Dear Editor-in-Chief,
Coronavirus disease 2019 (COVID-19) has caused a pandemic, and detailed information of adverse effects for its vaccines is to be timely needed. Adenovirus vector–based vaccines can be related to the development of venous thrombotic events including cerebral venous sinus thrombosis (CVST) associated with thrombocytopenia and platelet factor 4 (PF4)–heparin antibodies [1, 2]. Here, we report a case of CVST with prolonged mild headache after mRNA-based COVID-19 vaccination.
A man in his 50 s without remarkable medical history visited our department complaining of 1-week headache, which started 26–30 h after his second dose of tozinameran (Comirnaty® from Pfizer–BioNtech). He was evaluated by blood test, brain computed tomography (CT), and magnetic resonance imaging (MRI).
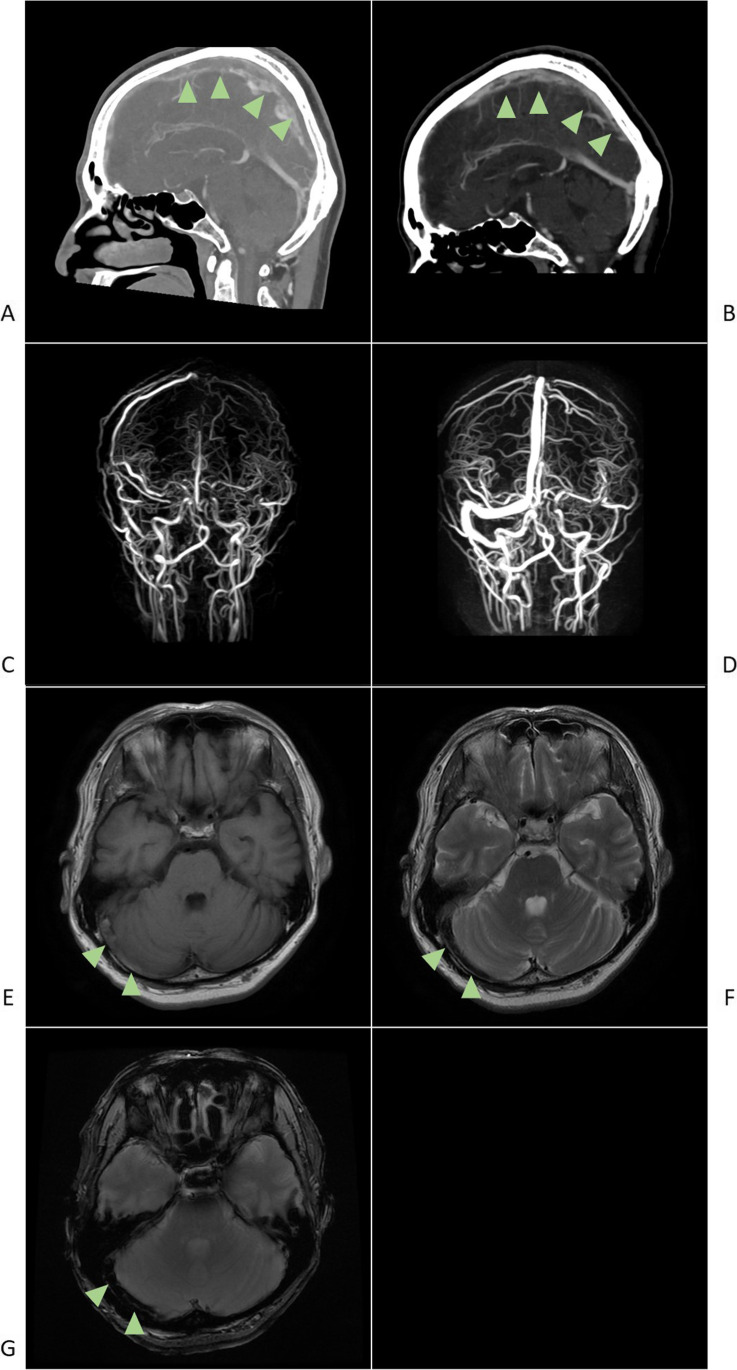
His headache was a combination of pulsative and tension types, predominantly felt around the bilateral occipital area. Nonetheless, it was mild; thus, he could still continue his office work, with over-the-counter pain reliever medication. No other common adverse events of vaccination were noted. On admission, he was alert and had no neurological deficit. His platelet count was normal (271,000/µL [reference range: 158,000–348,000]), but his D-dimer level was elevated (6.01 µg/mL [reference value: < 1.00]). Brain CT and MRI (Fig. 1), which were both conducted 7 days after second vaccination, showed thrombosis in the superior sagittal sinus, right transverse sinus, right sigmoid sinus, and right internal jugular vein [3]. There were no remarkable abnormalities in brain parenchyma. Nasopharyngeal swab samples were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) according to the polymerase chain reaction test. Blood tests for the risk factors of venous thrombosis such as antiphospholipid antibody and protein S/C showed no remarkable abnormality, and the screening test for antibodies against PF4–heparin was negative. After heparin treatment, his headache was relieved in 2 weeks, with sinus thrombosis partially disappearing.
Fig. 1.
a Sagittal view of the enhanced CT imaging of the brain showing CVST in superior sagittal sinus (arrow heads). b Sagittal view of the enhanced CT imaging of the brain showing partially disappearing CVST, 1 week after the admission (arrow heads). c MRV showing CVST in the superior sagittal sinus, right transverse sinus, right sigmoid sinus, and right internal jugular vein. d MRV showing partially disappearing CVST, 2 weeks after the admission. e MRI of the thrombosed right transverse sinus showing slight hyperintensity on the T1-weighted image (arrow heads). f MRI of the thrombosed right transverse sinus showing hypointensity on the T2-weighted image (arrow heads). g MRI of the right transverse sinus showing hypointensity on the T2*-weighted sequence (arrow heads). CT, computed tomography; CVST, cerebral venous sinus thrombosis; MRI, magnetic resonance imaging; MRV, magnetic resonance venography
CVST after SARS-CoV-2 vaccines are divided into a CVST in the setting of the vaccine-induced immune thrombotic thrombocytopenia syndrome (VITT), mainly related to adenovirus vector–based vaccines, and a CVST without VITT characteristics, related also to mRNA-based vaccines [4]. This case was a CVST not related to VITT.
In the UK (https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions), USA (Vaccine Adverse Event Reporting System [VAERS]: https://wonder.cdc.gov/vaers.html), Europe (http://www.adrreports.eu/en/index.html), and Japan (https://www.mhlw.go.jp/stf/shingi/shingi-kousei_284075.html), the occurrence of CVST after mRNA-based COVID-19 vaccinations has been reported to the authorities. In the analysis of thrombotic events reported to the WHO VigiBase, CVST, as well as PE and DVT, occurred after the injection of mRNA-based and adenovirus vector vaccines [5], but their causal relationship was not supported in a US epidemiologic study [6]. However, mild CVST events, as seen in our patient, might be possibly excluded in these analyses because headache may be too mild to be spontaneously reported. Therefore, our case suggests that the precise relationship between CVST and COVID-19 vaccination remains unclear, and its evaluation should include mild cases. Although excessive concern to post-vaccination headache is inappropriate, we recommend performing brain CT or MRI to evaluate CVST for patients whose headache lasts for > 1 week after vaccination, even for mild ones.
Declarations
Consent for publication
Written informed consent was obtained from the patient.
Conflict of interest
TY reports grants from Takeda Pharmaceutical Company, Ltd; grants from Daiichi Sankyo Company, Ltd; grants and personal fees from Pfizer Japan Inc.; and grants and personal fees from Mitsubishi Tanabe Pharma Corporation. The other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See I, Su JR, Lale A et al (2021) US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 325:2448–2456 [DOI] [PMC free article] [PubMed]
- 3.Isensee C, Reul J, Thron A. Magnetic resonance imaging of thrombosed dural sinuses. Stroke. 1994;25:29–34. doi: 10.1161/01.STR.25.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez van Kammen M, Aguiar de Sousa D, Poli S et al (2021) Characteristics and outcomes of patients with cerebral venous sinus thrombosis in SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. JAMA Neurol 78:1314–1323 [DOI] [PMC free article] [PubMed]
- 5.Smadja DM, Yue Q-Y, Chocron R, et al. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58:2100956. doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlowski C, Rincón-Hekking J, Awasthi S, et al. Cerebral venous sinus thrombosis is not significantly linked to COVID-19 vaccines or non-COVID vaccines in a large multi-state health system. J Stroke Cerebrovasc Dis. 2021;30:105923. doi: 10.1016/j.jstrokecerebrovasdis.2021.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]