Abstract
Background & Aims
Mucosal-associated invariant T (MAIT) cells are innate-like T cells restricted by major histocompatibility complex-related molecule 1 (MR1) and express a semi-invariant T cell receptor. Previously, we reported the activation status of circulating MAIT cells in patients with ulcerative colitis (UC) was associated with disease activity and that these cells had infiltrated the inflamed colonic mucosa. These findings suggest MAIT cells are involved in the pathogenesis of inflammatory bowel disease. We investigated the role of MAIT cells in the pathogenesis of colitis by using MR1−/− mice lacking MAIT cells and a synthetic antagonistic MR1 ligand.
Methods
Oxazolone colitis was induced in MR1−/− mice (C57BL/6 background), their littermate wild-type controls, and C57BL/6 mice orally administered an antagonistic MR1 ligand, isobutyl 6-formyl pterin (i6-FP). Cytokine production of splenocytes and colonic lamina propria lymphocytes from mice receiving i6-FP was analyzed. Intestinal permeability was assessed in MR1−/− and i6-FP-treated mice and their controls. The effect of i6-FP on cytokine production by MAIT cells from patients with UC was assessed.
Results
MR1 deficiency or i6-FP treatment reduced the severity of oxazolone colitis. i6-FP treatment reduced cytokine production in MAIT cells from mice and patients with UC. Although MR1 deficiency increased the intestinal permeability, i6-FP administration did not affect gut integrity in mice.
Conclusions
These results indicate MAIT cells have a pathogenic role in colitis and suppression of MAIT cell activation might reduce the severity of colitis without affecting gut integrity. Thus, MAIT cells are potential therapeutic targets for inflammatory bowel disease including UC.
Keywords: MAIT Cell, Mucosal Immunity, Inflammatory Bowel Disease, Gut Integrity
Abbreviations used in this paper: CD, Crohn’s disease; DAI, disease activity index; FITC, fluorescein isothiocyanate; iNKT, invariant natural killer T cells; i6-FP, isobutyryl 6-formyl pterin; IBD, inflammatory bowel disease; IFN, interferon; IL, interleukin; LPL, lamina propria lymphocyte; MAIT cells, mucosal-associated invariant T cells; MR1, major histocompatibility complex-related molecule 1; NIH, National Institutes of Health; PBMC, peripheral blood mononuclear cells; RL-7-Me, 7-methyl-8-D-ribityllumazine; TCR, T cell receptor; TNF, tumor necrosis factor; UC, ulcerative colitis; WT, wild-type
Graphical abstract
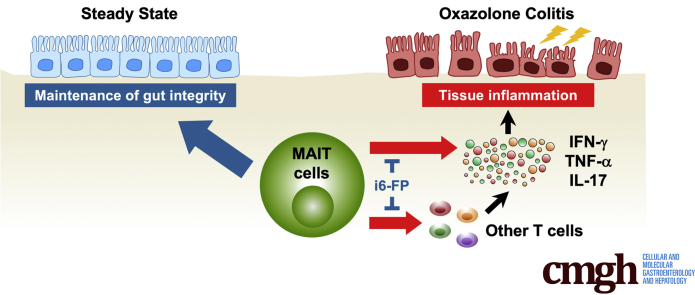
Summary.
Using a murine model of ulcerative colitis, we demonstrated mucosal-associated invariant T cells enhanced tissue inflammation of colitis and inhibition of mucosal-associated invariant T cell activation with a major histocompatibility complex-related molecule 1 ligand reduced the severity of colitis.
Inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory conditions of the gastrointestinal tract. IBD is thought to result from a dysregulated immune response to gut microbiota due to genetic and environmental factors.1,2 Although the exact etiology of IBD is not fully understood, recent advances in IBD therapy have highlighted the critical roles of cytokines and immune cells in the pathogenesis of IBD.3 Neutralizing antibodies specific for tumor necrosis factor (TNF)-α have been widely used for the treatment of IBD. Janus kinase inhibitors that inhibit the production of multiple cytokines have proven to be effective in IBD. The effectiveness of anti-adhesion molecules for IBD suggests lymphocytes, especially naïve and effector memory T cells recruited to the gut tissue, contribute to inflammation in IBD.4,5
Mucosal-associated invariant T (MAIT) cells are innate-like T cells that express a semi-invariant T cell receptor (TCR) α, Vα7.2-Jα33 in humans and Vα19-Jα33 in mice, which are restricted by major histocompatibility complex-related molecule 1 (MR1).6 MAIT cells recognize MR1 ligands derived from the riboflavin (vitamin B2) metabolic pathway7, 8, 9 of microbes, and expand in the periphery in the presence of commensal flora.10,11 MAIT cells were named after their preferential location in mucosal tissues and have a critical role in the maintenance of intestinal homeostasis. Similar to other innate-like T cells, including invariant natural killer T (iNKT) cells, MAIT cells are rapidly activated and secrete large amounts of cytokines including TNF-α, interferon (IFN)-γ, and interleukin (IL)-17 as well as perforin and granzyme B.12, 13, 14, 15, 16 Therefore, MAIT cells are thought to have important roles in first-line immune responses. Previously, our group reported the frequency of MAIT cells was reduced in the peripheral blood of patients with UC and that the activated status of MAIT cells was associated with disease activity. In addition, we demonstrated that MAIT cells accumulated in the inflamed mucosa of patients with UC.17 A recent single-cell analysis of colonic T cells in UC revealed the upregulated gene expression of molecules related to the activation of MAIT cells.18 These findings suggest that MAIT cells may be involved in the pathogenesis of IBD. However, the role of MAIT cells in the context of colitis has not been elucidated. In the present study, we report that MAIT cells have a pathogenic role in oxazolone colitis, a murine model of IBD.
Results
Oxazolone Colitis is Ameliorated by MR1 Deficiency
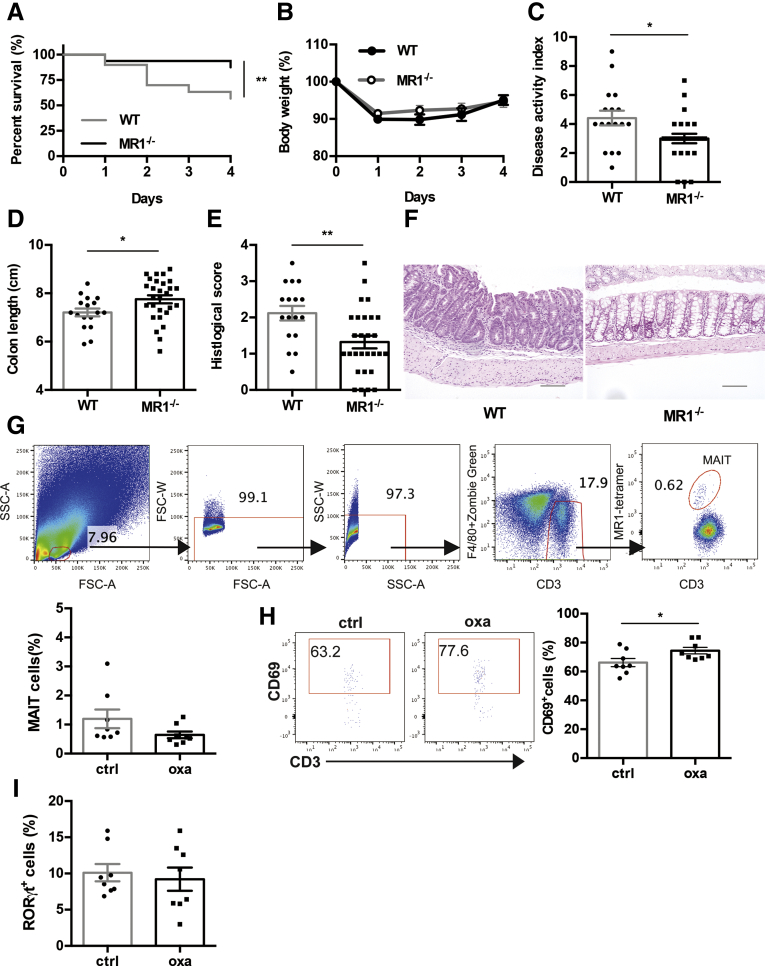
To investigate whether MAIT cells are involved in the pathogenesis of IBD, oxazolone colitis was induced in MR1−/− mice lacking MAIT cells and their littermate wild-type (WT) mice. MR1−/− and WT mice developed colitis, but the survival rate in MR1−/− mice was higher compared with that of WT mice (Figure 1A). The body weight was reduced in MR1−/− and WT mice, and there was no significant difference between these groups (Figure 1B). There was less colon shortening and the disease activity index (DAI) was lower in MR1−/− mice compared with WT mice (Figure 1C, D). Histologic analysis revealed MR1 deficiency reduced inflammation in the colon as shown by the decreased histologic scores in MR1−/− mice compared with WT mice (Figure 1E, F). We assessed whether colonic MAIT cells were activated in colitis-induced mice. Although the frequency of MAIT cells in lamina propria lymphocyte (LPL) of the large intestine was not affected by the induction of colitis, the frequency of CD69-expressing MAIT cells was increased in colitis-induced mice compared with control mice (Figure 1G, H). MAIT cells can be classified into IFN-γ-producing MAIT 1 and IL-17-producing MAIT 17 cells.19, 20, 21 Thus, we also investigated MAIT cells for the expression of RORγt, a marker of MAIT 17 cells. The frequency of RORγt+ cells among MAIT cells in LPL was 9.2%. However, this was not increased by the induction of colitis (Figure 1I). Although the frequency of MAIT cells in the colon was unchanged, these cells were activated by the induction of oxazolone colitis, and MR1 deficiency reduced the severity of colitis.
Figure 1.
Effect of MR1 deficiency on oxazolone-induced colitis. Oxazolone-induced colitis was induced in MR1−/− (n = 32) and littermate WT control mice (n = 30). Survival rate (A) and percent of body weight from day 0 (B) are shown after the intrarectal administration of oxazolone. (C-E) DAI, colon length, and histologic score at sacrifice are shown. (F) Representative pictures of hematoxylin and eosin-stained sections of colon from MR1−/− and WT mice. (Scale bars, 100 μm). (G-I) Flow cytometric evaluation of the frequencies of total MAIT cells, CD69+ cells among MAIT cells and RORγt+ cells among MAIT cells 24 hours after oxazolone (oxa) or control (ctrl) administration intrarectally (n = 8). (G) LPL were gated on using a forward scatter area (FSC-A) versus side scatter area (SSC-A) plot. Single cells were selected by using FSC-A versus FSC-width (FSC-W) and SSC-A versus SSC-width (SSC-W) plots. Zombie green positive dead cells and F4/80 positive cells were excluded to gate out cells that had bound to antibodies and tetramers non-specifically. MAIT cells were identified as CD3+ MR1/5-OP-RU tetramer+ cells. (H) Representative flow cytometry profiles of CD69-stained MAIT cells are shown. Significance was determined using the Log-rank (Mantel-Cox) test (A) and Welch’s t-test (B-E, G-I). ∗P < .05. Data are represented as the mean ± standard error of the mean in each group. Each symbol represents data from an individual mouse.
Inhibition of MAIT Cell Activation Reduces the Severity of Colitis
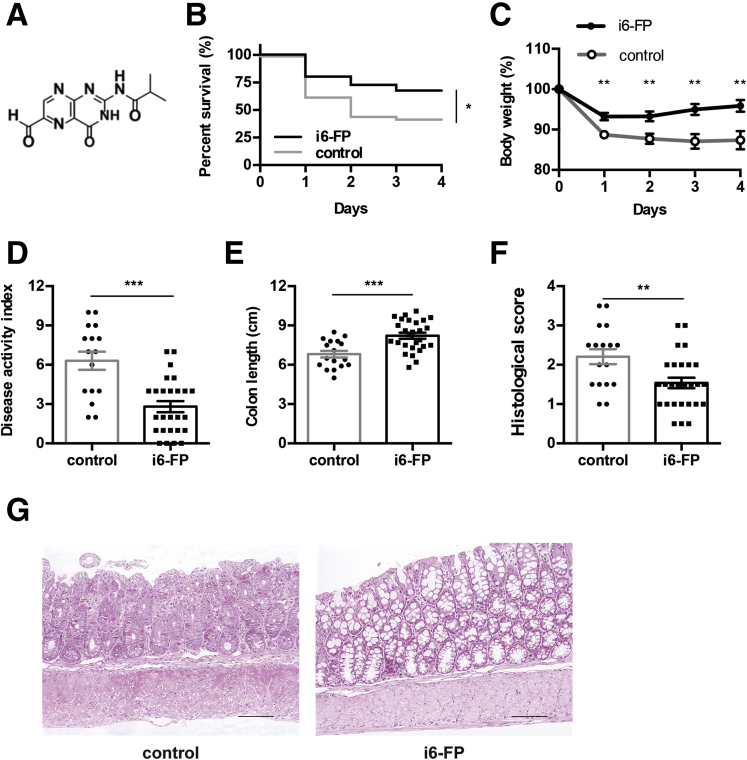
Next, we investigated whether the inhibition of MAIT cell activation decreased the severity of colitis. Previously, our group synthesized an MR1 ligand, isobutyl 6-formyl pterin (i6-FP) (Figure 2A), and demonstrated that MAIT cell activation was suppressed in mice orally treated with i6-FP by an MR1-dependent mechanism.22 Next, oxazolone colitis was induced in WT mice treated with i6-FP orally every other day starting on the day of colitis induction. i6-FP treatment increased the survival rate and reduced body wasting and colon shortening in these mice (Figure 2B-D). The DAI and histologic score of i6-FP mice were lower than those of vehicle control mice (Figure 2E-G). These results indicate that the inhibition of MAIT cell activation suppressed the disease severity of oxazolone colitis.
Figure 2.
Effect of i6-FP administration on oxazolone-induced colitis. (A) Chemical structure of i6-FP is shown. Oxazolone-induced colitis was induced in WT mice with or without i6-FP treatment. Survival rate (B) and percent of body weight from day 0 (C) are shown after the intrarectal administration of oxazolone in mice treated with i6-FP (n = 40) or control vehicle (n = 40). (D-F) DAI, colon length, and histologic score at sacrifice are shown. (G) Histologic hematoxylin and eosin stained sections from i6-FP-treated mice and control mice. One representative image from each group is shown (scale bars, 100 μm). Data are represented as the mean ± standard error of the mean in each group. Significance was determined using the Log-rank (Mantel-Cox) test (B) and the Welch’s t-test (C-F). ∗P < .05, ∗∗P < .01. Each symbol represents data from an individual mouse.
i-6FP Administration Suppresses Cytokine Production From MAIT Cells In Vivo
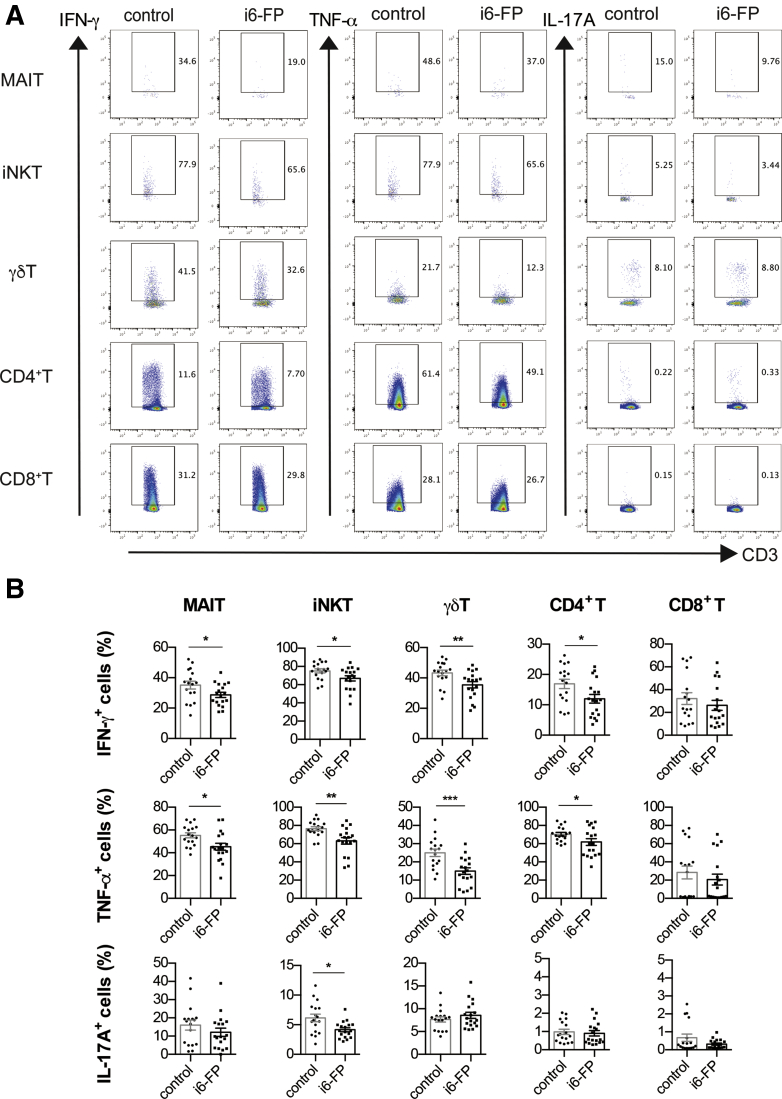
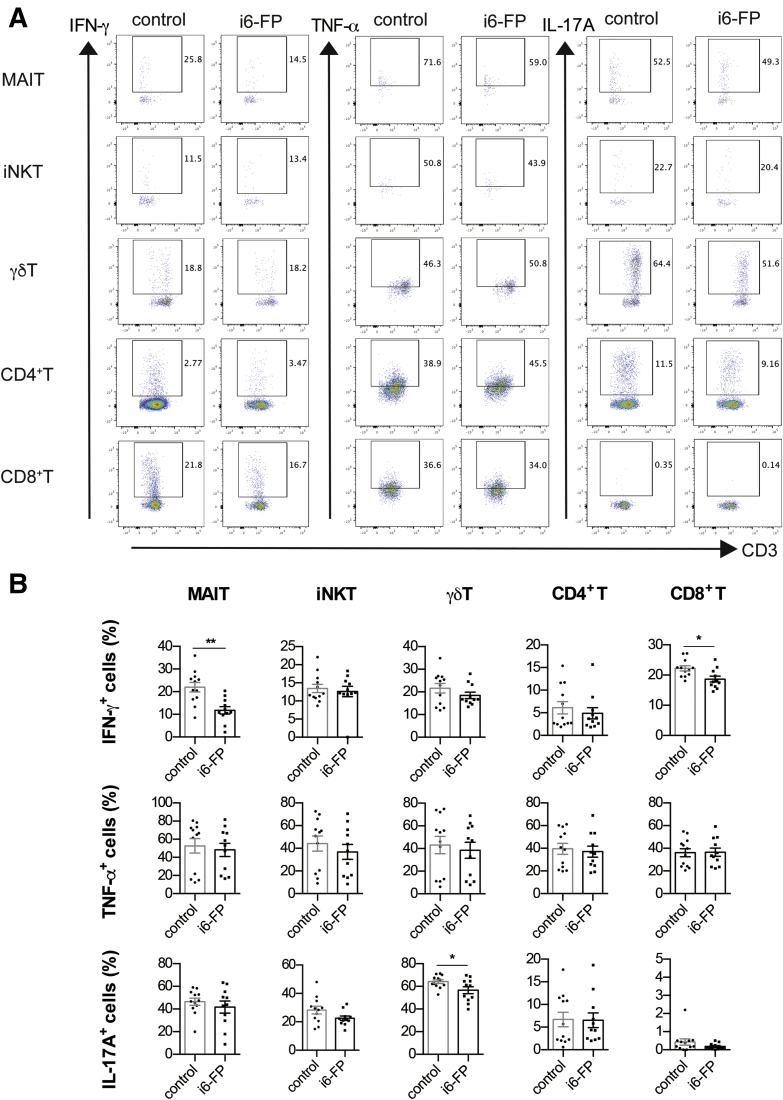
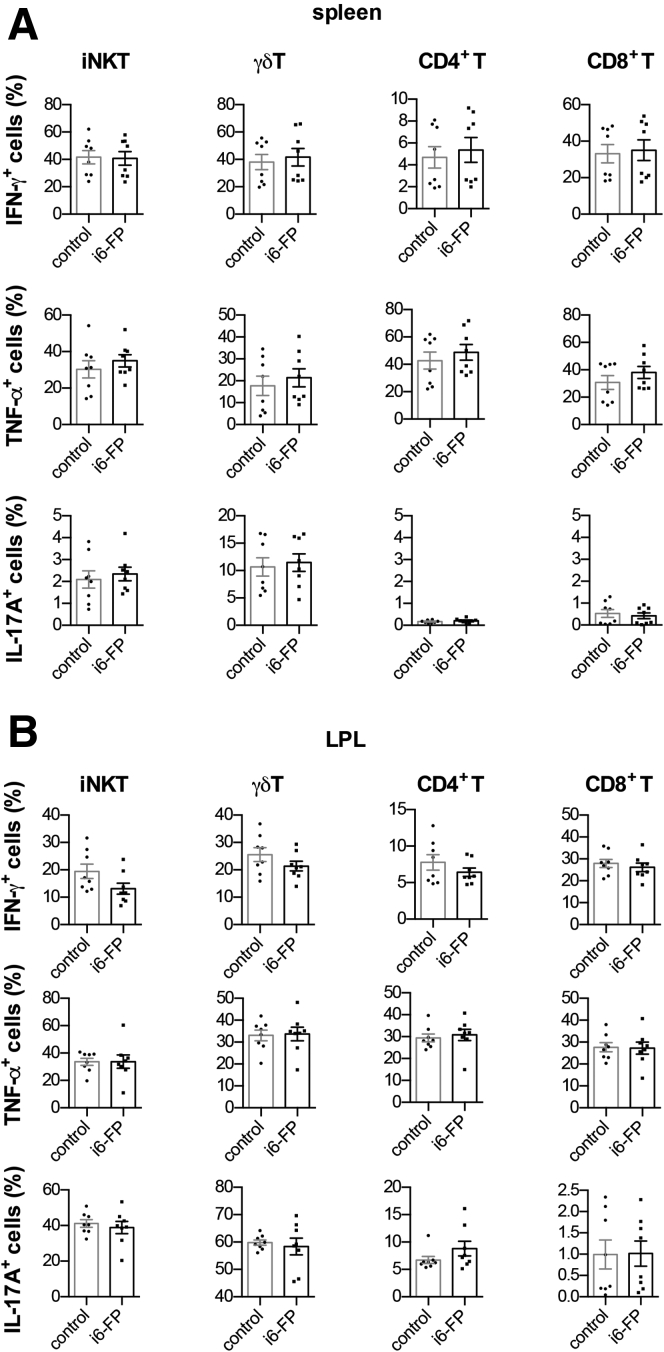
To elucidate the mechanism by which i6-FP treatment decreased the severity of colitis, we analyzed the cytokine-producing capacity of MAIT cells as well as other innate-like T and conventional T cells from i6-FP-treated mice. The capacity of MAIT cells in the spleen from i6-FP-treated mice to produce proinflammatory cytokines such as IFN-γ and TNF-α was decreased compared with MAIT cells in vehicle control mice. In addition, IFN-γ and TNF-α production by γδ T cells, iNKT cells, CD4+ T cells, and IL-17A production by iNKT cells were also decreased in i6-FP-treated mice (Figure 3A, B). We also assessed the effect of i6-FP treatment on the cytokine producing capacity of LPL in the large intestine. i6-FP treatment decreased IFN-γ production by MAIT and CD8+ T cells, and IL-17A production by γδ T cells (Figure 4A, B). The suppression of MAIT cell activation by i6-FP treatment appeared to decrease cytokine production by CD8+ other innate-like T and γδ T cells and the inhibitory effect of i6-FP on these cells was not observed in i6-FP treated-MR1−/− mice lacking MAIT cells (Figure 5A, B), which suggests that i6-FP treatment indirectly suppressed these cells via the inhibition of MAIT cell activation. These results indicate that the inhibition of MAIT cell activation reduced cytokine production in MAIT cells as well as other innate-like T cells and CD4+ T cells.
Figure 3.
Cytokine production by innate-like T cells and T cells in mice treated with i6-FP. IFN-γ, TNF-α, and IL-17A intracellular staining of MAIT cells, iNKT cells, γδ T cells, and CD4+ and CD8+ T cells in splenocytes from mice in the control group (n = 17) or i6-FP-treated mice (n = 18) 6 hours after phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation are shown. Representative flow cytometry profiles (A) and the percentages of indicated cytokine-producing cells (B). Data are represented as the mean ± standard error of the mean in each group. Significance was determined using Welch’s t-test. ∗P < .05, ∗∗P < .01. ∗∗∗P < .001. Each symbol represents data from an individual mouse.
Figure 4.
Cytokine production by innate-like T cells and T cells in mice treated with i6-FP. IFN-γ, TNF-α, and IL-17A intracellular staining of MAIT cells, iNKT cells, γδ T cells, and CD4+ and CD8+ T cells in LPL from mice in the control group (n = 12) or i6-FP-treated mice (n = 12) and representative flow cytometry profiles 6 hours after phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation are shown. Representative flow cytometry profiles (A) and the percentages of indicated cytokine-producing cells (B). Data are represented as the mean ± standard error of the mean in each group. Significance was determined using Welch’s t-test. ∗P < .05, ∗∗P < .01. ∗∗∗P < .001. Each symbol represents data from an individual mouse.
Figure 5.
Cytokine production by innate-like T and T cells in from MR1−/−mice treated with i6-FP. IFN-γ, TNF-α, and IL-17A intracellular staining of MAIT cells, iNKT cells, γδ T cells, and CD4+ and CD8+ T cells in splenocytes (A) and LPL (B) from MR1−/− mice treated with i6-PF (n = 8) or control vehicle (n = 8) 6 hours after phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation are shown. Data represent the mean ± SEM of each group. Significance was determined using Welch’s t-test. Each symbol represents data from an individual mouse.
Inhibition of MAIT Cell Activation With i6-FP Does Not Affect Gut Integrity
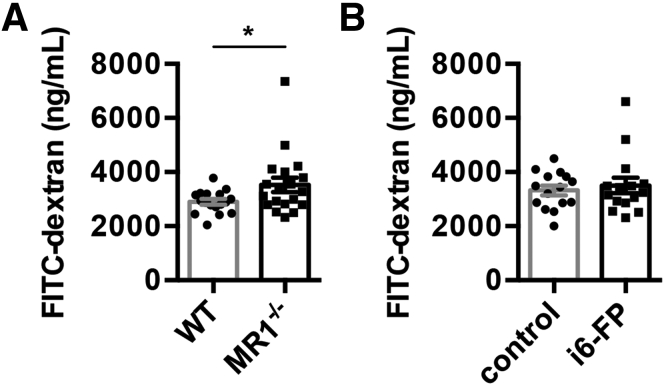
A previous study showed that MAIT cells have an important role in the maintenance of gut barrier integrity in NOD mice.23 To test the permeability of the gut, MR1−/− and WT mice were orally administered fluorescein isothiocyanate (FITC)-dextran and the plasma concentration of FITC-dextran was measured 4 hours later. As expected, the level of FITC-dextran was higher in MR1−/− mice compared with WT mice (Figure 6A), suggesting the lack of MAIT cell may cause an increase in intestinal permeability. Next, to evaluate whether intestinal permeability was increased by the inhibition of MAIT cell activation, WT mice were orally treated with i6-FP or control vehicle every other day, and on day 5, FITC-dextran was administered. There was no significant difference in the plasma FITC-dextran concentration between i6-FP-treated and control mice (Figure 6B). These results indicate that MAIT cells contribute to the maintenance of gut integrity and that the short-term inhibition of MAIT cell activation by i6-FP does not affect intestinal permeability.
Figure 6.
Intestinal permeability in MR1-deficient and i6-FP-treated mice. (A) FITC-dextran concentration in the plasma 4 hours after the oral administration of FITC-dextran to MR1−/− (n = 16) and littermate WT (n = 15) (A), or WT mice with (n = 15) or without i6-FP treatment (n = 15) (B). Data are represented as the mean ± SEM in each group. Significance was determined using Welch’s t-test. ∗P < .05. Each symbol represents data from an individual mouse.
Cytokine Production by MAIT Cells From Patients With UC is Suppressed by i6-FP
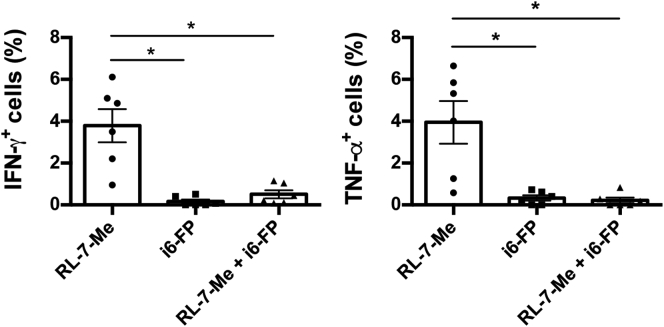
Previously, we demonstrated that in vitro treatment with a stimulatory MR1 ligand, 7-methyl-8-D-ribityllumazine (RL-7-Me), induced the expression of an activation marker, CD69, on murine MAIT cells, which was suppressed by the presence of i6-FP. However, it is not known whether i6-FP inhibits the activation and cytokine production of human MAIT cells. Peripheral blood mononuclear cells (PBMCs) isolated from patients with UC were treated with RL-7-Me in the presence or absence of i6-FP. MAIT cells produced IFN-γ and TNF-α upon stimulation with RL-7-Me. As expected, cytokine production was not observed in MAIT cells treated with i6-FP. However, i6-FP treatment reduced the frequency of IFN-γ or TNF-α positive MAIT cells stimulated with RL-7-Me (Figure 7). These results indicate that i6-FP treatment may also reduce the cytokine production of MAIT cells in patients with UC.
Figure 7.
Cytokine production by MAIT cells treated with RL-7-Me with or without i6-FP. PBMCs from patients with UC were treated with RL-7-Me for 16 hours in the presence or absence of i6-FP. The percentages of IFN-γ- and TNF-α-positive cells among MAIT cells are shown. Data are represented as the mean ± standard error of the mean in each group. Significance was determined using analysis of variance with Tukey’s multiple comparisons test. ∗P < .05. Each symbol represents data from an individual subject.
Discussion
As previously reported by us and others, the frequency of MAIT cells in the peripheral blood of patients with UC and CD was significantly reduced compared with healthy donors.17,24,25 Furthermore, MAIT cells accumulated in the inflamed mucosa of patients with UC and CD, suggesting they might be associated with inflammation of the mucosal tissues in IBD.17,25 However, the role of MAIT cells in colitis is poorly understood.
The severity of oxazolone colitis in MR1-deficient mice or mice treated with i6-FP was lower than that in control mice, suggesting MAIT cells have a pathogenic role in the setting of colitis. The pathophysiology of oxazolone colitis was originally thought to be mediated by Th2 cytokines.26,27 However, Iijima et al reported that the severity of oxazolone colitis in IL-4−/− and IFN-γ−/− mice was significantly reduced.28 Moreover, Watabe et al recently reported that IL-17, in addition to IFN-γ and IL-4, was also increased in association with the severity of oxazolone colitis.29 These findings indicate that various cytokines may contribute to the pathogenesis of oxazolone colitis, similar to that in human UC.2,30 MAIT cells have high cytokine-producing capacity, and we observed a decrease in the production of cytokines including IFN-γ and TNF-α by MAIT cells in the splenocytes and LPL of mice treated with i6-FP. In addition, a similar reduction of cytokine production was also observed in γδ T cells, iNKT cells, and CD4+ T and CD8+ T cells in mice treated with i6-FP. Therefore, we assumed that the amelioration of oxazolone colitis by i6-FP treatment was not only related to the suppressive effect on MAIT cell activation but was also because of secondary effects on other cell types by inhibiting the activation of MAIT cells. These results suggest that MAIT cells have a pathogenic role in oxazolone colitis mainly via the production of Th1 cytokines.
Gut barrier dysfunction is known to be related to the pathogenesis of colitis. Although the pathogenesis of IBD is poorly understood, impaired barrier function due to genetic and environmental factors may contribute to the onset and course of the disease.1,31 Indeed, increased intestinal permeability was reported in patients with IBD, especially those who developed a flare.32 A recent study reported that the enhanced severity of colitis in mice induced by the administration of dextran sodium sulfate was associated with increased intestinal permeability related to a deficiency in the Mediterranean fever gene, which controls barrier function via IL-18.33 MAIT cells are activated by microbe-derived antigens and produce cytokines including IL-17A, which is important in maintaining gut integrity.34 The expressions of the claudin 4 and claudin 8 genes, key components of tight junctions, were downregulated in MR1–/– mice.35 Transcriptome analysis of human and murine MAIT cells showed a tissue repair profile, and tissue repair genes including furin, CCL3, and IL-26 were upregulated in human MAIT cells upon their activation.36,37 Rouxel et al showed that MAIT cells have a key role in maintaining gut integrity in NOD mice.23 In this regard, we also observed increased intestinal permeability in MR1–/– mice compared with WT mice in the current study. Thus, the constitutive deficiency of MAIT cells may induce inflammation in the gut because of increased intestinal permeability. However, MR1-deficient mice do not show any apparent spontaneous symptoms of colitis, presumably because of the lack of MAIT cells. These findings indicate that MAIT cells contribute to the maintenance of gut barrier by several factors including IL-17A but act as major effector cells in tissue inflammation in the colon.
The suppression of MAIT cell activation by treatment with i6-FP did not elicit increased intestinal permeability. Rather, the inhibition of MAIT cell activation with short-term i6-FP treatment decreased the disease severity of oxazolone colitis compared with MR1 deficiency. Although there was no difference in body wasting between the MR1 and WT groups, i6-FP treatment reduced weight loss. Differences in the DAI, colon shortening, and histologic scores between i6-FP and control treatment groups were more apparent than between the MR1–/– and WT groups. This may be related to the effect of i6-FP, which reduces intestinal inflammation without affecting intestinal permeability.
The overactivation of CD4+ T cells, including dysregulated responses against commensal bacteria, can cause intestinal inflammation in mice and humans.1 The infiltration of activated CD4+ T cells into the lamina propria was observed in patients with IBD and murine models of colitis. Innate-like T cells including iNKT cells, δγT cells, and MAIT cells are enriched in these tissues and have important roles in tissue inflammation. A critical role of iNKT cells was shown in the development of oxazolone colitis.38 i6-FP treatment reduced the production of TNF-α and IFN-γ by MAIT cells as well as other innate-like T cells and CD4+ T cells. Thus, the inhibition of MAIT cell activation might further reduce the production of inflammatory cytokines by other innate-like T cells and CD4+ T cells. Although the role of δγT cells in oxazolone-induced colitis is not known, IL-17A produced by δγT cells was important for the protection of the intestinal barrier.34 IL-17 production by innate-like T cells and other T cells except iNKT cells was not decreased by treatment with i6-FP. Thus, the marked effect of i6-FP treatment on colitis may be partially due to its ability to strongly inhibit the production of Th1 cytokines by innate-like T cells and T cells.
The results of this study indicate that MAIT cells contribute to the pathogenesis of oxazolone colitis. Of note, we demonstrated that the inhibition of MAIT cell activation using i6-FP suppressed the severity of colitis, which was associated with the reduced cytokine-producing capacity of MAIT cells, innate T cells, and T cells. Previously, we demonstrated that the activated status of MAIT cells positively correlated with disease activity in patients with UC, and that MAIT cells accumulated in inflamed mucosa. In vitro cytokine production by MAIT cells from patients with UC was reduced by treatment with i6-FP. This suggests MAIT cells have a key role in the pathogenesis of human colitis and that they might be a novel therapeutic target for IBD.
Methods
Mice
C57BL/6J mice were obtained from the Sankyo Labo Service Corporation (Tokyo, Japan). MR1−/−mice on the C57BL/6 background were provided by S. Gilfillan (Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO). Mice were maintained under specific pathogen-free conditions in a room with controlled temperature (21–23°C) and a 12-hour light/dark cycle. Mice were fed chow, CRF-1 (Oriental Yeast Co, Ltd, Tokyo), and water ad libitum. Experimental procedures were performed in accordance with the institutional guidelines of Juntendo University (2020164/1200). All mice used for the experiments were male and between the ages of 8 and 20 weeks.
Induction of Colitis
Male MR1−/− mice on the C57BL/6J background and their littermate MR1+/+ controls maintained in our facility and male C57BL/6J mice obtained from the vendor were used for the murine model of IBD, oxazolone colitis. Hereafter, the littermate MR1+/+ in our facility and C57BL/6J from the vendor are referred to as WT mice. MR1−/− mice and their littermate WT mice were used at 13 to 18 weeks of age, and WT mice from the vendor were used at 16 to 20 weeks of age. Oxazolone colitis was induced in these mice by a previously described protocol with a minor modification.29 Briefly, mice were sensitized with 150 μL of 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone; Sigma-Aldrich, St. Louis, MO) at a concentration of 3% in 100% ethanol by skin painting on day 0. Control mice were painted with 100% ethanol on day 0. On day 5, MR1−/− and littermate WT mice were administered intrarectally with 200 μL of 1% oxazolone in 50% ethanol, and WT mice were administered intrarectally with 200 μL of 0.2% oxazolone in 50% ethanol. In some experiments, the large intestines were collected 24 hours after the intrarectal administration of oxazolone or ethanol control to analyze the expressions of CD69 and RORγt on MAIT cells in the LPL population. We optimized the age of animals and the concentration of intrarectally administered oxazolone because the colitis induced in our animal facility was more severe than usual, and the susceptibility of mice from the vendor was even higher than that of mice intercrossed and maintained in our facility. Therefore, the survival rate during the induction of colitis was assessed. The DAI was determined using previously reported methods with minor modifications.26 Briefly, the DAI is calculated from the sum of 3 parameters as follows: body weight, 0 to 4 (0, no loss; 1, 1%–5% loss; 2, 5%–10% loss; 3, 10%–20% loss; 4, <20% loss); stool consistency, 0 to 2 (0, normal; 1, loose; 2, diarrhea); and presence of blood in the stools, 0 to 2 (0, normal; 1, occult bleeding; 2, gross bleeding). DAI in mice that died before study termination were excluded from the analysis. Mice were then euthanized for histologic assessment.
Histologic Analysis
Tissue sections of colons were fixed in 10% formalin. Paraffin-embedded sections were stained with hematoxylin and eosin. Histologic scores were calculated by the sum of 4 parameters as follows: infiltration of mononuclear cells, 0 to 2 (0, normal; 1, mild; 2, moderate); reduction of goblet cell, 0 to 1 (0, no; 1, yes); crypt inflammation, 0 to 2 (0, normal; 1, cryptitis; 2, crypt abscess), and epithelial damage (0, normal; 1, erosion; 2, ulcer), following methods previously described with a minor change.28,29,38
Flow Cytometry
The large intestine was harvested from mice 24 hours after the administration of oxazolone or ethanol. Colonic LPL were isolated from the large intestine using a Lamina Propria Dissociation Kit, gentleMACS Dissociator (Miltenyi Biotec, North Rhine-Westphalia, Germany), and Percoll density-gradient centrifugation. Cells were stained using the Zombie Green Fixable Viability Kit (BioLegend, San Diego, CA) and then incubated with combinations of the following monoclonal antibodies: anti-CD3-Alexa700, anti-F4/80-FITC, and anti-CD69-PE-Cy7 (BioLegend). mMR1 tetramers loaded with 5-OP-RU-BV421 were used (National Institutes of Health [NIH] tetramer core facility at Emory University).9 All data were acquired on a FACS LSRFortessa (BD Biosciences) and analyzed by FlowJo software (TreeStar Inc, Ashland, OR).
Suppression of MAIT Cell Activation
i6-FP was synthesized by SundiaMediTech Company, Ltd (Shanghai, China). Mice orally received i6-FP (15 mg/kg) or control vehicle (10% dimethylsulfoxide in phosphate buffered saline) on the day of the induction of colitis, and 0, 2, 4, and 6 days later. Eight- to thirteen-week-old male WT or MR1−/− mice received 15 mg/kg of i6-FP or control vehicle orally on days 0, 2, and 4. On day 5, the spleen and large intestine were harvested for experiments related to the intracellular cytokine staining of splenocytes or measurement of intestinal permeability.
Intracellular Staining and Flow Cytometry
Spleens and large intestines were harvested from mice treated with i6-FP or control vehicle, splenocytes were isolated by homogenization, and erythrocytes were cleared with ammonium-chloride-potassium lysing buffer. LPL were isolated as described above. Splenocytes and LPL (2 × 106 cells/well) were cultured in 96-well flat-bottom plates in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin (all from Thermo Fisher Scientific, Waltham, MA). Splenocytes were stimulated with phorbol 12-myristate 13-acetate (50 ng/mL, Sigma-Aldrich) and ionomycin (500 ng/mL, Sigma-Aldrich) for 6 hours. GolgiPlug (0.67 μg/ml, BD Biosciences, San Jose, CA) was added during the final 3 hours. Cells were stained using the Zombie Green Fixable Viability Kit (BioLegend) and then incubated with combinations of the following monoclonal antibodies: anti-CD3-Alexa700, anti-F4/80-FITC, anti-TCR-γδ-PerCP-Cy5.5 (BioLegend), anti-CD4-APC-H7, and anti-CD8α-V500 (BD Biosciences). mCD1d tetramers loaded with PBS-57-APC and mMR1 tetramers loaded with 5-OP-RU-BV421 were used (NIH tetramer core facility at Emory University).9 After staining the cell-surface antigens, intracellular staining was performed using the BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences) and anti-IFN-γ-PE-Cy7 or anti-IFN-γ-PE, anti-TNF-α-PE-Cy7, and anti-IL-17A-PE-Cy7 or anti-IL-17A-PE (BioLegend) and RORγt staining was performed using Foxp3 / Transcription Factor Staining Buffer Set (Thermo Fisher Scientific) and anti-RORγt-PE(Thermo Fisher Scientific). All data were acquired on a FACS LSRFortessa and analyzed by FlowJo software.
Analysis of Intestinal Permeability
After overnight starvation, mice were orally administered FITC-dextran (Sigma-Aldrich) (44 mg per 100 g body weight) as previously described.23 Four hours later, blood was collected and centrifuged at 3000 × g for 20 minutes at 4°C. Plasma was diluted with water, and the fluorescence intensity was measured by Flex Station 3 (Molecular Devices, San Jose, CA).
Human Samples
Six patients with UC (Table 1) participated in this study. The clinical activity index of patients was evaluated using the Lichtiger index, and the endoscopic severity was determined using the Ulcerative Colitis Endoscopic Index of Severity. Peripheral blood was drawn after obtaining informed consent in accordance with the local ethical committee guidelines of Juntendo University. PBMCs were isolated from whole blood by density-gradient centrifugation using BD Vacutainer CPT Mononuclear Cell Preparation Tubes with Sodium Heparin (BD Biosciences). PBMCs were cryopreserved in cryopreservation medium (Bambanker; Nippon Genetics, Tokyo, Japan) until used.
Table 1.
Characteristics of the Patients Included in the Study
| Characteristic | Patients with UC (n = 6) |
|---|---|
| Mean age ± SD, y | 47.7 ± 13.3 |
| Sex (male/female) | 4/2 |
| Mean duration of disease ± SD, y | 12.0 ± 11.6 |
| Disease location, n | |
| Proctitis | 2 |
| Left-sided colitis | 1 |
| Extensive colitis | 3 |
| Ongoing treatments, n | |
| 5-ASA | 5 |
| Azathioprine, 6-MP | 2 |
| Anti-TNF therapy | 2 |
| Anti-α4β7 integrin therapy | 1 |
| PSL | 2 |
| Median Lichtiger index (range) | 1 (0-1) |
| Median UCEIS (range) | 1.5 (0-3) |
| Mean CRP ± SD, mg/dL | 0.17 ± 0.21 |
5-ASA, 5-Aminosalicylic acid; CRP, C-reactive protein; 6-MP, mercaptopurine; PSL, prednisolone; SD, standard deviation; TNF, tumor necrosis factor; UC, ulcerative colitis; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
Ex Vivo Culture and Intracellular Cytokine Staining of Human PBMCs
PBMCs (1 × 106cells/well) were incubated with or without i6-FP (100 μM) for 30 minutes in RPMI 1640, and incubated for 16 hours in 100 μM of 7-methyl-8-D-ribityllumazine (RL-7-Me) synthesized as previously described.9 GolgiPlug (0.67 μg/ml, BD Biosciences) was also added for the final 16 hours. The cells were stained using a Zombie Yellow Fixable Viability Kit (BioLegend), and combinations of the following monoclonal antibodies were used for the cell-surface and intracellular staining: anti-CD3-APC-H7 (BD Biosciences), anti-CD19-V500 (BD Biosciences), hMR1 tetramers loaded with 5-OP-RU-BV421 (NIH tetramer core facility at Emory University), IFN-γ-PE-Cy7 (eBioscience), and anti-TNF-α-PE-Cy7 (BD Biosciences). All data were acquired on a FACS LSRFortessa (BD Biosciences) and analyzed by FlowJo software (TreeStar Inc).
Statistical Analysis
All data were analyzed using GraphPad Prism (GraphPad, Inc, San Diego, CA). Statistical differences were analyzed with the Log-rank (Mantel-Cox) test or Welch’s t-tests or 1-way analysis of variance with Tukey’s multiple comparisons test.
Acknowledgments
The authors thank S. Gilfillan (Washington University School of Medicine, St. Louis, MO) for MR1 knockout mice, the NIH tetramer core facility for CD1d and MR1 tetramers, Yoichiro Iwakura for a material supply, Hirokazu Sasaki and Daiki Yamada for technical support, and J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
CRediT Authorship Contributions
Yusuke Yasutomi, MS (Conceptualization: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead)
Asako Chiba, MD, PhD (Conceptualization: Lead; Funding acquisition: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Lead)
Keiichi Haga, MD, PhD (Conceptualization: Supporting; Investigation: Supporting; Resources: Supporting)
Goh Murayama, MD, PhD (Investigation: Supporting; Methodology: Supporting; Validation: Supporting; Writing – review & editing: Supporting)
Ayako Makiyama, MD, PhD (Methodology: Supporting)
Taiga Kuga, MD (Investigation: Supporting)
Mamoru Watanabe, MD, PhD (Supervision: Supporting; Writing – review & editing: Supporting)
Ryuichi Okamoto, MD, PhD (Supervision: Supporting)
Akihito Nagahara, MD, PhD (Supervision: Supporting; Writing – review & editing: Supporting)
Takashi Nagaishi, MD, PhD (Funding acquisition: Supporting; Methodology: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Sachiko Miyake, MD, PhD (Conceptualization: Lead; Funding acquisition: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported in part by Japan Society For the Promotion of Science (Grant-in-Aid for Scientific Research (B) 17H04218 to SM, (B) 20H03658 to TN and (C) 17K09983 to AC, and Grant-in-Aid for Young Scientists (B) 17K15970 to KH), a collaborative research fund program for women researchers from Juntendo University funded by Initiative for the implementation of the diversity research environment from Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Promotion and Mutual Aid Corporation for Private Schools of Japan (Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools). Writing assistance was provided by J. Ludovic Croxford, PhD, from Edanz Group (https://en-author-services.edanz.com/ac) for English editing, which was funded by JSPS KAKENHI.
References
- 1.Abraham C., Cho J.H. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace K.L., Zheng L.B., Kanazawa Y., Shih D.Q. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neurath M.F. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 4.Wyant T., Yang L., Fedyk E. In vitro assessment of the effects of vedolizumab binding on peripheral blood lymphocytes. MAbs. 2013;5:842–850. doi: 10.4161/mabs.26392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zundler S., Klingberg A., Schillinger D., Fischer S., Neufert C., Atreya I., Gunzer M., Neurath M.F. Three-dimensional cross-sectional light-sheet microscopy imaging of the inflamed mouse gut. Gastroenterology. 2017;153:898–900. doi: 10.1053/j.gastro.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Tilloy F., Treiner E., Park S.H., Garcia C., Lemonnier F., de la Salle H., Bendelac A., Bonneville M., Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., Williamson N.A., Purcell A.W., Dudek N.L., McConville M.J., O’Hair R.A., Khairallah G.N., Godfrey D.I., Fairlie D.P., Rossjohn J., McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 8.Patel O., Kjer-Nielsen L., Le Nours J., Eckle S.B., Birkinshaw R., Beddoe T., Corbett A.J., Liu L., Miles J.J., Meehan B., Reantragoon R., Sandoval-Romero M.L., Sullivan L.C., Brooks A.G., Chen Z., Fairlie D.P., McCluskey J., Rossjohn J. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 9.Corbett A.J., Eckle S.B., Birkinshaw R.W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H., Williamson N.A., Strugnell R.A., Van Sinderen D., Mak J.Y., Fairlie D.P., Kjer-Nielsen L., Rossjohn J., McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 10.Le Bourhis L., Guerri L., Dusseaux M., Martin E., Soudais C., Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 12.Dusseaux M., Martin E., Serriari N., Peguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 13.van Wilgenburg B., Scherwitzl I., Hutchinson E.C., Leng T., Kurioka A., Kulicke C., de Lara C., Cole S., Vasanawathana S., Limpitikul W., Malasit P., Young D., Denney L., Stop HCV Consortium. Moore M.D., Fabris P., Giordani M.T., Oo Y.H., Laidlaw S.M., Dustin L.B., Ho L.P., Thompson F.M., Ramamurthy N., Mongkolsapaya J., Willberg C.B., Screaton G.R., Klenerman P. MAIT cells are activated during human viral infections. Nat Commun. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ussher J.E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T.H., Klenerman P., Willberg C.B. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahimpour A., Koay H.F., Enders A., Clanchy R., Eckle S.B., Meehan B., Chen Z., Whittle B., Liu L., Fairlie D.P., Goodnow C.C., McCluskey J., Rossjohn J., Uldrich A.P., Pellicci D.G., Godfrey D.I. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bourhis L., Martin E., Peguillet I., Guihot A., Froux N., Core M., Levy E., Dusseaux M., Meyssonnier V., Premel V., Ngo C., Riteau B., Duban L., Robert D., Huang S., Rottman M., Soudais C., Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 17.Haga K., Chiba A., Shibuya T., Osada T., Ishikawa D., Kodani T., Nomura O., Watanabe S., Miyake S. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol. 2016;31:965–972. doi: 10.1111/jgh.13242. [DOI] [PubMed] [Google Scholar]
- 18.Corridoni D., Antanaviciute A., Gupta T., Fawkner-Corbett D., Aulicino A., Jagielowicz M., Parikh K., Repapi E., Taylor S., Ishikawa D., Hatano R., Yamada T., Xin W., Slawinski H., Bowden R., Napolitani G., Brain O., Morimoto C., Koohy H., Simmons A. Single-cell atlas of colonic CD8(+) T cells in ulcerative colitis. Nat Med. 2020;26:1480–1490. doi: 10.1038/s41591-020-1003-4. [DOI] [PubMed] [Google Scholar]
- 19.Koay H.F., Gherardin N.A., Enders A., Loh L., Mackay L.K., Almeida C.F., Russ B.E., Nold-Petry C.A., Nold M.F., Bedoui S., Chen Z., Corbett A.J., Eckle S.B., Meehan B., d’Udekem Y., Konstantinov I.E., Lappas M., Liu L., Goodnow C.C., Fairlie D.P., Rossjohn J., Chong M.M., Kedzierska K., Berzins S.P., Belz G.T., McCluskey J., Uldrich A.P., Godfrey D.I., Pellicci D.G. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 20.Mak J.Y., Xu W., Reid R.C., Corbett A.J., Meehan B.S., Wang H., Chen Z., Rossjohn J., McCluskey J., Liu L., Fairlie D.P. Stabilizing short-lived Schiff base derivatives of 5-aminouracils that activate mucosal-associated invariant T cells. Nat Commun. 2017;8:14599. doi: 10.1038/ncomms14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salou M., Legoux F., Gilet J., Darbois A., du Halgouet A., Alonso R., Richer W., Goubet A.G., Daviaud C., Menger L., Procopio E., Premel V., Lantz O. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J Exp Med. 2019;216:133–151. doi: 10.1084/jem.20181483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murayama G., Chiba A., Suzuki H., Nomura A., Mizuno T., Kuga T., Nakamura S., Amano H., Hirose S., Yamaji K., Suzuki Y., Tamura N., Miyake S. A critical role for mucosal-associated invariant T cells as regulators and therapeutic targets in systemic lupus erythematosus. Front Immunol. 2019;10:2681. doi: 10.3389/fimmu.2019.02681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rouxel O., Da Silva J., Beaudoin L., Nel I., Tard C., Cagninacci L., Kiaf B., Oshima M., Diedisheim M., Salou M., Corbett A., Rossjohn J., McCluskey J., Scharfmann R., Battaglia M., Polak M., Lantz O., Beltrand J., Lehuen A. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol. 2017;18:1321–1331. doi: 10.1038/ni.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiejima E., Kawai T., Nakase H., Tsuruyama T., Morimoto T., Yasumi T., Taga T., Kanegane H., Hori M., Ohmori K., Higuchi T., Matsuura M., Yoshino T., Ikeuchi H., Kawada K., Sakai Y., Kitazume M.T., Hisamatsu T., Chiba T., Nishikomori R., Heike T. Reduced numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1529–1540. doi: 10.1097/MIB.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 25.Serriari N.E., Eoche M., Lamotte L., Lion J., Fumery M., Marcelo P., Chatelain D., Barre A., Nguyen-Khac E., Lantz O., Dupas J.L., Treiner E. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasaian M.T., Page K.M., Fish S., Brennan A., Cook T.A., Moreira K., Zhang M., Jesson M., Marquette K., Agostinelli R., Lee J., Williams C.M., Tchistiakova L., Thakker P. Therapeutic activity of an interleukin-4/interleukin-13 dual antagonist on oxazolone-induced colitis in mice. Immunology. 2014;143:416–427. doi: 10.1111/imm.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller F., Fuss I.J., Nieuwenhuis E.E., Blumberg R.S., Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 28.Iijima H., Neurath M.F., Nagaishi T., Glickman J.N., Nieuwenhuis E.E., Nakajima A., Chen D., Fuss I.J., Utku N., Lewicki D.N., Becker C., Gallagher T.M., Holmes K.V., Blumberg R.S. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–482. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watabe T., Nagaishi T., Tsugawa N., Kojima Y., Jose N., Hosoya A., Onizawa M., Nemoto Y., Oshima S., Nakamura T., Karasuyama H., Adachi T., Watanabe M. B cell activation in the cecal patches during the development of an experimental colitis model. Biochem Biophys Res Commun. 2018;496:367–373. doi: 10.1016/j.bbrc.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Nagaishi T., Watanabe M. In: Crohn’s disease and ulcerative colitis: from epidemiology and immunobiology to a rational diagnostic and therapeutic approach. Baumgart D., editor. Springer; New York: 2017. Paradigm of T cell differentiation in IBD. [Google Scholar]
- 31.Neurath M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
- 32.Kiesslich R., Duckworth C.A., Moussata D., Gloeckner A., Lim L.G., Goetz M., Pritchard D.M., Galle P.R., Neurath M.F., Watson A.J. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma D., Malik A., Guy C.S., Karki R., Vogel P., Kanneganti T.D. Pyrin inflammasome regulates tight junction integrity to restrict colitis and tumorigenesis. Gastroenterology. 2018;154:948–964.e8. doi: 10.1053/j.gastro.2017.11.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.S., Tato C.M., Joyce-Shaikh B., Gulen M.F., Cayatte C., Chen Y., Blumenschein W.M., Judo M., Ayanoglu G., McClanahan T.K., Li X., Cua D.J. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varelias A., Bunting M.D., Ormerod K.L., Koyama M., Olver S.D., Straube J., Kuns R.D., Robb R.J., Henden A.S., Cooper L., Lachner N., Gartlan K.H., Lantz O., Kjer-Nielsen L., Mak J.Y., Fairlie D.P., Clouston A.D., McCluskey J., Rossjohn J., Lane S.W., Hugenholtz P., Hill G.R. Recipient mucosal-associated invariant T cells control GVHD within the colon. J Clin Invest. 2018;128:1919–1936. doi: 10.1172/JCI91646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinks T.S.C., Marchi E., Jabeen M., Olshansky M., Kurioka A., Pediongco T.J., Meehan B.S., Kostenko L., Turner S.J., Corbett A.J., Chen Z., Klenerman P., McCluskey J. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep. 2019;28:3249–3262.e5. doi: 10.1016/j.celrep.2019.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leng T., Akther H.D., Hackstein C.P., Powell K., King T., Friedrich M., Christoforidou Z., McCuaig S., Neyazi M., Arancibia-Carcamo C.V., Hagel J., Powrie F., Oxford IBD Investigators. Peres R.S., Millar V., Ebner D., Lamichhane R., Ussher J., Hinks T.S.C., Marchi E., Willberg C., Klenerman P. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 2019;28:3077–3091.e5. doi: 10.1016/j.celrep.2019.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brozovic S., Nagaishi T., Yoshida M., Betz S., Salas A., Chen D., Kaser A., Glickman J., Kuo T., Little A., Morrison J., Corazza N., Kim J.Y., Colgan S.P., Young S.G., Exley M., Blumberg R.S. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]