Abstract
Disseminated histoplasmosis is a life-threatening condition in immunocompromised patients. The majority of healthy persons have benign disease not requiring treatment. However, in persons living with HIV, mortality is high and accurate diagnosis is paramount. We present a case of a 48-year-old HIV-positive woman who presented with haematuria and flank pain. She had a history of recurrent urinary tract infection and nephrolithiasis with obstructive hydronephrosis. During cystoscopy, a bladder lesion was found. Pathological evaluation demonstrated abundant intracellular organisms with apparent budding. Subsequent urine histoplasma antigen was negative. Given the high index of suspicion for histoplasmosis based on the surgical pathology findings and epidemiological history, the patient was started immediately on antifungal therapy. One week later, PCR results of the bladder lesion confirmed the presence of Histoplasma capsulatum. This case highlights a rare presentation of genitourinary histoplasmosis and the utility of surgical pathology evaluation and PCR for diagnosis.
Keywords: HIV / AIDS, medical management, infections, haematuria
Background
Histoplasma capsulatum is a species of dimorphic fungus that causes infection, which naturally occurs throughout the world. In the USA, it is endemic in Mississippi and Ohio River valleys.1 A majority of infected persons are asymptomatic or have mild symptoms that do not require treatment.2 However, in immunocompromised patients, there is a high probability of disseminated disease which can become life-threatening.3 This widespread form has been associated with an estimated mortality of 70%.4 Extrapulmonary histoplasmosis is an AIDS-defining opportunistic infection. The HIV Medicine Association of the Infectious Diseases Society of America recommends primary prophylaxis for persons with a CD4 count less than 150 cells/µL with high risk because of occupational exposure or living in a community with hyperendemic rate.5 The most common presentation in persons living with HIV is disseminated histoplasmosis with pulmonary infiltrate, lymphadenopathy and hepatosplenomegaly. However urinary histoplasmosis has not been well described in the HIV population. Genitourinary histoplasmosis is very rarely reported,6 7 and the few cases reported in the literature have mostly been in immunocompetent patients. This can be a diagnostic challenge as most cases of haematuria associated with abdominal pain and flank pain are caused by bacterial infection.8 Most urine cultures are not sent routinely for fungal stains. The majority of laboratories hold specimen for a short duration, an average of 3–5 days, which is insufficient to identify this dimorphic fungus.6 9 Even when yeast is identified in urine culture, it is most typically Candida species.10 The most common diagnostic method for histoplasmosis is the detection of urine antigen. Multiple studies have shown that this testing modality has high sensitivity and specificity in the range of 96%–98% in persons living with HIV who have disseminated disease.11 12 The published cases that are culture positive with also negative urine antigen test are extremely rare in this group. However, given the high mortality rate, it is prudent that other diagnostic methods need to be obtained if the suspicion of infection remains high.
Case presentation
The case was a 48-year-old woman with a history of HIV with a CD4 count of 16 cells/µL and chronic treatment-naïve hepatitis C with quantitative RNA PCR of 3 878 765 IU/mL. She presented to the urology clinic with a 5-day history of haematuria, dysuria and right flank pain. Her medical history was significant for recurrent nephrolithiasis with obstruction requiring nephrostomy and ureteral stents. A comprehensive review of systems was negative except for a generalised rash and itching of 2-year duration. She was diagnosed with HIV 13 years prior with a long-standing history of poor adherence to antiretroviral therapy. She had no documented history of any previous opportunistic infections and no clinical relevant mutations on prior drug resistance test. One month prior to presentation, the patient was started on Biktarvy and she reported good adherence. She had a history of polysubstance abuse with cocaine and intravenous heroin, which she quit 5 years before. She has never travelled outside the USA. She was originally from Indiana but had been living in Florida for the past 19 years. She reported travelling to Mississippi 3 years before where she worked as a truck driver for 3 months. She appeared well and in no distress. Blood pressure was 122/75 mm Hg, pulse rate was 95 beats per minute, temperature was 36.8°C (98.2°F) and respiratory rate was 16 breaths per minute. Her physical examination revealed tenderness in the right lower abdominal quadrant and right costovertebral angle. She had diffuse papular lesions with excoriations sparing her face and upper back.
Investigations
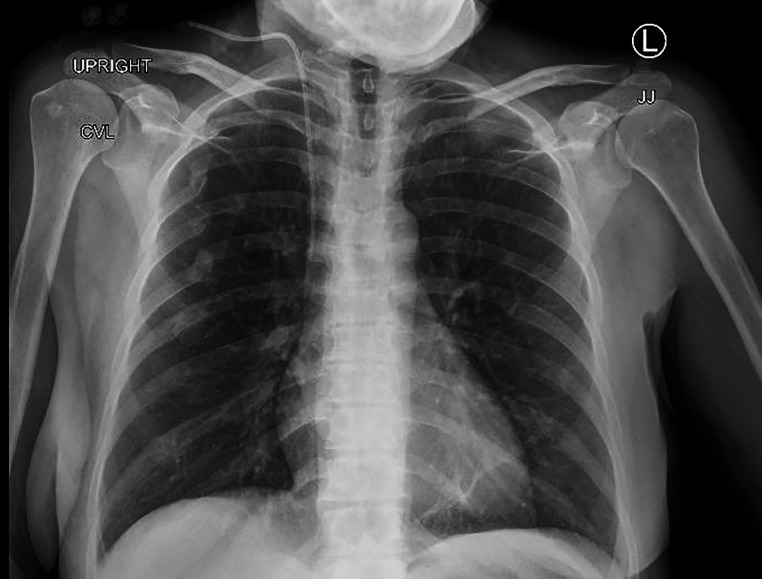
In the outpatient clinic, a urinalysis and microscopy revealed elevated red blood cells, positive nitrites, numerous leucocytes and protein of 100 mg/dL. A CT scan of the abdomen and pelvis (figure 1) showed right hydronephrosis and focal soft tissue thickening of bladder along the right posterior aspect near the ureteropelvic junction (figure 2). She was directly admitted to the hospital for cystoscopy with stent placement. In hospital, her laboratory studies showed a complete blood count: white blood cell count of 3.1×109/L (reference range 4.0–10.0×109/L), haemoglobin of 107 g/L (reference range 120–160 g/L) and platelets of 100×109/ L (reference range 150–450 ×109/L). Comprehensive metabolic panel showed aspartate aminotransferase of 155 IU/L (reference range 0–37 IU/L), alanine aminotransferase of 57 IU/L (reference range 0–35 IU/L), creatinine of 1.42 mg/dL (reference range 0.38–1.02 mg/dL) and blood urea nitrogen of 24 mg/dL (reference range 6–21 mg/dL). Her chest X-ray was normal with no enlarged lymph nodes and no effusion or infiltrates (figure 3). During cystoscopy, there was difficulty in identifying the right ureteral orifice. A diffuse bladder lesion was noted overlying the right trigone and lateral bladder wall. A transurethral resection was performed, with residual abnormal mucosa remaining at the conclusion of the procedure. The resected tissue was submitted for surgical pathological analysis. The patient was referred to Interventional Radiology for placement of a percutaneous nephroureteral stent to relieve the ureteral obstruction.
Figure 1.
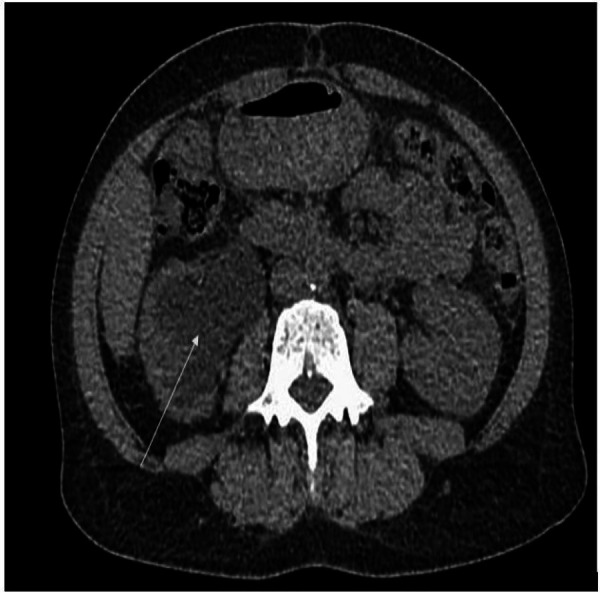
CT scan of the abdomen and pelvis with right hydronephrosis.
Figure 2.
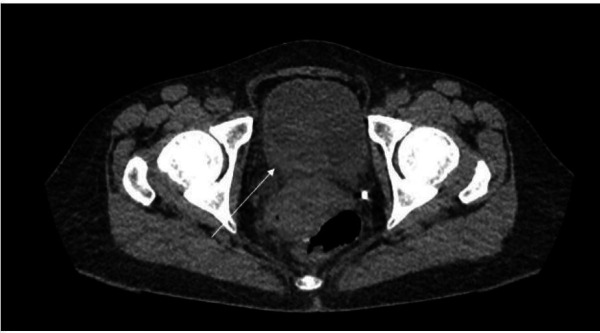
CT scan of the abdomen and pelvis with focal soft tissue thickening along the right posterior aspect of the bladder near the ureteropelvic junction.
Figure 3.
Chest X-ray with normal heart size, no lung infiltrates, no effusion and no lymphadenopathy.
A concurrent urine culture showed mixed growth of three or more organisms, which were not further identified by the microbiology laboratory. Surgical pathology assessment of the bladder lesion revealed abundant spherical intracellular organisms (figure 4), using a GMS (Grocott-Gomori Methamine Silver) stain. This highlighted multiple foci of apparent budding (figure 5). Given these features, the possibility of bladder involvement by histoplasmosis was raised as the most likely aetiology.
Figure 4.
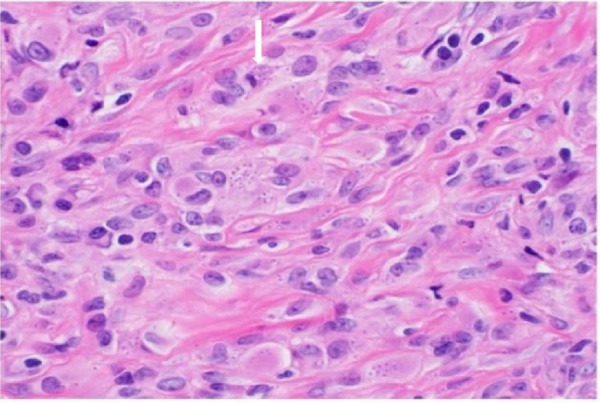
Surgical pathology bladder biopsy. H&E stain, with high-power field showing abundant intracellular organisms within histiocytes.
Figure 5.
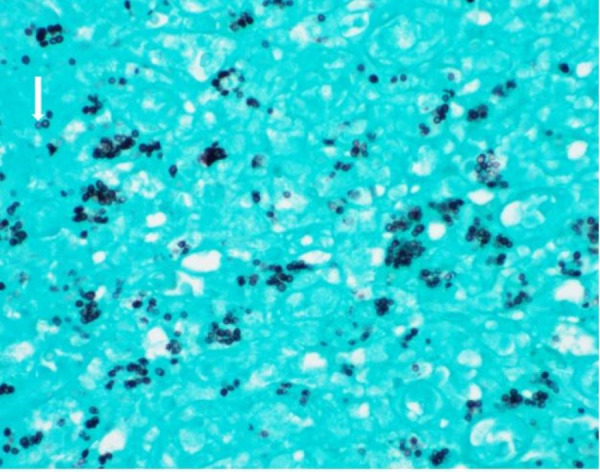
Surgical pathology bladder biopsy. Grocott-Gomori Methamine Silver stain highlighting intracellular organisms with multiple foci of apparent budding.
Unfortunately, given the lack of suspected infectious process at the time of cystoscopy, the bladder specimen was not sent for cultures. However, given the high suspicion based on the histological findings, PCR testing on the formalin-fixed paraffin-embedded tissue was performed. In addition, subsequent work-up included negative urine fungal culture, negative blood fungal culture, negative serum histoplasmosis antibodies, and negative urine histoplasmosis antigen from a clean catch specimen and nephrostomy tube. One week later following the initial pathological evaluation, the PCR test came back positive for the presence of H. capsulatum.
Differential diagnosis
In this patient with a very low CD4 count and intracellular yeast on surgical pathology, a broad differential diagnosis was considered. Due to overlapping features, it can be challenging to speciate some infectious process based on histological assessment alone. While other organisms such as Pneumocystis jirovecii can have a similar histological appearance, our patient was on dapsone prophylaxis, and the lack of concurrent respiratory symptoms made this diagnosis less likely. Other dimorphic fungi such as blastomycosis may present as budding yeast; however, the intracellular nature of these organisms and the smaller size essentially excluded this possibility. Given her lack of international travel, leishmaniasis and Talaromyces marneffei were excluded. Histoplasmosis remained high on the differential, given that she travelled to an endemic area, the low CD4 count and the characteristic budding yeast on pathology.
Treatment
Due to the high mortality rate in patients with HIV with disseminated histoplasmosis, treatment was started immediately with posaconazole extended-release capsules despite the negative urine antigen while the confirmatory PCR testing was pending. We chose posaconazole as the initial therapy because the patient requested to leave hospital secondary to social and housing issues. There was concern that with the dietary restrictions with oral itraconazole we may not be able to achieve therapeutic level in this patient given her history of non-adherence and outpatient clinic follow-up. Itraconazole capsule needs to be taken with a full meal for absorption and 2 hours before and 1 hour after antacids.
Outcome and follow-up
She was seen in the outpatient Infectious Disease clinic 1 month after hospital discharge. Her haematuria had resolved, but she continued with chronic dysuria. Three months later, she was readmitted with a 3-day history of fever, chills and right flank pain. She was found to have polymicrobial bacteraemia. Blood cultures revealed methicillin-susceptible Staphylococcus aureus, Enterobacter cloacae complex and Klebsiella pneumoniae. The source was considered to be pyelonephritis in the setting of her nephrostomy tube. She was treated with a 2-week course of cefazolin and cefepime. She completed 12 months of posaconazole with no evidence of persistent disseminated histoplasmosis.
Discussion
Disseminated histoplasmosis in patients with AIDS most commonly manifests with lymphadenopathy, hepatosplenomegaly, fever, night sweats, weight loss, cough and dyspnoea.6 Histoplasmosis can involve any organ of the body, but the most commonly affected areas are lungs, gastrointestinal tract, central nervous system and adrenal glands; genitourinary involvement is exceedingly rare. Most of the cases have been identified incidentally on postmortem series.9 The uniqueness of our case was that this patient was symptomatic with gross haematuria and abdominal pain. Review of the literature showed three cases in which individuals presented with gross haematuria; however, all of these patients were immunocompetent.13
The gold standard for the diagnosis of histoplasmosis is culture from the involved tissue; however, this takes at least 4–6 weeks and the overall sensitivity is low. In patients with HIV with disseminated disease, blood culture is positive only in 50% of cases.11 In contrast, urine histoplasma antigen has a detection rate of 95%–97%,11 12 making it the most preferred diagnostic test. It was unexpected that our patient would have two negative urine histoplasma antigen tests. The patient was also negative for serum histoplasma antibodies. It has been recognised that in patients with AIDS, diagnostic yield of serum histoplasma antibodies can be as low as 70% and some individuals fail to mount any serological response.14 Histopathology is helpful with the demonstration of intracellular yeast, but this can be easily confused with other organisms including P. jirovecii, Candida glabrata and Talromyces marneffi. Special stains such as Periodic acid–Schiff or GMS with an experienced pathologist are helpful. In our case, after the pathologist used a GMS stain that highlighted the intracellular organisms and accentuated the budding, they were confident that this was histoplasmosis. Given her history of travelling to an endemic area in Mississippi, we started her on active therapy despite her negative urine antigen. Tissue biopsy material was sent for PCR for confirmation of our diagnosis. Several real-time PCR assays have been developed to detect H. capsulatum. The rapidity of these tests with average results in 3 hours makes it extremely attractive in diagnosing histoplasmosis. Real-time PCR assay has 95.4% sensitivity and 96% specificity in respect to tissue and blood cultures.15 However, this test is expensive and is not available in most laboratories.
The recommended treatment for disseminated histoplasmosis by the Infectious Disease Society of America is amphotericin B for 4 weeks, followed by oral itraconazole.16 Histoplasma is highly susceptible to posaconazole in in vitro studies and has been used successfully in salvage therapy. Comparison between itraconazole and posaconazole has shown no statistical difference in disease outcome.17 Therefore, posaconazole is a reasonable alternative agent. She was requesting to leave hospital immediately; hence, amphotericin B was not an option as it requires clinical monitoring. She had complete recovery after 12 months of posaconazole.
There is a high probability that if a biopsy of the bladder lesion was not performed, she would have continued to progress with untreated histoplasmosis with potentially fatal outcome. The unique challenge in her case was the absence of any other symptoms or clinical features to suggest other organ involvement. She had thrombocytopenia and anaemia, which could have been a manifestation of bone marrow involvement. However, a review of records showed that this was long-standing and could be easily attributed to her underlying HIV and hepatitis C. To further complicate this clinical picture, she had a history of recurrent urinary tract infection and nephrolithiasis. Therefore, it was easy to attribute her urinary symptoms to this. Our case highlights how tissue PCR can be used in patients testing negative for urine antigen and serology.
Patient’s perspective.
I came to the hospital for my kidneys because of the blood from my stones. I did not know I have an infection. I did not feel sick. I do not know where this infection came from. I took the tablets three times a day for that whole year and now finished. I am happy that infection is gone and hope it never comes back.
Learning points.
Histoplasmosis in patients with AIDS may not always present with classic features of disseminated disease or pulmonary symptoms.
Epidemiological history is key in making a clinical diagnosis and obtaining differentials.
When the pretest probability of disease is high, one test in isolation may not confirm the diagnosis.
PCR is a great adjunctive tool in the diagnosis of histoplasmosis.
Footnotes
Contributors: CTJ designed and conceptualised the case reports and was involved in clinical care, data analysis and interpretation of results and drafting of initial manuscript. NI was involved in clinical care, data analysis and interpretation and revision of manuscript for intellectual content. MF was involved in interpretation of surgical pathology and revision of manuscript for intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Manos NE, Ferebee SH, Kerschbaum WF. Geographic variation in the prevalence of histoplasmosis sensitivity. Icon Dis Chest 1956;6:649–68. [DOI] [PubMed] [Google Scholar]
- 2.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007;20:115–32. 10.1128/CMR.00027-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couppie P, Sobesky M, Christine A. Histoplasmosis and acquired immunodeficiency syndrome. Clin Infect Dis 2004;38:134–8. 10.1086/379770 [DOI] [PubMed] [Google Scholar]
- 4.Wheat LJ, Chetchotisakd P, Williams B, et al. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin Infect Dis 2000;30:877–81. 10.1086/313824 [DOI] [PubMed] [Google Scholar]
- 5.New Panel on Opportunistic Infections in Adults and Adolescents with HIV . Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the centers for disease control and prevention, the National Institutes of health, and the HIV medicine association of the infectious diseases Society of America. Histoplasmosis 2019. [Google Scholar]
- 6.Parsons RJ, Zarafonetis CJ. Histoplasmosis in man, report of seven cases and a review of 71 cases. Arch Intern Med 1945;75:1–23. 10.1001/archinte.1945.00210250008001 [DOI] [Google Scholar]
- 7.Orr WA, Mulholland SG, Walzak MP. Genitourinary tract involvement with systemic mycosis. J Urol 1972;107:1047–50. 10.1016/S0022-5347(17)61204-7 [DOI] [PubMed] [Google Scholar]
- 8.Vranic SM, Zatric N, Rebic V, et al. The most frequent isolates from outpatients with urinary tract infection. Mater Sociomed 2017;29:17–20. 10.5455/msm.2017.29.17-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friskel E, Klotz SA, Bartholomew W, et al. Two unusual presentations of urogenital histoplasmosis and a review of the literature. Clin Infect Dis 2000;31:189–91. 10.1086/313904 [DOI] [PubMed] [Google Scholar]
- 10.Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of Funguria in hospitalized patients. Clin Infect Dis 2000;30:14–18. 10.1086/313583 [DOI] [PubMed] [Google Scholar]
- 11.Baddley JW, Sankara IR, Rodriquez JM, et al. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis 2008;62:151–6. 10.1016/j.diagmicrobio.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 12.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011;53:448–54. 10.1093/cid/cir435 [DOI] [PubMed] [Google Scholar]
- 13.Pasqualotto AC, Oliveira FM, Severo LC. Histoplasma capsulatum recovery from the urine and a short review of genitourinary histoplasmosis. Mycopathologia 2009;167:315–23. 10.1007/s11046-009-9182-z [DOI] [PubMed] [Google Scholar]
- 14.Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine 1990;69:361. 10.1097/00005792-199011000-00004 [DOI] [PubMed] [Google Scholar]
- 15.Babady NE, Buckwalter SP, Hall L, et al. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 2011;49:3204–8. 10.1128/JCM.00673-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases Society of America. Clin Infect Dis 2007;45:807–25. 10.1086/521259 [DOI] [PubMed] [Google Scholar]
- 17.Bobbili K, Akram S, Sundareshan V, et al. Comparison of posaconazole and itraconazole for treatment of histoplasmosis. Open Forum Infectious Disease 2016;3:1646. 10.1093/ofid/ofw172.1346 [DOI] [Google Scholar]