Highlights
-
•
A novel α1,2-fucosyltransferase was cloned from Thermosynechococcus.
-
•
Tsα1,2-FT catalyzes one-pot multi-enzyme synthesis of lacto-N-fucopentaose-I.
-
•
LNFP-I was verified to promote the growth of intestinal probiotics.
Keywords: Carbohydrate, Enzymatic synthesis, Fucosyltransferase, Fucosylated oligosaccharides, Lacto-N-fucopentaose I, Thermosynechococcus
Abstract
A novel bacterial α 1–2-fucosyltransferase (α 2FT) from Thermosynechococcus sp. NK55a (Ts2FT) has been discovered and characterized. It shares 28–62% protein sequence homology to α 2FTs reported previously. The Ts2FT was cloned as an N-terminal His6-tagged recombinant protein (His6-Ts2FT) and expressed in E. coli BL21 (DE3). It was expressed at a level of 6.2 mg/L culture after induction with 0.05 mM of isopropylβ-d-1-thiogalactoside (IPTG) at 16 °C for 20 h. It showed the optimal activity at a reaction temperature of 40 °C and pH of 7.0. The presence of a Mg2+ improved its catalytic efficiency. Ts2FT displayed a strict acceptor specificity and could recognize only β1–3-galatoside acceptors. It was used efficiently for one-pot multienzyme synthesis of fucosylated oligosaccharides. One of the products, lacto-N-fucopentaose I was shown to promote the growth of intestinal probiotics including those belonging to Acidobacteria, Actinobacteria, Proteobacteria, Planctomycetes, and Chloroflexi.
1. Introduction
Naturally occurring fucose-containing carbohydrates (fucosides) play important roles. For example, fucosides on cell surface are involved in many important biological processes such as fertilization, inflammation, and cancer metastasis (Gao et al., 2020). On the other hand, fucose-containing human milk oligosaccharides (HMOS) have shown to be prebiotics (Walsh, Lane, van Sinderen, & Hickey, 2020). Nevertheless, the functional study of fucose-containing glycans is limited due to the lack of access to biologically relevant structurally defined compounds in sufficient amounts. Among fucosides, HMOS deserve considerable attention due to their benefits for breast-fed infants. These fucosylated oligosaccharides exhibit bifidogenic effect in the gut, which enrich beneficial microorganisms and inhibit undesirable microorganisms. In addition, they play a significant role in the prevention of respiratory, urinary tracts, and gut infectious diseases of infants (Cheng et al., 2021, Walker and Iyengar, 2015). Especially, fucosylated HMOS have been reported to be growth stimulating factors for selected Bifidobacteria strains (Zhao et al., 2017). They have also been shown to exhibit protective activity against infections from enteric pathogens by inhibiting the binding of their surface adhesins and the toxins that they release (Newburg, Ruiz-Palacios, Mekibib, Prasoon, Jareen, Guerrero, & Morrow, 2004).
Various chemical and enzymatic strategies have been developed to obtain structurally defined synthetic oligosaccharides. Chemical synthesis of carbohydrates is challenging due to the numerous protection/deprotection steps, the large amounts of toxic reagents, and harsh reaction conditions required (Petschacher and Nidetzky, 2016, Zeuner and Meyer, 2020, Zhao et al., 2017). Enzymatic synthesis using glycosyltransferases or glycosidases from prokaryotes and eukaryotes does not require harsh chemicals and is economically friendly. It is an alternative strategy for commercial production of structurally complex carbohydrates. Fucosyltransferases (FTs) are inverting glycosyltransferases that catalyze the transfer of l-fucose from donor guanosine-5′-diphosphate β-l-fucose (GDP-fucose) to acceptors for the formation of α -fucosides. They have been found in both prokaryotic and eukaryotic organisms. Based on the types of linkages that they form, FTs are classified into α 1–2, α 1–3, α 1–4, α 1–3/4, α 1–6, and O-fucosyltransferases (Pang et al., 2007). All α 1–2 fucosyltransferases (α 2FTs) characterized so far belong to carbohydrate active enzyme (CAZy) glycosyltransferase family 11 (http://www.cazy.org/fam/acc_GT.html) and are responsible for catalyzing the transfer of l-fucose from GDP-fucose to galactose in the acceptor to form an α 1–2-linkage in the Fuc α 2Gal-containing products.
Lacto-N-fucopentaose I (LNFP-I) is one of fucosylated HMOS that has been reported to provide various health benefits for breast-fed infants. It is a promising pharmaceutical and nutraceutical ingredient. There is therefore an urgent need for its large-scale production. Extraction of LNFP-I from cow’s milk is not feasible due to its low concentration and extremely high cost (Bode et al., 2016, Phipps et al., 2021). Enzymatic synthesis of LNFP-I has been achieved using bacterial α 2FTs. Seven bacterial α 2FTs have been cloned and characterized so far including Escherichia coli O126 WbgL (EcWbgL), E. coli O128 WbsJ (EcWbsJ), E. coli O86:B7 WbwK (EcWbwK), E. coli O86:K62:H2 WbnK (EcWbnK), E. coli O127:K63(B8) WbiQ (EcWbiQ), Helicobacter pylori FutC (HpFutC or Hp2FT), and Thermosynechococcus elongatus BP-1 (Te2FT) (Baumgärtner et al., 2013, Engels and Elling, 2014, Li et al., 2008a, Li et al., 2008b, Pettit et al., 2010, Zhao et al., 2017). In addition, Helicobacter mustelae α 1–2-fucosyltransferase (Hm2FT) has been cloned and used for enzymatic and chemoenzymatic synthesis (Xiao et al., 2016, Ye et al., 2019), but its biochemical characterization has not been reported. Their expression levels have room for further improvement. Here, the identification and characterization of a new α 2FT from thermophilic cyanobacterium Thermosynechococcus sp. NK55a (Ts2FT) are reported. It has been used as an efficient catalyst in a one-pot multienzyme (OPME) fucosylation system for synthesizing LNFP-I and other fucosides.
2. Materials and methods
2.1. Bacteria strains, plasmids, and materials
E. coli BL21 (DE3), plasmid vector pET15b, and nickel-nitrilotriacetic acid agarose (Ni-NTA agarose) were purchased from Sangon (Shanghai, China). Spin miniprep kit and gel extraction kit were obtained from Bioman (Fuzhou, China). The restriction enzymes NdeI and BamHI, Taq DNA polymerase, and T4 DNA ligase were from Beyotime (Shanghai, China).
2.2. Cloning of His6-Ts2FT
The procedures were carried out similarly to that described previously (Zhao et al., 2017). Briefly, the gene encoding Ts2FT (GenBank accession number NC_023033.1) was amplified from genomic DNA of Thermosynechococcus sp. NK55a by polymerase chain reactions (PCRs) using primers designed with reference (Albermann, Piepersberg, & Wehmeier, 2001). The forward primer was 5′-CATATGATATTATTTTGGTC-3′ and the reverse primer was 5′-GGATCCTTATTTATCAATAACGAAGG-3′. The PCR reaction was carried out with an initial cycle of 30 s at 95 °C, followed by 30 cycles of 30 s at 95 °C, 30 s at 56 °C, and 1 min at 68 °C. The final extension was accomplished by incubating the reaction mixture at 68 °C for 10 min. The PCR product and the vector plasmid pET15b were digested with NdeI and BamHI restriction enzymes, purified using the DNA Purification Kit (Beyotime, Shanghai, China), and ligated using T4 DNA ligase. The resulting mixture was transformed into E. coli BL21 (DE3) competent cells and plated on a Luria-Bertani (LB)-agar plate containing 100 μg/mL ampicillin. Selectively colonies were cultured and plasmids were extracted and assayed by restriction mapping and DNA sequencing.
2.3. Overexpression and purification of His6-Ts2FT
E. coli BL21 (DE3) cells harboring the recombinant plasmid were cultured in LB medium at 37 °C. Once the OD600 reached 0.6, the cells were inducted by adding isopropyl-1-thio-β-d-galactosylpyranoside (IPTG) to reach a final concentration of 0.05 mmol/L. After incubation at 16 °C for 20 h, cells were collected by centrifugation (9391×g) for 10 min and stored at −80 °C until next step. The cell pellets were disrupted by sonication in lysis buffer (pH 8.0, 100 mmol/L Tris-HCl containing 0.1% Triton X-100) on ice. The cell lysate was clarified by centrifugation at 15871×g for 20 min at 4 °C. And the supernatant was purified using a Ni-NTA column, washed with 8 column volumes of binding buffer (5 mmol/L imidazole, 0.5 mol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5) and 10 column volumes of washing buffer (50 mmol/L imidazole, 0.5 mol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5), and then eluted with elution buffer (200 mmol/L imidazole, 0.5 mol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5). Protein expression was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
2.4. Fucosyltransferase activity studies
A one-pot multienzyme (OPME) fucosylation system was used for α 2FT activity screening (Fig. 1). Assays were carried out in a total volume of 10 μL in Tris-HCl buffer (200 mM, pH 8.0) containing 1.5 mmol/L adenosine-triphosphate (ATP), 1.5 mmol/L guanosine-triphosphate (GTP), 10 mmol/L MgCl2, 1 mmol/L l-fucose, 1 mmol/L acceptor, Bacteroides fragilis l-fucokinase/GDP-fucose pyrophosphorylase (BfFKP, 0.2 μg) (Yi et al., 2009), Pasteurella multocida inorganic pyrophosphatase (PmPpA, 0.2 μg) (Lau et al., 2010), and the recombinant His6-Te2FT (0.3 μg). The acceptor substrates Gal β 1–3GalNAc α ProN3, Gal β 1–3GalNAc β ProN3, Gal β 1–3GlcNAc α ProN3, and Gal β 1–3GlcNAc β ProN3 were synthesized as described previously (Yu et al., 2010). The others (Table 1) were purchased from Elicityl (Crolles, France). These constituents were then mixed and incubated at 37 °C for 20 h. After incubation, the same volume of ethanol was added and the mixture was centrifuged. The supernatant was diluted 5 times with ddH2O and submitted for mass spectrometry analysis.
Fig. 1.
One-pot multienzyme (OPME) synthesis of fucosylated oligosaccharides. Enzymes used: (a) BfFKP; (b) BfFKP; (c) His6-Ts2FT.
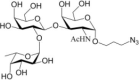
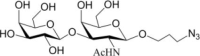
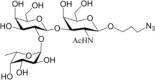
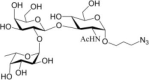
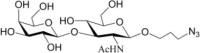
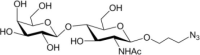
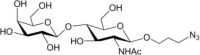
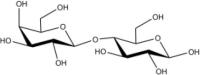
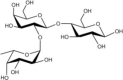
Table 1.
Acceptor substrate specificity of His6-Ts2FT. “−”, no activity detected.
| Acceptors | Products | % Conversion |
|---|---|---|
 |
40 | |
 |
 |
25 |
 |
 |
36 |
 |
 |
28 |
 |
 |
30 |
 |
 |
10 |
 |
 |
10 |
 |
– | |
 |
 |
– |
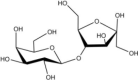 |
 |
– |
2.5. Effects of temperature, pH, and metal ions on the activities of His6-Ts2FT
The samples were analyzed by a Shimadzu Prominence LC-20A system equipped with a membrane online degasser, a temperature control unit and a fluorescence detector. A reverse-phase Premier C18 column (250 × 4.0 mm i.d., 5 μm, Shimadzu) was used. The mobile phase was acetonitrile/water (65:35), 1% formic acid at 40 °C at a flow rate of 0.2 mL/min. Glycan-containing fractions were analyzed by mass spectrometry. The activities of His6-Ts2FT at different temperatures (15–60 °C, with an increase of 5 °C from the starting point) were determined in a total volume of 10 μL in Tris-HCl buffer (200 mmol/L, pH 8.0) containing 1 mmol/L GDP-fucose, 1 mmol/L lacto-N-tetraose (LNT), 20 mmol/L MgCl2, and the recombinant enzyme (1.5 μg). All reactions under different temperatures were proceeded for 10 min, and subsequently stopped after boiling for 2 min. The activities of His6-Ts2FT in the presence of different pH ranging from 3.0 to 9.0 were assayed under the similar conditions at 40 °C with different buffers including citric acid-Na2HPO4 for pH 3.0–4.0, MES for pH 5.0–6.5, and Tris-HCl for pH 7.0–9.0. Metal cations effect assays were carried out similarly at pH 7.0 in Tris-HCl buffer (200 mmol/L) and 40 °C with the exception of using different concentrations (5 mmol/L, 10 mmol/L, and 20 mmol/L) of MgCl2 or in the presence of 10 mmol/L ethylenediaminetetraacetic acid (EDTA). The reaction without EDTA and metal ions was used as a control.
2.6. One-pot three-enzyme synthesis of LNFP-I
The synthesis was carried out in Tris-HCl buffer (8 mL, 100 mM, pH 7.5) containing LNT (50 mg, 1 equiv.), l-fucose (1.3 equiv.), ATP (1.3 equiv.), and GTP (1.3 equiv.), MgCl2 (20 mM), BfFKP (1.5 mg), PmPpA (1.0 mg), and His6-Ts2FT (2.0 mg). The reaction mixture was incubated at 40 °C for 1–2 days in an incubator shaker with agitation at 80 rpm. The product formation was monitored by mass spectrometry. When the reaction reached completion, the same amount of cold ethanol was added and kept on ice for 30 min and then the mixture was centrifuged. The supernatant was dried and purified by a BioGel P-2 gel filtration column. The fractions containing the pure product were combined, freeze-dried, and stored at −80 °C. The product identity and purify were confirmed by nuclear magnetic resonance analysis.
2.7. Caenorhabditis elegans strain and experimental design
E. coli OP50 and living C. elegans N2 strain were obtained from Shangyuan Biotechnology Co. Ltd. (Fuzhou, China). The worms at the same growth period were lysed by sodium hypochlorite. E. coli OP50 was cultured in LB medium at 37 °C for overnight and 1 mL of the resulting E. coli OP50 culture was added to each nematode growth medium (NGM) plate (Seo, Kingsley, Walker, Mondoux, & Tissenbaum, 2018). Fifty or more cultured C. elegans worms were added to each freshly prepared NGM plate and cultured at 20 °C for 72 h until the L4 stage before the addition of LNFP-I at 0.2 mg/mL for triplicates. The LNFP-I was dissolved in the Dulbecco’s Modified Eagle Medium (DMEM). Samples with the same volume of DMEM were used as the negative control.
2.8. DNA extraction and sequencing
The C. elegans intestinal microbial DNA was extracted by MOBIO PowerSoil DNA Isolation Kit (3bio Technolog, Shanghai, China) and used as templates for PCR amplification. The primers used to amplify the bacterial 16S rRNA fourth hypervariable (V4) region were 5′-GTGCCAGCMGCCGCGGTAA-3′ and 5′-GGACTACHVGGGTWTCTAAT-3′. PCR conditions were as follows: pre-denaturation at 94 °C for 5 min followed by 32 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 60 s. A final extension was at 72 °C for 17 min. The PCR products were sequenced using an Illumina Miseq PE 250 high-throughput sequencing platform. Bioinformatic analysis of sequencing results was processed using QIIME (Quantitative Insights into Microbial Ecology Release 1.8.0) software.
2.9. Statistical analysis
All data described above were presented as mean ± SD. One-way analysis of variance (ANOVA) was employed to compare the average means of multiple groups. P-value less than 0.05 and 0.01 was considered statistically to be significant.
3. Results and discussion
3.1. Cloning and analysis of His6-Ts2FT
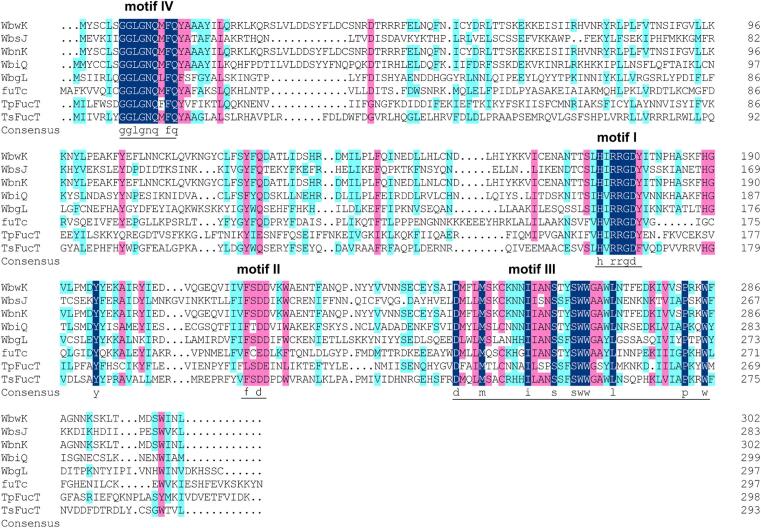
The gene encoding Ts2FT was successfully cloned in pET15b vector (Fig. S1) and DNA sequencing confirmed that its sequence was identical to that reported for Thermosynechococcus sp. NK55a α 1–2-fucosyltransferase (GenBank accession number AHB87954.1). Ts2FT, a member of the glycosyltransferase family GT11 in the CAZy database (Coutinho et al., 2003, Li et al., 2008a), showed 62% amino acid identity to Te2FT (Petschacher and Nidetzky, 2016, Zhao et al., 2017) and a low level (28.0–29.7%) of sequence identities to other characterized α 2FTs. GT11 family α 2FTs have been identified from diverse species from human and other mammals to bacteria and virus (Bing, Simala-Grant, & Taylor, 2006). The sequence alignment revealed that Ts2FT contained several conserved motifs Ⅰ–Ⅳ which were identified to be containing catalytic sites (Fig. 2). The highly conserved motif I containing H152*R154R155*D157 was the potential binding site of the donor GDP-fucose (Ihara et al., 2007). Moreover, the R154 shared by α 2FTs was likely to interact with the phosphate group of GDP-fucose directly. R154, R155, and D157 were found to have great significance on enzyme activities of α 2FTs (Li et al., 2008a).
Fig. 2.
Alignment of Ts2FT (GenBank: AHB87954.1), T. elongatus Te2FT (UniProtKB: Q8DK72, GenBank: BAC08546.1), H. pylori FutC (UniProtKB:A4L7J1), E. coli O86:B7 WbwK (GenBank:AAO37719.1), E. coli O86:K62:H2 WbnK (UniProtKB:Q58YV9), E. coli O126 WbgL (UniProtKB: A6M9C2), E. coli O127:K63 WbiQ (UniProtKB:Q5J7C6), and E. coli O128:B12 WbsJ (UniProtKB:Q6XQ53). The sequence alignment of the investigated genes indicates four common motifs I–IV. The highly conserved motif I (H162xR164R165xD167) suggests a potential binding site for GDP-fucose. Residues R164 and D167 were indicated to play critical roles in donor binding and enzyme activity.
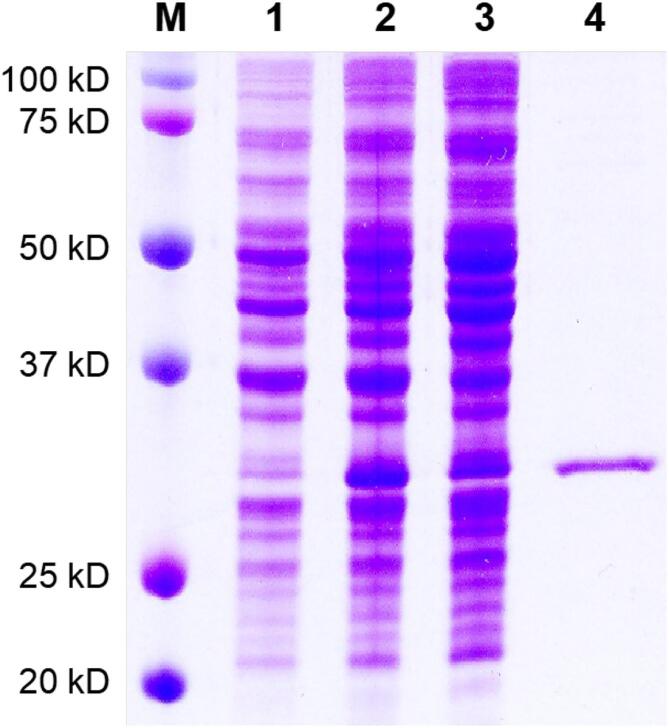
3.2. SDS-PAGE of His6-Ts2FT
The expression of His6-Ts2FT was carried out in LB medium at 16 °C for 20 h after induction with 0.05 mmol/L IPTG. SDS-PAGE (12%) showed that the fusion recombinant protein His6-Ts2FT was successfully purified using Ni-NTA column (Fig. 3). The apparent molecular weight of the purified protein was approximate 35 kDa, matching to its calculated molecular weight. The expression level of His6-Ts2FT was 6.2 mg/L culture, which was lower than Te2FT but higher than other reported α 2FTs (Engels and Elling, 2014, Zhao et al., 2017).
Fig. 3.
SDS-PAGE analysis of His6-Ts α 1,2FT expression and purification. Lanes: M, protein standards; 1, whole cell extraction before induction; 2, whole cell extraction after induction; 3, cell lysate after induction; 4, Ni2+-column purified protein.
3.3. Determination of acceptor substrate specificity of His6-Ts2FT
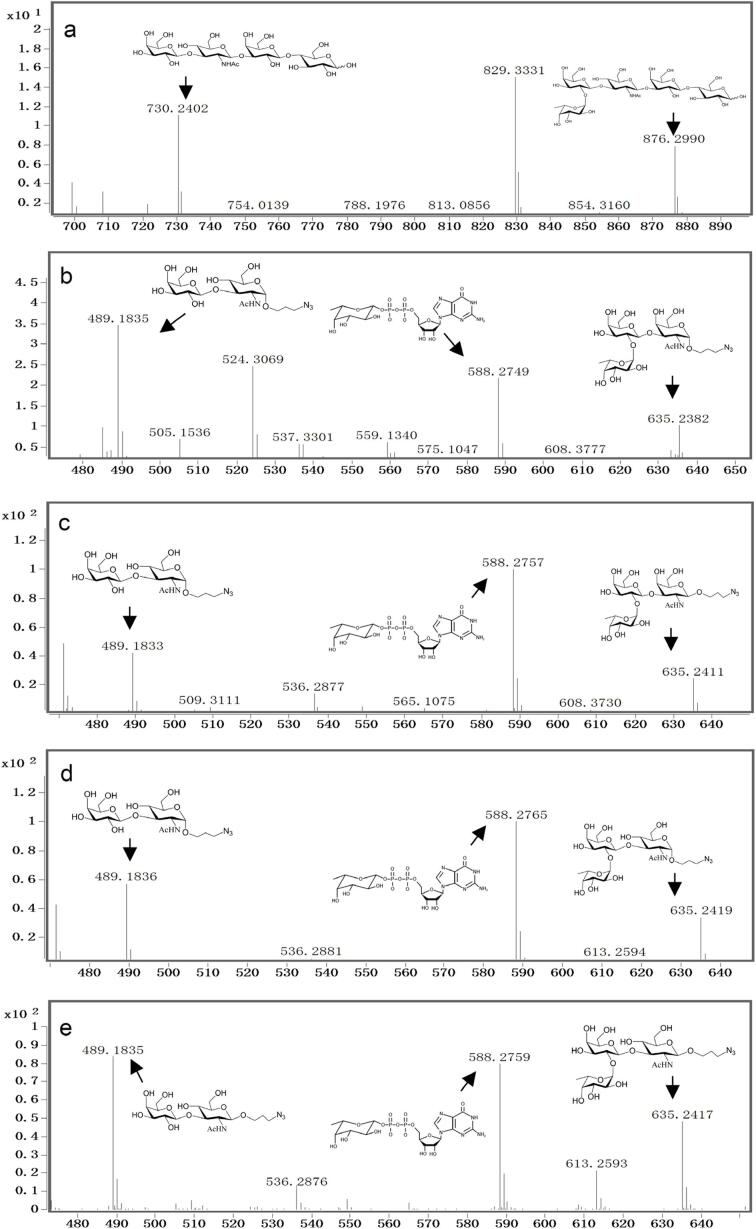
Common acceptors for FTs other than O-fucosyltransferases are galactosides. A panel of ten glycosides with different lengths and various glycosyl linkages were tested as potential acceptor substrates for the purified His6-Ts2FT (Table 1). The results showed that five of the galactosides tested were well suited acceptor substrates for His6-Ts2FT (representative mass spectrometry chromatograms are shown in Fig. 4). In general, Ts2FT was highly active toward LNT and other β 1–3-galactoside acceptors. Among these acceptors, the highest conversion (>98%) was observed for Galβ1–3GlcNAcβProN3. β1–4-Galactosides such as LNnT, LacNAcβProN3, LacβProN3, and lactose were not suitable acceptor substates for His6-Ts2FT.
Fig. 4.
Mass spectrum of the products synthesized by Ts2FT with acceptor substrates. LNT (a), Galβ1–3GalNAc α ProN3 (b), Galβ1–3GalNAcβProN3 (c), Galβ1–3GlcNAc α ProN3 (d), Galβ1–3GlcNAcβProN3 (e).
3.4. Effects of temperature, pH, and metal ions on His6-Ts2FT activity
Temperature profile studies of His6-Ts2FT carried out by varying reaction temperatures from 15 °C to 60 °C showed that it was active in a wide temperature range of 30–50 °C with an optimal catalytic efficiency observed at 40 °C (Fig. S2a). The pH profile studies using LNT as the acceptor indicated that His6-Ts2FT was active in the broad pH range from 6.0 to 8.0 (Fig. S2b). An optimum pH was found at pH 7.0. The effects of EDTA and divalent metal cation Mg2+ on Ts2FT activity were investigated (Fig. S2c). The results revealed that enzyme activity was at approximately the same level in the addition of 10 mmol/L EDTA, and it was increased to about two-fold with 20 mmol/L of Mg2+. Crystal structural analysis has shown that the catalytic domains of glycosyltransferase fall into GT-A, GT-B, GT-C, and CstII folds (Breton et al., 2006, Moremen and Haltiwanger, 2019). GT-A fold shares a DxD or ExD motif and a divalent metal is required for activity. GT-B fold enzymes contain two Rossmann-like folded domains and do not require divalent cations for activity. In comparison, X-ray crystal structures of GT11 family enzymes have not been reported. However, the data suggest that Ts2FT has probably the metal-dependent catalytic mechanism.
3.5. Enzymatic synthesis of α 1–2-linked fucoside LNFP-I
LNFP-I was synthesized in a preparative scale (57.3 mg) with an excellent 95% yield using a one-pot multienzyme (OPME) reaction containing GDP-fucose, LNT, and purified Ts2FT. In order to confirm the linkage and the structure, the purified LNFP-I was characterized by 1H- and 13C- nuclear magnetic resonance analysis (Data not shown). The spectra obtained were consistent to those reported previously (Zhao et al., 2017). Ts2FT exhibited narrow acceptor specificity. Ts2FT acted well on type 1 (Galβ1–3GlcNAc-), type 3 (Galβ1–3GalNAc α -), and type 4 (Galβ1–3GalNAcβ-) acceptors but not lactose and lactulose, both having β-d-Gal residue at the non-reducing end. The preference of Ts2FT for Galβ1–3- compared to Galβ1–4- structures is in agreement with the properties of FutC from H. pylori and Te2FT from T. elongatus (Wang et al., 1999, Zhao et al., 2017). LNFP-I is an abundant HMOS in pooled human milk (Kunz et al., 2000, Torres Roldan et al., 2020) which has shown high potential to regulate intestinal probiotics. The one-pot multienzyme synthesis system presented here is an economically feasible and efficient approach for the synthesis of LNFP-I, which can be readily adapted for large-scale industrial production (Zhao et al., 2017).
3.6. Effect of LNFP-I on gut microbiota composition of C. elegans
The variation in gut microbiota of C. elegans treated with or without the addition of LNFP-I was analyzed using principal component analysis (PCA). The score plots indicated that LNFP-I treatment significantly altered the composition of the intestinal flora compared with the group feeding normal diet (NFD) (Fig. 5a). To further investigate the different alterations of intestinal flora, the differences in the relative abundance among groups were determined using the linear discriminant analysis (LDA) score (Fig. 5b). The threshold on the logarithmic LDA score for discriminative features is set to 4.0. Hierarchical clustering also verified that gut microbiota was divided into two different groups (Fig. 5c). Compared to the NFD group, LNFP-I-treated group was featured by a significant increase of Acidobacteria, Actinobacteria, Proteobacteria, Planctomycetes, and Chloroflexi. However, the higher abundances of Bacteroidetes were obtained in the NFD group. The fucosylated milk oligosaccharide LNFP-I treatment has significantly altered the composition of gut flora. Further investigations are required to reveal the specific mechanism of probiotics from LNFP-I.
Fig. 5.
Effect of LNFP-I on the composition of gut microbiota. Principal component analysis plots (a), linear discriminant analysis (LDA) effect size (LEfSe) comparison of gut microbiota between NFD and LNFP-I-treated groups (b), and heatmap comparison and hierarchical clustering dendrogram (c).
4. Conclusions
In this work, a new bacterial α 2FT from Thermosynechococcus was identified, purified, and characterized. The homology of amino acid sequence displayed 28–62% to the previously published α 2FTs. This result revealed that Ts2FT had the well novelty. The yield of Ts2FT was 6.2 mg/L with the condition of induction temperature at 16 °C and IPTG concentration with 0.05 mM. The optimum temperature and pH of Ts2FT were 40 °C and 7.0, respectively. Mg2+ can improve its catalytic efficiency. With respect to substrate specificity, Ts2FT had obvious catalytic activities on lacto-N-tetraose, Galβ1–3GalNAc α ProN3, Galβ1–3GalNAcβProN3, Galβ1–3GlcNAc α ProN3, and Galβ1–3GlcNAcβProN3. LNFP-I synthesized by lacto-N-tetraose as receptor through the one-pot multienzyme fucosylation system could observably promote the growth of intestinal probiotics, and LNFP-I administration dramatically increased the abundance of Acidobacteria, Actinobacteria, Proteobacteria, Planctomycetes, and Chloroflexi. To date, there have been only reported eight prokaryotic α 2FTs. This study has already expanded the library of α 2FT and provided a strong support for the synthesis and biological research of fucosylated oligosaccharides.
CRediT authorship contribution statement
Ruting Zhong: Investigation, Formal analysis, Writing – original draft. Luying Gao: Writing – review & editing. Zhengxin Chen: Writing – review & editing. Sinan Yuan: Formal analysis. Xi Chen: Writing – review & editing. Chao Zhao: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by Double First-Class Construction Plan (KSYLX013) of Fujian Agriculture and Forestry University. The project was also supported by Key Project of the Natural Science Foundation of Fujian Province (2020J02032).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100152.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Albermann C., Piepersberg W., Wehmeier U.F. Synthesis of the milk oligosaccharide 2-fucosyllactose using recombinant bacterial enzymes. Carbohydrate Research. 2001;334(2):97–103. doi: 10.1016/s0008-6215(01)00177-x. [DOI] [PubMed] [Google Scholar]
- Baumgärtner F., Seitz L., Sprenger G.A., Albermann C. Construction of Escherichia coli strains with chromosomally integrated expression cassettes for the synthesis of 2'-fucosyllactose. Microbial Cell Factories. 2013;12:40. doi: 10.1186/1475-2859-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing M., Simala-Grant J.L., Taylor D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;115(12):E2791–E2800. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- Bode L., Contractor N., Barile D., Pohl N., Prudden A.R., Boons G.-J., et al. Overcoming the limited availability of human milk oligosaccharides: Challenges and opportunities for research and application. Nutrition Reviews. 2016;74(10):635–644. doi: 10.1093/nutrit/nuw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton C., Snajdrová L., Jeanneau C., Koca J., Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16(2):29R–37R. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- Cheng L., Akkerman R., Kong C., Walvoort M.T.C., de Vos P. More than sugar in the milk: Human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects. Critical Reviews in Food Science and Nutrition. 2021;61(7):1184–1200. doi: 10.1080/10408398.2020.1754756. [DOI] [PubMed] [Google Scholar]
- Coutinho P.M., Deleury E., Davies G.J., Henrissat B. An evolving hierarchical family classification for glycosyltransferases. Journal of Molecular Biology. 2003;328(2):307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- Engels L., Elling L. WbgL: A novel bacterial alpha1,2-fucosyltransferase for the synthesis of 2'-fucosyllactose. Glycobiology. 2014;24(2):170–178. doi: 10.1093/glycob/cwt096. [DOI] [PubMed] [Google Scholar]
- Gao X., Wu D., Wen Y., Gao L., Liu D., Zhong R., et al. Antiviral effects of human milk oligosaccharides: A review. International Dairy Journal. 2020;110:104784. doi: 10.1016/j.idairyj.2020.104784. [DOI] [Google Scholar]
- Ihara H., Ikeda Y., Toma S., Wang X.C., Suzuki T., Gu J.G., et al. Crystal structure of mammalian alpha1,6-fucosyltransferase, FUT8. Glycobiology. 2007;17(5):455–466. doi: 10.1093/glycob/cwl079. [DOI] [PubMed] [Google Scholar]
- Kunz C., Rudloff S., Baier W., Klein N., Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annual Review of Nutrition. 2000;20(1):699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- Lau K., Thon V., Yu H., Ding L., Chen Y., Muthana M.M., et al. Highly efficient chemoenzymatic synthesis of beta1-4-linked galactosides with promiscuous bacterial beta1-4-galactosyltransferases. Chemical Communications. 2010;46(33):6066–6068. doi: 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Liu X.W., Shao J., Shen J., Jia Q., Wen Y.i., et al. Characterization of a novel alpha1,2-fucosyltransferase of Escherichia coli O128_b12 and functional investigation of its common motif. Biochemistry. 2008;47(1):378–387. doi: 10.1021/bi701345v. [DOI] [PubMed] [Google Scholar]
- Li M., Shen J., Liu X.W., Shao J., Yi W., Chow C.S., et al. Identification of a new α 1,2-fucosyltransferase involved in O-antigen biosynthesis of Escherichia coli O86_b7 and formation of H-type 3 blood group antigen. Biochemistry. 2008;47(44):11590–11597. doi: 10.1021/bi801067s. [DOI] [PubMed] [Google Scholar]
- Moremen K.W., Haltiwanger R.S. Emerging structural insights into glycosyltransferase-mediated synthesis of glycans. Nature Chemical Biology. 2019;15(9):853–864. doi: 10.1038/s41589-019-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg, D. S., Ruiz-Palacios, G. M., Mekibib, A., Prasoon, C., Jareen, M. D., Guerrero, M. D. L., & Morrow, A. L. (2004). Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology, 14(3), 253-263. [DOI] [PubMed]
- Pang P.-C., Tissot B., Drobnis E.Z., Sutovsky P., Morris H.R., Clark G.F., et al. Expression of bisecting type and Lewisx/Lewisy terminated N-glycans on human sperm. Journal of Biological Chemistry. 2007;282(50):36593–36602. doi: 10.1074/jbc.M705134200. [DOI] [PubMed] [Google Scholar]
- Petschacher B., Nidetzky B. Biotechnological production of fucosylated human milk oligosaccharides: Prokaryotic fucosyltransferases and their use in biocatalytic cascades or whole cell conversion systems. Journal of Biotechnology. 2016;235:61–83. doi: 10.1016/j.jbiotec.2016.03.052. [DOI] [PubMed] [Google Scholar]
- Pettit N., Styslinger T., Mei Z., Han W., Zhao G., Wang P.G. Characterization of WbiQ: An alpha1,2-fucosyltransferase from Escherichia coli O127:K63(B8), and synthesis of H-type 3 blood group antigen. Biochemical and Biophysical Research Communications. 2010;402(2):190–195. doi: 10.1016/j.bbrc.2010.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps K.R., Lynch B., Stannard D.R., Gilby B., Baldwin N., Mikš M.H., et al. Genotoxicity and neonatal subchronic toxicity assessment of a novel mixture of the human-identical milk oligosaccharides lacto-N-fucopentaose I and 2'-fucosyllactose. Journal of Applied Toxicology. 2021;41(4):632–649. doi: 10.1002/jat.4071. [DOI] [PubMed] [Google Scholar]
- Roldan V.D.T., Urtecho M.S., Gupta J., Yonemitsu C., Cárcamo C.P., Bode L., Ochoa T.J. Human milk oligosaccharides and their association with late-onset neonatal sepsis in Peruvian very-low-birth-weight infants. American Journal of Clinical Nutrition. 2020;112(1):106–112. doi: 10.1093/ajcn/nqaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y., Kingsley S., Walker G., Mondoux M.A., Tissenbaum H.A. Metabolic shift from glycogen to trehalose promotes lifespan and healthspan in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(12):E2791–E2800. doi: 10.1073/pnas.1714178115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W.A., Iyengar R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatric Research. 2015;77(1-2):220–228. doi: 10.1038/pr.2014.160. [DOI] [PubMed] [Google Scholar]
- Walsh C., Lane J.A., van Sinderen D., Hickey R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. Journal of Functional Foods. 2020;72:104074. doi: 10.1016/j.jff.2020.104074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Boulton P.G., Chan N.W.C., Palcic M.M., Taylor D.E. Novel helicobacter pylori alpha1,2-fucosyltransferase, a key enzyme in the synthesis of Lewis. Microbiology. 1999;145:3245–3253. doi: 10.1099/00221287-145-11-3245. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Guo Y., Liu Y., Li L., Zhang Q., Wen L., et al. Chemoenzymatic synthesis of a library of human milk oligosaccharides. The Journal of Organic Chemistry. 2016;81(14):5851–5865. doi: 10.1021/acs.joc.6b00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Xia H., Sun N.a., Liu C.-C., Sheng A., Chi L., et al. Reprogramming the enzymatic assembly line for site-specific fucosylation. Nature Catalysis. 2019;2(6):514–522. [Google Scholar]
- Yi W., Liu X., Li Y., Li J., Xia C., Zhou G., et al. Remodeling bacterial polysaccharides by metabolic pathway engineering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4207–4212. doi: 10.1073/pnas.0812432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Thon V., Lau K., Cai L.i., Chen Y.i., Mu S., et al. Highly efficient chemoenzymatic synthesis of β1-3-linked galactosides. Chemical Communications. 2010;46(40):7507. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner B., Meyer A.S. Enzymatic transfucosylation for synthesis of human milk oligosaccharides. Carbohydrate Research. 2020;493:108029. doi: 10.1016/j.carres.2020.108029. [DOI] [PubMed] [Google Scholar]
- Zhao C., Wu Y., Liu X., Liu B., Cao H., Yu H., et al. Functional properties, structural studies and chemo-enzymatic synthesis of oligosaccharides. Trends in Food Science & Technology. 2017;66:135–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.