrRNS27 | 8.72 | 26.51 |
Bold italic is basic RNasesT2 and italic color is acidic RNasesT2
In silico analysis of the RNaseT2 gene family expression profile in legumes and Arabidopsis
RNaseT2 genes exist in the genome of all organisms. Analysis of gene expression indicated that these genes were expressed in different tissues and developmental stages; however, the levels of accumulation varied among the different tissues (MacIntosh et al. 2010). Also, RNaseT2 genes are regulated with biotic and abiotic stresses (Luhtala and Parker 2010). To investigate the expression patterns of RNaseT2 genes between different organs and compare them with Arabidopsis RNaseT2 genes, expression data of four legumes were retrieved from relevant databases. Expression data were shown as heat maps for each species (Fig. 5). Four genes of A. thaliana were expressed in almost every tissue. RNS2 gene has the highest expression level among all Arabidopsis RNaseT2 genes in the studied tissues. The level of RNS1 transcript was the highest in 3-weeks-old seeds and flowers compared to other tissues (Fig. 5a).
Fig. 5.
Gene expression profiles of RNaseT2 in four legumes and A. thaliana. Transcriptom data of RNaseT2 genes were obtain from the BAR resource for A. thaliana (a), G. max (b) and M. thruncatula (e); Lotus Base for L. japonicus (c); PvGEA for P. vulgaris (d). Heat map of RNaseT2 genes expression profiles obtain for each plant separately. RPKM values are represented by color key bottom of each heat map. (d: day, w: week, HAI: hours after inoculation, WT: wild type)
In G. max, GmaRNS6 is the only gene expressed in all the tissues studied at a considerable level, and GmaRNS10 was the only gene with no expression in any of the tissues. Most RNaseT2 genes had no expression in nodules of soybean except for GmaRNS3, GmaRNS4, GmaRNS5, and GmaRNS6, which have a moderate expression level. Furthermore, these genes were the only ones that were expressed simultaneously in the root and nodules. GmaRNS9 was expressed exclusively in the root, root tip, and root hair (Fig. 5b). On average, soybean RNaseT2 were more expressed in the roots and root hair compared to other tissues.
In L. japonicus, expression data were only available for four RNaseT2 genes, including LjRNS1, LjRNS2, LjRNS4, and LjRNS5 at Lotus Base server. They were expressed in the different tissues and had a high level of expression in 20-day-old seeds, flowers, and flowers under 5 mM nitrate treatment. Both LjRNS1, LjRNS2 had a considerable expression in almost all the tissues studied (Fig. 5c). In P. vulgaris, PhvuRNS1, PhvuRNS2, PhvuRNS3, and PhvuRNS4 were highly expressed in all studied tissues. PhvuRNS6 expression was limited to 140-mg seeds and PhvuRNS11 and PhvuRNS12 were only expressed in 12-day roots. A significant expression level of PhvuRNS8 was demonstrated in 140 mg-Seed (under treatment of NO3-Fertilizer). However, the PhvuRNS6, PhvuRNS9, and PhvuRNS10 had no expression in the tissues evaluated. In general, it can be observed that the expression level of RNaseT2 genes in P. vulgaris were low compared with other genes of this family in other plants studied (Fig. 5d).
Moreover, among all 27 RNaseT2 genes in M. truncatula, only expression data of 8 genes were available in BAR resource. All demonstrated genes of M. truncatula in Fig. 5e, was considerably expressed in all the tissues studied except for MedtrRNS20 and MedtrRNS21, which expression mostly limited to 36-day seed and mature nodule. In addition, the MedtrRNS3 showed the highest level of expression in all tissues by a very large margin. MedtrRNS1, MedtrRNS3, MedtrRNS5, and MedtrRNS25 were almost expressed at the same level in root and nodule (Fig. 5e). These expression patterns could indicate a hypothesized conservation of RNaseT2 gene function between legumes and Arabidopsis. Also, the expression of these genes in root, root hair, and nodule, indicating that they could have a role in nodulation. These expression patterns of some RNaseT2 genes in legume species suggested a putative role of these genes in legumes.
Quantitative expression of GmaRNS9 gene in G. Max in nutritional stress and nodulation
According to our previous study, GmaRNS9 had significant expression in the root tips compared to the shoot tip of soybean (Mirzaei et al. 2014, 2017). Moreover, according to in silico expression analysis, GmaRNS9 was expressed exclusively in root and root hairs. So, we selected GmaRNS9 gene for further studies. To evaluate the possible role of NARK gene and nutrients in the expression of this gene, we analyzed the GmaRNS9 expression in soybean root tip under nutrient stress (nitrate and phosphate starvation) and nodulation treatments in root tip of a wild type (commercial cultivar Bragg (maturity group 4) and its near-isogenic supernodulating mutant, nts382 (deficient in systemic AON).
GmaRNS9 expression is affected in the root tip by nodulation
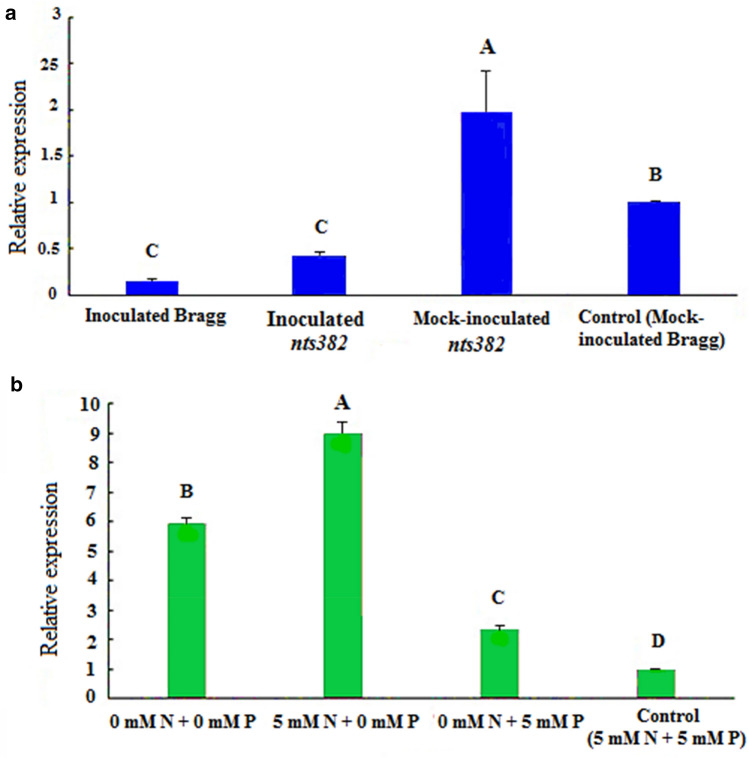
Steady-state mRNA level of GmaRNS9 was investigated in root tip samples of inoculated and un-inoculated soybean plants. First, roots of planted soybeans were examined for the presence and absence of nodules. It was observed that the roots of mock-inoculated plants with B. japonicum were completely free of nodules, but on the roots of the inoculated plants, the nodules were formed (Fig. 6). GmaRNS9 was expressed in all samples but at various levels (Fig. 7). Its expression was found to be in the highest and lowest in mock-inoculated nts382 and inoculate Bragg, respectively.
Fig. 6.
Root phenotype and nodule formation of the soybean wild type, Bragg, and nts382 mutant in presence and absence of B. japonicum (CB 1809)
Fig. 7.
Expression analysis of GmaRNS9 gene at the root tip of soybean. a Expression of GmaRNS9 gene at the root tip of soybean Bragg and nts382 affected by nodulation. b Expression of GmaRNS9 gene at root tip of soybean Bragg in nitrate and phosphate stresses. Error bars indicate SE
Interestingly, GmaRNS9 expression was higher in both un-inoculated Bragg and nts382 mutant compared to the inoculated ones. GmaRNS9 expression in mock-inoculated nts382 was about five times higher than inoculated nts382 and 14 times higher than inoculated Bragg. GmaRNS9 was expressed in mock-inoculated nts382, about 2 times higher than control (mock-inoculated Bragg) (Fig. 7a). Duncan’s mean comparison test showed a significant difference in the expression of this gene in the soybean samples. According to this, expression of GmaRNS9 gene in the root tip of mock-inoculated nts382 had a significant difference compared to the control (mock-inoculated Bragg) and other samples (P ≤ 0/05). Also, GmaRNS9 expression was reduced in inoculated Bragg compared to the control (mock-inoculated Bragg), and it was statistically significant (P ≤ 0/05). However, there was no statistically significant difference in GmaRNS9 expression in inoculated nts382 compared to inoculated Bragg. In general, after inoculation with B. japonicum, GmaRNS9 expression was significantly reduced in both Bargg and nts382 (Fig. 7a).
GmaRNS9 expression is impacted in root tip under nitrate and phosphate stress
Environmental stresses are the obstacle for the growth and yield of plants. As a legume plant, Soybean can accumulate phosphorus and especially nitrogen nutrients through biological nitrogen fixation system (Wang et al. 2015). Abiotic stresses such as nutrients stress negatively affect soybean production (Schmutz et al. 2010). On the other hand, RNaseT2 gene family members were expressed in biotic and abiotic stresses, particularly nutrient stress (MacIntosh et al. 2010). These genes act in phosphate starvation, and when phosphorus is limiting, they can scavenge phosphates from ribonucleotides (Kock et al. 2006; Luhtala and Parker 2010; MacIntosh et al. 2010). Hence, we examined the expression pattern of GmaRNS9 in root tips under nutrient stressed (0 phosphorous, 0 nitrogen) and normal conditions. We observed that GmaRNS9 exhibited significantly higher expression under nutrient stress treatment compared to the control (treated with 5mMN + 5mMP). Interestingly, the highest level of GmaRNS9 expression was observed under phosphate starvation compared to other treatments. Its expression in simultaneous nitrate and phosphate starvation, phosphate starvation alone, and nitrate starvation alone increased by about six, nine and two times compared to the control (treated with 5mMN + 5mMP), respectively. In other words, nutrient stress, especially phosphate, led to a significant increase in the expression of GmaRNS9 (Fig. 7b).
Discussion
The RNaseT2 family is known as transferase type RNases and is highly conserved among all organisms. In plant, RNaseT2 genes are divided into two main groups, S-RNase and S-like RNase, and involve in a variety of biological functions, including production of tRNA-derived sRNAs, participation in the cellular housekeeping salvage pathway, defense activities and function in gametophytic self-incompatibility in several plant families (Irie 1999; Deshpande and Shankar 2002; MacIntosh and Castandet 2020). Previous reports have analyzed the evolutionary relationship of RNaseT2 genes in plants such as rice and Arabidopsis (Igic and Kohn 2001; MacIntosh et al. 2010). Nevertheless, there has been no genome-wide analysis of RNaseT2 genes in the legume family, which is important in terms of feed, food and biofuel crops. Also, legumes acquire nitrogen through the mechanism of nodulation and atmospheric nitrogen fixation, which reduce agricultural dependence on nitrogen fertilizers (Mirzaei et al. 2017). Furthermore, the effect of nitrogen and phosphorus stress and nodulation on these genes in legumes are still not clear. Therefore, genome-wide identification and comprehensive phylogenetic study of the RNaseT2 family in legume species will yield new information about the functions of these proteins.
In this study, a total of 60 RNaseT2 genes were identified in four legume species, and in each legume species, RNaseT2 gene number varied from seven (L. japonicus) to 27 (M. truncatula) (Table 1). Except for Lotus with 7 RNaseT2 genes and comparable to the number of Arabidopsis RNaseT2 genes, the number of RNaseT2 genes in other legumes studied was two to three times higher than Arabidopsis. This was likely because legume genomes have been shaped by extensive large-scale gene duplications (Roalson and McCubbin 2003; Schmutz et al. 2010). In addition, this variation of number in legume species demonstrates that genome size does not explain the difference in the number of RNaseT2 genes in legume species. G. max, which has the largest genome size among species studied (about 1.1–1.15 Gb), contains 13 RNaseT2 genes, while M. truncatula with a genome of 860 Mb contains 27 RNaseT2 genes (Chen et al. 2020; Shultz et al. 2006).
Our phylogenetic analyses indicated that RNaseT2 proteins were divided into five clades. According to the phylogenetic tree, S-like RNases clustered in one clade, clade V, which was consistent with other studies (Moller et al. 1987; Igic and Kohn 2001). S-RNases were distributed in clades I–IV. The study of the genomic characteristics and intron–exon structure of the RNaseT2 genes suggested that the RNases in clade V had the highest number of exons (8–10 exons) except for LjRNS3 and LjRNS4 (Igic and Kohn 2001; MacIntosh et al. 2010). However, other clades had genes with two or three exons, except for MedtrRNS11 with 13 exons and MedtrRNS24 with only one exon in clade II and MedtrRNS12 with five exons in clade IV (Table 1; Fig. 3).
Generally, the highest homology and identity of RNaseT2 proteins in the legumes studied with Arabidopsis was seen in clade V which have similar characteristics and are also related to S-like RNases (MacIntosh et al. 2010; MacIntosh and Castandet 2020). All RNasesT2 of A. thaliana in this clade are conserved and have both CASI and CAS II (Taylor and Green 1991) (Fig. 1). However, in this clade, some of RNaseT2 proteins of legumes lack one or two active histidines in CASI and CASII (Fig. 2). While almost all proteins of clade II (except for MedtrRNS16 and MedtrRNS26) and clade III have both conserved residues. In clade I, all proteins lack two active histidines. These observations suggested that RNaseT2 genes in legume and Arabidopsis probably originated from a common ancestor, but in some cases in legumes, they lost one or both conserved histidine residues during evolution due to genome duplications and mutation events (MacIntosh et al. 2010) (Table 2; Figs. 2, 3, 4).
RNaseT2 are secretory proteins, and signal peptides would target these proteins to the secretory pathway (Sato and Egami 1957). According to the phylogenetic tree, proteins with similar or the same domains are placed together in one clade. For instance, all RNaseT2 proteins in clade I have a signal peptide and RNaseT2 domain. Also, most proteins in clades IV and V have a signal peptide and RNaseT2 domain except for MedtrRNS12 and PhvuRNS7 in clade IV and LjRNS3, PhvuRNS2, GmaRNS9 GmaRNS10 in clade V. However, some of RNaseT2 proteins only have RNaseT2 domain but lack signal peptide, such as the ones in clades II and III (Figs. 1, 4). It was anticipated that these proteins might have lost their ability to secrete and be localized. In addition, we found novel domains, GTP-EF Tu and transmembrane helix regions in MedtrRNS11 and thioredoxin domain in MedtrRNS13. GTP-EF Tu is a domain with GTP binding, and GTPase activity is involved in conformational alteration mediated by the hydrolysis of GTP to GDP in the elongation phases of mRNA translation (Moller et al. 1987). The transmembrane helix region is a membrane-spanning domain with a hydrogen-bonded helical configuration. Most transmembrane segments usually contain alpha helix transmembrane domains (TMDs) and are significantly enriched in aliphatic hydrophobic residues. TMDs, known as integral proteins, also span the hydrophobic core of the lipid bilayer (Sharpe et al. 2010). In addition, two RNaseT2 domains were found in MedtrRNS11, indicating that it may be related to segmental duplication that occurred in M. truncatula. MedtrRNS13 protein has an unusual domain called thioredoxin (Fig. 4). Thioredoxin is a class of small redox proteins that have been found in all living organisms. It plays a role in many critical biological processes, including redox signalling (Holmgren and Bjornstedt 1995; Holmgren 1989). Generally, the identification of extra domains in some RNaseT2 proteins is interesting and may give them some new functions that need further investigation.
Moreover, it could be an artefact of genome assembly or annotation. Our work was in silico, and the results obtained from the domains of RNaseT2 were based on Smart and SignalP databases. Therefore, qRT-PCR analysis could be useful for more accurate identification.
Most RNaseT2 enzymes are secretory RNases and therefore, they are found extracellularly or in compartments of the endomembrane system that would minimize their contact with cellular RNA (MacIntosh et al. 2001). In addition, it has also been shown that OsRNS3 and OsRNS2 of rice and RNS2 of Arabidopsis are intracellular enzymes that are located in the vacuole, the ER, or both (MacIntosh et al. 2010; MacIntosh and Castandet 2020). We found that RNasesT2 of Arabidopsis and the legumes studied were present in different cellular portions and extracellularly, consistent with previous studies (MacIntosh et al. 2001; Thompson and Parker 2009; Huang et al. 2015). The phylogenetic tree showed that RNasesT2 placed in clade III have no signal peptide and were located in intracellular portions (Table 3; Figs. 3, 4). In addition, based on domain information, cellular localization, and phylogenetic studies, it was possible that the lack of a signal peptide, which was observed in some of RNasesT2, was related to their intracellular position. Interestingly, GmaRNS9 without signal peptide (in clade V) in addition to be intracellular, were predicted to be extracellular proteins. However, based on the observed results of PSORT, it could be expected that RNasesT2 in legumes are mostly extracellular or present in nuclear, vacuoles, chloroplasts, and cytosol (Table 3; Fig. 3). These RNases subfamily may participate in conserved cellular damage mechanisms and determine cell survival process (Thompson and Parker 2009).
Generally, the RNaseT2 family has members with a wide range of pH preferences (MacIntosh et al. 2010; Ramanauskas and Igic 2017). Our results showed that clade V which consisted of S-like RNases are acidic with pI less than 7. Only GmaRNS6, LjRNS3, and LjRNS4 in this clade are basic RNases. Besides, clades, I, II, III and IV which contain the S-RNases are mostly basic (with an average pI of 7.86). However, only MedtrRNS20 (clade IV), MedtrRNS26 (clade II), PhvuRNS6 (clade III), GmaRNS4 and GmaRNS13 (clade I) have acidic pI (Table 4; Fig. 3). This indicated that legume RNasesT2 also had wide range of pH preferences and this was consistent with previous reports on the plant RNaseT2 family. Irie (1999) showed that S-like RNase proteins are mostly acidic RNases. Furthermore, it was observed that S-RNases are basic proteins (Deshpande and Shankar 2002; Cruz‐Garcia et al. 2003). The computed average of pI for the RNaseT2 studied was 6.99 (nearly 7). Therefore, it seemed that these proteins could precipitate in alkaline buffers. Moreover, most clades I and V proteins have an instability index of more than 40 and were predicted to be unstable. RNasesT2 of the legumes studied in clades III and IV were expected to be stable (instability index < 40) except for PhvuRNS11 and PhvuRNS12 (in clade III), and MedtrRNS15 (in clade IV) (Table 4; Fig. 3).
The analysis of gene expression of yeast and plant RNasesT2 indicates that they are expressed constitutively in all tissues and during developmental stages (Taylor et al. 1993; MacIntosh et al. 2001, 2010; Hillwig 2009; Huang et al. 2015). Also, these enzymes could have a housekeeping role (MacIntosh et al. 2010). In this study, we conducted an in silico analysis of RNasesT2 gene expression profiles in four legumes and A. thaliana and observed that all genes were expressed in various tissues and had high expression levels in some tissues such as root, seed and flower (Fig. 5). This suggested the potential roles of RNasesT2 genes in plant growth and development and a vital function for RNases that has preserved these enzymes throughout evolution.
According to the phylogenetic tree and expression of genes obtained from databases, it could be argued that all genes in clade I (RNasesT2 genes of G. max) showed more accumulation level in nodules and roots. The expression data of almost all RNasesT2 genes in clade II was not yet available in gene expression databases, indicating their potential for further studies. As the largest clade, Clade V contained genes with very high expression level in almost all tissues and had the same expression pattern as each other genes and with their homologous genes of Arabidopsis, which were in the same clade. This suggests that the expression patterns of these were conserved between Arabidopsis and the legume species studied. Gene expression levels were most significant in the roots of P. vulgaris and soybeans, and nodules from M. truncatula. Therefore, it could be explained that these genes are likely involved in the process of rooting and nodulation in legume plants. However, the high accumulation of gene expression in L. japonicus flower tissues, suggesting additional function for RNasesT2 genes in this species (Figs. 3, 5).
In addition to in silico studies, special laboratory research is needed to more accurately identify the RNaseT2 genes in the legumes and the roles and patterns of expression of these genes in normal or stress conditions. So, we analyzed the effect of nodulation, and nutrient stresses (phosphate and nitrate starvation) on the expression of GmaRNS9 in the roots tip of two soybean types (WT Bragg and nts382, supernodulating nark mutant). GmaRNS9 expression was influenced by nodulation and nutrient stresses (Fig. 5). GmaRNS9 was expressed in mock-inoculated Bragg and nts382. However, its expression was significantly higher in nts382 compared to WT Bragg (Fig. 7a). Interestingly, GmaRNS9 expression was significantly suppressed by inoculation with B. japonicum in both Bragg and nts382 (Fig. 7a). The GmNARK is the main nodule auto-regulation receptor kinase in soybean. It belongs to the LRR receptor kinase protein family and functions in controlling nodule numbers (Mirzaei et al. 2014, 2017). The nts382 is defective in its GmNARK and cause a supernodulating phenotype (Gresshoff et al. 2015). Collectively, these findings suggested that the GmaRNS9 expression was likely to be controlled by the NARK gene. Furthermore, we predicted that the expression of this gene could also be affected by nod factors, which might mean that this factors may have a suppressing effect on the expression of this gene.
RNaseT2 genes are involved in scavenging phosphates from ribonucleotides in phosphate starvation (Nurnberger et al. 1990; Abel et al. 2000). In this study, we found that phosphate and nitrate deficiency leaded to induction of GmaRNS9 expression in the root tip of soybean. GmaRNS9 expression was upregulated in response to phosphate starvation (P: 0 mM/N: 5 mM) up to nine times than the control (P: 5 mM/N: 5 mM). However, GmaRNS9 expression was also higher in plant under nitrate deficiency alone (P: 5 mM/N: 0 mM) but it was significantly lower (up to two times) than the control (P: 5 mM/N: 5 mM). These findings suggest that GmaRNS9 may involve in degrading and cleaving RNA to provide P and N for plant. Our results were consistent with the function of RNaseLE and RNase LX in tomato, RNS1, and RNS2 in Arabidopsis and PvRNS3 in P. vulgaris (Luhtala and Parker 2010; MacIntosh and Castandet 2020; Diaz-Baena et al. 2021).
In our study, the result of the multiple alignments of the conserved regions showed that a number of proteins in G. max, P. vulgaris and M. truncatula could lose their RNase activity due to mutation in conserved His in the active sites (CASI and/or CASII) (Figs. 1, 2). However, these genes are homologous to the Arabidopsis RNaseT2 genes, which are fully conserved in the CASI and CASII regions. So, these observations suggested that these alterations could be due to mutation or resulted from several duplications during the evolution of RNaseT2 in legumes. According to our and also previous studies, lack of RNase activity is not related to biological roles of these genes under biotic and abiotic stresses, nodulation and nitrogen fixation in legumes. For instance, OsRNS4, OsRNS5, and OsRNS7 in rice have defense responses while losing most conserved residues and RNases activity (MacIntosh et al. 2010).
Moreover, other studies have revealed that the ribonuclease activity is unnecessary for the antimicrobial roles of several members of the RNase A family in animals (Rosenberg 1995; Torrent et al. 2009). In fact, these biological roles are probably due to their protein properties such as pI, cellular localization, signal peptides, and domains, not their RNase activity. In general, future laboratory studies including qRT-PCR in legumes can help us better understand the expression patterns of these genes and their biological roles in stresses, nodulation process, and nitrogen fixation.
Overall, 60 RNaseT2 gene were identified in four legume species, with 13 genes in G. max, 27 in M. truncatula, 13 in P. vulgaris, and seven in L. japonicus. They belong to S-RNase and S-like RNase subfamily. All S-like RNases are acidic and were clustered in one clade, clade V. S-RNases are mostly basic and placed in four clades, I to IV. Our results demonstrated a great degree of diversification for RNaseT2 genes in the species studied. In addition to RNaseT2 domain, we found three novel domains, GTP-EF Tu and transmembrane helix regions in MedtrRNS11, thioredoxin domain in MedtrRNS13. Some of legume RNaseT2 lost one or both conserved histidine residues in the active sites (CASI and CASII) during evolution due to genome duplications and mutations events (Figs. 1 and 2). Moreover, they were dissimilar in biochemical features and expressed more under stress in pod, flower, and root tissues of legumes. In addition, RNasesT2 of the legumes studied were present in different cellular compartments and extracellularly.
Furthermore, legume RNaseT2 genes were expressed in different conditions and tissues and had the highest expression level in some tissues such as root, seed, and flower (Fig. 5). Expression of GmaRNS9, an S-like RNase, was influenced by nodulation and nutrient stresses. We suggested that GmaRNS9 expression was likely to be controlled by the NARK gene as well as nod factors. GmaRNS9 expression was upregulated in response to phosphate starvation, suggesting GmaRNS9 may be involved in degrading and cleaving RNA to provide P and N for the plant. Since the legumes come in a symbiosis relationship with bacteria collectively called rhizobium for nitrogen fixation, it would be valuable to study the function of these genes in this relationship (Libault et al. 2010b; Mirzaei and Azizkhani 2019).
Acknowledgements
The authors acknowledge to Institute of Science and High Technology and Environmental Sciences and Graduate University of Advanced Technology for providing necessary support and facilities to carry out the present research work.
Author contributions
SM contributed to the conception, interpretation, and supervision of the research. MT-M contributed to supervision of the research. NA conducted experiments and evaluated results. SM. and NA contributed to the writing and editing of the manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Contributor Information
Negin Azizkhani, Email: n.azizkhani@student.kgut.ac.ir.
Saeid Mirzaei, Email: s.mirzaei@kgut.ac.ir.
Masoud Torkzadeh-Mahani, Email: m.torkzadeh@kgut.ac.ir.
References
- Abel S, Nurnberger T, Ahnert V, Krauss G-J, Glund K. Induction of an extracellular cyclic nucleotide phosphodiesterase as an accessory ribonucleolytic activity during phosphate starvation of cultured tomato cells. Plant Physiol. 2000;122(2):543–552. doi: 10.1104/pp.122.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KL, Collins K. Several RNase T2 enzymes function in induced tRNA and rRNA turnover in the ciliate Tetrahymena. Mol Biol Cell. 2012;23(1):36–44. doi: 10.1091/mbc.e11-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, De Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40(W1):W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, MacIntosh GC. Degradation of cytosolic ribosomes by autophagy-related pathways. Plant Sci. 2017;262:169–174. doi: 10.1016/j.plantsci.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008;55(3):504–513. doi: 10.1111/j.1365-313X.2008.03519.x. [DOI] [PubMed] [Google Scholar]
- Bodenhausen N, Reymond P. Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol Plant Microbe Interact. 2007;20(11):1406–1420. doi: 10.1094/MPMI-20-11-1406. [DOI] [PubMed] [Google Scholar]
- Chen H, Zeng Y, Yang Y, Huang L, Tang B, Zhang H, Hao F, Liu W, Li Y, Liu Y. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat Commun. 2020;11(1):1–11. doi: 10.1038/s41467-020-16338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcilius L, Hastwell AH, Zhang M, Williams J, Mackay JP, Gresshoff PM, Ferguson BJ, Payne RJ. Arabinosylation modulates the growth-regulating activity of the peptide hormone CLE40a from soybean. Cell Chem Biol. 2017;24(11):1347–1355. doi: 10.1016/j.chembiol.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Coskan A, Dogan K. Symbiotic nitrogen fixation in soybean. London: IntechOpen; 2011. [Google Scholar]
- Cruz-Garcia F, Hancock CN, McClure B. S-RNase complexes and pollen rejection. J Exp Bot. 2003;54(380):123–130. doi: 10.1093/jxb/erg045. [DOI] [PubMed] [Google Scholar]
- De Leeuw M, Roiz L, Smirnoff P, Schwartz B, Shoseyov O, Almog O. Binding assay and preliminary X-ray crystallographic analysis of ACTIBIND, a protein with anticarcinogenic and antiangiogenic activities. Acta Crystallogr Sect F. 2007;63(8):716–719. doi: 10.1107/S1744309107034483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande RA, Shankar V. Ribonucleases from T2 family. Crit Rev Microbiol. 2002;28(2):79–122. doi: 10.1080/1040-840291046704. [DOI] [PubMed] [Google Scholar]
- Diaz-Baena M, Delgado-García E, Pineda M, Galvez-Valdivieso G, Piedras P. S-Like Ribonuclease T2 genes are induced during mobilisation of nutrients in cotyledons from common bean. Agronomy. 2021;11(3):490. doi: 10.3390/agronomy11030490. [DOI] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, Von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protocols. 2007;2(4):953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Indrasumunar A, Hayashi S, Lin MH, Lin YH, Reid DE, Gresshoff PM. Molecular analysis of legume nodule development and autoregulation. J Integr Plant Biol. 2010;52(1):61–76. doi: 10.1111/j.1744-7909.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM. Legume nodulation: the host controls the party. Plant Cell Environ. 2019;42(1):41–51. doi: 10.1111/pce.13348. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The proteomics protocols handbook. Totowa: Humana Press; 2005. pp. 571–607. [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40(D1):D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresshoff PM, Hayashi S, Biswas B, Mirzaei S, Indrasumunar A, Reid D, Samuel S, Tollenaere A, van Hameren B, Hastwell A. The value of biodiversity in legume symbiotic nitrogen fixation and nodulation for biofuel and food production. J Plant Physiol. 2015;172:128–136. doi: 10.1016/j.jplph.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Gundampati R, Sharma A, Kumari M, Debnath M. Extracellular poly (A) specific ribonuclease from Aspergillus niger ATCC 26550: purification, biochemical, and spectroscopic studies. Process Biochem. 2011;46(1):135–141. doi: 10.1016/j.procbio.2010.07.029. [DOI] [Google Scholar]
- Guruprasad K, Reddy BB, Pandit MW. Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng Des Sel. 1990;4(2):155–161. doi: 10.1093/protein/4.2.155. [DOI] [PubMed] [Google Scholar]
- Haud N, Kara F, Diekmann S, Henneke M, Willer JR, Hillwig MS, Gregg RG, MacIntosh GC, Gartner J, Alia A. rnaset2 mutant zebrafish model familial cystic leukoencephalopathy and reveal a role for RNase T2 in degrading ribosomal RNA. Proc Natl Acad Sci. 2011;108(3):1099–1103. doi: 10.1073/pnas.1009811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillwig MS. Regulation, function, and evolution of T2 RNases. Ames: Iowa State University; 2009. [Google Scholar]
- Hillwig MS, Contento AL, Meyer A, Ebany D, Bassham DC, MacIntosh GC. RNS2, a conserved member of the RNase T2 family, is necessary for ribosomal RNA decay in plants. Proc Natl Acad Sci. 2011;108(3):1093–1098. doi: 10.1073/pnas.1009809108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264(24):13963–13966. doi: 10.1016/S0021-9258(18)71625-6. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Bjornstedt M. [21] Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Yanai K, Takagi M, Yano K, Wakabayashi E, Sanda A, Mine S, Ohgi K, Irie M. Primary structure of a base non-specific ribonuclease from Rhizopus niveus. J Biochem. 1988;103(3):408–418. doi: 10.1093/oxfordjournals.jbchem.a122284. [DOI] [PubMed] [Google Scholar]
- Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier C, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(Suppl_2):W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kawamata T, Horie T, Tsugawa H, Nakayama Y, Ohsumi Y, Fukusaki E. Bulk RNA degradation by nitrogen starvation-induced autophagy in yeast. Eur Mol Biol Org Press. 2015;34(2):154–168. doi: 10.15252/embj.201489083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic B, Kohn JR. Evolutionary relationships among self-incompatibility RNases. Proc Natl Acad Sci. 2001;98(23):13167–13171. doi: 10.1073/pnas.231386798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai A. Thermostability and aliphatic index of globular proteins. J Biochem. 1980;88(6):1895–1898. doi: 10.1093/oxfordjournals.jbchem.a133168. [DOI] [PubMed] [Google Scholar]
- Irie M. Structure-function relationships of acid ribonucleases: lysosomal, vacuolar, and periplasmic enzymes. Pharmacol Ther. 1999;81(2):77–89. doi: 10.1016/S0163-7258(98)00035-7. [DOI] [PubMed] [Google Scholar]
- Itagaki T, Motoyoshi N, Kobayashi H, Ogawa Y, Hirose D, Inokuchi N. Cloning and characterization of ribonuclease T2 gene (RNHe30) from the basidiomycete, Hericium Erinaceum. Mycoscience. 2013;54(3):188–197. doi: 10.1016/j.myc.2012.09.011. [DOI] [Google Scholar]
- Kariu T, Sano K, Shimokawa H, Itoh R, Yamasaki N, Kimura M. Isolation and characterization of a wound-inducible ribonuclease from Nicotiana glutinosa leaves. Biosci Biotechnol Biochem. 1998;62(6):1144–1151. doi: 10.1271/bbb.62.1144. [DOI] [PubMed] [Google Scholar]
- Kawata Y, Sakiyama F, Tamaoki H. Amino-acid sequence of ribonuclease T2 from Aspergillus oryzae. Eur J Biochem. 1988;176(3):683–697. doi: 10.1111/j.1432-1033.1988.tb14331.x. [DOI] [PubMed] [Google Scholar]
- Kazibwe Z, Soto-Burgos J, MacIntosh GC, Bassham DC. TOR mediates the autophagy response to altered nucleotide homeostasis in an RNase mutant. J Exp Bot. 2020;71(22):6907–6920. doi: 10.1093/jxb/eraa410. [DOI] [PubMed] [Google Scholar]
- Kisa D, Ozturk L, Doker S, Gokce I. Expression analysis of metallothioneins and mineral contents in tomato (Lycopersicon esculentum) under heavy metal stress. J Sci Food Agric. 2017;97(6):1916–1923. doi: 10.1002/jsfa.7995. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Itagaki T, Inokuchi N, Ohgi K, Wada T, Iwama M, Irie M. A new type of RNase T2 ribonuclease in two Basidiomycetes fungi, Lentinus edodes and Irpex lacteus. Biosci Biotechnol Biochem. 2003;67(10):2307–2310. doi: 10.1271/bbb.67.2307. [DOI] [PubMed] [Google Scholar]
- Kock M, Theierl K, Stenzel I, Glund K. Extracellular administration of phosphate-sequestering metabolites induces ribonucleases in cultured tomato cells. Planta. 1998;204(3):404–407. doi: 10.1007/s004250050273. [DOI] [Google Scholar]
- Kock M, Stenzel I, Zimmer A. Tissue-specific expression of tomato Ribonuclease LX during phosphate starvation-induced root growth. J Exp Bot. 2006;57(14):3717–3726. doi: 10.1093/jxb/erl124. [DOI] [PubMed] [Google Scholar]
- Lers A, Khalchitski A, Lomaniec E, Burd S, Green PJ. Senescence-induced RNases in tomato. Plant Mol Biol. 1998;36(3):439–449. doi: 10.1023/A:1005993024161. [DOI] [PubMed] [Google Scholar]
- Lers A, Sonego L, Green PJ, Burd S. Suppression of LX ribonuclease in tomato results in a delay of leaf senescence and abscission. Plant Physiol. 2006;142(2):710–721. doi: 10.1104/pp.106.080135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43(D1):D257–D260. doi: 10.1093/nar/gku949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libault M, Thibivilliers S, Bilgin D, Radwan O, Benitez M, Clough S, Stacey G. Identification of four soybean reference genes for gene expression normalization. Plant Genome. 2008 doi: 10.3835/plantgenome2008.02.0091. [DOI] [Google Scholar]
- Libault M, Joshi T, Benedito VA, Xu D, Udvardi MK, Stacey G. Legume transcription factor genes: what makes legumes so special? Plant Physiol. 2009;151(3):991–1001. doi: 10.1104/pp.109.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libault M, Farmer A, Brechenmacher L, Drnevich J, Langley RJ, Bilgin DD, Radwan O, Neece DJ, Clough SJ, May GD. Complete transcriptome of the soybean root hair cell, a single-cell model, and its alteration in response to Bradyrhizobium japonicum infection. Plant Physiol. 2010;152(2):541–552. doi: 10.1104/pp.109.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libault M, Farmer A, Joshi T, Takahashi K, Langley RJ, Franklin LD, He J, Xu D, May G, Stacey G. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010;63(1):86–99. doi: 10.1111/j.1365-313X.2010.04222.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luhtala N, Parker R. T2 Family ribonucleases: ancient enzymes with diverse roles. Trends Biochem Sci. 2010;35(5):253–259. doi: 10.1016/j.tibs.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh GC, Castandet B. Organellar and secretory ribonucleases: major players in plant RNA homeostasis. Plant Physiol. 2020;183(4):1438–1452. doi: 10.1104/pp.20.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh GC, Bariola PA, Newbigin E, Green PJ. Characterization of Rny1, the Saccharomyces cerevisiae member of the T2 RNase family of RNases: unexpected functions for ancient enzymes? Proc Natl Acad Sci. 2001;98(3):1018–1023. doi: 10.1073/pnas.98.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh GC, Hillwig MS, Meyer A, Flagel L. RNase T2 genes from rice and the evolution of secretory ribonucleases in plants. Mol Genet Genomics. 2010;283(4):381–396. doi: 10.1007/s00438-010-0524-9. [DOI] [PubMed] [Google Scholar]
- Mirzaei S, Azizkhani N. The value of biodiversity in the legume family and its benefits for human lives. In: Islam S, Jeyabalan Sangeetha DT, Hong Ching G, editors. Biodiversity and conservation. Boca Raton: Chemical Rubber Company Press; 2019. pp. 27–54. [Google Scholar]
- Mirzaei S, Batley J, Ferguson BJ, Gresshoff PM. Transcriptome profiling of the shoot and root tips of S562L, a soybean GmCLAVATA1A mutant. Atlas J Biol. 2014;3(1):183–205. doi: 10.5147/ajb.v3i1.30. [DOI] [Google Scholar]
- Mirzaei S, Batley J, El-Mellouki T, Liu S, Meksem K, Ferguson BJ, Gresshoff PM. Neodiversification of homeologous CLAVATA1-like receptor kinase genes in soybean leads to distinct developmental outcomes. Sci Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-08252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller W, Schipper A, Amons R. A conserved amino acid sequence around Arg− 68 of Artemia elongation factor 1α is involved in the binding of guanine nucleotides and aminoacyl transfer RNAs. Biochimie. 1987;69(9):983–989. doi: 10.1016/0300-9084(87)90232-X. [DOI] [PubMed] [Google Scholar]
- Mun T, Bachmann A, Gupta V, Stougaard J, Andersen SU. Lotus base: an integrated information portal for the model legume Lotus japonicus. Sci Rep. 2016;6(1):1–18. doi: 10.1038/srep39447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger T, Abel S, Jost W, Glund K. Induction of an extracellular ribonuclease in cultured tomato cells upon phosphate starvation. Plant Physiol. 1990;92(4):970–976. doi: 10.1104/pp.92.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke JA, Iniguez LP, Fu F, Bucciarelli B, Miller SS, Jackson SA, McClean PE, Li J, Dai X, Zhao PX. An RNA-Seq based gene expression atlas of the common bean. BMC Genomics. 2014;15(1):1–17. doi: 10.1186/1471-2164-15-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonechnikov K, Golosova O, Fursov M, Team U. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166–1167. doi: 10.1093/bioinformatics/bts091m. [DOI] [PubMed] [Google Scholar]
- Ramanauskas K, Igic B. The evolutionary history of plant T2/S-type ribonucleases. PeerJ. 2017;5:e3790. doi: 10.7717/peerj.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38(Suppl_1):D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DE, Ferguson BJ, Hayashi S, Lin Y-H, Gresshoff PM. Molecular mechanisms controlling legume autoregulation of nodulation. Ann Bot. 2011;108(5):789–795. doi: 10.1093/aob/mcr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalson EH, McCubbin AG. S-RNases and sexual incompatibility: structure, functions, and evolutionary perspectives. Mol Phylogenet Evol. 2003;29(3):490–506. doi: 10.1016/S1055-7903(03)00195-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF. Recombinant human eosinophil cationic protein: ribonuclease activity is not essential for cytotoxicity. J Biol Chem. 1995;270(14):7876–7881. doi: 10.1074/jbc.270.14.7876. [DOI] [PubMed] [Google Scholar]
- Sahu BB, Baumbach JL, Singh P, Srivastava SK, Yi X, Bhattacharyya MK (2017) Analysis of the fusarium virguliforme transcriptomes induced during infection of soybean roots suggests that enzymes with hydrolytic activities could play a key role in root necrosis. Paper presented at the International Plant & Animal Genome, San Diego [DOI] [PMC free article] [PubMed]
- Sato K, Egami F. Studies on ribonucleases in takadiastase. I. J Biochem. 1957;44(11):753–767. doi: 10.1093/oxfordjournals.jbchem.a126717. [DOI] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37(5):501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci. 1998;95(11):5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2003;299(5603):109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- Sharpe HJ, Stevens TJ, Munro S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell. 2010;142(1):158–169. doi: 10.1016/j.cell.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz JL, Kurunam D, Shopinski K, Iqbal MJ, Kazi S, Zobrist K, Bashir R, Yaegashi S, Lavu N, Afzal AJ. The soybean genome database (SoyGD): a browser for display of duplicated, polyploid, regions and sequence tagged sites on the integrated physical and genetic maps of Glycine max. Nucleic Acids Res. 2006;34(Suppl_1):D758–D765. doi: 10.1093/nar/gkj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G. The soybean: botany, production and uses. Wallingford: Centre for Agriculture and Bioscience International; 2010. [Google Scholar]
- Skoog B, Wichman A. Calculation of the isoelectric points of polypeptides from the amino acid composition. Trends Anal Chem. 2013;5:82–83. doi: 10.1016/0165-9936(86)80045-0. [DOI] [Google Scholar]
- Solis-Miranda J, Fonseca-García C, Nava N, Pacheco R, Quinto C. Genome-wide identification of the CrRLK1L subfamily and comparative analysis of its role in the legume-rhizobia symbiosis. Genes. 2020;11(7):793. doi: 10.3390/genes11070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. Applying the bootstrap in phylogeny reconstruction. Stat Sci. 2003;1:256–267. doi: 10.1214/ss/1063994980. [DOI] [Google Scholar]
- Tanaka N, Arai J, Inokuchi N, Koyama T, Ohgi K, Irie M, Nakamura KT. Crystal structure of a plant ribonuclease, RNase LE. J Mol Biol. 2000;298(5):859–873. doi: 10.1006/jmbi.2000.3707. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Green PJ. Genes with homology to fungal and S-gene RNases are expressed in Arabidopsis thaliana. Plant Physiol. 1991;96(3):980–984. doi: 10.1104/pp.96.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CB, Bariola PA, Raines R, Green P. RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc Natl Acad Sci. 1993;90(11):5118–5122. doi: 10.1073/pnas.90.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J Cell Biol. 2009;185(1):43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrent M, De la Torre BG, Nogués VM, Andreu D, Boix E. Bactericidal and membrane disruption activities of the eosinophil cationic protein are largely retained in an N-terminal fragment. Biochem J. 2009;421(3):425–434. doi: 10.1042/BJ20082330. [DOI] [PubMed] [Google Scholar]
- Vidalino L, Monti L, Haase A, Moro A, Acquati F, Taramelli R, Macchi P. Intracellular trafficking of RNASET2, a novel component of P-bodies. Biol Cell. 2012;104(1):13–21. doi: 10.1111/boc.201100092. [DOI] [PubMed] [Google Scholar]
- Wang G, Zeng H, Hu X, Zhu Y, Chen Y, Shen C, Wang H, Poovaiah B, Du L. Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant Soil. 2015;386(1):205–221. doi: 10.1007/s11104-014-2267-6. [DOI] [Google Scholar]
- Ye Z-H, Droste DL. Isolation and characterization of cDNAs encoding xylogenesis-associated and wounding-induced ribonucleases in Zinnia elegans. Plant Mol Biol. 1996;30(4):697–709. doi: 10.1007/BF00019005. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wang Y, He Y, Zhou J, Li Y, Liu Q, Xie X. Overexpression of an S-like ribonuclease gene, OsRNS4, confers enhanced tolerance to high salinity and hyposensitivity to phytochrome-mediated light signals in rice. Plant Sci. 2014;214:99–105. doi: 10.1016/j.plantsci.2013.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.