Abstract
Background & Aims
One of the features of ulcerative colitis (UC) is a defect in the protective mucus layer. This has been attributed to a reduced number of goblet cells (GCs). However, it is not known whether abnormal GC mucus secretion also contributes to the reduced mucus layer. Our aims were to investigate whether GC secretion was abnormal in UC and exists as a long-term effect of chronic inflammation.
Methods
Colonoids were established from intestinal stem cells of healthy subjects (HS) and patients with UC. Colonoids were maintained as undifferentiated (UD) or induced to differentiate (DF) and studied as three-dimensional or monolayers on Transwell filters. Total RNA was extracted for quantitative real-time polymerase chain reaction analysis. Carbachol and prostaglandin E2 mediated mucin stimulation was examined by MUC2 IF/confocal microscopy and transmission electron microscopy.
Results
Colonoids from UC patients can be propagated over many passages; however, they exhibit a reduced rate of growth and transepithelial electrical resistance compared with HS. Differentiated UC colonoid monolayers form a thin and non-continuous mucus layer. UC colonoids have increased expression of secretory lineage markers ATOH1 and SPDEF, along with MUC2 positive GCs, but failed to secrete mucin in response to the cholinergic agonist carbachol and prostaglandin E2, which caused increased secretion in HS. Exposure to tumor necrosis factor α (5 days) reduced the number of GCs, with a greater percentage decrease in UC colonoids compared with HS.
Conclusions
Chronic inflammation in UC causes long-term changes in GCs, leading to abnormal mucus secretion. This continued defect in GC mucus secretion may contribute to the recurrence in UC.
Keywords: Ulcerative Colitis, Goblet Cell, Mucus Layer, Colonoids
Abbreviations used in this paper: ATOH1, atonal homolog 1; cAMP, cyclic adenosine monophosphate; Cch, carbachol; DF, differentiated; 3-D, three-dimensional; GC, goblet cell; HS, healthy subjects; IBD, inflammatory bowel disease; NDM, non-differentiated medium; Ngn 3, Neurogenin 3; PCR, polymerase chain reaction; PGE2, prostaglandin E2; SEM, standard error of the mean; SPEDF, SAM pointed domain containing Ets transcription factor; TEER, transepithelial electrical resistance; TEM, transmission electron microscopy; TNF, tumor necrosis factor; UC, ulcerative colitis; UD, undifferentiated
Graphical abstract
Summary.
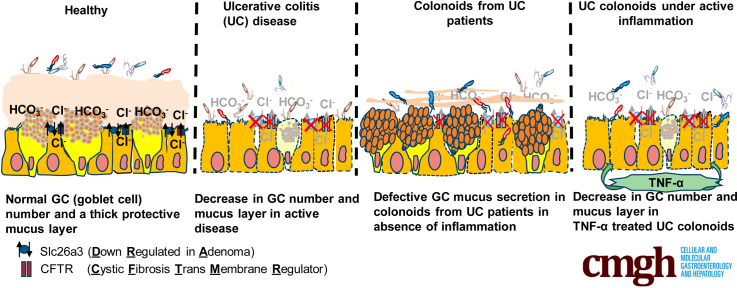
Our results suggest that the abnormal mucus layer in UC patients is due to the effect of an active inflammatory environment to reduce the number of goblet cell (GCs), as well as due to long-term changes in stimulated mucin secretion that persist even in the absence of inflammatory cells.
Ulcerative colitis (UC) is a chronic relapsing colonic disorder. A frequent colonic abnormality in UC is a reduced mucus layer secreted by goblet cells (GCs). Mucus layer defects contribute to the UC pathophysiology by triggering immune responses and/or allowing increased and more proximate exposure to luminal bacteria, both of which can lead to further reduced barrier maintenance, mucosal damage, defective absorption, and increased fluid secretion. The mucus layer is secreted by GCs, which primarily occur in differentiated (DF) colonocytes. Secretion of pro-inflammatory cytokines in UC contributes to the destruction of the epithelial barrier including the mucus layer.1, 2, 3, 4 However, even in the absence of endoscopic signs of active inflammation, the intestinal mucosa of UC patients in remission has a defective mucus layer and histologic changes including branching of crypts, thickened muscularis mucosa, Paneth cell metaplasia, and neuroendocrine cell hyperplasia.5 These changes suggest that the recovered intestine is permanently altered even after the inflammation has resolved. In fact, the intestinal epithelium of UC in remission has an expression profile that is significantly different from that found in healthy mucosa, which includes increases in expression of REG4, S100P, SERPINB5, DEFB1, and AQP3 and decreases in SLC16A1and AQP8 expression.6,7 Importantly, these genes modulate epithelial cell growth, sensitivity to apoptosis, and immune function.7 Other studies have shown that intestinal epithelium of inflammatory bowel disease (IBD) patients can harbor persistent alterations in gene expression or DNA methylation despite complete endoscopic and histologic remission.2,8,9 These changes could contribute to the frequent disease recurrences that are common in UC.7,10, 11, 12 Altogether these results support the view that changes in the mucosa of patients with UC persist long after the inflammation has resolved.
We hypothesized that a long-term consequence of colonic inflammation in UC is abnormal GC function that includes reduced stimulated mucus secretion. To test this hypothesis, we used an ex vivo human organoid/colonoid model made from healthy subjects (HS) and from active and inactive mucosa of UC patients. Our results suggest that UC colonoids, which lack the presence of inflammatory cells, maintain an abnormal GC phenotype, with a reduced mucus layer due to defective cholinergic/prostaglandin E2 (PGE2) induced mucus secretion but with an increase in the number of GCs. Exposure of UC colonoids to tumor necrosis factor (TNF)-α reduced the number of GCs, which occurred to a greater extent than in HS colonoids. Our results suggest that the abnormal mucus layer in UC is due to the effects of an active inflammatory environment to reduce the number of GCs as well as due to long-term changes in stimulated mucin secretion that persist even in the absence of inflammatory cells and exist in colonoids made from active and inactive UC.
Results
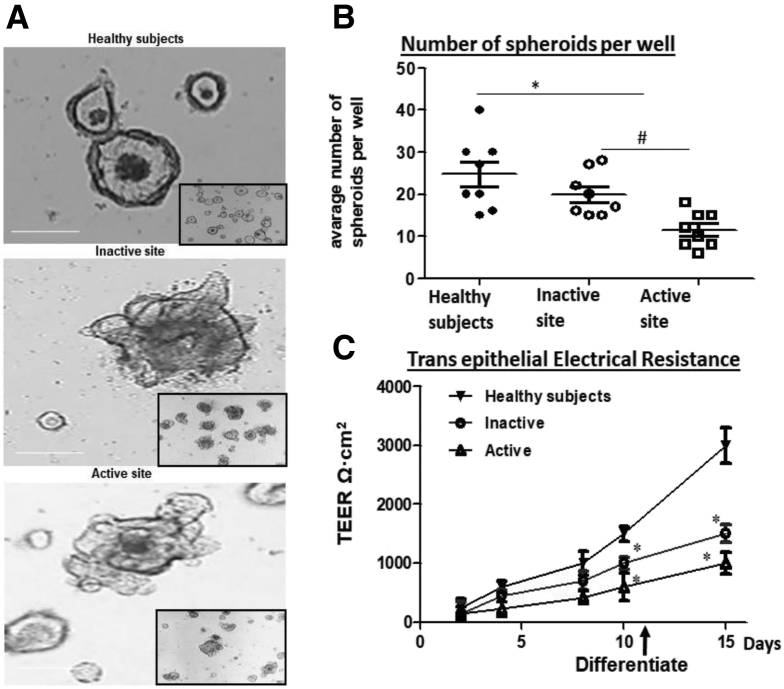
UC Derived Colonoids Can Be Grown in Culture Over Multiple Passages but They Exhibit a Reduced Growth Rate
Human UC patient-derived colonoids were propagated and compared with site-matched HS colonoids. Similar to colonoids grown from HS, UC colonoids formed three-dimensional (3D) spheroids and could be passaged at least 40 times. Figure 1A shows the phase-contrast images of colonoids from HS and UC patients 5 days after splitting. Morphologically, UC colonoids had more budding structures compared with HS. However, when the growth of 3D spheroids was quantitated by measuring the number of spheroids per well after each split over time and for multiple passages, active disease UC colonoids grew slowly and formed fewer spheroids compared with inactive UC and HS (Figure 1B). We have demonstrated that human colonoids can be grown as 2D monolayers.13 The progress of monolayer formation was monitored daily by a steady increase in transepithelial electrical resistance (TEER) (Figure 1C). The monolayers were maintained in the undifferentiated (UD) crypt-like state by growth in Wnt3A, RSPO1, and Noggin, whereas withdrawal of growth factors (Wnt3A and RSPO1) drove differentiation by 5 days. As shown in Figure 1C, active and inactive UC colonoids were delayed in establishing confluency and had lower TEER (inactive UC: UD, 700 Ω·cm2 ± 60; DF, 1500 Ω·cm2 ± 60; n = 10, P ≤ .05 vs HS; active UC: UD, 600 Ω·cm2 ± 60; DF, 1200 Ω·cm2 ± 80; n = 10, P ≤ .05 vs HS) compared with monolayers from HS (UD, 1200 Ω·cm2 ± 55; DF, 2500 Ω·cm2 ± 55; n = 10) measured at post-plating day10 for UD and day 15 for DF colonoids (Figure 1D). The slow growth of colonoids in 3D and 2D monolayer formation suggests that there are sustained differences within the epithelial stem cell compartment of the UC vs HS mucosa.
Figure 1.
UC colonoids have differences in growth compared with colonoids from HS.(A) A representative bright field image of 3D colonoids from HS and inactive and active sites of UC patients. (B) Number of 3D spheroids per well from HS and inactive and active sites of UC patients. Quantitation of spheroids was made 2 days after splitting. (C) Changes in TEER of colonoid monolayers from HS (black triangle), inactive UC (circle), and active (triangle) UC. Spheroids and monolayers from each subject were analyzed at least 3 times and used as n = 1. ∗P < .05 vs HS, #P < .05 vs inactive UC. Scale bar = 20 μm.
Colonoids Derived From UC Tissue Form a Thin Mucus Layer and Have Defective Barrier/Mucosal Integrity
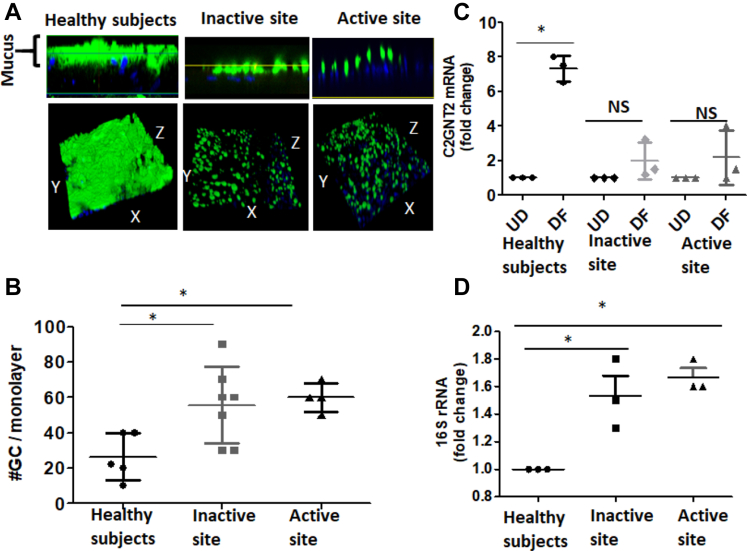
Active UC tissues have a reduced mucus layer, and many UC colons have a reduced number of mucus containing GCs.13 Similarly, colonoid monolayers made from the tissue derived from either inactive or active sites of UC lacked a uniform mucus layer; instead, they have a thin and non-uniform mucus layer (Figure 2A). We further analyzed the number of GCs in these monolayers by counting MUC2 positive cells per monolayer. Surprisingly, DF UC monolayers from both active and inactive sites had a significantly higher number of GCs compared with monolayers from HS (Figure 2B). The primary component of the mucus layer is MUC2, an extensively O-glycosylated molecule that forms polymeric sheets to which luminal bacteria attach and which provides a food source for the microbiota.8,14 O-glycans contribute to about 80% of its mass and therefore are an important determinant of mucus properties. O-glycosylation of MUC2 occurs post-translationally in the Golgi apparatus. The primary enzymes in this process are the core 1 β1,3-galactosyltransferase (C1galt1), core 2 β1, 6N-acetylglucosaminyltransferases (C2GnTs), and core 3 β1,3-N-acetylglucosaminyltransferase (C3GnT).15 The mRNA levels of several enzymes responsible for glycosylation of mucin dimers were measured including C1galt1, C2GnT, and C3GnT. Of the enzymes tested, C2GNT2 did not increase with differentiation of UC colonoids as occurred in HS colonoids. Similar results were seen in colonoids from inactive and active sites of UC patients (Figure 2C). In contrast, mRNAs of C1galt1 and C3GnT were not significantly different from HS (data not shown).
Figure 2.
UC colonoids have defects in mucus secretion and barrier function.(A) Methanol–Carnoy’s fixed DF colonoid monolayers stained with MUC2 (green), nucleus (blue). Representative confocal XZ (above) and 3D-XYZ (below) projections depicting the MUC2 layer in colonoids monolayer is shown. (B) Average number of GCs expressed after 5 days of differentiation of colonoid monolayers. (C) Differences in mRNA expression of C2GNT2 mRNA after differentiation of monolayers from HS and inactive and active UC sites. (D) Bacterial 16S rRNA expression in colonoids after 8-hour infection of DF monolayers. (A) and (B) Multiple areas of monolayers from each subject were analyzed. (C) and (D) n = 3 monolayers from each group were analyzed at different times. Results are shown as mean ± SEM. ∗P < .05 vs HS. Scale bar = 20 μm.
We further investigated the barrier (mucosal) integrity by exposing DF colonoid monolayers with intact mucus to apical Escherichia coli (1 × 106 colony-forming units/mL) (8 hours) and performed 16S bacterial rRNA based real-time polymerase chain reaction (PCR) analysis on total RNA extracted from monolayers. An increased amount of bacterial 16S rRNA was present in monolayers from UC patients (inactive and active) as compared with HS, suggesting that UC colonoids have a defective mucus barrier (Figure 2D).
Activation of Secretory Lineage Differentiation in UC Compared With Non–IBD Controls
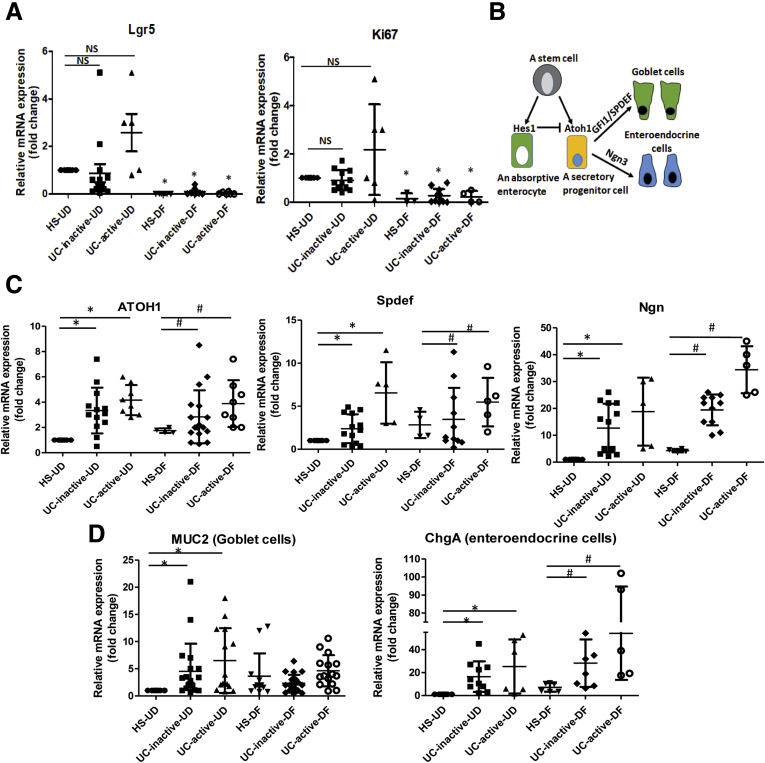
To investigate the differentiation status and GC-related gene expression in UC colonoids, quantitative PCR expression analysis of a selected panel of genes was performed in UD and DF colonoids from HS and UC patients. The expressions of the stem cell gene Lgr5 and cell proliferation marker Ki67 were slightly but not significantly increased in both inactive and active UC colonoids compared with HS (Figure 3A). Nonetheless, the expression of both Lgr5 and Ki67 decreased with DF of UC colonoids as in HS. In addition, the expression of genes associated with mucus-producing GCs was determined. Shown in Figure 3B are results for a transcription factor, atonal homolog 1 (ATOH1), which is a gatekeeper that controls the fate of intestinal progenitors. Intestinal progenitors with reduced Notch activity express high levels of ATOH1 and commit to a secretory lineage fate (Figure 3B). Therefore, ATOH1 expression in UD and DF colonoids was measured. Both active and inactive UC colonoids in UD, as well as DF, states had significantly higher expression of ATOH1 compared with HS (Figure 3C, left). The expression of transcription factors downstream of ATOH1 were also analyzed including SPDEF (SAM pointed domain containing Ets transcription factor) and Ngn3 (Neurogenin 3), which specify differentiation and maturation of GC and enteroendocrine cells, respectively (Figure 3B). Similar to ATOH1, the expressions of SPDEF and Ngn3 were significantly higher in both active and inactive UC colonoids compared with HS (Figure 3C, middle and right, respectively). The expression of MUC2 (GC marker) and ChgA (enteroendocrine cells) was also determined (Figure 3D). MUC2 message was increased in the UD colonoids from both active and inactive UC compared with HS, whereas the message was not significantly different between DF colonoids from each group. In contrast, ChgA transcripts followed a pattern of up-regulation in UD as well as in DF UC colonoids from active and inactive UC compared with HS.
Figure 3.
Differential gene expression profiles in UD and DF colonoids from HS compared with UC patients: Relative mRNA levels of (A) proliferation genes, (B) schematic representation of absorptive and secretory pathways starting from a progenitor and the genes involved in this process, (C) secretory lineage genes, and (D) genes specific to different cell types, by quantitative PCR. Messenger RNA levels are normalized to 18S ribosomal RNA expression. Result is normalized to HS set as 1 and expressed as fold change. Results are shown as mean ± SEM. ∗P < .05 vs HS-UD; #P < .05 vs HS-DF; 3D colonoids from each subject were analyzed at least 2 times and used as n = 1.
UC Colonoids Differentially Express Ion Transport Proteins as Compared With HS
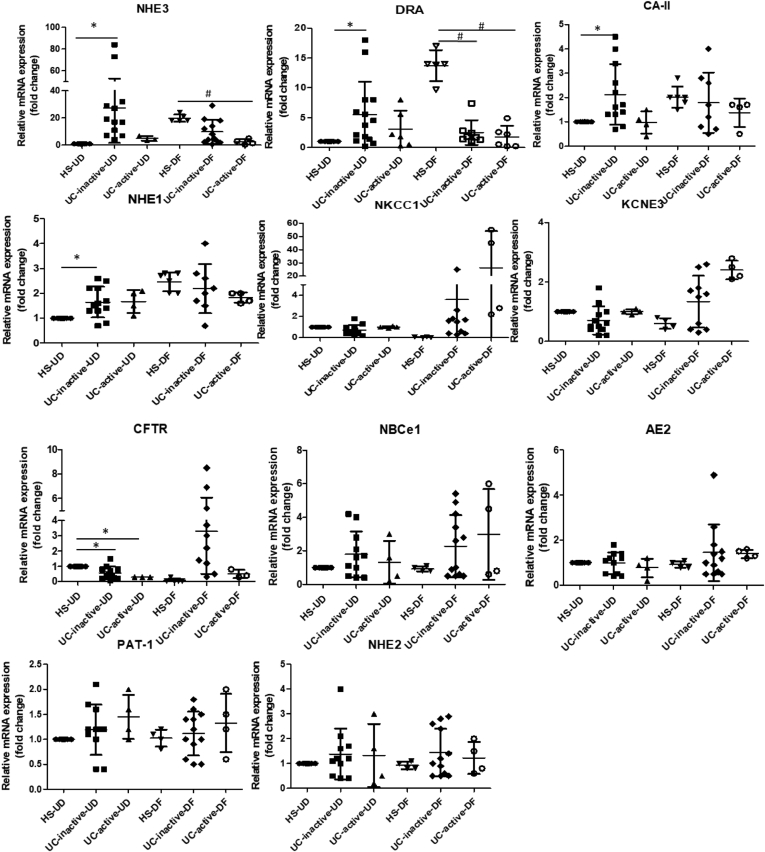
To further define the differentiation states of UC colonoids, mRNA expression of several ion transport proteins and a carbonic anhydrase isoform was determined. The ion transporters selected for study and the carbonic anhydrase isoform are known to play important roles in Cl- and HCO3- secretion, electroneutral Na+ absorption, and intracellular pH regulation under physiological and pathophysiological conditions and have been shown to change in expression with differentiation in intestinal epithelial cells.16 As shown in Figure 4, in colonoids from HS up-regulation occurred in sodium hydrogen exchanger-3 (NHE3) (18.4-fold), DRA (13.6-fold), CA2 (2.0-fold), and NHE1 (2.7-fold). In contrast, several ion transporters were down-regulated significantly after differentiation, including NKCC1 (20.1-fold), potassium channel, voltage-gated, subfamily E, regulatory subunit 3 (KCNE3) (4.2-fold), and CFTR (12-fold). In contrast, UC colonoids exhibited somewhat different mRNA expression patterns compared with HS. In the UD state, UC colonoids (inactive and active site) had significantly higher expression of NHE3 (inactive 27-fold; active 3.4-fold), DRA (inactive 5-fold; active 3.2-fold), and CA2 (inactive 2.1-fold; active 1.2-fold) and lower expression of CFTR (inactive 0.5-fold; active 0.3-fold). Differentiation failed to cause a significant change in the expression pattern of NHE3 and DRA. Importantly, when compared with DF HS, DF UC colonoids had significantly lower expression of NHE3 (inactive 2-fold; active 4-fold) and DRA (inactive 5.5-fold; active 8-fold). The mRNA levels of several other transporters were not significantly different between the groups: anion exchanger 2 (AE2), electroneutral Na+/HCO3- co-transporter 1 (NBCe1), NHE2, and putative anion transporter 1 (PAT-1). Overall this suggests that in UD conditions, UC colonoids were partially differentiated based on the increased mRNA expression pattern of NHE3, DRA, and CA-II and decreased CFTR expression. In contrast, in DF colonoids from inactive and active UC, there was no further or even reduced differentiation based on reduced NHE3 and DRA and a slight increase (not significant) in NKCC1 expression. These data show that the pattern of differentiation and expression of multiple ion transporters and a carbonic anhydrase isoform in UC colonoids are different from HS.
Figure 4.
The mRNA levels of selected ion transporters and carbonic anhydrase in UC colonoids compared with HS. The mRNA levels of selected ion transporters were determined by quantitative real-time PCR, and relative fold changes between UD and DF colonoids were calculated using 18S ribosomal RNA as endogenous control. Results are normalized to HS set as 1 and expressed as fold change. Spheroids from each subject were analyzed at least 3 times and used as n = 1. Means ± SEMs are shown. ∗P < .05 vs HS-UD; #P < .05 vs HS-DF.
Defects in Mucus Layer in UC Colonoids Are Not Due to Decreased Expression of Chloride/Bicarbonate Exchanger (DRA)
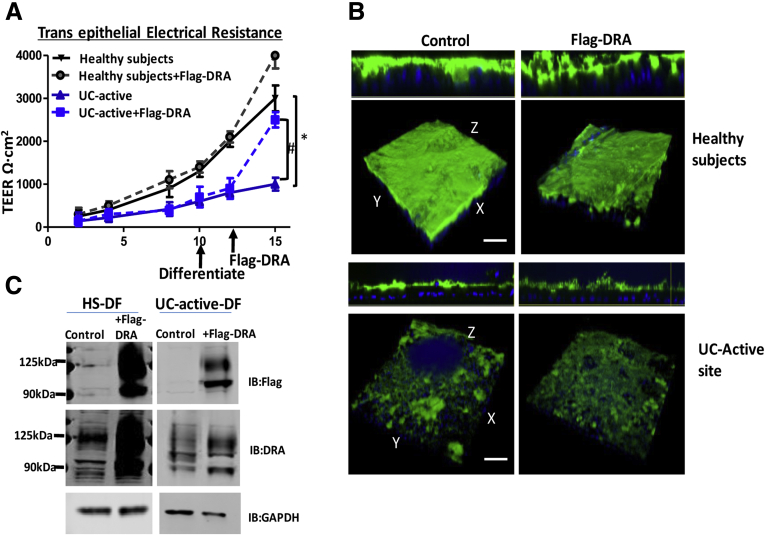
The presence of bicarbonate has been known to be critical for normal mucus secretion by its ability to sequester calcium from condensed mucins being discharged from GCs.17, 18, 19 Differentiated UC colonoids expressed GCs and failed to create a thick mucus layer, which suggested defective bicarbonate secreted into the apical domain in proximity to the mucus could be a possible etiologic feature responsible for the GC defect. Therefore, we investigated the effect of exogenously expressed chloride/bicarbonate exchanger DRA (Adeno-3xFlag-DRA) on mucus layer formation in UC colonoids. Interestingly, UC active monolayers showed a significant increase in TEER in inserts infected with Adeno-3xFlag-DRA (Figure 5A). Despite increasing the DRA expression to the endogenous level of DRA expression in HS, UC active monolayers failed to show any difference in the overall thickness of mucus (Figure 5B and C). These results indicate that the mucus secretion defect in UC monolayers is not due to a defect in DRA-dependent bicarbonate secretion.
Figure 5.
Defects in mucus layer in UC colonoids are not due to decreased expression of DRA.(A) Changes in TEER of colonoid monolayers from HS (black triangle), and active UC (blue triangle) infected with adevovirus-3xflag-DRA HS (grey circle) and active UC (blue square). (B) Methanol–Carnoy’s fixed differentiated colonoid monolayers from HS and UC active stained with MUC2 (green), nucleus (blue). Representative confocal XZ (above) and 3D-XYZ (below) projections depicting the MUC2 layer in colonoids monolayer are shown. (C) Protein lysate prepared from colonoid monolayers uninfected or infected with 3x-flag-DRA was subjected to Western blot analysis and probed with DRA for endogenous expression and flag for overexpressed DRA. Representative results from 3 independent experiments with similar results are shown. Results are shown as mean ± SEM. ∗P < .05 vs HS; #P < .05 vs UC control.
GCs in UC Colonoids Do Not Respond to Carbachol and PGE2 Mediated Mucin Secretion
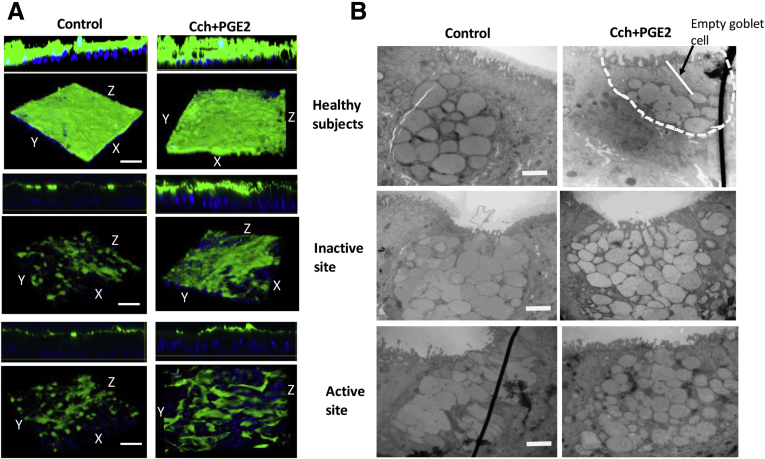
In addition to synthesizing MUC2, GCs release stored MUC2 granules in response to cholinergic plus cyclic adenosine monophosphate (cAMP)–related stimuli. Multiple studies have found that Ca2+ signaling is required for the release of mucin-filled vesicles.20,21 Following the known muscarinic cholinergic signaling pathway for mucin secretion, we treated UC monolayers with carbachol (Cch) (25 μmol/L) to elevate intracellular Ca2+ and with PGE2 (1 μmol/L) to elevate cAMP.22 In contrast to Cch/PGE2 induced mucin secretion and creation of a thick mucus layer in colonoids from HS, monolayers from both inactive and active UC did not respond to the treatment (Figure 6A). At the ultrastructure (transmission electron microscopy [TEM]) level, GCs in HS had the expected appearance of granule-filled vesicles located just apical to the nucleus (Figure 6B). After stimulation with Cch + PGE2, most of the GCs in HS exhibited cavitation at the apical side. In contrast, GCs in UC monolayers (both inactive and active) did not show any decrease in the mucin vesicles in response to the treatment. Overall, these results suggest that colonoids in UC can differentiate to GCs but have a compromised secretory function in response to cholinergic/cAMP stimulation.
Figure 6.
UC colonoids have defects in mucus secretion. Colonoid monolayers from HS and inactive and active UC sites were treated with Cch (25 μmol/L) + PGE2 (1 μmol/L) for 15 minutes and then analyzed. Representative image from each group is shown. (A) Methanol–Carnoy’s fixed monolayer stained for mucus layer, Muc2 (green), nucleus (blue). Scale bar = 20 μm. (B) TEM of GCs from control and Cch/PGE2 treated monolayers from different groups. Note empty area on apical side of GCs in HS, treated with Cch/PGE2, but not in UC. n = 3 monolayers from different subjects in each group. Scale bar = 500 nm.
TNF-α Treatment Reduces GC Number
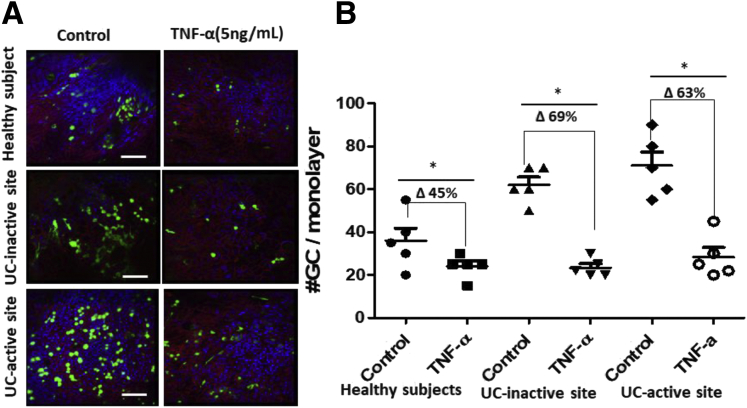
Because the colonoid model is devoid of any immune cells, we hypothesized that the differences in GC number in our model from those reported in UC patient tissue samples are because of the absence of active inflammatory cytokines secreted by immune cells in UC patients. To test this hypothesis, we differentiated monolayers from HS and UC patients in the presence of an inflammatory cytokine TNF-α (5 ng/mL for 5 days, refreshed with medium change at the second day of 5-day differentiation), followed by an analysis of MUC2 positive GCs per monolayer. A representative example is shown in Figure 7A, and quantitation of multiple monolayers is shown in Figure 7B; TNF-α treated monolayers had decreased numbers of MUC2 positive GCs in both HS (control: 40 ± 12; TNF-α: 22 ± 5.6) and UC colonoids (inactive control: 65 ± 15; TNF-α: 20 ± 12; active control: 71 ± 14; TNF-α: 26 ± 10). The percent change in the number of MUC2 positive GCs in UC was higher than in HS (UC inactive, 69%; UC active, 63%; HS, 45%). These results suggest that the decrease in mucus-filled GC number reported in UC patient tissue samples is dependent on the presence of active inflammatory cytokines.
Figure 7.
TNF-α (5 ng/mL, 5 days) treatment decreases GC number.(A) Monolayers from HS and UC inactive and active sites were differentiated alone or with TNF-α (5 ng/mL) for 5 days, and MUC2 positive GCs (green) were analyzed using confocal imaging. TNF-α (5 ng/mL) was refreshed during medium change at second day of 5-day period. (B) Average number of GCs expressed in untreated or TNF-α treated monolayers. Results are shown as means ± SEMs.∗P < .05 vs control/untreated monolayers, n = 3 separate monolayer from each group. Scale bar = 20 μm.
Discussion
In this study, we provide new mechanistic insight into the basis for the reduced mucus layer that is part of the pathophysiology of UC. Although a reduced number of GCs, as reported in many UC patients, is considered as the sole cause of the reduced mucus layer, our studies suggest that the reduced mucus layer seen in UC patients is related to both reduced number and reduced secretory function of the remaining GCs. Furthermore, we also provide evidence that the epithelial compartment in UC undergoes alterations and has reduced expression of the bicarbonate transporters DRA and CFTR. Reduction in luminal HCO3- is already known to contribute to the failure of the mucin to unfold.
Altered characteristics of epithelial cells in UC are thought to be largely due to the inflammatory environment. However, it was not known which of these changes revert to normal once the inflammation is removed, or whether some of them are imprinted in the epithelial compartment. In the present study, we took advantage of the ability to establish stem cell-derived colonoids from active and inactive areas of UC that could be passaged at least 40 times and studied them in both UD crypt-like and DF upper crypt and surface cell state to begin defining some of these long-term changes. Colonoids made from active and inactive areas of UC had properties distinct from colonoids made from the same colonic segments from healthy control subjects including the growth rate, which was slower in colonoids from active UC, and the TEER was significantly reduced in colonoids from both active and inactive UC. The reduced TEER is an indication of abnormal tight junctions and intestinal barrier function and duplicates a feature known to be present in patients with UC. Moreover, UC colonoids also showed decreased mucosal barrier integrity, which is based on an increased amount of bacterial 16S-rRNA compared with HS. This is partially supported by a previous study showing that active UC was associated with a thin mucus layer that was penetrable with beads of a size to mimic bacteria, indicating differences in mucus quality.23 UD active and inactive UC colonoids had increased mRNA expression of proteins normally present in DF colonocytes including NHE3, DRA, and CA-II but had reduced expression of CFTR, which is usually more highly expressed in the crypt; moreover, when the colonoids were exposed to conditions that led to differentiation in colonoids from HS, these genes either failed to increase or decreased in expression. MUC2 also behaved similarly and in a distinctly abnormal pattern, being increased in UD active and inactive UC colonoids, whereas there was no further increase with the application of differentiation conditions. Decreased expression of DRA is reported in various inflammatory diarrhea and in UC patients.24 Similarly, decreased mRNA expression of CFTR in UC colonoids is in accordance with reports from UC patients.25 These results are consistent with long-term effects of inflammation in UC colonoids exhibiting early differentiation, which fits with the reduced proliferation shown in Figure 1B. However, the mechanisms for these long-term changes have not been identified.
The UC colonoids had an increased number of GCs compared with HS colonoids. This was consistent with the increased level of ATOH1, a transcription factor that increases stem cell differentiation toward the secretory pathway. Moreover, there was also an increase in Ngn3 and ChgA mRNA in DF UC colonoids, another part of the ATOH1 driven secretory cell developmental pathway. Several studies have reported greater numbers of enteroendocrine cells in the colonic mucosa of patients with active UC, indicating similarity between the UC colonoid model and intact colon.26,27 Despite higher expression of GCs, both active and inactive UC colonoids formed a thin mucus layer, suggesting defects at the level of the signaling pathways or secretory machinery required for mucus secretion.
Secretion of mucin release from GC was examined by exposure to the muscarinic agonist Cch plus the cAMP agonist PGE2, which are known to cause mucin exocytosis.28 Formation of a functional mucus layer is a result of a complex multi-step process. It starts with an increase in intracellular Ca2+ in response to activation of muscarinic M3 receptors, which is followed by fusion of mucin-containing vesicles, compound exocytosis, and finally mucin unfolding via HCO3- exposure. Mucin release was markedly reduced in both active and inactive UC colonoids based on the thickness of mucus layer in colonoid monolayers and by examining the apical area of GCs by TEM (Figure 6). This has been previously shown in ex vivo human biopsies from active UC.29 Further studies are required to understand which of the multiple steps in mucus secretion is abnormal in UC. The second contributor to a thin mucin layer in UC colonoids is related to the dependence of mucus unfolding on luminal HCO3-. Although it is not known whether the HCO3- comes from adjacent epithelial cells or is more closely associated with the GCs, the mRNAs of both DRA and CFTR, the 2 major colonic apical HCO3- transporters, were significantly reduced in DF UC colonoids. The third likely contributor to abnormal GC mucin secretion is an abnormal expression of C2GnT2, an enzyme responsible for O-glycosylation of MUC2. C2GnT2 is highly expressed in the mouse small intestine and colon, and its deficiency reduces levels of core 2 and 4 O-glycans, as well as I-branching. Moreover, C2GnT2-/- mice exhibit increased susceptibility to dextran sulfate sodium–induced colitis.30 Additional studies are required to determine whether mucin glycosylation is abnormal in UC colonoids and to define the consequence of altered glycosylation on mucus layer formation.
A thin and defective mucus layer is a signature of UC, and this has been attributed to the reduced number of colonic GCs. In contrast, in our studies, UC colonoids had an increased number of GCs compared with HS colonoids. One of the limitations of studies with stem cell-derived intestinal organoids is that they only contain epithelial cells and lack the many additional cell types present in the normal intestine, including inflammatory and immune cells. Consequently, disease models using colonoids do not entirely duplicate the inflammatory or immune environment that plays a critical role in the pathophysiology of many gastrointestinal diseases including IBD. To deal with this limitation, co-culture with additional cell types has been developed, and the use of iPSC-derived organoids includes some of the additional intestinal mesenchymal cells. Based on this limitation, we hypothesized that the difference in GC number in UC tissue compared with UC colonoids might be due to a lack of the inflammatory environment. When colonoids were exposed to TNF-α for 5 days, there was a decrease in GC number in both HS and UC colonoids, with the reduction in number in the UC colonoids exceeding that in HS. This finding supports the interpretation that the reduced number of MUC2 positive GCs in UC is at least in part due to the local inflammatory environment. The conclusion from these studies is that the reduced protective mucus layer in UC is a consequence of both a reduced number of GCs, which appears to be a reversible inflammation-dependent phenomenon, and reduced mucin secretion by the remaining GCs, which appears to be a long-term aspect of the disease.
As demonstrated in a recent study, there are multiple types of GCs in the colon that are based on their location along the crypt-surface cell axis of the mouse and human colon.31 In the mouse colon, the most differentiated GCs, indicated by Ulex europaeus agglutinin I positivity and wheat germ agglutinin negativity, are localized on the surface epithelium between the crypts called intercrypt GC. Based on their gene expression profile these GCs show a functional profile with response to stress, cell differentiation, apoptosis, and protein transport. In humans, a fewer intercrypt GC population and mucus alteration are reported in both active and in remission UC.31 Thus, in addition to our findings, these results suggest that long-term inflammation in UC alters the GC population such that it differs from that in HS. Further studies are required to characterize the GC population in UC colonoids.
The current observations further support several recent studies that have suggested that epithelial cells from the involved colonic mucosa of patients with UC acquire a unique transcriptional signature that is maintained long after the acute inflammation has resolved, suggesting permanent epithelial cell changes.7 Epigenetic changes in genes from UC mucosa have been suggested related to pathways that affect antigen processing and presentation, cell adhesion, B- and T-cell receptor signaling, JAK-STAT signaling, and transforming growth factor-beta signaling. However, the extent and consequences of epigenetic changes in IBD have not been adequately characterized. However, because abnormal barrier function and a reduced protective mucus layer contribute to the initiation of IBD and potentially to recurrence, the presence of both characteristics in colonoids over multiple passages and in colonoids made from inactive as well as active UC tissue suggests that UC mucosa is primed for recurrence even in the absence of inflammation. We speculate that an approach to reverse these changes in UC colonoids has the potential to prevent UC recurrence and potentially to prevent the proximal spread of UC, concerning and unmet needs in UC management.
Materials and Methods
Chemicals and reagents were purchased from Thermo Fisher (Waltham, MA) or Sigma-Aldrich (St Louis, MO) unless otherwise specified. All authors have had access to the study data and reviewed and approved the final manuscript.
Patient Population and Biopsy Collection
Colonic biopsies were obtained from HS and UC patients (Table 1) undergoing colonoscopies or from patients having colonic surgery for refractory UC. In all cases, informed consent was obtained using an experimental protocol approved by the Johns Hopkins University Institutional Review Board (IRB# NA_00038329). All procedures were performed under approved guidelines and regulations. Intestinal biopsies were collected from the ascending colon, descending colon, or sigmoid colon of HS screened with colonoscopy for colorectal cancer or gastrointestinal symptoms and who had histologically normal colon. Seven UC patients had biopsies taken from area of uninvolved mucosa and/or active disease (paired biopsies from 4 patients and from inactive sites of 3 separate UC patients). Histologic status of the biopsies from colonoscopy or surgical samples is listed in Table 1. UC activity at the time of the colonoscopy was categorized according to the Mayo endoscopic subscore.32 Active UC was defined as Mayo endoscopic subscore of ≥1; inactive disease was defined as Mayo score of 0 in a previously involved segment. Colonoids were established via the Hopkins Conte Basic and Translational Digestive Diseases Research Core Center (NIH/NIDDK P30).
Table 1.
Clinical Descriptions and Origins of Biopsies of HS and Patients With UC
| Colon lines | Known ailment | Origin of biopsies |
|---|---|---|
| Patient 1 | UC inactive site | Descending colon |
| UC active site | Rectum | |
| Patient 2 | UC inactive site | Ascending colon |
| UC active site | Descending colon | |
| Patient 3 | UC inactive site | Sigmoid colon |
| UC active site | Rectum | |
| Patient 4 | UC inactive site | Transverse colon |
| UC active site | Sigmoid colon | |
| Patient 5 | UC inactive site | Sigmoid |
| Patient 6 | UC inactive site | Transverse colon |
| Patient 7 | UC inactive site | Descending colon |
| Healthy subject n = 2 | Normal | Ascending colon |
| Healthy subject n = 2 | Normal | Descending colon |
| Healthy subject n = 1 | Normal | Sigmoid colon |
Organoid Culture and Monolayer Formation
Human colonoid cultures and monolayers were established by using the methods reported previously.33,34 Human colonoids were maintained as cysts embedded in Matrigel (Corning #356231; Corning, NY) in 24-well plates and cultured in Wnt3A, Rspon, and Noggin containing growth medium or non-differentiated medium (NDM).34 Medium was replaced with fresh NDM every other day. Studies were carried out on passages 6–40. For a generation of a monolayer, colonoids were fragmented in Organoid Harvesting Solution (Trevigen, Gaithersburg, MD), and multiple wells were pooled together and resuspended in NDM after centrifugation. Colonoid fragments (in 100 μL NDM) were added onto 0.4 μm pore transparent polyester membrane 24-well cell culture inserts (Transwell; Corning, or Millipore, Burlington, MA) pre-coated with human collagen IV (30 μg/mL; Sigma-Aldrich). NDM (600 μL) was added to the bottom, and the cultures were incubated at 37 °C, 5% CO2. Monolayers were cultured in 5% CO2 atmosphere at 37°C. The growth medium was supplemented with Y-27632 (10 μmol/L) and CHIR99021 (10 μmol/L) during the first 2 days after seeding. The formation of colonoid monolayers was monitored by measurement of TEER. Once monolayers became confluent, the expansion medium was replaced with a differentiation medium that was made by substituting Wnt3A, R-spondin1, and SB202190 in the expansion medium with the base medium. Five days later, paired UD and DF enteroid monolayers were studied.
Quantitative Real-Time PCR
Total RNA was extracted from 3D cultures using the PureLink RNA Mini Kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. Complementary DNA was synthesized from 1 to 2 μg of RNA using SuperScript VILO Master Mix (Life Technologies). Quantitative real-time PCR was performed using Power SYBR Green Master Mix (Life Technologies) on a QuantStudio 12K Flex real-time PCR system (Applied Biosystems, Foster City, CA). Each sample was run in triplicate, and 5 ng RNA-equivalent complementary DNA was used for each reaction. Commercially available primer pairs from OriGene Technologies (Rockville, MD) were used. The following primer pairs were used: LGR5: HP207145; Ki67: HP206104; ATOH1: HP208359; NEUROG3: HP213982; SPDEF: HP210328; MUC2: HP206138; CHGA: HP205193; LYZ: HP200222; NHE3: HP207529; DRA: HP200096; CAII: HP200053; CFTR: HP200464; PAT1: HP232409; NHE1: HP206641; NKCC1: HP203742; KCNE3: HP208601; NBCE1: HP232301; AE2: HP206636; NHE2: HP206642; 18S rRNA: HP220445 (OriGene Technologies). The relative fold changes in mRNA levels of genes between DF organoids and UD organoids were determined by using the 2-ΔΔCT method with human 18S ribosomal RNA simultaneously studied and used as the internal control for normalization and shown in fold change compared with the HS-UD or HS-DF control.
Adenoviral 3xFlag-DRA Preparation, Purification, and Expression
Triple Flag-tagged human DRA was cloned into the adenoviral shuttle vector ADLOX.HTM under the control of a cytomegalovirus promoter. The virus was generated by transfection of CRE8 cells with ψ5 viral DNA and ADLOX.HTM/3Flag-DRA using Lipofectamine 2000 (Invitrogen, Waltham, MA). The crude adenoviruses were then propagated by infection in HEK 9-11 cells. Adenovirus was separated by CsCl gradient centrifugation and purified with a Sephadex G-25 column. Viral particle numbers were calculated as (A260 value) × (1.1 × 1012) × dilution. To test the expression of adenovirus (Adeno)-Flag-DRA, on the third day of differentiation colonoid monolayers were infected with viral particles diluted in DF medium by incubating at 37°C overnight. The next morning virus-containing medium was replaced with DF medium, and cells were allowed to grow for the next 2 days to complete 5-day differentiation.
Immunofluorescence Staining, Confocal and TEM Image Analysis
Analysis of MUC2 by immunofluorescence and confocal microscopy was carried out as previously reported.9 Briefly, human colonoid monolayers were fixed with Carnoy’s solution (90% [v/v] methanol, 10% [v/v] glacial acetic acid), washed 3 times with phosphate-buffered saline, permeabilized with 0.1% saponin, and blocked with 2% bovine serum albumin + 15% fetal bovine serum for 60 minutes (all Sigma-Aldrich), followed by overnight incubation with antibodies. For immunostaining in 3D organoids, staining was done in suspension. Briefly, recovered organoids were fixed in 4% paraformaldehyde in 10 mmol/L phosphate buffer (pH 7.4) for 30 minutes at 4°C and then washed 2× with phosphate-buffered saline. Organoids were permeabilized and stained in phosphate-buffered saline with 2% bovine serum albumin, 1% Triton X-100, and 1% saponin. Images were collected using ×20 or ×40 oil immersion objective on FV3000 confocal microscope (Olympus, Tokyo, Japan) with software (Olympus) and ImageJ software (NIH). Images were 3D-reconstructed using Volocity Image Analysis software (Improvision, Coventry, England). Primary antibody rabbit anti-MUC2 (Santa Cruz Biotechnology, Dallas, TX; Cat#sc7314) was used. All antibodies were diluted at 1:100. For quantitative analysis, the same settings were used to image across samples (eg, MUC2 staining). Mucin exocytosis and thickness were determined by measuring the MUC2 positive area above the epithelial surface. For electron microscopy, 2-mm sections were fixed in 1% osmium tetroxide and 1% uranyl acetate, dehydrated with ethanol and infiltrated with epoxy resin. Thin sections (80 nm) were cut and transferred to 200-mesh copper grids before staining with uranyl acetate and lead citrate. Grids were viewed on Hitachi (Tokyo, Japna) 7600 434 TEM operating at 80 kV, and digital images of the apical regions were captured with AMT 1K × 1K CCD camera.
Escherichia coli Strains and Infections
E coli H10407 strain was described previously.35,36 All antibiotics were purchased from Sigma Chemical Co (St Louis, MO). For colonoid infections, the strain was grown from frozen stocks (−80°C) at 37°C on Luria broth agar plates (Difco) 2 days before experiments. A day before infection, single colonies were inoculated in 5 mL Luria broth and grown overnight with vigorous shaking at 37°C. For infection, an overnight Luria broth culture was diluted 1:50 into fresh Luria broth and incubated at 37°C with agitation for 2 hours to achieve a log-phase culture (OD600 = 0.6). Subsequently, bacteria were adjusted to 108 colony-forming units/mL in sterile phosphate-buffered saline, and 10 μL (1×106) was added to the apical surface of colonoid monolayers with intact mucus. E coli infections were allowed to progress for 8 hours.
Immunoblotting
Transwell inserts with or without 3xFlag-DRA infection were rinsed 3 times with phosphate-buffered saline and harvested in phosphate-buffered saline by scraping. Cell lysate preparation and Western blot were performed as previously reported.37 Protein bands were visualized and quantitated using an Odyssey system and Image Studio software (LI-COR Biosciences, Lincoln, NE).
Statistical Analysis
Quantitative data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined using analysis of variance with Bonferroni’s post-test (Prism GraphPad, San Diego, CA) to compare groups including a minimum n = 3 replicates. A P value less than or equal to .05 was considered statistically significant.
Acknowledgments
The authors thank the Hopkins Conte Basic and Translational Digestive Diseases Research Core Center Integrated Physiology and Translational Research Enhancement Cores for their contributions to obtaining and maintaining colonoids.
Current address for Julie In: Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of New Mexico Health Science Center, Albuquerque, New Mexico.
CRediT Authorship Contributions
Varsha Singh (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Validation: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Kelli Johnson (Data curation: Supporting)
Jianyi Yin (Data curation: Supporting)
Sunny Lee (Methodology: Supporting)
Ruxian Lin (Data curation: Supporting)
Huimin Yu (Methodology: Supporting)
Julie In (Methodology: Supporting)
Jennifer Foulke-Abel (Methodology: Supporting)
Nicholas Zachos (Validation: Supporting)
Mark Donowitz (Writing – review & editing: Equal)
Yan Rong (Data curation: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institutes of Health grants R01-DK-116352, UO1-DK-10316, and P30-DK-89502 (the Hopkins Basic and Translational Research Digestive Diseases Research Core Center).
References
- 1.Nava P., Koch S., Laukoetter M.G., Lee W.Y., Kolegraff K., Capaldo C.T., Beeman N., Addis C., Gerner-Smidt K., Neumaier I., Skerra A., Li L., Parkos C.A., Nusrat A. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su L., Nalle S.C., Shen L., Turner E.S., Singh G., Breskin L.A., Khramtsova E.A., Khramtsova G., Tsai P.Y., Fu Y.X., Abraham C., Turner J.R. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zouiten-Mekki L., Serghini M., Fekih M., Kallel L., Matri S., Ben Mustapha N., Boubaker J., Filali A. [Epithelial cell in intestinal homeostasis and inflammatory bowel diseases] Med Sci (Paris) 2013;29:1145–1150. doi: 10.1051/medsci/20132912019. [DOI] [PubMed] [Google Scholar]
- 4.Jager S., Stange E.F., Wehkamp J. Inflammatory bowel disease: an impaired barrier disease. Langenbecks Arch Surg. 2013;398:1–12. doi: 10.1007/s00423-012-1030-9. [DOI] [PubMed] [Google Scholar]
- 5.Bjerrum J.T., Hansen M., Olsen J., Nielsen O.H. Genome-wide gene expression analysis of mucosal colonic biopsies and isolated colonocytes suggests a continuous inflammatory state in the lamina propria of patients with quiescent ulcerative colitis. Inflamm Bowel Dis. 2010;16:999–1007. doi: 10.1002/ibd.21142. [DOI] [PubMed] [Google Scholar]
- 6.Fenton C.G., Taman H., Florholmen J., Sorbye S.W., Paulssen R.H. Transcriptional signatures that define ulcerative colitis in remission. Inflamm Bowel Dis. 2021;27:94–105. doi: 10.1093/ibd/izaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planell N., Lozano J.J., Mora-Buch R., Masamunt M.C., Jimeno M., Ordas I., Esteller M., Ricart E., Pique J.M., Panes J., Salas A. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut. 2013;62:967–976. doi: 10.1136/gutjnl-2012-303333. [DOI] [PubMed] [Google Scholar]
- 8.Larsson J.M., Karlsson H., Sjovall H., Hansson G.C. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 9.In J., Foulke-Abel J., Zachos N.C., Hansen A.M., Kaper J.B., Bernstein H.D., Halushka M., Blutt S., Estes M.K., Donowitz M., Kovbasnjuk O. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol. 2016;2:48–62 e3. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dotti I., Mora-Buch R., Ferrer-Picon E., Planell N., Jung P., Masamunt M.C., Leal R.F., Martin de Carpi J., Llach J., Ordas I., Batlle E., Panes J., Salas A. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. 2017;66:2069–2079. doi: 10.1136/gutjnl-2016-312609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke J., Zhang H., Greger L., Silva A.L., Massey D., Dawson C., Metz A., Ibrahim A., Parkes M. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2128–2137. doi: 10.1002/ibd.22942. [DOI] [PubMed] [Google Scholar]
- 12.Narula N., Aruljothy A., Alshahrani A.A., Fadida M., Al-Saedi M., Marshall J.K., Rubin D.T., Christensen B. Histologic remission does not offer additional benefit for ulcerative colitis patients in endoscopic remission. Aliment Pharmacol Ther. 2020;52:1676–1682. doi: 10.1111/apt.16147. [DOI] [PubMed] [Google Scholar]
- 13.Gersemann M., Becker S., Kubler I., Koslowski M., Wang G., Herrlinger K.R., Griger J., Fritz P., Fellermann K., Schwab M., Wehkamp J., Stange E.F. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation. 2009;77:84–94. doi: 10.1016/j.diff.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Hollingsworth M.A., Swanson B.J. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 15.Thomsson K.A., Holmen-Larsson J.M., Angstrom J., Johansson M.E., Xia L., Hansson G.C. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology. 2012;22:1128–1139. doi: 10.1093/glycob/cws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin J., Tse C.M., Avula L.R., Singh V., Foulke-Abel J., de Jonge H.R., Donowitz M. Molecular basis and differentiation-associated alterations of anion secretion in human duodenal enteroid monolayers. Cell Mol Gastroenterol Hepatol. 2018;5:591–609. doi: 10.1016/j.jcmgh.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Lisle R.C. Pass the bicarb: the importance of HCO3- for mucin release. J Clin Invest. 2009;119:2535–2537. doi: 10.1172/JCI40598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen E.Y., Yang N., Quinton P.M., Chin W.C. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L542–L549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F., Yu Q., Li J., Johansson M.E., Singh A.K., Xia W., Riederer B., Engelhardt R., Montrose M., Soleimani M., Tian D.A., Xu G., Hansson G.C., Seidler U. Slc26a3 deficiency is associated with loss of colonic HCO3 (-) secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol (Oxf) 2014;211:161–175. doi: 10.1111/apha.12220. [DOI] [PubMed] [Google Scholar]
- 20.Abdullah L.H., Bundy J.T., Ehre C., Davis C.W. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol Lung Cell Mol Physiol. 2003;285:L149–L160. doi: 10.1152/ajplung.00359.2002. [DOI] [PubMed] [Google Scholar]
- 21.Wollman R., Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol. 2012;14:1261–1269. doi: 10.1038/ncb2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia M.A., Yang N., Quinton P.M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson M.E., Gustafsson J.K., Holmen-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjovall H., Hansson G.C. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohi H., Makela S., Pulkkinen K., Hoglund P., Karjalainen-Lindsberg M.L., Puolakkainen P., Kere J. Upregulation of CFTR expression but not SLC26A3 and SLC9A3 in ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G567–G575. doi: 10.1152/ajpgi.00356.2001. [DOI] [PubMed] [Google Scholar]
- 25.Villeda-Ramírez M.M.-G.D., Barreto-Zúñiga R., Jesus Yamamoto-Furusho. ABCC7/CFTR expression is down-regulated in patients with active ulcerative colitis. Preprint. 2020 [Google Scholar]
- 26.Hernandez-Trejo J.A., Suarez-Perez D., Gutierrez-Martinez I.Z., Fernandez-Vargas O.E., Serrano C., Candelario-Martinez A.A., Meraz-Rios M.A., Citalan-Madrid A.F., Hernandez-Ruiz M., Reyes-Maldonado E., Valle-Rios R., Feintuch-Unger J.H., Schnoor M., Villegas-Sepulveda N., Medina-Contreras O., Nava P. The pro-inflammatory cytokines IFNgamma/TNFalpha increase chromogranin A-positive neuroendocrine cells in the colonic epithelium. Biochem J. 2016;473:3805–3818. doi: 10.1042/BCJ20160390. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu R., Drew K., McAlindon M.E., Lobo A.J., Sanders D.S. Elevated serum chromogranin A in irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD): a shared model for pathogenesis? Inflamm Bowel Dis. 2010;16:361. doi: 10.1002/ibd.20982. [DOI] [PubMed] [Google Scholar]
- 28.Yang N., Garcia M.A., Quinton P.M. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. J Physiol. 2013;591:4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Post S., Jabbar K.S., Birchenough G., Arike L., Akhtar N., Sjovall H., Johansson M.E.V., Hansson G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68:2142–2151. doi: 10.1136/gutjnl-2018-317571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone E.L., Ismail M.N., Lee S.H., Luu Y., Ramirez K., Haslam S.M., Ho S.B., Dell A., Fukuda M., Marth J.D. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol. 2009;29:3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nystrom E.E.L., Martinez-Abad B., Arike L., Birchenough G.M.H., Nonnecke E.B., Castillo P.A., Svensson F., Bevins C.L., Hansson G.C., Johansson M.E.V. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. 2021:372. doi: 10.1126/science.abb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeder K.W., Tremaine W.J., Ilstrup D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 33.Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Noel G., Baetz N.W., Staab J.F., Donowitz M., Kovbasnjuk O., Pasetti M.F., Zachos N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep. 2017;7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans D.J., Jr., Evans D.G. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973;8:322–328. doi: 10.1128/iai.8.3.322-328.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foulke-Abel J., Yu H., Sunuwar L., Lin R., Fleckenstein J.M., Kaper J.B., Donowitz M. Phosphodiesterase 5 (PDE5) restricts intracellular cGMP accumulation during enterotoxigenic Escherichia coli infection. Gut Microbes. 2020;12 doi: 10.1080/19490976.2020.1752125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tse C.M., Yin J., Singh V., Sarker R., Lin R., Verkman A.S., Turner J.R., Donowitz M. cAMP stimulates SLC26A3 activity in human colon by a CFTR-dependent mechanism that does not require CFTR activity. Cell Mol Gastroenterol Hepatol. 2019;7:641–653. doi: 10.1016/j.jcmgh.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]