Abstract
Introduction
Coronavirus disease 2019 resulted in a 30% mortality rate in patients with thoracic cancer. Given that patients with cancer were excluded from serum antisevere acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccine registration trials, it is still unknown whether they would develop a protective antispike antibody response after vaccination. This prospective vaccine monitoring study primarily aimed to assess humoral responses to the SARS-CoV-2 vaccine in patients with thoracic cancer.
Methods
SARS-CoV-2–spike antibodies were measured using the Abbot Architect SARS-CoV-2 immunoglobulin G immunoassay before the first injection of BNT162b2 mRNA vaccine, at week 4, and 2 to 16 weeks after the second vaccine dose administration. The factors associated with antibody response were analyzed.
Results
Overall, 306 patients, with a median age of 67.0 years (interquartile range: 58–74), were vaccinated. Of these, 283 patients received two vaccine doses at 28-day intervals. After a 6.7-month median follow-up, eight patients (2.6%) contracted proven symptomatic SARS-CoV-2 infection, with rapid favorable evolution. Of the 269 serologic results available beyond day 14 after the second vaccine dose administration, 17 patients (6.3%) were still negative (<50 arbitrary units/mL, whereas 34 (11%) were less than 300 arbitrary units/mL (12.5th percentile). In multivariate analysis, only age (p < 0.01) and long-term corticosteroid treatment (p = 0.01) were significantly associated with a lack of immunization. A total of 30 patients received a third vaccine dose, with only three patients showing persistently negative serology thereafter, whereas the others exhibited clear seroconversion.
Conclusions
SARS-CoV2 vaccines were found to be efficient in patients with thoracic cancer, most of them being immunized after two doses. A third shot given to 1% of patients with persistent low antibody titers resulted in an 88% immunization rate.
Keywords: Lung cancer, COVID-19, SARS-CoV-2, mRNA vaccine, Antibody response
Introduction
Coronavirus disease 2019 (COVID-19) is associated with a dramatic 30% mortality rate in patients with thoracic cancer.1, 2, 3 The Chinese series reported mortality rates of 29% to 39%,4, 5, 6 compared with 0.7% to 8.0% case fatality rates in their general population.7, 8, 9, 10, 11 Patients with lung cancer should, therefore, be given priority for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccination. Nevertheless, active cancer condition and immunosuppressive therapy are included in the noninclusion criteria for SARS-CoV-2 vaccine registration trials, and scarcely anything is known about the vaccine effectiveness in cancer populations. Moreover, the antibody response after influenza vaccination was previously reported to be lower in patients with cancer versus healthy controls, especially concerning people aged 65 years and older.12 Notably, two doses of influenza vaccine were required in patients with cancer with ongoing chemotherapy or corticosteroid treatment to attain the same serum protection rate as in healthy controls, resulting in lower protection.13 Similarly, a meta-analysis on influenza vaccine effectiveness in patients with cancer exhibited significantly reduced seroconversion (greater than or equal to a fourfold rise) compared with vaccinated immunocompetent controls (0.31, 95% confidence interval [CI]: 0.22–0.43).14 Vaccination timing remains unclear as well. On chemotherapy, the midpoint between two cycles was empirically selected for the vaccine shot. Moreover, whereas immunotherapy has become an essential component of lung cancer treatment, only little is known concerning undesirable effects, although no short-term reactogenicity after influenza vaccination occurred in patients under immune checkpoint inhibitors (ICIs).15 Both mRNA vaccine registration trials reported 94% effectiveness against severe COVID-19 in healthy volunteers 14 or more days after the second booster (d 21 for Pfizer BNT162b216 or d 28 for Moderna mRNA-127317 , 18). Concerning adenovirus-based vaccines (one shot of Janssen Ad26.COV2.S19 or two doses of AstraZeneca ChAdOx1nCov-19 vaccines20 , 21), slightly lower rates were reported. Similar protection rates against severe COVID-19 were confirmed in real-life by population-based Israeli and Scottish studies for Pfizer BNT162b2 or ChAdOx1nCov-19.22 , 23 Yet, these data cannot be extrapolated to patients with cancer undergoing anticancer treatment. The duration of anti–SARS-CoV-2 spike (antispike [anti-S]) detection was at least 8 months in healthy volunteers.24 It is unclear whether such duration applies to patients with cancer receiving immunosuppressing drugs. In early January 2021, vaccination was made available in France. To increase first vaccine dose availability, French Health authorities recommended a 28-day interval for both mRNA vaccines, with a 42-day interval for healthy people—this delayed second dosing being debatable.25 , 26 Because of uncertainties concerning vaccination of patients with cancer, the observational COVID Vaccination in Onco-Hematology patients (COVIDVAC-OH) study (clinical.trials.gov NCT04776005) was launched, sponsored by Paris University Hospitals. This study sought to investigate the effectiveness of SARS-CoV-2 vaccination (mainly mRNA-based vaccines) in over 1100 consecutive patients with solid-tumor cancers or hematologic malignancies at the North-Paris University Cancer center. This report involved 306 patients with thoracic cancer.
Materials and Methods
Trial Design, Objectives, and Participants
We conducted a prospective study involving patients with thoracic cancer followed up in Bichat Hospital from January 26, 2021 to July 28, 2021. Patients diagnosed with thoracic cancer and deemed eligible (no known COVID-19 infection within the past 3 mo; life expectancy >3 mo; lack of known allergy to previous vaccines) were identified from medical records. They were contacted and offered to be vaccinated. If they accepted and, in the absence of contraindications, they have attended vaccination sessions in the outpatient clinic, according to priority sequencing, as follows: (1) elderly patients aged 75 years and older and those receiving chemotherapy; (2) patients receiving ICI; (3) patients with pneumonectomy or chronic radiation pneumonitis; (4) patients on oral tyrosine kinase targeted therapy (tyrosine kinase inhibitors [TKIs]); and then (5) patients without systemic therapy. They were given a written information leaflet on COVID-19 mRNA BNT162b2 vaccine, and on serologic and hematologic blood tests to be performed at first dose (day 0), at the second dose (d 28), and at least 2 weeks after the booster dose (d 42). All patients could oppose blood samplings and still undergo vaccine injections. Recommendations to keep facial masks and social distancing were given. All patients were registered to the National Health Insurance computed COVID-19 vaccine database, which included data on the national identification number, complete identity, main underlying conditions, and vaccine batch number. After blood sampling and vaccine injection, patients were followed up for 15 minutes under medical surveillance.27 This study was approved by the Paris-North institutional review board (number 00006477, approval N°CER-2021-72). In the case of COVID-19–suggestive symptoms, patients were instructed to promptly inform the medical team and perform nasopharyngeal swab testing for SARS-CoV-2 through reverse transcription–polymerase chain reaction (PCR). At the second vaccination visit, they were questioned regarding undesirable events after the first vaccination and any symptoms evoking COVID-19. Some patients (n = 16) were vaccinated by their general practitioner or in a government-certified vaccination center. No blood sampling was available for these patients on days 0 and 28. If they agreed, they underwent blood sampling in the period after the booster and were included in our study.
To set up the technical conditions for the SARS-CoV-2 antinucleocapsid (anti-N) index and anti-S antibody determination, 18 controls from Hôpital Bichat staff, without previous COVID-19 symptoms or PCR-proven SARS-CoV-2 infection, provided their written consent for blood sampling. This occurred on day 28 after injection of the first vaccine dose, whereas 13 patients underwent additional blood sampling at least 2 weeks after the administration of the second vaccine dose.
Study End Points
The primary end point was to assess humoral immunity against SARS-CoV-2 spike in patients with thoracic cancer after COVID-19 mRNA BNT162b2 vaccine injection and the booster dose. Some patients vaccinated outside our center who received Moderna mRNA-1273 (n = 1) or AstraZeneca ChAdOx1 nCoV-19 (n = 3) vaccines were also included.
Secondary end points were vaccination safety and clinical efficacy on the basis of reverse transcription-PCR–documented COVID-19 infection during the study and hospitalization or death from COVID-19. Phone safety consultations were scheduled every 3 weeks. Cell immunity to SARS-CoV-2 spike protein was evaluated using T-cell enzyme-linked immune absorbent spot (ELISpot) with lymphocyte subset counts, scheduled at day 28 and from day 42 after the first injection in 122 arbitrarily-designated patients.
Laboratory Analyses
SARS-CoV-2 anti-N and anti-S antibody titers were determined using Abbott Architect SARS-CoV-2 immunoglobulin G (IgG) and IgG Quant II (Abbott, Maidenhead, United Kingdom) and expressed as an index (cutoff: 0.49) and arbitrary units (AUs) (cutoff: 50 AU/mL), respectively. Pseudoneutralization assay was performed using iFlash-2019-nCoV neutralizing antibody (Nab) assay (YHLO, Shenzhen, People’s Republic of China), which assesses antibody-neutralizing capacity by competition with angiotensin-converting enzyme 2 (ACE2) receptor for binding to anti-S receptor-binding domain (RBD) (cutoff: 10 AU/mL). This assay correlated with SARS-CoV-2 in vitro cell microneutralization. This pseudoneutralization assay was validated against in vitro microneutralization of SARS-CoV-2 B strain. To this end, serial sera samples from nine healthy controls were decomplemented by heat inactivation, subjected to serial twofold dilution (1:25 to 1:12,800), and incubated with the virus (2 × 103 plaque-forming units/mL) for 60 minutes; Vero E6 cell suspension was then added, and a 4-day incubation was carried out until the microscopy examination was conducted on day 4 to assess the cytopathologic effects (article submitted for publication).
ELISpot Assay Methods
Overall, 122 and 74 patients who accepted larger blood sampling and received their vaccine injection in the morning (to enable peripheral blood mononuclear cells isolation procedure performed within the d) underwent CD3+ and CD4+ T-cell quantification at day 28 (before the second shot) and day 42 or beyond, respectively. On day 28, 115 patients out of 122 underwent successful determination of T-cell responses to SARS-CoV-2 vaccination, as assessed using interferon gamma ELISpot assay and described in the online Supplementary Material.
Statistical Analysis
All samples were deidentified and assigned an identification number with the sampling date. Sample processing and data analyses were performed, with all study personnel blinded to information concerning patients and samples. Deidentified data were exported from Microsoft Excel Version 2013 for Windows (Microsoft Corporation, 2013) to IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY) for statistical analysis.
Normality for each continuous variable was systematically checked in each subgroup (immunized and nonimmunized) by means of analyzing skewness and kurtosis, QQ plots, and Shapiro-Wilk’s testing. When the normality assumption was not verified in both subgroups, nonparametric tests were applied.
Pairwise between-group comparisons were performed using Pearson’s chi-square or Fisher’s exact tests for discrete variables, and Student’s t test or Mann-Whitney U tests for continuous variables. ORs and respective 95% CI were calculated using binary logistic regression. Hypothesis testing was two-tailed, with p values less than 0.05 considered statistically significant. In multivariable analysis, only variables exhibiting a p value less than or equal to 0.2 in univariable analyses were considered, except for T-cell counts and SARS-CoV-2–specific T-cells because of their small sample size. The assumptions of the logistic regression were thoroughly checked, as follows: (1) multicollinearity using Spearman’s rho bivariate correlation testing, (2) outliers’ identification using the Z-score method, and (3) log odds linearity using the Box-Tidwell method. Multivariable analysis was conducted using binary logistic regression using the Enter method, including variables exhibiting a significance threshold p value less than 0.20 yielded by the univariable analysis. The 50 AU/mL cutoff threshold for positivity of the Abbott assay was provided by the manufacturer. The 300 AU/mL value was data-driven, corresponding to the 12.5th distribution percentile of anti-S IgG titers after the injection of the first vaccine dose. This precise value has been recently reported to significantly discriminate high versus low risk of breakthrough SARS-CoV-2 infection in fully vaccinated people at 6 months postvaccination.28
Results
Participant Characteristics
From January 20, 2021 to June 1, 2021, overall, 325 patients with thoracic cancer who followed up in thoracic oncology and surgery departments were proposed anti–SARS-CoV-2 vaccination with Pfizer BNT162b2 mRNA vaccine. Initially, 36 (11%) declined the proposal. Of these, 17 eventually accepted to be vaccinated; nine of whom were vaccinated outside of our center but participated in serologic testing. Among them, three received either the AstraZeneca vaccine (n = 2) or Moderna vaccine (n = 1). Overall, 306 patients received their 28-day–spaced doses or underwent blood samplings between January 26, 2021 and May 17, 2021. Of the 306 patients, 43 were noted to have had a history of either symptomatic COVID-19 (>3 mo before first vaccine dose injection) or asymptomatic SARS-CoV-2 infection, as uncovered by detecting antinucleoplasmid viral protein N. Consequently, most of these patients only received one vaccine injection as recommended by French health authorities. Patient disposition is illustrated in Supplementary Figure 1 . Clinical follow-up was extended until September 30, 2021.
Patient clinical and demographic characteristics are provided in Table 1 . Overall, 181 patients (59.2%) were men and 285 (93.1%) had lung cancer with 260 (84.9%) being NSCLCs and 22 (7.2%) SCLCs, whereas 13 (4.4%) had pleural malignant mesothelioma. The median age was 67 years (interquartile range [IQR]: 58–74), with 41.2% comprising those older than 70 years. Most patients (57.2%) displayed late-stage disease, with 117 (38%) diagnosed within the past 12 months. The last treatment received within 3 months before administration of the first vaccine dose was chemotherapy (n = 74, 24.2%), given alone (51, 16.7%), with concurrent thoracic radiotherapy (n = 2), or combined with ICI (21, 6.9%); whereas, 49 patients (16%) received ICI alone, and 13.7% were treated with daily TKIs or maintenance bevacizumab. The last 141 patients (30.7%) had not received systemic treatment within the past 3 months. Overall, 37 patients (12.1%) displayed chronic radiation pneumonitis after radiochemotherapy for stage III lung cancer. History of thoracic surgery was recorded in 89 patients (29%), six of whom (1.95%) underwent pneumonectomy and 79 (25.8%) have had lobectomy or sublobar resection. There were 20 patients (6.5%) under oral corticosteroids for at least 3 weeks for immune-mediated ICI toxicity, pain, brain metastasis, or severe chronic obstructive pulmonary disease. Overall, 59.5% of patients were in complete or partial response at vaccination time, whereas 29.1% were only recently diagnosed with cancer or displayed progressive disease.
Table 1.
Patient Clinical and Demographic Characteristics at Baseline
| Patients (N = 306) | n (%) |
|---|---|
| Age (y) | |
| Median (range, y) | 67 (27–92) |
| <70 | 180 (58.9) |
| 70–79 | 95 (31) |
| ≥80 | 31 (10.1) |
| Sex | |
| Male | 181 (59.2) |
| Female | 125 (40.8) |
| Body mass index | |
| Median, kg/m2 (Q1–Q3) | 24.9 (21.8–27.9) |
| Histologic diagnosis | |
| Lung Non-SCC | 211 (68.9) |
| Lung SCC | 49 (16) |
| Lung NSCLC | 260 (84.9) |
| Lung SCLC | 22 (7.2) |
| Pleural mesothelioma | 13 (4.2) |
| Othersa | 11 (3.5) |
| Last treatment received <3 mo | |
| Chemo-based regimen | 74 (24.2) |
| Immunotherapy alone | 48 (15.7) |
| Oral TKI or bevacizumab alone | 43 (14) |
| No systemic treatment (radiotherapy, surgery, and complete response) | 141 (46.1) |
| Chronic radiation pneumonitis | |
| Yes | 37 (12.1) |
| No | 269 (87.9) |
| Previous thoracic surgery | 89 (29) |
| Pneumonectomy | 6 (1.95) |
| Lobectomy or sublobar resection | 79 (25.8) |
| Thymectomy or mediastinal tumor resection | 4 (1.3) |
| Duration of disease | |
| Median, mo (Q1–Q3) | 17.3 (6.4–35.3) |
| ≥12 mo | 189 |
| <12 mo | 117 |
| Long-term corticosteroid treatment | |
| Yes | 20 (6.5) |
| No | 286 (93.5) |
| Disease extent | |
| Metastatic | 175 (57.2) |
| Local or locoregional | 131 (42.8) |
| Disease status | |
| Response or stable | 211 (69.0) |
| Progressive | 95 (31.0) |
| T-lymphocyte count available at d 28 | 122 (39.9) |
| Median/mm3 (Q1–Q3) | 1129 (742–1434) |
| CD4+ count available at day 28 | 122 (39.9) |
| Median/mm3 (Q1–Q3) | 596 (345–853) |
| COVID–19 history before first vaccination | |
| Yes | 19 (6.2) |
| No | 287 (93.8) |
| Type of vaccine | |
| Pfizer BNT 162b2 | 302 |
| Moderna 1273 | 1 |
| AstraZeneca ChAdOx1 nCOVID–19 | 3 |
Chemo, chemotherapy; COVID-19, coronavirus disease 2019; Q, quartile; SCC, squamous cell cancer; TKI, tyrosine kinase inhibitor.
Five thymic carcinomas, four carcinoid tumors, one hemangioendothelioma, and one hamartochondroma.
Total lymphocyte counts were available for 122 patients (39.9%) on day 28, with a median T-lymphocyte (CD3+) count of 1129/mm3 (IQR: 742–1434), and median CD4+ T-cell count of 596/mm3 (IQR: 345–853).
Humoral Immune Response
Median follow-up was 202 (IQR: 195–244) days. Overall, 283 patients (92.5%) underwent serologic testing on day 28 after the first injection and received a second vaccine dose on that booster date. On day 28, a total of 248 samples from patients free of previous SARS-CoV-2 infection or with anti-N–negative IgG at day 0 or day 28 (n = 265) were available. The median anti-S IgG titer was 149.7 AU/mL (IQR: 21.9–436.1).
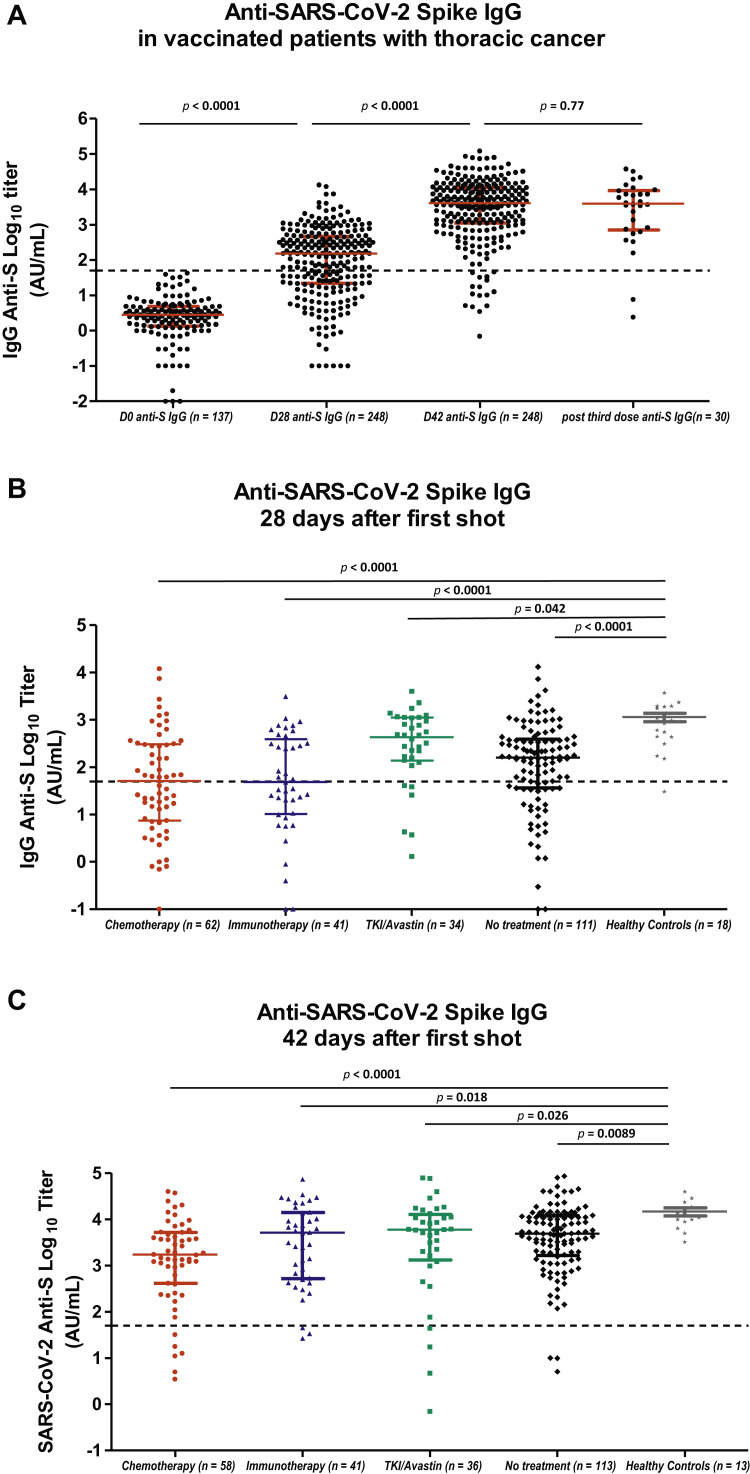
In patients without a history of symptomatic or asymptomatic COVID-19 history (thus, excluding 22 patients—17 with previous COVID-19 history, three with PCR-proven COVID-19 after the first dose, and two with anti-N IgG detected at d 0), a striking increase in antibody titers occurred between day 0 (137 patients with available serology) and day 28 (248 patients with available serology) after first vaccine dose administration (Fig. 1 A).
Figure 1.
Serologic response to COVID-19 vaccine BNT162b2 in COVID-19–free patients. (A) Anti-S IgG antibody titers at day 0 in 137 patients without previous history of COVID-19; at day 28 after one vaccine dose injection in 248 patients without previous history of COVID-19; beyond day 42 in 248 patients without previous history of COVID-19; beyond day 21 after third vaccine dose in 30 patients with available results. Large horizontal bars represent the median value, with short bars illustrating the values of the first (lower) and third (upper) quartiles. Mann-Whiney U test was applied for statistical comparison. (B) Anti-S IgG antibody titers at day 28 after the first vaccine dose, according to the systemic treatment received within the previous 3 months: chemotherapy, including chemoimmunotherapy (n = 62), immunotherapy alone (n = 41), oral TKI or bevacizumab single-agent therapy (n = 34), or without systemic treatment (n = 111). Anti-S IgG antibody titers at day 28 in 18 healthy controls are illustrated. Large horizontal bars represent the median value, with short bars illustrating the values of the first (lower) and third (upper) quartiles. Mann-Whiney U test was applied for statistical comparison. (C) Anti-S IgG antibody titers at day 42 or beyond after the first vaccine dose, according to the systemic treatment received within the previous 3 months: chemotherapy, including chemoimmunotherapy (n = 58), immunotherapy alone (n = 41), oral TKI or bevacizumab single-agent (n = 36), or without systemic treatment (n = 113). Anti-S IgG antibody titers at day 42 in 13 healthy controls were available. Large horizontal bars represent the median value, with short bars illustrating the values of the first (lower) and third (upper) quartiles. Mann-Whiney U test was applied for statistical comparison. Anti-S, anti-SARS-CoV-2 anti-spike antibody; AU, arbitrary unit; COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TKI, tyrosine kinase inhibitor.
Not all antibodies detected are able to efficiently neutralize the virus by impairing its binding to the ACE2 receptor expressed by respiratory cells. Nabs constitute a variable part of the anti-S antibodies. The neutralization activity was measured using a pseudoneutralization assay, assessing Nab capacity by means of competition with ACE2-receptor for binding to anti-S RBD. Supplementary Figure 2 depicts the correlation between anti-S IgG log10 titers and anti-S RBD pseudoneutralization log10 titers. A strong correlation was observed from anti-S IgG titer of 300 AU/mL (Spearman’s test, ρ = 0.92, p < 0.0001), supporting the neutralizing effect of serum anti-S IgG levels exceeding such cutoff.
At day 28, a total of 91 patients (32.3%) displayed no anti-S IgG (<50 AU/mL), whereas 165 patients (58.5%) exhibited only low titers (≤300 AU/mL).
By comparison, the median value of 18 healthy controls was 913 AU/mL (IQR: 438.3– 1859.3), with the antibody titer distribution of healthy controls significantly differing from that of patients treated with chemotherapy (p < 0.0001, Mann-Whitney U test), immunotherapy (p < 0.0001, Mann-Whitney U test), targeted therapy or bevacizumab (p = 0.043, Mann-Whitney U test), or those without systemic therapy within the past 3 months (p < 0.0001, Mann-Whitney U test) (Fig. 1 B). No significant differences in serum anti-S antibody titers were seen between chemotherapy- and immunotherapy-treated patients (p = 0.11).
The second booster dose was not administered to 24 patients because of cancer-related general condition alteration (n = 2), mild symptomatic PCR-proven COVID-19 infection after first vaccine dose administration (n = 3), history of symptomatic COVID-19 before vaccination supported by anti-N IgG detection (n = 17), and totally asymptomatic COVID-19 as reflected again by anti-N IgG detection (n = 2).
Serologic data were available for 269 patients (88%) at day 42, 2 to 9 weeks after second vaccine dose administration (median time interval: 52 d [IQR: 45–69]). Of the 306 patients, 37 could not undergo late serologic control because of altered general condition (n = 4), cancer-related death (n = 9), or patient refusal (n = 5); 19 patients exhibited COVID-19 at any time, with late serologic control deemed unnecessary by referent physicians.
Among patients free of previous SARS-CoV-2 infection or with anti-N–negative IgG at day 0 and day 28 (n = 265), 248 samples were available at day 42 (≥14 d after the second dose) with a median serum anti-S IgG titer of 4725 AU/mL (IQR: 1066–13,698), 300 AU/mL corresponding to 12.5th percentile.
Two to 9 weeks after administration of the second dose of the vaccine, an overall increase in serum anti-S IgG titers was noted (Fig.1 C) with a mean 1.4 to a twofold increase in the log10 anti-S IgG concentrations. However, 17 patients (6.3%) still exhibited negative serologic testing, whereas 34 (11%) displayed IgG concentrations less than or equal to 300 AU/mL, with 65 patients (24.1%) exhibiting antibody titers below the first quartile value of 1066 AU/mL.
The median serum anti-S IgG concentration in 13 healthy controls, within a median 57-day interval after the second vaccine dose administration, was 10,594 AU/mL (IQR: 8350–14,836). The titer distribution significantly differed from that observed in patients treated using chemotherapy (p = 0.0003, Mann-Whitney U test), immunotherapy (p = 0.013, Mann-Whitney U test), oral targeted therapy or bevacizumab (p = 0.02, Mann-Whitney U test), or those without systemic therapy within the past 3 months (p = 0.001, Mann-Whitney U test) (Fig. 1 C).
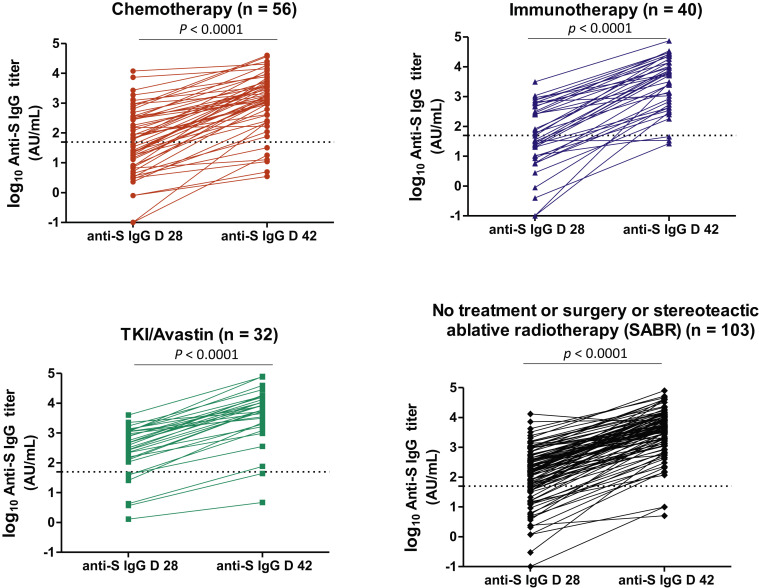
Considering the 231 patients with available data at both points, the anti-S IgG titers significantly rose between day 28 (first dose) and day 42 (second dose), irrespective of systemic treatments received (Fig. 2 ), with higher titers observed in previously COVID-19–infected patients (n = 31) (Supplementary Fig. 3). In patients for whom serology was available at day 28 after the first (sole) vaccine, an increase by two logs in anti-S IgG antibodies was recorded, with these antibodies remaining high in 14 patients on later samplings.
Figure 2.
Anti-S IgG antibody titers at D 28 and D 42 according to the different systemic treatments received. The horizontal dashed lines along the x axis indicate the limit of detection (positivity cutoff) provided by the manufacturer (log10 50 AU/mL). A nonparametric two-tailed pairwise comparison was performed using the Wilcoxon matched-pairs signed-rank test. Anti-S, anti-SARS-CoV-2 anti-spike antibody; AU, arbitrary unit; D, day; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SABR, stereoteactic ablative radiotherapy; TKI, tyrosine kinase inhibitor.
During the 6-month follow-up from late January 2021 to July 2021, eight patients (2.6%) experienced mildly symptomatic PCR-proven COVID-19 symptoms. In four patients, these symptoms respectively occurred at days 4, 6, 12, and 20 after the first dose of the vaccine was administered, and in the remaining four at days 33, 35, 42, and 65 after the second dose, respectively. Only one patient with thymic carcinoma (serum anti-S IgG titers at 300.4 AU/mL two days before positive SARS-CoV-2 PCR testing) was hospitalized because of his frail condition, yet not requiring oxygen supply. He was discharged a week later.
Safety
No anaphylactic reaction occurred among the 306 patients, with a total of 587 vaccine doses administered. Safety data were available for 278 patients (90.1%), without significant safety concerns. One-third of patients (n = 98) did not report symptoms after the first injection. Reported undesirable effects were transitory pain, injection-site swelling, or grade 1 injection-site erythema lasting less than 24 hours. More frequent undesirable effects of mild intensity were reported after the second vaccination in two-thirds of patients (n = 201), including injection-site erythema, pain, local injection-site swelling, and mild fever (<38.5°C), all lasting less than 36 hours. Flu-like symptoms, chills, and fatigue lasting less than 48 hours were reported in 25 cases (8%). In one patient, a spectacular grade 2 urticarial reaction occurred, resolving with oral antihistamines less than 3 days of booster dosing. Another patient reported a large grade 2 local reaction manifesting within 24 hours after the second injection as large annular erythema plaques with pain, fatigue, and mild fever (38°C), with spontaneous resolution within 8 days. No vaccine-related death occurred.
Clinical and Biological Variables Associated With Lack of Immunization
We analyzed the correlation between serologic titers using different cut-points (≤50 AU/mL; ≤300 AU/mL) and the main clinical, demographic, and biological variables in the whole population with two mRNA vaccine injections (n = 283) (Supplementary Table 1 A–D) and the 244 patients with no history of COVID-19 (Supplementary Table 2 A–D).
Briefly, at day 28, either with the 50 AU/mL or the 300 AU/mL cutoff, age (Supplementary Fig. 4 A and C), male sex, chemotherapy-based treatment within the past 3 months, immunotherapy as single-therapy within the past 3 months, or long-term corticosteroid treatment were significantly associated with negative (<50 AU/mL) or low (<300 AU/mL) serum anti-S IgG levels in univariable analyses (Supplementary Table 2 A).
Conversely, in 122 patients with such analyses, at 50 AU/mL cutoff, every 100 units/mm3 increase in day 28 T-lymphocyte (CD3+) counts (p < 0.01), and day 28 CD4+ T-cell counts (p = 0.01) (Supplementary Fig. 4 B), were associated with higher seroconversion probability, whereas this was not the case for both T-cell subsets with the 300 AU/mL cutoff. In 111 and 108 patients with these analyses, day 28 interferon-γ–specific T-cell response to SARS-CoV-2 spike, measured by the ELISpot assay, was significantly associated with higher seroconversion probability on day 28 at 50 AU/mL and 300 AU/mL cutoffs, respectively (OR = 0.97, 95% CI: 0.94–0.99, p = 0.04 and OR = 0.96, 95% CI: 0.93–0.98, p < 0.001, respectively).
Multivariable logistic regression analyses (Supplementary Table 2 A for 50 AU/mL cutoff and Supplementary Table 2 B for 300 AU/mL cutoff) confirmed that age was the only variable with significant impact when using both cutoffs (adjusted OR [aOR] = 1.05, 95% CI: 1.02–1.08, p < 0.0001 and aOR = 1.04, 95% CI: 1.02–1.07, p = 0.001, respectively), with a 5% or 4% increase in nonimmunization risk at day 28 for each additional year of age. Long-term corticosteroid use (aOR = 3.29, 95% CI: 0.96–11.28, with borderline significance p = 0.06), chemotherapy as last treatment (aOR = 3.46, 95% CI: 1.19–10.01, p = 0.02), or single-agent ICI treatment within last 3 months (aOR = 4.18; 95% CI: 1.36–12.82, p = 0.01), were independently associated with antibody titers of at least 50 AU/mL at day 28. In addition to age, only male sex (aOR = 2.35, 95% CI: 1.30–4.26, p = 0.005) was independently associated with day 28 anti-S IgG titers of less than or equal to 300 AU/mL.
Considering day 42 (after second vaccination injection) for both cutoffs of less than or equal to 50 AU/mL and less than or equal to 300 UA/mL, in univariable analyses, the variables significantly associated with negative serology risk included age, chemotherapy as last treatment received, lack of disease control, and chronic corticosteroid use (Supplementary Tables 2 C and D). Conversely, at both 50 AU/mL and 300 AU/mL cutoffs, in 68 patients with available data, each 100 units/mm3 increase in day 42 CD3+ T-cell (OR = 0.59, 95% CI: 0.39–0.89, p = 0.01 and OR = 0.72, 95% CI: 0.58–0.89, p < 0.01, respectively) or CD4+ T-cell counts (OR = 0.05, 95% CI: 0.004–0.54, p = 0.01 and OR = 0.49, 95% CI: 0.30–0.80, p < 0.01, respectively) was associated with a higher seroconversion probability.
In multivariable analyses, at both cutoffs, age (aOR = 1.10, 95% CI: 1.04–1.17, p < 0.01 and aOR = 1.07; 95% CI: 1.03–1.12, p < 0.01; respectively), and long-term corticosteroid use (aOR = 4.59, 95% CI: 0.96–21.95, p = 0.05 and aOR = 5.04; 95% CI: 1.38–18.49, p = 0.01, respectively), were significantly associated with day 42 negative serology (≤50 AU/mL) or low (<300 AU/mL) serum anti-S IgG levels.
Chemotherapy as the last treatment received was not retained in the model for the 50 AU/mL cut-point. However, at 300 AU/mL cutoff, chemotherapy as the last treatment received (aOR = 2.55, 95% CI: 0.90–7.28, p = 0.08), although close to significance, failed to predict a lower probability of seroconversion in patients without COVID-19 history, whereas it did predict such lack of seroconversion in the whole series of patients (Supplementary Table 1 D) (aOR = 3.14, 95% CI: 1.08–9.13, p = 0.03).
Third Vaccine Dose
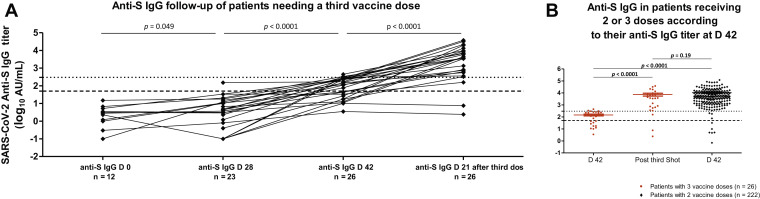
Serial serologic tests were performed in 10 patients exhibiting low antibody titers postvaccine boosting (≤300 AU/mL). Such cutoff for proposing a third vaccine dose was chosen on the basis of the large Israeli study, which reported that lower anti-S titers were associated with significantly more breakthrough infections in vaccine recipients. In seven patients, the antibody titers decreased over time (n = 6) or remained stable (n = 1), within a 13 to 52-day period, whereas the three others displayed a slight increase over 300 AU/mL within 59 days after the second dose. Two patients still presented less than 1066 AU/mL at 53, and 47 days after the booster dose, remaining at less than the 25th percentile. Seven of these patients were still receiving chemotherapy-based treatment (n = 5) or corticosteroids (n = 2), with three receiving neither chemotherapy nor corticosteroids.
On day 42, 30 patients exhibiting anti-S IgG titers of at least 300 AU/mL were proposed a third vaccine from day 28 after the second shot. Of these, two experienced cancer-related condition deterioration; therefore, they underwent no serology control after the third injection. A serology assay was available beyond day 21 after the third injection in 26/30 remaining (results still awaited for two). At the time of analysis, none of these patients displayed symptomatic COVID-19. In 26 patients (86.7%) with serologic tests available at day 28 after the third shot (Fig. 3 A), 19 (73%) exhibited a dramatic rise in anti-S IgG titers, exceeding 3500 AU/mL, whereas four (15.4%) displayed a moderate increase beyond 300 AU/mL cut-point but less than 1000 AU/mL (Fig. 3 B). Therefore, 88.5% exhibited seroconversion. In all, persistently negative anti-N IgG was found, excluding any recent SARS-CoV-2 infection. Among these, three had been receiving corticosteroids for several weeks, which were continued at the third vaccination. Only three patients did not respond to the third vaccination, being either totally negative (n = 2, <50/AU/mL) or exhibiting low anti-S antibodies. Among them was a 92-year-old patient (still <50 AU/mL after the second booster), on monthly azacytidine for chronic myelomonocytic leukemia type 2, with a complete molecular response. The two other patients, displaying 47 and 157 AU/mL, respectively, were treated using either chemoimmunotherapy or ICI alone. These two latter patients, aged 87 and 65 years, had hematologic conditions (hypogammaglobulinemia, monoclonal IgG peak), possibly explaining their poor immunization response.
Figure 3.
(A) Evolution from D 0 of anti-S IgG antibody titers after a third vaccine dose injection in 26 patients with titers below 300 AU/mL on sampling after the second dose. The lower dashed line along the x axis indicates the limit of detection (positivity cutoff) provided by the manufacturer (log10 50 AU/mL). The upper dashed line along the x axis indicates log10 300 AU/mL cutoff. Statistical comparison used Mann-Whiney U test. (B) Comparison of anti-S IgG antibody titers at D 42 with titers after the third vaccine dose in 26 patients and with D 42 titers of 222 patients who received two doses. Statistical comparison used Mann-Whiney U test. Anti-S, anti-SARS-CoV-2 anti-spike antibody; AU, arbitrary units; COVID-19, coronavirus disease 2019; D, day; IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
COVID-19 vaccines were made available in France in January 2021. Nothing was known on COVID-19 vaccination efficacy in patients with poor immune conditions, including patients with metastatic lung cancer, especially those under systemic corticosteroids or cytotoxic chemotherapy. Though patients with lung cancer were reported to have a high risk of COVID-19–related mortality in published series, lethality systematically exceeding 30% of infected patients,1 , 3 , 29 we observed only eight mild COVID-19 cases among our 306 vaccinated patients (2.6%). Such observation strongly supports the efficacy of mRNA COVID-19 vaccines used in 98.4% of our population. The patient acceptance rate of systematic vaccination was in line with previous reports, with only 11% initial refusals.30 Reactogenicity was weak, without short-term serious adverse effects in this real-life setting. We did not observe specific safety concerns in ICI-treated patients, especially regarding immune-related adverse effects, as reported by Israeli teams.31 Moreover, our study emphasized that seroconversion monitoring could be useful in immunosuppressed patients. In this population, the efficacy of the first vaccine dose was much lower than that reported in vaccine registration trials, with one-third of patients displaying negative serologic testing (≤50 AU/mL) at day 28, whereas three-quarters exhibited less than 25th percentile serologic titer distribution. These data are in line with the results from prospective studies involving a mixed population with solid cancers and hematologic malignancies.32 , 33 Although there has been no clear cutoff for antibody titers predicting protection against severe COVID-19, a 300 AU/mL cutoff was presented here to correlate well with the pseudoneutralization assay as a readout for antiviral efficacy. Similarly, a recent Israeli study reported, on the basis of 5141 vaccine recipients, that such value was able to discriminate between a 2.3% risk of breakthrough SARS-CoV-2 infection (for people with lower titers) and a low risk of 0.2% (for vaccinated people with higher titers), 6 months after the vaccine injections.28 We, thus, selected this value as protection cutoff against SARS-CoV-2 infection in our patients.34 Let us keep in mind that the recently described delta strain (one of the variant strains of concern, which currently represents more than 90% of sequenced viral isolates in France35) was reported to be 40% to 80% more transmissible than the alpha strain36 , 37; it is a viral burden being 1000-fold higher than other strains.38 It is, thus, crucial to define serologic correlates confirming the protection of immunocompromised patients. Although a strong relationship between mean neutralization levels and reported protection was evidenced in a recent meta-analysis,34 Nabs are not the only described correlates for protection against viral infection because specific anti–SARS-CoV-2 memory T and B-cells have also been reported to play an important role.39 However, several authors described waning specific T-cell immunity (specifically against the delta variant strains of concern) in parallel to humoral immunity waning over time, which especially occurred in elderly people.40
With this in mind, our study provided strong evidence for keeping the initially established intervals between two vaccine shots for patients with cancer. These patients displayed a delay in their immunization process, with lower levels of protective circulating vaccination-induced antibodies versus healthy vaccinated controls. Conversely, a reassuring observation has been the booster injection’s remarkable efficacy, with only 6.0% of thoracic patients with cancer still displaying negative serology at day 42, whereas only a certain percentage exhibited antibody titers of at least 300 AU/mL. The two characteristics independently associated with poor immunization, irrespective of the cut-point chosen, were age and long-term corticosteroid use. Concerning age, a lower immunization rate was identified in octogenarians, along with a 5% decreased probability per year to reach protective immunization. Regarding long-term corticosteroid use, the lower immunization may probably be explained by either lower total T-cell and CD4+ T-lymphocyte counts or T-cell–specific responses to spike protein. The adverse impact of age on the ability to induce vaccine-related protective humoral responses was already highlighted in studies involving octogenarians.40, 41, 42 While clearly delaying the immunization process as previously reported,32 , 43 , 44 cytotoxic chemotherapy was also associated with higher low-immunization rates at day 42 as well.
The limitations of our study are threefold. First, although this report involves, to the best of our knowledge, the largest series of patients with thoracic cancer receiving anti–SARS-CoV-2 mRNA vaccines that have been published to date, the sample size of the different patient subsets remains limited. Indeed, a limited number of patients accepted larger blood samplings for assessing immune correlates, thereby preventing the predictive analysis of specific T-cell responses at day 42 owing to a lack of statistical power.
Second, a possible limitation to the outcome of breakthrough infection in our vaccinated patients was accounted for by the decrease in SARS-CoV-2 virus circulation to less than 2000 new cases in France by mid-June 2021, versus 38,000 new cases in mid-March 2021. However, a dramatic rise in infections occurred again in late July, resulting in greater than 20,000 new daily cases until the end of August 2021; although there was a decline thereafter, we did not observe more symptomatic infections during this summer period.
Third, because of the observational study design, we did not perform systematic recurrent rhinopharyngeal swabs for SARS-CoV-2 molecular diagnosis. Therefore, we possibly did not capture the asymptomatic infection events. Nevertheless, we did not observe anti-N seroconversion events during the follow-up period until September 2021. On the basis of this, we believe that we did not miss a large body of such asymptomatic infection events.
As detailed herein, a third vaccine could contribute to appropriate seroprotection in patients still poorly immunized after the administration of two vaccine doses. Overall, 92% of patients were found to benefit from a third shot, reflected by substantial increases in anti-S IgG antibodies, with only very few patients left without sufficient protection. A limitation to this observation is the group’s small sample size. Such outcomes must be confirmed in larger-scale studies involving patients with solid tumors. Notably, a third vaccine dose is still being debated in patients with hematologic malignancies or solid organ transplantation.45, 46, 47, 48, 49, 50 This latter statement is supported by the fact that three of our patients with negative serology after third vaccine dose administration were, indeed, suffering from underlying hematologic conditions. Consequently, patients with lymphocyte function defects because of lymphoid cancers or lymphocyte-depleting treatments47 may exhibit lower benefits from a third vaccination dose.
CRediT Authorship Contribution Statement
Gérard Zalcman, Valérie Gounant, Diane Descamps, Luis Teixeira;
supervision: Gérard Zalcman, Valérie Gounant: Conceptualization.
Gérard Zalcman, Céline Namour, Zohra Brouk, Ghassen Soussi, Valérie Gounant, Valentine Marie Ferré: Data curation.
Gérard Zalcman, Ghassen Soussi, Valérie Gounant, Eric Vicaut, Valentine Marie Ferré: Formal analysis.
Gérard Zalcman, Luis Teixeira, Héloïse Flament, Diane Descamps, Eric Vicaut: Funding acquisition.
Gérard Zalcman, Valérie Gounant, Ghassen Soussi, Sandra Assoun, Alexandra Bizot, Céline Namour, Zohra Brouk: Investigation.
Valentine Marie Ferré, Charlotte Charpentier, Héloïse Flament, Nadhira Fidouh, Gilles Collin, Diane Descamps: Biological analyses.
Ghassen Soussi, Eric Vicaut: Statistical analyses.
Gérard Zalcman; Writing - original draft.
Gérard Zalcman, Valérie Gounant, Ghassen Soussi, Valentine Marie Ferré, Héloïse Flament, Diane Descamps, Eric Vicaut: Writing - review & editing.
Valérie Gounant, Valentine Marie Ferré, Ghassen Soussi, Charlotte Charpentier, Héloïse Flament, Nadhira Fidouh, Gilles Collin, Céline Namour, Sandra Assoun, Alexandra Bizot, Zohra Brouk, Eric Vicaut, Luis Teixeira, Diane Descamps, Gérard Zalcman: Final MS approbation.
Acknowledgments
The academic authors retained editorial control. The Assistance Publique-Hôpitaux de Paris funded the study without participating to study design, data collection, data analysis, data interpretation, or report writing.
Footnotes
Drs. Gounant and Ferré contributed equally to this work.
Disclosure: Dr. Zalcman reports receiving research grants from Roche-France, Bristol-Myers Squibb, and Takeda outside of the submitted work; perceived fees from Bristol-Myers Squibb, AstraZeneca, Pfizer, and Boehringer Ingelheim outside the submitted work; and reimbursement for international meetings assistance from AbbVie, Merck Sharp & Dohme, AstraZeneca, Bristol-Myers Squibb, and Roche-France. Dr. Gounant reports receiving personal fees from Merck Sharp & Dohme, Chugai, Novartis, and Boehringer Ingelheim; personal fees and nonfinancial support from AstraZeneca, Bristol-Myers Squibb, Takeda, and Pfizer; and grants, personal fees, and nonfinancial support from Roche all outside of the submitted work. Dr. Descamps reports receiving fees from Viiv-healthcare, Gilead-Sciences, and Janssen-Cilag outside of the submitted work. The remaining authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2021.10.015.
Supplementary Data
:
References
- 1.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid patients with cancer and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whisenant J., Trama A., Torri V., et al. TERAVOLT: thoracic cancers international COVID-19 collaboration. Cancer Cell. 2020;37:742–745. doi: 10.1016/j.ccell.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garassino M., Whisenant J., Huang L., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang K., Sheng Y., Huang C., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Levin A., Hanage W., Owusu-Boaitey N., Cochran K., Walsh S., Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35:1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerowitz-Katz G., Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mi Y., Huang T., Zhang J., et al. Estimating the instant case fatality rate of COVID-19 in China. Int J Infect Dis. 2020;97:1–6. doi: 10.1016/j.ijid.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins R., Charles E., Mehaffey J. Socio-economic status and COVID-19-related cases and fatalities. Public Health. 2020;189:29–134. doi: 10.1016/j.puhe.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rondy M., Larrauri A., Casado I., et al. 2015/15 seasonal vaccine effectiveness against hospitalisation with influenza A(H1N1)pdm09 and B among elderly people in Europe: results from the I-MOVE+ project. Euro Surveill. 2017;22:30580. doi: 10.2807/1560-7917.ES.2017.22.30.30580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hottinger A., George A.C., Bel M., et al. A prospective study of the factors shaping antibody responses to the AS03-adjuvanted influenza A/H1N1 vaccine in cancer outpatients. Oncologist. 2012;17:436–445. doi: 10.1634/theoncologist.2011-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck C., McKenzie B., Hashim A., Harris R.C. University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group, Nguyen-Van-Tam JS. Harris R and University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group N-V-T, JS. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis. 2012;206:1250–1259. doi: 10.1093/infdis/jis487. [DOI] [PubMed] [Google Scholar]
- 15.Bersanelli M., Giannarelli D., De Giorgi U., et al. INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: a multicenter prospective observational study (INVIDIa-2) J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack F., Thomas S., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L., Anderson E., Rouphael N., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baden L., El Sahly H., Essin B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voysey M., Clemens S., Madhi S., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voysey M., Costa Clemens S., Madhi S., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milman O., Yelin I., Aharony N., et al. Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals. Nat Med. 2021;27:1367–1369. doi: 10.1038/s41591-021-01407-5. [DOI] [PubMed] [Google Scholar]
- 23.Vasileiou E., Simpson C., Shi T., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widge A., Rouphael N., Jackson L., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2020;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson J., Sewell H., Stewart M. Delayed second dose of the BNT162b2 vaccine: innovation or misguided conjecture? Lancet. 2021;397:879–880. doi: 10.1016/S0140-6736(21)00455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadire S., Wachter R., Lurie N. Delayed Second Dose versus Standard Regimen for COVID-19 Vaccination. N Engl J Med. 2021;384:e28. doi: 10.1056/NEJMclde2101987. [DOI] [PubMed] [Google Scholar]
- 27.Castells M., Phillips E. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kertes J., Baruch Gez S., Saciuk Y., et al. Effectiveness of the mRNA BNT162b2 vaccine six months after vaccination: findings from a large 7 Israeli HMO. medRxiv. https://www.medrxiv.org/content/10.1101/2021.09.01.21262957v1 Accessed September 15, 2021.
- 29.Luo J., Rizvi H., Preeshagul I., et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Noia V., Renna D., Barberi V., et al. The first report on coronavirus disease 2019 (COVID-19) vaccine refusal by patients with solid cancer in Italy: early data from a single-institute survey. Eur J Cancer. 2021;153:260.e4. doi: 10.1016/j.ejca.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Addeo A., Shah P., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1–8. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palich R., Veyry M., Marot S., et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32:1051–1053. doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury D., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 35.Alizon S., Haim-Boukobza S., Foulongne V., et al. Rapid spread of the SARS-CoV-2 Delta variant in some French regions, June 2021. Euro Surveill. 2021;26:2100573. doi: 10.2807/1560-7917.ES.2021.26.28.2100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies N., Abbott S., Barnard R., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones T., Biele G., Mühlemann B., et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021;373:eabi5273. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidd M., Richter A., Best A., et al. S variant SARS CoV 2 lineage B1.1.7 is associated with significantly higher viral loads in samples tested by Thermo Fisher TaqPath RT qPCR. J Infect Dis. 2021;223:1666–1670. doi: 10.1093/infdis/jiab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner J., O’Halloran J., Kalaidina E., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tober-Lau P., Schwarz T., Vanshylla K., et al. Long-term immunogenicity of BNT162b2 vaccination in the elderly and in younger health care workers. Lancet Respir Med. 2021;9:E104–E105. doi: 10.1016/S2213-2600(21)00456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier D.A., Ferreira I.A.T.M., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iacono D., Cerbone L., Palombi L., et al. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021;12:1253–1255. doi: 10.1016/j.jgo.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barriere J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavriatopoulou M., Terpos E., Kastritis E., et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med. 2021;234:1–5. doi: 10.1007/s10238-021-00746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyarsky B., Werbel W., Avery R., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim S., Campbell N., Johnson M., et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8:e542–e544. doi: 10.1016/S2352-3026(21)00199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herishanu Y., Avivi I., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic Lymphocyti leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Oekelen O., Gleason C., Agte S., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
: