Abstract
Osteosarcoma (OS) is the most common primary bone tumor in children and adolescents. It is an aggressive tumor with a tendency to spread to the lung, which is the most common site of metastasis. Patients with advanced OS with metastases have poor prognoses despite the application of chemotherapy, thus highlighting the need for novel therapeutic targets. The tumor microenvironment (TME) of OS is confirmed to be essential for and supportive of tumor growth and dissemination. The immune component of the OS microenvironment is mainly composed of tumor-associated macrophages (TAMs). In OS, TAMs promote tumor growth and angiogenesis and upregulate the cancer stem cell-like phenotype. However, TAMs inhibit the metastasis of OS. Therefore, much attention has been paid to investigating the mechanism of TAMs in OS development and the progression of immunotherapy for OS. In this article, we aim to summarize the roles of TAMs in OS and the major findings on the application of TAMs in OS treatment.
Keywords: Tumor-associated macrophage, Osteosarcoma, Tumor microenvironment
1. Introduction
Osteosarcoma (OS) is an aggressive tumor that occurs mainly in children and young adults. OS treatment strategies include chemotherapy and surgery, which provide 75% of patients with 5-year nonmetastatic disease survival. However, the 5-year survival rate for patients with metastatic disease is less than 30% (Danieau et al., 2019). In recent decades, various new drugs and treatment methods have been used to treat OS, but the overall survival rate of patients with metastatic disease has not been effectively improved (Whelan and Davis, 2018). At present, research on the molecular level of OS is focused on finding potential therapeutic targets.
The tumor microenvironment (TME) of OS is a very specialized, complex, and highly dynamic environment that is composed of numerous components, such as bone cells, mesenchymal stromal cells (MSCs), vascular cells, macrophages, and extracellular matrix (ECM) (Corre et al., 2020). The bone cells, vascular cells, and MSCs communicate with each other, which ensures bone homeostasis in a normal physiological state (Heymann et al., 2019). However, tumor cells can take advantage of the bone physiological microenvironment to survive and grow. OS and the TME act upon each other through multiple cytokines, chemokines, and soluble growth factors (Alfranca et al., 2015).
The immune component of the OS microenvironment is mainly composed of tumor-associated macrophages (TAMs) (Inagaki et al., 2016). TAMs promote OS development and progression via multiple pathways, by activating and protecting cancer stem cells (CSCs) and promoting angiogenesis. However, it is noteworthy that although TAMs have been shown to inhibit OS metastasis in previous studies, there have been no studies showing the mechanism. The purpose of this review is to summarize the roles of TAMs in OS and the major findings regarding the utilization of TAMs in OS treatment.
2. TAMs
Macrophages can engulf and digest foreign substances and also clear harmful matters such as tumor cells. Based on the conditions of the internal environment, such as the presence of chemokines, cytokines, and other factors secreted by tumor cells, MSCs, and immune cells, and the presence of local anoxia, inflammation, or high levels of lactic acid, monocytic cells in the blood are recruited to the TME and become TAMs (Lopez-Yrigoyen et al., 2020). Numerous studies have reported that TAMs have a close relationship with tumor development and always suggest a poor prognosis for patients with malignancies such as human breast, gastric, liver, oral, ovarian, bladder, and thyroid cancers, non-small-cell lung carcinoma (NSCLC), and Hodgkin's lymphoma (Zhang et al., 2012; Yin et al., 2017; Zhao et al., 2017; Tian et al., 2019).
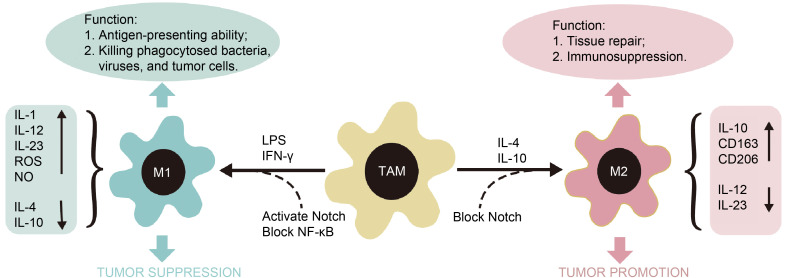
TAMs are induced to polarize into M1 and M2 types by the local microenvironment (Fig. 1); stimuli such as lipopolysaccharide (LPS) and interferon-γ (IFN-γ) could induce TAMs to polarize into M1 type. Interleukins (IL-1, IL-12, and IL-23), reactive oxygen species (ROS), and nitric oxide (NO) are highly expressed in M1-type TAMs. In contrast, IL-4 and IL-10 are expressed at low levels. In the TME, M1-type TAMs show strong antigen presentation abilities, which mainly serve to kill phagocytosed bacteria, viruses, and tumor cells, thereby inhibiting the occurrence and development of tumors. IL-4 or IL-10 could promote the polarization of TAMs to the M2 type. M2-type TAMs exhibit high expression of IL-10, the scavenger receptor cluster of differentiation 163 (CD163), and the mannose receptor CD206; they also exhibit low expression of IL-12 and IL-23. M2-type TAMs have weaker antigen presentation capabilities than M1-type TAMs and play important roles in tissue repair, immunosuppression, and tumor growth promotion (Xuan et al., 2015). It is worth noting that M2-type TAMs are further divided into four major subtypes based on their roles (M2a, M2b, M2c, and M2d). M1- and M2-type TAMs are just two extreme polarizations of TAMs; this classification has been used in recent studies and reviews (Gensel and Zhang, 2015).
Fig. 1. TAM polarization and the function of M1-type and M2-type TAMs. TAM, tumor-associated macrophage; M1, M1-type TAM; M2, M2-type TAM; IL, interleukin; LPS, lipopolysaccharide; IFN, interferon; ROS, reactive oxygen species; NO, nitric oxide; NF-κB, nuclear factor-κB; CD, cluster of differentiation.
Because TAMs affect various aspects of cancer progression, these cells are a new target for clinical therapeutic research. In addition, an increasing number of studies have shown that a high density of TAMs is associated with poor prognosis and positively correlated with the proliferation of a variety of cancer cells (Bingle et al., 2002; Han et al., 2019; Tiainen et al., 2020). By reducing the number of macrophages in tumor tissue, for example by blocking the recruitment of monocytes or clearing TAMs already present in tumor tissue, the growth of the primary tumor and the number of metastatic sites could be significantly reduced. In addition, attempts have been made to reprogram TAMs into inflammatory M1 macrophages, neutralize the tumor-associated products of TAMs, and use TAMs to introduce anticancer drugs into the tumor environment (Ngambenjawong et al., 2017). Thus, there is still much research potential for TAM-centered treatment strategies.
3. TAMs in OS
3.1. Effect of TAMs on OS growth
In most malignancies, TAMs are the M2 type, and can directly or indirectly promote tumor cell growth by secreting growth factors such as epidermal growth factor (EGF) (Klämbt, 2000; Lim et al., 2018). M2-type TAMs can promote endometrial cancer cell proliferation by upregulating the expression of cyclin D1 and inhibit breast cancer cell apoptosis by promoting B-cell lymphoma-2 (Bcl-2) expression (Hu et al., 2015; Yang et al., 2015). In OS, both M1- and M2-type TAMs are involved in tumor growth. Inducible nitric oxide synthase (iNOS, an M1-polarized macrophage marker) and CD163 (an M2-polarized macrophage marker) have been found to be correlated with the mitotic index, and CD163+ TAMs have been experimentally demonstrated to cause enhanced T cell depletion in OS patients (Dumars et al., 2016; Han et al., 2016). The recruited macrophages at the OS site were M2-type TAMs, which was demonstrated by implanting human OS into a mouse model. Moreover, tumor growth was decreased when these TAMs were deleted with specific macrophage-eliminating liposomes (Xiao et al., 2014). These experiments indicate that M2-type TAMs promote the development of OS. The M2-type polarization of TAMs is regulated by long noncoding RNAs (lncRNAs). LncRNA RP11-361F15.2 further affects the growth of OS cells by promoting development of cytoplasmic polyadenylation element-binding protein 4 (CPEB4)-mediated OS and M2-like polarization of TAMs in OS through miR-30c-5p. This RP11-361F15.2/miR-30c-5p/CPEB4 loop is a potential therapeutic strategy for the treatment of OS (Yang et al., 2020). However, IL-10-induced polarization of M2-type TAMs also inhibited OS cell growth when OS cell lines were cultured with cetuximab. Therefore, it is believed that M2-type TAMs may also inhibit the growth of OS under the action of some factors. M1-type TAMs polarized by lipid cell wall tripeptide together with IFN-γ could also inhibit OS cell growth (Pahl et al., 2014).
The growth of OS is regulated by a variety of signal transduction pathways and molecules. Eukaryotic translation elongation factor 1 delta (EEF1D) is highly expressed in OS and promotes proliferation of OS by promoting the protein kinase B (Akt)-mammalian target of rapamycin (mTOR) and Akt-Bad signaling pathways (Cheng et al., 2018). The phosphoinositide-3-kinase (PI3K)/Akt signaling pathway plays an important role in OS cells and the lncRNA LINC00628 can inhibit proliferation, invasion, and migration of OS cells by inactivating this pathway and promoting apoptosis (He et al., 2018). However, the mechanism of the effect of TAMs on OS growth needs to be studied further.
3.2. Effect of TAMs on angiogenesis in OS
TAMs secrete vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), IL-8, other proangiogenic factors, and various chemokines, including chemokine (C-X-C motif) receptors (CXCR2, CXCR4, and CXCR12), chemokine (C-X-C motif) ligands (CXCL3, CXCL4, and CXCL8–CXCL10), and chemokine (C-C motif) ligands (CCL2–CCL5). In addition, TAMs can secrete matrix metalloproteinases (MMPs), disrupt vascular stability, and induce tumor migration and metastasis (Rahma and Hodi, 2019). Studies have found these effects in a variety of malignant tumors, including glioblastoma (Peterson et al., 2016), breast cancer (Osinsky et al., 2011), and gastric cancer (Wu et al., 2012). Hypoxia is the main driving force for angiogenesis, and macrophages cover the hypoxic area of the tumor. Also, hypoxia inducible factor-1 (HIF-1) can redirect macrophages to tumor cells. TAMs regulate tumor angiogenesis by promoting the expression of VEGF in tumor tissues, which is an important factor associated with poor patient prognosis. In addition, HIF-1 can promote VEGF transcription (Lewis and Hughes, 2007; Murdoch et al., 2008). In breast cancer in which the HIF-1α gene was knocked out, TAMs were polarized toward the M2 type, but the angiogenic capacity of the tumor tissue was diminished (Werno et al., 2010). These findings require further study. In the OS microenvironment, IL-34 can enable M2 macrophages to accumulate in OS tissues and promote OS angiogenesis (Ségaliny et al., 2015). There have been few relevant studies of TAMs in OS neovascularization, and the mechanism should be further investigated.
Angiogenesis in OS is regulated by a variety of growth factors and signaling pathways. Li et al. (2019) found that overexpression of prolyl-hydroxylase-4 promotes endothelial cell neovascularization by stimulating transforming growth factor-β (TGF-β) expression in OS cells. Furthermore, the chemokine CCL5 increases VEGF expression via the protein kinase C δ (PKCδ)/c-Src/HIF-1α signaling pathway, promoting VEGF-dependent tumor angiogenesis in OS (Li et al., 2019). Therefore, VEGF is the most important influencing factor associated with OS angiogenesis. Infiltrating TAMs in OS tissues may induce OS neovascularization by promoting VEGF expression, a hypothesis which has not yet been experimentally investigated.
3.3. TAMs and OS metastasis
Tumor metastasis refers to the process in which a tumor expands to other sites distant from the primary tumor through various routes such as lymphatic vessels, blood vessels, or directly spreading and continuing to grow. Macrophages are present in large numbers in metastatic tumor lesions (Joyce and Pollard, 2009), and their roles in metastasis have gradually been recognized in recent years. Epithelial-mesenchymal transition (EMT) is the initial stage of tumorigenesis and metastasis (Liu et al., 2020). TAMs, MMP2, MMP7, and MMP9 released by tumor cells can degrade the matrix and induce the migration of tumor cells (Binnemars-Postma et al., 2018; Perry et al., 2018; Hegab et al., 2019). During tumor metastasis, TAMs degrade the ECM by upregulating MMP levels, and stimulate EMT by activating a variety of signal transduction pathways. Among them, M2-type TAMs activate the growth arrest-specific gene 6 (Gas6)/Axl-nuclear factor-κB (NF-κB) signal transduction pathway, promote EMT, and induce oral cancer metastasis (Lee et al., 2014). CCL18, a common chemokine released by M2 macrophages, is related to the proliferation and invasion phenotype of tumor cells. In breast cancer, M2-type TAMs can secrete the chemokine CCL18, which downregulates microRNA-98 (miRNA-98) and miRNA-27b gene expression, thus activating EMT and promoting metastasis (Lin et al., 2015).
Macrophages are highly plastic and can be polarized into M1 and M2 types in response to different stimuli. Imbalance of the M1/M2 ratio is a pathological sign of many inflammatory diseases, and has an important relationship with tumor prediction, metastasis, and prognosis (Jayasingam et al., 2020). Generally, highly infiltrating TAMs in most malignancies are mainly M2-type cells and can promote tumor metastasis. However, in high-grade OS, TAMs have the characteristics of both M1- and M2-type cells, and the higher the number of these M1/M2-type TAMs, the lower the probability of metastasis in OS patients and the longer the survival time, suggesting that TAMs that inhibit OS metastasis may be mainly characterized as M1-type TAMs (Buddingh et al., 2011). In nonmetastatic OS, M1-type TAM infiltration is significantly increased compared with that in metastatic OS. CD146+ cells are significantly increased in metastatic OS, and CD163+ M2 macrophages are positively correlated with CD146+ cells (Dumars et al., 2016). These results indicate that M1-type TAMs inhibit OS metastasis, while M2-type TAMs promote OS metastasis by expressing cellular factors. This effect may therefore be accompanied by a shift in the TAM phenotype during OS development.
In addition, TAMs promote OS cell migration and invasion by upregulating cyclooxygenase-2 (COX-2), MMP9, and phosphorylated signal transducer and activator of transcription-3 (p-STAT3); Han et al. (2019) observed that in OS cells co-cultured with TAMs, overexpression of COX-2 increased the expression of p-STAT3, which further promoted OS cell metastasis. However, no relationship has been found with TAM polarization, so this aspect requires further investigation and exploration.
3.4. Upregulation of the cancer stem cell-like phenotype by TAMs in OS
In the context of tumors, CSCs exhibit self-renewal and plasticity, as well as the ability to reconstitute heterogeneous tumor cell populations. CSCs have been found in various human cancers, such as breast, brain, colon, and pancreatic cancers, head and neck cancers, and melanoma (Bighetti-Trevisan et al., 2019). CSCs exhibit high metastatic potential and are resistant to traditional anticancer therapies. The resistance mechanism conferred by CSCs is mainly caused by factors such as cell quiescence, accumulation of adenosine triphosphate (ATP)-binding cassette (ABC) transporters, disruption of apoptosis, epigenetic reprogramming, and metabolism (Talukdar et al., 2016). CSCs were first isolated from OS in 2005, and surface markers unique to mesenchymal stem cells, such as CD133, CD177, and Sro-1, were highly expressed in CSCs (Gibbs et al., 2005; Brown et al., 2017). The CSC-like phenotype in OS is regulated by multiple pathways. The TGF-β1 and Notch signal transduction pathways can upregulate the CSC-like phenotype of OS, while the Wnt signal transduction pathway can inhibit upregulation of the CSC-like phenotype of OS (Yan et al., 2016). In OS cells, M2-type TAMs upregulate CSC markers (CD133, CXCR4, Nanog, and Oct4) by increasing OS cell numbers (Shao et al., 2019).
In most malignancies, TAMs promote CSC-like phenotype upregulation through different signal transduction pathways (Wan et al., 2014). One of the TGF-β1 signal transduction pathways is important in promoting the upregulation of the CSC-like phenotype in hepatocellular carcinoma, and TAMs induce EMT and upregulate the CSC-like phenotype by activating TGF-β1 (Fan et al., 2014). In addition, TAMs can also upregulate CSC-like phenotypes such as Scal-1 and ABCG2 by regulating the EGF receptor (EGFR)/STAT3/SRY-related HMG box-2 (SOX-2) signal transduction pathway (Balanis and Carlin, 2017). Therefore, it is also possible that TAMs in OS promote EMT and upregulate CSC-like phenotypes by activating signal transduction pathways. The mechanism by which TAMs upregulate the CSC-like phenotype of OS and the effect of CSCs on the polarization of TAMs in OS are not yet known.
3.5. Utilization of TAMs in OS treatment
In recent years, with the mechanistic study of TAMs and tumor progression, TAM-centered treatment regimens have received much attention. TAMs can produce two opposing effects during cellular chemotherapy and radiotherapy, both antagonizing antitumor activity by coordinating tumor promotion and tissue repair responses and promoting antitumor effects (Mantovani et al., 2017). Previous studies have shown that effective TAM-centered treatments include converting TAMs in the TME to M1-type TAMs, neutralizing the original tumor products of TAMs, introducing anticancer drugs into the tumor environment using TAMs, blocking the recruitment of monocytes in tumor tissues, and reducing the number of TAMs in tumors (Ngambenjawong et al., 2017). However, there have been few studies on TAMs for immune-targeted OS therapy.
A variety of chemical drugs have been found to inhibit the growth and metastasis of OS through TAMs (Table 1) (Uehara et al., 2019). Mifamurtide is an effective immunomodulator and has been approved in Europe for the treatment of nonmetastatic OS. Moreover, mifamurtide can inhibit cellular proliferation and induce OS differentiation by switching macrophage polarization toward a TAM-like intermediate M1/M2 phenotype (Punzo et al., 2020). Metformin can induce a reduction in myeloid-derived suppressor cells (MDSCs) and the transition of M2-type TAMs to M1-type TAMs in the TME, inhibiting the growth of OS (Uehara et al., 2019). Esculetin prevents adverse effects as well as the growth and metastasis of OS to the lung and liver through dual effects, inhibiting the differentiation of M2-type TAMs and abrogating the prevention of G1 cell cycle progression in tumor cells (Kimura and Sumiyoshi, 2015). Resveratrol and 2,3- and 4,4'-dihydroxystilbenes inhibit lymphangiogenesis by regulating the activation and differentiation of M2-type TAMs, ultimately inhibiting OS development and metastasis (Kimura and Sumiyoshi, 2016; Kimura et al., 2016). It was found that all-trans retinoic acid (ATRA) inhibited OS metastasis by inhibiting the M2 polarization of TAMs, and inhibited the colony-forming ability and replication of OS cells promoted by M2-type TAMs. The effects of ATRA are not dependent on the conventional M2-like polarized STAT3/6 or CCAAT/enhancer-binding protein β (C/EBPβ) signaling that regulates macrophages. On the other hand, ATRA significantly reduced the number of enhanced CSC markers (CD133, CXCR4, Nanog, and Oct4) (Zhou et al., 2017; Shao et al., 2019). These results indicate that ATRA is a chemical drug that can be used to treat OS by preventing M2 polarization of TAMs. The current study suggested a positive role for TAMs in the treatment of OS. TAMs can be applied in clinical practice as tools for adjuvant cell therapy and immunotherapy, and there is still a great deal of research potential for TAM-centered treatment strategies.
Table 1.
Summary of the TAM-centered functions of chemical drugs in OS
| Chemical drug | TAM-centered function | Role in OS | Reference | |
|---|---|---|---|---|
| Mifamurtide | Switching macrophage polarization towards a TAM-like intermediate M1/M2 phenotype | Inhibiting cellular proliferation and inducing OS differentiation | Punzo et al., 2016 | |
| Metformin | Inducing reduction of myeloid-derived suppressor cells and transition of M2-type TAMs to M1-type | Inhibiting growth of OS | Uehara et al., 2019 | |
| Esculetin | Inhibiting differentiation of M2 macrophages in TAM and inhibiting prevention of G1 phase | Inhibiting growth and metastasis of OS to the lung and liver | Kimura et al., 2015 | |
| Resveratrol | Regulating activation and differentiation of M2-type TAM | Inhibiting development and metastasis of OS | Kimura and Sumiyoshi, 2016 | |
| 2,3- and 4,4'-dihydroxystilbenes | Regulating activation and differentiation of M2-type TAM | Inhibiting development and metastasis of OS | Kimura et al., 2016 | |
| ATRA | Inhibiting M2 polarization of TAM | Inhibiting colony-forming and division-forming ability of OS cells | Zhou et al., 2017; Shao et al., 2019 |
OS, osteosarcoma; TAM, tumor-associated macrophage; ATRA, all-trans retinoic acid.
4. Conclusions
TAMs play an essential role in promoting OS growth and angiogenesis and in upregulating the OS cancer stem cell-like phenotype. The mechanisms of the latter two effects need more research. However, unlike other tumors, M1-type TAMs inhibit metastasis of OS. Reducing TAMs by various methods in order to inhibit the occurrence and development of OS is currently a research hotspot. Research on the role of TAMs in OS is still in the initial stage and faces many challenges. What is the major phenotype of TAMs in OS and is it related to the OS stage and differentiation degree? How does the OS microenvironment affect the TAM phenotype and what is the interaction mechanism between TAMs and OS? These and other similar questions call for supplementary studies and further experimentation.
Acknowledgments
This work was supported by the Natural Science Foundation of Hebei Province (No. H2019206309), China.
Funding Statement
This work was supported by the Natural Science Foundation of Hebei Province (No. H2019206309), China.
Author contributions
Yi ZHAO and Helin FENG designed the review. Benzheng ZHANG, Qianqian ZHANG, and Xiaowei MA searched references. Yi ZHAO and Benzheng ZHANG collated and summarized references. Yi ZHAO, Benzheng ZHANG and Helin FENG wrote the manuscript. All authors approved the final manuscript.
Compliance with ethics guidelines
Yi ZHAO, Benzheng ZHANG, Qianqian ZHANG, Xiaowei MA, and Helin FENG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Alfranca A, Martinez-Cruzado L, Tornin J, et al. , 2015. Bone microenvironment signals in osteosarcoma development. Cell Mol Life Sci, 72(16): 3097-3113. 10.1007/s00018-015-1918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanis N, Carlin CR, 2017. Stress-induced EGF receptor signaling through STAT3 and tumor progression in triple-negative breast cancer. Mol Cell Endocrinol, 451: 24-30. 10.1016/j.mce.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bighetti-Trevisan RL, Sousa LO, Castilho RM, et al. , 2019. Cancer stem cells: powerful targets to improve current anticancer therapeutics. Stem Cells Int, 2019: 9618065. 10.1155/2019/9618065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE, 2002. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol, 196(3): 254-265. 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- Binnemars-Postma K, Bansal R, Storm G, et al. , 2018. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J, 32(2): 969-978. 10.1096/fj.201700629R [DOI] [PubMed] [Google Scholar]
- Brown HK, Tellez-Gabriel M, Heymann D, 2017. Cancer stem cells in osteosarcoma. Cancer Lett, 386: 189-195. 10.1016/j.canlet.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Buddingh EP, Kuijjer ML, Duim RAJ, et al. , 2011. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res, 17(8): 2110-2119. 10.1158/1078-0432.ccr-10-2047 [DOI] [PubMed] [Google Scholar]
- Cheng DD, Li SJ, Zhu B, et al. , 2018. EEF1D overexpression promotes osteosarcoma cell proliferation by facilitating Akt-mTOR and Akt-Bad signaling. J Exp Clin Cancer Res, 37: 50. 10.1186/s13046-018-0715-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre I, Verrecchia F, Crenn V, et al. , 2020. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells, 9(4): 976. 10.3390/cells9040976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieau G, Morice S, Rédini F, et al. , 2019. New insights about the Wnt/β-catenin signaling pathway in primary bone tumors and their microenvironment: a promising target to develop therapeutic strategies? Int J Mol Sci, 20(15): 3751. 10.3390/ijms20153751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumars C, Ngyuen JM, Gaultier A, et al. , 2016. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget, 7(48): 78343-78354. 10.18632/oncotarget.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QM, Jing YY, Yu GF, et al. , 2014. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett, 352(2): 160-168. 10.1016/j.canlet.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Gensel JC, Zhang B, 2015. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res, 1619: 1-11. 10.1016/j.brainres.2014.12.045 [DOI] [PubMed] [Google Scholar]
- Gibbs CP, Kukekov VG, Reith JD, et al. , 2005. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia, 7(11): 967-976. 10.1593/neo.05394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QL, Shi HG, Liu F, 2016. CD163+ M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int Immunopharmacol, 34: 101-106. 10.1016/j.intimp.2016.01.023 [DOI] [PubMed] [Google Scholar]
- Han Y, Guo W, Ren TT, et al. , 2019. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett, 440-441: 116-125. 10.1016/j.canlet.2018.10.011 [DOI] [PubMed] [Google Scholar]
- He R, Wu JX, Zhang Y, et al. , 2018. LncRNA LINC00628 overexpression inhibits the growth and invasion through regulating PI3K/Akt signaling pathway in osteosarcoma. Eur Rev Med Pharmacol Sci, 22(18): 5857-5866. 10.26355/eurrev_201809_15915 [DOI] [PubMed] [Google Scholar]
- Hegab AE, Ozaki M, Kameyama N, et al. , 2019. Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts. J Pathol, 249(2): 193-205. 10.1002/path.5290 [DOI] [PubMed] [Google Scholar]
- Heymann MF, Lézot F, Heymann D, 2019. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell Immunol, 343: 103711. 10.1016/j.cellimm.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Hu HL, Bai HS, Pan HX, 2015. Correlation between TAMs and proliferation and invasion of type I endometrial carcinoma. Asian Pac J Trop Med, 8(8): 643-650. 10.1016/j.apjtm.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Hookway E, Williams KA, et al. , 2016. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin Sarcoma Res, 6: 13. 10.1186/s13569-016-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasingam SD, Citartan M, Tang TH, et al. , 2020. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol, 9: 1512. 10.3389/fonc.2019.01512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW, 2009. Microenvironmental regulation of metastasis. Nature Rev Cancer, 9(4): 239-352. 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, 2015. Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur J Pharmacol, 746: 115-125. 10.1016/j.ejphar.2014.10.048 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, 2016. Resveratrol prevents tumor growth and metastasis by inhibiting lymphangiogenesis and M2 macrophage activation and differentiation in tumor-associated macrophages. Nutr Cancer, 68(4): 667-678. 10.1080/01635581.2016.1158295 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, Baba K, 2016. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res, 36(1): 137-148. [PubMed] [Google Scholar]
- Klämbt C, 2000. EGF receptor signalling: the importance of presentation. Curr Biol, 10(10): R388-R391. 10.1016/S0960-9822(00)00485-1 [DOI] [PubMed] [Google Scholar]
- Lee CH, Liu SY, Chou KC, et al. , 2014. Tumor-associated macrophages promote oral cancer progression through activation of the Axl signaling pathway. Ann Surg Oncol, 21(3): 1031-1037. 10.1245/s10434-013-3400-0 [DOI] [PubMed] [Google Scholar]
- Lewis CE, Hughes R, 2007. Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res, 9(3): 209. 10.1186/bcr1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Liu Q, Tian J, et al. , 2019. Angiogenesis process in osteosarcoma: an updated perspective of pathophysiology and therapeutics. Am J Med Sci, 357(4): 280-288. 10.1016/j.amjms.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Lim B, Woodward WA, Wang XP, et al. , 2018. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat Rev Cancer, 18(8): 485-499. 10.1038/s41568-018-0010-y [DOI] [PubMed] [Google Scholar]
- Lin XR, Chen LJ, Yao YD, et al. , 2015. CCL18-mediated down-regulation of miR98 and miR27b promotes breast cancer metastasis. Oncotarget, 6(24): 20485-20499. 10.18632/oncotarget.4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Sun J, Yu W, et al. , 2020. Quantum dot-pulsed dendritic cell vaccines plus macrophage polarization for amplified cancer immunotherapy. Biomaterials, 242: 119928. 10.1016/j.biomaterials.2020.119928 [DOI] [PubMed] [Google Scholar]
- Lopez-Yrigoyen M, Cassetta L, Pollard JW, 2020. Macrophage targeting in cancer. Ann N Y Acad Sci, 242: 119928. 10.1111/nyas.14377 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Marchesi F, Malesci A, et al. , 2017. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol, 14(7): 399-416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, et al. , 2008. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer, 8(8): 618-631. 10.1038/nrc2444 [DOI] [PubMed] [Google Scholar]
- Ngambenjawong C, Gustafson HH, Pun SH, 2017. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev, 114: 206-221. 10.1016/j.addr.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinsky S, Bubnovskaya L, Ganusevich I, et al. , 2011. Hypoxia, tumour-associated macrophages, microvessel density, VEGF and matrix metalloproteinases in human gastric cancer: interaction and impact on survival. Clin Transl Oncol, 13(2): 133-138. 10.1007/s12094-011-0630-0 [DOI] [PubMed] [Google Scholar]
- Pahl JHW, Kwappenberg KMC, Varypataki EM, et al. , 2014. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-γ. J Exp Clin Cancer Res, 33: 27. 10.1186/1756-9966-33-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Muñoz-Rojas AR, Meeth KM, et al. , 2018. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med, 215(3): 877-893. 10.1084/jem.20171435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TE, Kirkpatrick ND, Huang YH, et al. , 2016. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci USA, 113(16): 4470-4475. 10.1073/pnas.1525349113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo F, Bellini G, Tortora C, et al. , 2020. Mifamurtide and TAM-like macrophages: effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget, 11(7): 687-698. 10.18632/oncotarget.27479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahma OE, Hodi FS, 2019. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res, 25(18): 5449-5457. 10.1158/1078-0432.ccr-18-1543 [DOI] [PubMed] [Google Scholar]
- Ségaliny AI, Mohamadi A, Dizier B, et al. , 2015. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer, 137(1): 73-85. 10.1002/ijc.29376 [DOI] [PubMed] [Google Scholar]
- Shao XJ, Xiang SF, Chen YQ, et al. , 2019. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol Sin, 40(10): 1343-1350. 10.1038/s41401-019-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Emdad L, Das SK, et al. , 2016. Evolving strategies for therapeutically targeting cancer stem cells. Adv Cancer Res, 131: 159-191. 10.1016/bs.acr.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Tiainen S, Masarwah A, Oikari S, et al. , 2020. Tumor microenvironment and breast cancer survival: combined effects of breast fat, M2 macrophages and hyaluronan create a dismal prognosis. Breast Cancer Res Treat, 179(3): 565-575. 10.1007/s10549-019-05491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian ZQ, Hou XJ, Liu WT, et al. , 2019. Macrophages and hepatocellular carcinoma. Cell Biosci, 9: 79. 10.1186/s13578-019-0342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Eikawa S, Nishida M, et al. , 2019. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol, 31(4): 187-198. 10.1093/intimm/dxy079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan SS, Zhao ED, Kryczek I, et al. , 2014. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology, 147(6): 1393-1404. 10.1053/j.gastro.2014.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werno C, Menrad H, Weigert A, et al. , 2010. Knockout of HIF-1α in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis, 31(10): 1863-1872. 10.1093/carcin/bgq088 [DOI] [PubMed] [Google Scholar]
- Whelan JS, Davis LE, 2018. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol, 36(2): 188-193. 10.1200/jco.2017.75.1743 [DOI] [PubMed] [Google Scholar]
- Wu H, Xu JB, He YL, et al. , 2012. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol, 106(4): 462-468. 10.1002/jso.23110 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zhang XP, Wu YY, et al. , 2014. Inhibition of macrophage polarization prohibits growth of human osteosarcoma. Tumour Biol, 35(8): 7611-7616. 10.1007/s13277-014-2005-y [DOI] [PubMed] [Google Scholar]
- Xuan WJ, Qu Q, Zheng B, et al. , 2015. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukocyte Biol, 97(1): 61-69. 10.1189/jlb.1A0314-170R [DOI] [PubMed] [Google Scholar]
- Yan GN, Lv YF, Guo QN, 2016. Advances in osteosarcoma stem cell research and opportunities for novel therapeutic targets. Cancer Lett, 370(2): 268-274. 10.1016/j.canlet.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Yang CX, He LY, He PQ, et al. , 2015. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol, 32(2): 14. 10.1007/s12032-014-0352-6 [DOI] [PubMed] [Google Scholar]
- Yang D, Liu KY, Fan L, et al. , 2020. LncRNA RP11-361F15. 2 promotes osteosarcoma tumorigenesis by inhibiting M2-like polarization of tumor-associated macrophages of CPEB4. Cancer Lett, 473: 33-49. 10.1016/j.canlet.2019.12.041 [DOI] [PubMed] [Google Scholar]
- Yin SC, Huang JY, Li Z, et al. , 2017. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PLoS ONE, 12(1): e0170042. 10.1371/journal.pone.0170042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QW, Liu L, Gong CY, et al. , 2012. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE, 7(12): e50946. 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XX, Qu JK, Sun YC, et al. , 2017. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget, 8(18): 30576-30586. 10.18632/oncotarget.15736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Xian M, Xiang SF, et al. , 2017. All-trans retinoic acid prevents osteosarcoma metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol Res, 5(7): 547-559. 10.1158/2326-6066.cir-16-0259 [DOI] [PubMed] [Google Scholar]