Abstract
The growing interest in the possibilities of modulating macrophages in inflammatory diseases with therapeutic purpose has prompted the development of new approaches for the treatment of periodontitis. This randomized add-on open preliminary clinical study evaluated the short-term effects of L-arginine or L-ornithine as an adjuvant to scaling and root planing (SRP) in patients with chronic periodontitis.
Materials and methods
Seventy-five periodontitis patients were recruited and monitored clinically and immunologically at baseline (before SRP) and 30 ± 5 days after SRP. All patients were assigned by stratified randomization to SRP (SRP only, n = 25), Arg (SRP + L-arginine, n = 25) or Control (SRP + L-ornithine, n = 25) Group. The medicines were used according to available instructions for 10 and 15 days, respectively. During the study, all patients were on a stable diet, without changing their rations and regiments. As immunological monitoring immunohistochemical study of CD68+ and CD163 + single positive gingival macrophages for 5 patients per group in the same time-point was conducted. The data were statistically analyzed.
Results
Reduction of periodontal pocket depth (PPD) and bleeding on probing (BoP) was observed in all groups, with significant between-group differences for BoP in the Arg Group (p < 0.0001) at 30 days. The SRP and Arg groups demonstrated nonsignificantly increased density of CD68+ and CD163 + cells. The Orn Group showed an increase in the density of CD68+ and CD163 + macrophages at intragroup (p = 0.0066 and p < 0.0001) and between-group levels (p = 0.001 and p < 0.0001), and these changes corresponded to clinical PPD and BoP reduction. In the Arg and Orn groups at 30 days, CD163 + macrophages significantly predominated over CD68+ (p = 0.013, p < 0.0001).
Conclusion
The use of L-arginine and L-ornithine as an adjunct to SRP promotes additional limited immunological benefit in the treatment of periodontitis. Metabolic stimulation with L-ornithine, but not L-arginine, is preferable for CD163+ Mφs subpopulation in periodontitis-affected gingiva.
Keywords: CD163+ macrophage, CD68+ macrophage, Clinical trial, L-arginine, L-ornithine, Macrophages-targeted therapy, Periodontitis
CD163 + macrophage, CD68 + macrophage, Clinical trial, L-arginine, L-ornithine, Macrophages-targeted therapy, Periodontitis.
1. Introduction
Periodontitis is a multifactorial immune-mediated disease, which develops as a disturbance of immune and inflammatory response to dysbiotic subgingival biofilm [1]. There are many clinical studies devoted to the optimization of the treatment of periodontitis [2, 3, 4]. The search for new approaches indicates the important social significance of periodontitis and dissatisfaction with current treatment results. Since 1976, when Page & Schroeder described the histopathologic events in periodontitis development, myeloid cells and lymphocytes involved in the initiation and progression of the disease still have been actively studied [5, 6, 7, 8, 9, 10]. However, the role of specific macrophage subsets in periodontitis remains largely unexplored [11].
Macrophages (Mφs) play a significant role in the immune system, both as antimicrobial effector cells and as immunoregulatory cells, which induce, suppress, or modulate adaptive immune responses. Compelling evidence from studies of recent years demonstrates that Mφs are heterogeneous and undergo heterogeneous phenotypic changes in response to microenvironmental stimuli. The M1 inflammatory type response and the M2 repair type response are best known as two extreme examples [12, 13]. In different diseases, the M1/M2 ratio can change, as does the expression profile of these cells, which transform to the pathogenic ones [13, 14]. In periodontitis, Mφs should be responsible for certain levels of inflammation maintenance and tissue remodeling in locus morbi.
Modulation of immune responses by nutrients is an important area of study in cellular biology and clinical sciences in the context of cancer therapies and anti-pathogen-directed immune responses in health and disease [15]. Cellular metabolism is a key factor in determining the fate and functions of immune cells. Immune cells have diverse functions, so it is perhaps unsurprising that they have different nutrient and energy demands [16]. For example, M1 Mφs exhibit a broken TCA cycle and have a pro-inflammatory role. By contrast, M2 Mφs undergo β-oxidation to produce anti-inflammatory responses [17]. M1 and M2 Mφs have differences in arginine metabolic pathways: M1 cells expressed inducible nitric oxide synthase (iNOS), and M2 – type-I arginase (Arg-I). At the same time, both arginine metabolic pathways cross-inhibit each other on the level of the respective arginine break-down products [12].
L-arginine regulation of macrophage physiology has been known for a long time [16, 18]. In addition, L-ornithine is involved in the regulation of macrophage functions [19, 20]. Hence, there are many examples of L-arginine and ornithine usage as metabolic regulators of Mφs. Recent trials checked L-arginine supplementation in patients with severe asthma and revealed that higher arginine availability index was associated with lower exacerbation events [21]. Meanwhile, the general use of ornithine has the purpose to support liver function and wound recovery [22, 23, 24]. Systematic pharmacokinetic and pharmacodynamic studies have focused on experimental models of liver failure in patients and resulted in the mechanisms that underpin the effective ammonia-lowering actions of L-ornithine L-aspartate, but little is known about the mechanisms of macrophages modulation.
Despite the confirmed disturbance of the Mφs ratio in periodontitis, attempts to modify them for treatment purposes have not been made. However, understanding the Mφs’ answer in periodontitis-affected gingiva on metabolic stimulation by L-arginine and L-ornithine is important as it opens new possibilities for targeted therapy.
This randomized add-on open preliminary clinical study evaluated the short-term effects of L-arginine or L-ornithine as an adjuvant to scaling and root planing (SRP) in patients with chronic periodontitis.
2. Material and methods
2.1. Patient population
Seventy five patients with periodontitis at stages II-III and grade B (38 women and 37 men, 51% and 49%, respectively) were selected from the population referred to the Department of Postgraduate Education for Dentists of Poltava State Medical University (PSMU, Poltava, Ukraine). All eligible patients (ClinicalTrials.gov ID: NCT05042024) were thoroughly informed of the nature and potential risks and benefits of their participation in the study and signed an informed consent form. The study protocol was reviewed and approved by the ethical committee of Poltava State Medical University (November 27th, 2019, No. 177b). The research was conducted in full accordance with the Helsinki Declaration of 1975, as revised in 2013.
Precise sample size calculation was not performed because of preliminary research nature of the study.
2.2. Inclusion and exclusion criteria
Periodontitis was diagnosed by using the criteria of the Classification of Periodontal and Peri-Implant Diseases and Conditions 2017. Stage II of periodontitis was diagnosed in the presence of 3–4 mm interdental clinical attachment level (CAL) at the site of greatest loss, 4 to maximum 5 mm periodontal pocket depth (PPD), the radiographic bone loss at the root coronal third, and no tooth loss due to periodontitis. Stage III was diagnosed in the presence of ≥5 mm interdental CAL, the radiographic bone loss extending to the middle or apical third of the root, ≤4 teeth loss due to periodontitis. In all cases, periodontitis had a generalized pattern (>30% of teeth involved) and grade B as patterns of the progression, based on indirect evidence (radiographic bone loss expressed as a percentage of root length divided by the age of the subject was from 0.25 to 1.0).
Inclusion criteria were as follows: 1) the presence of periodontitis; 2) good general health; 3) at least 19 remaining teeth, 4) written informed consent forms.
Exclusion criteria were: 1) antibiotics or anti-inflammatory medications use within the preceding 3 months; 2) periodontal therapy within the previous 6 months; 3) purulent exudation from periodontal pockets; 4) pregnancy and breastfeeding; 5) the presence of severe, uncontrolled (decompensated) diseases of the internal organs, or neuropsychiatric disorders; 6) the presence of other conditions that determined the inability of the patient to understand the nature and possible consequences of the study.
2.3. Experimental design and treatment protocol
The present work was the original research study. Patients were grouped by stratified randomization into three groups: the SRP Group (patients received conventional periodontal therapy including scaling and root planing as a full-mouth procedure, n = 25); the Arg Group (patients received oral L-arginine aspartate (Yuria-Pharm, Ukraine) at a dose of 1 g t.i.d. for 10 days after conventional periodontal therapy, n = 25); and the Orn Group (patients received oral L-ornithine aspartate (Farmak, Ukraine) at a dose of 3 g t.i.d. for 15 days after conventional periodontal therapy, n = 25). We used L-arginine and L-ornithine according to instructions for these medicines available for use in Ukraine. During the study, all patients were on a stable diet, without changing their rations and regiments.
All patients were clinically examined before periodontal treatment and after 1month ±5 days.
All patients received clinical monitoring at baseline, at 30 ± 5 days by the same examiner.
2.4. Clinical monitoring of patient continues to obtain long-term results
2.4.1. Examiner calibration
The Kappa coefficient greater than or equal to 0.85 was used for examiner calibration. Ten patients with at least five teeth with PPD and CAL ≥5 mm on proximal sites were selected. Each patient was examined twice by a manual periodontal probe (0106.DT06.CP10, Den Tag, Italy), at a 48-hour interval between the first and second assessments.
2.4.2. Clinical measurements
For all participants, gender and age were recorded, and periodontal parameters such as PPD, CAL and bleeding on probing (BoP) measurements were taken from six periodontal sites on all teeth except for the third molars. PPD and CAL were measured to the nearest 1 mm. BoP was recorded based on the presence or absence of bleeding after probing. PPD was measured from the free gingival margin to the bottom of the periodontal pocket. CAL was measured from the cementoenamel junction to the base of the periodontal pocket. All probing measurements were performed using a manual periodontal probe (0106.DT06.CP10, Den Tag, Italy).
2.5. Immunological monitoring (nested)
2.5.1. Collection of gingival tissue samples
For the precise immunohistochemical study, the gingival biopsy of approximately 3 × 3 mm was excised under local anesthesia before treatment and 1 month later in 5 selected patients per group. Biopsies were obtained in the same time-points, from a single site displaying the deepest pocket around suitable dental and periodontal procedures. Removal of these tissue biopsies did not interfere with the initial treatment plan or influence upon the expected clinical outcomes. After collection, biopsies were fixed in a 4% formalin solution for 24 h of fixation, dehydrated, and embedded in paraffin.
2.5.2. Immunohistochemistry and antibodies
Paraffin sections, 2–3 μm in thickness were deparaffinized and dehydrated. Heat-induced epitope retrieval in citrate buffer, pH 6, was performed by successive heating the slides in the microwave oven, then allowed to cool, rinsed with phosphate-buffered saline (PBS), incubated with blocked reagent, rinsed, and incubated with mouse monoclonal CD68 macrophage antibodies (1:30, clone PG-M1, Diagnostic BioSystems, The Hague, The Netherlands) or anti-CD163 (1:100, clone 10D6, Diagnostic BioSystems, The Hague, The Netherlands). Then sections were stained with the 2-steps Mouse/Rabbit PolyVue Plus™ HRP/DAB Detection System (Diagnostic BioSystems, The Hague, The Netherlands), and counterstained with Mayer's haemalaun. PBS was used as a negative control, the lymph node tissue – as a positive control.
2.5.3. Evaluation of immunohistochemical staining
CD68+ and CD163 + single positive Mφs were estimated by counting the number of the cells by light microscope ×400 in intensive infiltrative areas, 5 regions from each slice were selected, and all 5 counts were taken for statistics. We counted immunopositive Mφs in the areas of cell infiltration, since they are directly related to inflammation. The number of cells per 10 000 μm2 was calculated as immunopositive cell density. Photos were obtained using the light microscope Axio Lab.A1 (Carl Zeiss, Göttingen, Germany) (×400).
2.5.4. Outcome variables
Mean changes in PD, CAL and BoP at 30 ± 5 days post-SRP were defined as the primary outcome variables.
Mean changes at 30 ± 5 days post-SRP in CD68+ and CD163 + macrophages density in gingiva, calculated as the number of immunopositive cells per 10 000 μm2 were defined as the secondary outcome measures.
2.6. Statistical analysis
Means of PPD and CAL were calculated for sites with PD > 4mm (affected sites). Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, San Diego, USA) by means of descriptive statistics, χ2 test, one-way ANOVA and nonparametric ANOVA tests for multiple comparisons: Friedman – for dependent variables, Kruskal-Wallis test – for independent variables, with post-hoc analyzing. For descriptive statistics of cells numbers, the percentile ranges were also used because of non-normal distributions. The CD68+/CD163 + ratio was assessed by inter-group t-tests comparisons and Spearman correlation checking. P values of <0.05 were considered statistically significant in all of the analyses.
The null hypothesis tested was that L-arginine and L-ornithine have no influences on CD68+ and CD163+ Mφs densities when supplementing the treatment of periodontitis.
3. Results
All patients successfully completed the study. Figure 1 shows the study design. The demographic characteristics did not show any difference between groups. L-arginine and L-ornithine were well tolerated by all participants without any observed or self-reported adverse effects.
Figure 1.
Flowchart of the study design.
3.1. Clinical monitoring
All patients in the study had a confirmed diagnosis of periodontitis of II-III stages. At the baseline, all groups demonstrated similar periodontal clinical parameters (Table 1). In each group, reduction of PPD and BoP from the baseline to 1 month was observed, with significant between-group differences only for BoP in the Arg Group (Table 1). CAL changes did not reach statistical significance.
Table 1.
Comparative analysis of clinical periodontal indices over time and between groups.
| Group and patients distribution | Time-point | PPD (>4mm) mean, mm | CAL (sites with PD > 4mm) mean, mm | BoP mean, % | Comparisons between groups |
|---|---|---|---|---|---|
|
SRP Group Ages from 25 to 54 (mean 40); females (n = 12), males (n = 13) |
0 | 4.87 ± 0.30 | 5.11 ± 0.39 | 74 | ns |
| 1 month | 4.4 ± 0.28 | 5.13 ± 0.46 | 17 | BoP Conv > BoP Arg, χ2 test, p < 0.0001 | |
|
Arg Group Ages from 40 to 48 (mean 45); females (n = 13) males (n = 12) |
0 | 4.89 ± 0.46 | 5.26 ± 0.51 | 67 | ns |
| 1 month | 4.49 ± 0.47 | 5.28 ± 0.67 | 9 | BoP Arg < BoP Conv, χ2 test, p < 0.0001 | |
|
Orn Group Ages from 25 to 50 (mean 41); females (n = 13) males (n = 12) |
0 | 4.8 ± 0.41 | 5.09 ± 0.54 | 83 | ns |
| 1 month | 4.37 ± 0.41 | 4.99 ± 0.67 | 13 | BoP Orn > BoP Arg, χ2 test, p = 0.013 | |
|
Comparisons between examination periods |
0 > 1 month within each group, p < 0.0001 | ns p = 0.63 |
χ2 test, p < 0.0001 0 > 30 | - | |
Values shown in Table are mean ± SD (standard deviation); PD – probing depth; CAL – clinical attachment level; BoP – bleeding on probing; ns – non-significant; nonparametric one-way ANOVA (p < 0.0001) and χ2 tests results.
3.2. Immunological monitoring
Cells infiltrate in periodontitis-affected gingiva preferentially occupied areas near the sulcular/junctional epithelium. Fragments of the periodontal ligament were observed closer to the sulcular epithelium with the neighboring inflammatory cells infiltrate. The immunopositive cells were observed mainly in the infiltrated regions and were dispersed. The oral part of the epithelium was mostly located on a relatively dense lamina propria, and demarcated with more fibrous tissue.
General characteristics of CD68+ and CD163 + single positive Mφs were as follows: immunoreactivity was observed in the form of brown granular staining, which revealed some dendritic shape of cells. CD68+ and CD163+ Mφs localized subepithelially and usually in infiltrates. In addition, immunopositive cells density, especially CD163+, demonstrated a wide range of distribution.
At baseline, the between-group comparison showed no statistically significant differences in CD68+ and CD163+ Mφs levels (Table 2).
Table 2.
Comparative analysis of immunopositive macrophages density over time, intra- and between-groups.
| Group | Baseline | 1 month | Comparisons among time-points | |
|---|---|---|---|---|
| CD163+ macrophages | ||||
| SRP Group | 3.6 ± 4.7p2p{0–11.8} | CD163 + macrophages сorrelates with CD68 + macrophages, Spearman R = 0.35, p = 0.0056 | 4.6 ± 5.3p2p{0–15.8}p2pCorrelates with CD68 + macrophages, Spearman R = 0.45, p = 0.0452 | ns |
| Arg Group | 3.7 ± 2.9p2p{0.8–9.1} | 8.0 ± 5.8p2p{1.5–19.6}p2pDominates over CD68 + macrophages, p < 0.0001 | ns | |
| Orn Group | 3.7 ± 2.5p2p{1.9–8.6} | 8.8 ± 2.8p2p{6.0–13.2}p2pDominates over CD68 + macrophages, p < 0.013 | Friedman test, p < 0.0001; p2p0<1 month | |
| comparisons between groups | ns | Kruskal-Wallis test, p < 0.0001; Conv < Orn | - | |
| CD68+ macrophages | ||||
| SRP Group | 1.6 ± 1.5p2p{0–3.9} | CD68 + macrophages сorrelates with CD163 + macrophages, Spearman R = 0.35, p = 0.0056 | 3.3 ± 3.4p2p{0–7.1} | ns |
| Arg Group | 2.0 ± 0.9p2p{1.1–3.3} | 2.2 ± 1.96p2p{0–5.0} | ns | |
| Orn Group | 1.96 ± 1.2p2p{1.0–3.7} | 5.4 ± 4.2p2p{1.1–9.9} | Friedman test, p = 0.0066; p2p0<1 month | |
| comparisons between groups | ns | Kruskal-Wallis test, p = 0.001; Arg < Orn | - | |
Values shown in the table are Mean ± SD and the percentile ranges of {P10 −P90} 80%, because of non-normal distribution of variables; ns – non-significant; nonparametric ANOVA, t-tests and Spearman correlation results.
We revealed slight/nonsignificant predominance of CD163+ Mφs density over CD68+ in infiltrates before and after SRP treatment. Furthermore, a significant correlation was observed between CD163+ and CD68+ Mφs at the level of the total sample before treatment (Spearman R = 0.35, p = 0.0056) and in the SRP Group after treatment (Spearman R = 0.45, p = 0.0452), but we have insufficient evidence of correlation in two others groups (Table 2).
After L-arginine administration, the representation density of CD163+ Mφs increased nonsignificantly, but significantly exceeded the SRP Group before treatment (Table 2, Figure 2). CD68+ Mφs density increased als o nonsignificantly (Table 2, Figure 3). At the same time, CD163+ Mφs density significantly predominated over CD68+ (Wilcoxon matched-pairs signed-rank test, p < 0.0001).
Figure 2.
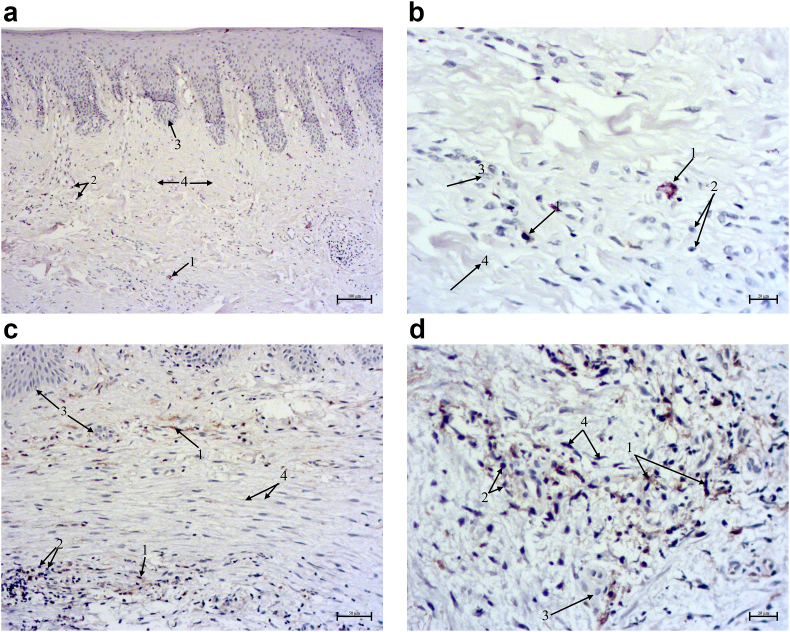
CD163+ Mφs in periodontitis-affected gingiva before and after treatment. a. CD163+ Mφs before treatment (1), infiltrating cells (2), epithelial cells (3). b. CD163+ Mφs after l-arginine (1), fibrous tissue (2), epithelial cells (3). c. Increased density of CD163+ Mφs after l-ornithine (1), infiltrating cells (2), epithelial cells (3). Contrasting: Mayer's haemalaun, mg.×400. d. Kruskal-Wallis test comparisons & post-hoc of CD163+ Mφs density, p < 0.0001.
Figure 3.
CD68+ Mφs in periodontitis-affected gingiva before and after treatment. a. CD68+ Mφs before treatment (1), infiltrating cells (2), epithelial cells (3), mg.×200. b. CD68+ Mφs after l-arginine (1), infiltrating cells (2), epithelial cells (3), mg.×400. c. CD68+ Mφs after l-ornithine (1), infiltrating cells (2), epithelial cells (3), periodontal ligament cells (4). Contrasting: Mayer's haemalaun, mg.×200. d. Kruskal-Wallis test comparisons & post-hoc of CD68+ Mφs density, p = 0.0010.
In the Orn Group, the density of CD163+ Mφs increased and significantly exceeded the SRP group (p < 0.0001). The density of CD68+ Mφs also increased and significantly exceeded the Arg Group (p = 0.001), but not the SRP Group (Figure 3). Meanwhile, the CD163+ Mφs density predominated over CD68+ (p = 0.013) (Table 2).
Accordingly, L-arginine had a weak effect on immunopositive Mφs, except for the CD68+/CD163 + ratio, and L-ornithine had a clear effect in the form of CD68+ and CD163+ Mφs density increase. Moreover, in the CD68+/CD163 + ratio, the CD163 + cells became significantly predominant in both Arg and Orn Groups.
A common feature after periodontitis treatment with amino acids was histological massive areas of fibrous stroma in the gingiva with decreased infiltration areas (Figure 4). The decreasing of CD68+ Mφs (Figure 4 a,b) and the increasing of CD68+ Mφs (Figure 4c,d) density in periodontitis-affected gingiva after l-ornithine administration were observed.
Figure 4.
Immunostaining of periodontitis-affected gingiva after l-ornithine administration: visible predominance of CD163+ Mφs over CD68+. Contrasting: Mayer's haemalaun, (1) single positive cells, (2) infiltrating cells, (3) epithelial cells, (4) connective tissue. a. Rare CD68+ Mφs in the fibrous area of the gingiva, mg.×100. b. CD68+ Mφs in the fibrous area, mg.×400. c. Increased density of CD163+ Mφs in the fibrous area of the gingiva, mg.×200. d. CD163+ Mφs in the fibrous area, mg.×400.
CD68+ and CD163+ Mφs representation density changes corresponded to clinical PPD and BoP reduction, although we did not confirm any statistical correlation.
4. Discussion
This randomized add-on open preliminary short-term clinical study evaluated the effects of L-arginine or L-ornithine on the non-surgical treatment of individuals with chronic periodontitis by analyzing clinical and immunological parameters. The results obtained for PPD, CAL and BoP, demonstrate similar reduction for all study groups, with significant between-group differences only for BoP in the Arg Group. These findings have insufficient evidence of the clinical significance of amino acids medicines in periodontal treatment. However, additional benefit was found by analyzing immunological parameters, which were CD68+ and CD163 + single positive Mφs representation density. The results show that the L-ornithine administration, as an adjunct to SRP, promoted additional limited immunological benefit in assessments at 30 ± 5 postoperative days. Although we have not received the full clinical effect and observations continue for monitoring the long-term effects. Other studies revealed the clinical benefits later [3, 4, 25].
The growing interest in the possibilities of modulating macrophages in inflammatory diseases with therapeutic purpose has prompted the development of new approaches for the treatment of periodontitis. The research is of a reconnaissance nature to a large extent. A global idea is to find out whether the metabolic modulation of local macrophages can change periodontal inflammation to less active and the response to treatment of the periodontitis to more predictable.
Specific types of inflammatory responses in the gingiva are necessary to initiate the destruction of the connective tissue attachment apical to the cement-enamel junction [26], and gingival inflammation is regarded as a necessary prerequisite for the subsequent development of periodontitis [27], which justified the choice of the gum for the study. The inter-relationships between health, gingivitis and periodontitis are complex and depend on the features of dental biofilm, the proportionality of the host's immune-inflammatory response and its ability to resolve inflammation [28]. The question about the opportunity to correct the host's immune-inflammatory response has not been resolved until today [26]. It is possible that Mφs, as a central regulatory instrument in the immunity [29], can be a key in this response. The findings of Zhou LN. et al. (2019) demonstrated that the gingival biopsies from patients with chronic periodontitis had more M1 cells (F4/80 + iNOS + cells) and higher M1/M2(F4/80 + CD206 + cells) ratio as compared to healthу gingiva, which positively correlates with clinical periodontal depth and significantly higher TNF-α, IFN-γ, IL-6 and IL-12 levels [6], thus emphasizing the role of M1 in the deterioration of periodontitis. On the other hand, immunofluorescence study of Garaicoa-Pazmino C. et al. (2019), performed using a combination of CD68 (macrophages), iNOS (M1), and CD206 (M2), showed similar levels of M1 and M2 Mφs to those observed in healthy tissues without changes in polarization, although without M1-and M2-related cytokine assessment [8]. In addition, a recent review [7] demonstrated that both subsets of Mφs can contribute to periodontitis at least because such pathogen as P.gingivalis can selectively tolerise regulatory M2 with little effect on pro-inflammatory M1 Mφs. This leaves the questions unsettled about the functional state of the phenotypically M1 M2 Mφs. Perhaps an attempt to influence the phenotype of Mφs together with changes in clinical signs of periodontitis will shed light on their functions. However, the assessment of advancements in periodontology over the past quarter-century does not mention the attempts of influence on macrophage modulation as a therapeutic approach [10]. Therefore, this study is one of the first in the field of macrophages-targeted therapy in periodontitis.
The suggestion that L-arginine metabolism could be involved in the regulation of Mφs phenotypes arose from early studies with murine Mφs [30, 31]. The comprehensive review suggested that arginase including Arg-I and Arg-II causes L-arginine deficiency, resulting in decreased NO production from iNOS in Mφs (M2 type function). Arg-I and Arg-II share the same L-arginine metabolizing function but seem to exert distinct effects on Mφs [32]. Another review speculated that M2/Arg-I Mφs might be more efficient in the induction of extracellular arginine depletion [12]. Ornithine itself can further feed into the important downstream pathways of polyamine and proline syntheses, which are important for cellular proliferation and repair, which is a predominant function of M2 Mφs [12]. Ornithine is transformed by ornithine decarboxylase (ODC), which limits M1 activation and macrophage anti-microbial activities by chromatin modification [19]. Therefore, we selected L-arginine and L-ornithine for studying as agents to promote M1 and M2 Mφs functions, respectively.
This field is characterized by Mφs classification problem, particularly in vivo, which originates from a gap in the knowledge of the several intermediate polarization statuses between the M1 and M2 extremes. Moreover, the detailed features of metabolic reprogramming crucial for macrophage polarization are largely unknown [33]. Therefore, we used the immunohistochemistry technique to determine the density of CD68+ and CD163 + cells as preliminarily morphological equivalents of different subpopulations of Mφs. CD68 and CD163 molecules are scavenger receptors [34]. Not only are some scavenger receptors more highly expressed in M2 cells than in M1 cells, but also the presence of some receptors contributes to the polarization programme of these cells. CD163 is instrumental in promoting an anti-inflammatory phenotype in M2 Mφs [35]. It can sequester and thus inactivate pro-inflammatory molecules such as TNF-related weak inducer of apoptosis (TWEAK) [36], and attenuates haemoglobin-associated damage that is a source of inflammation [37, 38]. Nevertheless, scavenger receptors can contribute to pro-inflammatory Mφ responses in certain contexts [34, 39]. Meanwhile, CD68 is a differentiation marker of hematopoietic cells of the monocyte/macrophage lineage. It is mostly found in the late endosomal compartment in Mφs and dendritic cells, with a limited expression on resting cells. CD68 plays a minor role in the binding and uptake of oxidized lipoproteins and apoptotic cells by Mφs [40]. But in general, not much is known about Mφs phenotype related to periodontitis.
Our major finding was a pronounced effect of the L-ornithine which significantly increased representation density of CD68+ and CD163+ Mφs with the predominance of CD163+ Mφs. Results about increased CD163+ Mφs fit into the modern concept about the anti-inflammatory effect of L-ornithine and its metabolites on Mφs [12, 41, 42]. In the present study the density of CD68+ Mφs although significantly increased, but did not significantly differ from the reference SRP Group. Nevertheless, this suggests that СD68+ Mφs, together with CD163+ Mφs, contribute to the resolution of inflammation in periodontitis.
L-arginine administration slightly increased the density of CD68+ and CD163+ Mφs, without inter- and between groups significance. The findings partially consist with mentioned above probabilities about M1 promotion by arginine, because arginase can limit arginine availability for NO synthesis, as demonstrated by pharmacological arginase inhibition in different types of macrophages [12, 32]. However, the statistically significant predominance of CD163+ Mφs density over CD68 + after L-arginine administration prompts a suggestion about its slight CD163+ Mφs promotion also.
In total, observed wide range distribution of CD68+ and CD163+ Mφs density indicates a high variability of individual response. To summarize, the integration of hundreds of signals, including metabolic, ultimately defines a macrophage's behavior, including those associated with periodontitis. Therefore we assume that the outcomes obtained with L-arginine or L-ornithine cannot be generalized.
Significant prevalence of CD163+ Mφs density after macrophage-targeted treatment, followed by massive areas of fibrous tissue in the gingiva, and difference from a slight predominance of CD163+ Mφs after SRP treatment, together with the data about the possibility to skew macrophages function for improved wound healing [43], can be evidence of M2 Mφs healing function.
One of the limitations of the study was the use of Mφs markers, which can be partially overlapped in pathology. In addition, it is not fully clarified whether CD68+ and CD163+ Mφs represent terminally differentiated cells, or whether these cells are plastic and can adapt their function according to new environmental cues. The above-mentioned fact is associated with another limitation, which was the time of the second biopsy, chosen with account for the timing of periodontal healing [44]. Hence, the long-term effect on periodontal status and the exact time of L-arginine and L-ornithine effects on Mφs should be clarified.
5. Conclusions
The use of L-arginine and L-ornithine as an adjunct to SRP promotes additional limited immunological benefit in the treatment of periodontitis. Metabolic stimulation with L-ornithine, but not L-arginine, is preferable for CD163+ Mφs subpopulation in periodontitis-affected gingiva. According to clinical improvement, CD163+ Mφs promotion corresponds to M2 polarization in periodontitis. The long-term results are necessary in order to obtain evidence of the new possibilities of macrophages-targeted therapy to improve periodontitis treatment.
Declarations
Author contribution statement
Viktoriia I. Shynkevych, Svitlana V. Kolomiiets: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Igor P. Kaidashev: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work is part of the research project No. 0120U101151 “The contribution of components of the molecular clock in the impairment of periodontal tissues in its inflammatory diseases for the development of methods for prevention and treatment” funded by the Ministry of Health of Ukraine.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Papapanou P.N., Sanz M., Buduneli N., et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45(Suppl 20):S162–S170. doi: 10.1111/jcpe.12946. [DOI] [PubMed] [Google Scholar]
- 2.Anusha D., Chaly P.E., Junaid M., et al. Efficacy of a mouthwash containing essential oils and curcumin as an adjunct to nonsurgical periodontal therapy among rheumatoid arthritis patients with chronic periodontitis: a randomized controlled trial. Indian J. Dent. Res. 2019;30:506–511. doi: 10.4103/ijdr.IJDR_662_17. [DOI] [PubMed] [Google Scholar]
- 3.[a] D'Aiuto F., Gkranias N., Bhowruth D., et al. TASTE Group. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lan. Diab. Endocrin. 2018 Dec;6(12):954–965. doi: 10.1016/S2213-8587(18)30038-X. [DOI] [PubMed] [Google Scholar]; [b] D'Aiuto F., Gkranias N., Bhowruth D., et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Erratum in: Lan. Diab. Endocrin. 2019 Mar;7(3):e3. doi: 10.1016/S2213-8587(18)30038-X. PMID: 30472992. [DOI] [PubMed] [Google Scholar]
- 4.Invernici M.M., Salvador S.L., Silva P.H.F., et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: a randomized clinical trial. J. Clin. Periodontol. 2018;45(10):1198–1210. doi: 10.1111/jcpe.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinkevich V.I., Kaĭdashev I.P. The role of immune cells factors in the remodeling of gingiva at chronic generalized periodontal disease. Stomatologiia. 2012;91(1):23–27. [PubMed] [Google Scholar]
- 6.Zhou L.N., Bi C.S., Gao L.N., et al. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. 2019;25:265–273. doi: 10.1111/odi.12983. [DOI] [PubMed] [Google Scholar]
- 7.Shynkevych V.I., Kaidashev I.P. Contribution of macrophage subpopulations to the pathogenesis of chronic periodontitis in humans and perspectives for study. Review of the literature. Zaporozhye medical journal. 2019;21(1):137–143. [Google Scholar]
- 8.Garaicoa-Pazmino C., Fretwurst T., Squarize C.H., et al. Characterization of macrophage polarization in periodontal disease. J. Clin. Periodontol. 2019;46(8):830–839. doi: 10.1111/jcpe.13156. [DOI] [PubMed] [Google Scholar]
- 9.Allam J.P., Duan Y., Heinemann F., et al. IL-23-producing CD68+ macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J. Clin. Periodontol. 2011;38:879–886. doi: 10.1111/j.1600-051X.2011.01752.x. [DOI] [PubMed] [Google Scholar]
- 10.Slots J. Periodontitis: facts, fallacies and the future. Periodontol. 2017;75(1):7–23. doi: 10.1111/prd.12221. 2000. [DOI] [PubMed] [Google Scholar]
- 11.Almubarak A., Tanagala K.K.K., Papapanou P.N., et al. Disruption of monocyte and macrophage homeostasis in periodontitis. Front. Immunol. 2010;11:330. doi: 10.3389/fimmu.2020.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rath M., Müller I., Kropf P., et al. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon S., Plüddemann A., Estrada F.M. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol. Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh T. Functional diversity of disorder-specific macrophages. Rinsho Ketsueki. 2018;59(6):805–811. doi: 10.11406/rinketsu.59.805. [DOI] [PubMed] [Google Scholar]
- 15.Ramalho R., Rao M., Zhang C., et al. Immunometabolism: new insights and lessons from antigen-directed cellular immune responses. Semin. Immunopathol. 2020;42(3):279–313. doi: 10.1007/s00281-020-00798-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedia-Mehta N., Finlay D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019;10(1):2123. doi: 10.1038/s41467-019-10015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angajala A., Lim S., Phillips J.B., et al. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front. Immunol. 2018;12(9):1605. doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nouwen L.V., Everts B. Pathogens MenTORing macrophages and dendritic cells: manipulation of mTOR and cellular metabolism to promote immune escape. Cells. 2020;9(1):161. doi: 10.3390/cells9010161. Published 2020 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardbower D.M., Asim M., Luis P.B., et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc. Natl. Acad. Sci. U. S. A. 2017;114(5):E751–E760. doi: 10.1073/pnas.1614958114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moinard C., Caldefie F., Walrand S., et al. Involvement of glutamine, arginine, and polyamines in the action of ornithine alpha-ketoglutarate on macrophage functions in stressed rats. J. Leukoc. Biol. 2000;67(6):834–840. doi: 10.1002/jlb.67.6.834. [DOI] [PubMed] [Google Scholar]
- 21.Liao S.Y., Showalter M.R., Linderholm A.L., et al. l-Arginine supplementation in severe asthma. JCI Insight. 2020;5(13) doi: 10.1172/jci.insight.137777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simsek B., Çakatay U. Could ornithine supplementation be beneficial to prevent the formation of pro-atherogenic carbamylated low-density lipoprotein (c-LDL) particles? Med. Hypotheses. 2019;126:20–22. doi: 10.1016/j.mehy.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Campion D., Giovo I., Ponzo P., et al. Dietary approach and gut microbiota modulation for chronic hepatic encephalopathy in cirrhosis. World J. Hepatol. 2019;11(6):489–512. doi: 10.4254/wjh.v11.i6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito N., Seki S., Ueda F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels-A randomized, double-blind, placebo-controlled trial. Mar. Drugs. 2018 Dec 3;16(12):482. doi: 10.3390/md16120482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loos B.G., Needleman I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020;47:61–71. doi: 10.1111/jcpe.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami S., Mealey B.L., Mariotti A., et al. Dental plaque–induced gingival conditions. J. Clin. Periodontol. 2018;45(Suppl 20):S17–S27. doi: 10.1111/jcpe.12937. [DOI] [PubMed] [Google Scholar]
- 27.Kinane D.F., Attström R. Advances in the pathogenesis of periodontitis. J. Clin. Periodontol. 2005;32(Suppl. 6):130–131. doi: 10.1111/j.1600-051X.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 28.Meyle J., Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol. 2015;69:7–17. doi: 10.1111/prd.12104. 2000. [DOI] [PubMed] [Google Scholar]
- 29.Brown B.N., Sicari B.M., Badylak S.F. Rethinking regenerative medicine: a macrophage-centered approach. Front. Immunol. 2014 Nov 4;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oswald I.P., Afroun S., Bray D., et al. Low response of BALB/c macrophages to priming and activating signals. J. Leukoc. Biol. 1992;52(3):315–322. doi: 10.1002/jlb.52.3.315. [DOI] [PubMed] [Google Scholar]
- 31.Dileepan K.N., Page J.C., Li Y., Stechschulte D.J. Direct activation of murine peritoneal macrophages for nitric oxide production and tumor cell killing by interferon-gamma. J. Interferon Cytokine Res. 1995;15(5):387–394. doi: 10.1089/jir.1995.15.387. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z., Ming X.F. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014;5:533. doi: 10.3389/fimmu.2014.00533. Published 2014 Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Santa F., Vitiello L., Torcinaro A., et al. The role of metabolic remodeling in macrophage polarization and its effect on skeletal muscle regeneration. Antioxidants Redox Signal. 2019;30(12):1553–1598. doi: 10.1089/ars.2017.7420. [DOI] [PubMed] [Google Scholar]
- 34.Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13(9):621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 35.Fabriek B.O., Dijkstra C.D., van den Berg T.K. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Bover L.C., Cardó-Vila M., Kuniyasu A., et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J. Immunol. 2007;178(12):8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- 37.Schaer D.J., Alayash A.I., Buehler P.W. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxidants Redox Signal. 2007;9(7):991–999. doi: 10.1089/ars.2007.1576. [DOI] [PubMed] [Google Scholar]
- 38.Philippidis P., Mason J.C., Evans B.J., et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004;94(1):119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 39.Yemchenko Y.O., Shynkevych V.I., Ishcheikin K.Y., et al. PPAR-gamma agonist pioglitazone reduced CD68+ but not CD163+ macrophage dermal infiltration in obese psoriatic patients. PPAR Res. 2020;2020:4548012. doi: 10.1155/2020/4548012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson K., El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimer's Dis. 2012;2012:489456. doi: 10.1155/2012/489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M., Borovikova L.V., Wang H., et al. Spermine inhibition of monocyte activation and inflammation. Mol. Med. 1999;5(9):595–605. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M., Wang H., Tracey K.J. Regulation of macrophage activation and inflammation by spermine: a new chapter in an old story. Crit. Care Med. 2000;28(4 Suppl):N60–N66. doi: 10.1097/00003246-200004001-00007. [DOI] [PubMed] [Google Scholar]
- 43.Kim S.Y., Nair M.G. Macrophages in wound healing: activation and plasticity. Immunol. Cell Biol. 2019;97(3):258–267. doi: 10.1111/imcb.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hämmerle C.H.F., Giannobile W.V. Biology of soft tissue wound healing and regeneration. Consensus Report of Group 1 of the 10th European Workshop on Periodontology. J. Clin. Periodontol. 2014;41(Suppl. 15):S1–S5. doi: 10.1111/jcpe.12221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.