Abstract
Background:
Anterior cruciate ligament reconstruction (ACLR) is often performed on an outpatient basis; thus, effective pain management is essential to improving patient satisfaction and function. Local infiltration analgesia (LIA) and femoral nerve block (FNB) have been commonly used for pain management in ACLR. However, the comparative efficacy and safety between the 2 techniques remains a topic of controversy.
Purpose:
To compare pain reduction, opioid consumption, and side effects of LIA and FNB after ACLR.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
A systematic search of MEDLINE, Embase, and Cochrane Library databases was performed to identify studies comparing pain on the visual analog scale (a 100-mm scale), total morphine-equivalent consumption, and side effects between the 2 techniques after ACLR at the early postoperative period. The LIA was categorized into intra-articular injection and periarticular injection, and subgroup analyses were performed comparing either intra-articular injection or periarticular injection with FNB. Two reviewers performed study selection, risk-of-bias assessment, and data extraction.
Results:
A total of 10 studies were included in this systematic review and meta-analysis. In terms of VAS pain scores, our pooled analysis indicated that FNB was significantly more effective at 2 hours postoperatively compared with LIA (mean difference, 8.19 [95% confidence interval (CI), 0.75 to 15.63]; P = .03), with no significant difference between the 2 techniques at 4, 8, and 12 hours postoperatively; however, LIA was significantly more effective at 24 hours postoperatively compared with FNB (mean difference, 5.61 [95% CI, −10.43 to −0.79]; P = .02). Moreover, periarticular injection showed a significant improved VAS pain score compared with FNB at 24 hours postoperatively (mean difference, 11.44 [95% CI, −20.08 to −2.80]; P = .009), and the improvement reached the threshold of minimal clinically important difference of 9.9. Total morphine-equivalent consumption showed no difference between the 2 techniques, and side effects were unable to be quantified for the meta-analysis because of a lack of data.
Conclusion:
Compared with FNB, LIA was not as effective at 2 hours, comparable within 12 hours, and significantly more effective at 24 hours postoperatively for reducing pain after ACLR. Total morphine-equivalent consumption showed no significant differences between the 2 techniques.
Keywords: anterior cruciate ligament, femoral nerve block, knee, local infiltration analgesia, pain control
Anterior cruciate ligament (ACL) rupture is the most common sports injury of the knee, with an estimated annual incidence of 200,000 ruptures in the United States, a rate that is steadily increasing. 8,31,40 Postoperative pain management is essential after ACL reconstruction (ACLR), as the procedure is now frequently performed on an outpatient basis in many countries. 8,16,25,29,31,40 In addition, effective postoperative pain management leads to faster recovery, efficient rehabilitation, and patient satisfaction, potentially resulting in successful outcomes after ACLR. 5,16,31,43
Perioperative pain management after ACLR has been a topic of interest that is still a matter of debate. 31 Various attempts involving administration of opioids, multimodal drug administration, cryotherapy compression or machine, mobilization strategies, local infiltration analgesia (LIA), and femoral nerve block (FNB) have been considered. 26,31,37,42,44 Among those pain management strategies, the use of FNB has recently gained popularity as an effective outpatient procedure in terms of satisfactory pain reduction, as well as a reduction in the need for opioids in the early postoperative period. 3,12,14,28,31 However, FNB is not without side effects, and it can cause complications such as nerve or vascular injury, residual quadriceps weakness, and insufficient coverage of nerve block of hamstring autograft donor site. 2,15,18,32,41 In addition, FNB requires preoperative regional block time with special equipment such as ultrasonography.
Intra-articular injection, which is one of the traditional LIA modalities, has provided effective pain relief after knee surgery for decades. 6,38 Recently, several studies have demonstrated that satisfactory pain reduction and a low complication rate could be achieved after periarticular injection. 14,17 Also, liposomal bupivacaine [LB]), a nonopioid local anesthetic, was developed to provide a longer-acting LIA agent. However, there have been conflicting opinions on the efficacies of LIA and FNB for pain management 21,22,39 ; these conflicting results may be a result of the differences in detailed protocols. Owing to these conflicting results, we were inspired to perform a systematic review and meta-analysis to compare the efficacy and safety between LIA and FNB techniques after ACLR.
The aim of this study was to compare LIA versus FNB for effective pain control, total opioid consumption, and side effects in patients undergoing ACLR during the period up to 48 hours postoperatively. Our hypothesis was that pain management using LIA would lead to no significant difference in early pain reduction, opioid consumption, and side effects compared with FNB and might be an alternative option for FNB in patients after ACLR.
Methods
Literature Search
This systematic review and meta-analysis was designed according to Cochrane Review Methods. The protocol of review was registered in the International Prospective Register of Systematic Reviews, and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline 24 was used in the process of article identification and information extraction. A systematic search using the PubMed (MEDLINE), Embase, and Cochrane Library databases was performed for articles published between database inception and July 10, 2020, using an a priori search strategy. “Anterior cruciate ligament,” “anterior cruciate ligament reconstruction,” “ACLR,” “local infiltration analgesia,” “LIA,” “local injection,” “peri-articular injection,” “intra-articular injection,” “femoral nerve block,” and “FNB” were used as key words, aided by the use of Boolean operators “AND” or “OR.” The bibliographies of initially retrieved studies were manually cross-checked to find additional relevant articles that could have been missed via the electronic search. No language restriction was applied.
Study Selection
Two investigators (D.K.L. and J.-H.K.) independently screened titles and abstracts of retrieved articles; full manuscripts were reviewed if the abstract provided insufficient data for study inclusion. Studies were included in the current study based on the condition that they met the criteria for patients, intervention, comparator, outcomes, and study design. Specifically, (1) patients: patients receiving ACLR; (2) intervention: application of LIA for ACLR; (3) comparator: application of FNB for ACLR; (4) outcomes: visual analog scale (VAS) for pain, total morphine consumption, and side effects including nausea and vomiting, sedation, and pruritis; (5) study design: randomized controlled trials (RCTs) and comparative studies. Exclusion criteria consisted of (1) conference or (2) clinical trial abstracts, (3) insufficient statistics or inability to reproduce statistics, (4) the involvement of arthroscopic surgeries other than ACLR, (5) no direct comparison of LIA and FNB, (6) involvement of other nerve blocks, and (7) lack of assessment of VAS.
Assessment of Methodological Quality
The same 2 investigators independently assessed the methodological quality of each study using the methodological index for non-randomized studies (MINORS) checklist 34 which consisted of 12 items to assess quality of comparative studies. Each item was assigned a score of 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate), for a maximum score of 24 for a comparative study. A score of <15 was considered poor quality; 15 to 19, moderate quality; and >19, good quality. 45 Also, the Cochrane Handbook for Systematic Reviews of interventions was used to evaluate the risk of bias of the included RCTs. 10 This risk assessment was based on the following types of bias: selection, performance, detection, reporting, attrition, and other. Any discrepancies in scores between the 2 reviewers were resolved via discussion.
Data Extraction
The same 2 investigators independently collected available data from included studies, and any disagreement was resolved via discussion. We collected basic characteristics including sample size, patient age, sex, body mass index, study design, graft choice, operation time, and main findings of each study. In addition, we noted the detailed protocol of pain management for each group, including anesthesia type, method of LIA (intra-articular or periarticular injection), choice of drug and dosage, and specific postoperative pain management at the postanesthesia care unit (PACU) and after PACU. Relevant outcome measures included scores on a 100-mm VAS pain spectrum, total morphine-equivalent consumption, and side effects (eg, nausea and vomiting, sedation, and pruritis). We converted all VAS scores to a 100-mm scale.
Statistical Analysis
The intended primary outcome of the systematic review and meta-analysis was the mean difference (MD) in VAS pain scores between LIA and FNB after ACLR for early postoperative pain management. The MD in total morphine-equivalent consumption and the pooled incidences of side effects were analyzed for secondary outcomes in the current meta-analysis. The LIA was divided into intra-articular injection group and periarticular injection group. Subgroup analyses comparing either intra-articular or periarticular injection with FNB regarding primary and secondary outcomes were additionally performed. Publication bias was not assessed because it was not considered necessary if there were <10 studies in a comparison. 10 For continuous data, the MD with 95% confidence interval (CI) was used to calculate the effect interval. For discontinuous data, odds ratio with 95% CI was used to weight the effect interval. Heterogeneity was assessed by estimating the proportion of between-study inconsistencies because of actual differences between studies using the I 2 statistic. 23 Random-effects meta-analysis was performed to pool outcomes across included studies. Forest plots were used to show outcomes, pooled estimate of effect, and overall summary effect of each study and were constructed using RevMan Version 5.4 (The Cochrane Collaboration) and Open Meta-Analyst (http://www.cebm.brown.edu/openmeta). Meta-analysis, including >3 pooled studies, was regarded as valid for inclusion in the current analysis. Metaregression analysis was performed to assess the effects of age, operation time, subgroup of LIA, and graft choice on VAS pain scores. Statistical significance was set at P < .05.
Results
Identification of Studies
The initial electronic search yielded 568 studies, with 1 study added after additional manual searching. After removing 279 duplicate studies, 290 studies remained. Of these, 252 were excluded after reading the title or abstract because of irrelevance to the research question, and 28 were excluded after full-text review. In the final assessment, 10 studies were included for systematic review (Figure 1).
Figure 1.

PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for identification and selection of studies. ACLR, anterior cruciate ligament reconstruction; FNB, femoral nerve block; LIA, local infiltration analgesia; VAS, visual analog scale.
Study Characteristics and Methodological Quality Assessment
Of the 10 included studies, 8 11,12,14,20 –22,28,39 were RCTs, and the other 2 were comparative studies that were designed prospectively 17 and retrospectively 30 (Appendix Table A1). A total of 400 knees and 388 knees with LIA and FNB, respectively, were included. In terms of grafts used in ACLR, hamstring autografts were used in 5 studies 11,12,14,17,39 ; bone--patellar tendon—bone (BPTB) autografts were used in 3 studies 20 –22 ; BPTB autografts, hamstring autografts, or posterior tibialis allografts were used in 1 study 28 ; and no information was provided in another study 39 (Appendix Table A2). Studies had a median MINORS score of 20 (range, 16-24) with 4 moderate- and 6 good-quality studies (Appendix Table A1). Quality assessment for bias based on guidelines previously provided is shown in Appendix Figure A1. Publication bias was absent, though the paucity of included studies limited its statistical significance.
Primary Outcome: VAS Pain Scores
Pain on VAS was evaluated at 2, 4, 8, 12, 24, and 48 hours after ACLR comparing LIA (intra-articular or periarticular injection) with FNB in included studies. At 2 hours after ACLR, 6 studies (439 patients) 11,12,20,21,30,39 reported VAS pain scores comparing the 2 groups, showing that the pooled MD was significantly improved in FNB compared with LIA (MD, 8.19 [95% CI, 0.75 to 15.63]; P = .03). Of the 6 studies, 5 11,20,21,30,39 reported intra-articular injection comparing FNB, showing significantly improved VAS in FNB versus intra-articular injection (MD, 8.86 [95% CI, 0.46 to 17.26]; P = .04) (Figure 2). At 4 hours after ACLR, 4 studies 11,12,20,22 (368 patients) reported VAS comparing LIA with FNB. No significant differences were found between LIA and FNB (MD, −2.18 [95% CI, −8.55 to 4.18]; P = .50) (Figure 2).
Figure 2.
Forest plots of the included studies showing improvement in visual analog scale pain scores at (A) 2 hours and (B) 4 hours between LIA and FNB after ACLR. LIA was categorized into subgroups of intra-articular injection and periarticular injection, and each subgroup was compared with FNB. Squares represent the mean difference in outcomes, with the size of the square being proportional to the sample size. ACLR, anterior cruciate ligament reconstruction; FNB, femoral nerve block; IA, intra-articular injection; IV, inverse variance; LIA, local infiltration analgesia; PAI, periarticular injection.
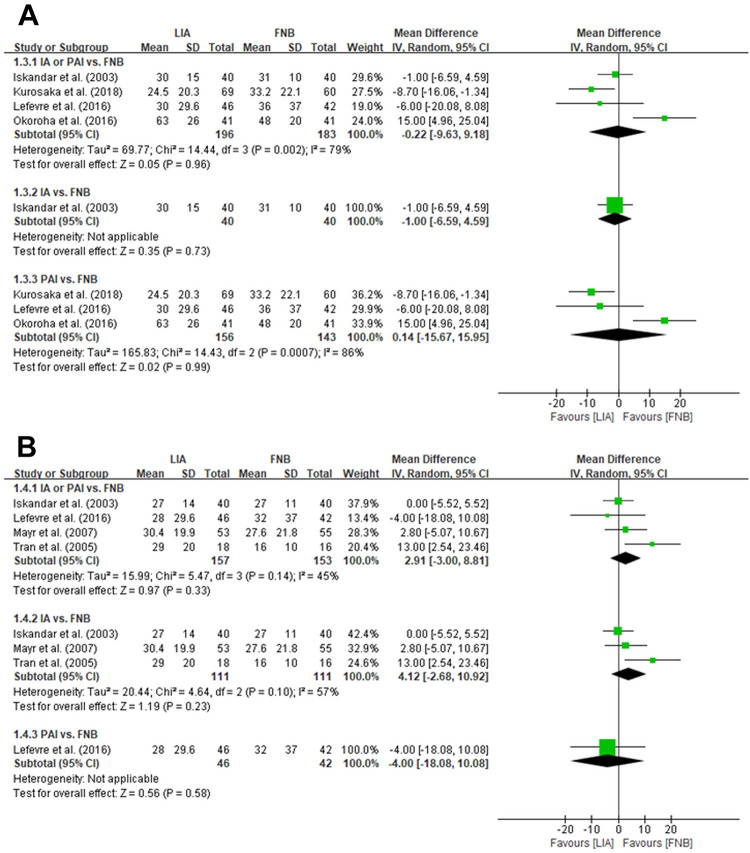
At 8 hours after ACLR, 4 studies (380 patients) 11,14,17,28 that reported VAS scores found no significant differences between LIA and FNB (MD, −0.22 [95% CI, −9.63 to 9.18]; P = .96) (Figure 3). At 12 hours after ACLR, 4 studies (311 patients) 11,17,20,39 that reported VAS scores found no significant differences between LIA and FNB (MD, 2.91 [95% CI, −3.00 to 8.81]; P = .33) (Figure 3).
Figure 3.
Forest plots of the included studies showing improvement in visual analog scale pain scores at 8 hours (A) and 12 hours (B) between LIA and FNB after ACLR. LIA was categorized into subgroups of intra-articular injection and periarticular injection, and each subgroup was compared with FNB. Squares represent the mean difference in outcomes, with the size of the square being proportional to the sample size. ACLR, anterior cruciate ligament reconstruction; FNB, femoral nerve block; IA, intra-articular injection; IV, inverse variance; LIA, local infiltration analgesia; PAI, periarticular injection.
At 24 hours after ACLR, 7 studies (573 patients) 11,12,14,17,20 –22 reported VAS comparing LIA (intra-articular or periarticular injection) with FNB, showing that the pooled MD was significantly improved in LIA compared with FNB (MD, −5.61 [95% CI, −10.43 to −0.79]; P = .02). Of these 7 studies, 4 11,20 –22 compared intra-articular LIA injection with FNB and found significantly better VAS scores for intra-articular injection (MD, −2.71 [95% CI, −4.95 to −0.47]; P = .02). The remaining 3 studies 12,14,17 comparing periarticular LIA injection with FNB showed significantly improved VAS scores in the periarticular injection compared with FNB (MD, −11.44 [95% CI, −20.08 to −2.80]; P = .009) (Figure 4).
Figure 4.
Forest plots of the included studies showing improvement in visual analog scale pain scores at 24 hours between LIA and FNB after ACLR. LIA was categorized into subgroups of intra-articular injection and periarticular injection, and each subgroup was compared with FNB. Squares represent the mean difference in outcomes, with the size of the square being proportional to the sample size. ACLR, anterior cruciate ligament reconstruction; FNB, femoral nerve block; IA, intra-articular injection; IV, inverse variance; LIA, local infiltration analgesia; PAI, periarticular injection.
At 48 hours after ACLR, 3 studies 12,14,17 reported VAS scores between the 2 groups; however, meta-analysis was not performed because of a lack of adequate data. Kurosaka et al 14 compared LIA (periarticular injection) with FNB and reported a significant difference in pain scores (mean, 22.1 ± 16.7 for periarticular injection vs 31.6 ± 17.4 for FNB; P = .002). However, Kristensen et al 12 and Lefevre et al 17 reported no significant difference in pain scores when comparing LIA with FNB.
Secondary Outcomes: Morphine-Equivalent Consumption
Six studies 11,12,14,21,30,39 provided information about total morphine-equivalent consumption after ACLR. No significant differences were found between LIA (intra-articular injection or periarticular injection) and FNB (MD, 2.55 mg [95% CI, −1.19 to 6.29 mg]; P = .18). Of these 6 studies, 4 11,21,30,39 reported total morphine-equivalent consumption comparing intra-articular injection with FNB and showed no significant difference (MD, 4.21 mg [95% CI, −0.26 to 8.68 mg]; P = .07) (Figure 5).
Figure 5.
Forest plots of the included studies showing improvement in total morphine-equivalent consumption (mg) between LIA and FNB after ACLR. LIA was categorized into subgroups of intra-articular injection and periarticular injection, and each subgroup was compared with FNB. Squares represent the mean difference in outcomes, with the size of the square being proportional to the sample size. ACLR, anterior cruciate ligament reconstruction; FNB, femoral nerve block; IA, intra-articular injection; IV, inverse variance; LIA, local infiltration analgesia; PAI, periarticular injection.
Secondary Outcomes: Side Effects
Side effects were reported in 5 studies. 11,12,14,17,39 Pooled data were unusable for a valid meta-analysis because only 2 studies 11,39 reported sufficient data. Among side effects, nausea and vomiting, sedation, and pruritis were reported in studies. Regarding nausea and vomiting, Iskandar et al 11 showed a significantly higher rate of nausea in intra-articular injection (27.5%) than FNB (7.5%); Tran et al 39 reported the incidence of vomiting with significantly higher rate in intra-articular injection (61.1%) than FNB (18.8%); however, 3 other studies 12,14,17 demonstrated that complication rates of nausea or vomiting were not significantly different between periarticular injection and FNB. In terms of sedation, Iskandar et al demonstrated a significantly higher rate in intra-articular injection (20%) than FNB (2.5%); however, Tran et al found no difference between the 2 groups (intra-articular injection, 78%; FNB, 50%). No differences in the incidence of pruritis were reported in the 2 studies. 14,39
Metaregression Analysis
Patient age, operation time, subgroup of LIA, and choice of graft were not significantly associated with pain management at 2 and 24 hours, which indicated no differences between the intra-articular and periarticular injection techniques (Appendix Table A3).
Discussion
In our meta-analysis of pain management for ACLR, the results suggested that patients treated with LIA have increased pain levels 2 hours postoperatively, usually in the PACU, compared with those treated with FNB. However, after leaving the PACU, patients treated with LIA had comparable pain control within 12 hours, even significantly improving pain levels 24 hours postoperatively. Postoperative opioid consumption was not significantly different between the 2 techniques. Side effects such as nausea or vomiting, sedation, and pruritis remain elusive, as it was difficult to perform a meta-analysis because of lack of studies.
Pain as recorded on a 100-mm VAS was the primary outcome studied in our meta-analysis. Our pooled data indicated LIA was not effective 2 hours postoperatively, comparable within 12 hours postoperatively, but significantly more effective 24 hours postoperatively for reducing pain after ACLR, as compared with FNB analgesia. However, the outcome of high heterogeneity should be considered when reviewing the results. Preoperative injection of FNB provided significant benefit by controlling pain in terms of VAS 2 hours postoperatively, usually in the PACU. This might be because of time difference of analgesic injection between the techniques, as FNB was injected preoperatively whereas LIA was administered before wound closure. The time interval between the 2 techniques might have affected the pain level at 2 hours postoperatively because it might not have been long enough for LIA to reach adequate titers of analgesic agents. In addition, the LIA showed similar 12,17 or better 14 pain management at 48 hours postoperatively in 3 included studies, although meta-analysis was not performed because of limited data. Successful pain management after ACLR at operation day potentially leads to improvements in sleep, opioid consumption, and patient satisfaction, which is especially important for patients undergoing ACLR on an outpatient basis. 17,28,31
The morphine-equivalent consumption was considered a secondary outcome in the current meta-analysis. Opioids including patient-controlled analgesia administration was usually used for postoperative pain management. Opioid-related complications, such as nausea, vomiting, sedation, pruritis, hypotension, respiratory depression, and loss of consciousness, brought on delayed rehabilitation, as well as prolonged hospitalization. 9,13,19 Previous studies have shown that patients treated with FNB demonstrated significantly less opioid consumption than those treated with LIA, mostly via intra-articular injection, after ACLR. 11,39 However, recent meta-analyses comparing LIA versus FNB after total knee arthroplasty have not found any differences in morphine consumption. 19,33,46 Our pooled data showed no significant differences of the morphine-equivalent consumption between the 2 techniques, which is consistent with the recent meta-analyses regarding pain management in total knee arthroplasty. 19,33,46
Previous studies have investigated solely intra-articular injection of LIA for pain management after ACLR 7,11,20 –22,39 ; however, recent investigations have focused on periarticular injection of LIA as an alternative option to FNB. 12,14,17,28 Drug agents of intra-articular injection interact with pain fibers in synovium that is inflamed, whereas drug agents of periarticular injection block the pain fiber in the traumatic soft tissue, involving skin, infrapatellar fat pad, capsule, synovium, and graft harvest donor site. 21,22,39 Regional diffusion of periarticular injection would bring the analgesic and anesthetic into contact with small rami of the femoral, sciatic, and obturator nerves; thus, periarticular injection acts at a more peripheral level of blocking the same pain pathway as the FNB. In addition, posterior capsule and hamstring autograft donor site are not covered by femoral nerve innervation; in such a case, LIA could cover those regions. At 24 hours postoperatively, intra-articular injection technique reduced pain level by 2.71, whereas, periarticular injection technique decreased pain level by 11.44 compared with FNB, which is above the minimal clinically important difference of 9.9. 27,36 Considering the disadvantages of FNB including potential vascular injury, quadriceps atrophy, nerve injury, and the need for special anesthetist and ultrasound equipment, the LIA technique, especially periarticular injection with or without intra-articular injection, could be an attractive option for replacing FNB for pain management after ACLR. 2,15,18,32,41 Moreover, recently developed long-acting (72 hours) local formulation, called LB, has potentially extended LIA in ACLR. 1,4 Investigations focusing on periarticular injection are needed to draw conclusions regarding its efficacy of pain management after ACLR in the future.
This study has some limitations. First, although there were 10 studies in our meta-analysis, 2 studies were not RCTs, and the sample size of included studies in our meta-analysis was small. Second, heterogeneity in analgesic techniques, drug choice and dosage, surgical techniques, and ACL graft choices among included studies may be a potential risk of bias. Third, side effects could not be analyzed in the meta-analysis because of insufficient existing data. In addition, the number of investigations on periarticular injection was not enough to provide the efficacy of periarticular injection and meaningful result of subgroup analysis of periarticular injection versus intra-articular injection. More studies are needed to determine the side effect and the efficacy of periarticular injection. Fourth, our comparison of LIA versus FNB technique was limited in application because of the recently increasing choice of adductor canal blocks. Fifth, we did not compare other modalities of pain management such as cryotherapy. Sixth, we could not compare the length of hospital stay or describe whether ACLR was performed in an outpatient clinic because only 1 study 14 in the included studies reported available data for the length of hospital stay and also only 1 study 21 clearly stated that ACLR was performed in an outpatient clinic. Nevertheless, we hope that our results will provide useful information for clinicians who prefer to perform ACLR as an outpatient procedure. Last, a motor-sparing saphenous nerve block was not assessed in this review because only 1 RCT 35 had existed when we performed a systematic search for relevant studies. We hope our results will contribute to performing RCTs of up-to-date methods such as comparing between motor-sparing saphenous nerve block and periarticular injection in the future.
Conclusion
Compared with FNB, LIA was not as effective at 2 hours, comparable within 12 hours, and significantly more effective at 24 hours postoperatively for reducing pain after ACLR. Total morphine-equivalent consumption showed no significant differences between the 2 techniques.
APPENDIX
Table A1.
General Characteristics of the Included Studies a
| Characteristics, LIA Group/FNB Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study (Year) | Study Design | Knees, n | Age, y | Male, % | BMI | Graft Choice | Tourniquet Use | Operation Time, min | Main Findings | MINORS |
| Santana (2019) 30 | RCS | 50/50 | 15.1/15.6 | 46/54 | 24.8/26.7 | NR | NR | NR | LIA = FNB < FNB + ScNB (pain and opioid use) | 16 |
| Kurosaka (2018) 14 | RCT | 69/60 | 27.1/25.5 | 39.1/46.7 | 22.4/22.8 | Hamstring auto | NR | 93/92 | LIA > FNB (pain and opioid use) | 21 |
| Lefevre (2016) 17 | PCS | 46/42 | 30.1/28.8 | 63.0/64.3 | 33.0/33.4 | Hamstring auto | NR | 38.8/42.2 | LIA = FNB (pain) LIA < FNB (opioid use) |
20 |
| Okoroha (2016) 28 | RCT | 41/41 | 27.6/27 | 61.0/58.5 | 26.5/26.0 | BPTB auto/ hamstring auto/ PT allo |
NR | NR | LIA = FNB (pain, sleep quality, and satisfaction) | 22 |
| Kristensen (2014) 12 | RCT | 28/27 | 29.3/25.6 | 75.0/25.0 | 25.6/23.7 | Hamstring auto | NR | 64/65 | LIA = FNB (pain, opioid use, adverse effects) | 24 |
| Mayr (2007) 20 | RCT | 53/55 | 32.0 | 57.3 | NR | BPTB auto | Yes | 62.8 | LIA = FNB (pain) | 17 |
| Tran (2005) 39 | RCT | 18/16 | 15/15 | 50/18.8 | NR | Hamstring auto/ Achilles tendon allo |
Yes | 205/221 | LIA < FNB (pain and opioid use) | 20 |
| Mehdi (2004) 22 | RCT | 25/25 | 24/26 | NR | 25/26 | BPTB auto | Yes | 72/72 | LIA = FNB (pain) | 17 |
| Iskandar (2003) 11 | RCT | 40/40 | 28.3/26.8 | 77.5/70.0 | NR | Hamstring auto | Yes | 46.2/48.4 | LIA < FNB (pain and opioid use) | 19 |
| McCarty (2001) 21 | RCT | 30/32 | NR | NR | NR | BPTB auto | Yes | NR | LIA = FNB (pain and opioid use) |
22 |
a allo, allograft; auto, autograft; BMI, body mass index; BPTB, bone--patellar tendon—bone; FNB, femoral nerve block; LIA, local infiltration analgesia; MINORS, methodological index for non-randomized studies; NR, not reported; PCS, prospective comparative study; PT, posterior tibialis; RCS, retrospective comparative study; RCT, randomized controlled trial; ScNB, sciatic nerve block.
Table A2.
Detailed Pain Management of Included Studies a
| LIA | FNB | Postoperative | ||||
|---|---|---|---|---|---|---|
| Study (Year) | Anesthesia | Method | Drug Dose | Drug Dose | PACU | After PACU |
| Santana (2019) 30 | G/A | IAI | 20 mL: bupi 0.25% | 20 mL: ropi 0.2% | Morphine as needed | NR |
| Kurosaka (2018) 14 | G/A | PAI (infrapatellar fat pad, medial and lateral synovial/capsule above meniscus, hamstring harvest, portals, incisions) | 44 mL: ropi 7.5 mg/mL, morphine 10 mg/mL, MPS 40 mg, ketoprofen 20 mg/mL, epi 1 mg/mL | Ropi based on patients: 20 mL, 2.5 mg/mL (n = 44); 10 mL, 2.5 mg/mL (n = 7); 20 mL, 3.75 mg/mL (n = 5); or 30 mL, 2.5 mg/mL (n = 4) | PCA fentanyl pump | COX-2-selective NSAIDs po; no oral narcotics |
| Lefevre (2016) 17 | G/A or S/A | PAI (skin incisions and hamstring harvest) | 3-4 ampules of 20 mL: ropi 2 mg/mL | 20 mL: ropi 0.475% | Morphine IV if VAS >30 | Paracetamol 825 mg qid, tramadol 37.5 mg qid, naproxen 550 mg bid, pregabalin 150 mg qd, po; no oral narcotics |
| Okoroha (2016) 28 | G/A | PAI (graft harvest sites, soft tissue dissection sites, portal, and incision sites) | 20 mL: LB (266 mg) + 10 mL NS | 40 mL: bupi 0.5% | Hydromorphone 0.5 mg every 10 min as needed for pain with maximum of 5 doses | Discharged home the day of surgery with 5 mg hydrocodone and 325 mg AAP |
| Kristensen (2014) 12 | G/A | PAI (surgical wound sites) | 20 mL: ropi 2 mg/mL + epi 5 µg/mL | 20 mL: ropi 2 mg/mL | Fentanyl 50 µg/mL IV; AAP 1 g po; morphine 5-10 mg po if VAS >30; fentanyl 50 µg/mL IV if VAS >50 | Discharged 4 h postoperatively with 18 T of AAP 500 mg and 6 T of morphine 10 mg |
| Mayr (2007) 20 | G/A | IAI | Fentanyl 0.1 mg + 8 mL: bupi 0.5% | 20 mL: prilocaine 1% + 20 mL bupi 0.5% | NR | Oxycodone 20 mg bid, ibuprofen 1200 mg/d po |
| Tran (2005) 39 | G/A | IAI | 1 mL/kg: bupi 0.25% + morphine 5 mg + clonidine 1 µg/kg, 15 min before tourniquet inflation | Max 40 mL: 0.5 mL/kg bupi 0.125% + 1:200,000 epi + clonidine 1 µg/kg | PCA: ketorolac 0.5 mg/kg IV if VAS >30; morphine 50 µg/kg bolus IV if pain persists | Ketorolac IV qid, morphine IV as needed, oxycodone, AAP po |
| Mehdi (2004) 22 | G/A | IAI + PAI (wounds) | 10 mL: bupi | 30 mL: bupi 0.375% or 40 mL bupi 0.25% | NR | Diclofenac 50 mg tid |
| Iskandar (2003) 11 | G/A | IAI | 20 mL: ropi 1% | 20 mL: ropi 1% | PCA pump: morphine increments of 2 mg every 5 min until VAS ≤30 | Propacetamol 2 g, ketoprofen 100 mg IV every 8 h |
| McCarty (2001) 21 | G/A | IAI | 50 mL: (bupi 0.25%) + lidocaine 1% with epi (1:200,000); morphine of 5 mg in 5 mL NS |
20 mL: bupi 0.5% with epi | Ketorolac 30 mg IV; morphine 2 mg IV, as needed | Ketorolac 10 mg qid and hydrocodone 5 mg/AAP 500 mg, po |
a AAP, acetaminophen; bid, twice a day; bupi, bupivacaine; epi, epinephrine; FNB, femoral nerve block; G/A, general anesthesia; IAI, intra-articular injection; IV, intravenously; LIA, local infiltration analgesia; LB, liposomal bupivacaine; max, maximum; MPS, methylprednisolone; NR, not reported; NS, normal saline; NSAID, nonsteroidal anti-inflammatory drug; PACU, postanesthesia care unit; PAI, periarticular injection; PCA, patient-controlled analgesia; po, by mouth; qd, once a day; qid, 4 times a day; ropi; ropivacaine; S/A, spinal anesthesia; T, tablets;tid, 3 times a day; VAS, visual analog scale.
Table A3.
Metaregression Analysis for Influence of Age, Operation Time, LIA Subgroup, and Graft Choice on VAS Pain Scores a
| Variable | β Coefficient (95% CI) | SE | P |
|---|---|---|---|
| VAS pain at 2 h | |||
| Age | −2.148 (−4.582 to −0.285) | 1.242 | .084 |
| Operation time | −0.055 (−0.158 to 0.047) | 0.052 | .289 |
| LIA subgroup IAI vs PAI |
−17.886 (−44.506 to 8.734) | 13.582 | .188 |
| Graft choice Hamstring vs BPTB autograft |
−3.853 (−28.231 to 20.525) | 12.438 | .757 |
| VAS pain at 24 h | |||
| Age | 0.375 (−1.465 to 2.215) | 0.939 | .689 |
| Operation time | 0.129 (−0.228 to 0.486) | 0.182 | .479 |
| LIA subgroup IAI vs PAI |
3.279 (−2.940 to 9.498) | 3.173 | .301 |
| Graft choice Hamstring vs BPTB autograft |
−1.984 (−11.179 to 7.211) | 4.692 | .672 |
a BPTB, bone--patellar tendon—bone; IAI, intra-articular injection; LIA, local infiltration analgesia; PAI, periarticular injection; VAS, visual analog scale.
Figure A1.

(A) Risk-of-bias graph showing the reviewers’ judgment about each risk-of-bias item present as percentages across all included studies. (B) Risk-of-bias summary showing the reviewers’ judgment about each risk-of-bias item for each included study.
Footnotes
Final revision submitted June 16, 2021; accepted July 26, 2021.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530–536. [DOI] [PubMed] [Google Scholar]
- 2. Bushnell BD, Sakryd G, Noonan TJ. Hamstring donor-site block: evaluation of pain control after anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(7):894–900. [DOI] [PubMed] [Google Scholar]
- 3. Choi S, McCartney CJ, van der Vyver M. Femoral nerve block does provide significant analgesia after anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(11):1417. [DOI] [PubMed] [Google Scholar]
- 4. Chughtai M, Cherian JJ, Mistry JB, Elmallah RD, Bennett A, Mont MA. Liposomal bupivacaine suspension can reduce lengths of stay and improve discharge status of patients undergoing total knee arthroplasty. J Knee Surg. 2016;29(3):224–227. [DOI] [PubMed] [Google Scholar]
- 5. Davey MS, Hurley ET, Anil U, et al. Pain management strategies after anterior cruciate ligament reconstruction: a systematic review with network meta-analysis. Arthroscopy. 2021;37(4):1290–1300.e1296. [DOI] [PubMed] [Google Scholar]
- 6. Denti M, Randelli P, Bigoni M, Vitale G, Marino MR, Fraschini N. Pre- and postoperative intra-articular analgesia for arthroscopic surgery of the knee and arthroscopy-assisted anterior cruciate ligament reconstruction: a double-blind randomized, prospective study. Knee Surg Sports Traumatol Arthrosc. 1997;5(4):206–212. [DOI] [PubMed] [Google Scholar]
- 7. Frost S, Grossfeld S, Kirkley A, Litchfield B, Fowler P, Amendola A. The efficacy of femoral nerve block in pain reduction for outpatient hamstring anterior cruciate ligament reconstruction: a double-blind, prospective, randomized trial. Arthroscopy. 2000;16(3):243-248. [DOI] [PubMed] [Google Scholar]
- 8. Hettrich CM, Dunn WR, Reinke EK, Spindler KP. MOON Group. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hida T, Yukawa Y, Ito K, et al. Intrathecal morphine for postoperative pain control after laminoplasty in patients with cervical spondylotic myelopathy. J Orthop Sci. 2016;21(4):425–430. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March 2011. The Cochrane Collaboration; 2014. [Google Scholar]
- 11. Iskandar H, Benard A, Ruel-Raymond J, Cochard G, Manaud B. Femoral block provides superior analgesia compared with intra-articular ropivacaine after anterior cruciate ligament reconstruction. Reg Anesth Pain Med. 2003;28(1):29-32. [DOI] [PubMed] [Google Scholar]
- 12. Kristensen PK, Pfeiffer-Jensen M, Storm JO, Thillemann TM. Local infiltration analgesia is comparable to femoral nerve block after anterior cruciate ligament reconstruction with hamstring tendon graft: a randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):317-323. [DOI] [PubMed] [Google Scholar]
- 13. Kunopart M, Chanthong P, Thongpolswat N, Intiyanaravut T, Pethuahong C. Effects of single shot femoral nerve block combined with intrathecal morphine for postoperative analgesia: a randomized, controlled, dose-ranging study after total knee arthroplasty. J Med Assoc Thai. 2014;97(2):195–202. [PubMed] [Google Scholar]
- 14. Kurosaka K, Tsukada S, Nakayama H, et al. Periarticular injection versus femoral nerve block for pain relief after anterior cruciate ligament reconstruction: a randomized controlled trial. Arthroscopy. 2018;34(1):182-188. [DOI] [PubMed] [Google Scholar]
- 15. Lareau JM, Robbins CE, Talmo CT, Mehio AK, Puri L, Bono JV. Complications of femoral nerve blockade in total knee arthroplasty and strategies to reduce patient risk. J Arthroplasty. 2012;27(4):564–568. [DOI] [PubMed] [Google Scholar]
- 16. Lefevre N, Klouche S, de Pamphilis O, Devaux C, Herman S, Bohu Y. Postoperative discomfort after outpatient anterior cruciate ligament reconstruction: a prospective comparative study. Orthop Traumatol Surg Res. 2015;101(2):163–166. [DOI] [PubMed] [Google Scholar]
- 17. Lefevre N, Klouche S, de Pamphilis O, Herman S, Gerometta A, Bohu Y. Peri-articular local infiltration analgesia versus femoral nerve block for postoperative pain control following anterior cruciate ligament reconstruction: prospective, comparative, non-inferiority study. Orthop Traumatol Surg Res. 2016;102(7):873–877. [DOI] [PubMed] [Google Scholar]
- 18. Luo TD, Ashraf A, Dahm DL, Stuart MJ, McIntosh AL. Femoral nerve block is associated with persistent strength deficits at 6 months after anterior cruciate ligament reconstruction in pediatric and adolescent patients. Am J Sports Med. 2015;43(2):331–336. [DOI] [PubMed] [Google Scholar]
- 19. Ma J, Zhang W, Yao S. Liposomal bupivacaine infiltration versus femoral nerve block for pain control in total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2016;36(pt A):44–55. [DOI] [PubMed] [Google Scholar]
- 20. Mayr HO, Entholzner E, Hube R, Hein W, Weig TG. Pre- versus postoperative intraarticular application of local anesthetics and opioids versus femoral nerve block in anterior cruciate ligament repair. Arch Orthop Trauma Surg. 2007;127(4):241–244. [DOI] [PubMed] [Google Scholar]
- 21. McCarty EC, Spindler KP, Tingstad E, Shyr Y, Higgins M. Does intraarticular morphine improve pain control with femoral nerve block after anterior cruciate ligament reconstruction? Am J Sports Med. 2001;29(3):327-332. [DOI] [PubMed] [Google Scholar]
- 22. Mehdi SA, Dalton DJ, Sivarajan V, Leach WJ. BTB ACL reconstruction: femoral nerve block has no advantage over intraarticular local anaesthetic infiltration. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):180-183. [DOI] [PubMed] [Google Scholar]
- 23. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–129. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moon DK, Jo HS, Lee DY, et al. Anterior cruciate ligament femoral-tunnel drilling through an anteromedial portal: 3-dimensional plane drilling angle affects tunnel length relative to notchplasty. Knee Surg Relat Res. 2021;33(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murgier J, Cassard XJO. Cryotherapy with dynamic intermittent compression for analgesia after anterior cruciate ligament reconstruction: preliminary study. Orthop Traumatol Surg Res. 2014;100(3):309–312. [DOI] [PubMed] [Google Scholar]
- 27. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424–429. [DOI] [PubMed] [Google Scholar]
- 28. Okoroha KR, Keller RA, Marshall NE, et al. Liposomal bupivacaine versus femoral nerve block for pain control after anterior cruciate ligament reconstruction: a prospective randomized trial. Arthroscopy. 2016;32(9):1838-1845. [DOI] [PubMed] [Google Scholar]
- 29. Park YG, Ha CW, Park YB, et al. Is it worth to perform initial non-operative treatment for patients with acute ACL injury? A prospective cohort prognostic study. Knee Surg Relat Res. 2021;33(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santana L, Lovejoy JF, Kiebzak G, Day J, Atanda A, Jr, Mandel D. Comparison of pain scores and medication usage between three pain control strategies for pediatric anterior cruciate ligament surgery. Cureus. 2019;11(8):e5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Secrist ES, Freedman KB, Ciccotti MG, Mazur DW, Hammoud S. Pain management after outpatient anterior cruciate ligament reconstruction: a systematic review of randomized controlled trials. Am J Sports Med. 2016;44(9):2435–2447. [DOI] [PubMed] [Google Scholar]
- 32. Sharma S, Iorio R, Specht LM, Davies-Lepie S, Healy WL. Complications of femoral nerve block for total knee arthroplasty. Clin Orthop Relat Res. 2010;468(1):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh PM, Borle A, Trikha A, Michos L, Sinha A, Goudra B. Role of periarticular liposomal bupivacaine infiltration in patients undergoing total knee arthroplasty---a meta-analysis of comparative trials. J Arthroplasty. 2017;32(2):675–688.e671. [DOI] [PubMed] [Google Scholar]
- 34. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 35. Stebler K, Martin R, Kirkham KR, Lambert J, De Sede A, Albrecht E. Adductor canal block versus local infiltration analgesia for postoperative pain after anterior cruciate ligament reconstruction: a single centre randomised controlled triple-blinded trial. Br J Anaesth. 2019;123(2):e343-e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927–932. [DOI] [PubMed] [Google Scholar]
- 37. Terai S, Zantop C, Zantop T. The effect of combined cryotherapy/compression versus cryotherapy following ACL recon in return-to-sports after 3 months. Orthop J Sports Med. 2020;8(9 suppl 7):2325967120S2325900548. [Google Scholar]
- 38. Tierney GS, Wright RW, Smith JP, Fischer DA. Anterior cruciate ligament reconstruction as an outpatient procedure. Am J Sports Med. 1995;23(6):755–756. [DOI] [PubMed] [Google Scholar]
- 39. Tran KM, Ganley TJ, Wells L, Ganesh A, Minger KI, Cucchiaro G. Intraarticular bupivacaine-clonidine-morphine versus femoral-sciatic nerve block in pediatric patients undergoing anterior cruciate ligament reconstruction. Anesth Analg. 2005;101(5):1304–1310. [DOI] [PubMed] [Google Scholar]
- 40. Vermeijden HD, Yang XA, van der List JP, DiFelice GS, Rademakers MV, Kerkhoffs G. Trauma and femoral tunnel position are the most common failure modes of anterior cruciate ligament reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2020;28:3666–3675. [DOI] [PubMed] [Google Scholar]
- 41. Widmer B, Lustig S, Scholes CJ, et al. Incidence and severity of complications due to femoral nerve blocks performed for knee surgery. Knee. 2013;20(3):181–185. [DOI] [PubMed] [Google Scholar]
- 42. Wu CL, Bronstein RD, Chen JM, Lee DH, Rouse LM. Postoperative analgesic requirements in patients undergoing arthroscopic anterior cruciate ligament reconstruction. Am J Orthop (Belle Mead NJ). 2000;29(12):974–978. [PubMed] [Google Scholar]
- 43. Yung EM, Brull R, Albrecht E, Joshi GP, Abdallah FW. Evidence basis for regional anesthesia in ambulatory anterior cruciate ligament reconstruction, part III: local instillation analgesia---a systematic review and meta-analysis. Anesth Analg. 2019;128(3):426–437. [DOI] [PubMed] [Google Scholar]
- 44. Zantop T, Zantop C, Hönninger A, Hauner D, Warminski PJ. The effect of combined cryotherapy/compression versus cryotherapy following ACL reconstruction with regard to return-to-sports 3 months after surgery. Orthop J Sports Med. 2020;8(5 suppl 4):2325967120S2325900320. [Google Scholar]
- 45. Zhang K, Crum RJ, Samuelsson K, Cadet E, Ayeni OR, de Sa D. In-office needle arthroscopy: a systematic review of indications and clinical utility. Arthroscopy. 2019;35(9):2709–2721. [DOI] [PubMed] [Google Scholar]
- 46. Zhang LK, Ma JX, Kuang MJ, Ma XL. Comparison of periarticular local infiltration analgesia with femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. J Arthroplasty. 2018;33(6):1972–1978.e1974. [DOI] [PubMed] [Google Scholar]