Abstract
Background
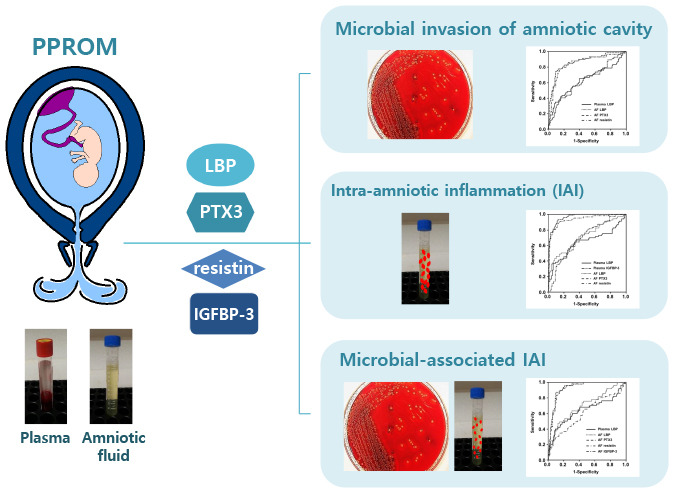
We sought to determine whether lipopolysaccharide binding protein (LBP), pentraxin 3, resistin, and insulin-like growth factor binding protein (IGFBP)-3 in plasma and amniotic fluid (AF) can predict microbial invasion of the amniotic cavity (MIAC), intra-amniotic inflammation (IAI), and microbial-associated IAI in women with preterm premature rupture of membranes (PPROM).
Methods
This was a retrospective cohort study involving 168 singleton pregnant women with PPROM. AF obtained via amniocentesis was cultured and assayed for interleukin (IL)-6 to define IAI and for IL-8 to compare with AF biomarkers. Plasma samples were collected at the time of amniocentesis, and C-reactive protein (CRP) levels in serum were compared with plasma biomarkers. The stored plasma and AF samples were assayed for LBP, pentraxin 3 (PTX3), resistin, and IGFBP-3 by ELISA.
Results
Multivariate logistic regression analysis revealed that: 1) elevated plasma and AF levels of LBP were independently associated with increased risks of MIAC, IAI, and microbial-associated IAI; 2) elevated AF, but not plasma, PTX3, and resistin levels were independently associated with increased risks of MIAC, IAI, and microbial-associated IAI; 3) decreased IGFBP-3 levels in the plasma were independently associated with only IAI, whereas those in the AF were associated with only microbial-associated IAI. Among the tested biomarkers, AF PTX3 and resistin had the highest predictive performance for MIAC, IAI, and microbial-associated IAI (area under the curves [AUC] = 0.85–0.95), which is similar to the performance of AF IL-8. The AUCs of the plasma LBP and IGFBP-3 were similar to that of serum CRP with respect to IAI.
Conclusion
Maternal plasma LBP and IGFBP-3 are potential biomarkers for the non-invasive identification of IAI in women with PPROM, with a similar accuracy to the serum CRP level. AF LBP, PTX3, resistin, and IGFBP-3 may be involved in the intra-amniotic inflammatory responses in PPROM complicated by MIAC.
Keywords: Amniotic Fluid, Intra-Amniotic Inflammation, IGFBP-3, Inflammatory Mediators, Microbial Invasion of Amniotic Cavity, Plasma, Preterm Premature Rupture of Membranes
Graphical Abstract
INTRODUCTION
Preterm prelabor rupture of membranes (PPROM) is a major obstetric complication worldwide that contributes largely to severe neonatal morbidity, mortality, and long-term neurodevelopmental disability.1,2 Specifically, PPROM complicates approximately 3% of all pregnancies, and is a direct antecedent to 25–30% of all preterm births.3,4,5 Importantly, evidence suggests that the presence of subclinical microbial invasion of the amniotic cavity (MIAC) and intra-amniotic inflammation (IAI) in PPROM poses additional risks for delivery latency and significant complications related to prematurity in children, such as pulmonary and neurodevelopmental sequelae.6,7,8,9 Considering the high prevalence of subclinical MIAC (25–55%) and IAI (35–52%) in PPROM,7,10 more precise and early prenatal methods (particularly those that use non-invasive measurements) are required to identify women at risk for these conditions.
Currently, analyses of amniotic fluid (AF) samples obtained via amniocentesis are generally considered the gold standard approach for identifying MIAC/IAI in PPROM. In particular, interleukin (IL)-6, -8, and matrix metalloproteinase (MMP)-8 and -9 in the AF have been shown to be the strongest predictors of intra-amniotic infectious and inflammatory conditions in PPROM.11,12,13,14 Nevertheless, their measurement in current practice may be of limited use due to their invasiveness (i.e., amniocentesis) and relatively low sensitivity.13,15 On the other hand, lipopolysaccharide (LPS) binding protein (LBP), pentraxin 3 (PTX3), and resistin are known to be important mediators involved in inflammatory and immunological processes during infection.16,17,18 In fact, previous cross-sectional studies found higher levels of LBP, PTX3, and resistin in AF from women with PPROM or preterm labor complicated by MIAC/IAI.19,20,21 However, these observations have not yet been validated in a different independent cohort, and these cross-sectional studies were not designed to evaluate the predictive performance of these biomarkers in the AF, leading to their limited clinical use. Moreover, despite the fact that the assessment of markers in the maternal blood may serve as a non-invasive, desirable, and inexpensive approach for the expectant management of PPROM women, these inflammatory biomarkers have not been studied sufficiently in plasma samples from women with PPROM in relation to MIAC/IAI. Insulin-like growth factor binding protein (IGFBP)-3 has been shown to have both pro-angiogenic and anti-angiogenic properties,22 while inflammation and angiogenesis are capable of enhancing each-others' actions.23,24 However, currently, no information is available on the role of IGFBP-3 as a predictor of MIAC/IAI in the AF and maternal blood of patients with PPROM. Thus, for the aforementioned reasons, we selected these four analytes for this study. The aim of this study was to determine whether LBP, PTX3, resistin, and IGFBP-3 in the plasma and AF can predict MIAC, IAI, and microbial-associated IAI in women with PPROM and to assess the correlation of each of these four proteins in the AF with the corresponding proteins in the plasma.
METHODS
Study design and participants
This was a retrospective cohort study involving singleton pregnant women between the gestational age of 24+0 to 33+6 weeks who were admitted to the Seoul National University Bundang Hospital (Seongnam, Korea) with a diagnosis of PPROM, between June 2004 and October 2018. This study was carried out by searching a comprehensive database of women with PPROM admitted to the high-risk pregnancy unit of our hospital during the study period. Eligible participants were identified according to the following inclusion criteria: 1) trans-abdominal amniocentesis conducted to evaluate the AF for infection or inflammation; 2) a live fetus; and 3) availability of aliquots of AF and plasma samples for analysis. Participants were excluded if they had 1) multiple gestations; 2) evidence of clinical chorioamnionitis at presentation; 3) active labor, defined as cervical dilation of 4 cm or more by sterile speculum examination; and 4) a fetus with major congenital anomalies. PPROM was diagnosed based on visual inspection using a sterile speculum examination to verify the pooling of AF in the vagina or fluid leakage from the cervix in association with a positive nitrazine test. The primary outcome measures were MIAC and IAI. We also conducted additional analyses for microbial-associated IAI.
Biological sample collection and processing
Ultrasound-guided transabdominal amniocentesis was performed under aseptic conditions at the time of admission. The AF samples were then immediately transported to the microbiology laboratory to culture microorganisms, such as aerobic/anaerobic bacteria and genital mycoplasmas (e.g., Mycoplasma hominis and Ureaplasma urealyticum), according to previously described methods.25 The left-over AF samples were centrifuged at 1,500 ×g for 10 minutes, aliquoted in 1.5 mL polypropylene tubes, and stored at −70°C until analysis. Clinicians had access to the AF culture results. Using an enzyme-linked immunosorbent assay (ELISA) human IL-6 DuoSet Kit (R&D System, Minneapolis, MN, USA), IL-6 levels in the AF were assessed in order to identify IAI, and AF IL-8 levels were measured for comparison with the AF biomarkers studied. We described the measurements of IL-6 and -8 concentrations in the AF in detail in the Supplementary Materials section.
At the time of amniocentesis, serum C-reactive protein (CRP) level was measured in the maternal serum samples as part of the hospital protocol using methods that have been previously described.25,26 Any remaining blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes, centrifuged at 1,500 ×g for 10 minutes, after which the supernatant was aliquoted and stored at −70°C until future use.
Analysis of IGFBP-3, LBP, PTX3, and resistin in the plasma and AF samples
The stored plasma and AF samples were assayed using ELISA kits for LBP, PTX3, resistin, and IGFBP-3 (DuoSet ELISA from R&D System, Minneapolis, MN, USA), according to the manufacturer's instructions. The ranges for the protein standard curves and their dilution ratios are described in detail in the Supplementary Materials section. The intra- and inter-assay coefficients of variation (CVs) were < 10% for all analyzed proteins, except for the inter-assay CVs of plasma resistin (13.2%).
Clinical management of PPROM and definition of various factors
PPROM was defined as clinically confirmed spontaneous rupture of the fetal membranes occurring before the onset of labor and at < 37 weeks of gestation. The management of PPROM has been previously described in detail6,7,27 and is also described in the Supplementary Materials section.
MIAC was defined as the presence of a positive AF culture for bacteria, fungi, and/or Mycoplasma hominis or Ureaplasma spp. IAI in PPROM pregnancies was defined as an AF IL-6 level ≥ 2.6 ng/mL based on previous reports.7,28 Microbial-associated IAI was defined as the presence of MIAC along with an elevated AF IL-6 concentration (≥ 2.6 ng/mL). Diagnosis of acute HCA was based on the presence of acute inflammatory changes in any of the placental tissue samples (amnion, chorion-decidua, chorionic plate, and umbilical cord) as described in a previous study.29 Clinical chorioamnionitis was diagnosed based on the criteria proposed by Gibbs et al.30 It has been described in detail in the Supplementary Materials section.
Statistical analyses
Comparison of continuous data was performed using the Mann-Whitney U test or Student's t-test based on the results of the Shapiro-Wilk test for normality, and comparison of categorical data was performed using the χ2 test or Fisher's exact test, as appropriate. Subsequently, multivariate logistic regression analyses were conducted to assess the independent relationship between the levels of each protein in the plasma and AF and each outcome measure, after controlling for baseline covariates (i.e., gestational age at sampling and parity), with a P value < 0.05 during univariate analysis. Receiver-operating characteristic (ROC) analyses were performed for each protein in the plasma and AF, where significant associations were noted with the outcome measures to determine the optimal cutoff value (giving the maximum sum of sensitivity and specificity) and predictive values. Thereafter, pairwise comparisons of areas under the curves (AUCs) of different proteins in plasma and AF were conducted using the method proposed by DeLong et al.31 Linear correlations of each protein level between the two compartments were analyzed using the Spearman rank correlation test. The statistical analyses of data were carried out using SPSS version 25.0 (IBM SPSS Inc., Chicago, IL, USA). The significance level was set at 0.05 (two-tailed) for all statistical analyses.
Ethics statement
The Institutional Review Board at Seoul National University Bundang Hospital approved this study (IRB no. B-1105/128–102), and written informed consent was obtained from all study participants for the amniocentesis procedure, for the collection and use of biological samples, and for the use of their clinical data for medical research.
RESULTS
Clinical characteristics of the study population
In total, 168 consecutive women with PPROM who met all the inclusion criteria were included in the present study. The prevalence of MIAC and IAI was 35.7% (60/168) and 41.6% (70/168) of women, respectively. Microbial-associated IAI (both MIAC and IAI) was present in 29.7% (50/168) of the women. The most common microorganisms identified in the AF from 60 cases were genital mycoplasmas [U. urealyticum (n = 46) and/or M. hominis (n = 33)], and a detailed description of the types of microorganisms isolated from AF is provided in Supplementary Results. Polymicrobial findings were observed in 34 of the 60 MIAC patients (56.6%).
The demographic and clinical characteristics of the women according to the presence or absence of MIAC, IAI, and microbial-associated IAI are presented in Table 1. Women with MIAC, IAI, and microbial-associated IAI had significantly lower gestational age at sampling, lower gestational age at delivery, and higher levels of serum CRP than those without these conditions. Moreover, women with IAI and microbial-associated IAI were more parous than those without these conditions (Table 1). A significant correlation was found between resistin levels in the plasma and AF (r = 0.157, P = 0.045), whereas LBP, PTX3, and IGFBP-3 levels in the plasma were not correlated with the corresponding protein levels in the AF (all variables, r = 0.128–0.150, P = 0.053–0.100).
Table 1. Demographic and clinical characteristics of the study population according to the presence or absence of MIAC, IAI, and microbial-associated IAI in women with preterm premature rupture of membranes.
| Variables | MIAC | P value | IAI | P value | Microbial-associated IAI | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 60) | Negative (n = 108) | Positive (n = 70) | Negative (n = 98) | Positive (n = 50) | Negative (n = 118) | ||||
| Maternal age, yr | 32.0 ± 3.7 | 31.0 ± 3.9 | 0.088 | 31.7 ± 3.6 | 31.1 ± 4.0 | 0.349 | 31.9 ± 3.8 | 31.2 ± 3.9 | 0.269 |
| Nulliparity | 40.0 (24/60) | 52.8 (57/108) | 0.113 | 37.1 (26/70) | 56.1 (55/98) | 0.016 | 36.0 (18/50) | 53.4 (63/118) | 0.040 |
| Gestational age at sampling, wk | 29.7 ± 2.7 | 30.7 ± 2.6 | 0.010 | 29.4 ± 2.7 | 31.1 ± 2.4 | < 0.001 | 29.5 ± 2.6 | 30.7 ± 2.6 | 0.002 |
| Gestational age at delivery, wk | 30.9 ± 2.4 | 33.1 ± 2.6 | < 0.001 | 31.7 ± 2.5 | 33.4 ± 2.3 | < 0.001 | 30.6 ± 2.3 | 33.0 ± 2.5 | < 0.001 |
| Serum CRP, mg/dL | 1.2 ± 1.5 | 0.7 ± 1.1 | 0.019 | 1.4 ± 1.7 | 0.5 ± 0.6 | < 0.001 | 1.4 ± 1.6 | 0.6 ± 1.0 | 0.003 |
| Use of tocolytic agents | 63.3 (38/60) | 57.4 (62/108) | 0.455 | 67.1 (47/70) | 54.1 (53/98) | 0.090 | 70.0 (35/50) | 55.1 (65/118) | 0.073 |
| Use of antibiotics | 98.3 (59/60) | 93.5 (101/108) | 0.261 | 95.7 (67/70) | 94.9 (93/98) | 1.000 | 98.0 (49/50) | 94.1 (111/118) | 0.438 |
| Use of antenatal corticosteroids | 93.3 (56/60) | 89.8 (97/108) | 0.577 | 92.9 (65/70) | 89.8 (88/98) | 0.590 | 92.0 (46/50) | 90.7 (107/118) | 1.000 |
| Clinical chorioamnionitis | 11.7 (7/60) | 8.3 (9/108) | 0.482 | 10.0 (7/70) | 9.2 (9/98) | 0.859 | 12.0 (6/50) | 8.5 (10/118) | 0.478 |
| Histological chorioamnionitisa | 72.7 (40/55) | 40.0 (38/95) | < 0.001 | 73.4 (47/64) | 36.0 (31/86) | < 0.001 | 76.1 (35/46) | 41.3 (43/104) | < 0.001 |
Data are given as mean ± standard deviation or % (n/N).
MIAC = microbial invasion of the amniotic cavity, IAI = intra-amniotic inflammation, CRP = C-reactive protein.
aData for the histologic evaluation of the placenta were only available in 150 of the 168 women because in 15 cases, delivery took place at another institution and in 3 cases, histologic evaluation of the placenta was not performed because of our institutional policy that only the placentas in cases of preterm delivery are to be sent for histopathologic examination or because of missing data for the histological chorioamnionitis.
Significant findings (P < 0.05) are indicated in bold fonts.
Various plasma proteins in relation to MIAC, IAI, and microbial-associated IAI
The median plasma levels of LBP were significantly higher in women with MIAC, IAI, and microbial-associated IAI than in women without these conditions (P < 0.01 for each, Table 2). These associations remained unchanged in multivariate logistic analysis after adjusting for gestational age at sampling and parity (Table 3). The plasma levels of IGFBP-3 were significantly lower in the presence of IAI (but not MIAC and microbial-associated IAI). Similarly, the multivariable analysis revealed that low plasma levels of IGFBP-3 were still significantly associated with IAI when adjusted for gestational age at sampling and parity. However, based on the univariate analyses, no differences in plasma PTX3 and resistin levels were found in relation to MIAC, IAI, and microbial-associated IAI in women with PPROM.
Table 2. Various proteins levels in plasma and amniotic fluid of the study population according to the presence or absence of MIAC, IAI, and microbial-associated IAI in women with preterm premature rupture of membranes.
| Variables | MIAC | P value | IAI | P value | Microbial-associated IAI | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive (n = 60) | Negative (n = 108) | Positive (n = 70) | Negative (n = 98) | Positive (n = 50) | Negative (n = 118) | ||||
| Plasma LBP, µg/mL | 7.11 ± 4.31 | 5.41 ± 3.10 | 0.006 | 7.18 ± 4.51 | 5.19 ± 2.63 | 0.001 | 7.33 ± 4.52 | 5.46 ± 3.08 | 0.004 |
| AF LBP, µg/mL | 0.38 ± 0.48 | 0.21 ± 0.21 | 0.006 | 0.42 ± 0.46 | 0.17 ± 0.15 | < 0.001 | 0.44 ± 0.51 | 0.21 ± 0.21 | < 0.001 |
| Plasma PTX3, ng/mL | 7.78 ± 5.16 | 8.99 ± 8.57 | 0.426 | 8.56 ± 7.01 | 8.56 ± 7.92 | 0.452 | 7.62 ± 5.30 | 8.96 ± 8.29 | 0.976 |
| AF PTX3, ng/mL | 19.31 ± 18.53 | 2.81 ± 8.26 | < 0.001 | 19.18 ± 18.38 | 1.19 ± 3.91 | < 0.001 | 22.39 ± 18.30 | 2.95 ± 8.53 | < 0.001 |
| Plasma resistin, ng/mL | 79.20 ± 133.25 | 53.11 ± 48.65 | 0.142 | 62.81 ± 53.09 | 61.98 ± 107.75 | 0.150 | 63.46 ± 55.47 | 61.84 ± 99.92 | 0.246 |
| AF resistin, ng/mL | 337.66 ± 359.11 | 70.21 ± 153.41 | < 0.001 | 349.14 ± 355.33 | 34.47 ± 34.96 | < 0.001 | 394.42 ± 367.45 | 69.30 ± 148.65 | < 0.001 |
| Plasma IGFBP-3, ng/mL | 813.80 ± 130.71 | 849.68 ± 158.40 | 0.067 | 789.45 ± 126.29 | 870.74 ± 156.38 | < 0.001 | 817.57 ± 109.02 | 845.04 ± 163.65 | 0.101 |
| AF IGFBP-3, ng/mL | 688.44 ± 358.31 | 771.72 ± 300.34 | 0.115 | 695.76 ± 364.27 | 775.10 ± 288.59 | 0.130 | 655.70 ± 366.43 | 778.31 ± 298.09 | 0.027 |
| AF IL-8, ng/mL | 19.76 ± 37.35 | 2.22 ± 5.56 | < 0.001 | 19.23 ± 34.74 | 0.79 ± 1.17 | < 0.001 | 23.48 ± 40.02 | 2.16 ± 5.35 | < 0.001 |
Data are given as mean ± standard deviation.
MIAC = microbial invasion of the amniotic cavity, IAI = intra-amniotic inflammation, LBP = lipopolysaccharide binding protein, AF = amniotic fluid, PTX3 = pentraxin 3, IGFBP = insulin-like growth factor-binding protein, IL = interleukin.
Significant findings (P < 0.05) are indicated in bold fonts.
Table 3. Relationship of various proteins in plasma and amniotic fluid with the presence of MIAC, IAI, and microbial-associated IAI, analyzed using multiple logistic regression.
| Predictors | MIACa | IAIb | Microbial-associated IAIb | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Plasma LBP, µg/mL | 1.132 (1.033–1.242) | 0.008 | 1.178 (1.066–1.302) | 0.001 | 1.148 (1.043–1.264) | 0.005 |
| AF LBP, µg/mL | 3.939 (1.197–12.963) | 0.024 | 37.140 (5.039–273.746) | < 0.001 | 9.167 (2.341–35.900) | 0.001 |
| AF PTX3, ng/mL | 1.120 (1.067–1.174) | < 0.001 | 1.390 (1.210–1.597) | < 0.001 | 1.132 (1.077–1.189) | < 0.001 |
| AF resistin, ng/mL | 1.022 (1.015–1.028) | < 0.001 | 1.034 (1.022–1.047) | < 0.001 | 1.006 (1.003–1.009) | < 0.001 |
| Plasma IGFBP-3, ng/mL | 0.997 (0.994–0.999) | 0.007 | ||||
| AF IGFBP-3, ng/mL | 0.998 (0.996–0.999) | < 0.001 | ||||
| Serum CRP, mg/dL | 1.328 (1.023–1.724) | 0.033 | 2.405 (1.521–3.803) | < 0.001 | 1.544 (1.162–2.050) | 0.003 |
| AF IL-8, ng/mL | 1.186 (1.104–1.273) | < 0.001 | 2.426 (1.685–3.493) | < 0.001 | 1.233 (1.140–1.333) | < 0.001 |
MIAC = microbial invasion of the amniotic cavity, IAI = intra-amniotic inflammation, LBP = lipopolysaccharide binding protein, AF = amniotic fluid, PTX3 = pentraxin 3, IGFBP = insulin-like growth factor-binding protein, CRP = C-reactive protein, IL = interleukin.
aAdjustment for gestational age at sampling; bAdjustment for gestational age at sampling and parity.
Significant findings (P < 0.05) are indicated in bold fonts.
Various AF proteins in relation to MIAC, IAI, microbial-associated IAI
AF LBP, PTX3, and resistin levels were significantly higher in women with MIAC, IAI, and microbial-associated IAI than in women without these conditions (P < 0.001 for each, Table 2), and these differences remained significant also when adjusted for gestational age at sampling and parity (Table 3). However, based on univariate analyses, the decreased AF IGFBP-3 levels were significantly associated with only microbial-associated IAI, but not with MIAC and IAI. Similarly, in the multivariate analysis, the aforementioned concentration difference of the AF IGFBP-3 remained significant even after controlling for baseline covariates (i.e., gestational age at sampling and parity) (Table 3).
Assessing the utility of the various biomarkers in the plasma and AF for the prediction of MIAC, IAI, and microbial-associated IAI
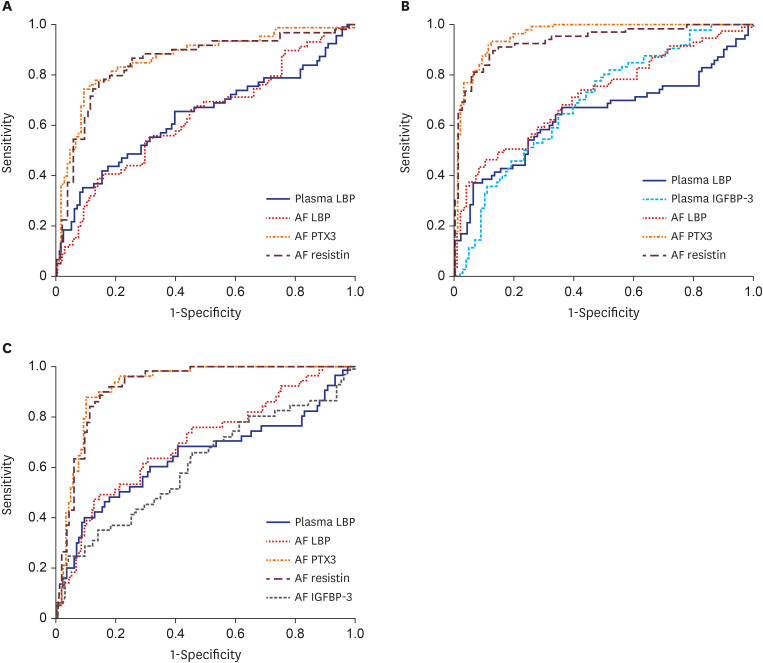
Table 4 presents the diagnostic values of various biomarkers in the plasma and AF with respect to predicting the presence of MIAC, IAI, and microbial-associated IAI, and Fig. 1 shows their ROC curves. The ROC curve analysis showed that the best cutoff value of plasma LBP was 5.62 µg/mL, with a sensitivity of 66.7% and a specificity of 53.7% for predicting the presence of MIAC and an AUC of 0.63 (Fig. 1A). For the identification of IAI, the AUC values of plasma LBP and IGFBP-3 were 0.65 and 0.69, respectively (Fig. 1B), and the AUC that identified microbial-associated IAI was 0.64 for plasma LBP (Fig. 1C). Moreover, the AUCs of the aforementioned plasma biomarkers were similar to those of the serum CRP with respect to the corresponding outcome variables shown to be statistically associated with all independent variables in the multivariate analysis (P = 0.363–0.713).
Table 4. Diagnostic indices of various biomarkers in plasma and amniotic fluid to predict MIAC, IAI, and microbial-associated IAI.
| Variables | Area (± SE) under the ROC curve | 95% CI | Cut-off valuea | Sensitivityb (95% CI) | Specificityb (95% CI) | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| MIAC | ||||||||
| Plasma LBP, µg/mL | 0.63 ± 0.05c | 0.54–0.72 | 5.62 | 66.7 (53.3–78.3) | 53.7 (43.9–63.4) | 44.4 | 74.4 | |
| AF LBP, µg/mL | 0.63 ± 0.05e | 0.54–0.72 | 0.18 | 61.0 (47.4–73.5) | 57.0 (47.1–66.5) | 43.9 | 72.6 | |
| AF PTX3, ng/mL | 0.86 ± 0.03d | 0.80–0.93 | 4.06 | 74.1 (61.0–84.7) | 90.7 (83.5–95.1) | 81.1 | 86.6 | |
| AF resistin, ng/mL | 0.85 ± 0.03d | 0.78–0.92 | 77.07 | 78.0 (62.3–87.7) | 85.0 (76.9–91.2) | 74.2 | 87.5 | |
| Serum CRP, mg/dL | 0.61 ± 0.05 | 0.52–0.70 | 0.37 | 58.3 (44.9–70.9) | 58.3 (48.5–67.8) | 43.8 | 71.6 | |
| AF IL-8, ng/mL | 0.86 ± 0.03 | 0.80–0.92 | 3.31 | 78.0 (65.3–87.7) | 89.8 (82.5–94.8) | 80.7 | 88.2 | |
| IAI | ||||||||
| Plasma LBP, µg/mL | 0.65 ± 0.05c | 0.55–0.74 | 5.86 | 67.1 (54.9–78.0) | 64.3 (54.0–73.7) | 57.3 | 73.3 | |
| AF LBP, µg/mL | 0.72 ± 0.04e | 0.64–0.80 | 0.15 | 73.9 (61.9–83.8) | 57.7 (47.3–67.7) | 55.4 | 75.7 | |
| AF PTX3, ng/mL | 0.96 ± 0.01d | 0.93–0.99 | 1.31 | 92.6 (83.7–97.6) | 87.6 (79.4–93.4) | 84.0 | 94.4 | |
| AF resistin, ng/mL | 0.94 ± 0.02d | 0.91–0.98 | 54.63 | 89.9 (80.2–95.8) | 87.6 (79.4–93.4) | 83.8 | 92.4 | |
| Plasma IGFBP-3, ng/mL | 0.69 ± 0.04c | 0.61–0.77 | 875.90 | 77.1 (65.6–86.3) | 53.1 (42.7–63.2) | 54.0 | 76.5 | |
| Serum CRP, mg/dL | 0.67 ± 0.04 | 0.59–0.76 | 0.39 | 62.9 (50.5–74.1) | 67.3 (57.1–76.5) | 57.9 | 71.7 | |
| AF IL-8, ng/mL | 0.97 ± 0.01 | 0.94–0.99 | 2.80 | 87.0 (76.7–93.9) | 96.9 (91.3–99.4) | 95.2 | 91.4 | |
| Microbial-associated IAI | ||||||||
| Plasma LBP, µg/mL | 0.64 ± 0.05c | 0.54–0.74 | 5.86 | 68.0 (53.3–80.5) | 59.3 (49.9–68.3) | 41.5 | 81.4 | |
| AF LBP, µg/mL | 0.70 ± 0.05e | 0.61–0.79 | 0.22 | 63.3 (48.3–76.6) | 69.2 (60.0–77.4) | 46.3 | 81.8 | |
| AF PTX3, ng/mL | 0.93 ± 0.02d | 0.90–0.97 | 4.06 | 88.5 (69.9–97.6) | 92.4 (84.2–97.2) | 79.3 | 96.1 | |
| AF resistin, ng/mL | 0.92 ± 0.02d | 0.88–0.96 | 77.07 | 89.8 (77.8–96.6) | 84.6 (76.8–90.6) | 71.0 | 95.2 | |
| AF IGFBP-3, ng/mL | 0.61 ± 0.05e | 0.51–0.71 | 750.32 | 65.3 (50.4–78.3) | 53.8 (44.4–63.1) | 37.2 | 78.8 | |
| Serum CRP, mg/dL | 0.65 ± 0.05 | 0.55–0.75 | 0.46 | 60.0 (45.2–73.6) | 66.9 (57.7–75.3) | 43.5 | 79.8 | |
| AF IL-8, ng/mL | 0.93 ± 0.02 | 0.89–0.97 | 3.31 | 89.8 (77.8–96.6) | 89.0 (81.9–94.0) | 77.2 | 95.5 | |
MIAC = microbial invasion of the amniotic cavity, IAI = intra-amniotic inflammation, SE = standard error, ROC = receiver-operating characteristic, CI = confidence interval, PPV = positive predictive value, NPV = negative predictive value, LBP = lipopolysaccharide binding protein, AF = amniotic fluid, PTX3 = pentraxin 3, CRP = C-reactive protein, IGFBP = insulin-like growth factor-binding protein, IL = interleukin.
aCut-off values corresponding to the highest sum of sensitivity and specificity; bValues are given as % (95% CI); c P > 0.05 compared to serum CRP by the method proposed by DeLong et al.; d P > 0.05 compared to AF IL-8 by the method proposed by DeLong et al.; e P < 0.001 compared to AF IL-8 by the method proposed by DeLong et al.
Fig. 1. (A) ROC curves of plasma LBP and AF LBP, PTX3, and resistin for predicting microbial invasion of the amniotic cavity. (B) ROC curves of plasma LBP and IGFBP-3, and AF LBP, PTX3, and resistin for predicting intra-amniotic inflammation. (C) ROC curves of plasma LBP and AF LBP, PTX3, resistin, and IGFBP-3 for predicting microbial-associated IAI.
LBP = lipopolysaccharide binding protein, AF = amniotic fluid, PTX3 = pentraxin 3, IGFBP = insulin-like growth factor-binding protein, ROC = receiver-operating characteristic.
The AUC values of the AF LBP, PTX3, and resistin for the prediction of MIAC were 0.63, 0.86, and 0.85, respectively (Fig. 1A). For the identification of IAI, the AUC values of the AF LBP, pentraxin 3, and resistin were 0.72, 0.96, and 0.94, respectively (Fig. 1B). Moreover, the AUCs that identified microbial-associated IAI were 0.61 for AF IGFBP-3, 0.70 for AF LBP, 0.93 for AF PTX3, and 0.92 for AF resistin (Fig. 1C). Differences in the AUCs between AF PTX3 and resistin were not statistically significant in predicting each of the three outcome variables. However, the AUCs for AF PTX3 and resistin were significantly larger than that for AF LBP for the prediction of each of the three outcome variables (P < 0.001 for each). Similarly, the pairwise AUC comparisons for AF IL-8 revealed that the AUCs of AF PTX3 and resistin were not significantly different from that of AF IL-8, whereas the AUC of AF LBP was significantly smaller than that of AF IL-8 for the prediction of each of the three outcome variables.
DISCUSSION
The principal findings of this study are as follows: 1) in women with PPROM, elevated plasma and AF levels of LBP were independently associated with increased risks of MIAC, IAI, and microbial-associated IAI; 2) elevated AF, but not plasma, PTX 3 and resistin levels were independently associated with increased risks for MIAC, IAI, and microbial-associated IAI; 3) decreased IGFBP-3 levels in the plasma were independently associated with only IAI, whereas those in the AF were associated with only microbial-associated IAI; 4) among the tested biomarkers, AF PTX3 and resistin have the highest predictive performance for MIAC, IAI, and microbial-associated IAI (AUC = 0.85–0.95; which is similar in performance to AF IL-8), whereas plasma LBP and IGFBP-3 performed similarly to the serum CRP level at predicting IAI; and 5) resistin levels in the plasma were significantly, but weakly, correlated with those in the AF samples, whereas the LBP, pentraxin 3, and IGFBP-3 levels in the plasma were not correlated with those in the AF samples. To our knowledge, the current study is the first to assess the levels of LBP, PTX3, resistin, and IGFBP-3 in the plasma of women with PPROM in relation to the presence of MIAC, IAI, and microbial-associated IAI.
In the current study, we demonstrated that AF PTX3 and resistin as invasive biomarkers have the best diagnostic performance for MIAC, IAI, and microbial-associated IAI, with good or excellent discriminatory power (AUC = 0.85–0.94, Table 4). In particular, these biomarkers showed similar diagnostic indices for the studied outcomes to those of AF IL-8, which is known to be the strongest marker for the identification of MIAC/IAI risk in women with PPROM.12,32 The present study also showed that LBP and IGFBP-3 (but not PTX3 and resistin) in the plasma may be potential novel non-invasive biomarkers for MIAC/IAI in pregnancies complicated by PPROM. Nevertheless, their clinical utility as non-invasive biomarkers is potentially limited due to their relatively small AUCs (0.63–0.69, Table 4), although their plasma levels demonstrated similar diagnostic performance as serum CRP. Collectively, our data suggest that the assessment of protein levels using maternal blood samples is of limited clinical value for the non-invasive identification of PPROM pregnancies complicated by MIAC/IAI.
An important finding that should be highlighted in this study is that LBP and IGFBP-3 are potential novel biomarkers for identifying IAI in plasma samples as well as AF. LBP, an acute-phase protein that is produced mainly by hepatocytes, binds with high affinity to LPS, transfers LPS to CD14 (macrophages and monocytes), and initiates the pro-inflammatory host response through the triggering of cytokine production.16 To our knowledge, only one cross-sectional study has been conducted that involved the AF. The aforementioned study was conducted by Espinoza et al.19 and they showed that levels of AF LBP were not significantly altered in PPROM patients with MIAC. Their results on AF LBP are different from those of the current study. The reason for the discrepancy may be attributable to the small sample size (n = 52) used in the previous study carried out by Espinoza et al.19 Additionally, a previous study involving plasma from women with PPROM, conducted by Chen et al.,33 reported significantly increased levels of plasma LPS in histological chorioamnionitis. Similarly, in this study, we demonstrated that plasma LBP is independently associated with increased risk for MIAC, IAI, and microbial-associated IAI, although their predictive values for these outcomes are unsatisfactory for clinical use (AUC = 0.63–0.70, Table 4). Given the reported significant association of HCA with MIAC/IAI in PPROM,7,32,34 the results of plasma LBP in the present study are in line with those in a previous report by Chen et al.33
IGFBP-3, the major IGFBP in the serum (responsible for approximately 80% of insulin-like growth factor [IGF]-1 and IGF-2 binding), is mainly produced by the liver Kupffer cells and regulates the biological activity of IGF, which is involved in mitogenic, anti-inflammatory, and anti-apoptotic action and idiopathic spontaneous preterm births.22,35,36 In particular, IGFBP-3 has been shown to inhibit IGF activity, thus inducing apoptosis and exerting both pro- and anti-inflammatory effects.22 Consistent with its known biology, in the context of systemic inflammation, DeBoer et al.37 showed that lower levels of serum IGFBP-3 and IGF-1 in children were associated with a mild degree of systemic inflammation (as evidenced by elevated CRP in the serum).37 Similarly, Lee et al.38 reported a significant association between the ratio of IGF-I/IGFBP-3 in the serum and inflammation in incident automated peritoneal dialysis patients. Furthermore, a previous study involving plasma from asymptomatic women in the mid-trimester has shown low IGFBP-3 levels in women with subsequent spontaneous preterm birth after 32 weeks.39 However, to date, there has been no report on the altered expression of IGFBP-3 in maternal plasma and AF during intra-amniotic infection-related and inflammatory conditions that occur during pregnancy. In this study, a high plasma IGFBP-3 level was significantly associated with only IAI, but not with MIAC and microbial-associated IAI, whereas the IGFBP-3 level in the AF was significantly associated with microbial-associated IAI only, but not MIAC and IAI.
PTX3, the prototypic long pentraxin, is an essential component of the humoral arm of innate immunity and acts as an acute phase response protein.17 This protein is mainly produced by macrophages, activated leukocytes, dendritic cells, and endothelial cells in response to primary inflammatory stimuli,17 in contrast to the short pentraxin CRP, which is mainly produced in hepatocytes in response to inflammatory mediators, particularly IL-6.40 Recent studies on AF have shown that PTX3 is the physiologic constituent of AF and its expression levels are increased in the AF during histological chorioamnionitis, MIAC, or IAI in pregnancies complicated by PPROM.20,41,42 These findings are in line with the results of our study on AF and suggest that PTX3 in AF may play an important role in the regulation of host response to intra-amniotic infection and inflammation. However, to date, few studies, especially those based on consecutive data, have been carried out to investigate the association between the altered expression of PTX3 in plasma and MIAC/IAI in the context of PPROM. Unlike previous studies on AF, we have demonstrated that the changes in PTX3 levels in plasma are not associated with intra-amniotic infection-related and inflammatory conditions that may occur during PPROM.
Resistin, also known as adipose tissue-specific secretory factor, is mainly secreted by inflammatory cells (e.g., monocytes and macrophages) in humans (although it is secreted solely from adipose tissues in rodents), and plays an important role in inflammatory processes.18 Resistin displayed potent pro-inflammatory properties that contribute to the increased expression of several pro-inflammatory cytokines (e.g., IL-1, IL-6, IL-12, and tumor necrosis factor-α) primarily through an nuclear factor kappa B-mediated pathway.18 In agreement with the biological characterization of resistin, a previous cross-sectional study on AF showed that high AF concentrations of resistin are associated with IAI in women with PPROM,21 which is consistent with the results of the present study. However, to date, no report has been published on the association between altered expression of resistin in the plasma and MIAC/IAI in pregnancies complicated by PPROM. Contrary to the results obtained from the AF analysis, the current study did not show that elevated resistin levels in plasma were significantly associated with MIAC/IAI in women with PPROM, even though several studies have shown a significant correlation between elevated blood resistin levels and systemic inflammation, which occurs during conditions such as acute pyelonephritis, atherosclerosis, and arthritis.43,44 The results of this study support the role of resistin in the regulation of local (but not systemic) infectious/inflammatory responses when the primary site of infection is the amniotic cavity.
The important question in the current study is: what are the differences in protein characteristics between useful (i.e., CRP, LBP, and IGFBP-3) and non-useful biomarkers (i.e., PTX3 and resistin; AF-based biomarkers) in the plasma for IAI detection? This is likely related to the location where a specific protein is produced and how much time has passed before the detection of proteins in the plasma after exposure to the infection and inflammation (peak plasma concentrations 6–8 hours for PTX3 vs. 36–48 hours for CRP),45 as well as how much time is required for the clearance of the protein from the blood. As potential plasma markers that reflect the infectious/inflammatory status of the amniotic cavity, CRP, LBP, and IGFBP-3 are proteins with common characteristics which are produced in the liver after in utero microbial exposure. In contrast, PTX3 and resistin are produced only by immune cells at the original site of infection (i.e., AF), and, thus, work only as mediators of local inflammatory response (i.e., AF-based biomarkers) in detecting MIAC/IAI,16,18,22,45 but may not be reflected in altered proteins in the blood.
The present study had several limitations that are worth mentioning. First, the MIAC was detected using conventional cultivation methods only, which did not include molecular techniques (for example, broad-range 16S rDNA polymerase chain reaction [PCR]), even though previous studies have reported the complementary role of PCR and culture-based methods for identifying microbes in the AF.46,47 Second, the study was retrospective and conducted in a single hospital, and the suggested cutoff values were not externally validated in independent cohorts, all of which may limit the generalizability of our findings. Third, we did not discover novel plasma-based biomarkers to replace serum CRP (a prototype marker of inflammation or infection) for the outcome measures examined here, as biomarkers identified in plasma samples (LBP and IGFBP-3) exhibited a predictive ability similar to that of the serum CRP.
In conclusion, we have shown that LBP and IGFBP-3 are potential novel biomarkers for identifying IAI in minimally invasive plasma samples from women with PPROM and that their accuracies are similar to that of the serum CRP level. AF LBP, PTX3, resistin, and IGFBP-3 may be involved in the intra-amniotic inflammatory responses in PPROM complicated by MIAC. Among the measured proteins, PTX3 and resistin in the AF are the best invasive biomarkers for identifying PPROM patients at risk for MIAC/IAI, with similar diagnostic performance as AF IL-8. Further studies are needed to confirm our findings in other cohorts and to explore the mechanistic role and clinical significance of LBP and IGFBP-3, which are significantly altered in plasma from PPROM pregnancies complicated by IAI.
Acknowledgments
We are grateful to the patients in the study. We thank our medical staffs for their assistance.
Footnotes
Funding: This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT; No. 2020R1F1A1048362). The funders had no role in the design of this study, data collection, data analyses, data interpretation, or in the writing of this manuscript.
Disclosure: The authors declare no conflict of interest.
- Conceptualization: Park KH.
- Data curation: Joo E, Kim YM, Ahn K, Hong S.
- Investigation: Joo E, Park KH.
- Formal analysis: Joo E, Park KH, Kim YM, Ahn K, Hong S.
- Funding acquisition: Park KH.
- Methodology: Joo E, Park KH, Kim YM.
- Validation: Joo E, Park KH, Ahn K, Hong S.
- Writing - original draft: Joo E, Park KH.
- Writing - review & editing: Joo E, Park KH, Kim YM, Ahn K, Hong S.
SUPPLEMENTARY MATERIAL
Supplemental Materials
References
- 1.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101(1):178–193. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 2.Manuck TA, Varner MW. Neonatal and early childhood outcomes following early vs later preterm premature rupture of membranes. Am J Obstet Gynecol. 2014;211(3):308.e1–308.e6. doi: 10.1016/j.ajog.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–419. doi: 10.1053/j.semperi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sae-Lin P, Wanitpongpan P. Incidence and risk factors of preterm premature rupture of membranes in singleton pregnancies at Siriraj Hospital. J Obstet Gynaecol Res. 2019;45(3):573–577. doi: 10.1111/jog.13886. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu A, Park KH, Oh KJ, Lee SY, Jeong EH, Park JW. Predictive value of combined cervicovaginal cytokines and gestational age at sampling for intra-amniotic infection in preterm premature rupture of membranes. Acta Obstet Gynecol Scand. 2013;92(5):517–524. doi: 10.1111/aogs.12073. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Kim SN, Oh KJ, Lee SY, Jeong EH, Ryu A. Noninvasive prediction of intra-amniotic infection and/or inflammation in preterm premature rupture of membranes. Reprod Sci. 2012;19(6):658–665. doi: 10.1177/1933719111432869. [DOI] [PubMed] [Google Scholar]
- 8.Hong JS, Park KH, Noh JH, Suh YH. Cervical length and the risk of microbial invasion of the amniotic cavity in women with preterm premature rupture of membranes. J Korean Med Sci. 2007;22(4):713–717. doi: 10.3346/jkms.2007.22.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung EY, Park KH, Han BR, Cho SH, Yoo HN, Lee J. Amniotic fluid infection, cytokine levels, and mortality and adverse pulmonary, intestinal, and neurologic outcomes in infants at 32 weeks' gestation or less. J Korean Med Sci. 2017;32(3):480–487. doi: 10.3346/jkms.2017.32.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 11.Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011;32(10):732–736. doi: 10.1016/j.placenta.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 12.Lee SM, Park KH, Jung EY, Kook SY, Park H, Jeon SJ. Inflammatory proteins in maternal plasma, cervicovaginal and amniotic fluids as predictors of intra-amniotic infection in preterm premature rupture of membranes. PLoS One. 2018;13(7):e0200311. doi: 10.1371/journal.pone.0200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung EY, Park KH, Han BR, Cho SH, Ryu A. Measurement of interleukin 8 in cervicovaginal fluid in women with preterm premature rupture of membranes: a comparison of amniotic fluid samples. Reprod Sci. 2017;24(1):142–147. doi: 10.1177/1933719116651149. [DOI] [PubMed] [Google Scholar]
- 14.Holmström E, Myntti T, Sorsa T, Kruit H, Juhila J, Paavonen J, et al. Cervical and amniotic fluid matrix metalloproteinase-8 and interleukin-6 concentrations in preterm pregnancies with or without preterm premature rupture of membranes. Fetal Diagn Ther. 2019;46(2):103–110. doi: 10.1159/000493207. [DOI] [PubMed] [Google Scholar]
- 15.Kacerovsky M, Musilova I, Hornychova H, Kutova R, Pliskova L, Kostal M, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2014;211(4):385.e1–385.e9. doi: 10.1016/j.ajog.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 16.Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem. 2001;268(16):4580–4589. doi: 10.1046/j.1432-1327.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- 17.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11(4):328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 18.Acquarone E, Monacelli F, Borghi R, Nencioni A, Odetti P. Resistin: a reappraisal. Mech Ageing Dev. 2019;178:46–63. doi: 10.1016/j.mad.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Espinoza J, Romero R, Chaiworapongsa T, Kim JC, Yoshimatsu J, Edwin S, et al. Lipopolysaccharide-binding protein in microbial invasion of the amniotic cavity and human parturition. J Matern Fetal Neonatal Med. 2002;12(5):313–321. doi: 10.1080/jmf.12.5.313.321. [DOI] [PubMed] [Google Scholar]
- 20.Cruciani L, Romero R, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Mazaki-Tovi S, et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med. 2010;38(2):161–171. doi: 10.1515/JPM.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21(12):902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slater T, Haywood NJ, Matthews C, Cheema H, Wheatcroft SB. Insulin-like growth factor binding proteins and angiogenesis: from cancer to cardiovascular disease. Cytokine Growth Factor Rev. 2019;46:28–35. doi: 10.1016/j.cytogfr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Front Oncol. 2019;9:1399. doi: 10.3389/fonc.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172(7):4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 25.Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Kim A. Intra-amniotic infection/inflammation as a risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. J Korean Med Sci. 2013;28(8):1226–1232. doi: 10.3346/jkms.2013.28.8.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Park KU. Relationship between maternal serum C-reactive protein, funisitis and early-onset neonatal sepsis. J Korean Med Sci. 2012;27(6):674–680. doi: 10.3346/jkms.2012.27.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HJ, Park KH, Kim YM, Joo E, Ahn K, Shin S. A protein microarray analysis of amniotic fluid proteins for the prediction of spontaneous preterm delivery in women with preterm premature rupture of membranes at 23 to 30 weeks of gestation. PLoS One. 2020;15(12):e0244720. doi: 10.1371/journal.pone.0244720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29(3):360–367. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung EY, Choi BY, Rhee J, Park J, Cho SH, Park KH. Relation between amniotic fluid infection or cytokine levels and hearing screen failure in infants at 32 wk gestation or less. Pediatr Res. 2017;81(2):349–355. doi: 10.1038/pr.2016.219. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145(1):1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 32.Kacerovsky M, Drahosova M, Hornychova H, Pliskova L, Bolehovska R, Forstl M, et al. Value of amniotic fluid interleukin-8 for the prediction of histological chorioamnionitis in preterm premature rupture of membranes. Neuroendocrinol Lett. 2009;30(6):733–738. [PubMed] [Google Scholar]
- 33.Chen FC, Sarioglu N, Büscher U, Dudenhausen JW. Lipopolysaccharide binding protein in the early diagnosis of intraamniotic infection of pregnant women with premature rupture of the membranes. J Perinat Med. 2009;37(2):135–139. doi: 10.1515/JPM.2009.004. [DOI] [PubMed] [Google Scholar]
- 34.Park JW, Park KH, Jung EY. Clinical significance of histologic chorioamnionitis with a negative amniotic fluid culture in patients with preterm labor and premature membrane rupture. PLoS One. 2017;12(3):e0173312. doi: 10.1371/journal.pone.0173312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockway HM, Kallapur SG, Buhimschi IA, Buhimschi CS, Ackerman WE, Muglia LJ, et al. Unique transcriptomic landscapes identified in idiopathic spontaneous and infection related preterm births compared to normal term births. PLoS One. 2019;14(11):e0225062. doi: 10.1371/journal.pone.0225062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch AM, Wagner BD, Deterding RR, Giclas PC, Gibbs RS, Janoff EN, et al. The relationship of circulating proteins in early pregnancy with preterm birth. Am J Obstet Gynecol. 2016;214(4):517.e1–517.e8. doi: 10.1016/j.ajog.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBoer MD, Scharf RJ, Leite AM, Férrer A, Havt A, Pinkerton R, et al. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition. 2017;33:248–253. doi: 10.1016/j.nut.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MJ, Shin DH, Ko KI, Koo HM, Kim CH, Doh FM, et al. Association between the ratio of insulin-like growth factor-I to insulin-like growth factor binding protein-3 and inflammation in incident automated peritoneal dialysis patients. Growth Horm IGF Res. 2013;23(5):170–174. doi: 10.1016/j.ghir.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Cooley SM, Donnelly JC, Collins C, Geary MP, Rodeck CH, Hindmarsh PC. The relationship between maternal insulin-like growth factors 1 and 2 (IGF-1, IGF-2) and IGFBP-3 to gestational age and preterm delivery. J Perinat Med. 2010;38(3):255–259. doi: 10.1515/jpm.2010.019. [DOI] [PubMed] [Google Scholar]
- 40.Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musilova I, Andrys C, Krejsek J, Drahosova M, Zednikova B, Pliskova L, et al. Amniotic fluid pentraxins: Potential early markers for identifying intra-amniotic inflammatory complications in preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2018;79(5):e12789. doi: 10.1111/aji.12789. [DOI] [PubMed] [Google Scholar]
- 42.Kacerovsky M, Tosner J, Drahosova M, Hornychova H, Andrys C. Pentraxin 3 in amniotic fluid as a marker of intra-amniotic inflammation in women with preterm premature rupture of membranes. Int J Gynaecol Obstet. 2010;108(3):203–206. doi: 10.1016/j.ijgo.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol. 2006;3(1):29–34. [PubMed] [Google Scholar]
- 44.Mazaki-Tovi S, Vaisbuch E, Romero R, Kusanovic JP, Chaiworapongsa T, Kim SK, et al. Hyperresistinemia - a novel feature in systemic infection during human pregnancy. Am J Reprod Immunol. 2010;63(5):358–369. doi: 10.1111/j.1600-0897.2010.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28(1):1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- 46.DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64(1):38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–125.15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials