Abstract
The Saccharomyces cerevisiae zip1 mutant, which exhibits defects in synaptonemal complex formation and meiotic recombination, triggers a checkpoint that causes cells to arrest at the pachytene stage of meiotic prophase. Overproduction of either the meiotic chromosomal protein Red1 or the meiotic kinase Mek1 bypasses this checkpoint, allowing zip1 cells to sporulate. Red1 or Mek1 overproduction also promotes sporulation of other mutants (zip2, dmc1, hop2) that undergo checkpoint-mediated arrest at pachytene. In addition, Red1 overproduction antagonizes interhomolog interactions in the zip1 mutant, substantially decreasing double-strand break formation, meiotic recombination, and homologous chromosome pairing. Mek1 overproduction, in contrast, suppresses checkpoint-induced arrest without significantly decreasing meiotic recombination. Cooverproduction of Red1 and Mek1 fails to bypass the checkpoint; moreover, overproduction of the meiotic chromosomal protein Hop1 blocks the Red1 and Mek1 overproduction phenotypes. These results suggest that meiotic chromosomal proteins function in the signaling of meiotic prophase defects and that the correct stoichiometry of Red1, Mek1, and Hop1 is needed to achieve checkpoint-mediated cell cycle arrest at pachytene.
Checkpoints maintain the integrity of the genome by ensuring the proper sequence of events during the cell division cycle (16). The dependency of later events in the cycle on the successful completion of earlier events prevents chromosome loss and missegregation leading to aneuploidy. Checkpoints also operate in meiosis, a specialized cell division that generates haploid gametes through two rounds of chromosome segregation.
Prior to the first meiotic division, homologous chromosomes pair, undergo high levels of genetic exchange, and become closely connected along their lengths by the synaptonemal complex (SC) (37). The SC consists of two lateral elements, corresponding to the individual chromosome cores, linked through a central region. Chromatin bridges, called chiasmata, form at the sites of recombination between homologs, and these connections persist after the SC has disassembled. Chiasmata ensure that chromosomes align on the meiosis I spindle such that homologs segregate to opposite poles at meiosis I. Correct reductional chromosome segregation also depends on synapsis (i.e., SC formation) between homologous chromosomes. The importance of recombination and synapsis to proper meiotic chromosome segregation is underscored by the existence of a checkpoint that monitors these processes and arrests cells at the pachytene stage of meiotic prophase in response to defects (4, 23, 26, 37, 51, 59).
Several yeast mutants delay or arrest at pachytene because of the checkpoint, including zip1, zip2, dmc1, and hop2 (4, 9, 23, 51). The zip1 mutant lacks a major component of the SC central region and arrests with homologously paired but unsynapsed chromosomes (51). zip1 mutant cells sustain wild-type levels of double-strand breaks (DSBs) (59), the initiators of meiotic recombination events; however, zip1 mutant cells are defective in processing double Holliday junctions into mature crossover products (49), and ∼10% of DSBs remain unrepaired (49, 59). Like zip1, the zip2 mutant arrests in pachytene with paired but unsynapsed chromosomes (9). The Zip2 protein is thought to act at sites of synaptic initiation to promote Zip1 assembly (9). The dmc1 mutant lacks a meiosis-specific homolog of the Escherichia coli RecA protein (4); dmc1 mutant cells exhibit hyperresected 5′ ends of DSBs (4) and defects in the progression from DSBs to double Holliday junctions (44). Also, chromosome synapsis is delayed in the dmc1 mutant (35). The hop2 mutant, like dmc1, is defective in DSB processing; in contrast to dmc1, however, hop2 mutant cells arrest with extensive synapsis between nonhomologous chromosomes (23). In the zip1, zip2, and dmc1 mutants, differences in yeast strain background determine whether cells arrest at pachytene or whether some cells complete sporulation after a delay in meiotic prophase progression (4, 9, 35, 49, 52).
Several observations indicate that the pachytene arrest of the zip1, zip2, dmc1, and hop2 mutants is due to the operation of a checkpoint rather than to a mechanical block in the meiotic cell cycle. First, the arrest of each of these mutants is alleviated by mutations that prevent the initiation of recombination and synapsis, and thereby prevent formation of the intermediates that trigger arrest (4, 8, 9, 23, 51). Second, pachytene arrest is bypassed by mutations in any one of several genes (RAD24, RAD17, MEC1) required to arrest the mitotic cell cycle in response to unrepaired DSBs and other types of DNA damage (26). Third, cells that arrest because of the pachytene checkpoint retain viability and can resume vegetative growth when returned to growth medium (4, 51).
Mutations in the meiosis-specific genes RED1, MEK1, and HOP1 allow zip1 to sporulate (59; K.-S. Tung and G. S. Roeder, unpublished data), although these mutations do not completely prevent the initiation of meiotic recombination (19, 31, 32). Red1 is a major component of SC lateral elements and the axial elements that serve as precursors to lateral elements (46). Hop1 colocalizes with Red1 to discrete sites on axial elements; however, Hop1 dissociates as these elements become incorporated into mature SCs (46). Mek1 is a meiosis-specific kinase that colocalizes with Red1 on meiotic chromosomes and phosphorylates Red1 (2, 11). Mek1-dependent phosphorylation of Red1 is required for wild-type levels of meiotic sister chromatid cohesion (2). Bypass of the zip1 arrest by deletion of RED1 or MEK1 led to the speculation that Red1 and Mek1 are required for the formation of a recombination structure that is monitored by the pachytene checkpoint machinery (59).
We have found that overproduction of either Red1 or Mek1, but not Hop1, suppresses checkpoint-induced arrest at pachytene. Red1 overproduction promotes nearly wild-type levels of sporulation in the zip1 mutant, whereas Mek1 overproduction promotes sporulation of a subset of zip1 mutant cells, after a delay. In each case, the checkpoint is inactivated without repairing all DSBs, suggesting that Red1 and Mek1 participate in checkpoint signaling. Cooverproduction of Red1 with Mek1 (or cooverproduction of Hop1 with either Red1 or Mek1) restores checkpoint function, indicating that the correct stoichiometry of these proteins is important for checkpoint function. In addition to inactivating the checkpoint, overproduction of Red1 decreases meiotic recombination in zip1 and in wild type and decreases homologous chromosome pairing in zip1. We speculate that Mek1 and Red1 function in checkpoint signaling and that alterations in Red1 phosphorylation allow defects in meiotic recombination and chromosome synapsis to escape detection by the checkpoint machinery.
MATERIALS AND METHODS
Plasmids.
Plasmids were constructed by using standard protocols (39) and were propagated in E. coli XL1-Blue (Stratagene). pB133 contains the EcoRI-PvuII fragment containing MEK1 from pB124 (32) in YEp352 (18). pJ16 contains the EcoRI-SalI fragment containing MEK1 from pB124 in the EcoRI-SalI sites of the YEp351 polylinker (18). pJ17 contains the EcoRI-SalI fragment containing RED1 from pB86 (46) in the EcoRI-SalI sites of the YEp351 polylinker. The XbaI fragment containing MEK1 from pJ16 was inserted at the XbaI site of pB86 to generate pJ14, which is YEp352 containing both MEK1 and RED1. pJ66, which is YEp352 containing both RED1 and HOP1, was generated by cloning the NheI (filled in)-SalI fragment containing HOP1 from pNH83-2 (21) into the SphI (filled in)-SalI sites of pB86. The NheI (filled in)-SalI fragment containing HOP1 from pNH83-2 was inserted into the SphI (filled in)-SalI sites of pB133 (containing the EcoRI-PvuII fragment of MEK1 in YEp352) to generate pJ70, which is YEp352 containing both MEK1 and HOP1. pAV62 contains the XbaI-EcoRI fragment containing RED1 in YEplac112 (14). pB64 is the XbaI-EcoRI fragment containing RED1 from pB8 (31) in YCp50 (45). The following plasmids have been described: pL15 for hop2::LEU2 (23), pMB116 for zip1::LYS2 (52), pR976 for THR1 (42), pR978 for spo13::ADE2 (31), pJC303-4 for CENIII::URA3 (10), and pNKY422 for dmc1::URA3 (4).
Yeast strains.
Table 1 lists the yeast strains used in this study. Strains were constructed and maintained by using standard procedures (45). Strains used for sporulation, nuclear division, gene conversion, and cytology are isogenic to BR2495 (31). Homozygous mutant strains were constructed by transforming the haploid parents of BR2495 (BR1919-8B and BR1373-6D [31]) with the appropriate plasmid(s) and then mating transformants. Disruptions were confirmed by Southern blot analysis (48). Wild-type and homozygous mutant diploids were then transformed with the appropriate multicopy or single-copy plasmids (see Table 1). BS223 and BS225 were constructed by first transforming haploids with pV180 (53) and then selecting for the red1::ura3-1 allele by plating haploids carrying the red1::URA3 and ura3-1 mutations on medium containing 5-fluoroorotic acid (7).
TABLE 1.
S. cerevisiae strains used in this study
| Straina | Genotype |
|---|---|
| BR2495 | MATa his4-280 leu2-27cyh10ade2-1ura3-1 trp1-1 |
| MATα his4-260 leu2-3,112 CYH10 ade2-1 ura3-1 trp1-289 | |
| BR1919-8B | MATα leu2-3,112 his4-260 ura3-1 ade2-1 thr1-4 trp1-289 |
| BR1373-6D | MATa leu2-27 his4-280 arg4-8 ura3-1 cyh10 ade2-1 thr1-1 trp1-1 |
| BS354 | BR2495 + pB86 (YEp352 RED1) |
| JM152 | BR2495 + YEp351 |
| JM474 | BR2495 + YEp352 |
| MY63 | BR2495 but homozygous zip1::LEU2 |
| JM153 | MY63 + YEp352 |
| JM154 | MY63 + pB133 (YEp352 MEK1) |
| JM155 | MY63 + pB86 (YEp352 RED1) |
| JM76 | MY63 + pNH83-2 (YEp24 HOP1) |
| JM107 | MY63 + YEp24 |
| JM64 | MY63 + pJ14 (YEp352 MEK1 RED1) |
| JM65 | MY63 + pJ70 (YEp352 MEK1 HOP1) |
| JM106 | MY63 + pJ66 (YEp352 RED1 HOP1) |
| PC674 | BR2495 but homozygous zip2::URA3 |
| JM156 | PC674 + YEp351 |
| JM158 | PC674 + pJ17 (YEp351 RED1) |
| JM157 | PC674 + pJ16 (YEp351 MEK1) |
| JM127 | BR2495 but heterozygous thr1-4/THR1 and homozygous dmc1::URA3 |
| JM159 | JM127 + YEp351 |
| JM161 | JM127 + pJ17 (YEp351 RED1) |
| JM160 | JM127 + pJ16 (YEp351 MEK1) |
| JM500 | BR2495 but homozygous hop2::LEU2 |
| JM541 | JM500 + YEp352 |
| JM542 | JM500 + pB133 (YEp352 MEK1) |
| JM543 | JM500 + pB86 (YEp352 RED1) |
| BS272 | BR2495 but spo13::ura3-1/spo13::ADE2 |
| JM117 | BS272 + YEp352 |
| JM119 | BS272 + YEp24 |
| JM116 | BS272 + pB86 (YEp352 RED1) |
| JM115 | BS272 + pB133 (YEp352 MEK1) |
| JM118 | BS272 + pNH83-2 (YEp24 HOP1) |
| BS206 | BR2495 but homozygous spo13::ADE2 zip1::LEU2 |
| JM112 | BS206 + YEp352 |
| JM113 | BS206 + YEp24 |
| JM111 | BS206 + pB86 (YEp352 RED1) |
| JM110 | BS206 + pB133 (YEp352 MEK1) |
| JM114 | BS206 + pNH83-2 (YEp24 HOP1) |
| JM212 | BS206 + pJ14 (YEp352 MEK1 RED1) |
| JM213 | BS206 + pJ70 (YEp352 MEK1 HOP1) |
| JM214 | BS206 + pJ66 (YEp352 RED1 HOP1) |
| BS394 | MATα HIS4 LEU2 CEN3THR1 spo13::ADE2ade2-1ura3-1trp1-289lys2 |
| MATa his4-260 leu2-3,112 CEN3::URA3 thr1-4 spo13::ADE2 ade2-1 ura3-1 trp1-289 lys2 | |
| BS395 | BS394 + YEplac112 |
| BS396 | BS394 + pAV62 (YEplac112 RED1) |
| JM289 | BS394 + pJ104 (YEplac112 MEK1) |
| BS397 | BS394 but homozygous zip1::LYS2 |
| BS398 | BS397 + YEplac112 |
| BS399 | BS397 + pAV62 (YEplac112 RED1) |
| JM290 | BS397 + pJ104 (YEplac112 MEK1) |
| JM445 | BR2495 but homozygous rad50-K181 |
| JM446 | JM445 + YEplac112 |
| JM447 | JM445 + pAV62 (YEplac112 RED1) |
| JM441 | BR2495 but homozygous rad50-K181 red1::LYS2 |
| JM442 | JM441 + YEplac112 |
| JM443 | JM441 + pAV62 (YEplac112 RED1) |
| JM437 | BR2495 but homozygous rad50-K181 zip1::LYS2 |
| JM438 | JM437 + YEplac112 |
| JM439 | JM437 + pAV62 (YEplac112 RED1) |
| BS223 | BR2495 but homozygous red1::ura3-1 zip1::LEU2 + pB86 (YEp352 RED1) |
| BS225 | BR2495 but homozygous red1::ura3-1 zip1::LEU2 + pB64 (CEN RED1 URA3) |
Reciprocal recombination and DSBs were measured in strains in which both haploid parents are isogenic to BR1919-8B. BR1919-8Ba that is His+ Leu+ was transformed with pR976 and pJC303-4. This strain and BR1919-8Bα were transformed either with pR978 (to generate the haploid parents of BS394) or with both pR978 and pMB116 (to generate the haploid parents of BS397) and then appropriate transformants were mated. Haploid strains that are rad50S::URA3 were mated to form JM445. Strains that are homozygous mutant rad50S::URA3 zip1::LYS2 (JM437) or rad50S::URA3 red1::LYS2 (JM441) were constructed from crosses between rad50S::URA3 haploids and zip1::LYS2 (51) or red1::LYS2 haploids (47).
Sporulation and nuclear division.
Three or four independent transformants from each strain were grown to saturation in 2 ml of 2× synthetic complete medium lacking either uracil or leucine. Cells (1.5 ml) were pelleted, resuspended in 2 ml of yeast extract-peptone-dextrose (45) supplemented with 60 μg of adenine per ml and 40 μg of uracil per ml, and grown for an additional 10 h. Samples (1.5 ml) of each culture were then collected, washed once with water, and resuspended in 10 ml of sporulation medium (2% potassium acetate) in 250-ml flasks. Cultures were incubated at 30°C with shaking. At the indicated time points, cells were analyzed for sporulation by light microscopy. Additionally, 180 μl of culture was removed at each time point and added to an Eppendorf tube containing 20 μl of 37% formaldehyde. After incubation at 4°C for 3 days, aliquots of each fixed culture were placed onto glass slides, allowed to air dry, washed in phosphate-buffered saline (39), and stained with 4′-6′-diamidino-2-phenylindole (DAPI). Nuclear division was assessed by fluorescence microscopy by using a Leica DMRB microscope. Sporulation and nuclear division were scored for 300 cells per culture per time point.
Recombination assays.
Gene conversion was measured as the frequency of prototroph formation in heteroallelic diploids after 3 days of sporulation. For each strain, three or four independent transformants were grown and sporulated as described above. Recombinants were selected on solid medium lacking uracil and histidine, uracil and threonine, or uracil and tryptophan to select for meiotic gene convertants carrying the overexpression plasmid. The meiotic frequency of prototroph formation was calculated for each culture; average values for each strain were then calculated. Physical isolation of spores was performed as described (33). Reciprocal recombination was measured in dyads produced from spo13 mutants as described (30).
DSB assay.
Strains were grown and sporulated as described above. After various time points in sporulation medium, cultures were harvested and analyzed by Southern blotting of pulsed-field gels (12). A DNA fragment containing THR4 (15) was labelled with 32P with the Redi-Prime II kit (Amersham) and was used as probe. Signal intensity was calculated by using Multi-Analyst software for the Bio-Rad Imaging Densitometer (Bio-Rad). The intensity of meiotic DSBs (corresponding to fragments smaller than the intact chromosome III fragments) was analyzed and compared to the total intensity of chromosome III DNA (DSBs plus intact chromosome III). The discrete band that migrates faster than the intact chromosome III corresponds to the fragment from the end of the chromosome to the THR4 DSB hotspot; smaller fragments are generated by DSBs centromere distal to the THR4 hotspot.
Cytology.
Meiotic nuclei were surface spread as described (9) and then incubated simultaneously with a chromosome III probe for fluorescent in situ hybridization (FISH) (9) and antibodies as described (23). Anti-Red1 antibodies (46) were used at a 1:100 dilution. For each strain, 50 spread nuclei that displayed anti-Red1 staining were scored for chromosome III pairing.
A modification of the terminal deoxynucleotidyltransferase-mediated nick end labeling (TUNEL) assay (17) was used to detect meiotic DSBs. Meiotic chromosomes were surface spread and then slides were incubated at 37°C with terminal deoxytransferase (Tdt) (Amersham Pharmacia Biotech Inc.) and nucleotides conjugated to digoxigenin (Boehringer Mannheim). Labeling was carried out according to the manufacturer's instructions, but for a longer time period (12 to 15 h). Slides were washed in 0.4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and then incubated with antitubulin antibody (YOL1/34; Sera-lab) at 1:50 dilution for 2 h at room temperature. Tubulin staining was detected by using secondary antibody coupled to fluorescein isothiocyanate (Jackson ImmunoResearch), and digoxigenin-labeled nucleotides were detected with antidigoxigenin antibody coupled to rhodamine (Boehringer Mannheim); chromosomal DNA was visualized with DAPI. Rabbit anti-Rad51 antibody (5) was used at a 1:400 dilution. For each strain, formation of Tdt-labeled foci or Rad51 foci was examined in at least 50 mononucleate cells and in at least 50 cells that exhibited meiotic spindles.
RESULTS
Overproduction of Red1 or Mek1 suppresses the zip1 sporulation defect.
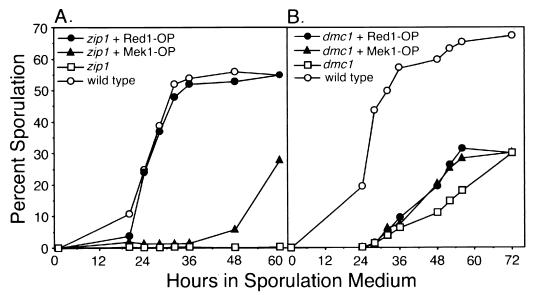
A screen for the Kluyveromyces lactis homolog of the ZIP1 gene identified the K. lactis RED1 gene as a high-copy-number suppressor of the zip1 sporulation defect (47). This observation raised the possibility that overexpression of the Saccharomyces cerevisiae RED1 gene might also allow the zip1 mutant to sporulate. To test this possibility, the RED1 gene was inserted into a plasmid containing the 2μm circle origin of DNA replication; such a plasmid is maintained in 20 to 50 copies per yeast cell (18). Overproduction of S. cerevisiae Red1 promotes wild-type levels of sporulation in the zip1 mutant in the BR2495 strain background (Fig. 1A; Table 2).
FIG. 1.
Sporulation of zip1 and dmc1 mutant strains overproducing Red1 or Mek1. (A) zip1 + Red1-OP, zip1 mutant overproducing Red1 (JM155); zip1 + Mek1-OP, zip1 mutant overproducing Mek1 (JM154); zip1, zip1 mutant carrying vector only (YEp352) (JM153); wild type, wild-type strain containing vector only (JM474). (B) dmc1 + Red1-OP, dmc1 mutant overproducing Red1 (JM161); dmc1 + Mek1-OP, dmc1 mutant overproducing Mek1 (JM160); dmc1, dmc1 mutant carrying vector only (YEp351) (JM159); wild type, wild-type strain containing vector only (JM152). Percent sporulation was calculated from triplicate cultures harvested at the times indicated; values shown are averages.
TABLE 2.
Overproduction of Red1 or Mek1 promotes sporulation of the zip1 mutant
| Strain | Mutant genotype | Sporulationa (%) | Nuclear divisionb (%) | Spore viabilityc (%) |
|---|---|---|---|---|
| JM474 | Wild type + YEp352 | 66.7 | 67.9 | 98.0% |
| JM153 | zip1 + YEp352 | <1.0 | <1.0 | NDd |
| JM107e | zip1 + YEp24 | <1.0 | <1.0 | ND |
| JM155 | zip1 + pB86 (YEp352 RED1) | 66.2 | 66.7 | 2.7 |
| JM76 | zip1 + pNH83-2 (YEp24 HOP1) | <1.0 | <1.0 | ND |
| JM154 | zip1 + pB133 (YEp352 MEK1) | 28.0 | 28.2 | 15.9 |
| JM64 | zip1 + pJ14 (YEp352 MEK1 RED1) | <1.0 | <1.0 | ND |
| JM65 | zip1 + pJ70 (YEp352 MEK1 HOP1) | <1.0 | <1.0 | ND |
| JM106 | zip1 + pJ66 (YEp352 RED1 HOP1) | <1.0 | <1.0 | ND |
Includes dyads, triads, and tetrads, measured after 72 h in sporulation medium.
Cells that have undergone meiosis I or meiosis I and II after 72 h in sporulation medium.
Based on dissection of 70 tetrads per strain.
ND, not determined.
Strain JM107 is the control for strain JM76; strain JM153 is the control for strains JM155, JM154, JM64, JM65, and JM106.
Because Red1 interacts with Hop1 and Mek1 (2, 11, 21), multicopy plasmids carrying HOP1 or MEK1 were tested for suppression of the zip1 sporulation defect. Hop1 overproduction fails to bypass the zip1 arrest (Table 2). Mek1 overproduction promotes sporulation of a subset of zip1 mutant cells, though with a delay (Fig. 1A; Table 2). However, overproduction of a mutant Mek1 protein that exhibits little or no protein kinase activity (Mek1-D290A [2]) does not promote sporulation of the zip1 mutant (data not shown), suggesting that excess Mek1 kinase activity contributes to the bypass of checkpoint-mediated arrest.
In strains in which the zip1 mutant sporulates (such as the fast-sporulating strain SK-1), spore viability is ∼50% (52, 55). In a strain in which the zip1 mutant arrests at pachytene (BR2495) (51), overproduction of Red1 or Mek1 promotes sporulation but decreases spore viability to ∼3 and 16%, respectively (Table 2).
Overproduction of Red1 or Mek1 promotes sporulation of mutants that arrest at the pachytene checkpoint.
To determine whether the overproduction phenotypes observed are specific to the zip1 mutant, the effect of Red1 or Mek1 overproduction was tested in other mutants that arrest at pachytene. Overproduction of either Red1 or Mek1 increases the sporulation frequency of the zip2 mutant (Table 3). In the dmc1 mutant, overproduction of either Red1 or Mek1 increases the rate of sporulation, but not the overall amount of sporulation (Fig. 1B; Table 3). The arrest of the hop2 mutant is partially suppressed by overproduction of Red1 or Mek1 (Table 3). In contrast, overproduction of Red1 or Mek1 does not promote sporulation of the sep1 mutant (data not shown), consistent with previous results indicating that sep1 arrest is triggered by signals different than those that activate the pachytene checkpoint (54). Mutants that arrest with meiotic recombination completed and chromosomes fully synapsed, such as top2 (38) and ndt80 (58), also do not sporulate when Red1 or Mek1 is overproduced (data not shown).
TABLE 3.
Overproduction of Red1 or Mek1 promotes sporulation of mutants that arrest at the pachytene checkpoint
| Strain | Mutant genotype | Sporulationa (%) | Nuclear divisiona (%) |
|---|---|---|---|
| JM156 | zip2 + YEp351b | 9.4 | 9.4 |
| JM158 | zip2 + pJ17 (YEp351 RED1) | 66.1 | 64.2 |
| JM157 | zip2 + pJ16 (YEp351 MEK1) | 32.0 | 27.6 |
| JM159 | dmc1 + YEp351 | 29.8 | 31.2 |
| JM161 | dmc1 + pJ17 (YEp351 RED1) | 29.8 | 28.0 |
| JM160 | dmc1 + pJ16 (YEp351 MEK1) | 30.1 | 33.0 |
| JM541 | hop2 + YEp352 | 0 | 0 |
| JM543 | hop2 + pB86 (YEp352 RED1) | 20.0 | 19.2 |
| JM542 | hop2 + pB133 (YEp352 MEK1) | 6.6 | 7.0 |
Measured after 72 h in sporulation medium.
Strain JM156 is the control for JM158 and JM157, strain JM159 is the control for JM161 and JM160, and strain JM541 is the control for JM543 and JM542.
Overproduction of Red1, but not Mek1, decreases meiotic recombination.
Most of the spores produced in zip1 mutant strains overproducing Red1 or Mek1 are inviable (Table 2), raising the possibility that recombination in these strains occurs at reduced levels. To test this possibility, both gene conversion and crossing over were measured in zip1 mutant strains overproducing Red1 or Mek1. Gene conversion was assayed by measuring prototroph formation in return-to-growth experiments, by using zip1 BR2495 strains. Crossing over was measured in the BR1919-8B strain background, in which the zip1 mutant sporulates, though sporulation is delayed and occurs with reduced efficiency relative to wild type. To improve the accuracy of recombination measurements, a spo13 mutation was introduced to improve spore viability. spo13 mutants undergo a single (predominantly equational) meiotic division to generate dyads containing diploid spores (22).
The zip1 spo13 double mutant displays approximately the same levels of gene conversion as wild-type strains (51); however, overproduction of Red1 in zip1 spo13 reduces gene conversion three- to fivefold (Table 4). Crossing over in the zip1 spo13 double mutant is decreased about twofold relative to the wild type (51, 55); crossing over in zip1 spo13 strains overproducing Red1 is decreased an additional 2.6-fold on average (Table 5). In a wild-type strain (i.e., ZIP1 spo13), Red1 overproduction decreases both gene conversion and crossing over approximately two- to threefold compared to a wild-type strain carrying vector only (Tables 4 and 5). These data indicate that Red1 overproduction decreases meiotic recombination both in the wild type and the zip1 mutant.
TABLE 4.
Overproduction of Red1 decreases gene conversion
| Strain | Mutant genotype | Prototrophsa
|
||
|---|---|---|---|---|
| Histidine | Threonine | Tryptophan | ||
| JM117 | spo13 + YEp352c | 41.0 | 2.2 | 1.9 |
| JM116 | spo13 + pB86 (YEp352 RED1) | 22.0 (1.8)b | 0.9 (2.4) | 1.0 (1.9) |
| JM115 | spo13 + pB133 (YEp352 MEK1) | 39.0 (1.0) | 2.2 (1.0) | 1.8 (1.0) |
| JM119 | spo13 + YEp24 | 37.0 | 2.4 | 2.2 |
| JM118 | spo13 + pNH83-2 (YEp24 HOP1) | 28.0 (1.3) | 1.6 (1.5) | 1.5 (1.5) |
| JM112 | spo13 zip1 + YEp352 | 36.4 | 1.7 | 6.1 |
| JM111 | spo13 zip1 + pB86 (YEp352 RED1) | 6.2 (5.6) | 0.4 (3.6) | 1.5 (4.7) |
| JM110 | spo13 zip1 + pB133 (YEp352 MEK1) | 35.5 (1.0) | 1.6 (1.1) | 4.3 (1.4) |
| JM212 | spo13 zip1 + pJ14 (YEp352 MEK1 RED1) | 25.2 (1.4) | 1.1 (1.5) | 3.8 (1.6) |
| JM213 | spo13 zip1 + pJ70 (YEp352 MEK1 HOP1) | 38.6 (0.94) | 1.3 (1.3) | 4.4 (1.4) |
| JM214 | spo13 zip1 + pJ66 (YEp352 RED1 HOP1) | 31.3 (1.2) | 1.3 (1.3) | 4.7 (1.3) |
| JM113 | spo13 zip1 + YEp24 | 35.0 | 1.7 | 7.0 |
| JM114 | spo13 zip1 + pNH83-2 (YEp24 HOP1) | 23.3 (1.5) | 1.2 (1.4) | 4.6 (1.5) |
Cells returned to growth after 72 h in sporulation medium. Values indicated are frequencies per 104 cells.
Values in parentheses indicate the fold decrease relative to that of the appropriate control strain.
Strain JM117 is the control for JM116 and JM115; strain JM119 is the control for JM118; strain JM112 is the control for JM111, JM110, JM212, JM213, and JM214; and strain JM113 is the control for JM114.
TABLE 5.
Red1 overproduction reduces crossing over in a zip1 mutant
| Strain | Mutant genotype | HIS4 LEU2 (cM)a | LEU2 MAT (cM)a | Spore viability (%)a |
|---|---|---|---|---|
| BS395 | spo13 + YEplac112 | 43.0 | 31.0 | 79.0 |
| BS396 | spo13 + pAV62 (YEplac112 RED1) | 19.2 (2.2)b | 17.3 (1.8) | 69.0 |
| JM289 | spo13 + pJ104 (YEplac112 MEK1) | 46.2 (0.93) | 32.9 (0.94) | 76.0 |
| BS398 | spo13 zip1 + YEplac112 | 24.8 | 19.0 | 44.1 |
| BS399 | spo13 zip1 + pAV62 (YEplac112 RED1) | 9.5 (2.6) | 7.1 (2.7) | 19.0 |
| JM290 | spo13 zip1 + pJ104 (YEplac112 MEK1) | 20.9 (1.2) | 16.5 (1.2) | 37.5 |
At least 100 dyads were analyzed per strain.
Strain BS395 is the control for BS396 and JM289; strain BS398 is the control for BS399 and JM290.
Among the total population of zip1 mutant cells overproducing Mek1, gene conversion and crossing over are not significantly decreased (Tables 4 and 5). However, since only a subset of zip1 mutant cells overproducing Mek1 sporulate, gene conversion was also measured among isolated spores. The frequencies of histidine, threonine, and tryptophan prototrophs in isolated spores are 93, 92, and 120%, respectively, of the levels of prototrophs in the total cell population. Thus, unlike Red1 overproduction, Mek1 overproduction bypasses zip1 arrest without decreasing meiotic recombination.
Overproduction of Red1 decreases DSBs.
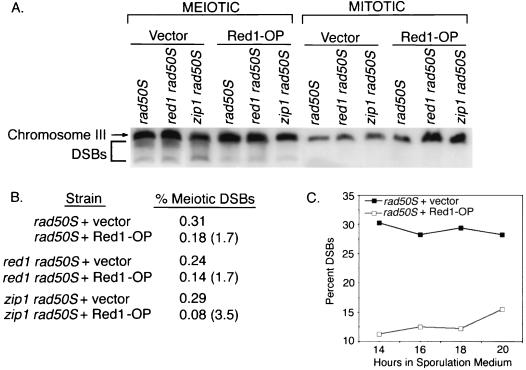
Red1 overproduction could decrease meiotic recombination either by reducing the number of recombination events initiated or by increasing the fraction of DSBs repaired by recombination between sister (rather than nonsister) chromatids. To distinguish these possibilities, DSB levels were assayed in strains carrying the rad50S mutation (1), which prevents DSB processing and results in the accumulation of broken DNA ends. DNA DSBs were analyzed by pulsed-field gel electrophoresis followed by Southern blotting and probing for DNA sequences from chromosome III; DSBs result in molecules that migrate faster than the intact chromosome (Fig. 2A). For each strain, the percent of total chromosome III DNA present in broken molecules was calculated; this frequency was then compared between strains containing either a multicopy RED1 plasmid or vector only (Fig. 2A).
FIG. 2.
Effect of Red1 overproduction on DSB formation. (A) Mitotic (0 h) and meiotic (15 h) cells were analyzed for chromosome III DSBs by pulsed-field gel electrophoresis and Southern blotting. Mutant strains used were as follows: rad50S plus vector (JM446), red1 rad50S plus vector (JM442), zip1 rad50S plus vector (JM438), rad50S overproducing Red1 (Red1-OP) (JM447), red1 rad50S overproducing Red1 (JM443), and zip1 rad50S overproducing Red1 (JM439). DSBs migrate below the position of the full-length chromosome III. Both a discrete band (representing the fragment from the end of the chromosome to the THR4 hot spot) and fragments corresponding to the products of cleavage at other DSB sites are observed. (B) Quantitation of the results shown in panel A. % Meiotic DSBs, the amount of chromosome III DNA in broken molecules as a percent of the total amount of chromosome III DNA. Numbers in parentheses indicate the fold decrease in percent DSBs of strains overproducing Red1 compared to strains carrying vector only. (C) DSBs in rad50S strains carrying either vector (JM446) or overproducing Red1 (JM447) were analyzed at several time points during meiosis. Percent DSBs, the amount of chromosome III DNA in broken molecules as a percent of the total amount of chromosome III DNA.
The levels of DSBs in rad50S and red1 rad50S cells are similar (Fig. 2A and B), as reported previously for the HIS4-LEU2 recombination hot spot (59). The level of DSBs in the zip1 rad50S strain is also similar to that observed in the rad50S and red1 rad50S strains (Fig. 2A and B). However, rad50S strains overproducing Red1 and red1 rad50S strains overproducing Red1 exhibit an approximately twofold reduction in DSBs compared to rad50S or red1 rad50S, respectively (Fig. 2A and B). The decrease in DSBs caused by Red1 overproduction is observed at several time points during meiosis (Fig. 2C). Furthermore, zip1 rad50S strains overproducing Red1 exhibit about a fourfold decrease in DSBs compared to zip1 mutant strains carrying the control vector (Fig. 2A and B). Red1 overproduction therefore decreases DSB levels in both rad50S and zip1 rad50S strains. The decrease in DSB formation observed when Red1 is overproduced approximates the decrease in meiotic recombination, suggesting that the decrease in DSBs is the cause of the reduction in meiotic recombination in strains overproducing Red1.
Red1 overproduction alters homologous chromosome associations in zip1.
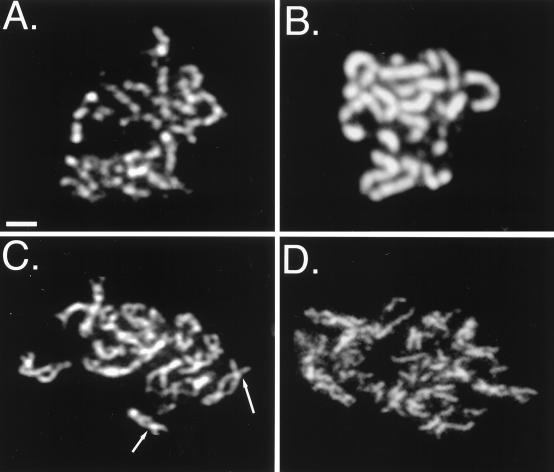
In a wild-type strain, Red1 overproduction alters the Red1 localization pattern from semicontinuous to fully continuous along chromosome axes (Fig. 3A and B) (46). The Red1 protein localizes continuously along chromosomes in the zip1 mutant, even when Red1 is not overproduced (Fig. 3C) (46). To investigate the effects of Red1 overproduction on the morphology of zip1 chromosomes, meiotic chromosomes were surface spread and stained with anti-Red1 antibodies. In zip1 mutant strains, the Red1-stained chromosome cores (corresponding to homologous chromosomes) are closely apposed at a few sites, called axial associations (Fig. 3C); these are sites where chromosome synapsis is thought to initiate (9, 35). In contrast to zip1 mutant strains containing the normal amount of Red1 (Fig. 3C), the chromosomes in zip1 mutant strains overproducing Red1 appear disorganized and fragmented (Fig. 3D). Axial elements are not obviously paired in zip1 mutant cells overproducing Red1, and axial associations are less evident (Fig. 3D).
FIG. 3.
Red1 overproduction alters meiotic chromosome morphology. (A) Spread nucleus from a wild-type strain carrying the YEp352 vector (JM152). (B) Spread nucleus from a wild-type strain overproducing Red1 (BS354). (C) Spread nucleus from the zip1 mutant carrying the YEp352 vector only (JM153); arrows indicate examples of axial associations. (D) Spread nucleus from a zip1 mutant overproducing Red1 (JM155). Scale bar, 1 μm.
The difference in chromosome morphology of zip1 mutant strains overproducing Red1 (compared to zip1) suggested a defect in homologous chromosome pairing. To measure pairing, FISH was carried out with DNA sequences from chromosome III to probe spread meiotic nuclei. zip1 red1 cells carrying RED1 on either a single-copy or a multicopy plasmid were surface spread, stained with anti-Red1 antibodies, and analyzed with FISH. Only cells that displayed Red1 staining were scored for pairing in order to eliminate cells from which the RED1-containing plasmid had been lost. In zip1 mutant strains carrying a single copy of RED1 (BS225), 90% of spread nuclei (45 of 50) contain a single FISH focus, indicating that the two copies of chromosome III are homologously paired. In contrast, overexpression of RED1 in zip1 (BS223) decreases homologous pairing of chromosome III to 10% (5 of 50); thus, Red1 overproduction substantially reduces pairing in the zip1 mutant. Overproduction of Red1 in a wild-type strain does not significantly affect homologous pairing of chromosome III (data not shown).
Some DSBs persist when the zip1 arrest is bypassed.
If unrepaired DSBs persist in zip1 mutant cells that arrest because of the checkpoint, and if unrepaired DSBs can trigger checkpoint-mediated arrest, then overproduction of Red1 or Mek1 might promote zip1 sporulation either by masking the DSB signal or by allowing DSBs to be repaired. To investigate these possibilities, spread meiotic chromosomes were prepared from zip1 strains carrying vector or a multicopy plasmid containing RED1 or MEK1. By using a modification of the TUNEL assay (17), DSBs were labeled in situ and then detected by indirect immunofluorescence. DSBs in chromosome spreads were labeled with digoxigenin-tagged nucleotides by using Tdt, which specifically incorporates nucleotides onto free 3′ hydroxyl ends of DNA (17). Incorporated digoxigenin-tagged nucleotides were detected by using antidigoxigenin antibody conjugated to rhodamine. The stage of the meiotic cell cycle was simultaneously monitored using antitubulin antibodies.
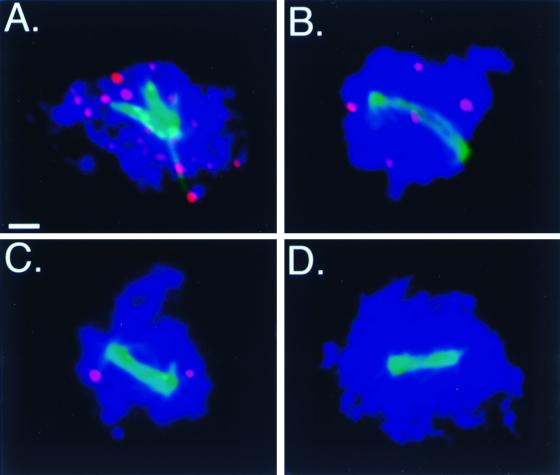
Although Red1 overproduction decreases the initial number of DSBs formed in the zip1 mutant, the number of Tdt-labeled foci present at pachytene, when cells are arrested, is similar for all strains analyzed. zip1 mutant cells in pachytene contain approximately 23.7 Tdt-labeled foci per nucleus, both in strains carrying vector only and in strains overproducing Red1 or Mek1 (Fig. 4A and data not shown). In contrast, zip1 mutant cells overproducing Red1 or Mek1 that progress past pachytene contain few or no Tdt-labeled foci. In zip1 mutant cells overproducing Red1 that contain a meiotic spindle, an average of 4.2 Tdt-labeled foci per nucleus is detected (Fig. 4B). zip1 mutant cells overproducing Mek1 that contain a meiotic spindle display an average of 1.7 Tdt-labeled foci per nucleus (Fig. 4C). In contrast, Tdt-labeled foci are not detected in cells prepared from the spo11 mutant (data not shown), which fails to form DSBs (8), or in wild-type cells that contain a meiotic spindle (Fig. 4D).
FIG. 4.
Most DSBs are repaired when the checkpoint is bypassed. Meiotic chromosomes were surface spread and then labeled in situ with Tdt (red) to detect DSBs. Antitubulin antibody (green) was used to visualize meiotic spindles (indicating that cells are no longer arrested at pachytene). Strains tested were as follows: (A) zip1 mutant carrying vector only (YEp352) (JM152), (B) zip1 mutant overproducing Red1 (JM155), (C) zip1 mutant overproducing Mek1 (JM154), (D) wild-type strain carrying vector only (YEp352) (JM152). Scale bar, 1 μm.
As an additional means to assay DSBs, Rad51 localization was examined in zip1 strains overproducing either Red1 or Mek1. The appearance and disappearance of Rad51 foci correlates with the appearance and disappearance of DSBs (13, 26), and Rad51 localization has been previously used as a marker for DSBs (26). In zip1 mutant cells overproducing Red1 or Mek1, cells that arrest at pachytene contain an average of 25.4 Rad51 foci per nucleus. However, in cells that contain a meiotic spindle, there is an average of 3.1 Rad51 foci per nucleus in zip1 mutant cells overproducing Red1 and an average of 1.0 Rad51 focus per nucleus in zip1 mutant cells overproducing Mek1. This indicates that most, but not all, DSBs are repaired in zip1 mutant cells that escape the checkpoint. Overproduction of Red1 or Mek1 thus appears to have two consequences: first, to allow the repair of many of the DSBs in zip1 mutant cells and, second, to inactivate the checkpoint such that the remaining DSBs are not detected.
Cooverproduction of Red1 and Mek1 restores the checkpoint.
Since Red1 and Mek1 interact with each other, overproduction of one of these proteins might impair checkpoint function by changing the stoichiometry of Red1 relative to Mek1. To test this possibility, zip1 mutant strains were transformed with a multicopy plasmid bearing both the RED1 and MEK1 genes. zip1 mutant strains overproducing both Red1 and Mek1 fail to sporulate, suggesting that the checkpoint is still active (Table 2). In principle, cooverproduction of Red1 and Mek1 might nonspecifically inhibit sporulation rather than restore checkpoint function. To address this possibility, sporulation was assayed in a wild-type strain in which Red1 and Mek1 are cooverproduced. In this control strain, sporulation occurs with wild-type kinetics and to wild-type levels, indicating that the multicopy plasmid carrying both MEK1 and RED1 is not deleterious to sporulation.
In the zip1 mutant, cooverproduction of Mek1 with Red1 also substantially eliminates the decrease in recombination conferred by Red1 overproduction alone (Table 4). In contrast, cooverproduction of a kinase-defective Mek1 mutant protein (Mek1-D290A) does not interfere with the ability of excess Red1 to bypass zip1 arrest (data not shown). Although overproduction of Hop1 has no effect on sporulation in zip1 mutant strains, cooverproduction of Hop1 with Red1 or Mek1 restores checkpoint function (Table 2). Overproduction of Hop1 also nearly eliminates the decrease in recombination resulting from Red1 overproduction. These results suggest that the stoichiometry of Red1, Mek1, and Hop1 is critical to checkpoint functioning.
DISCUSSION
Imbalance of meiotic chromosomal proteins inactivates the pachytene checkpoint.
Overproduction of Red1 or Mek1 specifically promotes sporulation of mutants that normally undergo checkpoint-mediated arrest at pachytene. The zip1, zip2, dmc1, and hop2 mutants all exhibit defects in both recombination and synapsis; however, the molecular signal that triggers arrest in these strains remains unknown.
In the zip1 mutant, most or all recombination intermediates arrest or delay as double Holliday junctions (49). The observation that mutation of PCH2 (41) or SWE1 (24) promotes sporulation of zip1 without substantially decreasing spore viability indicates that Holliday junctions are resolved when the checkpoint is inactivated. Thus, the unresolved Holliday junctions observed in zip1 appear to be the consequence, rather than the cause, of arrest.
It is not clear whether defects in synapsis activate checkpoint-induced arrest. Chromosomes in zip1 mutant cells fail to synapse (51), but form axial elements, which are SC precursors. Other mutants that form axial elements but not mature SCs, such as mer2 (34), do not arrest. Moreover, the mek1 mutant, which forms short stretches of SC (32), does not arrest. The observation that not all defects in SC assembly trigger a checkpoint response suggests that either the zip1 defect in synapsis is not the cause of arrest or only specific intermediates in SC assembly can trigger checkpoint-induced arrest.
Checkpoint-mediated arrest at pachytene may be activated by unrepaired DSBs; in mitotic cells, a single DSB is sufficient to cause arrest (40). In a total population of zip1 SK-1 cells, ∼10% of DSBs are unrepaired (49). However, the spore viability of SK-1 zip1 cells is 35 to 60% (52, 55), which is much higher than predicted based on the number of unrepaired DSBs, arguing that DSBs are repaired in zip1 mutant cells that sporulate. Consistent with this hypothesis, in the BR1919-8B strain background (in which a subset of zip1 mutant cells sporulate after a delay), zip1 mutant cells arrested at pachytene display approximately 20 to 25 Tdt-labeled foci, whereas zip1 mutant cells undergoing meiotic nuclear division have none (data not shown). There are two possible explanations for these results, depending on whether DSBs are the consequence or the cause of arrest. DSBs may be successfully repaired in a subset of cells, resulting in inactivation of the checkpoint and consequent cell division. Alternatively, a subset of cells may adapt to the checkpoint, and DSBs may be repaired as these cells progress through the cell cycle.
Overproduction of Red1 or Mek1 in zip1 mutant strains allows repair of a significant number of DSBs that would otherwise remain unrepaired. If overproduction of Red1 or Mek1 causes changes in sister chromatid cohesion, then perhaps DSBs can repair through intersister recombination. If only a small fraction (∼10%) of DSBs are repaired by intersister recombination, then the observed correlation between DSB levels and interhomolog recombination frequencies (referred to above) would still apply. Alternatively, if unrepaired DSBs are a consequence (rather than a cause) of checkpoint-induced arrest, then inactivation of the checkpoint by overproduction of Red1 or Mek1 might allow DSBs to resolve normally (i.e., through interhomolog recombination).
Overproduction of Red1 or Mek1 only partially suppresses the arrest of the dmc1 and the hop2 mutants, suggesting that these mutants generate different or additional signals for arrest or a stronger signal for arrest. Consistent with this hypothesis, a greater number of DSBs remain unrepaired in the dmc1 and hop2 mutants than in the zip1 mutant (4, 23, 44). Additionally, the DSBs that accumulate in the dmc1 mutant are hyperresected (4, 43). In the hop2 mutant, chromosomes are synapsed nonhomologously (23).
Detection of meiotic DSBs.
Meiotic DSBs are typically assayed by Southern blot analysis of restriction fragments or whole chromosomes (8, 12, 15, 50). Alternatively, the presence of meiotic DSBs has been assayed cytologically by using antibodies against the Rad51 protein (26). However, Southern blotting is not sensitive enough to detect very low levels of DSBs and cannot be used to assay DSBs in a subset of cells within a mixed population. Anti-Rad51 staining cannot be used to analyze rad51 mutant strains or other strains in which the Rad51 protein does not localize to chromosomes (13). In contrast, detection of meiotic DSBs by Tdt labeling is extremely sensitive and can be applied to all strain backgrounds.
In human fibroblasts, gamma irradiation induces Tdt- and Mre11-labeled foci, which are presumed to mark DSB sites (29). Mre11, Xrs2, and Rad50 colocalize on yeast meiotic chromosomes, and the localization of these proteins correlates with the presence of DSBs (56). However, though appearance and disappearance of Rad51 foci also correlates with DSBs, Rad51 does not colocalize extensively with Mre11 or Tdt-labeled foci either in human cells (29) or on spread meiotic chromosomes in yeast (data not shown). One explanation for the failure of colocalization between Rad51 and Mre11 is that these proteins localize to chromosomes with different timing: colocalization between Rad51 and Tdt foci might not be expected if those DSBs that have progressed to the stage of Rad51 localization are no longer capable of labeling by Tdt. Mre11 and Rad51 have distinct functions in the DSB repair process (29, 56).
Red1 overproduction antagonizes recombination in addition to suppressing pachytene arrest.
Certain non-null alleles of RED1, such as red1-2 (30) and red1-DraI (B. Rockmill, A. V. Smith, and G. S. Roeder, unpublished data), decrease meiotic recombination below the level observed for the red1 null mutant. Our data indicate that excess Red1 also antagonizes meiotic recombination, both in the wild type and in the zip1 mutant. In addition, excess Red1 decreases recombination in a specific non-null mek1 mutant, mek1-974 (21). Thus, Red1 overproduction appears to antagonize recombination in strains of different genotypes. Our results suggest that the mechanism by which excess Red1 decreases recombination is different from the way in which deletion of RED1 decreases recombination. In the red1 null mutant, DSBs are decreased to ∼10% of the wild-type level (44); however, in the red1 rad50S double mutant, DSBs are not decreased, indicating that rad50S is epistatic to red1 (59). In contrast, overproduction of Red1 decreases DSBs both in the wild type and in rad50S strains, suggesting that Red1 overproduction prevents DSB formation regardless of the status of RAD50.
In principle, Red1 overproduction might decrease recombination either by increasing the fraction of events repaired through sister chromatid exchange or by decreasing the number of DSBs formed. This work indicates that Red1 overproduction confers a decrease in DSBs. Furthermore, the decrease in DSBs approximates the decrease in gene conversion and crossing over. Thus, the reduction in meiotic recombination can be accounted for solely by a decrease in DSBs.
How might excess Red1 decrease DSBs? Red1 overproduction may block access of proteins required for meiotic recombination (e.g., Hop1) to meiotic chromosomes. In wild-type cells, Red1 and Hop1 colocalize on meiotic chromosomes (46) and are required for wild-type levels of DSBs (43, 44). When overproduced, Red1 displays continuous localization along chromosomes, which may prevent other proteins from interacting with the chromosome axes. Red1 interacts with itself in a two-hybrid assay (21), consistent with the idea that Red1 self-association (rather than association with Hop1 and Mek1) may be promoted when Red1 is present in excess.
Alternatively, or in addition, Red1 overproduction may decrease DSBs by affecting sister chromatid cohesion. Mek1-mediated phosphorylation of Red1 is required for sister chromatid cohesion (2); Red1 may be inefficiently phosphorylated if the ratio of Mek1 to Red1 is inappropriate. If sister chromatid cohesion provides the chromosome structure necessary for DSB formation, then a decrease in cohesion might contribute to a decrease in DSBs (44).
The decrease in DSBs might account for the observed decrease in homologous chromosome pairing in the zip1 mutant, if DSB formation or recombination intermediates are required for stable pairing (27, 36, 57). Although overproduction of Red1 decreases DSBs in both the wild type and the zip1 mutant, homologous chromosome pairing in a wild-type strain is not affected by Red1 overproduction. Perhaps the DSBs that form in the wild type are stabilized through recombination and SC formation. In zip1 mutant cells overproducing Red1, a lower level of DSBs, in conjunction with a deregulation of the distribution of recombination events among chromosomes (52), may result in a failure to stabilize pairing interactions. This might be particularly evident for smaller chromosomes, such as chromosome III, whose pairing was assayed by FISH.
Red1 and Mek1 as signals of meiotic prophase defects.
Red1 overproduction might promote zip1 mutant sporulation by decreasing meiotic recombination to a level below that required to activate the checkpoint. However, the following observations argue against this interpretation. First, a fourfold reduction in DSBs results in ∼60 DSBs per cell. In mitotic cells, a single unrepaired DSB is sufficient to trigger cell cycle arrest (40). Furthermore, wild-type meiotic cells do not sporulate until all DSBs have been repaired. Second, bypass of zip1 by Mek1 overproduction cannot be explained by a decrease in meiotic recombination.
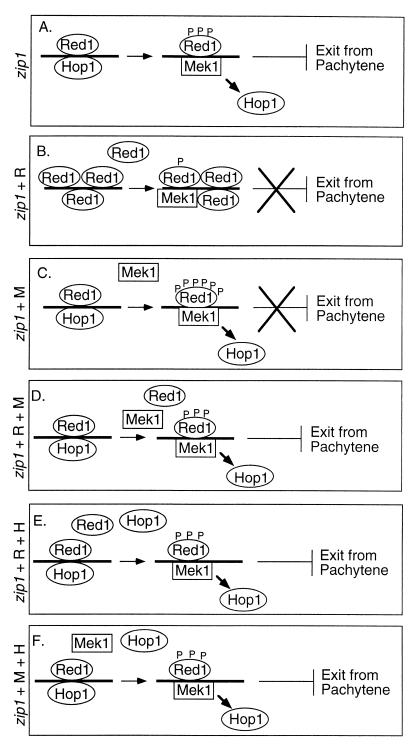
Previous work has demonstrated that Red1 remains phosphorylated and localized to meiotic chromosomes through pachytene (2), and recent data indicate that Red1 dephosphorylation is necessary for exit from pachytene (3). In the wild type, Red1 is dephosphorylated by the Glc7 phosphatase at the pachytene-diplotene transition as Red1 dissociates from chromosomes (3). However, in the zip1 mutant, cells arrest at pachytene with Red1 remaining phosphorylated and localized to chromosomes (3). Checkpoint-induced arrest of the zip1 mutant at pachytene is bypassed by inducing Red1 dephosphorylation by overproduction of Glc7 (3). Furthermore, Red1 does not become phosphorylated in certain mutants that inactivate the pachytene checkpoint pathway (3). We therefore speculate that the phosphorylated form of Red1 acts as an inhibitory signal to cell cycle progression (Fig. 5A).
FIG. 5.
Model for bypass of checkpoint-mediated arrest by overproduction of Red1 or Mek1. (A) In the zip1 mutant, continued Red1 phosphorylation serves as an inhibitory signal that prevents pachytene exit. (B) When Red1 is overproduced in the zip1 mutant, the excess Red1 protein may be inefficiently phosphorylated, resulting in little or no detectable signal to the checkpoint. (C) Overproduction of Mek1 in the zip1 mutant may result in excess or inappropriate phosphorylation of Red1, such that Red1 is not properly detected by the checkpoint machinery. (D) Cooverproduction of Mek1 and Red1 restores the appropriate phosphorylation of Red1, allowing proper checkpoint function. (E) Cooverproduction of Hop1 and Red1 may promote interaction between Red1 and Mek1, leading to Red1 phosphorylation. (F) Cooverproduction of Hop1 and Mek1 also may promote correct phosphorylation of Red1. P, phosphate group; R, Red1 overproduction; M, Mek1 overproduction; H, Hop1 overproduction.
When Red1 is overproduced (Fig. 5B), Red1 may be inefficiently phosphorylated. Proteins that normally detect phosphorylated Red1 may interact less well with unphosphorylated Red1, resulting in less-efficient detection of meiotic prophase defects. Also, zip1 mutant cells overproducing Red1 may emit a weaker signal to the checkpoint, since the total number of DSBs is decreased to one-fourth that of zip1 mutant cells containing vector only.
Overproduction of Mek1 might suppress the pachytene checkpoint if Red1 becomes hyperphosphorylated or if a greater fraction of Red1 molecules are phosphorylated. Analysis of Mek1-dependent phosphorylation of Red1 in vitro is consistent with this interpretation (data not shown). The amount of radioactive label incorporated into the Red1 protein in zip1 mutant strains overproducing Red1 is no greater than in the zip1 mutant alone, though zip1 mutant strains overproducing Red1 contain more Red1 protein. zip1 mutant strains overproducing Mek1 display similar amounts of Red1 protein, but increased phosphorylation of Red1 in vitro, compared to zip1 mutant alone. If the continued phosphorylation of Red1 is a signal that leads to checkpoint activation, then the Red1 protein might not be recognized by the checkpoint machinery if the ratio of phosphorylated to unphosphorylated Red1 is altered and/or if residues that are not normally phosphorylated become modified by Mek1 (Fig. 5C). Consistent with this interpretation, checkpoint-induced arrest at pachytene is not suppressed by overproduction of a kinase-defective Mek1 protein, although this mutant Mek1 protein still can bind to Red1 (2). Furthermore, cooverproduction of the mutant Mek1 protein with Red1 allows bypass of the checkpoint, suggesting that phosphorylation of Red1, rather than binding of Red1 to Mek1, is important for the checkpoint. It is possible that excess Mek1 binds to proteins required for the checkpoint, preventing the checkpoint proteins from detecting chromosomal defects. However, this interpretation requires the checkpoint proteins to be bound by wild-type Mek1, but not by the kinase-defective Mek1 mutant protein. Regulation of Red1 phosphorylation can explain this result: Red1 may be properly phosphorylated when the ratio of Mek1 to Red1 is in balance, even if both proteins are overproduced (Fig. 5D).
Unlike Red1 and Mek1, Hop1 overproduction fails to bypass pachytene arrest, perhaps because the Hop1 protein normally dissociates from chromosomes by late pachytene (46). However, cooverproduction of Hop1 with Red1 restores checkpoint function. If Red1 overproduction bypasses the checkpoint because Red1 is not sufficiently phosphorylated, then overproduction of Hop1 might counteract this by promoting Red1 phosphorylation (Fig. 5E). Mek1 may prefer to phosphorylate Red1 when it is associated with Hop1, and cooverproduction of Hop1 with Red1 might increase the ratio of Red1-Hop1 complexes on chromosomes relative to Red1-Red1 complexes.
How might overproduction of Hop1 counteract the effect of Mek1 overproduction? Both Red1 and Hop1 undergo Mek1-dependent phosphorylation in vitro (2). If overproduction of Mek1 permits checkpoint bypass because Red1 is hyperphosphorylated, then it is possible that excess Hop1 restores the normal level of Red1 phosphorylation by competing with Red1 as a substrate for Mek1 (Fig. 5F).
Analysis of red1 zip1 and mek1 zip1 mutant strains led to the suggestion that Red1 and Mek1 are required to form the complex of proteins and DNA recombination intermediates that is monitored by the checkpoint machinery (59). In principle, deletion of RED1 or MEK1 could promote zip1 sporulation because the structure that the checkpoint monitors (perhaps a recombination intermediate) is not formed. Alternatively, checkpoint bypass might occur because the proteins responsible for monitoring are absent. The present work argues that Red1 and Mek1 have a direct role in signaling meiotic prophase defects to the checkpoint machinery, possibly through Mek1-mediated phosphorylation of Red1. When Mek1 is overproduced in zip1 mutant strains, the wild-type number of recombination structures are formed, but monitoring is nonetheless disrupted. Suppression of the zip1 sporulation defect by Mek1 overproduction requires Mek1 kinase activity, which implies that Mek1 kinase activity or Red1 phosphorylation are important for proper monitoring. Furthermore, in the hop1 mutant, Mek1 fails to localize to chromosomes (2) or to phosphorylate Red1 (data not shown), and the checkpoint is inactive (K.-S. Tung and G. S. Roeder, unpublished data).
Involvement of meiotic chromosomal proteins in the pachytene checkpoint may be analogous to the role that replication enzymes, such as DNA polymerase ɛ, play in monitoring the completion of DNA replication at the S-phase checkpoint (28). Interestingly, the fission yeast kinase Cds1, which is homologous to Mek1 both within and outside of the kinase domain, is believed to respond to defects in DNA replication (25); Cds1 prevents activation of mitosis in the presence of incompletely replicated DNA (6, 60). Perhaps phosphorylated Red1 and/or Mek1 similarly prevent inappropriate exit from pachytene and entry into the meiosis I division in the presence of intermediates in recombination and synapsis.
ACKNOWLEDGMENTS
This study was conceived by A.V.S. Experiments were conducted by J.M.B. and A.V.S. We thank Seema Agarwal and Janet Novak for strains and Seema Agarwal, Erica Hong, and Beth Rockmill for helpful discussions throughout this work and comments on the manuscript. Anti-Rad51 antibody was generously provided by Doug Bishop.
This work was funded by the Howard Hughes Medical Institute and grant GM28904 from the United States Public Health Service to G.S.R. A.V.S. was supported in part by Postdoctoral Fellowship DRG-1239 from the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation.
REFERENCES
- 1.Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 2.Bailis J M, Roeder G S. Synaptonemal complex morphogenesis and sister-chromatid cohesion require Mek1-dependent phosphorylation of a meiotic chromosomal protein. Genes Dev. 1998;22:3551–3563. doi: 10.1101/gad.12.22.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailis J M, Roeder G S. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell. 2000;101:211–221. doi: 10.1016/S0092-8674(00)80831-4. [DOI] [PubMed] [Google Scholar]
- 4.Bishop D, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 5.Bishop D K. RecA homologs Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell. 1994;79:1081–1092. doi: 10.1016/0092-8674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 6.Boddy M N, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- 7.Boeke J D, Lacroute F, Fink G. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 8.Cao L, Alani E, Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- 9.Chua P R, Roeder G S. Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell. 1998;93:349–359. doi: 10.1016/s0092-8674(00)81164-2. [DOI] [PubMed] [Google Scholar]
- 10.Clarke L, Carbon J. Genomic substitutions of centromeres in Saccharomyces cerevisiae. Nature. 1983;305:23–28. doi: 10.1038/305023a0. [DOI] [PubMed] [Google Scholar]
- 11.de los Santos T, Hollingsworth N M. Red1p, a MEK1-dependent phosphoprotein that physically interacts with Hop1p during meiosis in yeast. J Biol Chem. 1999;274:1783–1790. doi: 10.1074/jbc.274.3.1783. [DOI] [PubMed] [Google Scholar]
- 12.Game J C, Sitney K C, Cook V E, Mortimer R K. Use of a ring chromosome and pulsed-field gels to study interhomolog recombination, double-strand DNA breaks and sister-chromatid exchange in yeast. Genetics. 1989;123:695–713. doi: 10.1093/genetics/123.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasior S L, Wong A K, Kora Y, Shinohara A, Bishop D K. Rad52 associates with RPA and functions with Rad55 and Rad57 to assemble meiotic recombination complexes. Genes Dev. 1998;12:2208–2221. doi: 10.1101/gad.12.14.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 15.Goldway M, Sherman A, Zenvirth D, Arbel T, Simchen G. A short chromosomal region with major roles in yeast chromosome III meiotic disjunction, recombination and double-strand breaks. Genetics. 1993;133:159–169. doi: 10.1093/genetics/133.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 17.Heatwole V M. TUNEL assay for apoptotic cells. Methods Mol Biol. 1999;115:141–148. doi: 10.1385/1-59259-213-9:141. [DOI] [PubMed] [Google Scholar]
- 18.Hill J E, Myers A M, Koerner T J, Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- 19.Hollingsworth N M, Byers B. HOP1: a yeast meiotic pairing gene. Genetics. 1989;121:445–462. doi: 10.1093/genetics/121.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingsworth N M, Goetsch L, Byers B. The HOP1 gene encodes a meiosis-specific component of yeast chromosomes. Cell. 1990;61:73–84. doi: 10.1016/0092-8674(90)90216-2. [DOI] [PubMed] [Google Scholar]
- 21.Hollingsworth N M, Ponte L. Genetic interactions between HOP1, RED1 and MEK1 suggest that MEK1 regulates assembly of axial element components during meiosis in the yeast Saccharomyces cerevisiae. Genetics. 1997;147:33–42. doi: 10.1093/genetics/147.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klapholz S, Esposito R E. Recombination and chromosome segregation during the single division meiosis in SPO12-1 and SPO13-1 diploids. Genetics. 1980;96:589–611. doi: 10.1093/genetics/96.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leu J-Y, Chua P R, Roeder G S. The meiosis-specific Hop2 protein of S. cerevisiae ensures synapsis between homologous chromosomes. Cell. 1998;94:375–386. doi: 10.1016/s0092-8674(00)81480-4. [DOI] [PubMed] [Google Scholar]
- 24.Leu J-Y, Roeder G S. The pachytene checkpoint in S. cerevisiae depends on Swe1-mediated phosphorylation of the cyclin-dependent kinase Cdc28. Mol Cell. 1999;4:805–814. doi: 10.1016/s1097-2765(00)80390-1. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay H D, Griffiths D J F, Edwards R J, Christensen P U, Murray J M, Osman F, Walworth N, Carr A M. S-phase specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1999;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lydall D, Nikolsky Y, Bishop D K, Weinert T. A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature. 1996;383:840–843. doi: 10.1038/383840a0. [DOI] [PubMed] [Google Scholar]
- 27.Nag D K, Scherthan H, Rockmill B, Bhargava J, Roeder G S. Heteroduplex DNA formation and homolog pairing in yeast meiotic mutants. Genetics. 1995;141:75–86. doi: 10.1093/genetics/141.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navas T A, Zhou Z, Elledge S J. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 29.Nelms B E, Maser R S, MacKay J F, Lagally M G, Petrini J H. In situ visualization of DNA double-strand break repair in human fibroblasts. Science. 1998;280:590–592. doi: 10.1126/science.280.5363.590. [DOI] [PubMed] [Google Scholar]
- 30.Rockmill B, Roeder G S. RED1: a yeast gene required for the segregation of chromosomes during the reductional division of meiosis. Proc Natl Acad Sci USA. 1988;85:6057–6061. doi: 10.1073/pnas.85.16.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockmill B, Roeder G S. Meiosis in asynaptic yeast. Genetics. 1990;126:563–574. doi: 10.1093/genetics/126.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockmill B, Roeder G S. A meiosis-specific protein kinase homolog required for chromosome synapsis and recombination. Genes Dev. 1991;5:2392–2404. doi: 10.1101/gad.5.12b.2392. [DOI] [PubMed] [Google Scholar]
- 33.Rockmill B, Lambie E, Roeder G S. Spore enrichment. Methods Enzymol. 1991;194:146–149. doi: 10.1016/0076-6879(91)94012-2. [DOI] [PubMed] [Google Scholar]
- 34.Rockmill B, Engebrecht J, Scherthan H, Loidl J, Roeder G S. The yeast MER2 gene is required for chromosome synapsis and the initiation of meiotic recombination. Genetics. 1995;141:49–59. doi: 10.1093/genetics/141.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockmill B, Sym M, Scherthan H, Roeder G S. Roles for two RecA homologs in promoting meiotic chromosome synapsis. Genes Dev. 1995;9:2684–2695. doi: 10.1101/gad.9.21.2684. [DOI] [PubMed] [Google Scholar]
- 36.Rockmill B, Roeder G S. Telomere-mediated chromosome pairing during meiosis in budding yeast. Genes Dev. 1998;12:2574–2586. doi: 10.1101/gad.12.16.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roeder G S. Meiotic chromosomes: it takes two to tango. Genes Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- 38.Rose D, Holm C. Meiosis-specific arrest revealed in DNA topoisomerase II mutants. Mol Cell Biol. 1993;13:3445–3455. doi: 10.1128/mcb.13.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Sandell L L, Zakian V A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 41.San-Segundo P A, Roeder G S. Pch2 links chromatin silencing to meiotic checkpoint control. Cell. 1999;97:313–324. doi: 10.1016/s0092-8674(00)80741-2. [DOI] [PubMed] [Google Scholar]
- 42.Schultes N P, Ellington A D, Cherry J M, Szostak J W. Saccharomyces cerevisiae homoserine kinase is homologous to prokaryotic homoserine kinases. Gene. 1990;96:177–180. doi: 10.1016/0378-1119(90)90250-u. [DOI] [PubMed] [Google Scholar]
- 43.Schwacha A, Kleckner N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994;76:51–63. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 44.Schwacha A, Kleckner N. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1136. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 45.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 46.Smith A V, Roeder G S. The yeast Red1 protein localizes to the cores of meiotic chromosomes. J Cell Biol. 1997;136:957–967. doi: 10.1083/jcb.136.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, A. V., and G. S. Roeder. Cloning and characterization of the Kluyveromyces lactis homologs of the Saccharomyces cerevisiae RED1 and HOP1 genes. Chromosoma, in press. [DOI] [PubMed]
- 48.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 49.Storlazzi A, Xu L, Schwacha A, Kleckner N. Synaptonemal complex (SC) component Zip1 plays a role in meiotic recombination independent of SC polymerization along the chromosomes. Proc Natl Acad Sci USA. 1996;93:9043–9048. doi: 10.1073/pnas.93.17.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun H, Treco D, Schultes N P, Szostak J W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989;338:87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- 51.Sym M, Engebrecht J, Roeder G S. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993;72:365–378. doi: 10.1016/0092-8674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- 52.Sym M, Roeder G S. Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell. 1994;79:283–292. doi: 10.1016/0092-8674(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 53.Sym M, Roeder G S. Zip1-induced changes in synaptonemal complex structure and polycomplex assembly. J Cell Biol. 1995;128:455–466. doi: 10.1083/jcb.128.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tishkoff D X, Rockmill B, Roeder G S, Kolodner R D. The sep1 mutant of Saccharomyces cerevisiae arrests in pachytene and is deficient in meiotic recombination. Genetics. 1995;139:495–509. doi: 10.1093/genetics/139.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tung K-S, Roeder G S. Meiotic chromosome morphology and behavior in zip1 mutants of Saccharomyces cerevisiae. Genetics. 1998;149:817–832. doi: 10.1093/genetics/149.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usui T, Ohta T, Oshiumi J, Tomizawa J, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 57.Weiner B M, Kleckner N. Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell. 1994;77:977–991. doi: 10.1016/0092-8674(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 58.Xu L, Ajimura M, Padmore R, Klein C, Kleckner N. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6572–6581. doi: 10.1128/mcb.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu L, Weiner B M, Kleckner N. Meiotic cells monitor the status of the interhomolog recombination complex. Genes Dev. 1997;11:106–118. doi: 10.1101/gad.11.1.106. [DOI] [PubMed] [Google Scholar]
- 60.Zeng Y, Forbes K C, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]