Abstract
The introduction of Cystic Fibrosis Trans Regulatory modulator (CFTRm) drugs has seen a transformation in Cystic Fibrosis (CF) treatment. This has led to a significant improvement in lung function and quality of life with the potential for a real impact on life expectancy.
Transient mild to moderate hepatic transaminitis is a well-known side effect of CFTRm drugs, which often improves on cessation and may not recur following the re-institution of the drug. We describe a case of transaminitis developing nine months after the initiation of Kaftrio, which progressed to liver necrosis despite stopping Kaftrio and took many months to resolve.
The patient had experienced significant improvement in lung function and overall health while on Kaftrio and deteriorated when it was stopped. He was keen to restart; however, Kaftrio was not reinstated due to the potential risk of acute liver failure.
Keywords: CF, Cystic Fibrosis; CFTRm, Transmembrane Regulator Protein Modulator; ALP, Alkaline Phosphatase; AST, Aspartate Transaminase; ALT, Alanine Transaminase; GGT, Gamma-Glutamyl Transferase; CMV, Cytomegalovirus; EBV, Epstein-Barr Virus; VZV, Varicella-Zoster Virus; FEV1, Forced Expiratory Volume
Keywords: Hepatic necrosis, CFTR modulators, Severe transaminitis, Liver injury, Adverse effect
1. Introduction
Cystic Fibrosis (CF) is the most common autosomal recessive disease affecting 6,225 adults in the UK [1]. Whilst treatment traditionally consisted of supportive management focusing on nutrition, airway clearance, and respiratory infections, the introduction of Cystic Fibrosis Transmembrane Regulator Protein Modulators (CFTRm) has revolutionized the management of CF.
Since the first CFTRm drug Ivacaftor for Class III gating mutations was developed, many more people with CF are benefitting from combinations of “potentiator” (Ivacaftor) and “corrector” (Lumacaftor, Tezacaftor, Elexacaftor). Kaftrio, consisting of Ivacaftor, Tazcaftor and Elexacaftor, was licensed in the UK in 2020 for patients with two ΔF508 (homozygous) mutations and subsequently a range of other mutations alone or in combination with ΔF508. Liver dysfunction is a well-established side effect of CFTR modulators affecting up to a quarter of patients in some studies. In contrast, transaminitis with a rise of more than three times the upper limit of normal is uncommon; if present, it is transient and rarely requires cessation of treatment [3].
Kaftrio has been shown to lead to significant improvement in sweat chloride, lung function [1] and weight, as well as a reduction in infective exacerbations and improved quality of life. The side effects of Kaftrio include dyspnoea, bronchospasm, abdominal pain, diarrhoea, rash, drug interactions and deranged liver functions (LFTs) [1], for which three monthly blood monitoring is recommended.
2. Case report
A 45-year-old Caucasian male with known Cystic Fibrosis (CF) (ΔF508/Q890X) was started on Kaftrio (then known as Trikafta) in December 2019 on a compassionate use program.
The patient was pancreatic insufficient, had insulin-requiring CF-related diabetes and was chronically colonized with Pseudomonas. He had no history of CF liver disease. He was on the full range of CF treatment, including Azithromycin, fat-soluble vitamins, cetirizine, inhalers (steroids and bronchodilators), insulin and DNAase (Pulmozyme).
A significant improvement was observed in FEV1 two months following the initiation of Kaftrio from 1.44 L Litres (34% of predicted) in Nov 2019 to 1.72 L (41% of predicted) in Feb 2020. He also started gaining weight, improving his BMI from 19.6 kg/m2 in Nov 2019 to 20.2 kg/m2 in Feb 2020. In addition, he felt better, had more energy, and resumed mountain biking.
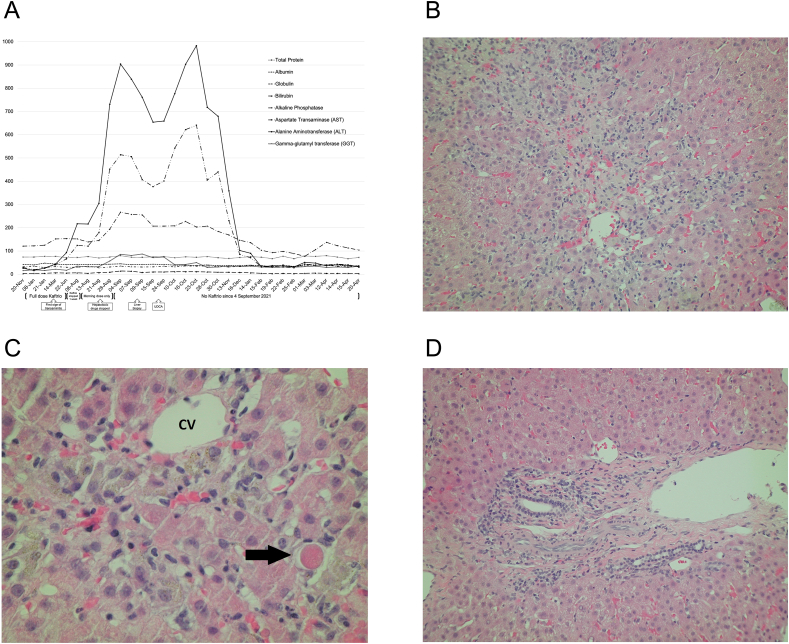
Seven months later, we detected early signs of deranged liver function tests (LFTs) (Fig. 1A) and repeated the measurements at six, seven, eight and nine weeks. These showed rapidly progressive and significant transaminitis. There was no abnormality of bilirubin or clotting time.
Fig. 1.
(A) Timeline of liver function tests (LFTs) that expands the duration of initiation to cessation of Kaftrio with the boxes represent significant events. (B) Medium power of peri-central necrosis. (C) High power of peri-central necrosis, showing hepatocytes that have died and a mixture of pigment-laden macrophages, sparse inflammatory cells, and an occasional apoptotic body. CV - central vein, arrow - apoptotic body. (D) Portal tract showing intact margin, expected primary contents (hepatic artery branch, portal vein branch and bile duct radicle), with minimal chronic inflammatory cells and peripheral ductular metaplasia.
Despite this, he was asymptomatic with no evidence of jaundice, pruritus, urine, or faecal colour changes. Physical examination did not reveal any abnormalities, and all physiological measurements were within the normal ranges. Kaftrio was stopped for one week at this point, with the view to restarting once LFTs improved. Unfortunately, the LFTs did not improve; however, they remained stable – ALP 139(153) U/L, AST 121(124) U/L, ALT 216(217) U/L.
As our patient was very keen to restart Kaftrio, the morning dose was restarted (no evening Kalydeko), which resulted in the worsening of transaminitis. Non-Invasive Liver Screen (NILS) and ultrasound of the liver did not show any abnormalities excluding the other causes of transaminitis, such as autoimmune or viral hepatitis (Hepatitis A, B, C, E, CMV, EBV, VZV), inflammatory or infiltrative disease. We consistently monitored clotting as a measure of liver synthetic function, and it remained within the normal range. Additionally, azithromycin, lansoprazole and nebulized levofloxacin were discontinued as these could have contributed to liver damage.
Following normal magnetic resonance imaging (MRI) of the liver and essentially normal NILS screen, Kaftrio was stopped altogether due to persistent worsening of LFTs.
As the transaminitis continued to worsen, we carried out a percutaneous liver biopsy which revealed extensive confluent necrosis with loss of cells in zone 3 areas, focally verging on bridging necrosis (Fig. 1B). There was only a little inflammation associated with this. Portal tracts were relatively unaffected, with only some showing mild lymphocytic inflammation, focal lymphocyte aggregates and focal interface hepatitis. Some portal tracts had peripheral ductular metaplasia, although there was no permanent fibrosis, and the contents were present and appeared normal (Fig. 1D). In keeping with this, multiple debris-laden macrophages were present around central vein areas. No other liver disease was identified. This relatively bland form of necrosis is characteristic of, but not specific for, a drug-related injury without background changes of typical CF liver disease.
Our patient had been well throughout until his Kaftrio was stopped. Whilst off the Kaftrio, he lost significant weight and lung function leading to hospital admission for intravenous antibiotics in February 2021. BMI fell back to 18.6 kg/m2 from 20.2 kg/m2 and FEV1 to 1.23L (29% predicted) from 1.72L (41% predicted). He repeatedly asked to be allowed to restart Kaftrio despite concerns of the CF team.
Although his liver function, including AST, ALT, GGT, and ALP, normalized, a Fibroscan showed a median score of 6.6 kPa, confirming liver fibrosis. In consultation with the local liver transplant team, it was felt that he should not restart Kaftrio in case of an unpredictable reaction resulting in acute hepatic necrosis and because his respiratory status mitigated against the possibility of an acute liver transplant.
We briefly tried Ursodeoxycholic acid (UCDA), but the transaminitis continued to get worse, and it upset his bowels and so was stopped. We also considered starting a course of steroids and treating for drug-induced hepatitis. However, our patient was not keen to take additional medications, and LFTs began to improve without their addition. We also considered a trial of introducing each element of Kaftrio one by one, but we faced difficulty accessing Tezacaftor and Elexacaftor individually.
3. Discussion
Kaftrio is the latest combination CFTRm therapy to be introduced in the UK. Although transaminitis has been observed in a small proportion of those taking Lumacaftor/Ivacaftor (Orkambi) or Tazacaftor/Ivacaftor (Symkevi/Symdeko), it appears more common in those taking Elexacaftor which is a component of Kaftrio. In addition, a rise in bilirubin has been previously considered a pointer of severity, although our patient's bilirubin remained within the normal range throughout this episode.
We had considered the possibility of cystic fibrosis-related liver disease (CFLD) due to its high prevalence, but no clinical, radiological or histopathological evidence suggested this diagnosis. Moreover, the temporal association between the introduction of the drug and liver dysfunction and improvement of liver function on cessation of the drug further supported the diagnosis of drug-induced hepatotoxicity.
The pathogenesis of CFTR modulator related liver dysfunction remains unclear; however, as metabolism occurs in the CYP3A system, hepatic injury from metabolites and interactions when given in combination has been suggested [2].
There are currently no established guidelines for managing CFTR modulator-induced liver injury, with no values that can be regarded as an absolute contraindication for reintroducing the drug. As a result, due to their significant benefits in patient's overall health, re-introduction is always considered. Strategies such as short-term discontinuation or restarting on a lower dose have been shown to effectively allow liver dysfunction to settle [3,4].
Our patient represents an extreme case of Kaftrio induced liver dysfunction with AST levels reaching 621U/L and ALT 983U/L (Fig. 1A), and maybe the first case of biopsy-proven hepatic necrosis without other obvious cause. Other centres (personal communication) have seen lesser transaminitis, and some are doing biopsies but none like this. Our patient concurred with our risk assessment, and sadly his CF has resumed its prior trajectory. His lung function remains stubbornly low (FEV1 less than 30% predicted), he is struggling to maintain weight and planning for a gastrostomy while being worked up for lung transplant. He can no longer ride his mountain bike.
4. Learning points
-
1.
CFTRm drugs commonly cause transient and relatively minor hepatic transaminitis but may progress to hepatic necrosis and fibrosis.
-
2.
There is a need for an evidence-based protocol for stopping and reintroducing these drugs in such cases.
-
3.
Given the potential life-prolonging benefits to the CF lung, evidence-based strategies to prevent, mitigate or treat hepatocellular injury and allow continuation of those drugs is urgently needed.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Contribution statement
AD and FE conceived the report. MS and MI drafted the manuscript. AA reviewed hepatology. AD reviewed histology. All authors reviewed and approved the final manuscript.
Declaration of competing interest
The authors have no competing interests to declare.
References
- 1.Taylor-Cousar J.L., Mall M.A., Ramsey B.W., McKone E.F., Tullis E., Marigowda G., et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res. 2019;5(2) doi: 10.1183/23120541.00082-2019. 00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. [Google Scholar]
- 3.Davies J.C., Moskowitz S.M., Brown C., Horsley A., Mall M.A., McKone E.F., et al. VX-659–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018;379(17):1599–1611. doi: 10.1056/NEJMoa1807119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor-Cousar J.L., Jain M., Barto T.L., Haddad T., Atkinson J., Tian S., et al. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J. Cyst. Fibros. 2018;17(2):228–235. doi: 10.1016/j.jcf.2017.09.012. [DOI] [PubMed] [Google Scholar]