Abstract
Background
This study sought to investigate which temporomandibular disorders (TMD) can be expected in patients with ankylosing spondylitis (AS) and to determine the combined impact of these conditions on the psychological status, chronic pain, and functional disability.
Material and Methods
A cross-sectional study composed of 30 patients between 18 and 65 years with ankylosing spondylitis was performed. The research protocol considered the evaluation of outcomes related to the ankylosing spondylitis (HLA-B27 antigen, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI) and Health Assessment Questionnaire – Spondylitis (HAQ-S)) and temporomandibular disorders (axis I and II of the Research Diagnostic Criteria for Temporomandibular Disorders – RDC/TMD). Descriptive analyses were applied to express the results.
Results
The sample presented both AS and TMD, most of them (24) were diagnosed with conventional AS (HLA-B27 positive). The BASDAI was scored as 7.70 (2.30) (high activity of AS disease). Functional disability represented by high scores of BASFI [7.00 (2.63)] and HAQ-S [1.79 (0.62)] demonstrates the severe impact of the disease on the daily routine and quality of life. According to RDC/TMD diagnostic criteria, 17 (57%) share the three groups of TMD, and 9 (30%) share two groups of TMD (Group I and III). Over 73% of the volunteers scored high levels of chronic pain (Grade III and IV) associated with a high depression scale score. The sample scored the somatization scale (with and without pain) as severe.
Conclusion
Patients with ankylosing spondylitis presented a high prevalence of temporomandibular disorder, most of them having the degenerative forms of TMJ disease. AS and TMD cause moderate to severe chronic pain and a negative impact on psychological status and functional capacities.
Keywords: spondyloarthritis, temporomandibular disorders, quality of life, chronic pain
Introduction
Ankylosing spondylitis (AS) is a chronic and immune-mediated enthesopathy of the axial skeleton that can progress to ossification and ankylosis of fibrocartilaginous joints.1–3 Although the estimated prevalence is low (0.12% in Latin America and 0.32% in North America),4 the disease is considered a public health problem due to being a potential cause of limitations in daily life, reduced motor activity, emotional impairment, and negative impact on quality of life.5–7
Some authors argued about the relationship between AS and temporomandibular disorders (TMD).8,9 Although not fully understood, destruction of joint capsule or components of the disc, synovitis in TMJ with a breakdown of the articular surfaces, and craniocervical postural changes due to AS are believed to be possible mechanisms underlying the TMJ involvement. Noteworthy, ankylosis is rare in the TMJ probably due to the presence of the intra-articular disc as a physical barrier.10
The prevalence of TMD in AS individuals vary across studies, mainly because of the population studied and the method used to assess the TMD. In general, scientific reports that considered the use of imaginological methods and objective and subjective symptoms to diagnose TMD found a prevalence between 30% and 37%.8,9,11 However, these methods did not follow standardized criteria and did not consider a comprehensive evaluation of the TMD. To overcome this deficiency, Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) system was proposed in 199212 and updated in 2016 for Diagnostic Criteria for Temporomandibular Disorders (DC/TMD).13 In the study of Bilgin et al5 who used the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for TMD diagnostic, it was found a TMD prevalence of 59% in AS individuals. Overall, higher values of the prevalence of TMD in AS individuals were found when the standardized diagnostic criteria were used.
The TMJ is an essential structure for well-performed mastication and other oral functions; thereby, its involvement causes an additional prejudice and vulnerability to individuals who suffer from ankylosing spondylitis. In line with this thought, a better understanding of the clinical manifestations of TMD in AS individuals as well as the combined effect of these conditions on psychosocial variables are relevant to establish a better description of the pattern of co-occurrence and the better approach to overcome severe and incapacitating consequences.
Given this background, this study sought to investigate which temporomandibular disorders can be expected in patients with ankylosing spondylitis and to determine the combined impact of these conditions on the psychological status, chronic pain, and functional disability.
Materials and Methods
This study was written in consonance with the recommendations of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.14
Study Design and Sample Eligibility
This cross-sectional study was performed in a sample of adults diagnosed with ankylosing spondylitis, who was evaluated between 2017 and 2018. This population was recruited from the Rheumatology Department of the Oswaldo Cruz University Hospital (HUOC), Recife-PE/Brazil. HUOC is a reference hospital for the treatment of ankylosing spondylitis in the Northeast region, especially in the state of Pernambuco (populational density: 9278 million people).
Consecutive sampling recruitment was accomplished by rheumatologists by spontaneous demand. To be eligible, patients must have ankylosing spondylitis diagnosed by HUOC rheumatologists using the modified New York criteria15 and aged between 18 and 65 years old. The exclusion criteria were patients:1 that did not change the medication in the previous 6 months,2 that have any pathology in the TMJ and adjacent tissues identified before ankylosing spondylitis diagnostic,3 who is in an acute state of the ankylosing spondylitis,4 who undergoing medications that affect bone metabolism,5 with neurological or cognitive deficits,6 presence of active dental or periodontal pain, and6 who had trauma in the head and neck region. Clinical and radiological evaluation for the diagnosis of AS was performed independently by Mariano MH and Rushansky E. Disagreements between them were resolved by a consensual method.
In the sample, it was evaluated outcomes related to the ankylosing spondylitis, temporomandibular disorders, self-perception of health, and sociodemographic factors. The outcomes centered in ankylosing spondylitis were the serotype of ankylosing spondylitis thought HLA-B27 genetic marker, the disease activity with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and the functional disability using the Bath Ankylosing Spondylitis Functional Index (BASFI) and quality of life using Health Assessment Questionnaire - Spondylitis (HAQ-S). The outcomes related to temporomandibular disorders were the diagnostic of temporomandibular disorders (axis I of the Research Diagnostic Criteria for Temporomandibular Disorders – RDC/TMD), self-reported TMD and bruxism/tightness, psychosocial disability (depression subscales and nonspecific physical symptoms), pain-related intensity and disability, and mandibular functioning limitation (axis II of RDC/TMD). Sociodemographic factors (age, sex, and race) were evaluated using data extracted from axis II of RDC/TMD.
Sample Size
Sample size forecast considered the two-sided hypothesis for one sample proportion according to the following formula:16
Where Z critical for α was 0.05 (type I error), Z critical for β was 0.20 (type II error),
Data Sources
HLA-B27 Marker Analysis
Blood tests for the HLA-B27 marker18 were made to differentiate between HLA-B27 positive and HLA-B27 negative ankylosing spondylitis.
Disease Activity of Ankylosing Spondylitis
Disease activity was measured using the cross-cultural adapted and validated for Brazilian Portuguese speaker’s version19 of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).20 This is a self‐administered instrument that includes six domains scored from 0 to 10 in a horizontal 10-cm visual analog scale (VAS), in which 10 score means very severe symptoms. Answers considered the last week of the volunteer. The domains assessed were1 fatigue,2 neck, back, and hip pain,3 peripheral joint pain and swelling,4 localized tenderness,5 severity of morning stiffness, and6 duration of morning stiffness. The overall score was calculated by the sum of the first four domains with the average of the fifth and sixth. This value was then divided by 5.
Functional Disability Caused by Ankylosing Spondylitis
Functional disability was estimated using two indices:
The cross-cultural adapted and validated for Brazilian Portuguese speaker’s version21 of Bath Ankylosing Spondylitis Functional Index (BASFI).22 BASFI consists of a self-assessment instrument designed with questions regarding physical function (Q1-8) and the patient’s ability to cope with everyday life (Q9-10). Answers considered the last week of the volunteer and were rated in a 10-cm VAS without demarcation. The word “easy” was anchored at the beginning of the line, the word medium at the middle, and the word “impossible” at the end. The overall score was calculated considering the mean of the 10 questions.
The short form of the Health Assessment Questionnaire23 modified by Daltroy et al24 for use in patients with Spondyloarthropathies (HAQ-S), and translated, cross-cultural adapted, and validated for use with Brazilian speakers.25 This instrument is composed of 20 items across eight categories of daily function as dressing and grooming (Q1-2), arising (Q3-4), eating (Q5-7), walking (Q8-9), hygiene (Q10-12), reach (Q13-14), grip (Q15-17), and common daily activities (Q18-20). Answers considered the last week of the volunteer. The difficulty in performing each activity was scored as without any difficulty (0), with some difficulty,1 with much difficulty, and unable to do.3 The overall score ranges from 3 (worst) to 0.
Research Diagnostic Criteria for Temporomandibular Disorders
Data collection and clinical examination were conducted according to the updated version of RDC/TMD Axis I and Axis II Tests.12 This instrument was used considering the translation and adaptation version for Brazilian Portuguese speakers.
Clinical physical examinations (Axis I) were performed to diagnose TMD. This instrument consists of 10 items in which it was performed muscle and joint palpation, testing of mandibular movements, and three subjective questions. Palpations were done with 2 lbs of pressure for extraoral muscles and 1 lb of pressure on the joints and intraoral muscles. Axis I was conducted by one calibrated researcher dentist (Souza RV), who was trained by a gold standard examiner in a sample of 10 volunteers. The kappa value was 0.79. Participants were diagnosed in three groups according to the TMD diagnostic algorithm applied to the Axis I of RDC/TMD summary of findings:12
Group I – Muscle Diagnosis: Ia - Myofascial pain; Ib - Myofascial pain with limited opening; Ic - No group I diagnosis.
Group II – Disc displacements on the (right and left) joint: IIa - Disc displacements with reduction; IIb - Disc displacements without reduction, with limited opening; IIc - Disc displacements without reduction, without limited opening; IId - No group II diagnosis.
Group III – Other joint conditions: IIIa - Arthralgia; IIIb - Osteoarthritis; IIIc - Osteoarthrosis; IIId - No group III diagnosis.
Biobehavioral questionnaire (Axis II) consists of 31 items that comprise the assessment of the following constructs: sociodemographic factors, self-reported TMD, self-perception health, pain-related intensity, and disability (Graded Chronic Pain Scale), Psychosocial disability (depression subscales and nonspecific physical symptoms), and mandibular functioning limitation:
Sociodemographic factors: Age (Q23), Sex (Q24), and race (Q25).
Self-reported TMD and bruxism/tightness: Opening of the Jaw (Q14a-b), Click or pop (Q15a), grating or grinding noise (Q15b), grind or clench the jaw when sleeping (Q15c), grind or clench the jaw during the day (Q15d), stiffness or painful jaw when waking up (Q15e), noise or ringing on the ears (Q15f), and uncomfortable bite feeling (Q15g). The options available for answers were Yes or No.
The pain-related intensity and disability (Graded Chronic Pain Scale): First, the Q3 evaluated the presence of chronic pain or not in the last 4 weeks. If no, the volunteer was graded as 0 (no disability). If yes, the Q7-Q9 corresponds to the analysis of Pain intensity; Q10-13 corresponds to the analysis of disability points. Chronic Pain was categorized as Grade I (low disability, low intensity), Grade II (low disability, high intensity), Grade III (high disability, moderately limiting), Grade IV (high disability, severely limiting).
Psychosocial disability was evaluated using questions related to Depression (Q20: 20 items - b, e, h, i, k, l, m, n, v, y, cc, dd, ee, f, g, q, z, aa, bb, ff), nonspecific physical symptoms with pain-related included (Q20: 12 items - a, c, d, j, o, p, r, s, t, u, w, x), and nonspecific physical symptoms with pain excluded (Q20: 7 items - c, r, s, t, u, w, x). A minimum of 2/3 answers were required to count the domain for each volunteer. The score for all items answered was: Not at all = 0; A little bit = 1; Moderately = 2; Quite a bit = 3; Extremely = 4. The score of each domain was obtained by the sum of the score of each item divided by the number of items answered. Depression index and nonspecific physical symptoms were categorized considering the following cut off points: normal (<0.535: Depression index; <0.500: Nonspecific physical symptoms (pain items included); and <0.428: Nonspecific physical symptoms (pain items excluded)), moderate (0.535–1.105: Depression index; 0.500–1.000: Nonspecific physical symptoms (pain items included); and 0.428–0.857: Nonspecific physical symptoms (pain items excluded)), and severe (>1.105: Depression index; >1.000: Nonspecific physical symptoms (pain items included); and >0.857: Nonspecific physical symptoms (pain items excluded)).26
The mandibular functioning limitation was accessed for item 19 of the questionnaire and provide information regarding subjects’ discomfort during some mandibular activities as chewing, drinking, brushing teeth, washing face, exercise, yawning, swallowing, talking, smiling or laughing, and facial expression. Answers were either “yes” or “no”. The score of this domain was obtained through the number of positive answers divided by the number of items answered. The number 1 represents the highest score.
The scoring protocol in the Axis II RDC/TMD questionnaire followed the international RDC/TMD consortium. Answers left out of the aforementioned description were excluded.
Data Analysis
Data were analyzed using the SPSS package for Windows, version 21.0 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were used to evaluate the quantitative and qualitative variables. For quantitative variables, sample distribution was verified using the Shapiro–Wilk test, and data were expressed as mean (standard deviation) or median (Interquartile range) according to the Gaussian distribution. Absolute and percentage frequencies were calculated for categorical variables.
Results
The study began with 36 volunteers; however, only 30 finished all steps of the research. Table 1 summarizes the sample characteristics, disease activity, and functional disability. The sex ratio male: female was 1.31:1.00 and the average age of volunteers was 49 (7.8) years. Half of the sample was diagnosed with the disease between 5 and 10 years ago. Except for one volunteer, the sample presented both AS and TMD conditions, most of them24 was diagnosed with conventional ankylosing spondylitis (HLA-B27 positive). The BASDAI was scored as 7.70 (2.30), which represents the presence of the disease activity. High values of BASFI and HAQ-S demonstrate the severe impact of the disease on the daily routine and quality of life.
Table 1.
Sample Characteristics, Ankylosing Spondylitis Activity, and Functional Disability
| Age, Yr – Mean (SD) | 49 (7.8) |
| Sex – nº. (%) | |
| Male | 17 (56.7%) |
| Female | 13 (43.3%) |
| Race – nº. (%)£ | |
| White | 14 (46.7%) |
| Non-white | 16 (53.3%) |
| Time Since Diagnosing of AS | |
| Lower than 5 years | 9 (30%) |
| 5–10 years | 15 (50%) |
| Higher than 10 years | 6 (20%) |
| Medicine Use – nº. (%) | |
| Etanercept | 16 (53.3%) |
| Adalimumab | 11 (36.7%) |
| Infliximab | 3 (10%) |
| Positive for HLA-B27 – nº. (%) | 24 (80%) |
| Disease Activity (BASDAI) – Median (IQR) β | |
| Fatigue | 8.00 (3.00) |
| Neck, back, and hip pain | 9.00 (2.00) |
| Peripheral joint pain and swelling | 7.00 (4.00) |
| Localized tenderness | 8.00 (3.00) |
| Morning stiffness severity | 8.00 (4.00) |
| Morning stiffness duration | 5.00 (7.00) |
| TOTAL SCORE | 7.70 (2.30) |
| Functional Disability (BASFI) – Median (IQR) β | |
| Physical function | 6.25 (2.66) |
| Ability to cope with everyday life | 8.75 (2.50) |
| TOTAL SCORE | 7.00 (2.63) |
| Functional disability (HAQ-S) – Mean (SD)σ | 1.79 (0.62) |
Notes: £Race was self-assessed; βScore scale ranged from 10 (worst) to 0. σScore scale ranged from 3 (worst) to 0.
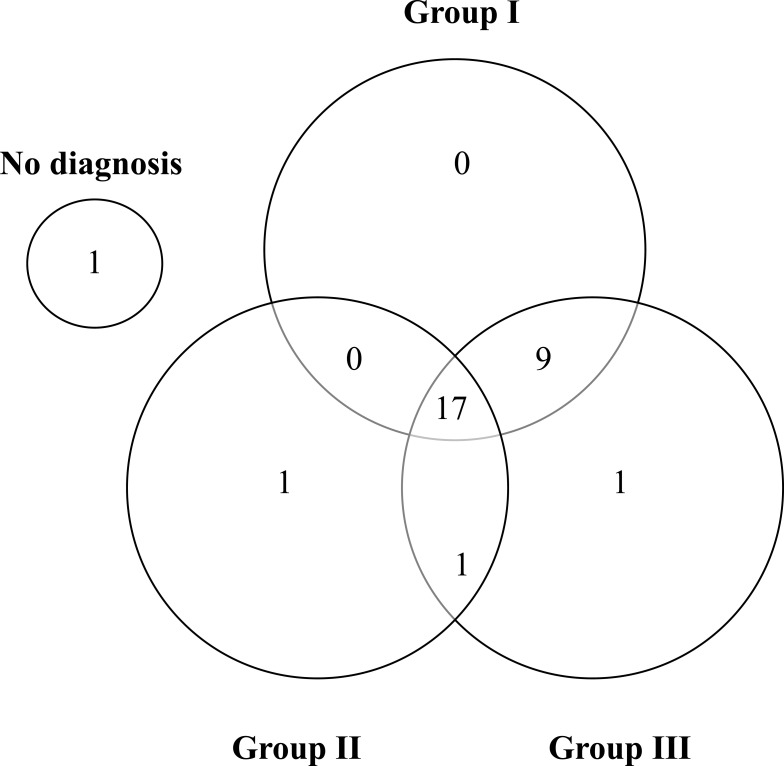
Figure 1 demonstrates that only one volunteer did not have TMD according to RDC/TMD diagnostic criteria. Of those individuals affected, 17 (57%) share the three groups of TMD, and 9 (30%) share two groups of TMD (Group I and III). The most prevalent TMJ subtypes of disorders were myofascial pain with a limited opening (53%) – Group I, disc displacement with reduction (right joint equal to 53% and left joint equal to 60%) – Group II, and arthralgia (right joint equal to 43% and left joint equal to 50%) – Group III (Table 2).
Figure 1.
Diagnostic distributions of the cases relative to TMJ Group I, II, and III. Group I – Muscle Diagnosis, Group II – Disc displacements on the (right and left) joint, and Group III – Other joint conditions (Arthralgia, osteoarthritis, and osteoarthrosis).
Table 2.
Prevalence of TMD Diagnoses According to the Axis I of RDC/TMD
| TMJ Subtypes | Yes n (%) | |
|---|---|---|
| Group I: Muscle Diagnosis | ||
| Ia: Myofascial pain | 10 (33.3) | |
| Ib: Myofascial pain with limited opening | 16 (53.3) | |
| Group II: Disc Displacements on the Joint | ||
| Right Joint Yes n (%) | Left Joint Yes n (%) | |
| IIa: Disc displacements with reduction | 16 (53.3) | 18 (60) |
| IIb: Disc displacements without reduction, with limited opening | 1 (3.3) | 1 (3.3) |
| IIc: Disc displacements without reduction, without limited opening | 0 | 0 |
| Group III: Other Joint Conditions | ||
| Right Joint Yes n (%) | Left Joint Yes n (%) | |
| IIIa: Arthralgia | 13 (43.3) | 15 (50) |
| IIIb: Osteoarthritis | 7 (23.3) | 8 (26.7) |
| IIIc: Osteoarthrosis | 3 (10) | 3 (10) |
The results in Table 3 indicated that over 73% of the volunteers scored high levels of chronic pain (Grade III and IV) associated with a high depression scale score (97% of the sample was graded as moderated or severe depression). The sample scored the somatization scale (with and without pain) as severe.
Table 3.
Prevalence of Pain-Related Intensity and Disability, Psychosocial Disability, and Mandibular Functioning Limitation According to Axis II of RDC/TMD
| Chronic Pain Gradeµ nº. (%) | |
| Grade 0 | 3 (10%) |
| Grade I | 2 (6.7%) |
| Grade II | 6 (20%) |
| Grade III | 11 (36.7) |
| Grade IV | 8 (26.7) |
| Depression nº. (%) | |
| Normal | 1 (3.3%) |
| Moderate | 11 (36.7%) |
| Severe | 18 (60%) |
| Nonspecific Physical Symptoms nº. (%) | |
| Total Scale | |
| Severeσ | 30 (100%) |
| Pain Excluded nº. (%) | |
| Severeσ | 30 (100%) |
| Mandibular Limitation – Mean (SD)β | 0.36 (0.19) |
Notes: µGraded Chronic Pain Scale: grade 0, no disability; I, low disability, low intensity; II, low disability, high intensity; III, high disability, moderately limiting; IV, high disability, severely limiting. σThe categories normal and moderated were not included in the table due to the absence of cases. βThe worst value for mandibular limitation was 1.
When asked regarding their TMD, the jaw locking and limitation in the jaw opening were the less frequent self-reported conditions. The symptoms related to sleep and awake bruxism/tightness as a grind or clench of the jaw when sleep and/or during the day were highly prevalent (Table 4).
Table 4.
Prevalence of Self-Reported TMD and Sleep and Awake Bruxism/Tightness According to Axis II of RDC/TMD
| Self-Reported TMD | nº. Yes (%) |
|---|---|
| Jaw locking | 10 (33.3%) |
| Limitation in the Jaw opening | 8 (26.7%) |
| Jaw click or pop | 25 (83.3%) |
| Grating or grinding noise | 22 (73.3%) |
| Noise or ringing on the ears | 19 (63.3%) |
| Uncomfortable bite feeling | 27 (90%) |
| Stiffness or painful jaw when waking up | 22 (73.3%) |
| Headaches or migraines | 24 (80%) |
| Bruxism/tightness | |
| Grind or clench the jaw when sleep | 13 (43.3%) |
| Grind or clench the jaw during the day | 19 (63.3%) |
Several volunteers considered their general health to be reasonable (18/60%) or bad (9/30%). The same trend was observed regarding oral health with 19/63% classified as reasonable and 5/17% as bad. The seek for treatment due to orofacial pain was observed in 3 volunteers (10%).
Discussion
The prevalence of TMD is approximately 5–12%;14 however, in individuals with AS the prevalence of TMD is seen to be higher.5,8,9 This epidemiological phenomenon can be linked with the musculoskeletal nature of both diseases and with the pathophysiology of inflammation in this group of individuals.27 In such a scenario, more important than determining the combined prevalence of both diseases is establish into the TMD taxonomy (Group I Muscle Disorders; Group II Disc Displacements; and Group III Arthralgia, Arthritis, Arthrosis) the distribution of TMD subtypes in these individuals and better understand their impact on the psychobiological aspects of life. Noteworthy, we are not able to find any study that provides a comprehensive understanding of the effect of TMD as an AS co-occurrence.
Based on the state-of-the-art in scientific literature and STARD recommendations,28 this study used validated methods to analyze AS (New York criteria and BASDAI) and TMD (RDC/TMD) axis I. Besides, the psychosocial impact of these conditions on individuals considered the use of instruments like BASFI and HAQ-S to qualify the functional disability due to AS. RDC/TMD axis II was used to qualify the psychosocial disability, functioning limitation, and chronic pain due to TMD. These instruments were broadly cited in the scientific literature because have a validity of the construct and were translated and validated in several languages, including Brazilian Portuguese.
In the study reported here, it was observed that almost all volunteers with AS have TMD according to RDC/TMD diagnostic criteria and most of them have more than one subtype of TMD (n = 26, 87%). Besides, it was found that a high prevalence of the most incapacitating forms of Group I (Myofascial pain with limited opening: 53%) and III (Degenerative joint disease – osteoarthritis and osteoarthrosis: ≈ 35%) TMD subtypes. Disk displacement with reduction was the most prevalent phenotype of TMD. When these results were compared with a sample of adults/elderly individuals without AS it was found an overall prevalence of approximately 31%. Disk displacement with reduction was the most prevalent phenotype of TMD with 25.9%. The overall degenerative joint disease (osteoarthritis and osteoarthrosis) was equal to 9.8%.29 Thus, the results of our study demonstrating that AS patients have a trend toward a great severity of TMD disease. Under this context, it must be highlighted that the concomitant occurrence of TMD and AS can increase the morbidity of patients.
Individuals in this research have high disease activity considering the internationally deemed as a BASDAI ≥ 4.30 Remarkably, patients included in this research use anti-tumor necrosis factor (anti-TNF), which is the currently recommended treatment for patients with persistent disease activity.31 A meta-analysis of 14 studies (3186 patients) with low to moderate risk of bias demonstrated that anti-TNF therapy can significantly improve disease activity and physical function in AS.32 In this context, caution is needed to interpret our data since the study design did not consider temporality; thus, the influence of any medicine in the improvement in both objective and subjective indicators of disease activity and patient functioning is out of the scope of this research.
The positivity of the HLA-B27 marker (n = 24, 80%) is in accordance with previous investigations that evidence 80–90% of the susceptibility to ankylosing spondylitis to the gene for HLA-B27.33 Although the mechanism underlying this association is unknown,34 HLA-B27 positive patients have more severe disease and systemic manifestation.35 Unfortunately, the nature of the research design places some restrictions on the understanding of the HLA-B27 inflammatory marker on the TMJ involvement in patients with AS. Of note, the understanding of the role of the genetic propensity and their respective impact on the clinical manifestation of TMD in AS patients can be elusive.
The functional limitation was assessed using BASFI and HAQ-S. The first one is related to physical function (bending, reaching, changing position, standing, turning, and climbing steps) and the ability to cope with everyday life. The second is related to the impairments in activities of daily living in patients with AS.36 Together, these instruments provide a comprehensive understanding of how incapacitating this condition can be. Individuals who suffer from AS have the potential of disability in several important dimensions of human life considering the biopsychosocial health model. Noticeably, the high score for the ability to cope with everyday life (8.75 (SD of 2.50)) can be a signal of an emotionally overwhelmed population.
Chronic pain is an element that attracts attention, mainly because of a high prevalence of the most severe and disabling forms of pain (Grade II to IV equal to 83%). This result contrasts with the Maringa Study performed in Brazil in a sample of healthy adults in the same country and of similar age. This research pointed out a lower prevalence of chronic pain (36% of the population regardless of the intensity or limitations due to pain).17 To better understand the impact of these outcomes in daily life, the moderate to severe levels of depression (97% of the sample) and severe levels of somatization related or not to pain (100% of the sample) highlight the need for treatment in this group of patients. Here, the call for action to encourage the creation of a comprehensive therapeutic structure that includes the dentist specialized in temporomandibular dysfunction in the rheumatological agenda. Of note, the seek for treatment due to orofacial pain was observed only in three volunteers (10% of the sample). Besides, in the pain-psychopathology network, the value of cognitive and emotional skills should be enhanced by the presence of mental health professionals.
A high prevalence of jaw-related functional limitation and parafunctional behaviors was found. Moreover, jaw locking and limitation in jaw opening were the less frequent self-reported conditions. These results have important implications for dental practice due to the potential complications like a limitation in masticatory function, swallowing, and impairment in social interaction due to prejudice in smiling, talk and sad or painful facial expressions. As a tendency in the literature, restriction of mandibular movements due to ankylosis was not find in any volunteers of our study. Based on a comprehensive image analysis criterion for RDC/TMD images, findings commonly observed in TMJ of AS patients were temporal flattening, abnormal condylar shape, erosions, sclerosis, disk alterations, and osteophytes.37 TMJ ankylosis is a rare condition in AS patients probably due to the presence of the articular disc between the bone surface.10
Most of the sample report that considers their own oral and general health as reasonable. This outcome must be seemed as relevant mainly in patients who were identified as non-responsive to treatments. Here, the patients’ beliefs about their illnesses can restrict adherence with the recommendation of health-care providers and potentially prejudice the compliance with treatment regimens. This trend was observed by Tolu et al38 who found that nearly three out of five AS patients were identified as at risk for non-adherence with the medication regimen. The self-perception of illness can disrupt the cognitive and emotional representations of diseases and point out to a population with a low sense of coherence related to their oral health.
Only three volunteers seek treatment due to orofacial pain. The key element above this outcome should be further investigated to improve treatment strategies targeted to this population. We can speculate three main reasons for this finding. Firstly, the co-occurrence of pain in other articulations possibly hides the importance of TMJ involvement. Secondly, the cultural low importance related to oral health when together with a medical problem. Thirdly, the lack of proper information regarding the own disease and the potential co-occurrence of TMD (as a separated disease entity).
This study has some strengths and limitations. Studies that consider psychosocial aspects of both AS and TMD are scarce in the scientific literature; thus, this study made a significant worldwide contribution to this research area and provide further background to the Brazilian Registry of Spondyloarthritides. Additionally, the use of validated instruments improves the quality of data, comparability with other realities, and provides a source for future research.
Cautionary notes include the lack of a control group with characteristics of individuals without rheumatic diseases, the cross-sectional design limits aspects related to the causality of TMD as an AS co-occurrence. Moreover, as this study only considered patients that used anti-TNF medicines, there is an overall heavy disease burden31 which may be a source of serious selection bias as the recruitment considered a hospital-based convenience sample. In this context, caution is needed to interpret our data since the study design did not consider temporality; thus, the influence of any medicine in the improvement in both objective and subjective indicators of disease activity and patient functioning is out of the scope of this research.
To overcome these limitations in a more complete clinical and research perspective, longitudinal and prospective cohort or case-control studies with a larger and randomized sample can be elusive to establish the potential psychosocial markers and better predict the natural course of TMD in individuals who suffer from AS.
Conclusions
Patients with ankylosing spondylitis presented a high prevalence of temporomandibular disorder, most of them having the degenerative forms of TMJ disease, bilaterally. AS and TMD cause moderate to severe chronic pain and a negative impact on psychological status and functional capacities.
Acknowledgments
We especially thank all volunteers for their valuable contribution to our research.
Funding Statement
The first-listed author was granted a Ph.D. Scholarship from Coordination from the Improvement of Higher Education Personnel (CAPES, Ministry of Education, Brazil).
Ethics and Consent
This study was approved by the Research Ethics Committee of the State University of Pernambuco - UPE (CAAE 15966819.1.0000.5207), which followed the Declaration of Helsinki and the regulatory guidelines and norms obey 466/12 resolution for research ethics in Brazil. All individuals who agreed to participate in this study signed an informed consent form, authorizing their participation.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest for this work.
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377(9783):2127–2137. doi: 10.1016/S0140-6736(11)60071-8 [DOI] [PubMed] [Google Scholar]
- 2.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. [DOI] [PubMed] [Google Scholar]
- 3.Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res. 2019;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53:650–657. [DOI] [PubMed] [Google Scholar]
- 5.Bilgin E, Bilgin E, Özdemir O, Kalyoncu U. Temporomandibular disorders in ankylosing spondylitis: a cross-sectional, monocentric study. Rheumatol Int. 2020;40:933–940. [DOI] [PubMed] [Google Scholar]
- 6.Alkan H, Yildiz N, Ardiç F. The correlations between disease specific quality of life, short form-36 and clinical variables in patients with ankylosing spondylitis. Arch Rheumatol. 2020;35:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogard E, Olofsson T, Bergman S, et al. Chronic pain and assessment of pain sensitivity in patients with axial spondyloarthritis: results from the SPARTAKUS cohort. J Rheumatol. 2020;jrheum.200872. doi: 10.3899/jrheum.200872 [DOI] [PubMed] [Google Scholar]
- 8.Helenius LM, Hallikainen D, Helenius I, et al. Clinical and radiographic findings of the temporomandibular joint in patients with various rheumatic diseases. A case–control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:455–463. [DOI] [PubMed] [Google Scholar]
- 9.Yildizer Keris E, Yaman SD, Demirag MD, Haznedaroglu S. Temporomandibular joint findings in patients with rheumatoid arthritis, ankylosing spondylitis, and primary Sjogren’s syndrome. J Investig Clin Dent. 2017;8:12255. [DOI] [PubMed] [Google Scholar]
- 10.Shadamarshan Rengasayee A, Roy Chowdhury SK, Sharma R, Padma Priya S. Novel hypotheses related to Temporomandibular joint derived from Ankylosing spondylitis. Med Hypotheses. 2020;144:110225. [DOI] [PubMed] [Google Scholar]
- 11.Ramos-Remus C, Major P, Gomez-Vargas A, et al. Temporomandibular joint osseous morphology in a consecutive sample of ankylosing spondylitis patients. Ann Rheum Dis. 1997;56:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomand Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 13.Schiffman E, Ohrbach R; International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache. 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 15.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. [DOI] [PubMed] [Google Scholar]
- 16.Chow S, Wang. Sample Size Calculations in Clinical Research. NY: Taylor & Francis; 2003:82–83. [Google Scholar]
- 17.Progiante PS, Pattussi MP, Lawrence HP, Goya S, Grossi PK, Grossi ML. Prevalence of temporomandibular disorders in an adult Brazilian community population using the research diagnostic criteria (Axes I and II) for temporomandibular disorders (The Maringá Study). Int J Prosthodont. 2015;28:600–609. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez O, Coto E, Martinez-Naves E, Choo SY, Lopez-Larrea C. Molecular typing of HLA-B27 alleles. Immunogenetics. 1992;36:277–282. [DOI] [PubMed] [Google Scholar]
- 19.Pimentel-Santos FM, Pinto T, Santos H, et al. Portuguese version of the bath indexes for ankylosing spondylitis patients: a cross-cultural adaptation and validation. Clin Rheumatol. 2012;31:341–346. [DOI] [PubMed] [Google Scholar]
- 20.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis disease activity index. J Rheumatol. 1994;21:2286–2291. [PubMed] [Google Scholar]
- 21.Cusmanich KG, Kowalski SC, Gallinaro AL, Goldenstein-Schainberg C, Souza LA, Gonçalves CR. Cross-cultural adaptation and validation of the Brazilian-Portuguese version of the Bath Ankylosing Spondylitis Functional Index (BASFI). Rev Bras Reumatol. 2012;52:733–741. [PubMed] [Google Scholar]
- 22.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis functional index. J Rheumatol. 1994;21:2281–2285. [PubMed] [Google Scholar]
- 23.Fries JF, Spits P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. [DOI] [PubMed] [Google Scholar]
- 24.Daltroy LH, Larson MG, Roberts NW, Liang MH. A modification of the Health Assessment Questionnaire for the spondyloarthropathies. J Rheumatol. 1990;17:946–950. [PubMed] [Google Scholar]
- 25.Shinjo SK, Gonçalves R, Kowalski S, Gonçalves CR. Brazilian-Portuguese version of the Health Assessment Questionnaire for Spondyloarthropathies (HAQ-S) in patients with ankylosing spondylitis: a translation, cross-cultural adaptation, and validation. Clin Rheumatol. 2007;26:1254–1258. [DOI] [PubMed] [Google Scholar]
- 26.Kim YK, Kim SG, Im JH, Yun PY. Clinical survey of the patients with temporomandibular joint disorders, using Research Diagnostic Criteria (Axis II) for TMD: preliminary study. J Craniomaxillofac Surg. 2012;40:366–372. [DOI] [PubMed] [Google Scholar]
- 27.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;375:1303. [DOI] [PubMed] [Google Scholar]
- 28.Bossuyt PM, Reitsma JB; Standards for Reporting of Diagnostic Accuracy, et al.. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Radiol. 2003;58:575–580. [DOI] [PubMed] [Google Scholar]
- 29.Valesan LF, Da-cas CD, Réus JC, et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin Oral Investig. 2021;25:441–453. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JD, Cunin P, Farrenq V, et al. Estimation of the bath ankylosing spondylitis disease activity index cutoff for perceived symptom relief in patients with spondyloarthropathies. J Rheumatol. 2006;33:79–81. [PubMed] [Google Scholar]
- 31.van der Heijde D, Sieper J; Assessment of SpondyloArthritis international Society, et al. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:905–908. [DOI] [PubMed] [Google Scholar]
- 32.Zong HX, Xu SQ, Tong H, Wang XR, Pan MJ, Teng YZ. Effect of anti-tumor necrosis factor α treatment on radiographic progression in patient with ankylosing spondylitis: a systematic review and meta-analysis. Mod Rheumatol. 2019;29:503–509. [DOI] [PubMed] [Google Scholar]
- 33.Thomas GP, Brown MA. Genetics and genomics in ankylosing spondylitis. Immunol Rev. 2010;233:162–180. [DOI] [PubMed] [Google Scholar]
- 34.Taurog JD. The role of HLA-B27 in spondyloarthritis. J Rheumatol. 2010;37:2606–2616. [DOI] [PubMed] [Google Scholar]
- 35.Usha SGK. Role of HLA B27 in diagnosis of seronegative spondyloarthropathies. Indian J Pathol Microbiol. 2007;50:908–913. [PubMed] [Google Scholar]
- 36.Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S47–58. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad M, Hollender L, Anderson Q, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolu S, Rezvani A, Karacan İ, et al. Self-reported medication adherence in patients with ankylosing spondylitis: the role of illness perception and medication beliefs. Arch Rheumatol. 2020;35:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]