Abstract
Scientists are working to identify prevention/treatment methods and clinical outcomes of coronavirus disease 2019 (COVID-19). Nutritional status and diet have a major impact on the COVID-19 disease process, mainly because of the bidirectional interaction between gut microbiota and lung, that is, the gut–lung axis. Individuals with inadequate nutritional status have a pre-existing imbalance in the gut microbiota and immunity as seen in obesity, diabetes, hypertension and other chronic diseases. Communication between the gut microbiota and lungs or other organs and systems may trigger worse clinical outcomes in viral respiratory infections. Thus, this review addresses new insights into the use of probiotics and prebiotics as a preventive nutritional strategy in managing respiratory infections such as COVID-19 and highlighting their anti-inflammatory effects against the main signs and symptoms associated with COVID-19. Literature search was performed through PubMed, Cochrane Library, Scopus and Web of Science databases; relevant clinical articles were included. Significant randomised clinical trials suggest that specific probiotics and/or prebiotics reduce diarrhoea, abdominal pain, vomiting, headache, cough, sore throat, fever, and viral infection complications such as acute respiratory distress syndrome. These beneficial effects are linked with modulation of the microbiota, products of microbial metabolism with antiviral activity, and immune-regulatory properties of specific probiotics and prebiotics through Treg cell production and function. There is a need to conduct clinical and pre-clinical trials to assess the combined effect of consuming these components and undergoing current therapies for COVID-19.
Key words: COVID-19, Immunity, Microbiota, Nutritional status, Viral infections
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), an infection which may affect the respiratory tract. The World Health Organization recognised COVID-19 as a pandemic in early 2020 owing to the rapid dissemination of the virus and the high virulence(1).
Most patients affected with COVID-19 are oligosymptomatic, having almost no symptoms or presenting them discreetly. However, damage caused by this virus may intensify due to an inadequate immune response(2). SARS-CoV-2 often causes respiratory tract infection symptoms, but it is not limited to impairing lung function; it can systematically affect the organism, undermining gastrointestinal(3), cardiovascular and renal functions(4), and even the nervous system(5).
Reports suggest that SARS-CoV-2 RNA may be detected in the stool of some COVID-19 patients(6,7), while another study has already reported that there is dysbiosis, meaning there are changes in the diversity and population of beneficial bacteria and that these are associated with the severity of COVID-19(8). These findings, associated with the gastrointestinal symptoms of this disease, point to an involvement of the gut–lung axis and an influence of the gut microbiota(6,7).
The ‘gut–lung axis’ refers to mutual interaction or a bidirectional effect between gut microbiota and lungs. Previous studies have shown that viral respiratory infections alter the commensal microbiota in both the gastrointestinal and the airway tracts of the host, probably through the blood, which transports endotoxins and microbial metabolites(9–11).
The gut microbiota varies depending on diet, lifestyle, antibiotics, genetic background and nutritional status(12). In this sense, obesity, hypertension and diabetes have been identified as risk factors for serious complications of COVID-19, although the underlying pathophysiology surrounding the manifestation of symptoms and clinical outcomes of the disease are not yet clear(13). There is evidence that dietetic modulation of the gut microbiota can influence viral transmission and disease progression(14).
Maintaining a healthy diet associated with the consumption of probiotics and prebiotics may be an important nutritional strategy as a prevention or as an adjuvant treatment for COVID-19(14), even in individuals with inadequate nutritional status and unbalanced gut microbiota(7,15). Probiotics and prebiotics play an important role in regulating the immune system through intestinal microbiota modulation, with increased proliferation of micro-organisms beneficial to health and reduced pathogenic micro-organisms, as well as by the systemic actions of the products of microbial metabolism such as short-chain fatty acids (SCFA)(15).
The immunomodulatory effect of probiotics and prebiotics on development and maturation of innate and adaptive immunity occurs through the secretion of cytokines such as interleukin 10 (IL-10) and transforming growth factor-β (TGF-β; control the regulatory T (Treg) cells development and the response of helper T cells (Th1/Th2)) for the control for releasing tumour necrosis factor-α (TNF-α), interferons and chemokines of immune cells. Treg cells are an important subpopulation of T cells responsible for suppressing the inflammation response and maintenance of immune system homeostasis(16–19).
Although vaccination is progressing in many countries, studies show that SARS-CoV-2 has mutated, with increased transmissibility and increased severity of COVID-19 cases(20). Considering that diet and nutritional status have been identified as important variables for the outcome of this infection(21), this narrative review analyses the main aspects of using probiotics and prebiotics as a nutritional strategy to improve the immune response against respiratory viral infections, considering prospects for COVID-19. We also considered the relationship between inflammatory responses and the clinical spectrum of COVID-19, ranging from asymptomatic to severe clinical presentations, and the influence of chronic diseases and nutritional status of individuals on disease prognosis.
In this sense, we sought to gather evidence and new insights into the role of consuming probiotic strains and prebiotic components in a single study, considering: the diversity of strains and compounds, doses, and administration time; the potential action mechanisms against COVID-19 related to the gut–lung axis; and the impact of nutritional status, the life cycle of subjects and the pros and cons of consuming probiotics and prebiotics in the progression of COVID-19. To date, no previous work has gathered this information.
Therefore, we conducted searches in research databases (PubMed, Cochrane Library, Scopus and Web of Science) using the keywords (prebiotic OR probiotic OR synbiotic OR dietary fibre OR polysaccharide OR oligosaccharide OR polyphenol OR phenolic OR Bifidobacterium OR Lactobacillus) AND (SARS OR SARS-CoV infection OR MERS OR MERS-CoV infection OR respiratory viral infection OR viral infection OR viral disease OR lung injury OR acute respiratory distress syndrome OR pneumonia OR ventilator-associated pneumonia) AND (immune system OR T cell OR Treg cell OR gut-lung axis OR dysbiosis OR intestinal dysbiosis OR gut dysbiosis OR symptoms OR abdominal pain OR cough OR diarrhoea OR fever OR headache OR nausea OR sore throat OR vomiting). Our search resulted in 260 studies published up to July 2021, and after removing duplicates and assessing eligibility, a total of 69 clinical studies were included in this narrative review.
Nutritional status and its relationship with the gut–lung axis as a risk factor for COVID-19 complications
The immune response among individuals generally varies according to genetic polymorphisms, which regulate the expression of cytokines, cytokine receptors, human leucocyte antigen and adhesion molecules. In addition, factors such as age, sex, stress, nutrient status and history of infections and vaccinations are important contributors to this response(22–24).
Individuals with chronic diseases such as obesity, hypertension and diabetes have undermined nutritional status, due to pre-existing chronic inflammation with high levels of inflammatory markers (C-reactive protein (CRP), IL-6 and TNF-α), a high degree of oxidative stress, and endothelial dysfunction caused by an imbalanced production of vasodilator and vasoconstrictor agents. These conditions combined with the COVID-19 pathophysiology overload the immune system and hinder the development of more effective responses(25,26), in turn increasing the risk of COVID-19 complications such as pneumonia and acute respiratory distress syndrome(27,28).
Experimental and clinical observations have suggested that the gut microbiota plays a key role in the pathogenesis of sepsis and acute respiratory distress syndrome(29), which further highlights the relevance of nutrition in the clinical outcome and remission of the symptoms of this disease. In addition, COVID-19, when combined with poor nutrition, is associated with requiring mechanical ventilation, longer length of stay in intensive care, and increased mortality rate(27).
Antibiotic therapy has been used to treat viral respiratory infections, including COVID-19. However, the use of antibiotics also causes dysbiosis(30), and this condition increases gut permeability, undermines healing and phagocytosis, reduces lymphocyte count (lymphopenia) and increases the production of pro-inflammatory cytokines(31).
There is evidence that the gut–lung axis plays a key role in the pathophysiology of COVID-19(6,7). Bacterial translocation was initially described to explain how a gut injury could lead to clinical manifestations of injuries at a distance in other organs, including in the lungs. The gut has extensive lymphoid tissue, and mesenteric lymph carries cytokines produced in the gut to the thoracic duct and then the lungs(32).
Previous studies have shown that respiratory infections such as influenza are associated with an alteration in the composition of the gut microbiota, which raises the question of whether SARS-CoV-2 could also harm the gut microbiota(33). SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) to infect alveolar and epithelial cells of the oesophagus and host enterocytes and cause local and/or systemic hyper-inflammation(34).
A recent study investigated the interaction between gut and SARS-CoV-2 through single-cell RNA sequencing, showing that the genes of the virus receptor binding domains and the modes of binding with the spike protein of this virus are probably constantly changing in the enterocytes(35). In addition, the SARS-CoV-2 genome data show specific mutations and genetic diversity according to the variability of individuals in different demographic regions, which could predict better-designed prophylactic measures among populations(36).
Due to the high degree of viral replication in the gut epithelium, the action of SARS-CoV-2 in the gastrointestinal system causes an increase in the occurrence of nausea, vomiting, diarrhoea and abdominal pain(34,37), which is further aggravated by dysbiosis(38). Other systemic symptoms such as fever, coughing, fatigue, myalgia, dyspnoea, head and throat pain, smell and taste loss, and anxiety generated by fear of the disease and social isolation lead to a marked reduction in appetite, which further impairs the patient’s nutritional status(39).
Inadequate nutritional status and high consumption of pro-inflammatory foods commonly present in a Westernised diet (namely a high content of saturated and trans fats, sodium, and sugars, and low in fibres, vitamins and minerals) are related to greater susceptibility to respiratory infections and a worse prognosis(12,21). This relationship may be due to the impact of consuming nutritionally inadequate diets on the individual’s nutritional status and gut health, as well as its interaction with the respiratory tract and the immune system(7,30).
On the other hand, adequate nutrition is a strong ally in promoting health, preventing and treating diseases(40). Several food components present in a Mediterranean diet (fibres, polyunsaturated fatty acids and phenolic compounds) have been demonstrated to reduce inflammation and improve immune response against respiratory infections(41).
Adherence to healthy eating habits may be considered a strategy to prevent severe cases of viral infections such as COVID-19. In this context, the inclusion of foods with prebiotic and probiotic properties in the diet may become an important tool for preventing mild to moderate COVID-19 cases, as these components can modulate the immune system through interaction with the gut microbiota and act directly or indirectly at the systemic level, including the respiratory(42), gastrointestinal(43), cardiovascular(44) and nervous system(45).
Probiotics
Action mechanisms and health benefits of probiotics
Probiotics are living micro-organisms which provide benefits to the host’s health when administered in adequate doses(43). These micro-organisms belong to different genera and species, both bacteria and yeast. Some lactic acid bacteria, in particular the Lactobacillus and Bifidobacterium genera, make up the vast majority of probiotics marketed either in pharmaceutical formulas or in foods, such as fermented milk, yoghurts, cheeses, kefir and kombucha(46).
The probiotics most frequently found in food products are Bifidobacterium (adolescentis, animalis, bifidum, breve and longum) and Lactobacillus (acidophilus, casei, fermentum, gasseri, johnsonii, paracasei, plantarum, rhamnosus and salivarius)(47). Some yeast strains are also believed to be probiotic, such as Saccharomyces boulardii (48). Despite the different metabolic characteristics of probiotic strains, which have not yet been fully elucidated, the ability to produce SCFA is a feature shared by many different probiotics and certainly plays a significant role in the host’s health(49).
SCFA are final products of the fermentation carried out by probiotics, either from endogenous compounds such as mucus and/or from non-digested carbohydrates in the diet, such as prebiotics(48). SCFA are organic acids which have one to six carbon atoms such as butyric, acetic and propionic acids, which are quantitatively and qualitatively produced depending on the production site in the large intestine, the gut microbiota composition, the substrates supplied by the diet and the presence of other metabolites(50).
These organic acids are energy sources for colonocytes and promote lower pH in the colon, which inhibits formation of high levels of secondary bile acids(51,52), increases the availability of calcium and magnesium(53), modulates the intestinal microbiota through selectively stimulating the growth of beneficial bacterial genera, induces proliferation of epithelial cells in the colon to maintain the integrity of the intestinal barrier, and decreases the translocation of inflammatory bacterial lipopolysaccharide(54).
SCFA act as anti-inflammatory mediators, thus stimulating the synthesis of anti-inflammatory cytokine IL-10 by macrophages, neutrophils and T cells, in addition to inhibiting the synthesis of inflammatory cytokines such as TNF-α and IL-6(49,55).
The main probiotic actions on the host’s health occur by inhibiting the proliferation of pathogens in the gut via the production of antimicrobial substances, mainly by Bifidobacterium and Lactobacillus genera, with consequent protection of the intestinal barrier, in addition to modulating the immune system through regulation of innate and adaptive immunity proteins(18,56,57). The specific probiotics can also act at the systemic level, in the central(58), cardiovascular(59), endocrine(60) and respiratory systems(61).
In this context, the consumption of probiotics has a potential preventive effect on COVID-19 complications, as they contribute to maintaining a healthy microbiota and reinforcing the intestinal barrier, increasing intestinal motility, and reducing pro-inflammatory states(56,62). Moreover, probiotic activity in different systems and axes of the organism which interact with the gut microbiota may be an effective strategy to alleviate the harmful effects of COVID-19.
The role of probiotics in the immune system and respiratory infections
Probiotics have been studied extensively in recent years and related to as an auxiliary tool in modulating humoral, cellular and non-specific immunity. The relationship between probiotics and the immune system is complex. Accordingly, it becomes even more important to understand the relationship between the consumption of probiotics, the modulation of immunity and its role in the respiratory system amid the COVID-19 pandemic.
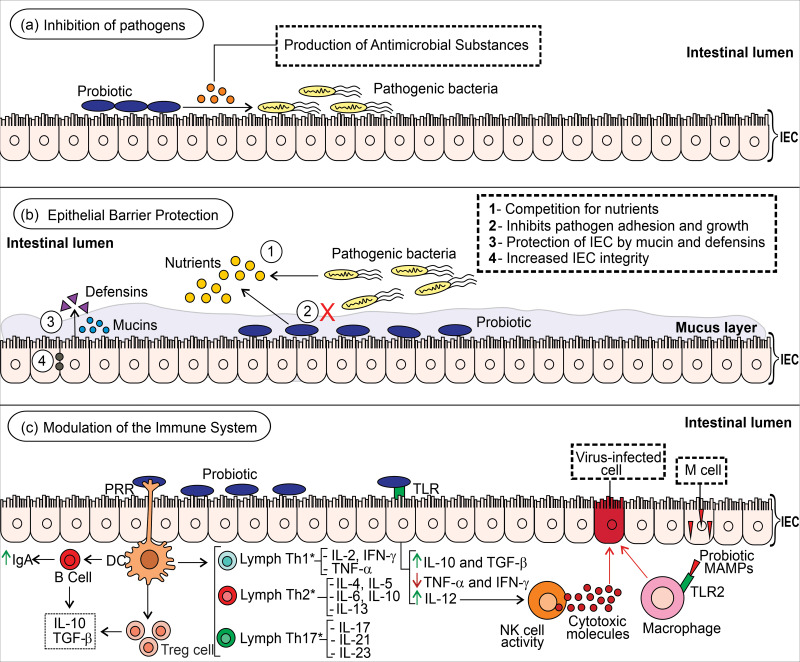
To date, few studies have been reported on the use of probiotics as an adjuvant in treating COVID-19(63–65). However, scientific evidence points to the immunomodulatory effect of probiotics in resolving viral infections which can be used as a model for COVID-19 cases (Fig. 1).
Fig. 1.
Potential mechanisms of probiotics on the intestinal immune system. (a) Probiotics secrete antimicrobial substances (i.e. bacteriocin and defensins) capable of inhibiting the action of pathogens in the intestinal epithelium. (b) These beneficial micro-organisms compete with pathogenic bacteria for the use of nutrients and for adhesion sites, which inhibits their growth and insertion in the intestinal epithelium. Probiotics can stimulate the synthesis and release of antimicrobial substances (i.e. defensins) by intestinal epithelial cells (IEC) as well as promote the expression and secretion of mucins which maintain the integrity of the IEC. (c) Probiotics bind with pathogen recognition receptors (PRR, i.e. toll-like receptor (TLR)) in dendritic cells, which stimulates B cells to produce immunomodulatory factors such as IgA, IL-10 and TGF-β; promotes differentiation of T cells in the respective T helper (Th) cells; and stimulates the activity of T-regulatory (Treg) cells which secrete anti-inflammatory IL-10 and TGF-β, regulating the immune system and controlling inflammation(16,67,170,171). The interaction of probiotics with IEC inhibits TNF-α and IFN-γ production, stimulates IL-10 and IL-12 production, and controls TGF-β function(18,19). The activation of NK cells by IL-12 activates the release of cytotoxic molecules capable of eliminating cells infected by viruses. Moreover, probiotic substances (i.e. microbe-associated molecular patterns (MAMPs)) cross the intestinal barrier mediated by M cells and promote phagocytic activity against virus-infected cells when recognised by innate receptors (i.e. TLR macrophage). It is worth highlighting that the effects differ among the probiotic strains. *The predominant or suppressed Th profile varies among probiotic strains.
Probiotics regulate systemic immune responses through antigen-presenting cells (i.e. macrophages and dendritic cells) present and activated in the mucosa. Mature antigen-presenting cells are activated by antigens (i.e. bacteria) and migrate to the mesenteric lymph node to differentiate lymphocytes with naive cluster of differentiation 4 (CD4) + Th0 into Treg or Th cells, but this will depend on the cytokine secretion pattern(66). Furthermore, the probiotic immunoregulatory and/or immunostimulatory effects are dependent on microbial strain, dose, administration time and nutritional status of the host(16,19,67).
Gut dysbiosis causes an innate response of the immune system through an increase in the expression of IL-15 and IL-12 cytokines and recruits natural killer (NK) cells responsible for the apoptosis of infected cells. However, the inflammatory immune response to COVID-19 cases is characterised by dysfunctional NK cells and increased macrophage activity which causes tissue damage. Microbe-associated molecular patterns derived from probiotic strains can be recognised by TLR2 present in macrophages and regulate their phagocytic activity to eliminate the pathogen(16,68,69).
The cell wall lipoproteins and lipoteichoic acid are microbe-associated molecular patterns of Bifidobacterium and Lactobacillus species which can modulate the immune system through binding with TLR2/TLR6 of the host. Lipoteichoic acid stimulates nitric oxide synthase action on the death of the pathogen-infected cell(16,70,71). The peptidoglycan hydrolase TgaA of Bifidobacterium spp. induces the production of IL-2 in the dendritic cell, which is the key cytokine in the development of Treg cells(18,72).
Probiotics strains can produce some metabolites with antiviral activity, such as acetic acid, lactic acid, γ-aminobutyric acid, plantaricin, bacteriocins (i.e. labyrinthopeptin A1, enterocin AAR-71 and erwiniocin NA4) and exopolysaccharides(73,74). Exopolysaccharides from Lactobacillus plantarum strain N4 (Lp) showed an inhibition effect on transmissible gastroenteritis coronavirus in vitro (75). A computational study demonstrated that plantaricin W’s anti-SARS-CoV-2 activity may be due to its stable binding to the ACE2 receptor of human, RNA-dependent RNA polymerase and residual binding domain of spike protein of SARS-CoV-2(74).
Human intestinal defensin 5 (HD5) is an α-defensin secreted by Paneth cells from the small intestine crypts, which has antimicrobial action. An in vitro study by Wang and colleagues(76) pointed out that HD5 cloaked several sites in the ligand-binding domain of ACE2 on enterocytes, mainly Asp30 and Lys31 residues on α-helix 1, which is essential for binding the SARS-CoV spike. They hypothesised that oral supplementation of specific probiotic strains would increase the number of Paneth cells and HD5 secretion to reduce infection of intestinal cells by SARS-CoV-2 and possibly maintain the balance of the local and systemic immune responses. However, studies must be conducted to investigate this hypothesis.
Studies with human and animal models which report effects from the use of probiotics on the immune system, viral infections, and risk factors for serious complications in COVID-19 are detailed in Supplementary Table S1. A study with female BALB/c mice infected with influenza A virus showed that the use of L. paracasei CNCM I-1518 at a dose of 2 × 108 colony-forming units (CFU)/d, for 7 d before and 10 d after viral infection, was able to modulate pulmonary immunity associated with better control of this infection, thereby enabling early activation of pro-inflammatory cytokines (IL-1α and IL-1β) and massive recruitment of immune cells into the lungs before influenza infection. The immune system pre-activation may induce faster clearance of the influenza virus(77).
A study of female BABL/c mice (6–8 weeks old) with influenza (H1N1 and H3N2) treated with Lactobacillus plantarum strain DK119 at a dose of 108–109 CFU/d, for 10 d before and 14 d after viral infection, is noteworthy, showing that Lactobacillus plantarum DK119 strain had a preventive and protective effect against infection by influenza viruses, probably increasing the innate immunity of dendritic cells and CD11c+ macrophages and antiviral cytokines (i.e. interferon-γ (IFN-γ) and IL-12), thus contributing to better control of pulmonary viral loads(78).
A study involving 190 healthy adults showed that the consumption of yoghurts with Lactobacillus gasseri SBT2055 strain probiotics at a dose of 100 g/d, consumed for 16 weeks, could activate innate and adaptive human immune responses (natural killer cell activity, myxovirus resistance A gene expression, IgG and IgA levels) after trivalent influenza vaccination, indicating the possible potential to prevent infections by influenza virus(79).
A meta-analysis has shown the relationship between the use of administered probiotics with doses ranging from 1 × 108 to 2 × 1010 CFU and a reduction in the severity of symptoms in cases of respiratory tract infection in children aged 3 months to 7 years and adolescents aged 7–18 years. This protocol also brought a decrease in the infection duration, mainly found in the formula containing Lactobacillus rhamnosus GG strain(80,81). Although COVID-19 cases are more prevalent in adults and older adults, children and adolescents are also susceptible to infection and death from SARS-CoV-2 and can transmit the virus(82–84). The results of the meta-analysis(80,81) indicate that a study protocol for the use of the Lactobacillus rhamnosus GG strain could be expanded to adult and juvenile populations for the prevention of COVID-19.
A pilot randomised clinical trial (RCT)(85) carried out in hospitals in Wuhan showed that oropharyngeal probiotic Streptococcus thermophilus ENT-K12 at a dose of 1 × 109 CFU administered daily for 1 month in ninety-eight healthy frontline medical staff (20–65 years of age) reduced the incidence of respiratory tract infections and time experiencing respiratory tract infections symptoms (sore and/or itchy throat, cough, oral ulcer and fever), eliminated the need for antibiotics or antivirals drugs, and shortened the days absent from work. However, this RCT has major limiting factors, including not being a blinded study, small number of participants, and few days of treatment, which were not sufficient to detect the preventive effect of infections by COVID-19, reducing its quality of evidence(85).
Two retrospective cohort studies by d’Ettorre et al.(63) and Ceccarelli et al.(64) reported the concomitant use of oral probiotic therapy (Bifidobacterium lactis DSM 32246 and DSM 32247 strains, Lactobacillus acidophilus DSM 32241, L. helveticus DSM 32242, L. paracasei DSM 32243, L. plantarum DSM 32244, L. brevis DSM 27961, Streptococcus thermophilus DSM 32345) at a dose of 2·4 × 1010 CFU/d and drug therapy (hydroxychloroquine, azithromycin, lopinavir-ritonavir or darunavir-cobicistat, and/or tocilizumab) for ±14 d in 28 and 112 Italian adults, respectively, with severe COVID-19 pneumonia to modulate the gut–lung axis. Patients treated with oral probiotic therapy showed remission of diarrhoea, fever, asthenia, headache, myalgia and dyspnoea; and no deaths or use of invasive mechanical ventilation were reported(63). Oral probiotic therapy increased the production of the nuclear factor erythroid 2p45-related factor 2 (Nrf2) and haem oxygenase-1 (HO-1), molecules with recognised antiviral activity(63). Furthermore, Ceccarelli et al.(64) showed that oral bacteriotherapy is an independent variable associated with a reduced risk for death in patients hospitalised with COVID-19.
A retrospective study evaluated the administration of probiotics in 311 patients with severe COVID-19 hospitalised at Wuhan Union Hospital(65). In addition to drug treatment (chloroquine phosphate, Arbidol and ribavirin interferon α inhalation or lopinavir/ritonavir), 123 patients received a combined dose of probiotics for an average time of 12·94 d. Probiotics were administered in a combined oral dose with tablets containing Bifidobacterium infantis, Lactobacillus acidophilus, Dung enterococcus and Bacillus cereus, 1·5 g tid; live combined Bifidobacterium longum, Lactobacillus bulgaricus and Streptococcus thermophiles tablets, 2 g tid; and live combined Enterococcus faecium and Bacillus subtilis capsules, 0·5 g tid. The use of probiotics could be an effective strategy in the treatment of COVID-19 patients to reduce the secondary infection and to moderate immunity(65).
The previously described trials(63–65) are observational, which limits extrapolating the outcomes to a larger population. Limitations include that these studies are non-blind, single-centre and not prospective, have a small number of participants, and did not randomise participants. However, the studies provide preliminary evidence that may guide future randomised controlled trials on prevention and/or treatment of COVID-19.
Probiotic treatment has been a promising field of research in the health sciences since specific probiotics (alone or combined with prebiotics) have the potential to modulate gut microbiota and immune responses in the host organism. It is seen that several probiotics strains, with emphasis on the genera Lactobacillus and Bifidobacterium, have a positive influence on innate immunity, exerting several antiviral properties, as well as on the protection of the respiratory system through controlling cytokine production and recruiting defence cells. As the lungs constitute the main action site of COVID-19, the use of specific probiotics becomes a promising tool in supporting the defence mechanisms in these organs.
Prebiotics
Action mechanisms and health benefits of prebiotics
The interest in foods and supplements with prebiotic properties has grown over the years, and its concept has undergone changes considering the scientific and clinical updates. Prebiotics are defined as a substrate which is selectively utilised by host micro-organisms that confer a health benefit. These compounds must not be degraded by the target host enzymes, and so they are fermented by the colon bacteria(86).
Most prebiotics are synthesised or isolated from plant polysaccharides and are oligosaccharides, such as: fructo-oligosaccharides (FOS), found in beetroot, asparagus, garlic, onions, chicory, wheat and banana; galacto-oligosaccharides (GOS), found in human milk and cow milk; isomalto-oligosaccharides, found in sugar cane and honey; xylo-oligosaccharides, found in fruits, vegetables, wheat bran and honey(87); inulin, found in wheat, tomatoes, garlic, barley and chicory roots; and resistant starch, found in raw potatoes, green bananas and grains. In addition to being natural sources, processed foods are often supplemented with prebiotics, such as dairy products, beverages, baby formulas, meats and bakery products(88).
Although prebiotics and probiotics have common action mechanisms, especially regarding the modulation of the gut microbiota, they differ in their composition and metabolism. Depending on the structure and composition, prebiotics can be used by specific bacteria as a source of carbon and energy(89). Prebiotics may have direct effects on gut epithelial cells and immune cells or indirect effect on the host’s health, serving as substrates for probiotics that promote immunomodulation, as they inhibit the growth of pathogenic bacteria and also improve digestion and absorption of essential nutrients(45,90).
Prebiotics intake is associated with benefits at the systemic level in the body, mediated by SCFA such as through regulation of various pathophysiological (i.e. inflammation) and metabolic processes (i.e. lipid and glucose metabolism), thus contributing to prevention or treatment of chronic diseases(91). Regular intake of prebiotics is also associated with beneficial effects on the renal(92), cardiovascular(93), nervous(94) and respiratory(33) systems.
Other non-carbohydrate substances such as phenolic compounds have also been accepted as potential prebiotics(86). Phenolics are secondary metabolites of plants and are present in various foods such as fruits, vegetables, teas, coffee, wines and chocolates(95). Phenolic compounds may also benefit the gut microbiota, exerting similar effects to prebiotics already well established in the pertinent literature(96). These compounds seem to interact with transcription factors, including nuclear factor κ-light-chain-enhancer of activated B cells (NF-kB) and Nrf2, exerting anti-inflammatory and antioxidant effects and immunomodulatory properties(44).
The role of prebiotics in the immune system and respiratory infections
There is evidence to support the effectiveness of using prebiotics in viral diseases, including respiratory diseases, although most studies have been conducted with individuals in their infancy(97–99). In this sense, associations between the effects of prebiotic consumption on the gut microbiota and immunity related to respiratory infections, especially on COVID-19 symptoms, constitute a challenge to be investigated(56).
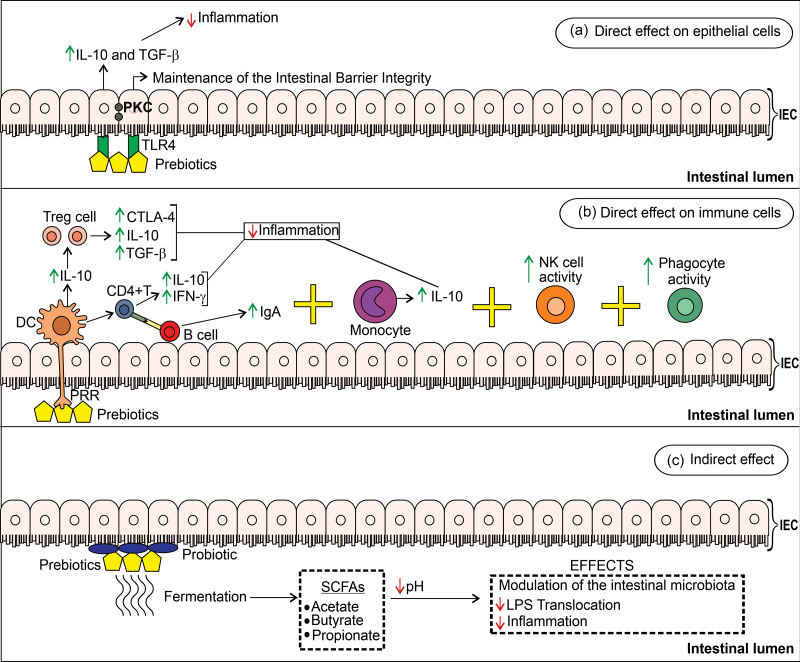
Prebiotics produce beneficial alterations in the immune system and the host’s health through direct and indirect mechanisms(99) (Fig. 2). Some non-clinical and clinical studies about the effects of prebiotics on the immune system, viral infections, and risk factors for serious complications in COVID-19 are detailed in Supplementary Table S2.
Fig. 2.
Potential mechanisms and effects of prebiotics on the intestinal immune system. (a) Prebiotics act directly on IEC by binding to their TLR4 and activate protein kinase C (PKC) to maintain the integrity of the intestinal barrier and regulate intestinal inflammation through synthesising inhibitory cytokines IL-10 and TGF-β. (b) The direct action of prebiotics on immune cells regulates the inflammatory response to the pathogen. Prebiotics bind to pathogen recognition receptors (PRR) on the surfaces of dendritic cells which secrete IL-10 to stimulate Treg cells to express cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) receptors, IL-10, and TGF-β. Dendritic cells activate CD4+ T cells to release IL-10 and IFN-γ (42,54). IFN-γ production by CD4+T cells helped in clearing influenza and dengue virus(172,173). CD4+ T cells interact with B cells and stimulate IgA production. Prebiotics can also bind to the TLR4 receptor for monocytes which secrete IL-10, and increase the activity of NK cells (involved in defence against viruses and tumour cells) and phagocytes (i.e. macrophages) to combat the offending agent. (c) Prebiotics reduce inflammation by indirect mechanisms when fermented by probiotic intestinal bacteria which produce short-chain fatty acids (SCFA)(174).
A case–control study investigated the association between dietary patterns and COVID-19 in 2884 front-line healthcare workers from six countries (France, Germany, Italy, Spain, UK, United States) who were screened based on substantial exposure to patients with COVID-19(100). Individuals who reported consuming plant-based diets or pescatarian diets, including prebiotics foods, were associated with lower odds of moderate-to-severe COVID-19. Despite the large sample size, diverse health professionals from different countries, and careful adjustment of potential confounding factors, this study relied on a self-report population predominantly composed of male physicians, without the inclusion of individuals affected by the most severe cases of COVID-19. This evidence points to the need to replicate the study both in women and in non-health professionals, with detailed data on the consumption of macro- and micronutrients to elucidate the associations between plant-based and pescatarian diets with the severity of COVID-19(100).
Studies on the administration of prebiotics in animal models with viral infections are scarce, since most publications address the prevention and treatment of rotavirus infection in childhood(101–103), with a few studies investigating the effect of prebiotics on other types of viral infections in adult rodents.
One study showed that inulin at a dose of 12 g for 2–4 weeks of treatment significantly increased the population of Bifidobacterium and Lactobacillus, which increased the SFCA production, thus modulating the gut microbiota(104). Prebiotics, such as wheat bran, GOS and FOS may raise butyrate levels, which in turn reduce inflammation and improve conditions in asthmatic patients(105). Butyrate alone cannot explain the effects of prebiotics on the gut immune system; propionate and acetate probably also play key roles in the regulation of expression of immune system genes(106).
Pectin and 1-kestose may induce the proliferation of Faecalibacterium prausnitzii, known for its anti-inflammatory effects(107), and pectin also increased the proliferation of Eubacterium eligens DSM 3376 in vitro, which increased the secretion of anti-inflammatory IL-10(108). GOS and FOS may cause IL-10 secretion in blood monocyte-derived dendritic cells stimulated by TLR4 binding(109).
Although it is clear that prebiotics have effects on the microbiota, such as modification, stimulation and antipathogenic effect, little is known about the specific action of each type of prebiotic in the various genera and species that make up the gut microbiota(106). This point can consequently be a limiting factor in prospecting the use of prebiotics in prevention and adjuvant treatment and in the period of COVID-19 remission. In this context, the concomitant use of prebiotics and probiotics in synbiotic formulation could provide more benefit in COVID-19 owing to synergic effects between components.
Emerging prebiotics, such as phenolic compounds, can act as antioxidants and direct enzyme inhibitors and may block virus–cell interaction(110). Luteoxanthin and violaxanthin from Urtica dioica (111), rutin(112) and neochlorogenic acid from Lianhuaqingwen (an herb product of traditional Chinese medicine)(113) can be potent ligands/inhibitors of ACE2 to prevent SARS-CoV-2/ACE2 binding. However, this ACE2 inhibitory function by phenolic compounds needs to be confirmed in humans with COVID-19, as well as whether the synergic interaction of these compounds would bring greater benefits in the treatment of this disease.
Another important point to investigate is whether synbiotic formulations of these phenolic compounds with oligosaccharides, as well as Bifidobacterium and Lactobacillus strains, could protect against infection by SARS-CoV-2 while controlling the inflammatory response. Various technologies (omics and bioinformatics) can be used to check the effectiveness and safety of new natural products with antiviral action.
Pros and cons of prebiotics and probiotics in the prevention and treatment of COVID-19
The cytokine storm which occurs in patients infected with COVID-19 contributes to worsening of symptoms and development of acute respiratory distress syndrome, pneumonia, sepsis and multiple organ dysfunction(114–117). Drug therapies have been tested to control the immune system; however, many medications have serious side effects which can cause dysbiosis in the gut–lung axis and worsen COVID-19 symptoms(118).
At this delicate moment in which the number of cases and deaths is still increasing in many countries despite beginning vaccination, researchers are looking for foods or bioactive compounds with potential functional properties and micro-organisms that offer prevention and benefits in the adjuvant treatment of COVID-19. To date, there are a few clinical studies which have evaluated the impact of consuming probiotics and prebiotics in people affected by COVID-19(63–65,119–121). However, there are no evaluations on introducing prebiotics and probiotics in the remission period of this disease. Some RCTs regarding probiotic and/or prebiotic use in COVID-19 are in progress and are registered in the ClinicalTrials.gov and International Clinical Trials Registry Platform (ICTRP) databases (ACTRN12620000480987; IRCT20101020004976N6; IRCT20200923048815N1; NCT04366089; NCT04366180; NCT04390477; NCT04399252; NCT04420676; NCT04507867; NCT04521322; NCT04621071; NCT04666116; NCT04734886; NCT04756466; NCT04793997; NCT04798677; NCT04813718; NCT04847349; NCT04877704; NCT04884776; NCT04937556; NCT04941703; and NCT04950803), or have been finalised but have not yet published their results (NCT04458519, NCT04517422 and NCT04854941).
Thus, considering the levels of scientific evidence, we sought RCTs and RCT meta-analyses which support the preventive and therapeutic potential of prebiotics, probiotics and their associations in signs and symptoms of viral infections. Through indirect evidence, we highlight studies on the effect of prebiotics, probiotics and synbiotics consumption on the incidence, duration and clinical outcomes related to viral infections, which can be promising in the pathophysiology of COVID-19 (Table 1). Only two RCT have been conducted to date, demonstrating direct evidence of the use of curcumin, a polyphenol which is considered an emerging prebiotic, in the treatment of COVID-19(119,120). Oral administration of curcumin (80–525 mg twice a day) for 2 weeks substantially reduced morbidity and mortality and improved recovery time in patients with mild, moderate and severe symptoms of COVID-19 (Table 1). The promising effects of this polyphenol were evaluated in a prospective open-label non-RCT which administrated nanocurcumin in soft gel (80 mg twice a day) for 2 weeks in patients (n = 21) aged 18–75 years with mild to moderate symptoms of COVID-19(121). The main effects found were resolution of fever, cough, tachypnoea, chill and myalgia; increased lymphocyte count and oxygen saturation; and shorter length of supplemental oxygen use and hospitalisation(121).
Table 1.
Effects of the consumption of prebiotics, probiotics and synbiotics on the incidence, duration and symptoms related to viral infections
| Prevention | ||||
|---|---|---|---|---|
| Prebiotic and probiotic (alone or combinations), and dose | Duration | Population (n) and age | Main results | References |
| B-GOS, 5·5 g/d | Up to 67 d | Healthy subjects (n = 159), >18 years old | Decreased the duration of diarrhoea and abdominal pain, significant potential in preventing the incidence and symptoms of traveller’s diarrhoea | (175) |
| GOS, FOS or butyrate, 1·5 to 10 g/d; L. rhamnosus GG, L. acidophilus, L. fermentum KLD or Saccharomyces boulardii CNCM I-745, 2·5 × 109 to 2 × 1011 CFU/d | Prebiotics: 42 d or more Probiotics: up to 28 d |
Healthy subjects (n = 7319), >18 years old | Only Saccharomyces boulardii CNCM I-745 decreased the incidence of traveller’s diarrhoea | (122) |
| Bifidobacterium longum BB536, 5 × 109 CFU/d | 10 months | Healthy children (n = 219), 2–6 years old | Decreased the duration of cough, sore throat, fever and runny nose | (176) |
| Bifidobacterium spp., Lactobacillus spp. and/or Streptococcus spp. + FOS, GOS and/or inulin, 28–3000 mg of prebiotic/d and 106 to 10 × 109 CFU of probiotic/d | 2 weeks to 1 year | Healthy subjects (n = 10 443) of all ages | Decreased the risk of developing a respiratory tract infection, mainly in adults | (123) |
| Bifidobacterium spp., Lactobacillus spp., Propionibacterium spp., Streptococcus spp. and/or α-Hemolytic streptococci, 2 × 108 to 1011 CFU/d | 14 d to 2 years | Healthy children (n = 4513), <18 years old | Decreased the numbers of days of respiratory tract infection per person | (81) |
| Bacillus subtilis, Bifidobacterium spp., Clostridium butyricum, Lactococcus spp., Lactobacillus spp., Leuconostoc cremoris, Saccharomyces spp., Streptococcus spp., 108 CFU three times daily to 2 × 1010 CFU twice daily | 7–5 weeks | Outpatients (n = 3631) of all ages | Probiotics, especially the Lactobacillus rhamnosus GG and Saccharomyces boulardii strains, reduced the risk of antibiotic-associated diarrhoea (ADD) by 51 % in outpatients of all ages. Doses >5 × 109 CFU/d were associated with fewer AAD events | (177) |
| Fermented milk with Lactobacillus casei ssp. Shirota, 1·5 × 1010 CFU/d | 6 months | Elderly person (n = 88), >60 years old | Reduced the number of days and mean duration of fever | (178) |
| Garlic extract, anthocyanins of blueberries, anthocyanins, flavonoids, flavonols, Iscucin Populi, isoflavones, pro-anthocyanidins, quercetin, Viscum Mali e planta tota, and l-theanine + epigallocatechin gallate, 0·2–1·2 g/d | 1–13 weeks | Healthy subjects (n = 727), 18–65 years old | Decreased the incidence (33 %) and sick-day count (40 %) of upper respiratory tract infections | (179) |
B., Bifidobacterium; B-GOS, Bimuno-galactooligosaccharides; CFU, colony-forming units; FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; HIV, human immunodeficiency virus; L., Lactobacillus; spp., species; ssp., subspecies.
Clinical studies generally have different forms (i.e. powder, capsules, tablets, soft gels or foods), doses (0·20–10 g/d) and durations of prebiotics administration (3 d to 43 weeks); as well as probiotics alone or mixed, with varying doses (106–2 × 1011 CFU/d) and administration time (5 d to 104 weeks) (Table 1). A large variation was similarly observed in the synbiotic administration of prebiotics (0·03–3 g/d) and probiotics (106–10 × 109 CFU/d) dose, as well as in the administration time (2–52 weeks).
We also noted that most studies have an experimental design with supplementation of prebiotics, probiotics or synbiotics, possibly due to the ability to administer doses that are better defined compared with the consumption of foods containing these components (Table 1). Some studies cited mild adverse events in healthy children and adults consuming prebiotics and probiotics, such as loose stools, abdominal pain, diarrhoea, constipation, vomiting, poor appetite, hives, rash and dry skin(81,122,123).
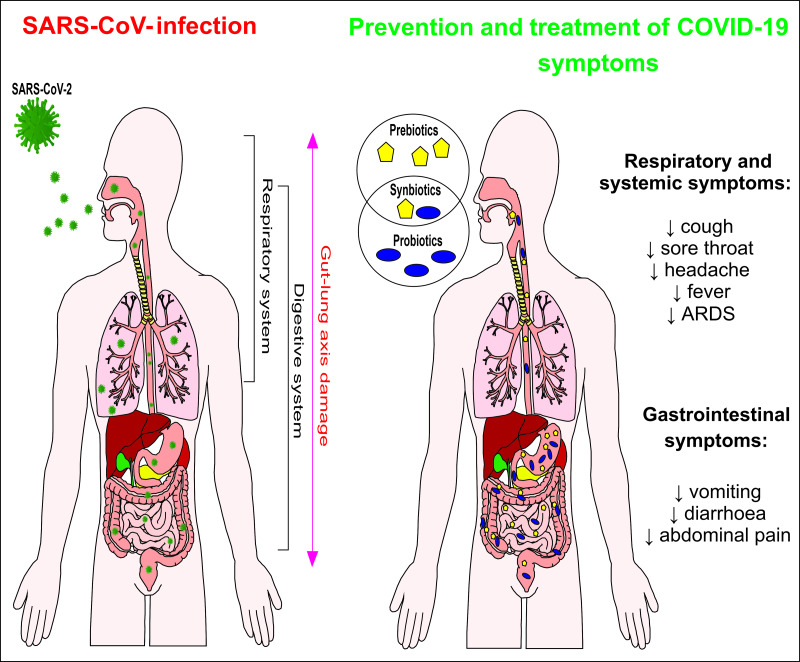
Despite variation in methodology and evidence, the studies previously cited in conjunction with data from some guidelines(124–126) point to beneficial effects of the consumption of prebiotics, probiotics and synbiotics, which may contribute to the prevention and treatment of some symptoms of COVID-19 and other viral infections (Fig. 3), provided they are associated with a healthy diet.
Fig. 3.
Potential effects of the consumption of prebiotics ( ), probiotics (
), probiotics ( ) or synbiotics (
) or synbiotics (
 ) against COVID-19 symptoms. ARDS, acute respiratory distress syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 (
) against COVID-19 symptoms. ARDS, acute respiratory distress syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 ( ).
).
Furthermore, probiotics use (Lactobacillus plantarum alone or in combination with other probiotics and Lactobacillus rhamnosus GG strain in combination with other probiotics) at an average dose of 5 × 109 CFU/d for 3–28 d has been associated with a reduction in infection complications such as ventilator-associated pneumonia in critically ill adult patients(127).
Probiotics (Lactobacillus spp., Bifidobacterium spp., Enterococcus faecealis, Clostridium butyricum, Bacillus mesentericus, Streptococcus faecalis, Pediacoccus pentosaceus and/or Leuconostoc mesenteroides) and synbiotics (different probiotic strains + GOS, FOS, β-glucan, inulin, pectin, resistant starch, oat fibre, oligofructose and/or malto-oligosaccharide) in a dosage range of 107–1011 CFU/d for 3–28 d reduced the duration of antibiotic therapy and the incidence of pneumonia, sepsis, abdominal distention, diarrhoea and other surgery-related complications(128). These beneficial effects were associated with the reduction in plasma levels of inflammatory markers such as CRP and IL-6, as well as an increase in blood concentration of acetic, butyric and propionic acids. However, the limited amount of data from the studies included in this meta-analysis(128) made it difficult to determine the most suitable probiotic strains and synbiotics profile to avoid complications related to surgery, as well as the best intervention time and ideal supplement dose.
Specific prebiotic and probiotic intake can minimise gastrointestinal symptoms of COVID-19 and the effects of using antibiotics which worsen these symptoms(129,130) and reconstitute the gut microbiome, along with consequent modulation of the immune system, a decrease in vulnerability to infections and increased number of resistance genes(19,131). Moreover, the inclusion of prebiotics and probiotics in the diet can be a potential therapeutic intervention against persistent health damage derived from antibiotics use such as the development of asthma and other respiratory diseases(132).
Some meta-analyses show the potential of prebiotics (including emerging ones, such as phenolic compounds), probiotics and synbiotics to modulate the immune system of healthy and sick people: AIDS, respiratory and skin allergies, chronic obstructive pulmonary disease, asthma, and other diseases. Prebiotics reduce CRP(133–135); probiotics also reduce CRP as well as TNF-α and IL-1β levels, and increase IL-10 levels, leucocyte count, NK cell count and activity, and T-cell and monocyte percentages(135–139); synbiotics reduce CRP and TNF-α levels and increase leucocyte, NK-cell, T-cell and monocyte counts(133,135,139). After a month of synbiotic mixture supplementation, there was an increase in CD4 count in HIV-infected women(140); however, Fu et al.(141) found no significant difference in CD4 counts in groups of patients treated with probiotics, prebiotics, synbiotics or placebo. It is not yet clear whether probiotic use in HIV-infected subjects can improve the systemic immune response(64).
Oesophageal cancer patients received daily synbiotics (Bifidobacterium breve Yakult, Lactobacillus casei Shirota, and galacto-oligosaccharides) at a dose of 3 g during 6 weeks of neoadjuvant chemotherapy and presented a lower frequency of severe lymphopenia, febrile neutropenia and diarrhoea(142), constituting clinical characteristics which are also seen in COVID-19 patients(143–146).
A therapeutic approach with prebiotics, probiotics and synbiotics can modulate other key points in the severity of COVID-19 cases: (a) increased production of Treg cells to control inflammation(147–149); (b) reduced ferritin synthesis and regulation of iron metabolism by polyphenols (natural iron chelators), helping to reduce inflammation and oxidative stress(150–152); (c) reduced D-dimer level involved in COVID-19 coagulopathy(153); (d) increase in immune efficacy of COVID-19 vaccine(154,155); and (e) reduced occurrence of persistent post-COVID-19 symptoms such as dyspnoea, tiredness, and joint and chest pain(156).
It is important to warn that the consumption of prebiotics should be planned according to the health status of the subject, as some prebiotics (i.e. GOS and FOS) cause increased intestinal osmolality and gastrointestinal symptoms in patients with irritable bowel syndrome(157). Also, some microbial strains can cause bacteraemia (e.g. Lactobacillus strains rhamnosus, acidophilus, casei and GG; Bacillus subtilis; Bifidobacterium longum and B. breve), sepsis (B. infantis) and fungaemia (i.e. Saccharomyces boulardii and S. cerevisiae) in immunocompromised subjects. Therefore, this situation requires adequate quality control of administered probiotic micro-organisms(158–160).
Another concern is that the global market still offers products with contaminants such as pathogenic bacteria which cause adverse effects to consumer health, mainly in vulnerable populations; incorrect identification of prebiotic components and probiotic strains; and reduced functional properties over product useful life. Thus, competent organisations must create rigorous regulations of production and marketing to guarantee the consumption of safe and effective products(86,161–163).
Traditionally consumed probiotics are generally recognised as safe (GRAS) or Qualified Presumption of Safety (QPS), according to the Food and Drug Administration (FDA) and European Food Safety Authority (EFSA), respectively(164,165). Even with the growing number of studies about health and immune benefits of probiotics, these claims are not yet approved by the FDA and EFSA. Prebiotics is also not yet a term recognised by the FDA or the European Union(86). Thus, assessing the safety of consuming prebiotics and probiotics which are intended to be used in a therapeutic category is essential; their safe intake level must be established, and they must obtain government approval for consumption(166). However, it is emphasised that the legislation or regulatory guidelines applied to prebiotics and probiotics vary significantly among countries, which in this time of the COVID-19 pandemic highlights the need to build globally uniform standards(167).
Future perspectives
Due to the urgency to find ways to prevent and minimise COVID-19 symptoms and its drug treatment associated with gut dysbiosis(7), the consumption of probiotics, prebiotics and synbiotics can be encouraged given the levels of scientific evidence which have demonstrated its beneficial effects on health, especially on the immune system and at the systemic level, provided they are combined with a healthy diet(168). It should also be considered that several fresh and processed foods, as well as supplements available on the market, have prebiotic and probiotic properties which can facilitate consumption even during social distancing(90). Walton et al.(14) emphasise that the dietetic approaches discussed have a scientific basis in addition to generally being safe and simple to implement. Despite vaccination and pharmaceutical treatments, the search for supporting strategies that reduce syndrome severity and duration can not only bring benefits to individuals affected by COVID-19 but also to health systems worldwide, mainly due to the emergence of new variants of the SARS-CoV-2 virus(14).
We emphasise that specialists (i.e. United Kingdom All-Party Parliamentary Group (APPG) on the Human Microbiome; International Society for Immunonutrition; International Scientific Association for Probiotics and Prebiotics; World Gastroenterology Organisation; Institute for the Advancement of Food and Nutrition Sciences: International Society of Microbiota; and others) in conjunction with government actions should plan and develop guidelines to inform the population about the proper use of prebiotics, probiotics and synbiotics in disease and health to avoid indiscriminate consumption. An example of this effort was made by APPG on the Human Microbiome when it sent the ‘Call for a government evaluation of the link between nutrition and the gut microbiome with respect to the COVID-19 pandemic’ for the Secretary of State for Health and Social Care of the United Kingdom. This statement points out the safe use of specific probiotics and/or prebiotics in COVID-19 treatment to achieve a healthy microbiota which benefits the human immune system(169).
In this scenario, future studies on animal models, as well as prospective, multicentre randomised, triple- or double-blind, placebo-controlled clinical studies, need to be conducted involving the administration of different probiotic strains and prebiotic components, either isolated or combined to obtain synergistic effects with the establishment of doses, intervention time and action mechanisms against COVID-19. The studies should focus on prevention, the adjuvant treatment of mild to moderate symptoms of COVID-19, and the remission period of this disease, and should also consider inter-individual variability, especially in individuals who have chronic diseases capable of altering the gut microbiota.
Acknowledgements
This work was supported by the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) – CAPES, Brazil (K.S.B., M.H.A.V., M.L.R.B. and J.G.d.A., grant number 0001) and the Consejo Nacional de Ciencia y Tecnología – Conacyt, Mexico, (J.G.d.A., grant number 921107). CAPES and Conacyt had no role in the design, analysis or writing of this article.
All authors contributed equally to the elaboration of the manuscript. K.S.B., J.G.d.A., M.H.A.V., M.L.R.B., M.B.S.B., R.O.P and J.S.A. contributed to the conceptualisation, data curation, investigation, methodology, and roles/writing – original draft. K.S.B. and J.S.A. supervised the project.
The authors have no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0954422421000317.
click here to view supplementary material
References
- 1. Cucinotta D & Vanelli M (2020) WHO declares COVID-19 a pandemic. Acta Biomed 91, 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Böhmer MM, Buchholz U, Corman VM, et al. (2020) Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect 20, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong SH, Lui RN & Sung JJ (2020) Covid-19 and the digestive system. J Gastroenterol Hepatol 35, 744–748. [DOI] [PubMed] [Google Scholar]
- 4. Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. (2020) COVID-19 and the cardiovascular system. Nat Rev Cardiol 17, 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger JR (2020) COVID-19 and the nervous system. J Neurovirol 26, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amirian ES (2020) Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int J Infect Dis 95, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhar D & Mohanty A (2020) Gut microbiota and Covid-19 possible link and implications. Virus Res 285, 198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang L, Gu S, Gong Y, et al. (2020) Clinical significance of the correlation between changes in the major intestinal bacteria species and COVID-19 severity. Engineering 6, 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enaud R, Prevel R, Ciarlo E, et al. (2020) The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dang AT & Marsland BJ (2019) Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 12, 843–850. [DOI] [PubMed] [Google Scholar]
- 11. Zuo T, Zhang F, Lui GCY, et al. (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159, 944.e8–955.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danneskiold-Samsøe NB, Barros HDFQ, Santos R, et al. (2019) Interplay between food and gut microbiota in health and disease. Food Res Int 115, 23–31. [DOI] [PubMed] [Google Scholar]
- 13. Maffetone PB & Laursen PB (2020) The perfect storm: coronavirus (COVID-19) pandemic meets overfat pandemic. Front Public Health 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walton GE, Gibson GR & Hunter KA (2020) Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19. Br J Nutr 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vernocchi P, Del Chierico F & Putignani L (2020) Gut microbiota metabolism and interaction with food components. Int J Mol Sci 21, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azad MAK, Sarker M & Wan D (2018) Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int 2018, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardy H, Harris J, Lyon E, et al. (2013) Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 5, 1869–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng W, Shen J, Bo T, et al. (2019) Cutting edge: probiotics and fecal microbiota transplantation in immunomodulation. J Immunol Res 2019, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters VBM, van de Steeg E, van Bilsen J, et al. (2019) Mechanisms and immunomodulatory properties of pre- and probiotics. Benef Microbes 10, 225–236. [DOI] [PubMed] [Google Scholar]
- 20. Dao TL, Hoang VT & Gautret P (2021) Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur J Clin Microbiol Infect Dis 40, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morais AHA, Aquino JS, da Silva-Maia JK, et al. (2021) Nutritional status, diet and viral respiratory infections: perspectives for SARS-CoV-2. Br J Nutr 125, 851–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calder PC & Kew S (2002) The immune system: a target for functional foods? Br J Nutr 88, Suppl. 2, S165–S177. [DOI] [PubMed] [Google Scholar]
- 23. Lippi G, Lavie CJ, Henry BM, et al. (2020) Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin Chem Lab Med 58, 1415–1422. [DOI] [PubMed] [Google Scholar]
- 24. Fakhouri EW, Peterson SJ, Kothari J, et al. (2020) Genetic polymorphisms complicate COVID-19 therapy: pivotal role of HO-1 in cytokine storm. Antioxidants 9, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwaifa IK, Bahari H, Yong YK, et al. (2020) Endothelial dysfunction in obesity-induced inflammation: molecular mechanisms and clinical implications. Biomolecules 10, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butler MJ & Barrientos RM (2020) The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun 87, 53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma R, Agarwal M, Gupta M, et al. (2020) Clinical characteristics and differential clinical diagnosis of novel coronavirus disease 2019 (COVID-19): epidemiology, pathogenesis, diagnosis, and therapeutics. In Coronavirus Disease 2019 (COVID-19), pp. 55–70 [Saxena SK, editor]. Singapore: Springer. [Google Scholar]
- 28. Yang X, Yu Y, Xu J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dickson RP (2016) The microbiome and critical illness. Lancet Respir Med 4, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolodziejczyk AA, Zheng D & Elinav E (2019) Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol 17, 742– 753. [DOI] [PubMed] [Google Scholar]
- 31. Calder PC, Carr AC, Gombart AF, et al. (2020) Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 12, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iyer R & Bansal A (2019) What do we know about optimal nutritional strategies in children with pediatric acute respiratory distress syndrome? Ann Transl Med 7, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Groves HT, Higham SL, Moffatt MF, et al. (2020) Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lamers MM, Beumer J, van der Vaart J, et al. (2020) SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muus C, Luecken MD, Eraslan G, et al. (2021) Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med 27, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bajaj A & Purohit HJ (2020) Understanding SARS-CoV-2: genetic diversity, transmission and cure in human. Indian J Microbiol 60, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henry BM, de Oliveira MHS, Benoit J, et al. (2020) Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Intern Emerg Med 15, 857–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levy M, Kolodziejczyk AA, Thaiss CA, et al. (2017) Dysbiosis and the immune system. Nat Rev Immunol 17, 219–232. [DOI] [PubMed] [Google Scholar]
- 39. Lechien JR, Chiesa-Estomba CM, de Siati DR, et al. (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277, 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rautiainen S, Manson JE, Lichtenstein AH, et al. (2016) Dietary supplements and disease prevention – a global overview. Nat Rev Endocrinol 12, 407–420. [DOI] [PubMed] [Google Scholar]
- 41. Messina G, Polito R, Monda V, et al. (2020) Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int J Mol Sci 21, 3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanders ME, Merenstein DJ, Reid G, et al. (2019) Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 16, 605–616. [DOI] [PubMed] [Google Scholar]
- 43. Hill C, Guarner F, Reid G, et al. (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11, 506–514. [DOI] [PubMed] [Google Scholar]
- 44. Delgado GTC & Tamashiro WMSC (2018) Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res Int 113, 183–188. [DOI] [PubMed] [Google Scholar]
- 45. Ahmadi S, Nagpal R, Wang S, et al. (2019) Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome-gut-brain axis modulation. J Nutr Biochem 67, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gallego CG & Salminen S (2016) Novel probiotics and prebiotics: how can they help in human gut microbiota dysbiosis? Appl Food Biotechnol 3, 72–81. [Google Scholar]
- 47. Zielińska D & Kolożyn-Krajewska D (2018) Food-origin lactic acid bacteria may exhibit probiotic properties: review. Biomed Res Int 2018, 5063185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kerry RG, Patra JK, Gouda S, et al. Benefaction of probiotics for human health: a review. J Food Drug Anal 26, 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanders ME, Benson A, Lebeer S, et al. (2018) Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol 49, 207–216. [DOI] [PubMed] [Google Scholar]
- 50. Ríos-Covián D, Ruas-Madiedo P, Margolles A, et al. (2016) Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Molska M & Reguła J (2019) Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davison JM & Wischmeyer PE (2019) Probiotic and synbiotic therapy in the critically ill: state of the art. Nutrition 59, 29–36. [DOI] [PubMed] [Google Scholar]
- 53. Graf D, Di Cagno R, Fåk F, et al. (2015) Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 26, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brosseau C, Selle A, Palmer DJ, et al. (2019) Prebiotics: mechanisms and preventive effects in allergy. Nutrients 11, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rooks MG & Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalantar-Zadeh K, Ward SA, Kalantar-Zadeh K, et al. (2020) Considering the effects of microbiome and diet on SARS-CoV-2 infection: nanotechnology roles. ACS Nano 14, 5179–5182. [DOI] [PubMed] [Google Scholar]
- 57. Qian L, Lu L, Huang L, et al. (2019) The effect of neonatal maternal separation on short-chain fatty acids and airway inflammation in adult asthma mice. Allergol Immunopathol 47, 2–11. [DOI] [PubMed] [Google Scholar]
- 58. Wang H, Lee I-S, Braun C, et al. (2016) Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil 22, 589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsu C-N, Lin Y-J, Hou C-Y, et al. (2018) Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 10, 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farzi A, Fröhlich EE & Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang H, Yeh C, Jin Z, et al. (2018) Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol 3, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harper A, Vijayakumar V, Ouwehand AC, et al. (2021) Viral infections, the microbiome, and probiotics. Front Cell Infect Microbiol 10, 596166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. d’Ettorre G, Ceccarelli G, Marazzato M, et al. (2020) Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med 7, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ceccarelli G, Borrazzo C, Pinacchio C, et al. (2020) Oral bacteriotherapy in patients with COVID-19: a retrospective cohort study. Front Nutr 7, 613928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Q, Cheng F, Xu Q, et al. (2021) The role of probiotics in coronavirus disease-19 infection in Wuhan: a retrospective study of 311 severe patients. Int Immunopharmacol 95, 107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fong FLY, Shah NP, Kirjavainen P, et al. Mechanism of action of probiotic bacteria on intestinal and systemic immunities and antigen-presenting cells. Int Rev Immunol 35, 179–188. [DOI] [PubMed] [Google Scholar]
- 67. Yousefi B, Eslami M, Ghasemian A, et al. (2019) Probiotics importance and their immunomodulatory properties. J Cell Physiol 234, 8008–8018. [DOI] [PubMed] [Google Scholar]
- 68. Merad M & Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Market M, Angka L, Martel AB, et al. (2020) Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front Immunol 11, 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jiang P, Yang W, Jin Y, et al. (2019) Lactobacillus reuteri protects mice against Salmonella typhimurium challenge by activating macrophages to produce nitric oxide. Microb Pathog 137, 103754. [DOI] [PubMed] [Google Scholar]
- 71. Lee I-C, van Swam II, Boeren S, et al. (2020) Lipoproteins contribute to the anti-inflammatory capacity of WCFS1. Front Microbiol 11, 1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ye C, Brand D & Zheng SG (2018) Targeting IL-2: an unexpected effect in treating immunological diseases. Signal Transduct Target Ther 3, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tiwari SK, Dicks LMT, Popov IV, et al. (2020) Probiotics at war against viruses: what is missing from the picture? Front Microbiol 11, 1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anwar F, Altayb HN, Al-Abbasi FA, et al. (2020) Antiviral effects of probiotic metabolites on COVID-19. J Biomol Struct Dyn 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang Y, Song H, Wang L, et al. (2017) Antiviral effects of a probiotic metabolic products against transmissible gastroenteritis coronavirus. J Prob Health 5, 1–6. [Google Scholar]
- 76. Wang C, Wang S, Li D, et al. (2020) Human intestinal defensin 5 inhibits SARS-CoV-2 invasion by cloaking ACE2. Gastroenterology 159, 1145–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Belkacem N, Serafini N, Wheeler R, et al. (2017) Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS ONE 12, e0184976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park M-K, Ngo V, Kwon Y-M, et al. (2013) Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 8, e75368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nishihira J, Moriya T, Sakai F, et al. (2016) Lactobacillus gasseri SBT2055 stimulates immunoglobulin production and innate immunity after influenza vaccination in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Funct Food Health Dis 6, 544–568. [Google Scholar]
- 80. Laursen RP & Hojsak I (2018) Probiotics for respiratory tract infections in children attending day care centers – a systematic review. Eur J Pediatr 177, 979–994. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Li X, Ge T, et al. (2016) Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine 95, e4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dong Y, Mo X, Hu Y, et al. (2020) Epidemiology of COVID-19 among children in China. Pediatrics 145, e20200702. [DOI] [PubMed] [Google Scholar]
- 83. Götzinger F, Santiago-García B, Noguera-Julián A, et al. (2020) COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 4, 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee B & Raszka WV Jr (2020) COVID-19 transmission and children: the child is not to blame. Pediatrics 146, e2020004879. [DOI] [PubMed] [Google Scholar]
- 85. Wang Q, Lin X, Xiang X, et al. (2021) Oropharyngeal probiotic ENT-K12 prevents respiratory tract infections among frontline medical staff fighting against COVID-19: a pilot study. Front Bioeng Biotechnol 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gibson GR, Hutkins R, Sanders ME, et al. (2017) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14, 491–502. [DOI] [PubMed] [Google Scholar]
- 87. Ashwinia A, Ramya HN, Ramkumara C, et al. (2019) Reactive mechanism and the applications of bioactive prebiotics for human health: review. J Microbiol Methods 159, 128–137. [DOI] [PubMed] [Google Scholar]
- 88. Neri-Numa IA, Arruda HS, Geraldi MV, et al. (2020) Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr Opin Food Sci 33, 98–107. [Google Scholar]
- 89. Singh RK, Chang H-W, Yan D, et al. (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Markowiak P & Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xavier-Santos D, Bedani R, Lima ED, et al. (2020) Impact of probiotics and prebiotics targeting metabolic syndrome. J Funct Foods 64, 103666. [Google Scholar]
- 92. McFarlane C, Ramos CI, Johnson DW, et al. (2019) Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr 29, 209–220. [DOI] [PubMed] [Google Scholar]
- 93. Delzenne NM, Olivares M, Neyrinck AM, et al. (2020) Nutritional interest of dietary fiber and prebiotics in obesity: lessons from the MyNewGut consortium. Clin Nutr 39, 414–424. [DOI] [PubMed] [Google Scholar]
- 94. Paiva IHR, Duarte-Silva E & Peixoto CA (2020) The role of prebiotics in cognition, anxiety, and depression. Eur Neuropsychopharmacol 34, 1–18. [DOI] [PubMed] [Google Scholar]
- 95. Amiot MJ, Riva C & Vinet A (2016) Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 17, 573–586. [DOI] [PubMed] [Google Scholar]
- 96. Moorthy M, Chaiyakunapruk N, Jacob SA, et al. (2020) Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: a systematic review of randomised controlled trials. Trends Food Sci Technol 99, 634–649. [Google Scholar]
- 97. Luoto R, Ruuskanen O, Waris M, et al. (2014) Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol 133, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shahramian I, Kalvandi G, Javaherizadeh H, et al. (2018) The effects of prebiotic supplementation on weight gain, diarrhoea, constipation, fever and respiratory tract infections in the first year of life. J Paediatr Child Health 54, 875–880. [DOI] [PubMed] [Google Scholar]
- 99. Ranucci G, Buccigrossi V, Borgia E, et al. (2018) Galacto-oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: a randomized clinical trial. Nutrients 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim H, Rebholz CM, Hegde S, et al. (2021) Plant-based diets, pescatarian diets and COVID-19 severity: a population-based case–control study in six countries. BMJ Nutr Prev Health 4, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Azagra-Boronat I, Massot-Cladera M, Knipping K, et al. (2018) Supplementation with 2’-FL and scGOS/lcFOS ameliorates rotavirus-induced diarrhea in suckling rats. Front Cell Infect Microbiol 8, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rigo-Adrover MDM, van Limpt K, Knipping K, et al. (2018) Preventive effect of a synbiotic combination of galacto- and fructooligosaccharides mixture with Bifidobacterium breve M-16V in a model of multiple rotavirus infections. Front Immunol 9, 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rigo-Adrover MDM, Knipping K, Garssen J, et al. (2019) Prevention of rotavirus diarrhea in suckling rats by a specific fermented milk concentrate with prebiotic mixture. Nutrients 11, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vandeputte D, Falony G, Vieira-Silva S, et al. (2017) Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 66, 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Anand S & Mande SS (2018) Diet, microbiota and gut-lung connection. Front Microbiol 9, 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gourbeyre P, Denery S & Bodinier M (2011) Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol 89, 685–695. [DOI] [PubMed] [Google Scholar]
- 107. Tochio T, Kadota Y, Tanaka T, et al. (2018) 1-kestose, the smallest fructooligosaccharide component, which efficiently stimulates as well as Bifidobacteria in humans. Foods 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chung WSF, Meijerink M, Zeuner B, et al. (2017) Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol 93, 1–9. [DOI] [PubMed] [Google Scholar]
- 109. Lehmann S, Hiller J, van Bergenhenegouwen J, et al. (2015) In vitro evidence for immune-modulatory properties of non-digestible oligosaccharides: direct effect on human monocyte derived dendritic cells. PLOS ONE 10, e0132304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chojnacka K, Witek-Krowiak A, Skrzypczak D, et al. (2020) Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J Funct Foods 73, 104146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Upreti S, Prusty JS, Pandey SC, et al. (2021) Identification of novel inhibitors of angiotensin-converting enzyme 2 (ACE-2) receptor from Urtica dioica to combat coronavirus disease 2019 (COVID-19). Mol Divers 2021, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. García-Iriepa C, Hognon C, Francés-Monerris A, et al. (2020) Thermodynamics of the interaction between the spike protein of severe acute respiratory syndrome coronavirus-2 and the receptor of human angiotensin-converting enzyme 2. Effects of possible ligands. J Phys Chem Lett 11, 9272–9281. [DOI] [PubMed] [Google Scholar]
- 113. Chen X, Wu Y, Chen C, et al. (2021) Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B 11, 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Robba C, Battaglini D, Pelosi P, et al. (2020) Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med 14, 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ragab D, Salah Eldin H, Taeimah M, et al. (2020) The COVID-19 cytokine storm; what we know so far. Front Immunol 11, 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gupta A, Madhavan MV, Sehgal K, et al. (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26, 1017–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chen Z & John Wherry E (2020) T cell responses in patients with COVID-19. Nat Rev Immunol 20, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Alhazzani W, Møller MH, Arabi YM, et al. (2020) Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 48, e440–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ahmadi, R , Salari, S , Sharifi, MD , et al. (2021) Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: a randomized triple-blind placebo-controlled clinical trial. Food Sci Nutr 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pawar KS, Mastud RN, Pawar SK, et al. (2021) Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol 28, 669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Saber-Moghaddam N, Salari S, Hejazi S, et al. (2021) Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial. Phytother Res 35, 2616–2623. [DOI] [PubMed] [Google Scholar]
- 122. McFarland LV & Goh S (2019) Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis 27, 11–19. [DOI] [PubMed] [Google Scholar]
- 123. Chan CKY, Tao J, Chan OS, et al. (2020) Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 11, 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. World Gastroenterology Organisation (2018) WGO Practice Guideline – Diet and the Gut. https://www.worldgastroenterology.org/guidelines/global-guidelines/diet-and-the-gut (accessed May 2020).
- 125. World Gastroenterology Organisation (2017) WGO Practice Guideline – Probiotics and Prebiotics. https://www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics (accessed May 2020).
- 126. Hojsak I, Fabiano V, Pop TL, et al. (2018) Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr 107, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Manzanares W, Lemieux M, Langlois PL, et al. (2016) Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care 19, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Skonieczna-Żydecka K, Kaczmarczyk M, Łoniewski I, et al. (2018) A systematic review, meta-analysis, and meta-regression evaluating the efficacy and mechanisms of action of probiotics and synbiotics in the prevention of surgical site infections and surgery-related complications. J Clin Med Res 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lin L, Jiang X, Zhang Z, et al. (2020) Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 69, 997–1001. [DOI] [PubMed] [Google Scholar]
- 130. Agamennone V, Krul CAM, Rijkers G, et al. (2018) A practical guide for probiotics applied to the case of antibiotic-associated diarrhea in the Netherlands. BMC Gastroenterol 18, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Guo Q, Goldenberg JZ, Humphrey C, et al. (2019) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 4, CD004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Becattini S, Taur Y & Pamer EG (2016) Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 22, 458–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. McLoughlin RF, Berthon BS, Jensen ME, et al. (2017) Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr 106, 930–945. [DOI] [PubMed] [Google Scholar]
- 134. Sahebkar A, Cicero AFG, Simental-Mendía LE, et al. (2016) Curcumin downregulates human tumor necrosis factor-α levels: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 107, 234–242. [DOI] [PubMed] [Google Scholar]
- 135. Zheng HJ, Guo J, Wang Q, et al. (2021) Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 61, 577–598. [DOI] [PubMed] [Google Scholar]
- 136. Maia LP, Levi YL de AS, do Prado RL, et al. (2019) Effects of probiotic therapy on serum inflammatory markers: a systematic review and meta-analysis. J Funct Foods 54, 466–478. [Google Scholar]
- 137. Tamtaji OR, Milajerdi A, Reiner Ž, et al. (2020) A systematic review and meta-analysis: the effects of probiotic supplementation on metabolic profile in patients with neurological disorders. Complement Ther Med 53, 102507. [DOI] [PubMed] [Google Scholar]
- 138. Pan H, Li R, Li T, et al. (2017) Whether probiotic supplementation benefits rheumatoid arthritis patients: a systematic review and meta-analysis. Proc Est Acad Sci Eng 3, 115–121. [Google Scholar]
- 139. Kazemi A, Soltani S, Ghorabi S, et al. (2020) Is probiotic and synbiotic supplementation effective on immune cells? A systematic review and meta-analysis of clinical trials. Food Rev Int 37, 491–537. [Google Scholar]
- 140. Kazemi A, Djafarian K, Speakman JR, et al. (2018) Effect of probiotic supplementation on CD4 cell count in HIV-infected patients: a systematic review and meta-analysis. J Diet Suppl 15, 776–788. [DOI] [PubMed] [Google Scholar]
- 141. Fu Y-S, Chu Q-S, Ashuro AA, et al. (2020) The effect of probiotics, prebiotics, and synbiotics on CD4 counts in HIV-infected patients: a systematic review and meta-analysis. Biomed Res Int 2020, 7947342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Motoori M, Yano M, Miyata H, et al. (2017) Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin Nutr 36, 93–99. [DOI] [PubMed] [Google Scholar]
- 143. Azkur AK, Akdis M, Azkur D, et al. (2020) Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 75, 1564–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tralongo AC & Extermann M (2020) Older patients with cancer and febrile neutropenia in the COVID-19 era: a new concern. J Geriatr Oncol 11, 1329–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Spencer HC & Wurzburger R (2020) COVID-19 presenting as neutropenic fever. Ann Hematol 99, 1939–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Feng X, Li S, Sun Q, et al. (2020) Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med 7, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chowdhury AH, Cámara M, Verma C, et al. (2019) Modulation of T regulatory and dendritic cell phenotypes following ingestion of Bifidobacterium longum, AHCC® and Azithromycin in healthy individuals. Nutrients 11, 2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Iemoli E, Trabattoni D, Parisotto S, et al. (2012) Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol 46, S33–S40. [DOI] [PubMed] [Google Scholar]
- 149. Dwivedi M, Kumar P, Laddha NC, et al. (2016) Induction of regulatory T cells: a role for probiotics and prebiotics to suppress autoimmunity. Autoimmun Rev 15, 379–392. [DOI] [PubMed] [Google Scholar]
- 150. Speer H, D’Cunha NM, Botek M, et al. (2019) The effects of dietary polyphenols on circulating cardiovascular disease biomarkers and iron status: a systematic review. Nutr Metab Insights 12, 1178638819882739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ferlazzo N, Visalli G, Cirmi S, et al. (2016) Natural iron chelators: protective role in A549 cells of flavonoids-rich extracts of Citrus juices in Fe(3+)-induced oxidative stress. Environ Toxicol Pharmacol 43, 248–256. [DOI] [PubMed] [Google Scholar]
- 152. Lakey-Beitia J, Burillo AM, La Penna G, et al. (2021) Polyphenols as potential metal chelation compounds against Alzheimer’s disease. J Alzheimers Dis 82, S335–S357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Stiksrud B, Nowak P, Nwosu FC, et al. (2015) Reduced levels of D-dimer and changes in gut microbiota composition after probiotic intervention in HIV-infected individuals on stable art. J Acquir Immune Defic Syndr 70, 329–337. [DOI] [PubMed] [Google Scholar]
- 154. Yeh T-L, Shih P-C, Liu S-J, et al. (2018) The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther 12, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lei W-T, Shih P-C, Liu S-J, et al. (2017) Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients 9, 1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Carfì A, Bernabei R, Landi F, et al. (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Schumann D, Klose P, Lauche R, et al. (2018) Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition 45, 24–31. [DOI] [PubMed] [Google Scholar]
- 158. Sotoudegan F, Daniali M, Hassani S, et al. (2019) Reappraisal of probiotics’ safety in human. Food Chem Toxicol 129, 22–29. [DOI] [PubMed] [Google Scholar]
- 159. Baud D, Dimopoulou Agri V, Gibson GR, et al. (2020) Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front Public Health 8, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Costa RL, Moreira J, Lorenzo A, et al. (2018) Infectious complications following probiotic ingestion: a potentially underestimated problem? A systematic review of reports and case series. BMC Complement Altern Med 18, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Vermeulen MJ, Luijendijk A, van Toledo L, et al. (2020) Quality of probiotic products for preterm infants: contamination and missing strains. Acta Paediatr 109, 276–279. [DOI] [PubMed] [Google Scholar]
- 162. Kolaček S, Hojsak I, Berni Canani R, et al. (2017) Commercial probiotic products: a call for improved quality control. A position paper by the ESPGHAN working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr 65, 117–124. [DOI] [PubMed] [Google Scholar]
- 163. Swanson KS, Gibson GR, Hutkins R, et al. (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17, 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Food and Drug Administration (2017) Regulatory Framework for Substances Intended for Use in Human Food or Animal Food on the Basis of the Generally Recognized as Safe (GRAS) Provision of the Federal Food, Drug, and Cosmetic Act: Guidance for Industry. https://www.fda.gov/media/109117/download (accessed November 2020).
- 165. EFSA Panel on Biological Hazards (BIOHAZ), Koutsoumanis K, Allende A, et al. (2020) Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 11: suitability of taxonomic units notified to EFSA until September 2019. EFSA J 18, e05965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kumar H, Salminen S, Verhagen H, et al. (2015) Novel probiotics and prebiotics: road to the market. Curr Opin Biotechnol 32, 99–103. [DOI] [PubMed] [Google Scholar]
- 167. Verma DK, Niamah AK, Patel AR, et al. Chemistry and microbial sources of curdlan with potential application and safety regulations as prebiotic in food and health. Food Res Int 133, 109136. [DOI] [PubMed] [Google Scholar]
- 168. Morais AHA, Passos TS, Maciel BLL, et al. (2020) Can probiotics and diet promote beneficial immune modulation and purine control in coronavirus infection? Nutrients 12, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. All-Party Parliamentary Group, Gibson GR & Calder PC (2020) Call for a Government Evaluation of the Link between Nutrition and the Gut Microbiome with Respect to the COVID-19 Pandemic. https://www.nutritionsociety.org/sites/default/files/attachments/page/call_for_evaluation_of_nutrition_covid.pdf (accessed May 2021).
- 170. Campbell C & Rudensky A (2020) Roles of regulatory T cells in tissue pathophysiology and metabolism. Cell Metab 31, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sharabi A, Tsokos MG, Ding Y, et al. (2018) Regulatory T cells in the treatment of disease. Nat Rev Drug Discov 17, 823–844. [DOI] [PubMed] [Google Scholar]
- 172. Tian Y, Seumois G, de-Oliveira-Pinto LM, et al. (2019) Molecular signatures of dengue virus-specific IL-10/IFN-γ co-producing CD4 T cells and their association with dengue disease. Cell Rep 29, 4482.e4–4495.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shinde T, Hansbro PM, Sohal SS, et al. (2020) Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms 8, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Corrêa-Oliveira R, Fachi JL, Vieira A, et al. (2016) Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology 5, e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Drakoularakou A, Tzortzis G, Rastall RA, et al. (2010) A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur J Clin Nutr 64, 146–152. [DOI] [PubMed] [Google Scholar]
- 176. Lau AS-Y, Yanagisawa N, Hor Y-Y, et al. (2018) Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Benef Microbes 9, 61–70. [DOI] [PubMed] [Google Scholar]
- 177. Blaabjerg S, Artzi DM & Aabenhus R (2017) Probiotics for the prevention of antibiotic-associated diarrhea in outpatients – a systematic review and meta-analysis. Antibiotics 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kushiro A, Shimizu K, Takada T, et al. (2019) Decreased number of days of fever detection and duration of fever with continuous intake of a fermented milk drink: a randomized, double-blind, placebo-controlled study of elderly nursing home residents. Biosci Microbiota Food Health 38, 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Somerville VS, Braakhuis AJ & Hopkins WG (2016) Effect of flavonoids on upper respiratory tract infections and immune function: a systematic review and meta-analysis. Adv Nutr 7, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Vulevic J, Tzortzis G, Juric A, et al. (2018) Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol Motil 30, e13440. [DOI] [PubMed] [Google Scholar]
- 181. Guo C, Lei M, Wang Y, et al. (2018) Oral administration of probiotic Lactobacillus casei Shirota decreases pneumonia and increases pulmonary functions after single rib fracture: a randomized double-blind, placebo-controlled clinical trial. J Food Sci 83, 2222–2226. [DOI] [PubMed] [Google Scholar]
- 182. Lee DK, Park JE, Kim MJ, et al. (2015) Probiotic bacteria, B. longum and L. acidophilus inhibit infection by rotavirus in vitro and decrease the duration of diarrhea in pediatric patients. Clin Res Hepatol Gastroenterol 39, 237–244. [DOI] [PubMed] [Google Scholar]
- 183. Das S, Gupta PK & Das RR (2016) Efficacy and safety of Saccharomyces boulardii in acute rotavirus diarrhea: double blind randomized controlled trial from a developing country. J Trop Pediatr 62, 464–470. [DOI] [PubMed] [Google Scholar]
- 184. Martami F, Togha M, Seifishahpar M, et al. (2019) The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: a randomized double-blind controlled trial. Cephalalgia 39, 841–853. [DOI] [PubMed] [Google Scholar]
- 185. Russo M, Coppola V, Giannetti E, et al. (2018) Oral administration of tannins and flavonoids in children with acute diarrhea: a pilot, randomized, control-case study. Ital J Pediatr 44, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hawkins J, Baker C, Cherry L, et al. (2019) Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: a meta-analysis of randomized, controlled clinical trials. Complement Ther Med 42, 361–365. [DOI] [PubMed] [Google Scholar]
- 187. Saint-Marc T, Blehaut H, Musial C, et al. (1995) AIDS-related diarrhea: a double-blind trial of Saccharomyces boulardii. Semaine Des Hopitaux 71, 735–741. [Google Scholar]
- 188. McFarland LV (2010) Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 16, 2202–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Irvine SL, Hummelen R & Hekmat S (2011) Probiotic yogurt consumption may improve gastrointestinal symptoms, productivity, and nutritional intake of people living with human immunodeficiency virus in Mwanza, Tanzania. Nutr Res 31, 875–881. [DOI] [PubMed] [Google Scholar]
- 190. Salminen MK, Tynkkynen S, Rautelin H, et al. (2004) The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV patients on antiretroviral therapy: a randomized, placebo-controlled, crossover study. HIV Clin Trials 5, 183–191. [DOI] [PubMed] [Google Scholar]
- 191. Trois L, Cardoso EM & Miura E (2008) Use of probiotics in HIV-infected children: a randomized double-blind controlled study. J Trop Pediatr 54, 19–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0954422421000317.
click here to view supplementary material