Introduction
Psoriasis is a complex, multifactorial immune-mediated skin disorder linked to the interleukin 17 (IL-17) signaling pathway, among others. Therapies such as ixekizumab, secukinumab, and brodalumab interrupt this IL-17 signaling, improving psoriasis with remarkable efficacy.1 While an increased occurrence of skin infections including Candida species, Staphylococcus aureus, and tinea infections have been documented with use of IL-17 inhibitors,2,3 brodalumab is the only IL-17 inhibitor for which the package insert specifically reports tinea versicolor infection. We report an unusual presentation of generalized tinea versicolor developing shortly after initiation of the IL-17 inhibitor ixekizumab for treatment of psoriasis.
Case report
A 52-year-old man employed as a cook had a longstanding history of psoriasis well controlled with ustekinumab therapy. However, new flares of psoriasis developed, and his therapy was changed to ixekizumab. At the 4-month follow-up, he reported a new, occasionally pruritic rash, which began 1 month after the first injection of ixekizumab. He denied having similar rashes in the past.
Physical examination showed hypopigmented and hyperpigmented, scaly macules and patches covering his chest, abdomen, and entire back with extension to the buttocks (Fig 1). Lesions extended up the neck into the scalp and over the extremities to the wrists and ankles (Fig 2, A and B). A scraping of scale for potassium hydroxide examination was floridly positive for fungal hyphae and spores (Fig 3) in a “spaghetti and meatball” pattern, confirming the clinical diagnosis of widespread tinea versicolor.4
Fig 1.
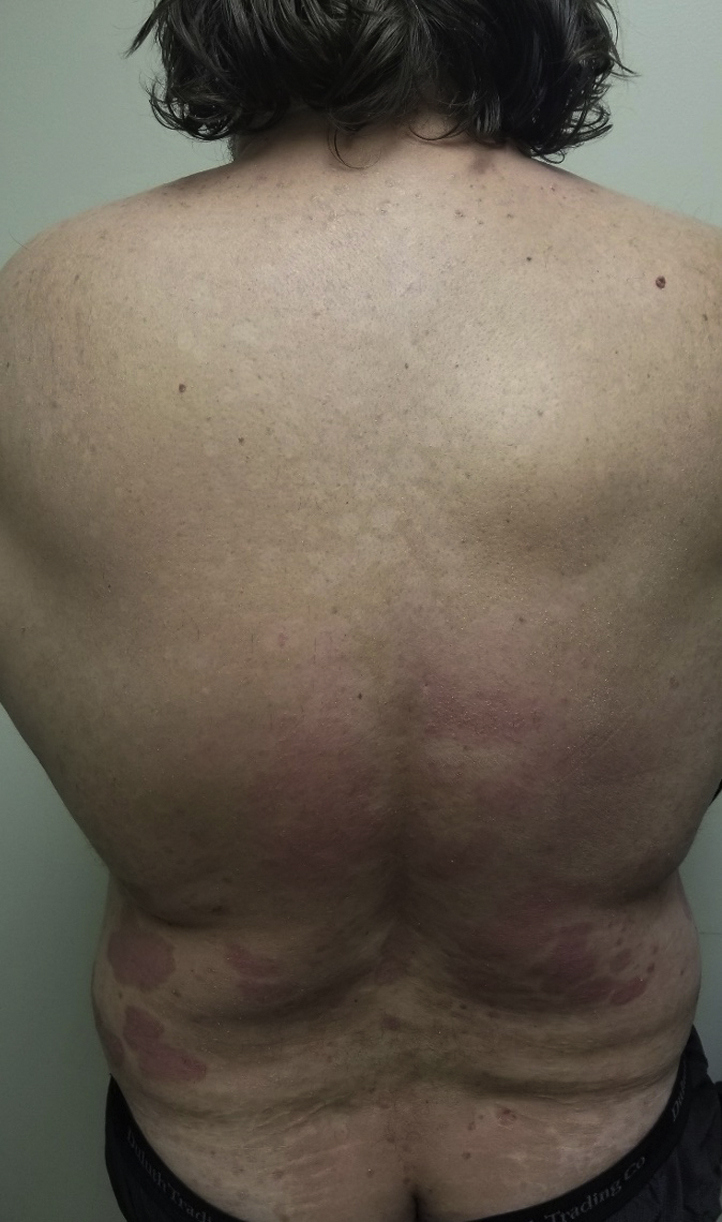
Hypopigmented scaly macules and patches extended from the shoulders to the lower mid back; similar hyperpigmented lesions extended from the lower back over the buttock. Psoriatic plaques persisted over the mid and lower back.
Fig 2.
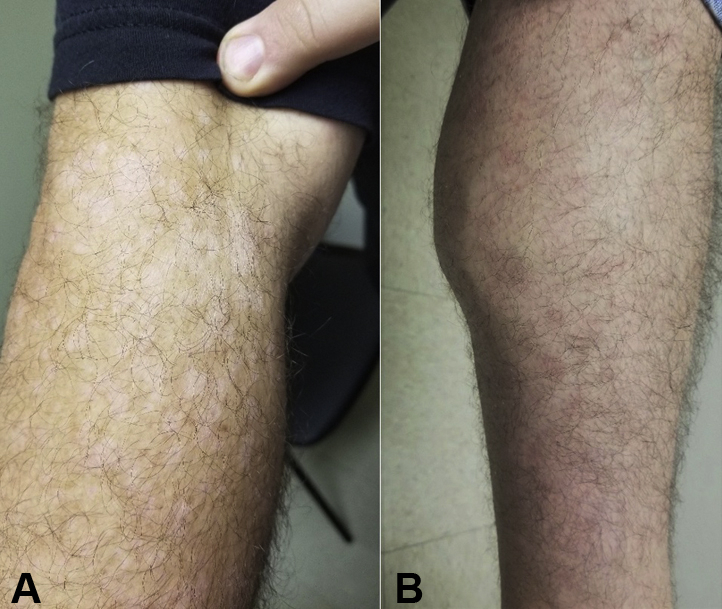
A, Diffuse hypopigmented scaly macules with minimal pinkness covered the right arm. B, Tan pinkish macules and small patches extended down to the distal left lower leg.
Fig 3.
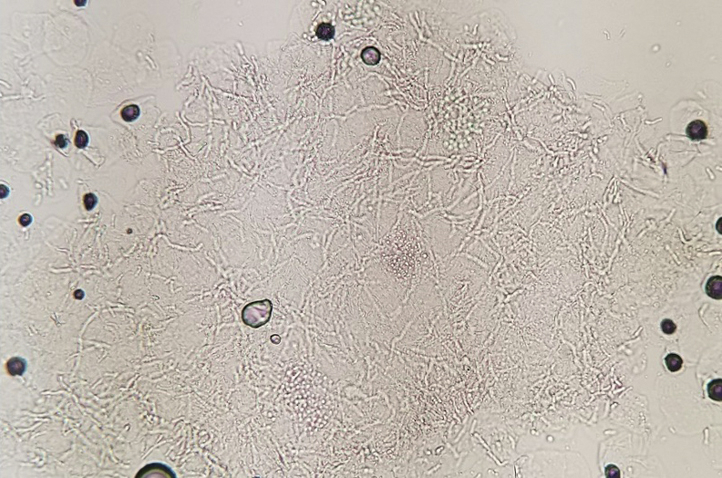
Potassium hydroxide preparation demonstrated the classic “spaghetti and meatballs” presentation of Malassezia overgrowth.
Treatment recommendations included ketoconazole 2% shampoo applied as a lather over the trunk, extremities, and scalp with instructions to rinse off after 5 minutes. This regimen was to be initially repeated 2 to 3 times per week, then once per month for maintenance. Fluconazole 300 milligrams once weekly for 2 weeks was also prescribed. Ixekizumab was continued. At the 6-month follow-up, the tinea versicolor was totally resolved.
Discussion
Tinea versicolor, also referred to as pityriasis versicolor, is a cutaneous fungal infection caused by Malassezia species. Erythematous to hyper- or hypopigmented macules and patches with a branny or powdery “furfuraceous” scale typically develop on the upper trunk and neck, extending into the scalp; generalized involvement is unusual. Older teenagers and young adults are most commonly affected. Warm, humid environments, such as a hot steamy kitchen in this case, are predisposing factors. Additionally, studies have demonstrated a potential for overgrowth of Malassezia species in immunocompromised patients.5 Specifically, individuals on chronic immunosuppressive medications including corticosteroids, azathioprine, and cyclosporine as well as those with immunosuppressive conditions such as human immunodeficiency virus, Cushing disease, pregnancy, and malnutrition have been shown to be at an increased risk of tinea versicolor.6,7
Ixekizumab, a humanized monoclonal IgG, binds to the IL-17 signaling molecule to prevent this molecule from binding to its receptor, thereby weakening the IL-17 immune response. IL-17 is normally responsible for stimulating and activating neutrophils; overactivation may contribute to the development of psoriasis. Common adverse reactions to this drug include injection site reactions and upper respiratory tract infections. Less frequent adverse reactions include nausea, rhinitis, urticaria, conjunctivitis, inflammatory bowel disease, angioedema, influenza, tinea infections, and oral candidiasis; tinea versicolor is not specifically listed as a potential adverse event in the ixekizumab package insert.4,8
Although some sources have found that microbiome diversity remains unchanged despite IL-17 inhibitor therapy, an increased risk of mild to moderate candidal infections is well recognized. In initial trials, candidal infections occurred in 3.3% of patients on ixekizumab, 1.7% of patients on secukinumab, and 4.0% of patients on brodalumab.2 As IL-17 has been shown to be particularly instrumental in maintaining antifungal immunity to both opportunistic fungal pathogens (eg, C. albicans) and commensal organisms (eg, Malassezia species), it follows that IL-17 blocking medications may predispose patients to develop tinea versicolor.9 Review of the package inserts for all biologic therapies currently approved for psoriasis revealed that, although many include “tinea infections” as a potential adverse event, only brodalumab, an IL-17 inhibitor, and risankizumab, an IL-23 inhibitor, specifically list tinea versicolor as a potential adverse event (Table I).2
Table I.
Biologic medications used in the treatment of psoriasis and review of their package inserts for reported tinea versicolor infection
| Drug | Inhibitor class | Tinea infection reported | Tinea versicolor infection reported |
|---|---|---|---|
| Brodalumab | IL-17 | Yes | Yes |
| Ixekizumab | IL-17 | Yes | Not listed |
| Secukinumab | IL-17 | Yes | Not listed |
| Guselkumab | IL-23 | Yes | Not listed |
| Risankizumab | IL-23 | Yes | Yes |
| Tildrakizumab | IL-23 | No | Not listed |
| Ustekinumab | IL-12/23 | No | Not listed |
| Adalimumab | TNF | No | Not listed |
| Certolizumab | TNF | No | Not listed |
| Etanercept | TNF | No | Not listed |
| Infliximab | TNF | No | Not listed |
IL, Interleukin; TNF, tumor necrosis factor.
Rapid onset of tinea versicolor following initiation of ixekizumab, an IL-17 inhibitor known to increase risk of cutaneous fungal infections, strongly implicates ixekizumab as the trigger for this impressive, generalized eruption in a middle-aged adult patient without prior history of the disease. While, to our knowledge, this occurrence has not been previously reported with ixekizumab, it is not a surprising consequence of IL-17 inhibition. Given the extensive involvement, both topical and systemic therapies were initiated. As ongoing ixekizumab therapy is anticipated, a maintenance regimen for prevention of tinea versicolor will likely be required. Providers should be aware of the potential increased risk of tinea versicolor when treating psoriasis with IL-17 inhibitors such as ixekizumab, especially as unusually widespread presentations of this disease may occur.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Ly K., Smith M.P., Thibodeaux Q., Reddy V., Liao W., Bhutani T. Anti IL-17 in psoriasis. Expert Rev Clin Immunol. 2019;15(11):1185–1194. doi: 10.1080/1744666X.2020.1679625. [DOI] [PubMed] [Google Scholar]
- 2.Saunte D.M., Mrowietz U., Puig L., Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177(1):47–62. doi: 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 3.Sparber F., Ruchti F., LeibundGut-Landmann S. Host immunity to Malassezia in health and disease. Front Cell Infect Microbiol. 2020;10:198. doi: 10.3389/fcimb.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karray M., McKinney W.P. StatPearls [Internet] StatPearls Publishing; 2021. Tinea versicolor.https://www.ncbi.nlm.nih.gov/books/NBK482500/ [PubMed] [Google Scholar]
- 5.Koike Y., Kuwatsuka S., Nishimoto K., Motooka D., Murota H. Skin mycobiome of psoriasis patients is retained during treatment with TNF and IL-17 inhibitors. Int J Mol Sci. 2020;21(11):3892. doi: 10.3390/ijms21113892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik N., Pujalte G.G., Reese S.T. Superficial fungal infections. Prim Care. 2015;42(4):501–516. doi: 10.1016/j.pop.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Euvrard S., Kanitakis J., Cochat P., Cambazard F., Claudy A. Skin diseases in children with organ transplants. J Am Acad Dermatol. 2001;44(6):932–939. doi: 10.1067/mjd.2001.113465. [DOI] [PubMed] [Google Scholar]
- 8.Farahnik B., Beroukhim K., Zhu T.H., et al. Ixekizumab for the treatment of psoriasis: a review of phase iii trials. Dermatol Ther (Heidelb) 2016;6(1):25–37. doi: 10.1007/s13555-016-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparber F., LeibundGut-Landmann S. Interleukin-17 in antifungal immunity. Pathogens. 2019;8(2):54. doi: 10.3390/pathogens8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]


