Abstract
Objective
To evaluate the utility of Golgi protein 73 (GP73) in the diagnosis of non-alcoholic steatohepatitis (NASH) and hepatic fibrosis (HF) staging.
Methods
Ninety-one patients with non-alcoholic fatty liver disease (NAFLD) were allocated to NAFL (n = 46) and NASH (n = 45) groups according to their NAFLD activity score (NAS), and there were 30 healthy controls. Serum GP73 was measured by ELISA, GP73 protein expression was evaluated using immunohistochemistry, and FibroScan was used to determine liver hardness.
Results
The serum GP73 concentrations of the NAFL and NASH groups were significantly higher than those of controls. GP73 expression in the liver of the patients gradually progressed from absent or low to moderate or high. Serum GP73 positively correlated with liver expression, and the serum and liver GP73 of the patients positively correlated with FibroScan value and HF stage. There was a strong positive correlation of the combination of alanine aminotransferase, gamma glutamyl transferase and GP73 with NASH. The combination of serum GP73 and FibroScan value was found to predict NASH (NAS > 4) and advanced HF (stage ≥2) in patients with NAFLD using receiver operating characteristic analysis.
Conclusion
Serum GP73 may be useful in the diagnosis of NASH and the staging of HF.
Keywords: Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, Golgi protein 73, hepatic fibrosis, NAFLD activity score, FibroScan, alanine aminotransferase, gamma glutamyl transferase
Introduction
Non-alcoholic fatty liver disease (NAFLD), which was first defined by Austrian pathologist Carl von Rokitansky in 1849, is a common clinical liver disease that is characterised by metabolic disorders.1,2 Its main pathological features are diffuse hepatocyte hypertrophy and vesicular hepatic steatosis. According to the presence or absence of inflammatory fibrosis and the severity of the disease, NAFLD is divided into simple non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), liver cirrhosis and hepatocellular carcinoma (HCC). 3 The mean worldwide prevalence of NAFLD in adults has now reached 25% 4 and that in China is 20.1%, making it the most common chronic liver disease. 5 This high prevalence is closely related to poor lifestyles. 6 According to data published in 2020, the proportion of patients in which NASH was a key factor in the aetiology of liver cancer in the United States, Europe and other developed countries increased 7.7-fold (from 2.1% to 16.2%) and the number of liver transplantations performed increased seven-fold over 14 years.7–9 Furthermore, the combined prevalence of NASH, and hepatitis B and C virus-induced liver disease is 63.5% in Asia.7–9 Previous studies have shown that the disease progresses from simple steatosis to more serious liver disease, such as NASH, in a significant number of patients, and 10% to 15% of patients with NASH ultimately develop liver cirrhosis and/or liver cancer.10–12 In addition, NAFLD can not only cause irreversible damage to the liver, but is also closely associated with the metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), arteriosclerotic cardiovascular disease and colorectal cancer.13,14 The aetiology of NAFLD is thought to involve genetic susceptibility, glycolipid toxicity and insulin resistance,15,16 but the exact aetiology remains unclear.
Fibrosis is the most important predictor of liver-related complications in NAFLD and has the closest relationship of any histological feature with mortality in patients with NASH. Therefore, it is very important for patients with chronic liver disease to have their hepatic fibrosis (HF) staged and for appropriate measures to be taken to ameliorate any HF.17–19 The diagnostic value of FibroScan for HF caused by various chronic liver diseases has been demonstrated. 20 However, in clinical practice, FibroScan cannot be used to accurately diagnose NAFLD or distinguish between NAFL and NASH, which makes it difficult to evaluate the progression of the disease in patients. Therefore, it would be of great value to identify a flexible, non-invasive and simple means of diagnosing NASH and evaluating HF in patients with NAFLD.
Golgi protein 73 (GP73) is a transmembrane protein of 73 kDa, also known as Golgi phosphoprotein 2. Diseased or damaged cells release GP73 into the extracellular space, which results in an increase in the serum concentration of GP73.21–23 GP73 was first identified in 2000 by Kladney et al. 24 during a screen for differentially expressed proteins in patients with adult giant cell hepatitis. A subsequent study showed that this protein is not expressed in normal hepatocytes, but highly expressed in patients with liver cirrhosis of various aetiologies. 22 Many studies have shown that GP73 has great diagnostic value for liver cirrhosis.25,26 However, it has also been reported that serum GP73 is of limited value for the evaluation and monitoring of disease progression, including in the identification of liver necroinflammation and fibrosis in patients with chronic hepatitis C virus infection. 27 Therefore, it is uncertain whether the concentration of GP73 is related to the severity of inflammation in NASH or HF.
We have shown (article submitted) that GP73 concentration is useful in the diagnosis and prognosis of chronic hepatitis B. However, the diagnostic value of GP73 in NAFLD and the related HF has been little studied to date. In the present study, we measured the concentrations of GP73 in the serum and liver of patients with NAFLD to evaluate the utility of GP73 for the diagnosis of NASH and the staging of HF. We show that the measurement of GP73 may provide a rapid, convenient, and accurate means of making an early diagnosis of NAFLD and staging HF.
Materials and Methods
Participants
Patients with NAFLD were enrolled at the Department of Infectious Diseases, General Hospital of Ningxia Medical University, between December 2016 and October 2019, and healthy individuals were enrolled to form a control group. According to The liver tissue activity in the pathological work guide of the NASH Clinical Research Network of the National Institutes of Health, 28 the NAFLD of the recruited patients was classified using a 0- to 8-point scale (the NAFLD activity score (NAS)), and HF was staged using the Brunt/NAFLD fibrosis score (NFS) as between F0 and F4. The basic clinical data were collected for each participant. All the participants provided their written informed consent, and the study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (approval no: 2020-392).
The inclusion criteria were: 1) presence of NAFLD but no history of excessive alcohol consumption (men <30 g/day and women <20 g/day) or other specific causes of fatty liver, such as obesity, T2DM or MetS, and no abnormal serological indices related to viral hepatitis; and 2) abdominal B-ultrasonography or liver biopsy results consistent with fatty liver disease.
The exclusion criteria were: 1) viral hepatitis A, B, C, D or E, or the presence of other viruses; 2) alcoholic liver disease, autoimmune hepatitis, hepatolenticular degeneration or other specific liver diseases that can cause fatty liver; 3) pregnancy or recent childbirth; 4) total parenteral nutrition, inflammatory bowel disease, celiac disease, hypothyroidism, Cushing’s syndrome, β-lipoprotein deficiency, lipoatrophic diabetes, Mauriac’s syndrome or other causes of fatty liver; 5) primary or metastatic HCC or tumours of other organs; and 6) severe mental or psychological disease, or severe organ dysfunction.
The reporting of this study conforms with the STROBE statement. 29
Sample collection
Venous blood samples were collected from all the participants (10 ml each) after they had fasted for >8 hours and serum was obtained by centrifugation. Liver biopsies were collected, fixed in buffered formalin and embedded in paraffin for sectioning and histological staining.
Measurement of serum GP73 concentration by ELISA
Serum GP73 concentration was measured using an ELISA kit (Beijing Rejing Biotechnology Co., Ltd., Beijing, China; Cat# R-KMLJr34981), according to the manufacturer’s instructions. The absorbance of the samples at 450 nm was measured using a microplate reader (Spectramax M2; Molecular Devices, Sunnyvale, CA, USA) The serum GP73 concentration was interpolated from a standard curve.
Immunohistochemistry
Liver sections were cut into 5-μm sections, then antibody retrieval was performed in an oven at 68°C for 12 hours. Primary rat anti-human GP73 antibody (MedChemExpress Inc., Monmouth Junction, NJ, USA; HY-10182) was added at a 1:50 dilution and incubated at 37°C for 60 minutes. After washing, secondary antibody reaction enhancement solution (Sigma Inc., San Francisco, CA, USA; A2228-104UL) was added and the sections were incubated at 37°C for 20 minutes, then the secondary antibody (Sigma Inc.; A2228-129UL) was added and the sections were further incubated at 37°C for 20 minutes. After haematoxylin counterstaining, hydrochloric acid/ethanol differentiation, and ethanol gradient dehydration, the sections were mounted and examined under an optical microscope. The expression of GP73 was defined as absent (−, no expression), low (+, fine brown particles), moderate (++, coarse brown particles) or high (+++, coarse dark brown particles).
Statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA) was used for data analysis. Normally distributed data are expressed as mean ± SD and were analysed using Student’s t-test. Non-normally distributed data are expressed as median (interquartile range) and were analysed using the Mann–Whitney U or Kruskal–Wallis H test. Categorical data are expressed as counts and were analysed using the chi-square test. Relationships between continuous variables were analysed using Spearman’s correlation. The relationship between serum GP73 concentration and liver fibrosis was analysed using receiver operating characteristic curve (ROC) analysis and the optimal cut-off value was calculated. The ROC curve was constructed using MedCalc 19.1 (MedCalc Software Ltd., Ostend, Belgium) and Prism 8.3.0 (GraphPad, San Diego, CA, USA) software. Data were regarded as statistically significant when P < 0.05.
Results
Clinical characteristics of the various clinical stages of NAFLD
Ninety-one patients with NAFLD and 30 healthy controls were enrolled. The patients comprised 46 with NAFL (35 men and 11 women, with NASs of 0–4, body mass indexes (BMIs) of 24.72 ± 2.50 kg/m2 and ages of 40.7 ± 11.3 years) and 45 with NASH (32 men and 13 women, with NASs of 5–8, BMIs of 27.59 ± 2.33 kg/m2 and ages of 35.9 ± 10.0 years). The basic clinical data for the participants are listed in Table 1. The BMI, alanine aminotransferase (ALT) activity and platelet count of the NASH group were higher than those of the NAFL group, but the participants in the former group were younger (all P < 0.05). There were no significant differences in sex distribution, serum aspartate aminotransferase (AST) activity, gamma glutamyl transferase (GGT) activity, alkaline phosphatase (ALP) activity, glucose concentration, triglyceride concentration or albumin concentration between the NAFL and NASH groups (Table 1).
Table 1.
Clinical characteristics of participants with NASH or NAFL (N = 91).
| Parameter | NAFL (n = 46) | NASH (n = 45) | P |
|---|---|---|---|
| Sex | |||
| M | 35 | 32 | 0.764 |
| F | 11 | 13 | |
| Age (years) | 40.67 ± 11.34 | 35.87 ± 9.97 | 0.040* |
| BMI (kg/m2) | 24.72 ± 2.50 | 27.59 ± 2.33 | 0.001* |
| ALT (U/L) | 53.00 (34.43, 91.15) | 78.80 (51.80, 98.75) | 0.028* |
| AST (U/L) | 36.30 (25.25, 55.10) | 41.50 (30.50, 57.35) | 0.074 |
| AST/ALT | 0.66 (0.49, 0.90) | 0.57 (0.46, 0.78) | 0.184 |
| GGT (U/L) | 45.00 (24.53, 69.80) | 51.40 (29.75, 94.65) | 0.289 |
| ALP (U/L) | 74.06 ± 22.98 | 78.64 ± 28.82 | 0.275 |
| GLU (mmol/L) | 5.45 ± 1.46 | 5.71 ± 1.51 | 0.296 |
| TG (mmol/L) | 3.59 ± 3.83 | 2.48 ± 2.05 | 0.264 |
| ALB (g/L) | 48.26 ± 3.44 | 49.10 ± 4.30 | 0.067 |
| PLT (×109/L) | 213.67 ± 53.92 | 244.21 ± 60.93 | 0.023* |
| NAFLD fibrosis score | 5.65 ± 0.76 | 6.83 ± 0.99 | 0.036* |
Data are counts or mean ± standard deviation. * P < 0.05 vs. the NAFL group. Data were analysed using Student’s t-test, the Mann–Whitney U-test, the Kruskal–Wallis H-test or the chi-square test, as appropriate.
NAFL(D): non-alcoholic fatty liver (disease); NASH: non-alcoholic steatohepatitis; BMI: body mass index; ALT: alanine aminotransferase activity; AST: aspartate aminotransferase activity; GGT: gamma glutamyl transferase activity; ALP: alkaline phosphatase activity; GLU: glucose; TG: triglyceride; ALB: albumin; PLT: platelet count.
Serum GP73 concentration in participants with the various clinical stages of NAFLD
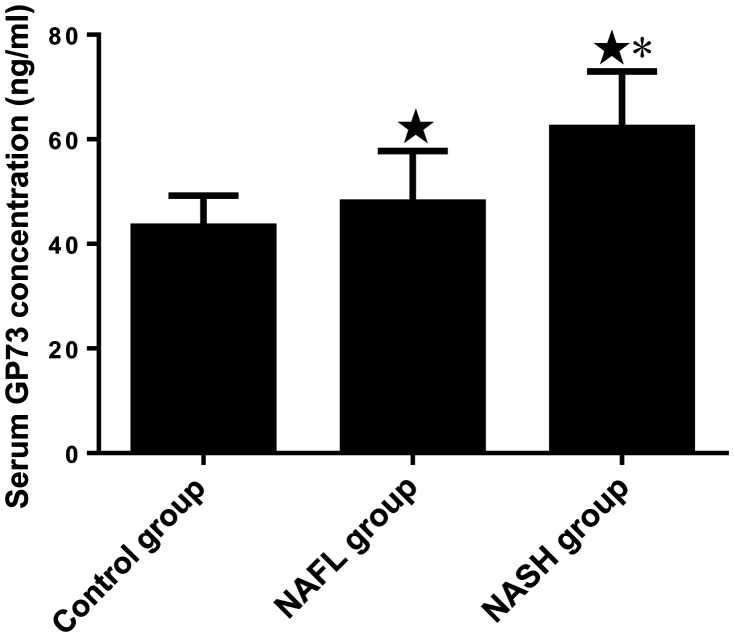
The serum GP73 concentrations of the control group, NAFL group and NASH group were 43.29 ± 5.94 ng/ml, 47.92 ± 9.83 ng/ml and 62.17 ± 10.79 ng/ml, respectively. The serum GP73 concentrations of the NAFL and NASH groups were significantly higher than that of the control group (P < 0.05), and that of NASH group was significantly higher than that of the NAFL group (P < 0.05) (Figure 1).
Figure 1.
Comparison of serum GP73 concentration in the control group and participants with NAFL disease at different clinical stages. ★P < 0.05 vs. the control group; *P < 0.05 vs. the NAFL group. N = 46, 45 and 30 for the NASH, NAFL and control groups, respectively.
GP, Golgi protein; NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis.
Serum GP73 concentrations in participants with differing severity of HF
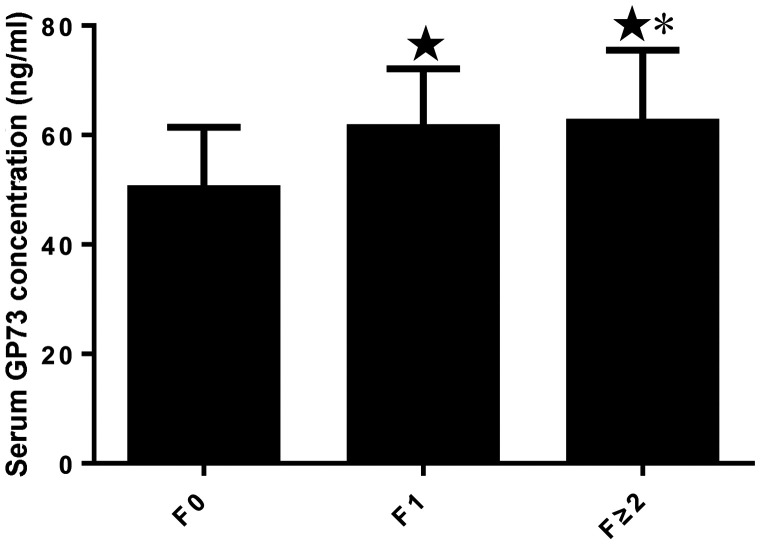
According to the fibrosis classification, 53 participants (58.2%) had F0 fibrosis, 29 (31.9%) had F1 fibrosis and 9 (9.9%) had F ≥ 2 fibrosis. The mean serum GP73 concentration in participants with F0 stage fibrosis was 50.20 ± 11.26 ng/ml, that for participants with F1 fibrosis was 61.38 ± 10.71 ng/ml and that for participants with F2 fibrosis was 62.34 ± 13.16 ng/ml (Figure 2). All of these were significantly different (P < 0.05).
Figure 2.
Serum GP73 concentration in participants with non-alcoholic fatty liver disease and differing severities of hepatic fibrosis (stages F0, F1 and F ≥ 2). ★P < 0.05 vs. F0; *P < 0.05 vs. F1. N = 53, 29 and 9 for F0, F1 and F ≥ 2, respectively.
GP, Golgi protein.
Protein expression of GP73 in the livers of participants with NAFLD
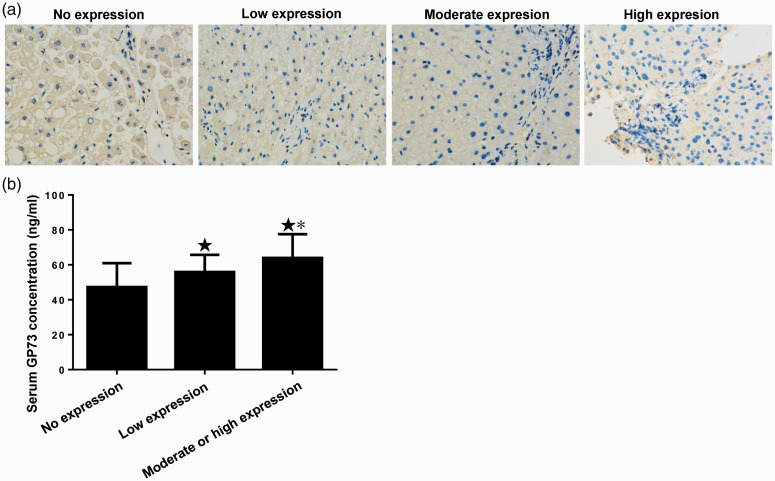
The expression of GP73 in the livers of the participants was assessed using immunohistochemistry. Representative micrographs of immunohistochemically stained sections are shown in Figure 3a. Twenty-three (28.6%) livers were negative for GP73 expression, 54 (45.1%) showed low expression and 14 (26.4%) showed moderate or high expression. The serum GP73 concentration in participants with no liver GP73 expression was 47.30 ± 13.66 ng/ml, that in participants with low liver GP73 expression was 55.90 ± 9.77 ng/ml and that in participants with medium or high liver GP73 expression was 63.95 ± 13.56 ng/ml (Figure 3b). The liver GP73 expression increased as the fibrosis became more severe (H = 14.487, P = 0.001 and as the liver GP73 expression increased, the corresponding serum GP73 concentration also increased (H = 16.053, P = 0.001). The expression of GP73 in the liver and the serum concentration were normally distributed, and there was a positive correlation between these (r = 0.402, P = 0.001).
Figure 3.
Levels of GP73 in the serum and liver. (a) Protein expression of GP73 in the livers of participants with non-alcoholic fatty liver disease. Representative photomicrographs of the immunohistochemistry study (400× magnification). (b) Serum GP73 concentrations in participants with no, low and moderate or high liver GP73 expression. ★P < 0.05 vs. the no expression group; *P < 0.05 vs. the low expression group. N = 23, 54 and 14, respectively.
GP, Golgi protein.
Relationships between serum GP73 concentration, liver GP73 expression and fibrosis stage in participants with NAFLD
The liver expression and serum GP73 concentration in participants with NAFLD positively correlated with fibrosis stage and with the FibroScan value (Table 2) (P < 0.01), which implies that serum GP73 is useful for the diagnosis of NAFLD and in the evaluation of the severity of HF.
Table 2.
Relationships of the liver GP73 expression or serum GP73 concentration with the stage of fibrosis or FibroScan value (N = 91).
| Hepatic fibrosis score |
FibroScan value |
|||
|---|---|---|---|---|
| R | P | R | P | |
| Liver tissue GP73 | 0.301 | 0.004 | 0.434 | 0.001 |
| Serum GP73 | 0.436 | 0.001 | 0.360 | 0.001 |
GP73: Golgi protein 73. Spearman’s correlation.
Diagnostic values of serum GP73 and FibroScan in participants with NAFLD and F ≥ 2
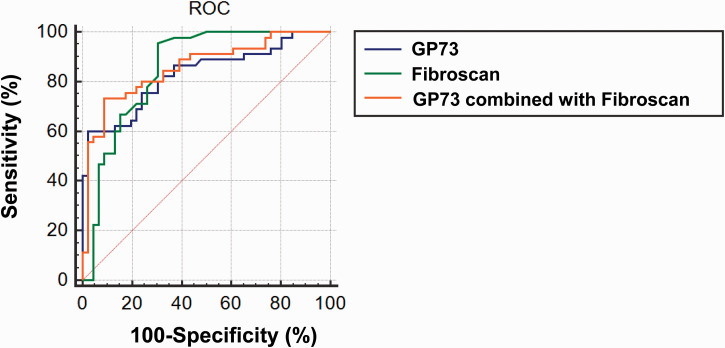
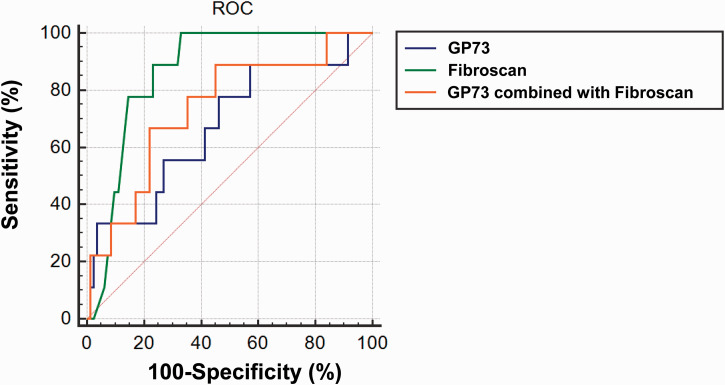
The pathological grade of patients with NAFLD is usually assessed using the NAS, with NAS > 4 being used to define NASH. We plotted ROC curves to evaluate the diagnostic value of serum GP73, FibroScan and their combination for NAFLD. As shown in Figure 4, for the diagnosis of NASH, the areas under the ROC curves for serum GP73 and FibroScan in participants with NAFLD were 0.830 and 0.852, respectively, and that for serum GP73 combined with FibroScan was 0.857. Therefore, serum GP73 and FibroScan, and especially a combination of the two, may be useful for the prediction of NASH (NAS > 4) during the early stages of the disease.
Figure 4.
ROC curve assessment of the use of serum G)73, FibroScan value and their combination for the early diagnosis of non-alcoholic steatohepatitis (NAFLD activity score > 4) in participants with NAFLD. N = 91.
ROC, receiver operating characteristic; GP, Golgi protein; NAFLD, non-alcoholic fatty liver disease.
For the identification of HF stage F ≥ 2, the optimal cut-off value for serum GP73 was found to be 50.2 ng/ml (sensitivity 88.9% and specificity 42.7%), that for FibroScan value was 7.0 kPa (sensitivity 100% and specificity 67.1%), and the sensitivity for a combination of GP73 and FibroScan were 88.9% and 70.7%, respectively (Figure 5). These results indicate that the use of serum GP73, FibroScan or a combination are sensitive methods of diagnosing NASH (NAS > 4) and identifying HF stage F ≥ 2.
Figure 5.
ROC curve assessment of the use of serum GP73, FibroScan value and their combination in the identification hepatic fibrosis stage F ≥ 2 in participants with non-alcoholic fatty liver disease. N = 91.
ROC, receiver operating characteristic; GP, Golgi protein.
Relationships of NFS and FibroScan value with NASH and HF stage, determined using a liver biopsy
The NFS and FibroScan value of participants with NAFLD positively correlated with NAS score (P < 0.05) (Table 3). There was also a positive correlation between NFS and HF stage (P < 0.05). These data indicate that NAS score could also be used as a predictor of liver fibrosis.
Table 3.
Relationships of NFS or FibroScan value with NAS or stage of hepatic fibrosis (N = 91).
| NFS |
FibroScan value |
|||
|---|---|---|---|---|
| R | P | R | P | |
| NAS | 0.555 | 0.001 | 0.609 | 0.001 |
| Stage of hepatic fibrosis | 0.212 | 0.043 | 0.376 | 0.001 |
Spearman’s correlation.
NAS: non-alcoholic fatty liver disease (NAFLD) activity score; NFS: NAFLD fibrosis score.
Relationships of serum ALT, GGT, and GP73 with NAS in participants with NAFLD
The ALT activity and serum GP73 concentration of the participants with NASH positively correlated with NAS (P < 0.05), but GGT did not (Table 4). In addition, there was a strong positive correlation between a combination of the three and NAS (P = 0.001).
Table 4.
Relationships of serum ALT, GGT, and GP73 with NAS in participants with NAFLD (N = 91).
| ALT |
GP73 |
GGT |
Combination of ALT, GP73 and GGT |
|||||
|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | |
| NAS | 0.232 | 0.027 | 0.571 | 0.001 | 0.112 | 0.292 | 0.621 | 0.001 |
Spearman’s correlation.
ALT: alanine aminotransferase activity; GGT: gamma glutamyl transferase activity; GP73: serum Golgi protein 73 concentration; NAFLD: non-alcoholic fatty liver disease; NAS: NAFLD activity score.
Discussion
Epidemiological studies have shown that NAFLD is now the most common chronic liver disease in the world,30–32 and its incidence is increasing year by year. It progresses through several pathological stages: NAFL, NASH and HF, cirrhosis and HCC, which seriously affect health and quality of life. 33 There have been many studies of potential non-invasive methods for the early diagnosis of HF, and several serum biomarkers have been identified for use in the diagnosis and staging of HF, including cytokeratin (CK)-18, CK-18 M30 segment, CK-18 M65 and GP73.34–36 In particular, we expect that GP73 will be adopted as a new serological index of HF in chronic liver disease. GP73 is a Golgi membrane protein that is expressed on the surface of epithelial cells and is principally involved in protein secretion, glycosylation and membrane transformation, and has important roles in signal transmission, cell differentiation and apoptosis. It is highly expressed in human epithelial cells, but rarely in hepatocytes.26,37 Kladney et al. 38 found that the expression of GP73 in the liver of patients with chronic progressive liver diseases, such as viral or non-viral hepatitis or cirrhosis, is ∼70 times higher than that of normal liver. In addition, Mao et al. 39 found that the GP73 expression in diseased livers is significantly higher than that in patients with non-liver tumours and healthy individuals. Subsequently, Shi et al. 40 showed that the serum GP73 concentration in patients with HCC is high, implying that GP73 may be of diagnostic value for HCC, and in combination with other serological and imaging data, it could improve the diagnosis rate. Therefore, in the present study, we aimed to determine whether serum GP73 concentration is high in chronic progressive liver disease, and thus whether it might be suitable for use as a non-invasive means of evaluating HF. We measured the serum concentration and liver protein expression of GP73 in patients with NAFLD and healthy controls, and found that the serum GP73 concentrations of the patients were significantly higher than those of healthy individuals and that the two parameters were positively correlated. Thus, the serum GP73 concentration in patients with NAFLD reflects the hepatocyte GP73 protein expression.
We constructed ROC curves to assess the sensitivity and specificity of the use of serum GP73 and FibroScan values and their combination for the diagnosis of NASH (NAS > 4) and substantial HF (F ≥ 2) in patients with NAFLD. We calculated optimal cut-offs for GP73 and FibroScan value that were consistent with the previous findings of Cao et al. 41
Most hepatocytes in patients with hepatic fibrosis are damaged, and this is associated with deteriorations in platelet-related parameters and prothrombin time, and a resulting increase in the risk of haemorrhage. Patients with hepatic fibrosis also show hypersplenism and activation of the mononuclear phagocyte system in the spleen, which results in the phagocytosis of platelets. Moreover, hepatitis virus can inhibit bone marrow megakaryocytes, which impairs platelet production, thereby reducing platelet count and their ability to aggregate, and increasing mean platelet volume and width.42,43 Liver damage is associated with increases in the serum activities of AST and ALT, and these are commonly used as markers of hepatocyte injury.44,45 These parameters are also used as a means of diagnosing NAFLD early, and the identification of a high-normal serum ALT activity is of great significance for the early prevention of NAFLD. 46 GGT is another liver enzyme and is involved in glutathione synthesis and catabolism, amino acid transport and detoxification.47,48 Exposure to pro-oxidative factors stimulates an antioxidant response and induces the production of GGT, thereby increasing the production of glutathione.49,50 An increase in GGT activity can also reflect the deposition of fat in the liver and visceral obesity, and visceral fat is associated with impaired insulin signalling in the liver, which promotes the development of NAFLD.51–53 Therefore, the diagnostic value of GP73 for NASH was assessed alongside those of these other parameters, and we found that a combination is useful for the early diagnosis of NASH.
The present study had several limitations. First, the sample size was relatively small. Therefore, a further study should be performed with a larger number of participants. Second, there were few participants with F ≥ 2, which may explain the small difference in serum GP73 between participants with F1 and F ≥ 2. Third, the biological function of GP73 and molecular mechanisms involved are not yet clear; therefore, additional studies are needed to remedy these deficiencies to provide further justification for the use of serum GP73 in the early diagnosis of HF.
In conclusion, serum GP73 may represent a useful serological parameter for the diagnosis and monitoring of liver disease, and serum GP73 or FibroScan value alone or in combination may be of value for the early diagnosis of liver fibrosis.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was supported by the Ningxia Natural Science Foundation (grant no. NZ17171).
ORCID iD: Huiping Sheng https://orcid.org/0000-0003-0638-7747
References
- 1.Perla FM, Prelati M, Lavorato M, et al. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children (Basel) 2017; 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan Y, Iloeje U, Li H, et al. Economic implications of entecavir treatment in suppressing viral replication in chronic hepatitis B (CHB) patients in China from a perspective of the Chinese Social Security program. Value Health 2008; 11: S11–S22. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol 2015; 7: 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niederseer D, Wernly S, Bachmayer S, et al. Diagnosis of non-alcoholic fatty liver disease (NAFLD) is independently associated with cardiovascular risk in a large Austrian screening cohort. J Clin Med 2020; 9: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Xue J, Chen P, et al. Prevalence of nonalcoholic fatty liver disease in mainland of China: A meta-analysis of published studies. J Gastroenterol Hepatol 2014; 29: 42–51. [DOI] [PubMed] [Google Scholar]
- 6.Meng G, Liu F, Fang L, et al. The overall computer/mobile devices usage time is related to newly diagnosed non-alcoholic fatty liver disease: a population-based study. Ann Med 2016; 48: 568–576. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019; 17: 748–755.e3. [DOI] [PubMed] [Google Scholar]
- 8.Haldar D, Kern B, Hodson J, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol 2019; 71: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 10.Vanni E, Bugianesi E, Kotronen A, et al. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis 2010; 42: 320–330. [DOI] [PubMed] [Google Scholar]
- 11.VanWagner LB, Wilcox JE, Ning H, et al. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: The CARDIA study. J Am Heart Assoc 2020; 9: e014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobeika C, Cauchy F, Poté N, et al. Short- and long-term outcomes of liver resection for intrahepatic cholangiocarcinoma associated with the metabolic syndrome. World J Surg 2019; 43: 2048–2060. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JZ, Zhou QY, Wang YM, et al. Prevalence of fatty liver disease and the economy in China: A systematic review. World J Gastroenterol 2015; 21: 5695–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang N, Wang Y, Chen X, et al. Bone turnover markers and probable advanced nonalcoholic fatty liver disease in middle-aged and elderly men and postmenopausal women with type 2 diabetes. Front Endocrinol (Lausanne) 2019; 10: 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji Y, Yin Y, Sun L, et al. The molecular and mechanistic insights based on gut-liver axis: Nutritional target for non-alcoholic fatty liver disease (NAFLD) improvement. Int J Mol Sci 2020; 21: 3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 17.Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol 2017; 9: 715–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ampuero J, Aller R, Gallego-Durán R, et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol 2020; 73: 740–741. [DOI] [PubMed] [Google Scholar]
- 19.Shi YW, Wang QY, Zhao XY, et al. Non-obese NAFLD patients may use lower liver stiffness cut-off to better assess fibrosis stages. J Dig Dis 2020; 21: 279–286. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Zheng L, Hu L, et al. Transient elastography (FibroScan) performs better than non-invasive markers in assessing liver fibrosis and cirrhosis in autoimmune hepatitis patients. Med Sci Monit 2017; 23: 5106–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao M, Wang L, Leung PSC, et al. The clinical significance of GP73 in immunologically mediated chronic liver diseases: Experimental data and literature review. Clin Rev Allergy Immunol 2018; 54: 282–294. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z, Liu L, Pan X, et al. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore) 2015; 94: e659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SL, Zeng C, Fang X, et al. Hepatitis B virus upregulates GP73 expression by activating the HIF-2alpha signaling pathway. Oncol Lett 2018; 15: 5264–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kladney RD, Bulla GA, Guo L, et al. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 2000; 249: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrero JA, Romano PR, Nikolaeva O, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol 2005; 43: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 26.Xu Z, Shen J, Pan X, et al. Predictive value of serum Golgi protein 73 for prominent hepatic necroinflammation in chronic HBV infection. J Med Virol 2018; 90: 1053–1062. [DOI] [PubMed] [Google Scholar]
- 27.Qian X, Zheng S, Wang L, et al. Exploring the diagnostic potential of serum Golgi protein 73 for hepatic necroinflammation and fibrosis in chronic HCV infection with different stages of liver injuries. Dis Markers 2019; 2019: 3862024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 2010; 7: 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 30.Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol 2020; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacifico L, Andreoli GM, D'Avanzo M, et al. Role of osteoprotegerin/receptor activator of nuclear factor kappa B/receptor activator of nuclear factor kappa B ligand axis in nonalcoholic fatty liver disease. World J Gastroenterol 2018; 24: 2073–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younossi ZM, Golabi P, De Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol 2019; 71: 793–801. [DOI] [PubMed] [Google Scholar]
- 33.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10: 686–690. [DOI] [PubMed] [Google Scholar]
- 34.Altaf B, Rehman A, Jawed S, et al. Association of liver biomarkers and cytokeratin-18 in nonalcoholic fatty liver disease patients. Pak J Med Sci 2020; 36: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng KI, Liu WY, Pan XY, et al. Combined and sequential non-invasive approach to diagnosing non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease and persistently normal alanine aminotransferase levels. BMJ Open Diabetes Res Care 2020; 8: e001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haidari F, Hojhabrimanesh A, Helli B, et al. An energy-restricted high-protein diet supplemented with beta-cryptoxanthin alleviated oxidative stress and inflammation in nonalcoholic fatty liver disease: a randomized controlled trial. Nutr Res 2020; 73: 15–26. [DOI] [PubMed] [Google Scholar]
- 37.Liang R, Chen XY, Ge LY, et al. Meta-analysis supports the diagnostic value of GP73 in primary liver cancer. Clin Res Hepatol Gastroenterol 2015; 39: e71–e72. [DOI] [PubMed] [Google Scholar]
- 38.Kladney RD, Cui X, Bulla GA, et al. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 2002; 35: 1431–1440. [DOI] [PubMed] [Google Scholar]
- 39.Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010; 59: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Chen J, Li L, et al. A study of diagnostic value of golgi protein GP73 and its genetic assay in primary hepatic carcinoma. Technol Cancer Res Treat 2011; 10: 287–294. [DOI] [PubMed] [Google Scholar]
- 41.Cao Z, Li Z, Wang H, et al. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int 2017; 37: 1612–1621. [DOI] [PubMed] [Google Scholar]
- 42.Sang C, Yan H, Chan WK, et al. Diagnosis of fibrosis using blood markers and logistic regression in southeast Asian patients with non-alcoholic fatty liver disease. Front Med (Lausanne) 2021; 8: 637652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vincentis A, Tavaglione F, Jamialahmadi O, et al. A polygenic risk score to refine risk stratification and prediction for severe liver disease by clinical fibrosis scores. Clin Gastroenterol Hepatol 2021: S1542-3565(21)00595-4. [DOI] [PubMed] [Google Scholar]
- 44.Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J 2017; 23: 13030/qt7rv7j80p. [PubMed] [Google Scholar]
- 45.Drori A, Rotnemer-Golinkin D, Avni S, et al. Attenuating the rate of total body fat accumulation and alleviating liver damage by oral administration of vitamin D-enriched edible mushrooms in a diet-induced obesity murine model is mediated by an anti-inflammatory paradigm shift. BMC Gastroenterol 2017; 17: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakkak R, Gauss CH, Bell A, et al. Short-term soy protein isolate feeding prevents liver steatosis and reduces serum ALT and AST levels in obese female Zucker rats. Biomedicines 2018; 6: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang CH, Ni XC, Chen BY, et al. Combined preoperative albumin-bilirubin (ALBI) and serum gamma-glutamyl transpeptidase (GGT) predicts the outcome of hepatocellular carcinoma patients following hepatic resection. J Cancer 2019; 10: 4836–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh J, Jones G, Mahan D. A case of idiopathic GGT elevation with acute hepatitis A. J Family Med Prim Care 2019; 8: 2542–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunutsor SK, Apekey TA, Seddoh D. Gamma glutamyltransferase and metabolic syndrome risk: A systematic review and dose-response meta-analysis. Int J Clin Pract 2015; 69: 136–144. [DOI] [PubMed] [Google Scholar]
- 50.Jahani V, Kavousi A, Mehri S, et al. Rho kinase, a potential target in the treatment of metabolic syndrome. Biomed Pharmacother 2018; 106: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 51.Neuman MG, Malnick S, Chertin L. Gamma glutamyl transferase–an underestimated marker for cardiovascular disease and the metabolic syndrome. J Pharm Pharm Sci 2020; 23: 65–74. [DOI] [PubMed] [Google Scholar]
- 52.Maev IV, Samsonov AA, Palgova LK, et al. Effectiveness of phosphatidylcholine as adjunctive therapy in improving liver function tests in patients with non-alcoholic fatty liver disease and metabolic comorbidities: real-life observational study from Russia. BMJ Open Gastroenterol 2020; 7: e000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao W, Tong J, Liu J, et al. The dose-response relationship between gamma-glutamyl transferase and risk of diabetes mellitus using publicly available data: A longitudinal study in Japan. Int J Endocrinol 2020; 2020: 5356498. [DOI] [PMC free article] [PubMed] [Google Scholar]