Abstract
An interplay between Ca2+/calmodulin-dependent protein kinase IIδc (CaMKIIδc) and late Na+ current (INaL) is known to induce arrhythmias in the failing heart. Here, we elucidate the role of the sodium channel isoform NaV1.8 for CaMKIIδc-dependent proarrhythmia. In a CRISPR-Cas9-generated human iPSC-cardiomyocyte homozygous knock-out of NaV1.8, we demonstrate that NaV1.8 contributes to INaL formation. In addition, we reveal a direct interaction between NaV1.8 and CaMKIIδc in cardiomyocytes isolated from patients with heart failure (HF). Using specific blockers of NaV1.8 and CaMKIIδc, we show that NaV1.8-driven INaL is CaMKIIδc-dependent and that NaV1.8-inhibtion reduces diastolic SR-Ca2+ leak in human failing cardiomyocytes. Moreover, increased mortality of CaMKIIδc-overexpressing HF mice is reduced when a NaV1.8 knock-out is introduced. Cellular and in vivo experiments reveal reduced ventricular arrhythmias without changes in HF progression. Our work therefore identifies a proarrhythmic CaMKIIδc downstream target which may constitute a prognostic and antiarrhythmic strategy.
Subject terms: Cardiology, Molecular medicine
In heart failure, increased CaMKII activity is decisively involved in arrhythmia formation. Here, the authors introduce the neuronal sodium channel NaV1.8 as a CaMKII downstream target as its specific knock-out reduces arrhythmias and improves survival in a CaMKII-overexpressing mouse model.
Introduction
Voltage-gated sodium channels (NaV) play a critical role in physiological cardiac conduction. NaV channels become inactive within a few milliseconds after activation under physiological conditions. However, in cardiac pathologies such as ischemia, hypoxia, oxidative stress, and heart failure (HF), some NaV remain persistently open or reopen, generating a small but persistent Na+ current, referred to as the late Na+ current (INaL)1–5. This current slows the repolarisation rate and thereby prolongs the action potential duration (APD). Augmented INaL may additionally cause Na+-dependent Ca2+ overload in cardiomyocytes, thereby playing an essential role for arrhythmogenesis and diastolic dysfunction1,2,6,7. Furthermore, Na+/Ca2+ overload caused by augmented INaL can give rise to early afterdepolarizations (EADs), delayed afterdepolarizations (DADs), and hence sustained triggered arrhythmias8–11. In the failing heart, increased INaL induces an influx of Na+ into the cardiomyocyte, which in turn stimulates Ca2+ influx via the reverse mode of Na+/Ca2+ exchanger (NCX)12,13. Cytosolic Ca2+ may now bind to calmodulin (CaM), forming a Ca2+/CaM complex, which activates Ca2+/calmodulin-dependent protein kinase IIδ (CaMKIIδ), a multifunctional serine/threonine protein kinase8,10,14,15. CaMKII is expressed in four isoforms α, β, γ, and δ. CaMKIIδ is the predominant isoform in heart16 while the δc isoform is mainly located in the cytosol. Once CaMKIIδc is activated, it may cause hyperphosphorylation of the ryanodine receptor 2 (RyR2) residing within the sarcoplasmic reticulum (SR)-sarcolemma junction, leading to spontaneous proarrhythmogenic SR-Ca2+ release events in HF13,17–20. Further, this augmented CaMKIIδc activity can also induce INaL augmentation by phosphorylating NaV channels10,14,21,22 leading to a vicious cycle between INaL and CaMKIIδc.
Besides NaV1.5 other NaV isoforms have been reported to be present in the heart. Theoretically, they could also generate INaL, induce APD prolongation, and spontaneous SR-Ca2+ release. In the previous few years, different reports have been published on the existence of noncardiac NaV in the heart. NaV1.8, a noncardiac tetrodotoxin-resistant NaV channel, is encoded by the SCN10A gene and was originally reported to be expressed in the dorsal root ganglion23. Certain genome-wide association studies reported an association of SCN10A with changes in ECG parameters but most importantly with cardiac arrhythmias such as atrial fibrillation and sudden cardiac death24–27. Later, the presence of NaV1.8 was detected in atria and further evidence came from studies conducted in mouse and rabbit cardiomyocytes investigating its involvement in cardiac electrophysiology28–30. Moreover, we recently reported that NaV1.8 mRNA and protein expression are upregulated in tissue from human hypertrophied and failing ventricles and that NaV1.8 contributes to INaL generation in the human heart under these pathological conditions31,32. However, NaV1.8 regulation, a potential interplay with pathologically increased CaMKIIδc activity in HF, and the role of NaV1.8 on HF progression and arrhythmias in vivo and in vitro remain elusive.
In this work, we describe a detrimental interaction of NaV1.8 with CaMKIIδc in human and murine failing cardiomyocytes. Moreover, we investigate the contribution of NaV1.8 to cellular electrophysiology in relation to enhanced CaMKIIδc activity and consequently show a reduction of arrhythmias which is paralleled by an improved survival due to NaV1.8 deletion in CaMKIIδc transgenic HF mice.
Results
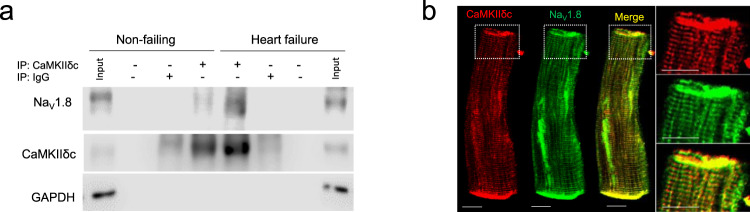
NaV1.8 and CaMKIIδc interaction in human HF
Since interaction between CaMKIIδc and NaV1.5 was shown previously, we hypothesized that CaMKIIδc interacts also with NaV1.8 and therefore performed co-immunoprecipitation using human ventricular tissue homogenates. Indeed, we found that CaMKIIδc associates with NaV1.8 in human non-failing as well as HF myocardium (Fig. 1a). Using immunofluorescence stainings, CaMKIIδc and NaV1.8 were found to be co-localized in isolated human cardiomyocytes (Fig. 1b). Of note, SCN10A mRNA expression in tissue from non-failing and HF hearts, as well as isolated cardiomyocytes from human HF hearts was found to be much lower than SCN5A. Further, RT-qPCR experiments revealed that a relevant part of SCN10A mRNA in the heart originates from cardiomyocytes (Supplementary information, Supplementary Figs. 1, 2).
Fig. 1. CaMKIIδc interacts with NaV1.8 in human myocardium and isolated cardiomyocytes.
a Co-immunoprecipitation of CaMKIIδc and NaV1.8 from left ventricular homogenates of human non-failing and failing hearts (NF: n = 7; HF: n = 7). b Co-localization of CaMKIIδc and NaV1.8 in human failing cardiomyocytes with immunofluorescence staining. Scale bar: 10 µm (staining was performed in cardiomyocytes isolated from five heart failure patients).
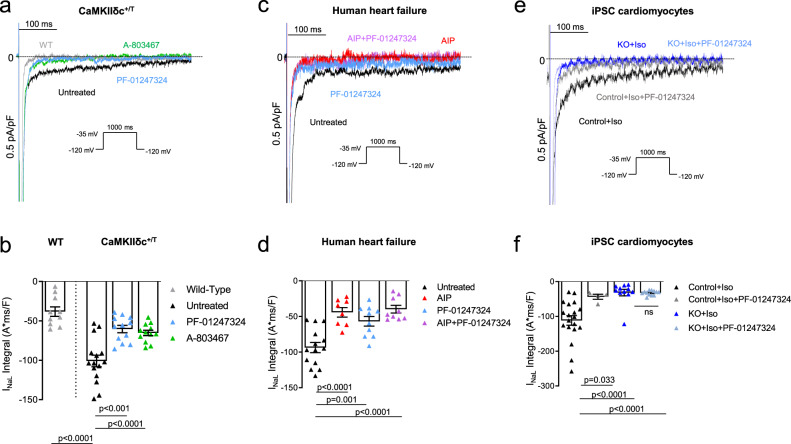
NaV1.8 inhibition reduces INaL in human failing, mouse CaMKIIδc transgenic cardiomyocytes, and in SCN10A knockout iPSC-cardiomyocytes
In functional experiments, we could show that INaL, induced by increased CaMKIIδc activity, was significantly reduced following pharmacological inhibition and genetical knockout of NaV1.8 in human and murine cardiomyocytes. In isolated murine CaMKIIδc+/T cardiomyocytes, INaL was augmented to ~150% compared to wildtype (WT), whereas the specific NaV1.8 blockers A-806734 or PF-01247324 reduced INaL by ~40% in the same background (CaMKIIδc+/T) (Fig. 2a, b). These results illustrate that CaMKIIδc-induced INaL can be clearly ameliorated by inhibiting NaV1.8. A current–voltage relationship of NaV1.8-dependent INaL in isolated ventricular myocytes from CaMKIIδc+/T mice is presented in the Supplementary information (Supplementary Fig. 3).
Fig. 2. Reduced INaL upon NaV1.8 inhibition in human failing and mouse CaMKIIδc transgenic cardiomyocytes, and in SCN10A knockout iPSC-cardiomyocytes.
a Original traces of INaL in WT and CaMKIIδc+/T mouse ventricular cardiomyocytes elicited using the protocol shown in the inset. b Mean data ± SEM along with individual values shown in the graph plotting WT (n = 10 cells/5 mice) and CaMKIIδc+/T (untreated: n = 15 cells/7 mice; A-806467: n = 12 cells/7 mice; PF-01247324: n = 12 cells/7 mice). Probability vs. untreated (One-way ANOVA with post hoc Bonferroni’s correction). c Original traces of INaL from human failing ventricular cardiomyocytes elicited using the protocol shown in the inset. d Mean data ± SEM along with individual values shown in the graph plotting (untreated: n = 14 cells/8 patients; AIP: n = 8 cells/5 patients; PF-01247324: n = 10 cells/6 patients; AIP + PF-01247324 = 9 cells/5 patients). Probability vs. untreated (One-way ANOVA with post hoc Bonferroni’s correction). e Original traces of INaL from human ventricular SCN10A knockout iPSC-cardiomyocytes elicited using the protocol shown in the inset. f Mean data ± SEM along with individual values shown in the graph plotting (control + Iso: n = 19 cells/3 cardiac differentiations; control + Iso + PF: n = 4 cells/3 differentiations; SCN10A knockout (KO) + Iso: n = 11 cells/3 differentiations; KO + Iso + PF-01247324 = 12 cells/3 differentiations). Probability vs. control + Iso (One-way ANOVA with post hoc Bonferroni’s correction).
In the human failing heart, CaMKIIδc and INaL are upregulated in parallel2,33. Therefore, we inhibited NaV1.8 by using PF-01247324 and compared its ability to reduce INaL to CaMKIIδc inhibition using autocamtide-2-related inhibitory peptide (AIP) in human failing ventricular cardiomyocytes. In addition, we blocked NaV1.8 and CaMKIIδc in parallel by exposing human failing cells simultaneously to PF-01247324 and AIP. INaL measurements demonstrated that NaV1.8 inhibition alone (PF-01247324) led to a ~40% decrease and CaMKIIδc inhibition by AIP to a ~53% reduction of INaL in human failing cardiomyocytes (Fig. 2c, d). However, preincubation with AIP and PF-01247324 together decreased INaL comparable to AIP alone suggesting that CaMKIIδc inhibition might already suppress NaV1.8-driven INaL. Further, peak INa measurements revealed, that INaL reduction due to NaV1.8 inhibition is not caused by a reduction of overall Na+ current (Supplementary information, Supplementary Fig. 4).
As the existence of NaV1.8 and its role in cardiomyocytes is still a matter of debate, we generated homozygous NaV1.8 knockout (KO) lines by using CRISPR-Cas9 in human induced pluripotent stem cells (iPSC) and differentiated these cells into 2-month-old cardiomyocytes. Sanger sequencing demonstrated frameshifts and premature stop codons on both alleles (Supplementary information, Supplementary Fig. 5). As the amplitude of INaL is relatively small under healthy conditions we used isoproterenol (Iso, 50 nmol/l) to enhance INaL. Control iPSC-cardiomyocytes treated simultaneously with Iso and PF-01247324 exhibited ~60% less INaL compared to Iso alone, (Fig. 2e, f). Most importantly, in NaV1.8 KO iPSC-cardiomyocytes INaL was reduced by ~70% compared to the control iPSC-cardiomyocytes. PF-01247324 did not exert any further impact on INaL in NaV1.8 KO iPSC-cardiomyocytes compared to untreated NaV1.8 KO cells, underlining the specificity of the drug.
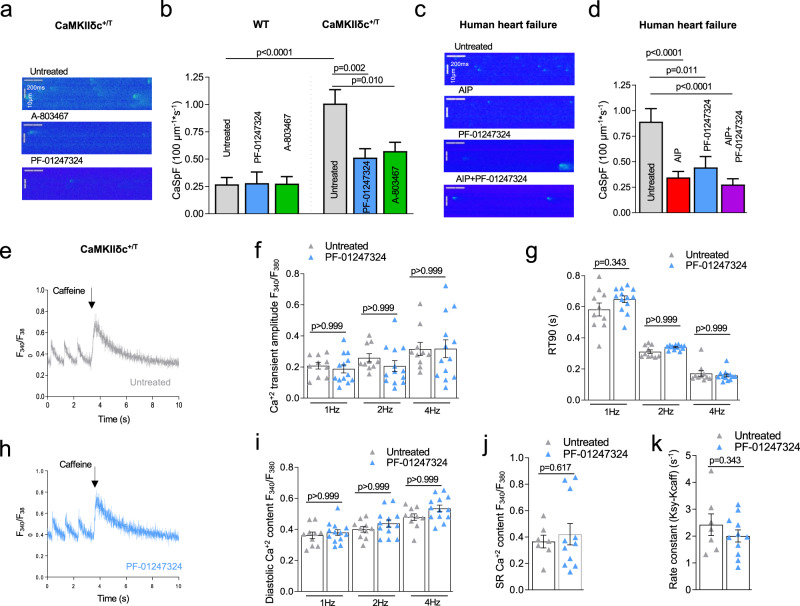
NaV1.8 modulates Ca2+ homeostasis under enhanced CaMKIIδc activity
In HF, enhanced INaL can potently induce proarrhythmic SR-Ca2+ release events8,11. Accordingly, we investigated whether inhibition of the NaV1.8-mediated INaL could attenuate the increase of the proarrhythmogenic SR-Ca2+ spark frequency (CaSpF) caused by overexpression of CaMKIIδc (Fig. 3a, b). We incubated CaMKIIδc+/T mouse cardiomyocytes with NaV1.8 inhibitors and measured the CaSpF. A ~50% reduction of CaSpF was observed in both NaV1.8 inhibitor groups compared to untreated cells (Fig. 3a, b). These results display that SR-Ca2+ leak due to increased CaMKIIδc expression and activity can be reduced by inhibiting NaV1.8.
Fig. 3. Effects NaV1.8 inhibition on intracellular Ca2+ handling.
a Representative line scan images of CaMKIIδc+/T ventricular cardiomyocytes. b CaSpF data shown as mean ± SEM for wildtype (WT) (untreated: n = 58 cells/4 mice; PF-01247324: n = 41 cells/4 mice; A-806467: n = 41 cells/4 mice) and CaMKIIδc+/T (untreated: n = 122 cells/8 mice; PF-01247324: n = 105 cells/7 mice; A-806467: n = 101 cells/8 mice). Data were analyzed by one-way ANOVA with post hoc Bonferroni’s correction. c Representative line scan images of human failing ventricular cardiomyocytes. d Data shown as mean ± SEM (untreated: n = 123 cells/14 patients; Autocamtide-2-related inhibitory peptide (AIP): n = 105 cells/15 patients; PF-01247324: n = 59 cells/10 patients; AIP + PF-01247324 = 89 cells/13 patients). Data were analyzed by one-way ANOVA with post hoc Bonferroni’s correction, Probability vs. untreated. e Representative Ca2+ transients stimulated at 1 Hz and caffeine-induced Ca2+ transients in ventricular cardiomyocytes from CaMKIIδc+/T under untreated conditions. f Mean data ± SEM show no effect of PF-01347324 treatment on Ca2+ transient amplitude at 1.0, 2.0, and 4.0 Hz stimulation (n = 13 cells/5 mice) compared to untreated (n = 10 cells/5 mice). g Ca2+-transient decay (90% of Ca2+-removal) RT90 was unchanged in PF-01347324-treated cells (n = 11 cells/5 mice) compared to untreated (n = 8 cells/5 mice). Data were presented as mean values ± SEM. h Representative Ca2+ transients stimulated at 1 Hz and caffeine-induced Ca2+ transients in ventricular cardiomyocytes from CaMKIIδc+/T treated with PF-01247324. i Diastolic Ca2+ after addition of PF-01347324 (n = 13 cells/5 mice) compared to untreated cells (n = 10 cells/5 mice) at different stimulation frequencies was unchanged (one-way ANOVA with post hoc Bonferroni’s correction, Fig. 3f, g, i). Data were presented as mean values ± SEM. j Mean and individual values ± SEM of caffeine-induced Ca2+ transients (untreated: n = 7 cells/4 mice, PF-01247324: n = 11 cells/5 mice) did not differ between the groups (Student’s t-test). k Ca2+-reuptake into the SR was not affected by inhibition of NaV1.8 (untreated: n = 7 cells/4 mice, PF-01247324: n = 11 cells/5 mice), analyzed by Student’s t-test. Data were presented as mean values ± SEM.
It is well known that inhibition of CaMKIIδc can attenuate SR-Ca2+ leak18,34. However, therapeutic general inhibition of CaMKIIδc in humans may not be suitable because of its pivotal involvement in different vital pathways such as learning processes35. We explored whether the NaV1.8 inhibitor PF-01247324 exerts similar effects comparable to the inhibition of CaMKIIδc. Incubation of human failing cardiomyocytes with either the CaMKIIδc inhibitor AIP or the NaV1.8 inhibitor PF-01247324 resulted in a similar reduction of CaSpF compared to untreated cells (Fig. 3c, d). Furthermore, blocking CaMKIIδc and NaV1.8 in parallel resulted in a significant reduction of CaSpF, comparable to AIP or PF-01247324 alone in human failing cardiomyocytes (Fig. 3c, d).
We further investigated whether NaV1.8 inhibition modulates the Ca2+ transient amplitude and SR-Ca2+ load in cardiomyocytes isolated from CaMKIIδc+/T mice. NaV1.8 inhibition using PF-01247324 did not pose any effect on either the Ca2+ transient amplitude or Ca2+ transient decay measured at different stimulation frequencies (Fig. 3e–h). Furthermore, diastolic Ca2+, SR-Ca2+ content, and SERCA2a activity were not affected by PF-01247324 (Fig. 3i–k). Similar effects of PF-01247324 on Ca2+ transient kinetics and SR-Ca2+ content were observed in cardiomyocytes isolated from WT mice (Supplementary information, Supplementary Fig. 6).
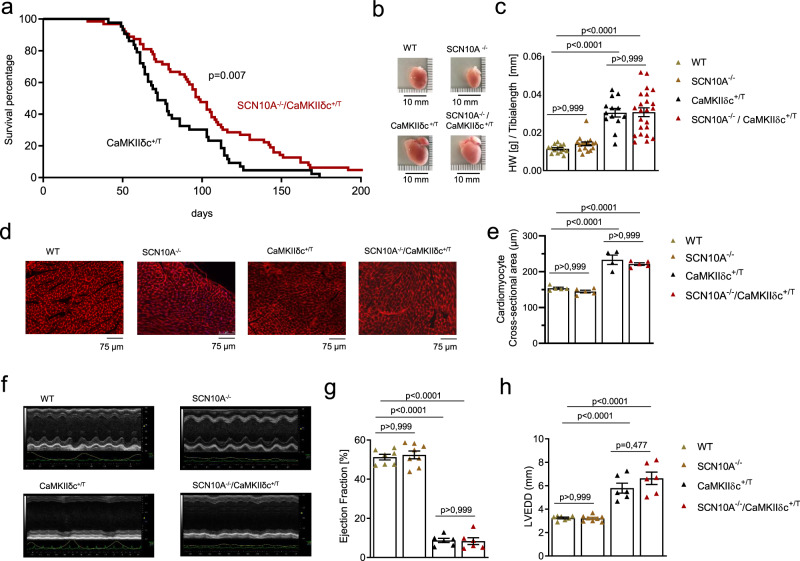
Scn10a knockout improves survival in CaMKIIδc+/T mice in the absence of structural ventricular changes
To study whether inhibition of NaV1.8 influences the development of HF, arrhythmogenesis, or survival in CaMKIIδc+/T mice, we crossbred these mice with NaV1.8 knockout mice. Interestingly, SCN10A−/−/CaMKIIδc+/T mice showed a significantly improved survival compared to CaMKIIδc+/T (median survival 98 vs. 72 days, 64 vs. 43 animals, Hazard Ratio 0.6) as assessed in blinded investigations. Specifically, CaMKIIδc+/T mice showed only a 37% survival at 12 weeks, whereas SCN10A−/−/CaMKIIδc+/T died at a slower rate, with 67% survival at this age (Fig. 4a). These data illustrate that NaV1.8 knockout is capable to counteract the lethal phenotype of CaMKIIδc overexpression to a relevant extent.
Fig. 4. Knockout of the Scn10a (NaV1.8) gene in CaMKIIδc+/T mice improves survival.
a Survival curve of CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T (43 vs. 64 animals, median survival 72 vs. 98 days, blinded analysis). Log-rank (Mantel–Cox test and Gehan–Breslow–Wilcoxon test (two-tailed analysis) were performed to calculate the survival percentage of mice. Probability vs CaMKIIδc+/T. b Hearts from WT, SCN10A−/−, CaMKIIδc+/T, and SCN10A−/−/CaMKIIδc+/T mice. c Ratio of heart weight to tibia length as a parameter of cardiac hypertrophy. CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T showed a significant increase in this ratio compared to WT and SCN10A−/− mice. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s correction. (N = hearts studied, WT = 14, SCN10A−/− = 16, CaMKIIδc+/T = 13, and SCN10A−/−/CaMKIIδc+/T = 23). Data were presented as mean values ± SEM. d Original histological wheat germ agglutinin staining from WT, SCN10A−/−, CaMKIIδc+/T, and SCN10A−/−/CaMKIIδc+/T mice. Scale bars = 75 µm. Stainings were produced from different sections and three different regions (basal, mid-ventricular, and apical) of each heart studied. e Cardiomyocyte cross-sectional-area (CSA) as a parameter for cellular hypertrophy. CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T showed a significant increase in CSA compared to WT and SCN10A−/− mice. CSA in CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T mice did not significantly differ. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s correction. N = hearts studied (>300 cardiomyocytes were studied per heart, from different sections and different regions (basal, mid-ventricular, apical), WT = 5 hearts, SCN10A−/− = 5 hearts, CaMKIIδc+/T = 4 hearts, SCN10A−/−/CaMKIIδc+/T = 5 hearts. Data were presented as mean values ± SEM. f Original echocardiography recordings from WT, SCN10A−/−, CaMKIIδc+/T, and SCN10A−/−/CaMKIIδc+/T at M-mode in 12–13- week-old mice. g Echocardiography recordings revealed a decrease in left ventricular ejection fraction (EF) in CaMKIIδc+/T (six mice) and SCN10A−/−/CaMKIIδc+/T (six mice) compared to WT (seven mice) or SCN10A−/− (eight mice) (p < 0.0001(one-way ANOVA with post hoc Bonferroni’s correction). Data were presented as mean values ± SEM. h Echocardiography recordings revealed a significant increase in left ventricular end-diastolic diameter (LVEDD) in CaMKIIδc+/T (six mice) and SCN10A−/−/CaMKIIδc+/T (six mice) compared to WT (seven mice) or SCN10A−/− (eight mice) (p < 0.0001). LVEDD was not significantly different in WT vs SCN10A−/− or CaMKIIδc+/T vs SCN10A−/−/CaMKIIδc+/T (one-way ANOVA with post hoc Bonferroni’s correction). Data were presented as mean values ± SEM.
Detailed investigations of hearts from SCN10A−/−/CaMKIIδc+/T double-mutant and CaMKIIδc+/T mice by the age of 12 weeks exhibited comparably enlarged heart chambers (Fig. 4b). Heart weight to tibia length ratio was similarly increased in double-mutant and CaMKIIδc+/T mice (Fig. 4c). To investigate whether NaV1.8 knockout influences CaMKIIδc-mediated hypertrophy we prepared histological heart stainings. CaMKIIδc overexpression led to an increase of cardiomyocyte cross-sectional area (CSA) compared to WT and SCN10A−/− in mouse left ventricles. However, in double-mutant mice CSA did not differ from CaMKIIδc+/T alone (Fig. 4d, e). In addition, Scn10a knockout did not change the expression of NaV1.5 (Scn5a) and CaMKIIδc on protein and mRNA levels in hearts from WT and CaMKIIδc+/T mice (Supplementary information, Supplementary Figs. 7, 8).
Serial echocardiography revealed a severe HF phenotype in CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T with a significant reduction of left ventricular ejection fraction (EF) (Fig. 4f, g). Moreover, significantly enlarged left ventricular end-diastolic diameters (LVEDD) were measured in CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T compared to WT or SCN10A−/− (Fig. 4f, h). There were no changes between WT and SCN10A−/− or CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T mice with respect to LVEDD and EF.
Reduction of proarrhythmic activity in SCN10A−/−/CaMKIIδc+/T cardiomyocytes
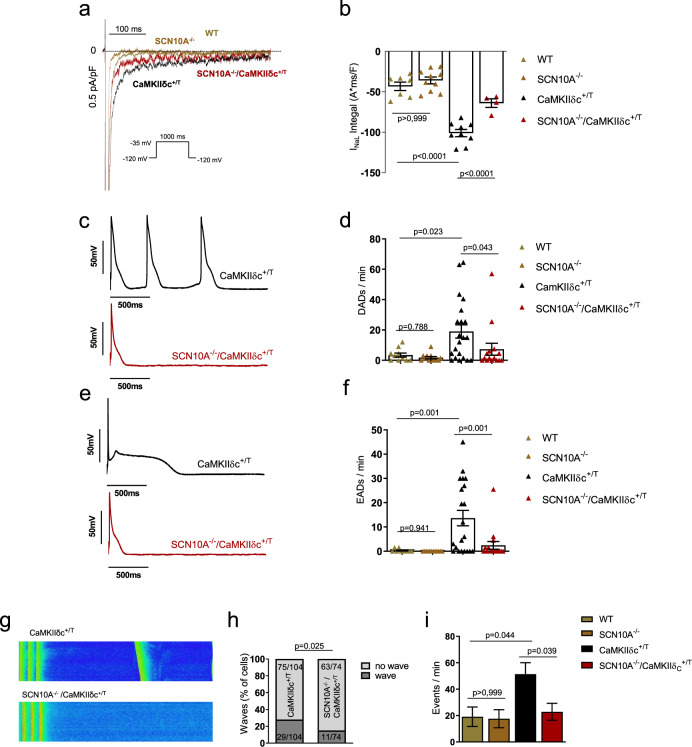
To test whether enhanced INaL in CaMKIIδc+/T mice can be ameliorated by genetic knockout of NaV1.8, we measured INaL in isolated mouse cardiomyocytes (Fig. 5a, b). While CaMKIIδc+/T cardiomyocytes showed significantly enhanced INaL, knockout of NaV1.8 resulted in a ~45% decrease in INaL in SCN10A−/−/CaMKIIδc+/T mice compared with CaMKIIδc+/T. Of note, at basal/unstimulated conditions, INaL did not differ between WT and SCN10A−/−.
Fig. 5. Knockout of Scn10a (NaV1.8) in CaMKIIδc+/T mice (SCN10A−/−/CaMKIIδc+/T) significantly reduces INaL and proarrhythmic triggers.
a Original traces of INaL in WT, SCN10A−/−, CaMKIIδc+/T, and SCN10A−/−/CaMKIIδc+/T mouse ventricular cardiomyocytes elicited using the protocol shown in the inset. b Mean data ± SEM along with individual values shown in the graph plotting (WT: n = 7 cells/4 mice, SCN10A−/− n = 10 cells/5 mice, CaMKIIδc+/T: n = 9 cells/5 mice; SCN10A−/−/CaMKIIδc+/T: n = 4 cells/4 mice), there was a significantly reduced INaL in SCN10A−/−/CaMKIIδc+/T cardiomyocytes compared to CaMKIIδc+/T. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s correction. c Original traces of action potentials showing triggered action potentials originating from delayed afterdepolarizations (DADs) in CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T cardiomyocytes. d Graph of mean data ± SEM along with individual values showing DADs per minute in WT (n = 10 cells/5 mice), SCN10A−/− (n = 12 cells/5 mice), CaMKIIδc+/T (n = 21 cells/5 mice) and SCN10A−/−/CaMKIIδc+/T (n = 15 cells/5 mice) cardiomyocytes. There were significantly less events of afterdepolarizations in SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T cardiomyocytes. Data were analyzed by one-way ANOVA with the post hoc two-stage step-up method of Benjamini, Krieger, and Yekutieli. e Original traces of action potential showing early afterdepolarizations (EADs) in CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T cardiomyocytes. f Graph of mean data ± SEM along with individual values showing EADs per minute in WT (n = 10 cells/5 mice), SCN10A−/− (n = 12 cells/5 mice), CaMKIIδc+/T (n = 21 cells/5 mice) and SCN10A−/−/CaMKIIδc+/T (n = 16 cells/5 mice) cardiomyocytes. There were significantly less events of afterdepolarizations in SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T cardiomyocytes. Data were analyzed by one-way ANOVA with the post hoc two-stage step-up method of Benjamini, Krieger, and Yekutieli. g Original confocal line scans images of CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T cardiomyocytes showing diastolic Ca2+ waves. h Percentage of cells exhibiting waves was significantly less in SCN10A−/−/CaMKIIδc+/T (n = 74 cells/7 mice) compared to CaMKIIδc+/T (n = 104 cells/9 mice). Data were analyzed by Chi-square test, two-tailed analysis. i Significantly decreased number of Ca2+ waves per minute in SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T. Data were analyzed by one-way ANOVA with post hoc Bonferroni’s correction. Cells/mice studied, WT: n = 48 cells/5 mice, SCN10A−/−: n = 52 cells/5 mice, CaMKIIδc+/T: n = 104 cells/9 mice; SCN10A−/−/CaMKIIδc+/T: n = 74 cells/7 mice. Data were presented as mean values ± SEM.
To evaluate whether chronic ablation of NaV1.8 in CaMKIIδc+/T mice may reduce proarrhythmic cellular activity, we performed electrophysiological measurements (Fig. 5c–f). Patch-clamp experiments revealed that CaMKIIδc+/T cardiomyocytes exhibited approximately fivefold more delayed afterdepolarizations/min (DADs) compared to WT and SCN10A−/−. Scn10a knockout reduced the fraction of CaMKIIδc-induced DADs/min by ~60% (Fig. 5c, d). Comparable observations were made regarding the occurrence of early afterdepolarizations (EADs, Fig. 5e, f). While WT and SCN10A−/− cardiomyocytes developed almost no EADs, 13.6 ± 3.2 EADs/min were recorded in CaMKIIδc+/T. NaV1.8 knockout caused an 80% reduction of EADs/min in these cells. A detailed description of the action potential characteristics of these measurements is provided in the Supplementary information (Supplementary Table 1).
Furthermore, to evaluate whether inhibition of NaV1.8 may decrease the number of cardiomyocytes exhibiting Ca2+-derived proarrhythmic events, we quantified the occurrence of Ca2+ waves in mouse ventricular cardiomyocytes. The fraction of cardiomyocytes developing Ca2+ waves was significantly less in SCN10A−/−/CaMKIIδc+/T versus CaMKIIδc+/T (14.8 vs 27.8%, Fig. 5g, h). However, some cardiomyocytes showed more than one Ca2+ wave. Therefore, we calculated the occurring events per minute. In CaMKIIδc+/T the frequency of Ca2+ waves was ~2.5-fold higher compared to WT and SCN10A−/− and was reduced by ~55% in cardiomyocytes from SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T (Fig. 5i). Of note, Ca2+ transient amplitude and Ca2+ extrusion from cytosol was unchanged between SCN10A−/−/CaMKIIδc+/T and CaMKIIδc+/T (Supplementary information, Supplementary Fig. 9). In summary, we describe a cellular rescue of the proarrhythmic CaMKIIδc+/T phenotype due to genetic ablation of NaV1.8, which is associated with improved animal survival.
Scn10a knockout reduces ventricular arrhythmias in CaMKIIδc+/T mice
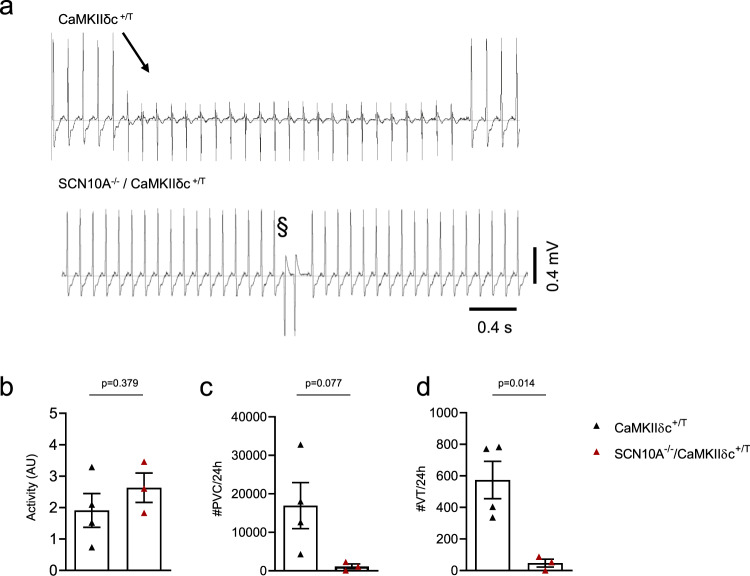
To further investigate whether the proarrhythmic potential of Scn10a knockout on the cellular level is translatable into the in vivo situation, we implanted telemetric monitors into 8 week old SCN10A−/−/CaMKIIδc+/T and CaMKIIδc+/T mice. After 10 days of recovery from surgery, telemetric measurements were performed twice a week for 24 h over a period of 2 weeks. Baseline ECG characteristics are presented in the Supplementary information (Supplementary Table 2). There was no relevant difference in overall physical animal activity between the groups (Fig. 6b). Blinded analysis revealed that SCN10A−/−/CaMKIIδc+/T showed a strong trend towards a reduction of premature ventricular contractions (PVC) by ~93% compared to CaMKIIδc+/T (Fig. 6a, c, p = 0.08). Most importantly, the incidence of ventricular tachycardia (VT) was significantly lower (91%) in SCN10A−/−/CaMKIIδc+/T (Fig. 6a, d). Therefore, the observed improved survival of SCN10A−/−/CaMKIIδc+/T-animals is associated with reduced ventricular arrhythmias.
Fig. 6. SCN10A−/−/CaMKIIδc+/T exhibit less in vivo arrhythmias compared to CaMKIIδc+/T mice.
a Original ECG traces from telemetry recordings of 10-week-old CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T mice showing ventricular arrhythmias. b Unchanged activity levels in SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T (CaMKIIδc+/T (four mice); SCN10A−/−/CaMKIIδc+/T (three mice), four individual recordings each). Data were presented as mean values ± SEM, analyzed by unpaired two-tailed Student’s t-test. c Mean values of premature ventricular contractions (PVCs) in SCN10A−/−/CaMKIIδc+/T (p = 0.08, Unpaired two-tailed Student’s t-test), CaMKIIδc+/T (four mice); SCN10A−/−/CaMKIIδc+/T (three mice), four individual recordings each. Data were presented as mean values ± SEM. d Reduction of ventricular tachycardia (VT) incidence in SCN10A−/−/CaMKIIδc+/T (p < 0.05, Unpaired two-tailed Student’s t-test), CaMKIIδc+/T (four mice); SCN10A−/−/CaMKIIδc+/T (three mice), four individual recordings each. Data were presented as mean values ± SEM.
Discussion
In our present study, we demonstrate that CaMKIIδc interacts with the neuronal sodium channel NaV1.8 in human ventricular cardiomyocytes. Using different approaches in human and mouse cardiomyocytes, we demonstrated the relevance of NaV1.8 for INaL generation in HF and that an enhanced CaMKIIδc, indeed, regulates this NaV1.8-driven INaL. Isolated cardiomyocytes from SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T mice exhibit reduced cellular arrhythmic events. While there was no change with respect to HF progression, i.e., similar left ventricular ejection fraction and chamber diameters, we found reduced ventricular arrhythmias and an improved animal survival of SCN10A−/−/CaMKIIδc+/T animals. Thus, we identified a modifiable proarrhythmic CaMKIIδc downstream target in the failing heart.
We recently found that NaV1.8 is upregulated and thereby contributes to INaL under conditions of HF and cardiac hypertrophy where CaMKIIδc activity is known to be enhanced31,32. Therefore, the aim of the current study was to investigate a potential crosstalk between NaV1.8 and CaMKIIδc and its consequences on INaL generation and cellular arrhythmogenety. Accordingly, we revealed an interaction of NaV1.8 and CaMKIIδc in human ventricular myocardium of both non-failing and HF samples. CaMKIIδc is known to also interact with NaV1.5 channels at the intercalated disc where NaV1.5 and CaMKIIδc are part of a macro complex with Ankyrin-G and ßIV-spectrin36. The interaction with CaMKIIδc in this complex influences NaV1.5 channel function in several cardiac disease states such as HF14,21. As NaV1.8 was previously shown to interact with Ankyrin-G in neurons37 in a similar fashion as it is known for NaV1.5 in cardiomyocytes, similar mechanisms of NaV1.8 interaction with CaMKIIδc like known for NaV1.5 are conceivable. Several Serin residues as well as CaMKII-binding consensus motifs were found to be conserved between NaV1.5 and NaV1.8 (for details see Supplementary Fig. 10).
In a variety of cardiac pathologies, enhanced CaMKIIδc activity and expression is a key contributor to maladaptive electrical remodeling and thereby promotes arrhythmias9,38–40. CaMKIIδc can influence INaL magnitude by phosphorylating NaV, which has been exclusively investigated for cardiac NaV1.5 before, whereas a possible CaMKIIδc-dependent regulation of NaV1.8 has not been investigated yet14,21,22. In our study, we investigated the contribution of NaV1.8 to INaL in an HF model that was exclusively induced by chronic CaMKIIδc overexpression. As previously shown, INaL was augmented in cardiomyocytes from CaMKIIδc+/T compared to WT21,40, while these effects were ameliorated by the application of specific NaV1.8 blockers. Therefore, at least a relevant part of CaMKIIδc-induced INaL appears to be driven by NaV1.8. Additional support for this conclusion comes from our INaL measurements from human failing cardiomyocytes where CaMKIIδc activity and INaL are both known to be increased in parallel2,13,16,21,41. Inhibition of either CaMKIIδc or NaV1.8 reduced INaL as separately demonstrated before21,31. However, simultaneous inhibition of NaV1.8 and CaMKIIδc had no additive effect compared to CaMKIIδc inhibition alone. Therefore, it can be assumed that the majority of NaV1.8-driven INaL was already abolished by CaMKIIδc inhibition and hence, seems to be CaMKIIδc-dependent. In addition, Scn10a knockout in CaMKIIδc+/T mice resulted in a reduction in INaL comparable to NaV1.8 inhibition upon specific blockers.
In previous studies, the functional relevance of NaV1.8 expression in cardiomyocytes was questioned as an application of specific blockers had no effects on peak INa and INaL in healthy and unstimulated cardiomyocytes42,43. These data are not in conflict with our findings, as we also did not observe differences in INaL magnitude in cardiomyocytes from healthy mice, while clear effects were evident under conditions of enhanced INaL either by chronic CaMKIIδc overexpression or isoproterenol treatment of iPSC-cardiomyocytes. Therefore, the interaction of NaV1.8 with enhanced CaMKIIδc activity might be necessary to generate meaningful effects on cardiomyocyte electrophysiology while NaV1.8 appears to play a negligible role in healthy cardiomyocytes. This establishes NaV1.8 to be a disease-specific target.
An augmentation of INaL was demonstrated to cause a Na+-dependent Ca2+ overload and spontaneous SR-Ca2+ release providing a substrate for cellular proarrhythmia8,15,30,44. We previously reported that inhibition of INaL by specifically targeting NaV1.8 is potent enough to reduce NCX reverse mode and thereby diastolic SR-Ca2+ leak11,31,32. In the present work, we inhibited either CaMKIIδc or/and NaV1.8 in failing humans and CaMKIIδc+/T mouse ventricular cardiomyocytes and correspondingly observed a decrease in INaL and diastolic SR-Ca2+ leak. A significant reduction of diastolic SR-Ca2+ release events was already prominent after NaV1.8 inhibition alone in failing human cardiomyocytes which was almost comparable to the effect caused by CaMKIIδc inhibition. Moreover, we did not observe a further reduction in SR-Ca2+ leak when AIP was co-administrated with the NaV1.8 blocker suggesting that effects of Nav1.8 inhibition on SR-Ca2+ leak act via indirect inhibition of CaMKIIδc as it was previously shown for INaL inhibition with TTX or Ranolazine8,15. The present findings, therefore confirm that NaV1.8 inhibition abrogates the vicious proarrhythmic cycle between enhanced INaL and CaMKIIδc.
Increased expression and enhanced activity of CaMKIIδc is not only known to increase proarrhythmic triggers in HF but is also associated with a strong HF phenotype and increased mortality in mice9,13,16,45. Our current findings illustrate that NaV1.8 plays a relevant role for arrhythmogenesis under conditions of enhanced CaMKIIδc activity. For deeper mechanistic analysis, we crossbred our CaMKIIδc+/T mice with Scn10a knockout mice. In fact, we observed an improved survival in these SCN10A−/−/CaMKIIδc+/T mice but still with an unchanged typical phenotype of dilated cardiomyopathy in these blinded investigations. There may be three potential explanations for the improved survival, which were either reduced lethal arrhythmias, pump failure, or a combination of both. As we could demonstrate that induction and progression of HF is not influenced by the additional Scn10a knockout we propose a reduction of augmented INaL with subsequent lower proarrhythmic activity to constitute the underlying mechanism of this improved survival. The link between enhanced INaL and increased arrhythmia risk is rather complex. On the one hand, enhanced INaL prolongs APD and may therefore give rise to the formation of EADs. On the other hand, enhanced INaL causes Na+ overload of the cardiomyocyte subsequently leading to Ca2+ overload by activating NCX reverse mode6,8,11,15. This may trigger the occurrence of diastolic SR-Ca2+ release and DADs due to enhanced CaMKIIδc-dependent RyR2 phosphorylation8,10,15. In our experiments, a reduction of INaL in SCN10A−/−/CaMKIIδc+/T compared to CaMKIIδc+/T cardiomyocytes was observed. This was clearly associated with a reduction in the occurrence of EADs, DADs and diastolic SR-Ca2+-release events, thereby illustrating that the CaMKIIδc-induced proarrhythmic phenotype can be ameliorated on the cellular level by NaV1.8 knockout.
In a recent study by our groups, we could demonstrate that selective inhibition of diastolic SR-Ca2+ leak by the compound Rycal S36 improves survival in an HF mouse model, where INaL and CaMKIIδc activity were also described to be enhanced10,46. As in our study, improved survival was caused by a significant reduction of malignant arrhythmias, while the severity of HF was unchanged. Likewise, we observed less ventricular arrhythmias in SCN10A−/−/CaMKIIδc+/T mice in vivo. It is well known that individuals with structural heart disease are at increased risk for sustained ventricular tachycardia and ventricular fibrillation47. Moreover, sustained ventricular tachycardia may degenerate to ventricular fibrillation47. In addition, clinical trials have shown an association of PVCs and ventricular tachycardia with adverse outcomes in patients with dilated cardiomyopathy47–49. The NaV1.8 knockout significantly reversed a relevant part of the arrhythmogenic CaMKIIδc transgenic substrate in vitro and in vivo which is known to be associated with sudden cardiac death. However, due to the sporadic nature of sudden cardiac death, we were not able to correlate mortality with ventricular arrhythmias in these CaMKIIδc transgenic mice as this is technically and ethically not feasible. We are also not aware of any other experimental trial that investigates such a high number of HF mice with implanted telemetric recorders during death struggle. Finally, a chicken or the egg question would remain, as detected rhythm disorders cannot be differentiated to be either caused primary by arrhythmia or secondary due to hypoxia.
Persistent CaMKIIδc hyperactivity in hearts of HF patients has several detrimental effects such as influencing myocardial remodeling and promoting arrhythmias33,39. Despite the fact that several CaMKIIδc inhibitors have been demonstrated to effectively reduce arrhythmogenesis in HF in vitro46,50, CaMKIIδc inhibition to manage cardiac arrhythmias has not been established in patients so far. This can be explained by the vast signaling network of this enzyme, which is required to keep a balance between pivotal off-target effects and the therapeutic benefits. Any other alternative downstream or upstream target of the CaMKIIδc pathway, which is involved in promoting arrhythmias may be a more specific therapeutic option. As it has consistently been shown that activation of CaMKIIδc is involved in enhancement of INaL and vice versa10,11,21,34,40 our proposed crosstalk between CaMKIIδc and NaV1.8 causing enhanced INaL in the presence of hyperactive CaMKIIδc may be a novel strategy to prevent detrimental CaMKIIδc-induced effects on cellular electrophysiology. Most importantly, we and others have shown that selective NaV1.8 inhibition does not influence either peak Na+ current or Na+ current kinetics and thereby cardiac conduction as another critical regulator of proarrhythmia11,29,31,42,51.
In summary, the results of our current study demonstrate that increased CaMKIIδc activity in HF contributes to enhanced INaL via interaction with NaV1.8. This augmented INaL generated by NaV1.8 detrimentally influences cellular electrophysiology by increasing RyR2-leakiness and can give rise to cellular proarrhythmic events in HF cardiomyocytes. Importantly, the results of the present study demonstrate that pharmacological inhibition and genetic ablation of NaV1.8 can specifically reverse these detrimental proarrhythmic effects in the human HF heart and in our SCN10A−/−/CaMKIIδc+/T mouse model. This is associated with improved animal survival. Therefore, targeting NaV1.8 as a specific substrate of increased CaMKIIδc activity may constitute a promising antiarrhythmic approach in HF which merits further translational investigation.
Methods
Human tissue samples
The study conforms to the declaration of Helsinki and was approved by the local ethics committee. All participants were informed about the study prior to inclusion. All patients signed informed consent. We obtained left ventricular tissues from explanted hearts of patients with end-stage HF (NYHA HF classification IV) who were undergoing heart transplantation. After explantation, the whole heart or myocardial tissues were immediately placed in a container having precooled cardioplegic solution containing (in mmol/L): NaCl 110, KCl 16, MgCl2 16, NaHCO3 16, CaCl2 1.2, and glucose 11. Myocardial samples for Western blot and co-immunoprecipitation were immediately frozen in liquid nitrogen and stored at −80 °C. The heart tissue for cell isolation was stored in cooled cardio-protective solution containing (in mmol/L): 156 Na+, 3.6 K+, 135 Cl−, 25 HCO3−, 0.6 Mg2+, 1.3 H2PO4−, 0.6 SO42−, 2.5 Ca2+, 11.2 glucose, and 10 2,3-butanedionmonoxime (BDM) aerated with 95% O2 and 5% CO2. We used healthy myocardium from healthy donor hearts that could not be transplanted for technical reasons as controls co-immunoprecipitation. Patient characteristics are presented in the Supplementary information (Supplementary Table 3). The study was approved by the local ethics committee of the University Medicine of Goettingen.
CaMKIIδc transgenic mice
CaMKIIδc transgenic (CaMKIIδc+/T) mice were generated using an α-MHC promoter13. We used 12- to 14-week-old mice for electrophysiology experiments. The animal investigations conform to the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (8th edition, revised 2011) and the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. The mouse study was approved by the local ethics committee of the University Medicine of Goettingen and the public authority on animal welfare.
Generation of SCN10A−/−/CaMKIIδc+/T mice
To generate the SCN10A−/−/CaMKIIδc+/T mouse model we crossbred CaMKIIδc+/T mice from a Black-Swiss strain with NaV1.8 knockout mice (SCN10A−/−) from a C57BL/6 strain. Eight weeks old male CaMKIIδc+/T mice were mated with 8 weeks old female SCN10A−/− mice. This breeding resulted in a genotype carrying a WT or transgenic (TG) allele of the CaMKIIδc gene and a heterozygote knockout of Scn10a. From this first generation, 8 weeks old male mice carrying a TG CaMKIIδc allele (CaMKIIδc+/T) were mated with 8 weeks old female mice carrying homozygous WT CaMKIIδc alleles and heterozygous for Scn10a gene. This breeding produced 25% mice with the desired genotype having heterozygous CaMKIIδc gene (CaMKIIδc+/T) and homozygous Scn10a knockout (SCN10A−/−/CaMKIIδc+/T). Mice carrying a heterozygote Scn10a gene were excluded from survival analysis and experimental studies. A detailed scheme of the crossbreeding is displayed in the Supplementary information (Supplementary Fig. 11). The breeding rooms were maintained at 20–22 °C with 50–60% humidity. Mice were housed in a room with a 12-h light/dark cycle with ad libitum access to water and food.
Survival of CaMKIIδc+/T mice was documented in a database of the local facility of animal experiments. A survival curve was prepared from this database and a comparison of survival was made between the groups CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T. Animals used for experiments were excluded from survival analysis. Before scarifying animals, body weight was recorded while after explantation heart weight and tibia length were measured to tabulate animal size. Some of the animals showed signs of heart failure with fluid retention while some had signs of terminal illness therefore, heart weight to tibia length ratio was analyzed instead of heart weight to body weight ratio. The mouse study was approved by the local ethics committee of the University Medicine of Goettingen and the public authority on animal welfare.
Generation of homozygous knockout iPSCs using CRISPR/Cas9 and directed differentiation into iPSC-cardiomyocytes
All procedures were performed according to the Declaration of Helsinki and were approved by the local ethics committee. Informed consent was signed by all tissue donors. Gene editing was conducted using two CRISPR guide RNAs (gRNA1: GTGACTCCGGAGTAAAGCGACGG and gRNA2: ACGGAAGTTGTTAGTTTCGAGG, designed with IDTdna.com design tool) targeting SCN10A exon1. About 2 × 106 wild-type iPSCs were electroporated with 2.5 µl (100 µM) of each gRNA, 5 µl tracrRNA (100 µM), 2 µl Cas9 protein (10 ng/l, IDT), and 1 µl electroporation enhancer (100 µM, IDT) using the Amaxa Nucleofection II Device (Lonza, program B-016) and the corresponding Human Stem Cell Nucleofection Kit (Amaxa, VPH-5022). Electroporated iPS cells were expanded and analyzed by Sanger sequencing (Microsynth). After additional singularization two identical homozygous SCN10A knockout clones (K62.1 and K62.4) were chosen for pluripotency analysis and cardiac differentiation. Two NaV1.8 knockout iPSC-lines (K62.1 and K62.4) and the corresponding isogenic control iPSC- line52 were cultured feeder-free and adherent by cultivation on Geltrex-coated cell culture dishes in the presence of chemically defined medium E8 (Life Technologies).
Directed cardiac differentiation of iPSCs was done by manipulation of the Wnt signaling pathway52. Briefly, iPSCs were cultured in E8 medium as a monolayer on Geltrex-coated 12-well plates to a confluence of 85–95%. Wnt signaling was initiated by a medium change to RPMI1640 GlutaMAX (Thermo Fisher Scientific) supplemented with human recombinant albumin, l-ascorbic acid 2-phosphate including the GSK3β inhibitor CHIR99021 (4 µmol/L, Millipore). After 48 h, cells were treated with fresh medium supplemented with the inhibitor of Wnt production 2 (IWP2) (5 µmol/L, Millipore) for 48 h. On day 6, the medium was changed to RPMI1640 GlutaMAX with 1x B27 with insulin (Thermo Fisher Scientific) with a medium change every 2–3 days. Cardiomyocytes were purified by using a metabolic selection for 2–4 days with 4 mmol/L lactate as carbon source after 15–20 days of differentiation53. Cells were cultured for 60 days and passaged onto glass-bottom FluoroDishes (WPI, 30 K/dish) by trypsinizing for 3 min at 37 °C. Cells settled for 7 days prior to further measurements with medium change every 2 days. IPSC-cardiomyocytes were analyzed 8–10 weeks after initiation of differentiation except when mentioned otherwise. Following differentiation, purity of iPSC-CM was determined by flow analysis (>90% cardiac TNT+) and qPCR of ventricular cardiomyocytes marker MLC2v. Four to five differentiation experiments into ventricular iPSC-CMs of two NaV1.8 knockout lines and the corresponding healthy isogenic control line were used.
Cardiomyocyte isolation
Human
Human myocardium was rinsed, cut into small pieces, and incubated at 37 °C in a spinner flask filled with Joklik-MEM solution (JMEM; AppliChem, Darmstadt, Germany) containing 1.0 mg/mL collagenase (type 1, 185 U/mg; Worthington Biochem, New Jersey, USA) and trypsin (2.5 g/L, Life Technologies, Carlsbad, California, USA). After 45 min of digestion, the supernatant was discarded, and the tissue was incubated with fresh JMEM solution containing only collagenase. The solution was incubated for 10 to 20 min until cardiomyocytes were disaggregated using a Pasteur pipette. The supernatant containing disaggregated cells was removed and centrifuged (700 r.p.m., 5 min). Fresh JMEM with collagenase was added to the remaining tissue. This procedure was repeated 4 to 7 times. After every step, the centrifuged cells were resuspended in JMEM medium containing (in mmol/L): 10 taurine, 70 glutamic acid, 25 KCl, 10 KH2PO4, 22 dextrose, 0.5 EGTA, and 10% bovine calf serum (pH 7.4 adjusted with KOH at room temperature). Only cell solutions containing elongated, non-granulated cardiomyocytes with cross-striations were selected for experiments, plated on laminin-coated recording chambers, and allowed to settle for 30 min.
Mouse
Explanted mouse hearts from male and female wild-type (WT), SCN10A−/−, CaMKIIδc+/T, and SCN10A−/−/CaMKIIδc+/T mice were retrogradely perfused with a nominally Ca2+-free Tyrode’s solution containing (in mmol/L): 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4 × 2H2O, 1.2 MgSO4 × 7H2O, 12 NaHCO3, 10 KHCO3, 10 HEPES, 30 taurine, 10 BDM, 5.5 glucose, and 0.032 phenol red (pH 7.4, with NaOH at 37 °C) using a Langendorff perfusion apparatus. Then, 0.05 mg/mL liberase TM (Roche Diagnostics, Mannheim, Germany), 0.014% trypsin, and 0.1 mmol/L CaCl2 were added to the perfusion solution. Once the heart became flaccid, ventricular tissue was removed, cut into small pieces, and dispersed. Ca2+ reintroductions were performed carefully by increasing Ca2+ stepwise from 0.1 to 0.4 mmol/L for the patch-clamp and to 1.6 mmol/L for Ca2+ spark experiments. Cells were plated on laminin-coated chambers, allowed to settle for 15 min, and then used for measurements.
Co-immunoprecipitation
Tissue homogenates from human ventricular myocardium were suspended in lysis buffer containing (1% CHAPS in RIPA buffer containing (in mmol/L): 50 Tris-HCl, 120 NaCl, 200 NaF, 1 Na3VO4, 1 DTT, (pH 7.4), and complete protease and phosphatase inhibitor cocktails (Roche Diagnostics). Protein concentration was determined by a BCA assay. CaMKIIδc was immunoprecipitated with rabbit polyclonal anti-CaMKIIδ antibody (3 μg/500 μg of protein; preincubation at 4 °C overnight, a gift from Prof. Donald M. Bers, Department of Pharmacology, University of California Davis, USA) and protein G Plus Agarose (prewashed and blocked with 5% BSA overnight at 4 °C; Pierce, #22851) for 2 h and 30 min at 4 °C. As a control, rabbit anti-control IgG (3 µg/500 µg protein Santa Cruz, sc-2027) was used and a negative control without antibody. The pellets were collected and washed with the RIPA buffer without CHAPS 3 times. The immunoprecipitated proteins were eluted in 2x Laemmli sample buffer containing β-mercaptoethanol (10 min at 70 °C). Supernatants were subjected to Western blotting to detect NaV1.8 (mouse monoclonal, LSBio, LS-C109037), CaMKIIδ (rabbit polyclonal, Thermo Scientific, PA5-22168), and GAPDH (mouse monoclonal, Biotrend, BTMC-A437-9).
Immunofluorescence staining of human isolated cardiomyocytes
Isolated human ventricular cardiomyocytes were allowed to attach the poly-l-lysine coated glass chambers for 30 to 45 min at room temperature. Cardiomyocytes were fixed in ice-cold 99.2% ethanol for 20 min at −20 °C. After fixation, cardiomyocytes were washed with phosphate buffer saline (1X PBS) and subsequently blocked and permeabilized with 0.5% Triton X-100 and 5% BSA (bovine serum albumin) in PBS at room temperature.
After blocking, cells were washed with PBS (3х 5 min) and incubated with a primary antibody: mouse monoclonal anti-NaV1.8 (1:50, LSBio, LS-C109037). The antibody was diluted in antibody diluent (Dako) containing 0.5% Triton X-100 and incubated overnight at 4 °C. On the next day, cells were washed with PBS (3х 10 min). After washing, cells were incubated for 1 h at room temperature in darkness with the fluorescent-conjugated secondary antibody: Alexa Fluor-488 goat anti-mouse (1:200, Life Technologies, A-11029). In the next step, cells were again incubated overnight at 4 °C with a primary antibody: rabbit polyclonal anti-CaMKIIδ (1:100, gift from D. M. Bers, Department of Pharmacology, University of California Davis, USA). After washing with PBS (3х 5 min) cells were incubated for 1 h at room temperature in darkness with a fluorescent-conjugated secondary antibody: Alexa Fluor-555 goat anti-rabbit (1:200, Life Technologies, A-21424). After washing, cells were covered with Vectashield HardSet Mounting Medium (Vector Laboratories) and viewed using an LSM 5 Pascal confocal microscope (Zeiss) with a 40x oil immersion objective.
Immunofluorescence of iPSCs and iPSC-cardiomyocytes
NaV1.8 KO-iPSCs and iPSC-CMs were fixated with 4% Histofix solution (Sigma) for 20 min at room temperature and subsequently blocked in 1% BSA overnight at 4 °C. The primary antibodies mouse anti-SSEA4 (1:200, Abcam), mouse anti-α-actinin (1:750, Sigma), rabbit anti-Titin-M8/M9 (1:750, MyoMedix) were incubated overnight at 4 °C. The fluorescently labeled secondary antibodies were added for 1 h at 37 °C (AF488 donkey anti-mouse IgG (1:1000, Invitrogen); AF555 donkey anti-rabbit IgG (1:750, Invitrogen). Images were acquired with the Axiovert 200 fluorescence microscope (Zeiss) and the Axiovision software (Rel 4.8).
Patch-clamp experiments
INaL measurements
To measure INaL in human and mouse ventricular cardiomyocytes, a ruptured whole-cell patch-clamp was performed at room temperature. The resistance of the pipette was between 2 and 3 mega-Ohm (MΩ) when filled with pipette solution containing (in mmol/L): 95 CsCl, 40 Cs-glutamate, 10 NaCl, 0.92 MgCl2, 5 Mg-ATP, 0.3 Li-GTP, 5 HEPES, 0.03 niflumic acid (to block Ca2+-activated chloride current), 0.02 nifedipine (to block Ca2+ current), 0.004 strophanthidin (to block Na+/K+ ATPase current) 1 EGTA, and 0.36 CaCl2 (free [Ca2+]i,100 nmol/L) (pH 7.2 with CsOH at room temperature). Cardiomyocytes were maintained in the bath solution containing (in mmol/L): 135 NaCl, 5 tetramethylammonium chloride, 4 CsCl, 2 MgCl2, 10 glucose, 10 HEPES (pH 7.4 with CsOH at room temperature). To minimize contaminating Ca2+ currents during INaL measurements, Ca2+ was omitted from the bath solution. INaL was measured only in those cardiomyocytes where a seal of more than 1 giga-Ohm was achieved and the access resistance remained <7 MΩ. When whole-cell patch configuration was achieved, cardiomyocytes were given a period of 3 min to be stabilized before conducting measurements. Then cardiomyocytes were held at −120 mV before depolarization to −35 mV for a duration of 1000 ms with 10 pulses and a basic cycle length of 2 s. INaL was measured as integral current amplitude between 100 and 500 ms and was normalized to the membrane capacitance. Mouse cardiomyocytes were incubated with either A-803467 (30 nmol/L, Sigma) or PF-01247324 (1 µmol/L, Sigma) for 15 min before starting INaL measurements. Human cardiomyocytes were incubated with autocamptide-2-related inhibitor peptide (AIP, 1 µmol/L), PF-01247324 (1 µmol/L, Sigma), or PF-01247324+AIP for 15 min. For control groups, cardiomyocytes were incubated for 15 min in a normal bath solution to equilibrate the measurements.
Current–voltage relationship of INaL
Ruptured whole-cell patch-clamp experiments were applied to establish the current–voltage relationship of INaL. The pipette resistance ranged between 1.5 and 3.5 MΩ when filled with the pipette solution containing (in mmol/L): 95 CsCl, 40 Cs-glutamate, 10 NaCl, 0.92 MgCl2, 5 Mg- ATP, 0.3 Li-GTP, 5 HEPES, 0.03 niflumic acid (to block Ca2+-activated chloride current), 0.02 nifedipine (to block Ca2+ current), 0.004 strophanthidin (to block Na+/K+ ATPase current), 1 EGTA, and 0.36 CaCl2 (free [Ca2+]i,100 nmol/L) (pH 7.2 with CsOH at room temperature). The bath solution contained (in mmol/L): 135 NaCl, 5 tetramethylammonium chloride, 4 CsCl, 2 MgCl2, 10 glucose, 10 HEPES (pH 7.4 with CsOH at room temperature). In some experiments, cardiomyocytes were incubated with PF-01247324 (1 μmol/L, Sigma) for 15 min before initiation of INaL measurements. After patch rupture cardiomyocytes were allowed to stabilize for at least 3 min. Currents were elicited using a voltage step protocol with 10 mV increments ranging from −120 to +30 mV (step duration: 1 s, holding potential: −120 mV). INaL was determined as the mean current density between 180 to 190 ms of each depolarization step. PF-sensitive INaL was calculated as the difference between the mean INaL densities of the vehicle and PF-treated cardiomyocytes at each membrane potential.
Peak Na+ current measurements
Ruptured-patch whole-cell voltage-clamp was used to measure INa in mouse cardiomyocytes with microelectrodes (2–3 MΩ, room temperature). Pipettes were filled with (in mmol/L): 80 CsCl, 40 Cs-glutamate, 5 NaCl, 0.92 MgCl2, 5 Mg-ATP, 0.3 Li-GTP, 5 HEPES, 0.03 niflumic acid (to block Ca2+-activated chloride current), 0.02 nifedipine (to block Ca2+ current), 0.004 strophanthidin (to block Na+/K+ ATPase current), 1 EGTA, and 0.36 CaCl2 (free [Ca2+]i,100 nmol/L) (pH 7.2 with CsOH at room temperature). Cardiomyocytes were maintained in the bath solution containing (in mmol/L): 5 NaCl, 135 tetramethylammonium chloride, 4 CsCl, 2 MgCl2, 10 glucose, 10 HEPES, 0.4 CaCl2 (pH 7.4 with KOH at room temperature). INa was measured only in those cardiomyocytes where a seal of more than 1 Giga-Ohm was achieved and the access resistance remained <7 MΩ. Mouse cardiomyocytes were incubated with PF-01247324 (1 µmol/L, Sigma) for 15 min before starting INa measurements. In all experiments, myocytes were mounted on the stage of a microscope (Nikon Eclipse TE2000-U). Liquid junction potentials were corrected with the pipette in the bath. Membrane capacitance and series resistance were compensated after patch rupture. Data were collected using Patchmaster 2.0 (HEKA Elektronik).
Membrane potential recordings
The whole-cell patch-clamp technique in current-clamp configuration was used to measure membrane potential in single isolated cardiomyocytes. Microelectrodes (3–5 MΩ, room temperature) were filled with (in mmol/L): 92 K-Aspartate, 48 KCl, 1 Mg-ATP, 10 HEPES, 0.02 EGTA, 0.1 GTP-Tris, and 4 Na2-ATP (pH 7.2, KOH). The bath solution contained (in mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH 7.4, NaOH). Action potentials were continuously elicited by square current pulses of 1–2 nA amplitude and 1–5 ms duration at increasing stimulation frequency (0.5–3 Hz). Access resistance was typically ~5–15 MΩ after patch rupture. Fast capacitance was compensated for in a cell-attached configuration. Recordings were commenced after cell stabilization, which was ~5 min after rupture. Data were collected using Patchmaster 2.0 (HEKA Elektronik) and was analyzed using LabChart 7.37 (ADInstruments). Afterdepolarizations such as EADs and DADs were counted under continuous stimulation with 0.5 Hz. An EAD was counted as an EAD when a renewed depolarization of at least 1 mV occurred before complete membrane repolarization. Criteria for counting DADs were similar, but after achieving complete membrane repolarization.
Ca2+ spark and Ca2+ wave measurement
Human and mouse ventricular cardiomyocytes were loaded with a Fluo-4AM (10 µmol/L for 15 min, Molecular Probes) at room temperature. For some experiments, either A-803467 (30 nmol/L for 15 min, Sigma) or PF-01247324 (1 µmol/L for 15 min, Sigma) was added to the loading buffer to inhibit NaV1.8. Cells were perfused with Tyrode’s solution containing (in mmol/L): 136 NaCl, 4 KCl, 0.33 NaH2PO4, 4 NaHCO3, 2 CaCl2, 1.6 MgCl2, 10 HEPES, 10 glucose (pH 7.4 adjusted with NaOH at room temperature) and the respective active agent. The cardiomyocytes were washed with Tyrode’s solution for 15 min for de-esterification of the Ca2+ dye. Ca2+ spark measurements were performed with a laser scanning confocal microscope (LSM 5 Pascal, Zeiss) using a 40× oil immersion objective. Fluo-4AM was excited by an argon ion laser (488 nm), and emitted fluorescence was collected through a 505-nm long-pass emission filter. Fluorescence images were recorded in the line scan mode with 512 pixels per line (width of each scanline: 38.4 µm) and a pixel time of 0.64 µs. One image consists of 10,000 unidirectional line scans, equating to a measurement period of 7.68 s. Experiments were conducted under resting conditions after loading the sarcoplasmic reticulum with Ca2+ by field stimulation at 1 Hz and 20 V. Ca2+ sparks were analyzed with the program SparkMaster for ImageJ. The mean spark frequency of the respective cell (CaSpF) resulted from the number of sparks normalized to cell width and scan rate (100 µm/s). Cardiomyocytes exhibiting major arrhythmic events (Ca2+ waves, spontaneous Ca2+ transients, and spark clouds) were excluded from the quantification of the Ca2+ spark. Cardiomyocytes showing Ca2+ waves were analyzed separately. The percentage of cardiomyocytes exhibiting Ca2+ waves was calculated and compared between the groups. Some cardiomyocytes showed more than one event therefore we calculated the events per time and represented this ratio as waves per minute. The time to the first arrhythmic event was measured in seconds.
Ca2+ transient measurements
Cardiomyocytes were isolated from CaMKIIδc+/T mouse ventricles, plated as described above, and incubated with a Fura-2AM loading buffer (10 µmol/L, Molecular Probes, Eugene, Oregon, USA) for 20 min. After staining, the cardiomyocytes were washed with experimental solution (as described above in the Ca2+ sparks measurement section, at room temperature) for 15 min before measurements were started to enable complete de-esterification of intracellular Fura-2AM and to allow cellular rebalance of Ca2+ cycling properties. During measurements, cardiomyocytes were continuously superfused with an experimental solution. Measurements were performed with a Motic AE31 microscope provided with a fluorescence detection system (ION OPTIX Corp.) at room temperature. Cells were excited at 340 and 380 nm and the emitted fluorescence was collected at 510 nm. The intracellular Ca2+ level was measured as the ratio of fluorescence at 340 and 380 nm (F340/F380 nm in ratio units, r.u.). Ca2+ transients were recorded at steady-state conditions under constant field stimulation (1.0, 2.0, and 4.0 Hz, 20 V). The recorded Ca2+ transients were analyzed with the software IONWizardR (ION OPTIX Corp.).
Analysis of Ca2+-transients from confocal line scans
From confocal line scans that were used for quantification of diastolic Ca2+ waves in cardiomyocytes from CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T mice Ca2+-kinetics were analyzed from the Ca2+-transients at the beginning of each line scan before 3 Hz steady-state stimulation was paused. Ca2+-transient amplitude (F, normalized to diastole, F0) and time to half-maximal decay (RT50), and time to 90% of maximal decay were calculated from confocal line scan images using ImageJ software.
Quantitative real-time PCR (RT-qPCR)
Human or mouse cardiac tissues, isolated human cardiomyocytes or iPSC-cardiomyocytes were snap-frozen in liquid nitrogen and stored at −80 °C. RNA was isolated by use of the ReliaPrepTM RNA Tissue Miniprep System (Promega). About 200 ng RNA was reverse transcribed into cDNA using standard protocols (QuantiNova Reverse Transcription Kit (QIAGEN) for mouse tissue and iScript cDNA Synthesis Kit (Bio-Rad) for human samples). For qPCR, 10 µL SYBR Green PCR Master Mix (Bio-Rad), 7 µL nuclease-free water, 1 µL forward and 1 µl reverse Primer, and 1 µL of cDNA were mixed. Q-PCR was carried out using the CFX ConnectTM Real-Time System (Bio-Rad). Forty cycles of 15 s at 95 °C followed by 1 min of 60 °C were used and fluorescence was measured after each cycle. After 40 cycles melt curve analysis was performed to ensure the specificity of the products. Thresholds cycles were evaluated and normalized to housekeeping genes and controls. A list of all primers used is presented in Supplementary Table 4.
Western blots
Ventricular tissue from WT, SCN10A−/−, CaMKIIδc+/T, and SCN10A−/−/CaMKIIδc+/T mice or pellets of iPSC-cardiomyocytes from WT and homozygous NaV1.8 knockout (KO) lines were homogenized in Tris buffer containing (in mmol/L): 20 Tris-HCl, 200 NaCl, 20 NaF, 1 Na3VO4, 1 DTT, 1% Triton X-100 (pH 7.4), and complete protease and phosphatase inhibitor cocktails (Roche Diagnostics). Protein concentration was determined by BCA assay (Pierce Biotechnology). Denatured tissue homogenates (10 min, 70 °C in 2% beta-mercaptoethanol) were separated on 8% SDS-polyacrylamide gels, then transferred to a nitrocellulose membrane, and incubated with the following primary antibodies: rabbit polyclonal anti-CaMKIIδ (1:5000, Thermo Scientific, PA5-22168), rabbit polyclonal anti-NaV1.5 (1:2000, Alomone labs, ASC-005), and mouse monoclonal anti-GAPDH (1:20000, BIOTREND, BTMC-A473-9) at 4 °C overnight. Secondary antibodies included HRP-conjugated goat anti-rabbit and goat anti-mouse (1:20000, Jackson Immunoresearch, 111-035-144 and 115-035-062, respectively). The membrane was incubated with secondary antibodies for 1 h at RT. ImmobilonTM Western Chemiluminescent HRP Substrate (Millipore) was used for chemiluminescent detection. Analysis was performed using Image Studio Lite Ver 5.2. The full scan blots for NaV1.5 and CaMKIIδ are shown in the Data Availability file.
Histology
Freshly explanted hearts were fixed in 4% buffered formaldehyde at 4 °C for 24 h. LV tissue was harvested, fixed in 4% buffered formaldehyde overnight, paraffin-embedded, sectioned (5 μm), and stained with fluorescein‐conjugated wheat germ agglutinin (WGA‐Alexa Fluor 594; Invitrogen, USA) for cross‐sectional area (CSA) assessment. At least 300 randomly selected cardiomyocytes per animal from different sections and different regions (basal, mid-ventricular, apical) of each heart were measured using the ImageJ software (Bethesda, USA).
Mouse echocardiography
For echocardiography, mice were anesthetized using 1.5% isoflurane, and echocardiography was performed using a VS-VEVO 660/230 (VisualSonics, Canada). During this procedure, the core temperature was maintained at 37 °C, and heart rates were kept consistent between experimental groups at 400–500 b.p.m. Electrocardiogram monitoring was obtained using hind limb electrodes. LV geometry and systolic function were assessed by using standard 2D parasternal long and short-axis views. The examiner was blinded towards group assignment.
Telemetric ECG recordings in mice
Mice were implanted with an intraperitoneal telemetric ECG transmitter (TA11ETA-F10, Data Sciences International) with its Cable in lead II configuration. After a postoperative recovery period of 10 days, we recorded ECGs during periods of normal activity (24 h continuous recording, twice a week for 2 weeks). ECG parameters were analyzed, and the QT interval was corrected (QTc) using Bazett’s formula, QTc = QT/(RR/100)1/2 established for mice with QT and RR expressed in ms. Premature ventricular contractions and ventricular tachycardia (>3 beats) were counted per 24 h and a medium value was per animal was calculated from all measurements. Further, physical activity was recorded by the telemetric monitors.
Statistics
All data were expressed as the mean ± standard error of the mean (SEM). Where appropriate, one-way ANOVA with multiple comparison tests (post hoc Bonferroni’s and two-stage step-up methods of Benjamini, Krieger, and Yekutieli correction) was used. Mixed-effects analysis with Holm–Sidak’s post hoc test was used to analyze I-V curves. Otherwise, Student’s unpaired t-tests were used. The Chi-Square test was used to compare the occurrence of diastolic Ca2+ waves. Log-rank (Mantel–Cox) test was used to compare survival between CaMKIIδc+/T and SCN10A−/−/CaMKIIδc+/T mice. Two-sided p ≤ 0.05 was considered significant.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Prof. John Wood (University College London) for providing us a pair of SCN10A+/− mice. We thank Timo Schulte, Yvonne Metz, Johanna Heine, Sabrina Koszewa, Manar El Kenani, and Sarah Zafar for technical assistance. This work was supported by the German Heart Foundation/German Foundation of Heart Research (to P.B., P.T., and S.S.); by the University Hospital Regensburg (ReForM C program) (to L.S.M. and S.S.); by the Marga und Walter Boll-Stiftung (to S.A. and S.S.); the College of Translational Medicine by the Ministry of Culture and Science, State of Lower Saxony (P.B.), the German Center for Cardiovascular Research (DZHK; to K.S.-B.), the Else Kröner-Fresenius Stiftung (N.H. and M.T.) and the Deutsche Forschungsgemeinschaft (DFG) through the International Research Training Group Award (IRTG) 1816 (to K.S.-B.; W.M. is a fellow under IRTG 1816) and SFB 1002 (to K.T., N.D., and G.H.).
Source data
Author contributions
P.B., S.A., and S.S. conceived the study. K.S.-B., P.B., and S.S. designed the experiments and wrote the manuscript. P.B., N.D., P.T., S.A., B.M., N.H., M.C.K., W.M., S.P., and M.T. carried out experimental work and analyzed the data. K.S.-B. designed the CRISPR experiments. J.G., H.M., and S.L.-H. acquired and provided human tissue. K.T. applied for the authorization to carry out animal experiments and helped to analyze in vivo data. J.M., S.W., L.S.M., and G.H. provided expertise and feedback. All authors discussed the results and had the opportunity to comment on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analyzed during this study are available within the Article and its Supplementary Information. All raw data supporting the findings from this study are available from the corresponding author upon reasonable request. Source data are provided with this paper. Any remaining raw data will be available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Robin Shaw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Philipp Bengel, Nataliya Dybkova, Petros Tirilomis, Katrin Streckfuss-Bömeke, Samuel Sossalla.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-26690-1.
References
- 1.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na(+) concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 2.Maltsev VA, et al. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 3.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur. J. Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. AJP Hear. Circ. Physiol. 2008;294:H2031–H2039. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- 5.Valdivia CR, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J. Mol. Cell. Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Sossalla S, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts — Role of late sodium current and intracellular ion accumulation. J. Mol. Cell. Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: Role of sustained inward current. Cell. Mol. Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer TH, et al. Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII. Cardiovasc. Res. 2015;107:184–196. doi: 10.1093/cvr/cvv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sag CM, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ. Hear. Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toischer K, et al. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J. Mol. Cell. Cardiol. 2013;61:111–122. doi: 10.1016/j.yjmcc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengel P, et al. Contribution of the neuronal sodium channel NaV1.8 to sodium- and calcium-dependent cellular proarrhythmia. J. Mol. Cell. Cardiol. 2020;144:35–46. doi: 10.1016/j.yjmcc.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Larbig R, Torres N, Bridge JHB, Goldhaber JI, Philipson KD. Activation of reverse Na+-Ca2+ exchange by the Na+ current augments the cardiac Ca2+ transient: evidence from NCX knockout mice. J. Physiol. 2010;588:3267–3276. doi: 10.1113/jphysiol.2010.187708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier LS, et al. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 14.Ashpole NM, et al. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J. Biol. Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sag CM, et al. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J. Mol. Cell. Cardiol. 2014;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ. Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 17.Wehrens, X. H. T., Lehnart, S. E., Reiken, S. R. & Marks, A. R. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 94, e61–e70 (2004). [DOI] [PubMed]
- 18.Kohlhaas M, et al. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ. Res. 2006;98:235–244. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 19.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 20.Fischer TH, et al. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation. 2013;128:970–981. doi: 10.1161/CIRCULATIONAHA.113.001746. [DOI] [PubMed] [Google Scholar]
- 21.Wagner S, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glynn P, et al. Voltage-gated sodium channel phosphorylation at Ser571 regulates late current, arrhythmia, and cardiac function in vivo. Circulation. 2015;132:567–577. doi: 10.1161/CIRCULATIONAHA.114.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie MD, et al. Genome- and phenome-wide analyses of cardiac conduction identifies markers of arrhythmia risk. Circulation. 2013;127:1377–1385. doi: 10.1161/CIRCULATIONAHA.112.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabbari J, et al. Common and rare variants in SCN10A modulate the risk of atrial fibrillation. Circ. Cardiovasc. Genet. 2015;8:64–73. doi: 10.1161/HCG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savio-Galimberti E, et al. SCN10A/Nav1.8 modulation of peak and late sodium currents in patients with early onset atrial fibrillation. Cardiovasc. Res. 2014;104:355–363. doi: 10.1093/cvr/cvu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gando I, et al. Functional characterization of SCN10A variants in several cases of sudden unexplained death. Forensic Sci. Int. 2019;301:289–298. doi: 10.1016/j.forsciint.2019.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Facer P, et al. Localisation of SCN10A gene product Nav1.8 and novel pain-related ion channels in human heart. Int. Heart J. 2011;52:146–152. doi: 10.1536/ihj.52.146. [DOI] [PubMed] [Google Scholar]
- 29.Yang T, et al. Blocking Scn10a channels in heart reduces late sodium current and is antiarrhythmic. Circ. Res. 2012;111:322–332. doi: 10.1161/CIRCRESAHA.112.265173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabel, S. et al. Inhibition of NaV1.8 prevents atrial arrhythmogenesis in human and mice. Basic Res. Cardiol. 115, 20 (2020). [DOI] [PMC free article] [PubMed]
- 31.Dybkova N, et al. Differential regulation of sodium channels as a novel proarrhythmic mechanism in the human failing heart. Cardiovasc. Res. 2018;114:1728–1737. doi: 10.1093/cvr/cvy152. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad S, et al. The functional consequences of sodium channel NaV1.8 in human left ventricular hypertrophy. ESC Hear. Fail. 2019;6:154–163. doi: 10.1002/ehf2.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin- dependent protein kinase in failing and nonfailing human hearts. Cardiovasc. Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 34.Neef S, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ. Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 35.Zalcman, G., Federman, N. & Romano, A. CaMKII isoforms in learning and memory: Localization and function. Front. Mol. Neurosci.11, 445 (2018). [DOI] [PMC free article] [PubMed]
- 36.Hund TJ, et al. A βIV-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J. Clin. Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montersino A, et al. Tetrodotoxin-resistant voltage-gated sodium channel Nav 1.8 constitutively interacts with ankyrin G. J. Neurochem. 2014;131:33–41. doi: 10.1111/jnc.12785. [DOI] [PubMed] [Google Scholar]
- 38.Koval OM, et al. Ca2+/calmodulin-dependent protein kinase ii-based regulation of voltage-gated na+ channel in cardiac disease. Circulation. 2012;126:2084–2094. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sossalla S, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ. Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 40.Sossalla S, et al. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδC can be reversed by inhibition of late Na+ current. Basic Res. Cardiol. 2011;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Undrovinas AI, Maltsev VA, Kyle JW, Silverman N, Sabbah HN. Gating of the late Na+ channel in normal and failing human myocardium. J. Mol. Cell. Cardiol. 2002;34:1477–1489. doi: 10.1006/jmcc.2002.2100. [DOI] [PubMed] [Google Scholar]
- 42.Casini S, et al. Absence of functional Nav1.8 channels in non-diseased atrial and ventricular cardiomyocytes. Cardiovasc. Drugs Ther. 2019;33:649–660. doi: 10.1007/s10557-019-06925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stroud DM, et al. Contrasting Nav1.8 activity in Scn10a−/− ventricular myocytes and the intact heart. J. Am. Heart Assoc. 2016;5:e002946. doi: 10.1161/JAHA.115.002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bengel, P., Ahmad, S. & Sossalla, S. Inhibition of late sodium current as an innovative antiarrhythmic strategy. Curr. Heart Fail. Rep. 14, 179–186 (2017). [DOI] [PubMed]
- 45.Dybkova N, et al. Overexpression of CaMKIIδc in RyR2R4496C+/− knock-in mice leads to altered intracellular Ca2+ handling and increased mortality. J. Am. Coll. Cardiol. 2011;57:469–479. doi: 10.1016/j.jacc.2010.08.639. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed BA, et al. Sarcoplasmic reticulum calcium leak contributes to arrhythmia but not to heart failure progression. Sci. Transl. Med. 2018;10:eaan0724. doi: 10.1126/scitranslmed.aan0724. [DOI] [PubMed] [Google Scholar]
- 47.Al-Khatib SM, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 48.Meinertz T, et al. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1984;53:902–907. doi: 10.1016/0002-9149(84)90522-8. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann T, et al. Mode of death in idiopathic dilated cardiomyopathy: a multivariate analysis of prognostic determinants. Am. Heart J. 1988;116:1455–1463. doi: 10.1016/0002-8703(88)90728-4. [DOI] [PubMed] [Google Scholar]
- 50.Neef, S., Mann, C., Zwenger, A., Dybkova, N. & Maier, L. S. Reduction of SR Ca2+ leak and arrhythmogenic cellular correlates by SMP-114, a novel CaMKII inhibitor with oral bioavailability. Basic Res. Cardiol. 112, 45 (2017). [DOI] [PubMed]
- 51.Payne CE, et al. A novel selective and orally bioavailable Nav 1.8 channel blocker, PF-01247324, attenuates nociception and sensory neuron excitability. Br. J. Pharmacol. 2015;172:2654–2670. doi: 10.1111/bph.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borchert T, et al. Catecholamine-dependent β-adrenergic signaling in a pluripotent stem cell model of Takotsubo cardiomyopathy. J. Am. Coll. Cardiol. 2017;70:975–991. doi: 10.1016/j.jacc.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 53.Tohyama S, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available within the Article and its Supplementary Information. All raw data supporting the findings from this study are available from the corresponding author upon reasonable request. Source data are provided with this paper. Any remaining raw data will be available from the corresponding author upon reasonable request.