Highlights
-
•
Rhodotorula mucilaginosa is predominant soil yeast capable of producing carotenoids.
-
•
Presence of glycerol in culture medium induces the biosynthesis of carotenoids by yeasts.
-
•
Temperatures above 30 °C sharply reduce carotenoid production in Rhodotorula mucilaginosa.
-
•
Yeast cells cultivated under white-light LED indicate higher production of carotenoids.
Keywords: Carotenoids, Optimization, β-carotene, Soil, Rhodotorula mucilaginosa
Abstract
Yeasts are alternative source of natural carotenoids, a group of colored terpenoids with various market applications. Carotenoid Production by yeast fermentation technology is greatly effective and proposes considerable benefits with large scale production, cost effectiveness and safety. In this study, four pigment-producing yeasts were isolated from forest park soils with the potential to produce carotenoids. Morphological, physiological, biochemical and molecular characterization indicates the isolates belong to Rhodotorula mucilaginosa. Carotenoid production was optimized by small scale cultivation. The optimum condition was 120 h of incubation at pH 6.0, 28 °C, white light irradiation, Yeast Extract Peptone Glycerol medium composed of 10 g/L yeast extract, 20 g/L peptone, 20 ml/L glycerol, yielding maximum content of 223.5 μg/g dry weight. The β-carotene content was confirmed by HPLC and FT-IR. The results suggested that soil yeasts are potential sources of carotenoids that could be utilized as a natural agent for industrial products.
Graphical abtract
1. Introduction
Today, the need for natural colors used in various industries such as pharmaceuticals, food, cosmetics and health leads the human to use natural resources. A wide range of colors from yellow and orange to red and purple in plants, animals and microorganisms are due to the presence of pigments, one of the most important of which is carotenoids. This pigment is one of the most important and valuable secondary metabolites with important physiological effects on the human body, including strengthening the immune system and reducing the risk of many diseases such as cancers [1, 2].
Carotenoids can arise from two major sources: natural or synthetic source. Natural colorants are those obtained from plants, animals and microorganisms while synthetic colorants are those artificially made from chemical substances [3]. The chemical production of carotenoids, in addition to the high cost, has several limitations such as the production of by-products with harmful effects on the environment and the possibility of toxicity. Therefore, today, the use of natural alternative resources with mass production ability instead of chemical products without side effects, is an important issue that has led consumers to prefer natural outputs of this valuable product to artificial ones.
Moreover, various species of microorganisms focused high consideration as alternative biosources [4] that producing carotenoids by them is considered to be industrially competitive. They have relatively higher growth velocity than plants and animals with such potential [5]. The same instability as those isolated from naturally grown plants is shown by pigments isolated from animal cells. Thus, microorganisms are better alternatives for novel biotechnologically derived colors. Regarding the microorganisms, naturally pigmented yeasts have potential to make high value of carotenoids like β-carotene, lycopene, torulene, astaxanthin, etc. Carotenoid-producing yeasts are highly indicated by Rhodotorula sp., Rhodosporidium sp., Sporobolomyces sp., Xanthophylomyces sp. [6, 7]. Carotenoids are significant for their high distribution, structural diversity, and different performances. More than 600 carotenoids were isolated and characterized from natural sources [8] which just some of them are commonly exploited on industrial scale.
Beta carotene is one of the main carotenoids which is one among them that were known to indicate provitamin A activity [9, 10]. Several researches were surveyed and correlate β-carotene and their disincentive impact on diseases involving cancer [11 – 13, 14, 15], photosensitivity disorders [16, 17], cardiovascular diseases [12, 18], age-related ones [19], [20], [21]. The global production of β-carotene has increased regarding highly utilizations in various regions. β-carotene as a great amount compound indicated a global market of $ 309 million in 2018 with an annual growth rate of 3.6% [23]. In addition, its health related role other utilization for β-carotene is as food, cosmetic and care products colorant [23, 24].
The presence of several profitable characteristics has made high interest in the industrial production of β-carotene. Although β-carotene in plants is natural, regarding low percentage in plants, today's plant extraction approaches are less usually utilized for industrial production of β-carotene [25]. Besides, large-scale β-carotene extraction from fruits and vegetables is not feasible based on economic, environmental and logistical constraints. In addition, natural β-carotene arisen from yeasts is considered to be secure due to nontoxic, non-carcinogenic and biodegradable in nature [25], [26], [27]. Hence, from industrial viewpoints, there is a requirement to advance a high throughput and cost-effective methods for high scale production of microbial β-carotene. Carotenoid making microorganisms like yeasts are plentiful in natural environment. Soil has a multiple and considerable ecosystem which can obtain microorganisms with biotechnological potential for producing β-carotene. This research aimed at screening and determination of carotenoid-producing yeasts from the soils of forest parks in Tehran urban green belt, capital city of Iran, optimization the nutritional and environmental parameters for their carotenoid production regarding β-carotene.
2. Materials and methods
2.1. The materials and reagents for cultures and biochemical-based reactions were purchased from Merck (Darmstadt, Germany)
2.2. Ecosystem, sampling, culture media, yeast isolation
Soil samples were gathered from forest parks, namely Sorkhe Hesar (35°38′18″N, 51°34′46″E), Khojir (35°24′36″N, 51°24′36″E), Lavizan (35°45′49.26″N, 51°30′3.5″E) and Chitgar (35°44′2.04″N, 51°12′34.92″E) in Tehran, Iran. These urban forests with dark color and rough texture due to the dominance of Coniferae, in a strategic location, play the role of preserving the green belt and determining the urban area of Tehran, which regulates the structural form and act as respiratory lungs for the city air. Topographically, they are very uneven and composed of plains, hills and highlands, and soils are classified as Entisols [28]. Sorkheh Hesar in altitude range of 1280–1493 m altitude sea level (a.s.l.) and Khojir in altitude range of 1200–2200 m a.s.l. are located in the eastern part of the city, Lavizan in altitude range of 1390–1590 m a.s.l. is located in the northeast and Chitgar in altitude of 1260–1290 m a.s.l. is located in the westernmost part of Tehran. The mean annual rainfall in the aforementioned forest parks is 275, 300, 250, and 242 mm, and the average annual temperature is 12.6, 11, 15 and 17.1 respectively, which would cause temperate semi-arid climate in these regions.
The surface of these forests is mostly densely planted with tree species, the predominant species of which are evergreen Coniferae, especially eldarica pine (Pinus eldarica), mountain pine (Pinus mugo) and arizona cypress (Cupressus arizonica). Of course, some parts have a variety of broad-leaf trees such as Ailanthus glandulosa, Cercis siliquastrum, Robinia pseudoacacia and Morus sp. but planting in almost all parks is done in single planting stands. Pinus needle-leaf sites were sampled randomly in February 2018. Samples were gathered with scraping of soil surface with sterile spatula and about 10 gr of soil were provided from a depth of 2–5 cm at a distance of 1 m from a pine tree trunk. The samples were transported to laboratory in a sterile plastic bag with an ice bag for subsequent analysis. To isolate pigmented yeast colonies with desired coloration of yellow to red from the soil samples, standard microbiological serial dilution and plating techniques were applied. All plates were incubated at 28 °C for 48–72 h. Chosen pigmented colonies are streaked on to suitable maintenance agar media for pure culture isolation and future determination. Potato Dextrose agar (PDA, potato infusion 200 g/L, Dextrose 20 g/L, Agar 20 g/L), Sabouraud Dextrose agar (SDA, Dextrose 40 g/L, peptone 10 g/L, Agar 15 g/L), Yeast Extract Peptone Glycerol (YPG, Yeast extract 10 g/L, Peptone 20 g/L, glycerol 20 mL/L, Agar 20 g/L), and Yeast Malt Extract agar (YMA, 3 g/L yeast extract, 3 g/L malt extract, 5 g/L, Peptone 5 g/L, Dextrose 10 g/L, Agar 20 g/L) were utilized to culture.
2.3. Determination and characterization of yeast isolates
To survey morphological and physiological features, the pure culture of chosen yeasts was applied according to the approaches explained by Kurtzman et al. [29]. Molecular characterization of promising isolates was handled based on ITS region for appropriate confirmation. Cell biomass was gathered from 3 mL of cell culture grown in YPG media which centrifuged in refrigerated centrifuge at 8000 rpm for 10 min and washed with distilled water. Genomic DNA was extracted applying DNP Kit (Sinaclon, High yield DNA purification Kit, Cat No: EX6071) considering manufacturer's instructions.
ITS area was amplified applying ITS 1 (5′-TCCGTAGGTGAACCTGCGG-3′) was the forward primer and ITS 4 (5′-TCCTCCGCTTTATTGATATGC-3′) was reverse primer (Metaboin International AG) [30]. Each 30 µl PCR mixture involving 15 µl Parstous (www.parstous.com) Master Mix (2X Taq Premix: involving Taq DNA Polymerase, reaction buffer, dNTPs mixture, protein stabilizer), 12 µl double distilled water (DDW), 1 µl each primer (10 µmol) and 1 µl genomic DNA. Thermal profile was as follows: initial denaturation at 95 °C for 2 min, 35 cycles of 95 °C for 1 min, 50 °C for 1 min, 72 °C for 1 min, and a final extension for 7 min at 72 °C. PCR products was analyzed by electrophoresis and visualized on 1% agarose gel stained with syber safe. Purified PCR products were sequenced in both directions by Bioneer Company (Republic of Korea).
2.4. Test for presence, fermentative production, extraction and identification of caretonoids
2.4.1. Analysis of optimal conditions for maximum carotenoid biosynthesis and biomass yields
Culture media including YM (3 g/L yeast extract, 3 g/L malt extract, 5 g/L peptone, Dextrose 10 g/L), YPG (20 g/L peptone, 10 g/L yeast extract, 20 mL/L glycerol), YPD (20 g/L peptone, 10 g/L yeast extract, Dextrose 20 g/L) and PDB (200 g/L potatoes, Dextrose 20 g/L) were utilized to find the suitable medium for pigment production. The isolates were grown in each medium in triplicates and cell growth was controlled for up to 120 h by assessing OD600 nm at various sampling times. Due to provided results, the graphs of optical density (OD) of cultures from cultivation time were provided. After measuring optical density and pigment production, YPG medium culture was chosen as suitable medium to optimize carotenoid production. In addition to culture medium which can regulate genes expression of interest and activate metabolic pathways significant for the production of pigments, some factors were influencing cell growth and pigmentation. After assessing isolates growth on chosen culture medium, subsequent impact of different factors on carotenogenesis was investigated and fermentation flasks were subjected to various cultivation conditions like incubation time, temperature, initial pH amount and light due to YPG liquid medium to identify the optimum condition for the pigmentation of isolates and carotenoid production. The parameters were adjusted due to the experimental design to assess biomass yields and carotenoid content in such conditions.
2.4.2. Fermentation condition
To obtain the inoculum, liquid YPG medium was inoculated by one loopful of yeast culture on agar plates. Triplicate cultures were grown in flasks containing 100 mL of medium on a rotary shaker, at 150 rpm for 72 h. The experiment was handled applying a series of Erlenmeyer flasks, shaken at 150 rpm for 120 h, each flask contained 90 ml YPG medium inoculated with inoculum constituting 10% of the culture volume.
2.4.3. Identification of biomass yield
By gravimetric approach after cultivation, the biomass yield was identified. 10 mL of the medium was centrifuged for 20 min at 8000 × g, the supernatant was poured off and biomass was washed twice with deionized water to delete any residual. For obtaining a constant mass, the wet cell biomass was dried at 105 °C. The results were computed in grams of dry weight per liter of culture medium (gd.w./L).
2.4.4. Identification of carotenoid content in biomass yield
The carotenoid content in yeasts biomass was spectrophotometrically identified. To this purpose, pigments were extracted with a mixture of 2 mL acetone (Romil, Cambridge) and 2 mL petroleum ether (Sigma Aldrich, Milan, Italy) preceded by the destruction of yeast cell wall by 2 mL of DMSO (Merck) pre-heated at 55 °C and glass beads (d = 0.5 mm) with vigorous vortex. The extracts were centrifuged and the supernatants were filtered through a 0.45 μm membrane filter and collected. The extracts were decanted into small separator funnels and 2 mL of saturated NaCl solution were added to the extracts. A clear-cut separation of the two phases appeared and the existence of carotenoid pigments was occurred as the upper petroleum ether layer was colored orange, pink or red. All methods were done under low-light conditions. Carotenoids were quantified using their tabulated absorption coefficients by spectrophotometry. The absorbance of colored ether fraction was estimated at λmax (450 nm) [31] utilizing a spectrophotometer (UV-1202 spectrophotometer; Shimadzu, Germany). The total carotenoid content was noted as β-carotene-equivalents [32] calculated using the following equation [33] and indicated as microgram per gram of dry substance.
A = Absorbance; V = Total extract volume; m = Dry cell biomass; (β-carotene Extinction Coefficient in petroleum ether).
2.4.5. Analysis of beta-carotene in R. mucilaginosa isolates carotenoid extracts
The Beta-carotene content of carotenoid extracts was analyzed by two methods; High Performance Liquid Chromatography (HPLC) and Fourier transform infrared spectroscopy (FT-IR).
2.4.5.1. Analysis of beta-carotene in R. mucilaginosa isolates carotenoid extracts by HPLC
The Carotenoids content was analyzed using HPLC, applying a liquid chromatograph, model d-14,163 (Knauer, Berlin, Germany) equipped with a UV‐visible photodiode detector. Analysis process was performed on a C18 column (Eurospher 100 A, 150 × 4.6 mm, 5 μm) at a wavelength of 450 nm. Mobile phase was a solvent mixture of ethyl acetate, isopropanol and acetonitrile and tetrahydrofuran (2:4:4 v/v/v) with a flow rate of 1 mL/min. The injection volume was 5 μL. Beta-carotene identification was based on the retention time of the standard (838 K406623, E. Merck, Darmstadt, Germany).
2.4.5.2. Analysis of beta-carotene in R. mucilaginosa isolates carotenoid extracts by FT-IR
Fourier transform infrared spectroscopy (FT-IR) analysis of extracted carotenoids was done as explained by Song [34], utilizing Thermo Scientific Nicolet iS5 FT-IR Spectrometer (Thermo Fisher Scientific, USA). To this purpose, the extracts were concentrated and pelleted with potassium bromide. Beta carotene from Merck (838 K406623, E. Merck, Darmstadt, Germany) was applied as standard. Available spectrum ranges were 400 – 4000 cm−1.
2.5. Gene submission
The sequences of amplified ITS regions were edited and contrasted to rDNA sequences. The segments were compared to deposited data of GenBank utilizing BLAST (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov/BLAST/). The obtained ITS sequences of the isolates were submitted to GenBank (accession numbers: MW433717 and MW465350).
2.6. Statistical analysis
The data were obtained from triplicate experiments were presented as mean ± standard deviation. The statistical analyses of experimental data were handled utilizing ANOVA followed by Tukey's multiple comparisons test to identify the significance of differences among average amounts, applying SPSS Statistic 16 software. p < 0.05 was statistically regarded significant.
3. Results and discussion
3.1. Characteristics of identified carotenoid-producing yeasts
The twenty five yeast isolates were purified and 20 pigmented yeast isolates were chosen. The other five isolates were avoided because their colors were white or white with red top and were regarded as non-pigmented yeasts. The cultural and microscopic features of chosen 20 yeast isolates were similar. Yeast isolates were sub cultured on agar plates to provide 2 days old yeast cultures. Sixteen pigmented yeast isolates were deleted because they weakly grew on agar. Among 25 isolates, only four (codified as M14, M22, M23, M24) had an orange to red color at high intensity with quick growth and high pigment making abilities were isolated from soil of the forest areas as pure cultures. Pure cultures were transferred to Yeast extract-peptone-glycerol agar medium because of high ability of yeast cells to survive on the medium as it was shown by measuring the absorbance at λ600 nm after 5 days; it is based on the high content of minerals and the presence of glycerol as a pigmentation stimulant factor in this medium. The investigated 4 cultures indicated similarity in major features. The isolates developed mucous, smooth surface with orange to red colored colonies on YPG agar plates. In microscopic examination, the isolated cells had oval or round shape with asexual reproduction, indicating multi-lateral budding and the absence of ascospores, occasionally a few rudimentary pseudohyphae. They have assimilated sugars like glucose, galactose, sucrose, maltose, melezitose, trehalose, raffinose. The assimilation of methanol and nitrate was negative. Growth at 37 °C was weak for all four isolates, because of adaptation with low average temperatures of the forest parks. Additional tests to classify the isolate to the levels of genus and species were done and the results are mentioned in Table 1. The studying features of yeast isolates agree well with those of Rhodotorula mucilaginosa.
Table 1.
The morphological, physiological and biochemical characteristics of isolated yeasts M14, M22, M23, M24.
| M14 | M22 | M23 | M24 | M14 | M22 | M23 | M24 | ||
|---|---|---|---|---|---|---|---|---|---|
| color | Orange | Red | Pink | Orange | Xylitol | + | + | − | + |
| Pseudohyfae | + | − | − | − | Ribitol | − | − | − | − |
| Ascopore | − | − | − | − | Galactitol | − | − | − | − |
| Mycelium | − | − | − | − | D-Mannitol | − | − | − | − |
| Conjugation | − | − | − | − | D-Glucitol | − | − | − | − |
| Glucose | + | + | + | + | Melibiosi | − | − | − | − |
| Galactose | + | + | + | + | D-Gluconate | + | + | + | + |
| Palatinose | + | + | + | + | DL-Lactate | + | + | + | + |
| Sucrose | + | + | + | + | Succinate | + | + | + | + |
| Maltose | + | + | + | + | Citrate | − | + | − | − |
| Cellobiose | − | − | − | − | Inositol | − | − | − | − |
| Trehalose | + | + | + | + | Hexadecane | − | − | − | − |
| Lactose | − | − | − | − | Nitrate | − | − | − | − |
| Melibiose | − | − | − | − | Vitamin-free | − | − | − | − |
| Raffinose | + | + | + | + | 2-Keto-d-gluconate | − | − | − | − |
| Melezitose | + | + | + | + | Saccharate | − | − | − | − |
| Inulin | − | − | − | − | D-Glucuronate | + | + | + | + |
| Soluble starch | − | − | − | − | 50% Glucose | − | − | − | − |
| D-Xylose | + | + | + | + | 10% NaCl/5% Glucose | − | − | − | − |
| Arabinose | − | − | − | − | Starch formation | − | − | − | − |
| α-m-d-Glucosideo | − | − | − | − | Urease | + | + | + | + |
| Glucose fermentation | − | − | − | − | Gelatin liquefaction | − | − | − | − |
| N-Acetyl-d-glucosamine | − | − | − | − | Thiamine-free | − | − | − | − |
| Methanol | − | − | − | − | Growth at 37 °C | + | + | + | + |
| Ethanol | − | − | − | − | Growth at 40 °C | − | − | − | − |
| Glycerol | + | + | + | + | Cycloheximide | − | − | − | − |
| Erythritol | − | − | − | − | Sedimentaion | + | + | + | + |
(-) Negative test, (+) Positive test.
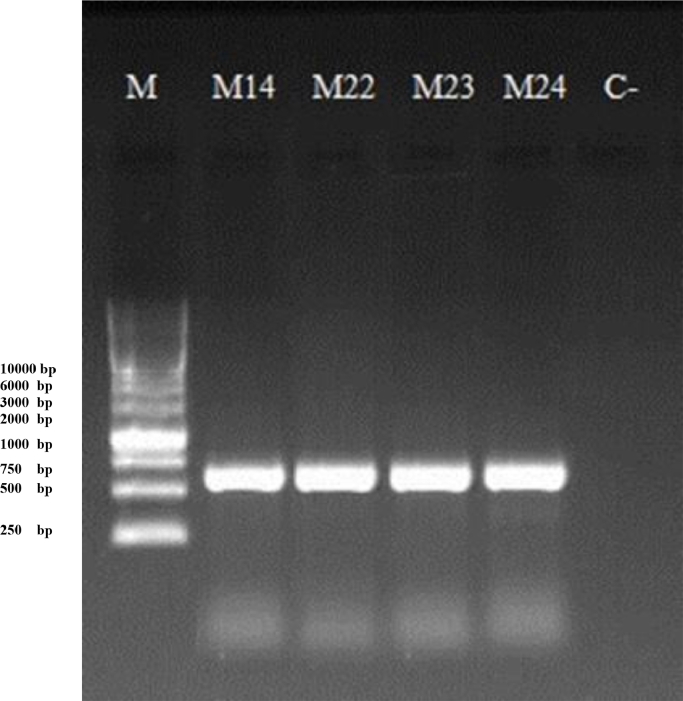
The results were not enough to classify the genus, hence Internal Transcribed Spacers (ITS) of ribosomal DNA sequence were amplified to approve isolates classification (Fig. 1). ITS sequence provided from isolates Rhodotorula sp. M14, M22, M23, M24 was compared to the sequences in GenBank database and indicated that the isolates had > 99% homology to Rhodotorula mucilaginosa.
Fig. 1.
Polymerase chain reaction products of isolated yeasts using primers ITS 1 and ITS 4 on 1% (w/v) agarose gel electrophoresis; lanes: (M) 1 Kb DNA Ladder; (M14, M22, M23, M24) PCR products of Rhodotorula mucilaginosa isolates; (C-) negative control.
Carotenoids making microorganisms are isolated from soil [35], cave [36], marine [37], and slattern crystallizer pond [38] environments. The soil is a multiple and considerable ecosystem which can prepare yeasts with biotechnological potential to produce carotenoids. Some yeast species can synthesize carotenoids, in particular, genera Rhodotorula which is one of major carotenoid-forming yeasts with predominant synthesis of β-carotene, torulene and torularhodin [39], [40], [41].
Producing carotenoid pigments in multiple natural isolates of genera Rhodotorula was investigated by others, like Rhodotorula mucilaginosa, Rhodotorula glutinis, Rhodotorula minuta, and Rhodotorula graminis [42]. Maldonade et al. isolated 5 yeasts strains of Rhodotorula mucilaginosa and Rhodotorula graminis from 242 samples of soil, leaves and fruits that can make carotenoid pigments [43]. Libkind et al. screened and determined caroteniod making yeast strains Rhodotorula mucilaginosa from natural environment of Patagonia [44]. El-Banna et al. isolated 46 yeasts from natural environments; all the strains belonged to Rhodotorula glutinis [45]. Griffin and Kernaghan isolated Rhodotorula mucilaginosa (GenBank submission number: MT028113.1) from upland forest in Nova Scotia, Canada, which has high similarity with ITS sequences and ecosystem parameters of this study. We isolated and determined soli yeasts that can make carotenoid pigment, due to morphological and physiological characterization, biochemical tests and ITS sequence, and our results indicated that all isolates was finally determined as Rhodotorula mucilaginosa.
3.2. Optimization of five effective parameters on total carotenoid and biomass yield in the isolates
3.2.1. Culture medium
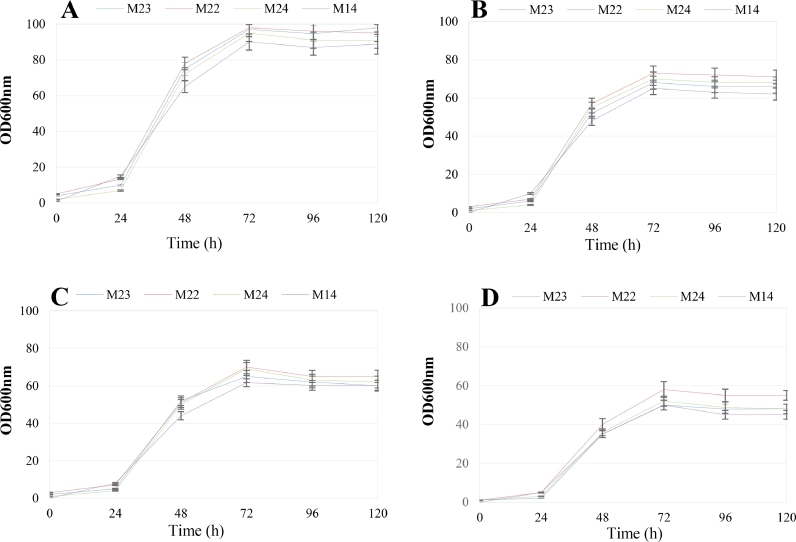
The impact of different culture media was analyzed to identify the optimal growth conditions, comparative efficiency of every culture medium for cells growth was indicated in Fig. 2. The profitability of pigment production is basically identified by the yield of cellular biomass in various media, in addition to carotenoid content in biomass, and the time of cultivation which is indicated in Table 2. The yeast isolates showed the ability to grow and biosynthesize carotenoids in the media. Biomass yield and content of carotenoids in biomass strongly depended on the composition of the culture medium. As detailed in Table 2, the isolates performed best in YPG broth, followed by YM broth, YPD broth and PDB broth. The differences showed YPG medium was considerably favored for growth and carotenogenesis of tested yeast isolates. The greatest biomass yield of determined isolates was provided in YPG medium (approximately 20 gd.w./L) that were considerably highest than those after cultivation in other media. In addition to yeast extract and peptone that obtain nitrogen, amino acids and vitamins, YPG medium contains glycerol as carbon source which are regarded efficient enhancers of the whole carotenoids production [46]. Due to the results, carotenoid content were considerably greatest in YPG medium (approximately 145 μg/gd.w) than in other media (approximately 85–96 μg/gd.w). It may be resulted from the reality that glycerol is the skeleton of the structure of triacylglycerol molecules, in which its presence in medium may induce carotenoids biosynthesis by yeasts [47]. It may be that glycerol is metabolized rapidly and passes through the cell membrane by facilitated diffusion [48] and causes stress inside the yeast cells, Therefore stimulating the production of carotenoids and other secondary metabolite [49, 50]. Therefore, based on other authors [51, 52] glycerol confirmed to be a well-suited substrate for carotenoids production. Saenge et al. [53] reported R. glutinis TISTR 5159 yeast culture in media containing ammonium sulfate as a source of nitrogen and 5% and 9.5% glycerol. The yield of cellular biomass was only 5.15– 5.65 gd.w./L, while the yield obtained in the present study in YPG medium was approximately 20.08–20.30 gd.w./L. The mixture of peptone and yeast extract with glycerol in optimized amounts permits the formation of a complex medium, which is a source of macro- and micronutrients required for isolates growth. Besides, high dextrose amounts in the medium can reduce the efficiency of carotenoid biosynthesis by Rhodotorula yeast [54]. Increased dextrose suppresses the production of carotenoids due to the incidence of the Crabtree effect in yeasts [54, 55]. Although yeasts of the genus Rhodotorula are regarded as Crabtree-negative [56], some strains may produce ethanol [57]. It is likely that reduction of carotenoid production expressed in YM, YPD and PBD broth media with added dextrose was due to the Crabtree effect.
Fig. 2.
Growth kinetics of Rhodotorula mucilaginosa isolates by measuring OD600 nm for up to 120 h in different culture media of; (A) YPG, (B) YM, (C) YPD, (D) PDB. Data are representative of three independent experiments.
Table 2.
Yield of cellular biomass (g/L) and total carotenoid content (μg/gd.w) in various media after 120 h of cultivation R .mucilaginosa yeasts.
| Culture medium | M14 | M22 | M23 | M24 | ||||
|---|---|---|---|---|---|---|---|---|
| Ybio | Tcar | Ybio | Tcar | Ybio | Tcar | Ybio | Tcar | |
| YPG | 20.07 ± 0.025 | 144.31 ± 0.032 | 20.296 ± 0.035 | 146.44 ± 0.045 | 20.22 ± 0.04 | 145.50 ± 0.045 | 20.15 ± 0.05 | 145 ± 0.05 |
| YM | 9.55 ± 0.040 | 95.90 ± 0.045 | 10.23 ± 0.04 | 96.22 ± 0.02 | 10.18 ± 0.060 | 96.01 ± 0.076 | 10.18 ± 0.04 | 96.23 ± 0.060 |
| YPD | 8.976 ± 0.035 | 90.29 ± 0.045 | 9.32 ± 0.045 | 91.78 ± 0.03 | 9.286 ± 0.032 | 91.15 ± 0.0450 | 9.13 ± 0.017 | 91.13 ± 0.042 |
| PDB | 6.95 ± 0.040 | 84.80 ± 0.040 | 7.186 ± 0.065 | 85.106 ± 0.065 | 7.10 ± 0.040 | 85 ± 0.075 | 7.16 ± 0.035 | 84.99 ± 0.07 |
(Ybio, Yield of cellular biomass (expressed as dry weight after 120 h growth); Tcar, totals carotenoid content, expressed as β-carotene equivalents).
The significant difference of the performance of the isolates were examined and it was noticed that in each medium separately, there is no significant (p < 0.05) difference between the isolates. Comparatively, isolate M22 was the best in production of cell biomass and carotenoids biosynthesis followed by M23, M24 and M14 in the all media.
3.2.2. Incubation time
The maximum pigment production was accessed at an incubation time of 120 h of cultures on shaker. Kinetics of cells growth and pigmentation in YPG broth showed that after 72 h, growth reached stationary phase in which the activity of pigment production was considerably detectable to continue at late stationary phase (120 h) [Fig. 2A]. Hewedy et al., 2009 noted that optimum incubation period for pigmentation in the yeast Rhodotorula is 120 h, while 24–72 h as optimum time was reported for most bacteria [58, 59]. Since carotenoid biosynthesis in yeasts start at the late logarithmic phase continuing in stationary phase [59], [60], [61], [62], to increase the yield of carotenoid in media, the time of cultivation should be extended.
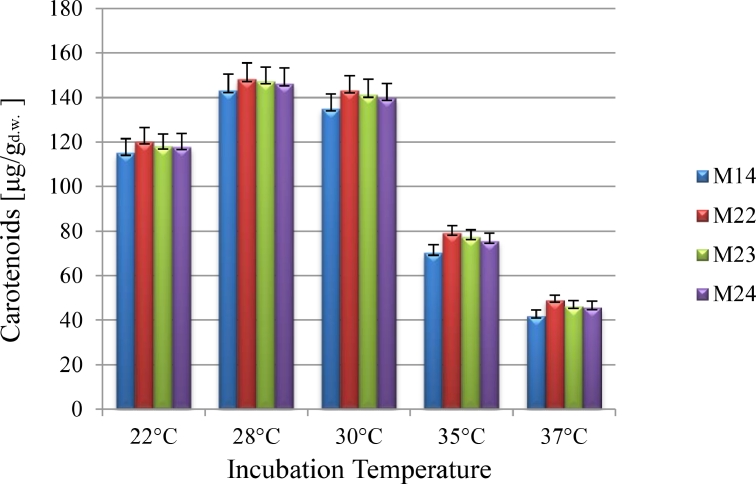
3.2.3. Incubation temperature
Carotenoids synthesis is highly influenced by the temperature of incubation which has to be monitored in fermentation, ranging from 22 to 37 °C were controlled for biomass and carotenoids production in isolates (Table 3). As shown in Fig. 3, maximal carotenoids production was obtained at 28 °C for all the isolates. Carotenoid formation rates increased by the increase in temperature up to 28 °C, and sharply reduced above 30 °C. It may be regarding the denaturation of enzyme system of microorganism in higher temperatures. The same result was reported for Rhodotorula mucilaginosa [63]. Malisorn and Suntornsuk reported optimum temperature for maximum Rhodotorula glutinis growth and carotenoids biosynthesis was 29 °C and 30 °C [64]. Since, temperature impact regulates enzyme concentration included in carotenoids production [65]; carotenoid levels could be monitored in cells.
Table 3.
The values of biomass (g/L), volumetric yield of carotenoids (mg/L) and volumetric carotenoids productivity (mg/L/h) at different temperature in YPG medium.
| Temperature | M14 | M22 | M23 | M24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ybio | YCar | QCar | Ybio | YCar | QCar | Ybio | YCar | QCar | Ybio | YCar | QCar | |
| 22°C | 15.80 ± 0.045 | 1.80 ± 0.0556 | 0.015 ± 0.005 | 16.55 ± 0.04 | 1.98 ± 0.01 | 0.016 ± 0.005 | 16.28 ± 0.036 | 1.90 ± 0.015 | 0.014 ± 0.003 | 16 ± 0.01 | 1.85 ± 0.01 | 0.014 ± 0.002 |
| 28°C | 18.31 ± 0.045 | 2.60 ± 0.0550 | 0.021 ± 0.003 | 20.55 ± 0.045 | 3.06 ± 0.035 | 0.024 ± 0.003 | 20.26 ± 0.040 | 2.97 ± 0.015 | 0.024 ± 0.0025 | 19.87 ± 0.02 | 2.85 ± 0.005 | 0.022 ± 0.001 |
| 30°C | 18.02 ± 0.072 | 2.43 ± 0.05 | 0.03 ± 0.02 | 19.72 ± 0.03 | 2.81 ± 0.040 | 0.022 ± 0.002 | 19.43 ± 0.035 | 2.73 ± 0.020 | 0.022 ± 0.0011 | 18.93 ± 0.026 | 2.66 ± 0.015 | 0.022 ± 0.001 |
| 35°C | 8.70 ± 0.03 | 0.60 ± 0.0503 | 0.004 ± 0.003 | 10.89 ± 0.04 | 0.85 ± 0.030 | 0.006 ± 0.003 | 10.62 ± 0.040 | 0.82 ± 0.026 | 0.006 ± 0.0015 | 9.95 ± 0.01 | 0.74 ± 0.02 | 0.006 ± 0.001 |
| 37°C | 5.01 ± 0.07 | 0.21 ± 0.050 | 0.001 ± 0.0005 | 6.75 ± 0.032 | 0.33 ± 0.04 | 0.003 ± 0.002 | 6.33 ± 0.025 | 0.29 ± 0.03 | 0.002 ± 0.001 | 5.86 ± 0.02 | 0.24 ± 0.02 | 0.002 ± 0.0015 |
(Ybio, Yield of cellular biomass; YCar, volumetric yield of carotenoids; QCar, volumetric carotenoids productivity).
Fig. 3.
The effect of temperature on carotenoids production by R. mucilaginosa isolates.
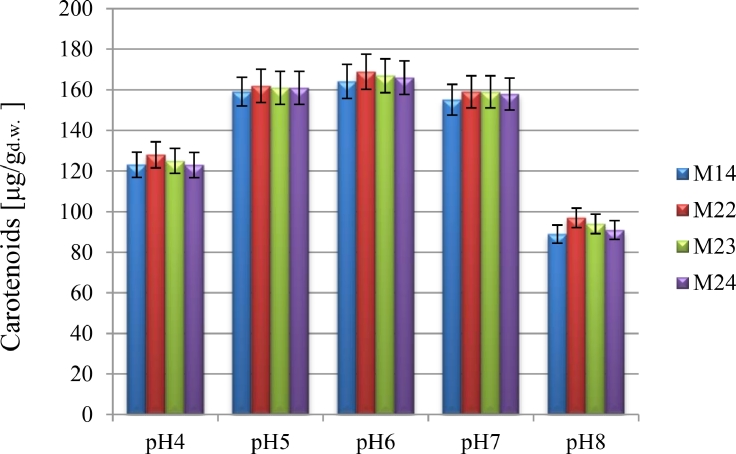
3.2.4. Culture medium pH
Other factor which influences carotenoid content in yeast biomass is the pH amount of culture media[[66], [67]]. The impact of culture medium pH on cell biomass and pigment biosynthesis was assessed at 28 °C in YPG medium (Fig. 4, Table 4). Due to Table 4, volumetric yields after the cultivation of R. mucilaginosa isolates in media at pH 5.0, 6.0 and 7.0 were almost similar and amounted to 3.31– 3.93 mg/L which resulted from the higher yields of cellular biomass and the content of whole carotenoids. Thus, the optimal pH amount of culture media was at pH 6.0 under our culture conditions. A similar optimal pH 6.0 was seen for carotenoid production in species of Rhodotorula mucilaginosa and Rhodotorula graminis [43]. Sharma and Ghoshal [63] reported that pH 6.1 yielded the highest biomass and carotenoid concentrations for R. mucilaginosa in their work. On the other hand, producing β-carotene by yeast Rhodotorula mucilaginosa and Rhodotorula acheniorum was favored at a lower pH 5.5 [67]. Given that alkalinization of a culture medium as a stressor affects the metabolism of yeast cells and absorption of nutrients and induces the expression of genes responsible for cellular glucose metabolism, over-synthesis of polysaccharides including trehalose instead of synthesizing lipids and carotenoids occurs inside the cell. Hence, the optimum pH for most Rhodotorola species is between 5 and 6 [68], [69], [70].
Fig. 4.
The effect of initial pH on carotenoids production by R. mucilaginosa isolates.
Table 4.
The values of biomass (g/L), volumetric yield of carotenoids (mg/L) and volumetric carotenoids productivity (mg/L/h) at different initial pHs at 28 °C in YPG medium.
| pH | M14 | M22 | M23 | M24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ybio | YCar | QCar | Ybio | YCar | QCar | Ybio | YCar | QCar | Ybio | YCar | QCar | |
| 4 | 16.68 ± 0.44 | 2.08 ± 0.02 | 0.016 ± 0.001 | 17.64±0.02 | 2.52 ± 0.02 | 0.022 ± 0.001 | 17.24 ± 0.015 | 2.15 ± 0.01 | 0.016 ± 0.001 | 16.96 ± 0.003 | 2.07 ± 0.006 | 0.017 ± 0.0001 |
| 5 | 21.93 ± 0.015 | 3.48 ± 0.025 | 0.028 ± 0.003 | 22.34±0.01 | 3.60 ± 0.015 | 0.03 ± 0.01 | 22.21 ± 0.005 | 3.57 ± 0.005 | 0.0296 ± 0.0005 | 22.20 ± 0.006 | 3.56 ± 0.005 | 0.029 ± 0.0002 |
| 6 | 22.62 ± 0.015 | 3.70 ± 0.015 | 0.033 ±0.025 | 23.30±0.005 | 3.93 ± 0.02 | 0.032 ± 0.002 | 23.04 ± 0.01 | 3.85 ± 0.01 | 0.032 ± 0.0005 | 22.88 ± 0.001 | 3.78 ± 0.007 | 0.031 ± 0.0004 |
| 7 | 21.4 ± 0.026 | 3.31 ± 0.020 | 0.027 ± 0.002 | 21.89±0.015 | 3.47 ± 0.01 | 0.028 ± 0.0005 | 21.9 ± 0.01 | 3.47 ± 0.015 | 0.028 ± 0.001 | 21.79 ± 0.0005 | 3.44 ± 0.005 | 0.028 ± 0.0004 |
| 8 | 12.25 ± 0.011 | 1.09 ± 0.026 | 0.007 ± 0.001 | 13.37±0.01 | 1.29 ± 0.015 | 0.02 ± 0.01 | 12.89 ± 0.015 | 1.2 ± 0.01 | 0.02 ± 0.01 | 12.55 ± 0.006 | 1.14 ± 0.0015 | 0.008 ± 0.0005 |
(Ybio, Yield of cellular biomass; YCar, volumetric yield of carotenoids; QCar, volumetric carotenoids productivity).
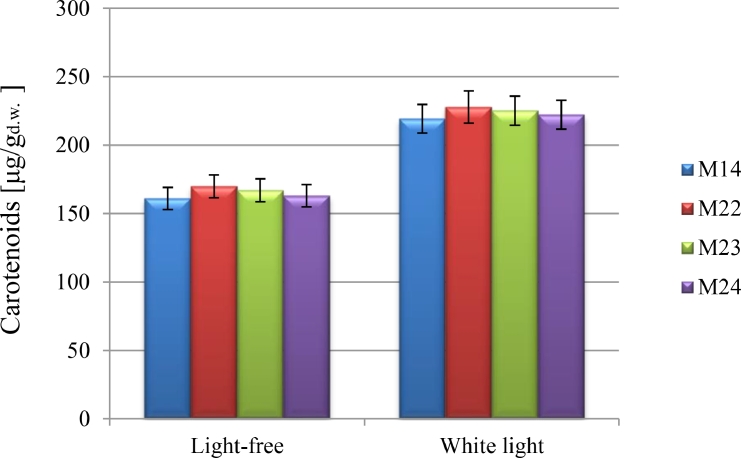
3.2.5. Light
Light was regarded a critical parameter for pigmentation; thus, it improves carotenogenesis which is a photoprotective mechanism to inhibit the cells from light which causes damage [71], [72], [73]. The cells cultivated under white-light LED (wavelengths of 395–530 nm) indicated higher production of carotenoids compared to LED-off cultivation. Then, illuminated phase changed the intensity of the cells’ color of the isolates and enhanced carotenoids content from 170 μg/gd.w. to 228 μg/gd.w. in culture conditions with pH 6.0 at 28 °C in YPG medium of hyper-pigmented R .mucilaginosa-M22 (Fig. 5). Due to the much lower of carotenoids content in biomass without illumination, the amount of volumetric biosynthesis yield was comparable with that provided in light condition. Based on table 5 data, the highest volumetric yield of carotenoid production (5.44 mg/L) was found after the cultivation of R. mucilaginosa-M22 yeast in medium exposure to white light with initial pH 6.0 at 28 °C. In these conditions, carotenoid biosynthesis productivity was 0.045 mg/L/h. Thus, for remain isolates YCar and QCar values did not reduce to less than 4.88 mg/L and 0.040 mg/L/h, respectively (Table 5). Some researchers have surveyed the utilization of artificial lighting like light-emitting diodes (LEDs) to advance the production of target compounds like carotenoids.
Fig. 5.
The effect of light on carotenoids production by R. mucilaginosa isolates.
Table 5.
The values of biomass (g/L), volumetric yield of carotenoids (mg/L) and volumetric carotenoids productivity (mg/L/h) under white-light LED illumination at 28 °C, initial pH 6.0 in YPG medium.
| Light | M14 | M22 | M23 | M24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ybio | YCar | QCar | Ybio | YCar | QCar | Ybio | YCar | QCar | Ybio | YCar | QCar | |
| Light-free | 22.61 ± 0.035 | 3.71 ± 0.01 | 0.026 ± 0.005 | 23.31 ± 0.01 | 3.93 ± 0.01 | 0.031 ± 0.0005 | 23.03 ± 0.01 | 3.84 ± 0.015 | 0.032 ± 0.001 | 22.87 ± 0.015 | 3.79 ± 0.006 | 0.031 ± 0.001 |
| White light | 22.29 ± 0.040 | 4.87 ± 0.01 | 0.041 ± 0.001 | 23.9 ± 0.01 | 5.45 ± 0.01 | 0.045 ± 0.0005 | 23.67 ± 0.015 | 5.32 ± 0.005 | 0.044 ± 0.0005 | 23.41 ± 0.01 | 5.22 ± 0.060 | 0.042 ± 0.001 |
(Ybio, Yield of cellular biomass; YCar, volumetric yield of carotenoids; QCar, volumetric carotenoids productivity).
Moreover, Yen and Zhang [74] assessed β-carotene production by Rhodotorula glutinis in a batch reactor equipped with two LED lamps which caused an increase of β-carotene concentration from 14.69 μg/g to 24.6 μg/g. Moliné et al. [75] reported that the hyper-pigmented of R .mucilaginosa strains indicated enhanced survival (250%) due to relation among carotenoids, ergosterol and cell UV-light resistance.
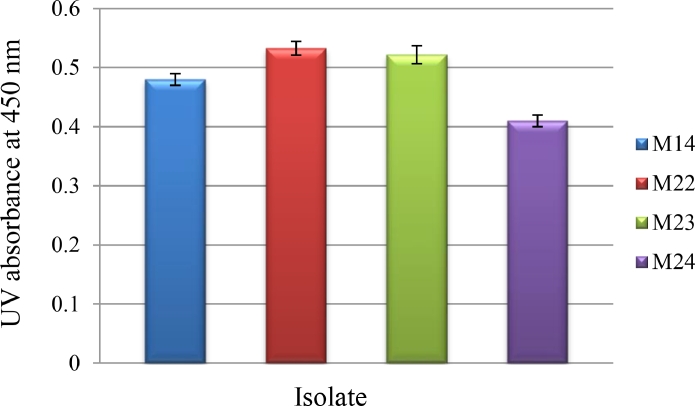
Rodriguez-Amaya et al. [76] and Delia [77] proposed the absorption spectra of β-carotene to be around 450 nm. Regarding absorption maxima of pigments extracted from isolates at 450 nm, isolate M22 produced higher amount of β-carotene when compared to other isolates, under optimized culture conditions (Fig. 6). The carotenoid content has not had a considerable difference among determined isolates (Fig. 5), while R. mucilaginosa-M22 mentioned the greatest amount (228 μg/g); R. mucilaginosa-M14 noted the lowest amount (219 μg/g). The whole carotenoid content highly alters in Rhodotorula sp. Perrier et al. [78] identified the whole carotenoid from Rhodotorula sp., and observed that the concentrations changed from 10 μg/g to 100 μg/g. Shih and Hang [79] also provided total carotenoids of Rhodotorula sp., noted as β-carotene, at a range from 52.2 to 131 μg/g. By various culture conditions, the value of cellular biomass highly changed from 5 to 23.90 gd.w./L and the whole carotenoids content changed from 42 to 228 mg/g. The yields from genus Rhodotorula provided in abovementioned studies were lower than those obtained that can be attributed to culture medium and other cultivation circumstances. El-Banna et al. [45] suggested that the content of carotenoids in yeast biomass could be regarded low for amount < 100 μg/gd.w., the average for 101–500 μg/gd.w., and high for > 500 μg/gd.w.
Fig. 6.
Carotenoid production by Rhodotorula mucilaginosa isolates under optimized culture conditions according to absorption spectra of β-carotene at 450 nm.
3.3. Analysis of produced beta-carotene by HPLC and FT-IR
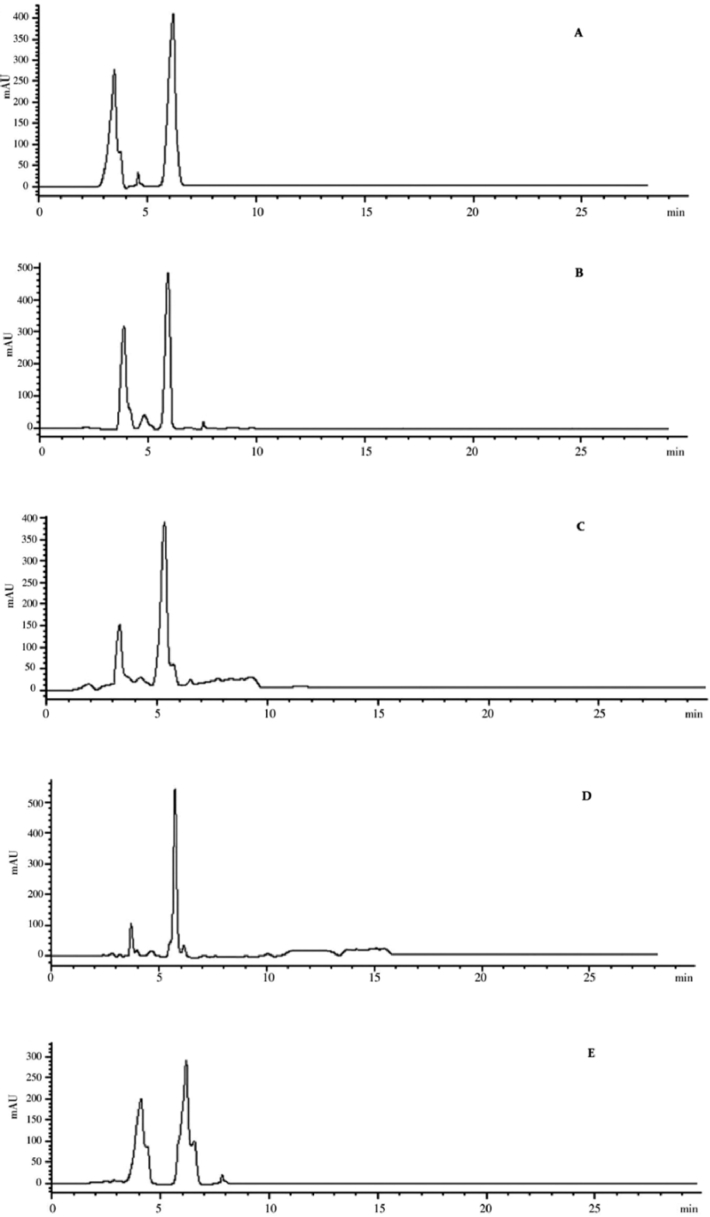
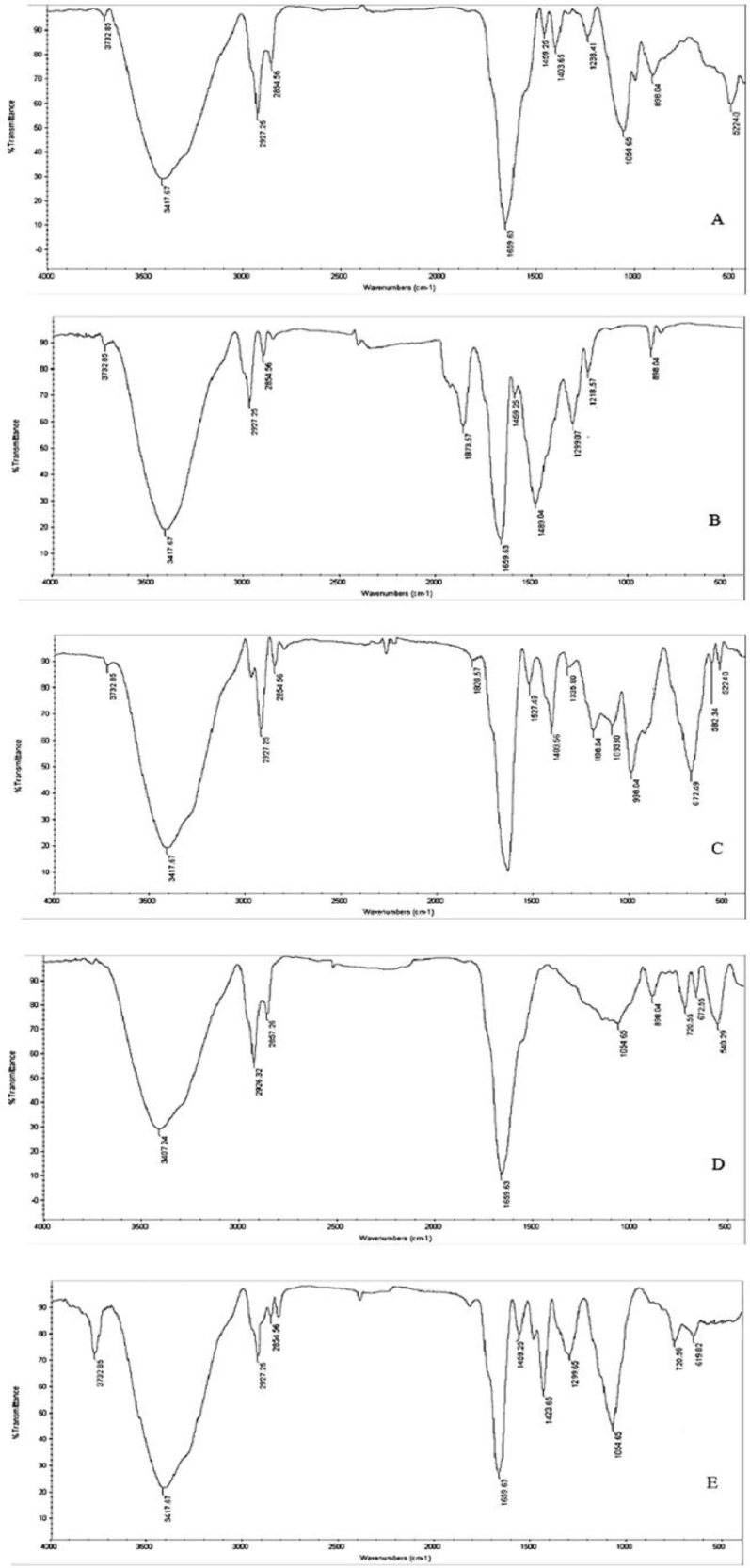
HPLC analysis (Fig. 7), retention time of standard β-carotene was 5.96 min. Extracted carotenoids from the isolates, peaks with the same retention time were recorded. Therefore, the extracted pigments were identified as β-carotene. On the other hand, FTIR absorption spectra of extracted pigments was dominated by two major and strong peaks at 3417 cm–1 (aromatic O—H), and 1659 cm–1 (aromatic C = C) and medium peaks at Vmax 2927(=C—H), 2654 (-C-H), 1459 (C–H) and 1054 (C—C) (Fig. 8). Different functional groups absorb the characteristic frequencies of IR radiations differently. FT-IR determined the functional groups of carotenoid extracts, and showed that pigments pattern are similar to chromatogram given by standard beta carotene.
Fig. 7.
HPLC chromatogram of the carotenoids from R. mucilaginosa isolates; (A) Standard beta-carotene, (B) R. mucilaginosa-M23, (C) R. mucilaginosa-M24, (D) R. mucilaginosa-M22, (E) R. mucilaginosa-M14.
Fig. 8.
FTIR profiling of carotenoid pigments produced by R. mucilaginosa isolates; (A) Standard beta-carotene, (B) R. mucilaginosa-M14, (C) R. mucilaginosa-M22, (D) R. mucilaginosa-M23, (E) R. mucilaginosa-M24.
Our achievements suggest that the tested yeast isolates of the R. mucilaginosa are average producers of carotenoids which will be one of the promising biofactories for the commercial production of carotenoids by optimizing culture conditions.
4. Conclusions
Rhodotorula mucilaginosa is predominant and abundant soil yeast in Tehran's forest parks which was indicated a potential candidate for carotenoids production, basically β-carotene. The research revealed that it is possible to maximize the content of carotenoid biosynthesis by yeast isolates when growing in YPG medium with optimal carotenogenesis conditions; an incubation time of 120 h, temperature of 28 °C, and pH 6.0 and exposure to white light. The cultural medium was composed by 20 g/L peptone, 10 g/L yeast extract, 20 mL/L glycerol. Under optimal conditions, an average of maximum carotenoid content of 223.5 μg/gd.w. was provided. Collectively, the findings indicate that yeast R. mucilaginosa could become a biosource of carotenoids as natural antioxidants that enable to cover the global requirement by pharmacutical, food, beverages, poultry, cosmetic and others industries.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors thank for Laboratory facility support provided by SRBIAU (Science and Research Branch of Islamic Azad University) to carry out this research work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.btre.2021.e00687.
Appendix. Supplementary materials
References
- 1.Li Z., Sun M., Li Q., Li A., Zhang C. Profiling of carotenoids in six microalgae (Eustigmatophyceae) and assessment of their beta-carotene productions in bubble column photobioreactor. Biotechnol. Lett. 2012;34(11):2049–2053. doi: 10.1007/s10529-012-0996-2. [DOI] [PubMed] [Google Scholar]
- 2.Cabral M.M.S., Cence K., Zeni J., Tsai S.M., Durrer A., Foltran L.L. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain by submerged fermentation. Eur. Food Res. Technol. 2011;233(1):159–166. doi: 10.1007/s00271-011-1510-0. [DOI] [Google Scholar]
- 3.Downham A., Collins P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000;35:5–22. doi: 10.1046/j.1365-2621.2000.00373.x. [DOI] [Google Scholar]
- 4.Perez-Fons L., Steiger S., Khaneja R., Bramley P.M., Cutting S.M., Sandmann G. Identification and the developmental formation of carotenoid pigments in the yellow/orange Bacillus spore-formers. Biochim. Biophys. Acta. 2011;1811(3):177–185. doi: 10.1016/j.bbalip.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Babitha S. In: Biotechnology For Agro-Industrial Residues Utilization. Singhee'Nigam P., Pandey A., editors. Springer Science; Republic of Korea: 2009. Microbial pigments; pp. 147–162. [Google Scholar]
- 6.Mannazzu I., Landolfo S., da Silva T.L., Buzzini P. Red yeasts and carotenoid production: outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015;31:1665–1673. doi: 10.1007/s11274-015-1927-x. [DOI] [PubMed] [Google Scholar]
- 7.Kot A.M., Bła zejak S., Gientka I., Kieliszek M., Bry´s J. Torulene and torularhodin: “New” fungal carotenoids for industry. Microb. Cell Fact. 2018;17:49. doi: 10.1186/s12934-018-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfander H. Birkhäuser Verlag; Basel: 1987. Key to Carotenoids, Second ed. [Google Scholar]
- 9.Ishida B.K., Bartley G.E. In: Encyclopedia of Human Nutrition. Caballero B., Allen L., Prentice A., editors. Elsevier; Oxford: 2005. Carotenoids: chemistry, sources and physiology; pp. 330–338. [Google Scholar]
- 10.Olsen J.A., Provitamin A. Function of carotenoids: the conversion of β-carotene into vitamin A. J. Nutr. 1989;119:105–108. doi: 10.1093/jn/119.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E., Ascherio A., Rimm E.B., Stampfer M.J., Colditz G.A., Willett W.C. Intake of carotenoids and retino in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995;87(23):1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 12.Gaziano J.M. Antioxidant vitamins and coronary artery disease risk. Am. J. Med. 1994;97(3):18–21. doi: 10.1016/0002-9343(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 13.Meyskens F.L., Manetta A. Prevention of cervical intraepithelial neoplasia and cervical cancer. Am. J. Clin. Nutr. 1995;62(6):1417–1419. doi: 10.1093/ajcn/62.6.1417S. [DOI] [PubMed] [Google Scholar]
- 14.Zheng W., Blot W.J., Diamond E.J., Norkus E.P., Spate V., Morris J.S., Comstock G.W. Serum micronutrients and the subsequent risk of oral and pharyngeal cancer. Cancer Res. 1993;53(4):795–798. [PubMed] [Google Scholar]
- 15.Jain M., Miller A.B., To T. Premorbid diet and the prognosis of women with breast cancer. J. Natl. Cancer Inst. 1994;86(18):1390–1397. doi: 10.1093/jnci/86.18.1390. [DOI] [PubMed] [Google Scholar]
- 16.Stahl W., Sies H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007;37(1):26–30. doi: 10.1007/s12033-007-0051-z. [DOI] [PubMed] [Google Scholar]
- 17.Mathews-Roth M.M. Cartenoids in erythropoietic protoporphyria and other photosensitivity diseases. Ann. N. Y. Acad. Sci. 1993;691(1):127–138. doi: 10.1111/j.1749-6632.1993.tb26164.x. [DOI] [PubMed] [Google Scholar]
- 18.Gaziano J.M., Hennekens C.H. The role of beta carotene in preventation of cardiovascular diseases. Ann. N.Y. Acad. Sci. 1993;691:148–155. doi: 10.1111/j.1749-6632.1993.tb26166.x. [DOI] [PubMed] [Google Scholar]
- 19.Seddon J.M., Ajani U.A., Sperduto R.D., Hiller R., Blair N., Burton T.C., Farber M.D., Gragoudas E.S., Haller J., Miller D.T., Yannuzzi L.A., Willett W. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. eye disease case-control study group. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 20.Semba R.D., Lauretani F., Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch. Biochem. Biophys. 2007;458(2):141–145. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayne S.T. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996;10(7):690–701. [PubMed] [Google Scholar]
- 23.Office for technology commercialization, University of minnesota, carotenoid biosynthesis for nutraceuticals and commercial food colorings. http://www.license.umn.edu/Products/Carotenoid-Biosynthesis-for-Nutraceuticals-and Commercial-Food-Colorings__Z05100.aspx, 2011 (accessed 4 October 2011).
- 24.Byrne J. Supply: beta carotene club and beyond. http://www.nutraingredients.com/Industry/Supply-Beta-carotene-club-and-beyond, 2011 (accessed 12 October 2011).
- 25.Ausich R.L. Commercial opportunities for carotenoid production by biotechnology. Pure. Appl. Chem. 1997;69:2169–2173. doi: 10.1351/pac199769102169. [DOI] [Google Scholar]
- 26.Dufosse L. Microbial production of food-grade pigments. Food Technol. Biotechnol. 2006;44(3):313–321. doi: 10.1016/b978-0-12-809633-8.13091-2. [DOI] [Google Scholar]
- 27.Kirti K., Amita S., Priti S., Kumar A.M., Jyoti S. Colorful world of microbes: carotenoids and their applications. Adv. Biol. 2014;(2014):1–13. doi: 10.1155/2014/837891. [DOI] [Google Scholar]
- 28.Soil Survey Staff . USDA-Natural Resources Conservation Service; Washington DC: 2014. Keys to Soil Taxonomy, Twelfth ed. [Google Scholar]
- 29.Kurtzman C.P., Fell J.W., Boekhout T., Robert V. Elsevier; Burlington, MA, USA: 2011. The Yeasts, a Taxonomic Study, Fifth ed. [Google Scholar]
- 30.White T.J., Bruns T., Lee S., Taylor J. In: PCR Protocols: a Guide to Methods and Applications. Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. Academic Press; San Diego: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- 31.Ravindar J., Arulselvi I., N Elangovan. Isolation and molecular characterization of butanol tolerant bacterial strains for improved biobutanol production. J. Environ. Biol. 2014;35(6):1131–1136. [PubMed] [Google Scholar]
- 32.Cutzu R., Coi A., Rosso F., Bardi L., Ciani M., Budroni M., Zara G., Zara S., Mannazzu I. From crude glycerol to carotenoids by using a Rhodotorula glutinis mutant. World J. Microbiol. Biotechnol. 2013;29:1009–1017. doi: 10.1007/s11274-013-1264-x. [DOI] [PubMed] [Google Scholar]
- 33.Davies B.H. Academic Press; London: 1976. Carotenoids. Cap. 2, In: Goodwin TW Chemistry and Biochemistry of Plant Pigments; pp. 39–65. [Google Scholar]
- 34.Song M.J., Bae J., Lee D.S., Kim C.H., Kim J.S., Kim S.W., et al. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J Biosci Bioengin. 2006;101(2):157–161. doi: 10.1263/jbb.101.157. [DOI] [PubMed] [Google Scholar]
- 35.Arulselvi I.P., Umamaheswari S., Sharma R.G., Kartik C., Jayakrishna C. Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. J. Food Process Technol. 2014;5:292. doi: 10.4172/2157-7110.1000292. [DOI] [Google Scholar]
- 36.Liu L., Zhou E.M., Jiao J.Y., Manikprabhu D., Ming H., Huang M.J. Hymenobacter mucosus sp. nov., isolated from a soil sample in karst cave. Int. J. Syst. Evol. Microbiol. 2015;65:4121–4127. doi: 10.1099/ijsem.0.000550. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.H., Kim Y.S., Choi T.J., Lee W.J., Kim Y.T. Paracoccus haeundaensis sp. nov., a gram-negative, halophilic, astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1699–1702. doi: 10.1099/ijs.0.63146-0. [DOI] [PubMed] [Google Scholar]
- 38.Anton J., Oren A., Benlloch S., Rodriguez-Valera F., Amann R., Rossello-Mora R. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Microbiol. 2002;52:485–491. doi: 10.1099/00207713-52-2-485. [DOI] [PubMed] [Google Scholar]
- 39.Davoli P., Mierau V., Weber R.W.S. Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Appl. Biochem. Microbiol. 2004;40(4):392–397. doi: 10.1023/B:ABIM.0000033917.57177.f2. [DOI] [PubMed] [Google Scholar]
- 40.Libkind D., van Broock M. Biomass and carotenoid pigment production by patagonian native yeasts. World J. Microbiol. Biotechnol. 2006;22:687–692. doi: 10.1007/s11274-005-9091-3. [DOI] [Google Scholar]
- 41.Maldonade I.R., Rodriguez-Amaya D.B., Scamparini A.R.P. Carotenoids of yeasts isolated from the Brazilian ecosystem. Food Chem. 2008;107(1):145–150. doi: 10.1016/j.foodchem200707075. [DOI] [Google Scholar]
- 42.Frengova G.I., Beshkova D.M. Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 2009;36(2):163–180. doi: 10.1007/s10295-008-0492-9. [DOI] [PubMed] [Google Scholar]
- 43.Maldonade I.R., Scamparini A.R.P., Rodriguez-Amaya D.B. Selection and characterization of carotenoid-producing yeasts from campinas region, Brazil. Braz. J. Microbiol. 2006;38:65–70. doi: 10.1590/S1517-83822007000100014. [DOI] [Google Scholar]
- 44.Libkind D., Sommaruga R., Zagarese H., Broock M.V. Mycosporines in carotenogenic yeasts. Syst. Appl. Microbiol. 2008;28(8):749–754. doi: 10.1016/j.syapm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.El-Banna A., Abd El-Razek A., El-Mahdy A. Isolation, identification and screening of carotenoid-producing strains of Rhodotorula glutinis. Food Nutr. Sci. 2012;3:627–633. doi: 10.4236/fns.2012.35086. [DOI] [Google Scholar]
- 46.Bhosale P., Gadre R.V. Optimization of carotenoids production from hyper-producing Rhodotorula glutinis mutant 32 by a factorial approach. Lett. Appl. Microbiol. 2001;33:12–16. doi: 10.1046/j.1472-765x.2001.00940.x. [DOI] [PubMed] [Google Scholar]
- 47.Ratledge C. Microbial oils and fats: an assessment of their commercial potential. Prog. Ind. Microbiol. 1982;16:119–206. [Google Scholar]
- 48.Makri A., Fakas S., Aggelis G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010;101:2351–2358. doi: 10.1016/j.biortech.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Marova I., Certik M., Breierova E. In: Biomass detection, Production and Usage. Matovic D., editor. In Tech; Rijeka, Croatia: 2011. Production of enriched biomass by carotenogenic yeasts – application of whole-cell yeast biomass to production of pigments and other lipid compounds; pp. 345–384. [DOI] [Google Scholar]
- 50.Petrik S., Marova I., Haronikova A., Kostovova I., Breierova E. Production of biomass, carotenoid and other lipid metabolites by several red yeast strains cultivated on waste glycerol from biofuel production – a comparative screening study. Ann. Microbiol. 2013;63:1537–1551. doi: 10.1007/s13213-013-0617-x. [DOI] [Google Scholar]
- 51.Chatzifragkou A., Makri A., Belka A., Bellou S., Mavrou M., Mastoridou M., Mystrioti P., Onjaro G., Aggelis G., Papanikolau S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy. 2011;36(2):1097–1108. doi: 10.1016/j.energy.2010.11.040. [DOI] [Google Scholar]
- 52.Taccari M., Canonico L., Comitini F., Mannazzu I., Ciani M. Screening of yeasts for growth on crude glycerol and optimization of biomass production. Biores. Technol. 2012;110:488–495. doi: 10.1016/j.biortech.2012.01.109. [DOI] [PubMed] [Google Scholar]
- 53.Saenge C., Cherisilp B., Suksaroge T.T., Bourtoom T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011;46(1):210–218. doi: 10.1016/j.procbio.2010.08.009. [DOI] [Google Scholar]
- 54.El-Banna A.A., El-Razek A.A.M., El-Mahdy A.R. Some factors affecting the production of carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr. Sci. 2012;3(1):64–71. doi: 10.4236/fns.2012.31011. [DOI] [Google Scholar]
- 55.Reynders M.B., Rawlings D.E., Harrison S.T.L. Demonstration of the Crabtree effect in Phaffia rhodozyma during continuous and fedbatch cultivation. Biotechnol. Lett. 1997;19(6):549–552. doi: 10.1023/A:1018341421122. [DOI] [Google Scholar]
- 56.Yuan S.L., Jian Y.W. Optimization of cell growth and carotenoid production of Xanthophyllomyces dendrorhous through statistic experiment design. Biochem. Eng. J. 2006;36(2):182–189. doi: 10.1016/j.bej.2007.02.014. [DOI] [Google Scholar]
- 57.Bura R., Vajzovic A., Doty S.L. Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: production of xylitol and ethanol. J. Ind. Microbiol. Biotechnol. 2012;39(7):1003–1011. doi: 10.1007/s10295-012-1109-x. [DOI] [PubMed] [Google Scholar]
- 58.Hewedy M.A., Ashour S.M. Production of a melanin like pigment by Kluyveromyces marxianus and Streptomyces chibaensis. Aust. J. Basic Appl. Sci. 2009;3(2):920–927. [Google Scholar]
- 59.Keneni A., Gupta V.K. Characterization of a red bacterium strain isolated from root nodule of faba bean (Vicia faba L.) for growth and pigment production. J. Adv. Lab. Res. Biol. 2011;2(3):138–146. [Google Scholar]
- 60.Ramirez J., Obledo N., Arellano M., Herrera E. Astaxanthin production by Phaffia rhodozyma in a fed-batch culture using a low cost medium feeding. e-Gnosis. 2006;4:1–9. [Google Scholar]
- 61.Goodwin T.W. Carotenoids in fungi and non-photosynthetic bacteria. Prog. Ind. Microbiol. 1972;11:29–88. [PubMed] [Google Scholar]
- 62.Latha B.V., Jeevaratnam K., Murali H.S., Manja K.S. Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DER-PDY from natural source. Indian J. Biotechnol. 2005;4:353–357. [Google Scholar]
- 63.Sharma R., Ghshal G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: a statistical approach. Biotechnol. Rep. 2020;25:e00407. doi: 10.1016/j.btre.2019.e00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malisorn C., Suntornsuk W. Optimization of β-carotene production by Rhodotorula glutinis DM28 in fermented radish brine. Bioresour. Technol. 2008;99:2281–2287. doi: 10.1016/j.biortech.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Hayman E.P., Yokoyama H., Chichester C.O., Simpson K.L. Carotenoid biosynthesis in Rhodotorula glutinis. J. Bacteriol. 1974;120:1339–1343. doi: 10.1128/JB.120.3.1339-1343.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin A.M., Chun L., Patel T.R. Growth parameters for the yeast Rhodotorula rubra grown in peat extracts. J. Ferm. Bioeng. 1993;76(4):321–325. [Google Scholar]
- 67.Nasrabadi M.R.N., Razavi S.H. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci. Biotechnol. 2011;20:445–454. doi: 10.1007/s10068-011-0062-1. [DOI] [Google Scholar]
- 68.Krzyściak P., Halska A., Macura A.B. Występowanie i chorobotwórczość grzybów Rhodotorula spp. Post. Mikrobiol. 2007;46(4):291–300. [Google Scholar]
- 69.Casado C., González A., Platara M., Ruiz A., Ariño J. The role of the protein kinase a pathway in the response to alkaline pH stress in yeast. Biochem. J. 2011;438(3):523–533. doi: 10.1042/BJ20110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serra-Cardona A., Canadell D., Ariño J. Coordinate responses to alkaline pH stress in budding yeast. Microbial. Cell. 2015;2(6):182–196. doi: 10.15698/mic2015.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodwin T.W. In: Carotenoids Part B: Metabolism, Genetic and Biosynthesis. Parker L., editor. Academic Press; San Diego: 1993. Biosynthesis of carotenoids: an overview, in: methods in enzymology; pp. 330–340. [Google Scholar]
- 72.Raja R., Haemaiswarya S., Rengasamy R. Exploitation of Dunalliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007;74:517–523. doi: 10.1007/s00253-006-0777-8. [DOI] [PubMed] [Google Scholar]
- 73.Weeks O.B., Saleh F.K., Wirahadikusumah M., Berry R.A. Photoregulated carotenoid biosynthesis in non-photosynthetic microorganisms. Pure Appl. Chem. 1973;35:63–80. doi: 10.1351/pac197335010063. [DOI] [PubMed] [Google Scholar]
- 74.Yen H.W., Zhang Z. Enacement of cell growth rate by light irradiation in the cultivation of Rhodotorula glutinis. Biores. Tech. 2011;102(19):9279–9281. doi: 10.1016/j.biortech.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 75.Moliné M., Flores M.R., Libkind D., del Carmen D.M., Farías M.E., van Broock M. Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: the role of torularhodin. Photochem. Photobiol. Sci. 2010;9:1145–1151. doi: 10.1039/c0pp00009d. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez-Amaya D.B. ILSI; Washington DC: 2001. A Guide to Carotenoid Analysis in Foods, First ed. [Google Scholar]
- 77.Delia B., Rodriguez-Amaya D.B., Kimura M. IFPRI and CIAT; Washington DC and Cali: 2004. HarvestPlus Handbook for Carotenoid Analysis, First ed. [Google Scholar]
- 78.Perrier V., Dubreucq E., Gayzy P. Fatty acid and carotenoid composition of Rhodotorula strains. Arch. Microbiol. 1995;164:173–179. doi: 10.1007/BF02529968. [DOI] [PubMed] [Google Scholar]
- 79.Shih C.T., Hang Y.D. Production of carotenoids by Rhodotorula rubra from Sauerkraut brine. Lebensm. Wiss. U. - Technol. 1996;29:570–572. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.