Abstract
Nonalcoholic fatty liver disease (NAFLD) patients with diabetes constitute a subgroup of patients with a high rate of liver-related complications. Currently, there are no specific drug recommendations for these patients. Metformin, a conventional insulin sensitizer agent, has been widely prescribed in patients with diabetes. Metformin treatment has been shown to be effective at alleviating hepatic lipogenesis in animal models of NAFLD, with a variety of mechanisms being deemed responsible. To date, most studies have enrolled diabetic patients who are treated with metformin, with the drug being taken continuously throughout the study. Although evidence exists regarding the benefits of metformin for NAFLD in preclinical studies, reports on the efficacy of metformin in adult NAFLD patients have had some discrepancies regarding changes in liver biochemistry and hepatic fat content. Evidence has also suggested possible effects of metformin as regards the prevention of hepatocellular carcinoma tumorigenesis. This review was performed to comprehensively summarize the available in vitro, in vivo and clinical studies regarding the effects of metformin on liver steatosis for the treatment of adult NAFLD patients with diabetes. Consistent reports as well as controversial findings are included in this review, and the mechanistic insights are also provided. In addition, this review focuses on the efficacy of metformin as a monotherapy and as a combined therapy with other antidiabetic medications.
Keywords: Diabetes mellitus, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common disease with increasing incidence worldwide. A large cohort in the United States showed a 5-fold increase in incidence from 1997 to 2014.1 Insulin resistance has a pivotal role in NAFLD development and progression2 and NAFLD patients with diabetes are a subgroup of patients with a high rate of liver-related complications.3 In response to insulin resistance, hyperinsulinemia occurs causing the augmentation of hepatic de novo lipogenesis pathways, resulting in hepatic steatosis and further hepatic inflammation.4 Currently, the treatment of NAFLD is markedly under investigation. To date, no medication has been approved to treat NAFLD and nonalcoholic steatohepatitis (NASH) by the Food and Drug Administration in the United States and there is no specific drug recommended for treating the subgroup of NAFLD patients with diabetes.
Metformin, an insulin sensitizer agent in the biguanide subclass, is a widely used drug in diabetic patients with a good safety profile. Since it involves multiple molecular mechanisms in glucose metabolism and anti-inflammatory effects,5 metformin is one of the most interesting medications for the possible treatment or control of NAFLD progression. A previous meta-analysis of randomized-controlled trials evaluated the treatment response of metformin in patients with NAFLD and NASH.6 It was concluded that metformin was not associated with liver histologic improvement in patients with histologic NASH. However, most of the patients enrolled in these studies were nondiabetic7-11 or patients with insulin resistance without established diabetes.12 Currently, reports on the effects of metformin among diabetic NAFLD patients have been inconsistent. Since most of the diabetes patients were receiving metformin, the beneficial effects of this treatment need to be elucidated.
In this review, we have comprehensively summarized findings from in vivo, in vitro, and clinical studies regarding metformin for the treatment of adult NAFLD patients with diabetes. Our review focuses on the efficacy of metformin in treating liver steatosis. Consistent and controversial reports regarding the mechanisms responsible for the effect of metformin on NAFLD development are also discussed. Relevant publications in the PubMed database were included in this review, the search terms used being “metformin” and “NAFLD,” “NASH” and “diabetes.” Only the articles published in English were reviewed.
EFFECTS OF METFORMIN ON LIPOGENESIS REDUCTION IN NAFLD: REPORTS FROM IN VITRO STUDIES
The findings from in vitro studies demonstrated that metformin could reduce lipid accumulation13 and de novo fatty acid synthesis.14-16 Several proteins have been shown to be essential to the regulation of hepatic de novo lipogenesis. For example, the enzyme acetyl-CoA carboxylase (ACC) catalyzes acetyl CoA into malonyl CoA, a precursor for fatty acid hepatic synthesis, ACC playing a vital role in a rate limiting step of lipogenesis.17 Phosphorylation of ACC via AMP-activated protein kinase (AMPK) inhibits the action of ACC, leading to inhibition of lipogenesis.18,19 Metformin increased inhibitory phosphorylation of ACC13 and induced hepatic Rho-kinase 1 (ROCK1) inhibition,16 resulting in AMPK activation and a decrease in lipogenic genes associated with de novo lipogenesis.16 Autophagy restoration via the sirtuin 1 (SIRT1) dependent pathway13 and signal transducer and activation of transcription 3 (STAT3) inhibition20 by metformin has been demonstrated. Anti-apoptotic activity,21 protection against lipid-induced necrotic cell death,21 reduction of oxidative stress21 and inflammatory markers20 were also shown in metformin treated cells. These in vitro reports are summarized in Supplementary Table 1.
EFFECTS OF METFORMIN ON LIPOGENESIS REDUCTION IN NAFLD: REPORTS FROM IN VIVO STUDIES
Several in vivo studies evaluated the effects of metformin on the reduction of hepatic fat content and the mechanism responsible. A variety of studies involving a range of dosages, routes, and durations of metformin treatment in genetically modified mice which exhibit features of hepatic steatosis, or dietary models of NAFLD rats or mice have been performed. Most of the studies demonstrate the effectiveness of intrahepatic lipid reduction by metformin.13,14,16,20,22-28 However, there are a few contradictory reports showing ineffectiveness of metformin treatment.15,29-31 The differences in the NAFLD models used causing the varying degrees of disease severity, and accompanying metabolic derangement could be responsible for the discrepancies. It is observable that among the studies showing negative effects, the models with more severe disease were used, including the use of mice feeding with higher percentage of fat in high fat diet (HFD),15 methionine- and choline-deficient diet,29 Zucker diabetic fatty rat,30 and Goto-Kakizaki rat fed with HFD.31 The dosing and route of metformin administration were also varied between studies, and this could potentially affect the drug absorption with all studies using intraperitoneal route administration showing positive effects.13,14,20
1. Effects of metformin on molecular mechanisms of hepatic steatosis (de novo lipogenesis reduction and increased fatty acid β-oxidation)
Metformin is known to activate AMPK.32 The inhibition of phosphorylation of ACC by AMPK resulting in de novo lipogenesis reduction is one of the most widely mentioned responsible mechanisms.14,16,22,24,30 An ACC knock-in mouse model had increased liver triglyceride (TG) content and increased liver fibrosis.14 Metformin treatment decreased hepatic lipogenesis and liver TG content in wild-type mice but not in ACC knock-in mice. These findings suggested that inhibition of phosphorylation of ACC by AMPK was essential in metformin action.14 Other studies added weight to this by demonstrating increasing AMPK activation and decreasing hepatic TG content in mice treated with metformin.16,22,24,30 It has been proposed that AMPK activation was mediated by ROCK1.16 Lipogenic gene expression of proteins involved in hepatic lipogenesis, including sterol regulatory element-binding protein 1 (SREBP-1c),16,23 ACC,23 fatty acid synthase (FAS)16,23 and stearoyl-CoA desaturase-1 (SCD1)16 were reduced with metformin treatment. It was speculated that these changes were related to the activation of AMPK.16,33
Leptin is an adipose tissue-produced peptide which decreases hepatic de novo fatty acid synthesis and promotes peroxisome proliferator-activated receptor gamma coactivator-1α (PPARα)-dependent fatty acid beta oxidation.34 Circulating leptin levels were found to be higher in NAFLD patients than controls35 and it was proposed that the blunted response of the liver to leptin action was related to hepatic steatosis.36 An enhanced leptin sensitivity by metformin is one of potential mechanisms underlying its steatosis alleviation effect.23 However, a study in Zucker diabetic fatty rats, those with missense mutation in the leptin receptor gene which develop early fatty liver, severe hyperlipidemia, and insulin resistance,37 showed that metformin had no effect on NAFLD.30 This finding may imply the leptin gene is essential for metformin treatment to be effective, or suggest that the extent of the effect of metformin was not enough in the case of a more severe and early onset of disease. Proteins involved in mitochondrial lipid oxidation were up-regulated following metformin treatment,38 suggesting the potential effect of metformin in increasing mitochondrial lipid oxidation. All these reports are summarized in Table 1.
Table 1.
Effects of Metformin on the Molecular Mechanisms of Hepatic Steatosis (De Novo Lipogenesis Reduction and Increased Fatty Acid β-Oxidation): Evidence from In Vivo Reports
| Author (year) | Model (age) | Method | Metformin (dose/route/duration) | Effects of metformin | Interpretations |
|---|---|---|---|---|---|
| Studies showing effective intrahepatic lipid reduction by metformin | |||||
| Fullerton et al. (2013)14 | Male C57BL/6J mice (6 weeks old) | - HFD |
|
|
Inhibitory phosphorylation of ACC by AMPK was essential for controlling lipid metabolism and metformin action. |
|
|
|
|||
| Karavia et al. (2015)24 | Male C57BL/6J mice (10–12 weeks old) | - Western diet |
|
|
Metformin induced phosphorylation of AMPK. |
| Guo et al. (2018)22 | Male C57BL/6J mice (3 weeks old) | - HFD |
|
|
Metformin activated AMPK in vivo. |
| Huang et al. (2018)16 | Male C57BL/6J mice (6 weeks old) | - HFD |
|
|
Metformin inactivated ROCK1, resulting in activation of AMPK signaling. |
|
|
||||
| Tang et al. (2016)23 | Male C57BL/6J mice (4–6 weeks old) | - HFD |
|
|
Metformin dose-dependently enhanced hepatic leptin sensitivity. |
|
|
||||
| Stachowicz et al. (2012)38 | Female C57BL/6J mice (8 weeks old) | - apoE-/- |
|
↑ SCAD, ECHD3, IPYR | Metformin up-regulated protein related fatty acid beta-oxidation. |
| Brandt et al. (2019)26 | Female C57BL/6J mice (6–8 weeks old) | - Fat-fructose-and cholesterol-rich diet |
|
|
Metformin improved liver histology and delayed the development of NAFLD. |
|
|
||||
| Studies showing ineffective intrahepatic lipid reduction by metformin | |||||
| Ford et al. (2015)15 | Male C57BL/6J mice (8 weeks old) | - HFD |
|
|
Metformin had no effect on hepatic TG content and AMPK activation. |
| Sui et al. (2019)30 | Male Zucker diabetic fatty rats (4–8 weeks old) | - fa/fa rats |
|
|
Metformin promoted AMPK activation and correction of gene expression associated with LepR mutation. |
ACC, acetyl-CoA carboxylase; ACC-DKI, ACC double knock-in mutation; AMPK, AMP-activated protein kinase; apoE-/-, apolipoprotein E knock-out mutation; ECHD3, enoyl-CoA hydratase domain-containing protein 3; FAS, fatty acid synthase; G6PDX, glucose-6-phosphate dehydrogenase X-linked; HFD, high fat diet; HMGCS1, 3-hydroxy-3-methylglutaryl-coA synthase 1; IGFBP1, insulin-like growth factor-binding protein-1; IP, intraperitoneal route; IPYR, inorganic pyrophosphatase; LepR, leptin receptor; L-ROCK1-/-, liver-specific ROCK1 deficient mice; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; p-ACC, phosphorylation of acetyl-CoA carboxylase; p-AMPK, phosphorylation of AMPK; PO, per os; ROCK1, Rho-kinase 1; SCAD, short-chain specific acyl-Co A dehydrogenase; SCD1, stearoyl-CoA desaturase-1; SREBP-1c, sterol regulatory element-binding protein 1; TC, total cholesterol contents; TG, triglyceride content; ↓, significant decrease; ↑, significant increase; ↔, no significant change.
2. Effects of metformin on hepatic inflammation, oxidative stress, and fibrosis
Tumor necrosis factor-α (TNF-α) is known to be a mediator of apoptosis and hepatotoxicity.39 It is also involved in NAFLD development and NASH progression.40 The results regarding TNF-α level upon metformin treatment are inconsistent, showing both reduction in20,25,26,31 and neutral TNF-α levels.28,29 Other inflammatory markers, including, inducible nitric oxide synthase (iNOS),25,26 interleukin-1β,20 transforming growth factor β (TGF-β),28 and CD6822 decreased upon metformin treatment. While the markers of inflammation decreased, the oxidative stress parameters glutathione and superoxide dismutase (SOD),25 and the antioxidant protein peroxiredoxin 6 (PRDX-6)38 increased after metformin treatment. These findings provide a potential mechanism for metformin in treatment of NAFLD by alleviating inflammation in the liver and decreasing oxidative stress. All these reports are summarized in Table 2.
Table 2.
Metformin Effects on Hepatic Inflammation, Oxidative Stress, and Fibrosis: Evidence from In Vivo Reports
| Author (year) | Model (age) | Method | Metformin (dose/route/duration) | Effects of metformin | Interpretations |
|---|---|---|---|---|---|
| Studies showing effective intrahepatic lipid reduction by metformin | |||||
| Stachowicz et al. (2012)38 | Female C57BL/6J mice (8 weeks old) | - apoE-/- | - 10 mg/kg/day/PO/16 weeks |
|
Metformin up-regulated antioxidant protein. |
| Guo et al. (2018)22 | Male C57BL/6J mice (3 weeks old) | - HFD |
|
|
Metformin reduced macrophage content in the liver. |
| Khalaf et al. (2019)25 | Male albino rats (200–250 g weight) | - 10% fructose in drinking water | - 300 mg/kg/day/PO/4 weeks |
|
Metformin reduced oxidative stress and inflammatory mediators. |
| Li et al. (2019)20 | Male C57BL/6J mice (6 weeks old) | - MCD diet | - 250 mg/kg/day/IP/4 weeks |
|
Metformin reduced hepatic inflammatory markers. |
| de Jesús Acosta-Cota et al. (2019)28 | Male Wistar rats (4 weeks old) | - 50% sucrose (wt/vol) in drinking water |
|
|
Metformin decreased hepatic cholesterol contents and TGF-β. |
| Brandt et al. (2019)26 | Female C57BL/6J mice (6–8 weeks old) | - Fat/fructose-and cholesterol-rich diet | - 300 mg/kg/day/PO/4 days |
|
Metformin treatment decreased inflammatory cell infiltration and TNF-α in the liver. |
| - 300 mg/kg/day/PO/6 weeks |
|
||||
| Studies showing ineffective intrahepatic lipid reduction by metformin | |||||
| Matafome et al. (2011)31 | Goto-Kakizaki rats (8 weeks old) | - HFD |
|
|
Metformin decreased TNF-α in liver. |
| Mahzari et al. (2019)29 | Male C57BL/6J mice (10 weeks old) | - MCD diet | - 250 mg/kg/day/PO/6 weeks |
|
Metformin did not improve NASH features and proteins associated with profibrotic pathways. |
apoE-/-, apolipoprotein E knock-out mutation; CRP, C-reactive protein; GSH, glutathione; HFD, high fat diet; IHC, immunohistochemical staining; IL, interleukin; IP, intraperitoneal route; iNOS, inducible nitric oxide synthase; MCD, methionine-and choline-deficient diet; MDA, malondialdehyde; NASH, nonalcoholic steatohepatitis; PRDX-6, peroxiredoxin 6; PO, per os; SOD, superoxide dismutase; TC, total cholesterol contents; TG, triglyceride content; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; ↓, significant decrease; ↑, significant increase; ↔, no significant change.
3. Direct degradation of intracellular lipid by autophagy induction
The induction of autophagy enables cells to reutilize their own constituents for energy, one of the approaches for NAFLD treatment.41 The downregulation of SIRT1 expression and autophagy induction in the liver of ob/ob mice were restored following treatment with metformin.13 Additionally, metformin was shown to inhibit the STAT3 pathway,20 the pathway in which inhibition also induced autophagy.42 All these reports are summarized in Table 3.
Table 3.
Metformin Effects on the Direct Degradation of Intracellular Lipid by Autophagy Induction: Evidence from In Vivo Reports
| Author (year) | Model (age) | Method | Metformin (dose/route/duration) | Effects of metformin | Interpretations |
|---|---|---|---|---|---|
| Studies showing effective intrahepatic lipid reduction by metformin | |||||
| Song et al. (2015)13 | C57BL/6J mice (8 weeks old) |
|
- 300 mg/kg/day/IP/4 weeks |
|
Metformin restored SIRT1 expression and induced autophagy. |
| Li et al. (2019)20 | Male C57BL/6J mice (6 weeks old) |
|
- 250 mg/kg/day/IP/4 weeks |
|
Metformin inactivated STAT3 signaling pathway and reversed autophagy inhibition. |
IP, intraperitoneal route; MCD, methionine-and choline-deficient diet; SIRT1, sirtuin 1; STAT3, signal transducer and activator of transcription 3; TG, triglyceride content; ↓, significant decrease; ↑, significant increase.
4. Other proposed mechanisms of metformin
Apolipoprotein A-I (ApoA-I) may be involved in the treatment effects of metformin as its deficiency increased mice sensitivity to diet-induced obesity43 and blunted the beneficial effect of metformin on liver lipid content.24 Metformin alters the enzymes and genes associated with NAFLD development. Deficiency of the enzyme glycine N-methyltransferase (GNMT) which has a crucial role in NAFLD development,44 was up-regulated upon treatment.38 It also induced transcriptome alteration which is negatively correlated with liver disease and injuries,22 and induced changes in gene expression associated with the NAFLD phenotypes.22
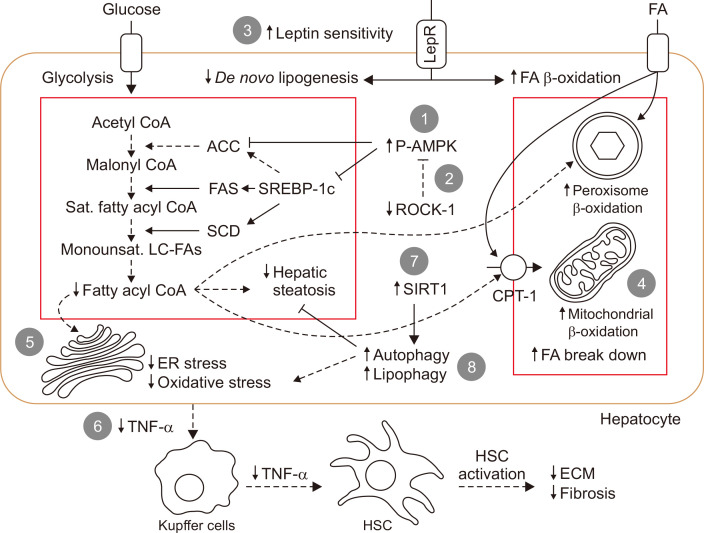
Intestinal dysbiosis and gut barrier function were found to be associated with the development of NAFLD.45 The mechanism behind the protective effects of metformin against NAFLD development could be partly due to the modulation of the population of intestinal microbiota,27 protection against tight junction protein loss,26 and the reduction of bacterial endotoxins.26,27 All these reports are summarized in Supplementary Table 2. A summary of in vivo and in vitro reports, regarding the mechanisms behind the action of metformin and lipogenesis reduction in the NAFLD model is also shown in Fig. 1.
Fig. 1.
Mechanism of action of metformin in nonalcoholic fatty liver disease. (A) Decrease in de novo lipogenesis: (1) AMPK activation and increase inhibitory phosphorylation of ACC; (2) inhibition of ROCK-1 by metformin resulting in inhibitory phosphorylation of ACC; and (3) increase in leptin sensitivity attenuates de novo lipogenesis pathway. Decreasing fatty acyl CoA also decreases hepatic steatosis, decreases lipid-induced ER stress and decreases substrate for FA β-oxidation. (B) Increase in FA β-oxidation: (3) increase in leptin sensitivity induces PPARα-dependent FA β-oxidation; (4) up-regulation of proteins involved in mitochondrial lipid oxidation by metformin results in increased FA breakdown and energy combustion. (C) Decrease in inflammation and HSC activation: (5) decreased lipid-induced ER stress and oxidative stress due to decreased de novo lipogenesis; (6) TNF-α reduction decreases Kupffer cell and HSC activation resulting in reducing inflammation and fibrosis in the liver. (D) Direct degradation of intracellular lipid: (7, 8) induction of autophagy by restoration of SIRT1 activity causing lipolysis by lysosome (lipophagy).
ACC, acetyl-CoA carboxylase; CPT-1, carnitine palmitoyltransferase; ECM, extracellular matrix proteins; ER, endoplasmic reticulum; FA, fatty acid; FAS, fatty acid synthase; HSC, hepatic stellate cells; LepR, leptin receptor; Monounsat. LC-FAs, monounsaturated long-chain FAs; P-AMPK, phosphorylated AMP-activated protein kinase; PPARα, peroxisome proliferator-activated receptor gamma coactivator-1α; ROCK1, Rho-kinase 1; Sat., saturated; SCD, stearoyl-CoA desaturase; SIRT1, sirtuin 1; SREBP-1c, sterol regulatory element-binding protein 1; TNF-α, tumor necrosis factor.
EFFECTS OF METFORMIN ON THE LIVER IN NAFLD PATIENTS WITH DIABETES
Preclinical studies showed remarkable improvement in liver histology and in the reduction of hepatic fat content following treatment with metformin as mentioned earlier. Therefore, metformin was expected to be a promising medication against NASH. However, metformin had limited impact in clinical studies among NAFLD or NASH patients without diabetes.7,8,12 The question remained to be answered is whether metformin treatment in NAFLD patients with diabetes could provide any clinical benefit since it is accepted as a safe medication and is widely used. Here we summarized available clinical reports on NAFLD patients with diabetes, including the effects of metformin as a monotherapy, comparison of metformin to other antidiabetic medications and as part of a combination treatment.
1. Effects of metformin as a monotherapy in NAFLD patients with diabetes
The efficacy of metformin monotherapy in the NAFLD population with diabetes has rarely been evaluated in a randomized-controlled study. The majority of the clinical studies were conducted with the primary aim of comparing the effects of metformin with other antidiabetic medications. No placebo-controlled study has been conducted at this time. In eight studies that reported the effects of metformin in diabetic NAFLD patients compared to the pretreatment baseline condition, the patients were treated with metformin at dosages ranging from 1,000 to 2,000 mg/day for 12 to 48 weeks.46-53 These studies had various methods of NAFLD diagnosis, including ultrasonographic assessment of hepatic steatosis, a quantitative ultrasonographic method, or a liver/spleen computed tomography ratio (L/S CT ratio) of less than 1. One study included the patients who underwent liver biopsy and were diagnosed as NASH.51
Metformin was shown to be beneficial in patients with NAFLD compared to baseline. Five studies showed that metformin treatment for 12 to 24 weeks reduced the body mass index (BMI), liver fat content, liver enzymes, and hemoglobin A1c (HbA1c) and improved insulin resistance in NAFLD patients with type 2 diabetes mellitus (T2DM).46-50 A prospective study in 11 patients with new-onset T2DM showed lower amounts of fat in the liver after 16 weeks of metformin treatment.53 However, one study reported inconsistent findings. In this small study with 16 participants treated with metformin for 24 weeks, increased liver fat content was demonstrated, and no beneficial effects of metformin were found on the BMI, transaminase level, and HbA1c.52 The possible cause of this conflicting result could be due partly to the limited number and type of patients enrolled. In this study, the enrolled patients were older than other studies (mean age of 60 years) with slightly lower baseline HbA1c compared to other studies (mean HbA1c of 7.4%). These patients’ characteristics suggest longer NAFLD disease duration and less insulin resistance in the enrolled patients. Future studies are needed to test this hypothesis.
Metformin treatment significantly decreased liver fibrosis evaluated by noninvasive measurement in NAFLD patients.46 However, inconsistent report exists. No significant improvement of fibrosis was demonstrated in histologic NASH patients (n=10) evaluated by liver biopsy.51 The number of patients included in this study was small, which could limit the study power. Since this study also enrolled both T2DM and impaired glucose tolerance patients, the mean HbA1c at baseline (5.8%) was lower than other studies. Several studies already showed that metformin was ineffective among patients without diabetes,7,8,12 thus further investigations in diabetes population are required. All these reports are summarized in Table 4.
Table 4.
Effects of Metformin on the Liver in Diabetic NAFLD Patients
| Author (year) | Populations | Method of NAFLD diagnosis | Design | Metformin, PO (dose/duration) | Major findings (compared to baseline) | Interpretations | |||
|---|---|---|---|---|---|---|---|---|---|
| Body anthropometry | Liver fat contents | Biochemistry | Liver fibrosis and histology | ||||||
| Studies showing benefit of metformin use | |||||||||
| Fan et al. (2013)47 | T2DM with NAFLD (n=68) | US | Randomized study | 1,000-2,000 mg/day/12 weeks |
|
- |
|
- | Metformin was able to improve hepatic enzymes. |
| Feng et al. (2017)48 | T2DM with NAFLD (n=29) | Quantitative US with IHF ≥10% | Randomized open-label study | 2,000 mg/day/24 weeks |
|
|
- | Metformin decreased liver aminotransferase level and hepatic fat content. | |
| Zhang et al. (2017)46 | T2DM with NAFLD (n=50) | CT (L/S CT ratio <1) | Randomized study | 1,500 mg/day/24 weeks |
|
|
|
|
Metformin was effective in reducing liver enzymes, fat content and hepatic fibrosis. |
| Yabiku et al. (2017)49 | Male T2DM with NAFLD (n=92) | US | Randomized study | 1,000 mg/days/24 weeks |
|
|
|
- | Metformin improved liver enzymes and hepatic fat content. |
| Tian et al. (2018)50 | T2DM with NAFLD (n=75) | US | Randomized study | 1,000-1,500 mg/day/ 12 weeks |
|
- |
|
- | Metformin decreased liver enzymes. |
| Zsóri et al. (2019)53 | New-onset T2DM (n=11) | CT | Prospective study | 1,000 mg/day/16 weeks |
|
↑ CT radiation absorption* |
|
- | Metformin treatment lowered the amount of fat in the liver of patients with new-onset T2DM. |
| Studies showing uncertain/negative results of metformin use | |||||||||
| Omer et al. (2010)51 | T2DM or IGT with NASH and elevated ALT (n=22) | Histologic diagnosis of NASH (NAS ≥5) | Open-label randomized study | 1,700 mg/day/48 weeks |
|
- |
|
|
Metformin did not improve transaminase levels and histological NASH score. |
| Shibuya et al. (2018)52 | T2DM with NAFLD (n=16) | US or CT | Randomized open-label study | 1,500 mg/day/24 weeks |
|
|
|
- | Metformin had no beneficial effects on transaminase level and hepatic fat content. |
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; HbA1c, hemoglobin A1c; HOME-IR, homeostatic model assessment for insulin resistance; IGT, impaired glucose tolerance; IHF, intrahepatic fat; L/S CT ratio, liver/spleen liver/spleen computed tomography (CT) ratio; LSM, liver stiffness measurement; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; PO, per os; T2DM, type 2 diabetes mellitus; US, ultrasonography; WC, waist circumference; ↓, significant decrease; ↑, significant increase; ↔, no significant change.
*An asterisk symbol in the major finding column indicates the primary outcomes in that report.
Similar to findings from preclinical studies, metformin use is associated with decreased liver fat content in diabetic patients. However, the effect is not as prominent as the effect shown in the rodent studies as the liver histology improvement was not replicated. It is unclear why there is a difference between animal and human studies. We speculate that this might be due to the uniform pattern of fatty phenotype in the animals studied, and the difference in the pharmacokinetics of metformin between species. Animal studies with an NAFLD model include genetically modified mice or mice fed with a diet promoting the development of a fatty liver. These models generate uniform fatty rodents and the effect of metformin might be seen more clearly than in a human study in which the participants had various degrees of severity of fatty liver and concurrent metabolic derangements. Metformin is taken up in the liver via organic cation transporter-1 (OCT1).54 Hepatic uptake of various drugs via this transporter has been shown to have differ between species, for example between mice and humans.55 To our knowledge, no previous research had explored the species difference of metformin uptake. Therefore, it remains unclear whether our speculation would impact the results. Future research should examine this issue as it is important when projecting animal research results to humans. Histologic outcome should be further evaluated in a larger NAFLD population with diabetes. The effect of long-term treatment of metformin on liver-related adverse events are currently unclear, knowledge surrounding this is desirable.
Most of the recent studies conducted in diabetic NAFLD patients enrolled the patients treated with metformin and allowed metformin continuation during the study. Some studies included both metformin users and nonusers. However, despite modest effects being observed, metformin monotherapy decreased liver transaminases and hepatic fat content. These effects were prominent during the 12 to 24 weeks after administration. Therefore, the conduction of clinical studies should consider this possible effect for patient selection to avoid confounding effects caused by metformin treatment.
2. Effects of metformin compared to other antidiabetic drugs in NAFLD patients with diabetes
In the past decades, new classes of antidiabetic drugs have been approved to be used in T2DM patients. Although metformin as a monotherapy has been shown to reduce hepatic steatosis and improve liver biochemistry in diabetic NAFLD patients, the magnitude of the benefits seems to be more subtle than the newer antidiabetic drugs. These newer agents include thiazolidinediones,49,51 glucagon-like peptide-1 (GLP-1) receptor agonists,47,48,50 and sodium-glucose co-transporter-2 (SGLT2) inhibitors.52
Sitagliptin, a dipeptidyl peptidase-4 inhibitor (DPP4i), had less potency than metformin in reducing liver enzymes.49 Gliclazide, a drug in sulfonylurea class, was able to decrease hepatic fat content but to a lower extent than that observed after metformin therapy.48 A summary of reports comparing the effects of metformin to other antidiabetic drugs on the liver in diabetic NAFLD patients is shown in Table 5.
Table 5.
Effects of Metformin and Other Antidiabetic Drugs on the Liver in Diabetic NAFLD Patients
| Author (year) | Populations | Method of NAFLD diagnosis | Design | Intervention | Major findings | Interpretations | |||
|---|---|---|---|---|---|---|---|---|---|
| Body anthropometry | Liver fat contents | Biochemistry | Liver fibrosis and histology | ||||||
| Fan et al. (2013)47 | T2DM with NAFLD (n=117) | US | Randomized study | Metformin (n=68) |
|
- |
|
- | Exenatide was more effective than metformin in improving hepatic enzymes. |
| Exenatide (n=49) for 12 weeks |
|
- |
|
- | |||||
| Yabiku et al. (2017)49 | Male T2DM with NAFLD (n=366) | US | Randomized study (compared to no treatment; n=91) | Metformin (n=92) |
|
|
|
- | Pioglitazone elicited greatest improvement of liver fat content and hepatic enzymes compared to metformin and Sitagliptin. |
| Pioglitazone (n=91) |
|
|
|
- | |||||
| Sitagliptin (n=92) for 6 months |
|
|
|
- | |||||
| Shibuya et al. (2018)52 | T2DM with NAFLD (n=32) | US or CT | Randomized open-label study | Metformin (n=16) |
|
|
|
- | Luseogliflozin was more effective than metformin in reducing liver fat deposition. |
| Luseogliflozin (n=16) for 6 months |
|
|
|
- | |||||
| Feng et al. (2017)48 | T2DM with NAFLD (n=87) | Quantitative US method with IHF ≥10% | Randomized open-label study | Metformin (n=29) |
|
|
- | Liraglutide provided greater improvement in liver function and IHF contents than metformin. | |
| Liraglutide (n=29) |
|
|
- | ||||||
| Gliclazide (n=29) for 24 weeks |
|
|
- | ||||||
| Tian et al. (2018)50 | T2DM with NAFLD (n=127) | US | Randomized study | Metformin (n=75) |
|
- |
|
- | Liraglutide was more effective than metformin in decreasing ALT levels. |
| Liraglutide (n=52) for 12 weeks |
|
- |
|
- | |||||
| Omer et al. (2010)51 | T2DM or IGT with NASH and elevated ALT (n=42) | Histologic diagnosis of NASH (NAS ≥5) | Open-label randomized study | Metformin (n=22) |
|
- |
|
|
Rosiglitazone was more effective than metformin in improving liver enzymes and histology. |
| Rosiglitazone (n=20) for 48 weeks |
|
- |
|
|
|||||
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; HbA1c, hemoglobin A1c; HOME-IR, homeostatic model assessment for insulin resistance; IGT, impaired glucose tolerance; IHF, intrahepatic fat; L/S CT ratio, liver/spleen liver/spleen computed tomography (CT) ratio; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; PO, per os; SC, subcutaneous injection; T2DM, type 2 diabetes mellitus; US, ultrasonography; WC, waist circumference; ↓, significant decrease; ↑, significant increase; ↔, no significant change.
*An asterisk symbol in the major finding column indicates the primary outcomes in that report.
3. Effects of combined treatment with metformin and other antidiabetic drugs in NAFLD patients with diabetes
A combination of metformin with other antidiabetic drugs in NAFLD patients with diabetes had been studied and reported. Since most of diabetic patients had previously received metformin, enrollment of these patients with other antidiabetic medications being added on was common across most of these studies. Addition of thiazolidinediones,51 GLP-1 receptor agonists,56,57 DPP4i57 and SGLT2i58 all provided additional benefit to metformin as a monotherapy. However, it should be noted that the synergistic effect of metformin added on to other antidiabetic medications has not yet been reported. Insulin glargine treatment did not improve NAFLD parameters further, as insulin treatment did not affect the insulin resistance nor body weight reduction.57 A summary of the reports regarding the effects of a combined treatment with metformin and other antidiabetic drugs on the liver in NAFLD patients with diabetes is shown in Table 6.
Table 6.
Effects of Combined Treatment with Metformin and Other Antidiabetic Drugs on the Liver in Diabetic NAFLD Patients
| Author (year) | Populations | Method of NAFLD diagnosis | Design | Intervention | Major findings | Interpretations | |||
|---|---|---|---|---|---|---|---|---|---|
| Body anthropometry | Liver fat contents | Biochemistry | Liver fibrosis and histology | ||||||
| Omer et al. (2010)51 | T2DM or IGT with NASH and elevated ALT (n=22) | Histologic diagnosis of NASH (NAS ≥5) | Open-label randomized study | Metformin+ Rosiglitazone (n=22) |
|
- |
|
|
Combination of metformin and rosiglitazone improved liver enzymes and liver histology. |
| Choi et al. (2018)58 | T2DM with NAFLD and elevated ALT (n=102) | US | Retrospective study | Metformin+ Dapagliflozin (n=50) |
|
- | - | Combination of metformin and dapagliflozin was more effective in improving liver biochemistry than a combination of metformin and DPP4i. | |
| Metformin+ Sitagliptin or Linagliptin (n=52) |
|
- | - | ||||||
| Cuthbertson et al. (2012)56 | T2DM and NAFLD treated with metformin (n=25) | 1H MRS with IHL >5.5% | Prospective single arm study | Exenatide (n=19) or Liraglutide (n=6) |
|
|
|
- | GLP1-RA added to metformin treatment decreased liver fat content and ALT. |
| Yan et al. (2019)57 | T2DM with NAFLD treated with metformin (n=65) | MRI-PDFF >10% | Randomized study | Liraglutide (n=18) |
|
|
|
|
Combination of Metformin with liraglutide and sitagliptin reduced IHL. |
| Sitagliptin (n=26) |
|
|
|
|
|||||
| Insulin glargine (n=21) |
|
|
|
|
|||||
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; DPP4i, dipeptidyl peptidase-4 inhibitors; FIB-4, fibrosis-4 index; GLP1-RA, glucagon-like peptide-1 receptor agonists; 1H MRS, proton magnetic resonance spectroscopy; HbA1c, hemoglobin A1c; HOME-IR, homeostatic model assessment for insulin resistance; IGT, impaired glucose tolerance; IHL, intrahepatocellular lipid; IL, interleukin; MRI-PDFF, magnetic resonance imaging-estimated proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; NFS, NAFLD fibrosis score; T2DM, type 2 diabetes mellitus; US, ultrasonography; WC, waist circumference; ↓, significant decrease; ↑, significant increase; ↔, no significant change.
*An asterisk symbol in the major finding column indicates the primary outcomes in that report.
EFFECTS OF METFORMIN ON HCC DEVELOPMENT
The use of metformin was associated with a reduced risk of hepatocellular carcinoma (HCC).59 Several epidemiological studies suggested that metformin had potential antitumor effect with potential effects in cancer prevention.60,61 A large matched-paired cohort conducted in Taiwan found that metformin was associated with HCC incidence reduction in patients with T2DM with a hazard ratio of 0.76 (0.67 to 0.85).62 In a mouse model of NASH and liver tumor, metformin decreased the proportion of tumor-carrying mice.63 However, this effect was not observed in the liver in mice that had already developed NAFLD.63 Another study of a HFD-fed, HCC model of transgenic zebrafish demonstrated the HFD enhanced malignancy-related histologic and morphologic features.64 Metformin treatment reduced liver size and reversed the diet-induced increase in steatosis, vessel formation, and inflammation and restored T cell infiltration.64 These results suggested potential benefits of metformin in the prevention of HFD-induced liver tumorigenesis and progression, especially if administered early prior to the onset of NAFLD. Further studies are needed to warrant this benefit of metformin as regards liver cancer.
CONCLUSION AND FUTURE PERSPECTIVES
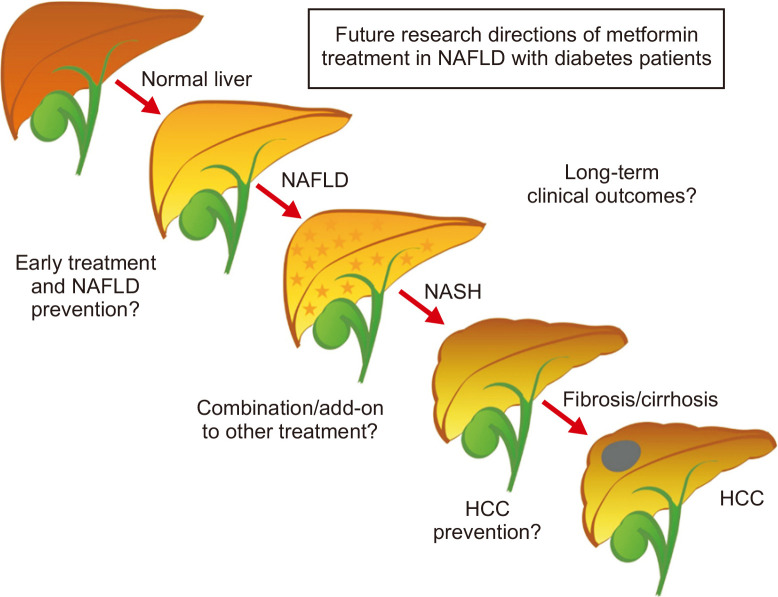
Metformin treatment was shown to be effective in alleviating hepatic lipogenesis in animal models of NAFLD through various mechanisms. However, in clinical studies, metformin could modestly reduce the BMI, liver fat content, and liver enzymes in NAFLD patients with diabetes. Despite these reports on benefits of metformin, some contradicting reports still exist. Combination treatments with other antidiabetic drugs, especially the drugs in the thiazolidinediones, GLP-1 receptor agonists and SGLT2 inhibitors groups demonstrated increased efficacy. Among diabetic patients with biopsy-proven NASH, currently available data from a small enrolled study suggested that metformin was not associated with histologic or liver fibrosis improvement. Further research with a larger sample size is warranted to confirm these findings. A long-term clinical study to evaluate liver-related complications, and a study to elucidate the role of metformin in HCC prevention are necessary. Summaries of the future directions are shown in Fig. 2. Nevertheless, there is a potential benefit in the continued use of metformin in NAFLD patients with diabetes, either alone or in combination with other antidiabetic drugs.
Fig. 2.
Future directions.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma.
Supplemental Materials
ACKNOWLEDGEMENTS
This work was supported by the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (N.C.), and the Chiang Mai University Center of Excellence Award (N.C.).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Lomonaco R, Bril F, Portillo-Sanchez P, et al. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care. 2016;39:632–638. doi: 10.2337/dc15-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haukeland JW, Konopski Z, Eggesbø HB, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 8.Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 10.Duseja A, Das A, Dhiman RK, et al. Metformin is effective in achieving biochemical response in patients with nonalcoholic fatty liver disease (NAFLD) not responding to lifestyle interventions. Ann Hepatol. 2007;6:222–226. doi: 10.1016/S1665-2681(19)31902-7. [DOI] [PubMed] [Google Scholar]
- 11.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 12.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic ateatohepatitis (NASH): a pilot trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song YM, Lee YH, Kim JW, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford RJ, Fullerton MD, Pinkosky SL, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468:125–132. doi: 10.1042/BJ20150125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Lee SH, Sousa-Lima I, et al. Rho-kinase/AMPK axis regulates hepatic lipogenesis during overnutrition. J Clin Invest. 2018;128:5335–5350. doi: 10.1172/JCI63562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc. 2016;91:452–468. doi: 10.1111/brv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34(Pt 2):223–227. doi: 10.1042/BST0340223. [DOI] [PubMed] [Google Scholar]
- 19.Viollet B, Foretz M, Guigas B, et al. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574(Pt 1):41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li YL, Li XQ, Wang YD, Shen C, Zhao CY. Metformin alleviates inflammatory response in non-alcoholic steatohepatitis by restraining signal transducer and activator of transcription 3-mediated autophagy inhibition in vitro and in vivo. Biochem Biophys Res Commun. 2019;513:64–72. doi: 10.1016/j.bbrc.2019.03.077. [DOI] [PubMed] [Google Scholar]
- 21.Geng Y, Hernández Villanueva A, Oun A, et al. Protective effect of metformin against palmitate-induced hepatic cell death. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165621. doi: 10.1016/j.bbadis.2019.165621. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Zhou Y, Cheng Y, et al. Metformin-induced changes of the coding transcriptome and non-coding RNAs in the livers of non-alcoholic fatty liver disease mice. Cell Physiol Biochem. 2018;45:1487–1505. doi: 10.1159/000487575. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, Li J, Xiang W, et al. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J Endocrinol. 2016;230:227–237. doi: 10.1530/JOE-16-0142. [DOI] [PubMed] [Google Scholar]
- 24.Karavia EA, Hatziri A, Kalogeropoulou C, et al. Deficiency in apolipoprotein A-I ablates the pharmacological effects of metformin on plasma glucose homeostasis and hepatic lipid deposition. Eur J Pharmacol. 2015;766:76–85. doi: 10.1016/j.ejphar.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 25.Khalaf HM, Ibrahim MA, Amin EF, Abdel-Tawab Ibrahim S, Abdel-Wahab S, Fouad YM. Allopurinol potentiates the hepatoprotective effect of metformin and vitamin E in fructose-induced fatty liver in rats. Clin Exp Hepatol. 2019;5:65–74. doi: 10.5114/ceh.2019.83159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt A, Hernández-Arriaga A, Kehm R, et al. Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci Rep. 2019;9:6668. doi: 10.1038/s41598-019-43228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin NR, Bose S, Wang JH, et al. Flos Lonicera combined with metformin ameliorates hepatosteatosis and glucose intolerance in association with gut microbiota modulation. Front Microbiol. 2017;8:2271. doi: 10.3389/fmicb.2017.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jesús Acosta-Cota S, Aguilar-Medina EM, Ramos-Payán R, et al. Therapeutic effect of treatment with metformin and/or 4-hydroxychalcone in male Wistar rats with nonalcoholic fatty liver disease. Eur J Pharmacol. 2019;863:172699. doi: 10.1016/j.ejphar.2019.172699. [DOI] [PubMed] [Google Scholar]
- 29.Mahzari A, Li S, Zhou X, et al. Matrine protects against MCD-induced development of NASH via upregulating HSP72 and downregulating mTOR in a manner distinctive from metformin. Front Pharmacol. 2019;10:405. doi: 10.3389/fphar.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sui Y, Kong X, Fan R, et al. Long-term treatment with metformin in the prevention of fatty liver in Zucker diabetic fatty rats. Diabetol Metab Syndr. 2019;11:94. doi: 10.1186/s13098-019-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matafome P, Louro T, Rodrigues L, et al. Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes Metab Res Rev. 2011;27:54–62. doi: 10.1002/dmrr.1157. [DOI] [PubMed] [Google Scholar]
- 32.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism. 2015;64:60–78. doi: 10.1016/j.metabol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59:30–43. doi: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 36.Fishman S, Muzumdar RH, Atzmon G, et al. Resistance to leptin action is the major determinant of hepatic triglyceride accumulation in vivo. FASEB J. 2007;21:53–60. doi: 10.1096/fj.06-6557com. [DOI] [PubMed] [Google Scholar]
- 37.Yokoi N, Hoshino M, Hidaka S, et al. A novel rat model of type 2 diabetes: the Zucker Fatty Diabetes Mellitus ZFDM rat. J Diabetes Res. 2013;2013:103731. doi: 10.1155/2013/103731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stachowicz A, Suski M, Olszanecki R, Madej J, Okoń K, Korbut R. Proteomic analysis of liver mitochondria of apolipoprotein E knockout mice treated with metformin. J Proteomics. 2012;77:167–175. doi: 10.1016/j.jprot.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 40.Kakino S, Ohki T, Nakayama H, et al. Pivotal role of TNF-α in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm Metab Res. 2018;50:80–87. doi: 10.1055/s-0043-118666. [DOI] [PubMed] [Google Scholar]
- 41.Singh R. Autophagy and regulation of lipid metabolism. Results Probl Cell Differ. 2010;52:35–46. doi: 10.1007/978-3-642-14426-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You L, Wang Z, Li H, et al. The role of STAT3 in autophagy. Autophagy. 2015;11:729–739. doi: 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karavia EA, Papachristou DJ, Liopeta K, Triantaphyllidou IE, Dimitrakopoulos O, Kypreos KE. Apolipoprotein A-I modulates processes associated with diet-induced nonalcoholic fatty liver disease in mice. Mol Med. 2012;18:901–912. doi: 10.2119/molmed.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-Chantar ML, Vázquez-Chantada M, Ariz U, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang R, Cheng K, Xu S, et al. Metformin and diammonium glycyrrhizinate enteric-coated capsule versus metformin alone versus diammonium glycyrrhizinate enteric-coated capsule alone in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus. Gastroenterol Res Pract. 2017;2017:8491742. doi: 10.1155/2017/8491742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702–708. doi: 10.1590/S0004-27302013000900005. [DOI] [PubMed] [Google Scholar]
- 48.Feng W, Gao C, Bi Y, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800–809. doi: 10.1111/1753-0407.12555. [DOI] [PubMed] [Google Scholar]
- 49.Yabiku K, Mutoh A, Miyagi K, Takasu N. Effects of oral antidiabetic drugs on changes in the liver-to-spleen ratio on computed tomography and inflammatory biomarkers in patients with type 2 diabetes and nonalcoholic fatty liver disease. Clin Ther. 2017;39:558–566. doi: 10.1016/j.clinthera.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Tian F, Zheng Z, Zhang D, He S, Shen J. Efficacy of liraglutide in treating type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. Biosci Rep. 2018;38:BSR20181304. doi: 10.1042/BSR20181304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 52.Shibuya T, Fushimi N, Kawai M, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438–442. doi: 10.1111/dom.13061. [DOI] [PubMed] [Google Scholar]
- 53.Zsóri G, Illés D, Ivány E, et al. In new-onset diabetes mellitus, metformin reduces fat accumulation in the liver, but not in the pancreas or pericardium. Metab Syndr Relat Disord. 2019;17:289–295. doi: 10.1089/met.2018.0086. [DOI] [PubMed] [Google Scholar]
- 54.Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- 55.Morse BL, Kolur A, Hudson LR, et al. Pharmacokinetics of Organic Cation Transporter 1 (OCT1) substrates in Oct1/2 knockout mice and species difference in hepatic OCT1-mediated uptake. Drug Metab Dispos. 2020;48:93–105. doi: 10.1124/dmd.119.088781. [DOI] [PubMed] [Google Scholar]
- 56.Cuthbertson DJ, Irwin A, Gardner CJ, et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One. 2012;7:e50117. doi: 10.1371/journal.pone.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan J, Yao B, Kuang H, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69:2414–2426. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi DH, Jung CH, Mok JO, Kim CH, Kang SK, Kim BY. Effect of dapagliflozin on alanine aminotransferase improvement in type 2 diabetes mellitus with non-alcoholic fatty liver disease. Endocrinol Metab (Seoul) 2018;33:387–394. doi: 10.3803/EnM.2018.33.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–891. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 60.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HJ, Lee S, Chun KH, et al. Metformin reduces the risk of cancer in patients with type 2 diabetes: an analysis based on the Korean National Diabetes Program Cohort. Medicine (Baltimore) 2018;97:e0036. doi: 10.1097/MD.0000000000010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018;38:2018–2027. doi: 10.1111/liv.13872. [DOI] [PubMed] [Google Scholar]
- 63.Tajima K, Nakamura A, Shirakawa J, et al. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am J Physiol Endocrinol Metab. 2013;305:E987–E998. doi: 10.1152/ajpendo.00133.2013. [DOI] [PubMed] [Google Scholar]
- 64.de Oliveira S, Houseright RA, Graves AL, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70:710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.