Highlights
-
•
Ultrasonic activity was investigated in polar and nonpolar organic solvents.
-
•
Ultrasonic/mechanical soil washing for PCBs removal was also investigated.
-
•
Ultrasonic washing had higher washing efficiency compared to conventional process.
-
•
Degradation of PCBs in the liquid phase was investigated in photolytic processes.
Abbreviations: PCBs, polychlorinated biphenyls; UVC, Ultraviolet C; TEF, toxic equivalency factor
Keywords: Polychlorinated biphenyls, Cavitation, Soil washing, Methanol, n-Hexane
Abstract
Ultrasonic soil washing processes using organic solvents were investigated for the development of novel remediation technologies for persistent organic pollutants (POPs)- contaminated soils. Aluminum foil erosion was first tested to understand sonophysical activity in water, methanol (polar) and n-hexane (nonpolar) in a 28 kHz double-bath-type sonoreactor. Significant sonophysical damage on the aluminum foil was observed at the antinodes for all solvents, and the order of degree of sonophysical damage was as follows: water > methanol > n-hexane. Subsequently, conventional (mechanical mixing only) and ultrasonic soil washing (mechanical mixing and ultrasound) techniques were compared for the removal of polychlorinated biphenyls (PCBs) from soil. Two types of contaminated soils, fresh (Soil A, C0 = 2.5 mg/kg) and weathered (Soil B, C0 = 0.5 mg/kg), were used and the applied soil-to-liquid (S:L) ratio was 1:5 and 1:10 for methanol and n-hexane, respectively. The polar solvent significantly increased washing efficiencies compared to the nonpolar solvent, despite the nonpolar nature of the PCBs. Washing efficiency was significantly enhanced in ultrasonic soil washing compared to conventional washing, owing to macro- and micro-scale sonophysical actions. The highest washing efficiencies of 90% for Soil A and 70% for Soil B were observed in the ultrasonic washing processes using methanol. Additionally, a single operation of the ultrasonic washing process was superior to two sequential processes with conventional mixing in terms of washing efficiency, consumption of washing agents, treatment of washing leachate, and operation time. Finally, the removal of PCBs in an organic solvent (methanol) was investigated in photolytic and sonolytic processes for the post-treatment of soil washing leachate. A photolysis efficiency of 80% was obtained within 60 min of UV exposure for intensities of 1.0, 2.0, and 4.0 W/cm2. The primary mechanism of PCBs degradation is photolytic dechlorination. In contrast, no degradation was detected in the sonolytic process, as the excess organic solvent acted as a strong radical scavenger.
1. Introduction
Contamination of soils and sediments with persistent organic pollutants (POPs) including dioxins and polychlorinated biphenyls (PCBs) has been reported for decades [1], [2], [3]. It is well known that POPs bioaccumulate through the food chain and are considered to be carcinogenic in humans [2], [3]. Novel remediation technologies, including thermal, biological, physical, and chemical treatments have been developed [3], [4], [5], [6] and a combination of thermal desorption (300–500 °C) and incineration (1,000–1,100 °C) has been used for rapid and complete treatment of POPs in large-scale remediation sites [7], [8], [9], [10]. In recent years, soil washing using organic solvents has been suggested as an alternative for the remediation of POPs-contaminated soils and sediments due to the high energy consumption and cost of thermal treatment [6], [11]. It has been reported that the treatment costs per m3 of contaminated soils are USD 44–252, USD 914–1,540, and USD 70–187 for thermal desorption, incineration, and soil washing, respectively [12]. Additionally, soil washing processes have high removal efficiency as a result of the high solubility of dioxins and PCBs in organic solvents [6].
In previous studies, various polar and nonpolar solvents including methanol [6], ethanol [13], [14], [15], acetone [16], isopropanol [17], and hexane [16] have been tested in soil washing processes for the remediation of dioxins and PCBs-contaminated soils and sediments. Some studies have also investigated the effect of natural solvents including fish oil [18], wine [19], and olive oil [20] on the removal of dioxins and PCBs from contaminated soils. It is generally known that the selection of an appropriate solvent depends on soil/sediment characteristics, including soil particle size and organic content, contaminant concentration, and the solubility of target organic pollutants in the solvents [4], [13], [15]. However, to the best of our knowledge, there is no rule of thumb for selecting organic solvents for the desorption of nonpolar pollutants from soils because contaminated soil systems are highly complicated and have different characteristics.
In recent years, the effectiveness of ultrasonic soil washing has been demonstrated for the remediation of soils contaminated with petroleum hydrocarbons and heavy metals, mainly using water-based washing agents, including pure water, surfactant solutions, and acidic solutions. The ultrasonic soil washing processes showed significantly higher removal efficiencies compared to the conventional soil washing processes [21], [22], [23], [24], [25]. This is attributed to ultrasonic desorption/extraction at the soil particle surface, as well as in particle pores and interiors via microscale sonophysical effects, including microjets, microstreaming, and shockwaves [21], [26], [27]. Since most studies have focused on acoustic cavitation phenomena and the resulting sonochemical/sonophysical effects in water and water-based solutions [28], [29], only a few studies have investigated ultrasonic soil washing processes using organic solvents [18], [19], [30]. Further research is necessary for the optimal design and operation of ultrasonic soil washing processes using organic solvents due to the lack of understanding of cavitation phenomena in organic solvents.
This study aimed to understand ultrasonic activity in organic solvents and the effect of ultrasound on organic solvent-based soil washing processes as a basic step towards designing an appropriate remediation technology for POPs-contaminated soils. PCBs were selected as the model soil pollutants in this study. A 28 kHz double bath sonoreactor and two commercial organic solvents, methanol (polar) and n-hexane (nonpolar), were used. Calorimetric energy and sonophysical activity were measured for the aforementioned solvents. The efficiency of PCBs removal from soils by conventional and ultrasonic soil washing processes using organic solvents was subsequently compared. Additionally, the degradation of separated PCBs in the liquid phase was examined using photolytic and sonolytic processes.
2. Experimental methods
2.1. Chemicals
Methanol (99.9 %, HPLC grade) and n-hexane (95.0 %, HPLC grade) were acquired from Samchun Chemical (KOR). Aroclor 1242 (1,000 μg/mL), Aroclor 1254 (1,000 μg/mL), and Aroclor 1260 (1,000 μg/mL) were acquired from Dr. Ehrenstorfer (UK).
2.2. Sonoreactor
A schematic of the double-bath-type ultrasonic system used in this study is shown in Fig. 1. It consisted of a rectangular acrylic sonoreactor (L × W × H: 25 cm × 25 cm × 21 cm), equipped with a 28 kHz ultrasonic transducer module (Mirae Ultrasonic Tech., KOR), an acrylic support plate with a circular hole at the center (L × W × H: 25 cm × 25 cm × 4 cm; D: 7 cm), and a 500 mL cylindrical glass vessel, which was submerged in the sonoreactor. The transducer module included nine transducers with a working power of 480 W. The sonoreactor was filled with 4 L of tap water, and fresh tap water was used for each experiment. An overhead stirrer with a Teflon-coated blade was used for mechanical mixing in the vessel [21], [25]. The mixing rate was 100 rpm.
Fig. 1.
A schematic of experimental setup used in this study.
2.3. Quantification of ultrasonic activity
The ultrasonic energy, termed as calorimetric power in this study, was calculated using the following calorimetric equation [21], [31]:
| (1) |
where Pcal is the ultrasonic/calorimetric energy; dT/dt is the rate of increase of the liquid temperature; Cp is the specific heat capacity of the liquid [water: 4.19 J/(g·K); methanol: 2.51 J/(g·K); n-hexane: 2.26 J/(g·K)]; and M is the mass of the liquid.
Aluminum foil erosion tests were conducted in water, methanol, and n-hexane. An aluminum foil in the rectangular frame was placed in the vessel perpendicular to the bottom of the vessel and then irradiated ultrasonically for 60, 120, and 180 s [24], [32], [33]. The frame was used to avoid moving of the foil by ultrasound and ultrasound-induced liquid flow. The foil was vertically trimmed from 1 cm above the bottom to the liquid surface. The volume of each solvent was 500 mL. The thickness of the aluminum foil was 16 μm. The damaged foil was dried, scanned, and visually analyzed.
2.4. PCBs contaminated soil
PCBs-contaminated soils were artificially prepared using sieved soil (less than2.000 mm) and PCBs solution, consisting of Aroclor chemicals (#1242 : #1254 : #1260 = 1 : 1 : 1) and n-hexane [34]. Basic physicochemical properties of the sieved soil are listed in Table 1. The mixture of the soil and PCBs solution was gently mixed and aged in a glass vessel for over three weeks. After aging, the solvent was gradually removed and homogeneously contaminated soils with a PCBs concentration of 2.5 mg/kg were obtained. PCBs-contaminated soils were artificially weathered by washing five times with tap water. The PCBs concentration in soils was 0.5 mg/kg after the weathering and drying processes. In this study, fresh PCBs-contaminated soil and weathered PCBs-contaminated soil were designated as “Soil A” and “Soil B,” respectively. The Korean strongest regulation level of PCBSs in soil is 1 mg/kg, according to the Soil Environment Conservation Act.
Table 1.
Physicochemical properties of the sieved soil used in this study.
| pH | Organic content(%) | CEC* (cmol/kg) |
Clay (%) |
Silt (%) |
Sand (%) |
Soil texture |
|---|---|---|---|---|---|---|
| 6.3 | 2.48 | 19.43 | 20.64 | 11.23 | 68.14 | Sandy Clay Loam |
*CEC: Cation exchange capacity
2.5. Soil washing tests
Conventional soil washing and ultrasound-assisted soil washing processes were compared using the PCBs-contaminated soils (Soil A and Soil B) [22], [25]. Two organic solvents, methanol and n-hexane, were selected as representative polar and nonpolar solvents and were used as washing agents considering the high persistence of PCBs in natural soils [35]. Methanol and n-hexane are solvents used for the extraction of dioxins and furans in the EPA method 1613 Revision B (1994). Soil-to-liquid (S:L) ratios (based on mass) of 1:5 and 1:10 were tested using 20 g of contaminated soil. For the conventional soil washing process, only mechanical mixing was applied to the slurry of the contaminated soil and organic solvent in the washing vessel, while ultrasound was additionally applied to the ultrasound-assisted soil washing process. Concentrations of PCBs in the soil were quantified using a gas chromatograph, equipped with an electron capture detector (NL/450 GC, Varian) according to the Korean standard method for soil pollution, considering the total peak area of 13 PCB congeners (#18, #28, #31, #44, #52, #101, #108, #138, #149, #153, #170, #180, #194) in the GC chromatogram [36].
2.6. Removal of PCBs in organic solvent
PCBs removal experiments were conducted in a photolytic process using a UVC (254 nm) lamp (TUV6W, Philips) and sonolytic process using a 20 kHz probe sonicator (VCX–750, Sonics & Materials Inc.). The PCBs solution, considered as artificial washing leachate in this study, consisted of Aroclor 1260 and methanol with an initial concentration of 0.5 mg/L. The concentration of PCBs in the liquid phase was quantified using the same gas chromatography system and the method described above.
3. Results and discussion
3.1. Ultrasonic activity in organic solvent
Ultrasonic power, also called calorimetric power, was compared for water, methanol, and n-hexane. The measured ultrasonic powers were 8.1 ± 1.3 W, 11.9 ± 2.0 W, and 11.7 ± 2.6 W and the temperature increase rates were 0.53 ℃/min, 1.13 ℃/min, and 1.37 ℃/min for water, methanol, and n-hexane, respectively. Because of lower specific heat ratios of organic solvents [water: 4.19 J/(g·K); methanol: 2.51 J/(g·K); n-hexane: 2.26 J/(g·K)], higher temperature increase rates and higher calorimetric powers were obtained for methanol and n-hexane. Toma et al. have measured ultrasonic power for water and various organic solvents with different specific heat ratios and have reported similar ultrasonic powers at 20 kHz and 500 kHz for all organic solvents investigated [37]. Additionally, they have checked the effect of vapor pressure, viscosity, and surface tension of organic solvents on the measured ultrasonic powers and have found no significant relationship between physical properties and the measured ultrasonic powers [37].
Sonophysical activity in solvents including water, methanol, and n-hexane was investigated using an aluminum foil erosion test. As shown in Fig. 2, different erosion patterns on the foil were recorded in water, methanol, and n-hexane. Dular and Osterman have summarized the cavitational erosion in the following three steps: 1) the formation of small deformations, such as shallow pits, 2) the accumulation of pits, such as a cluster of deep pits, and 3) material separation, such as the formation of holes in aluminum foil. As reported, the damage can be accelerated at sites where cavitational damage, such as pits and holes, are already formed. This is because the edge of the material acts as an amplifier of cavitation and sonophysical erosion [24], [38]. Erosion steps and related phenomena are shown in Fig. 2.
Fig. 2.
The cavitation-damaged aluminum foils for different solvents and irradiation times.
For water, cavitational damage to the foil, including a hole at the center of the foil and a cluster of pits around the hole were clearly observed. As the irradiation time elapsed, the hole expanded to the area where a cluster of pits previously existed. For organic solvents, including methanol and n-hexane, relatively typical damage patterns were observed. Less severe cavitational damage consisting mainly of the clusters of pits was recorded at the antinodes of the standing wave field [33], [38]. Although locations of most damages for the water case seemed to be related to the antinode position, it was difficult to recognize the form of the standing wave field in the foil images of water. Furthermore, the damaged area in both solvents seemed to be somewhat larger than that in water. Notably, cavitational erosion did not accumulate at the site where material separation occurred. The presence of a large hole at the center in the early stage of erosion might prevent more severe damage to the foil. Niemczewski have measured the cavitation intensity of water and 37 organic solvents, including methanol. He has reported that the highest intensity is obtained for water, and lower intensity levels (6–74%) were observed for organic solvents [39], [40]. Therefore, the order of degree of sonophysical damage was as follows: water > methanol > n-hexane. It should be noted that the order of the measured ultrasonic power was methanol (11.9 W) ≈ n-hexane (11.7 W) > water (8.1 W). The relationship between ultrasonic power and cavitational effects for different solvents requires further research.
Interestingly, damage that was more distinct was observed for methanol compared to n-hexane. Considering physicochemical properties of methanol and n-hexane, as shown in Table 2, cavitation events are more likely to be initiated in hexane than in methanol owing to lower viscosity, surface tension, and density [41], [42], [43], [44]. Brezhneva et al. have obtained very low cavitation intensities, which are quantified as measured sound pressures using a hydrophone system, in ethylene glycol compared to those in ethanol [44]. However, more effective cavitational effects via more vigorous implosion of the cavitation bubble can be obtained in a solvent with higher viscosity and surface tension once cavitation initiates [42]. This indicates that the applied ultrasonic power (or its sound pressure amplitudes) in the solution used in this study was sufficient to initiate cavitation events for both organic solvents, and more violent cavitational effects could be observed in methanol. This was confirmed by the fact that a noticeable damage was obtained in water with the highest density, viscosity, and surface tension among the three solvents in this study. Moreover, organic solvents can be favorable for initiating cavitation because of the higher solubility of gases in organic solvents than in water [44]. However, the higher vapor pressure of methanol and n-hexane can inevitably reduce the severity of the cavitational effects because of the cushioning effect [42], [43], [45]. Although the reasons for the difference in foil damage in water, methanol, and n-hexane were not clearly understood, it was expected that effective cavitational desorption would occur in the organic solvent-based ultrasonic soil washing process, and that higher ultrasonic desorption could be observed in methanol.
Table 2.
| Solvent | Density(g/mL) | Vapor pressure (Pa) |
Viscosity (mPa∙s) |
Surface tension (mN/m) |
Octanol-Water partition constant (log Kow) |
Dielectric constant (Permittivity)(ε) |
Sound speed (m/s) |
|---|---|---|---|---|---|---|---|
| Water | 1.00 | 103.50 | 0.890 | 72.06 | 80.1 (20 ℃) | 1,497 | |
| Methanol | 0.79 | 104.23 | 0.544 | 22.17 | −0.77 | 33.0 (20 ℃) | 1,116 (20 ℃) |
| n-Hexane | 0.66 | 104.30 | 0.300 | 17.88 | 4.00 | 1.89 (20 ℃) | 1,078 (20 ℃) |
3.2. Ultrasonic soil washing process
Effects of the organic solvents, S:L ratios, and ultrasound application on the desorption of PCBs in the soil washing processes were investigated for the two types of PCBs-contaminated soils (Soil A and Soil B), as shown in Fig. 3. Persistent organic pollutants, including PCBs and dioxins, are strongly adsorbed to organic matter in soil, and they are hardly desorbed from the soil using water because of their highly nonpolar nature. Based on the number and position of chlorine atoms in the two phenyl rings, 209 individual PCB congeners exist theoretically. PCBs with higher BZ numbers (more chlorine atoms) have higher Kow values. Some PCBs with 4 to 7 chlorine atoms are more toxic and considered as dioxin-like PCBs with toxicity equivalency factor ranging from 0.0003 to 0.1. The log Kow values of the 209 PCB congeners ranges from 4.46 to 8.18 [34], [48], [49]. In this study, PCBs desorption efficiencies of 3–12 % were observed in the soil washing processes using water. Removal of PCBs might be attributed to loss of fine particles, onto which organic pollutants are highly likely to adsorb, during the washing and soil/liquid separation processes [4].
Fig. 3.
Soil washing efficiencies in the conventional soil washing processes and ultrasonic soil washing processes using methanol and n-hexane for the S/L ratio of 1:5 and 1:10.
Generally, PCBs are readily soluble in nonpolar solvents. It was expected that n-hexane as a washing agent resulted in a higher washing efficiency than methanol. However, in this study, the use of methanol, a polar solvent, resulted in higher washing efficiencies than the use of n-hexane, a nonpolar solvent, in all cases. In the conventional washing processes (using only mechanical mixing), the washing efficiency of methanol increased by 20.5 % and 36.5 % (in terms of the average efficiency for both S:L ratios) for Soil A and Soil B, respectively, compared to that of n-hexane.
In previous studies, polar organic solvents and polar/nonpolar organic solvent mixtures have been suggested as optimal washing/extraction agents for the separation of PCBs from soils. Takigami et al. have selected isopropyl alcohol (polar; ε: 20.18 at 20 ℃) and obtained a PCBs extraction efficiency of 98.6 % after 10 repeated extraction processes [17]. Nam et al. have reported a high removal efficiency of over 90 % using a mixture of alkane (nonpolar) and alcohol (polar) from PCBs, polychlorinated dibenzodioxins, and polycyclic aromatic hydrocarbon-contaminated soils [50]. Majid et al. have compared n-hexane (nonpolar; ε: 1.89 at 20 ℃) and n-hexane/acetone (polar; ε: 21.01 at 20 °C) mixtures, and higher extraction efficiency was obtained using the mixture of n-hexane/acetone for Aroclor 1016 contaminated soil [16]. Kimbrough et al. have demonstrated that polar solvents were superior to nonpolar solvents in both Soxhlet extraction and ultrasonic extraction for PCBs removal from contaminated soils [51].
The use of polar organic solvents or polar/nonpolar organic solvent mixtures rather than nonpolar organic solvents for the separation of highly persistent organic pollutants such as PCBs from soils can be controversial, considering the extremely high nonpolar nature of these pollutants. To the best of our knowledge, no basic rules for selecting organic solvents in soil washing for the remediation of nonpolar pollutants such as PCBs and dioxins have been suggested owing to highly complicated soil systems and pollutants characteristics. However, some studies have provided clues for the necessity of polar solvents—1) soil, such as clay and soil organic matter, can be negatively charged; 2) the attraction for water increases as the size of soil particles decreases; 3) soil with higher organic content has higher water-holding capacity; and 4) water content in soil inhibits the contact between pollutants in soils and nonpolar solvents [4], [14], [51], [52], [53]. In this study, the water content in the soil was less than 0.15 %, and the proportion of clay was 20.6 % in the soil texture. Therefore, the reason for higher efficiency of pollutant removal by using methanol (polar solvent) in this study could be due to the presence of clay particles, which have large specific surface areas. Further research is required to understand the selection of appropriate solvents for the remediation of PCBs-contaminated soils.
The additional use of ultrasound in the conventional soil washing resulted in 13 %–20 % higher washing efficiencies for both n-hexane and methanol, as shown in Fig. 3. The highest washing efficiencies of over 90 % for Soil A and approximately 70 % for Soil B were obtained in the ultrasonic washing processes with methanol, because of the higher desorption ability and cavitational effects of the polar organic solvent as mentioned above with additional ultrasonic physical actions. Ultrasound could induce a more violent movement of soil particles in the washing liquid, and as a result, the collision and scrubbing among particles and between particles and the washing agent could increase at the macro scale. Moreover, the particles’ surfaces and pores underwent severe sonophysical actions, including microjets, shockwaves, and microstreaming via continuous cavitation events at the micro scale. Soil particle breakage into smaller particles could also occur via both macro- and micro-scale physical actions, and more surface area of particles could be exposed to these strong actions and washing agents. Consequently, pollutants that were strongly sorbed in deep pores, which were difficult to remove by conventional washing processes, could be removed along with achieving higher washing efficiencies using ultrasonic and cavitational actions [21], [22], [24], [25].
Notably, the macro-scale physical actions of ultrasound were very weak compared to those of mechanical mixing. No noticeable movement of soil particles was observed under only ultrasound irradiation (no mechanical mixing), and no significant desorption of pollutants was detected in the double bath system used in this study. This was because severe attenuation of ultrasound energy occurred in the layer packed with the soil particles at the vessel bottom when the ultrasound was irradiated upwards from the bottom of the sonoreactor. No significant damage to the aluminum foil placed above the soil layer was observed for water, methanol, and n-hexane under ultrasound irradiation only in this study. Therefore, ultrasonic and cavitational actions of soil particles could be significantly enhanced when particles were suspended in the liquid by mechanical mixing, and the space between particles was large enough for ultrasound to transmit in the less-packed slurry phase with less attenuation.
To further investigate the washing ability of methanol and n-hexane, two sequential washes were applied to Soil A (the S:L ratio was 1:5.) using a single solvent (methanol or n-hexane) and dual solvents (1st: methanol → 2nd: n-hexane or 1st: n-hexane → 2nd: methanol), as shown in Fig. 4. Noticeably, the polar solvent, methanol, played a major role in the desorption of PCBs from the soil compared to the nonpolar solvent n-hexane. No significant increase in the washing efficiency was observed after washing twice with n-hexane compared to the single washing with n-hexane alone. However, the washing efficiency increased by 13 % in sequential washing compared to a single wash with methanol, and the consecutive application of polar and nonpolar solvents enhanced the washing efficiency regardless of the order of methanol use in the sequential washing.
Fig. 4.
Comparison of soil washing efficiencies during one and two washes in the conventional soil washing processes. The S:L ratio is 1:5.
Interestingly, washing efficiencies of sequential conventional washing processes were lower than that of the single ultrasonic washing process when using methanol. This indicated that the ultrasonic washing processes were remarkably profitable in terms of the consumption of washing agents, the treatment of washing leachate, and the operation time compared to conventional washing processes [24], [25]. In our previous research on diesel-contaminated soils, the ultrasonic washing process resulted in a diesel removal efficiency of 75 %, which was obtained only after four sequential washings in the conventional washing process [24]. It has also been revealed that a larger enhancement induced by ultrasound was obtained under less washing favorable conditions for the removal of heavy metals from soils [25].
Following soil washing, the soils and washing liquid were separated, and the residual organic solvents in the soil could be removed by volatilization at relatively moderate temperature [54]. Although small amounts of PCBs were detected in the residual solvents in soil following the separation, most PCBs could be removed. If necessary, repeated soil washing can be done to meet the regulation levels.
3.3. Photolytic and sonolytic removal of PCBs
The removal of PCBs in the liquid phase was investigated to consider a post-washing leachate treatment system using photolytic (UVC) and sonolytic (20 kHz) processes. In this study, no significant removal of PCBs occurred during the sonolytic process. This might be due to an excess presence of the volatile solvent methanol, which could induce the cushioning effect as mentioned above and scavenge most of the generated radical species. Büttner et al. have compared the generation of main and minor sonochemical reaction products for various ratios of a water-methanol mixture and have reported that no sonochemical reaction is detected for the mixture conditions with over 80 % methanol [55]. Besides this, they have also mentioned that the temperature inside the cavitation bubble may decrease significantly as the methanol content increases [55]. Zhang and Hua have investigated the cavitational removal of PCBs in the aqueous phase using a 20 kHz sonication system. They have found that the removal follows pseudo-first-order reaction kinetics and the main removal mechanism is dechlorination [56].
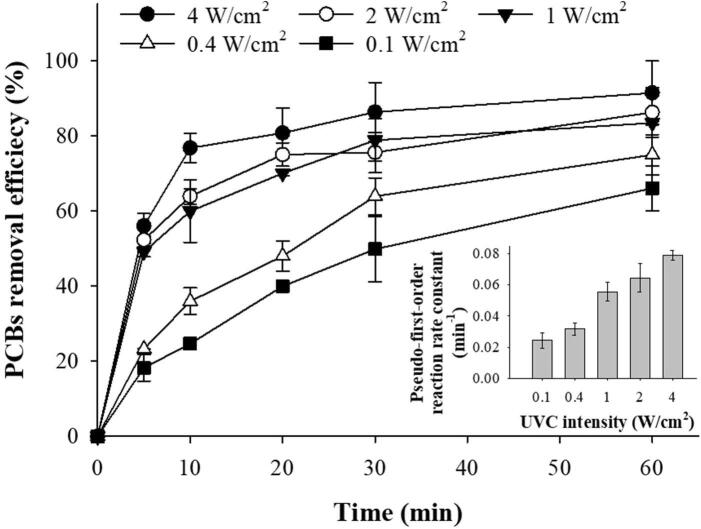
In the photolytic process, remarkable PCBs removal was observed at various intensities as shown in Fig. 5. A removal efficiency of over 80 % was obtained within 60 min of UVC irradiation for 1.0. 2.0 and 4.0 W/cm2. The average pseudo-first-order reaction rate constants were 0.0244, 0.0316, 0.0555, 0.0643, and 0.0788/min for 0.1, 0.4, 1.0, 2.0, and 4.0 W/cm2, respectively. The main removal mechanism by UV irradiation was suggested to be dechlorination, and it was revealed that the separation of chlorine atoms from PCBs occurred in the following order: ortho > meta > para [57], [58], [59]. In this study, the dechlorination of PCBs was also observed qualitatively and quantitatively using gas chromatography-mass spectrometry. The area of higher chlorinated congeners with 5–7 chlorines decreased drastically, while the area of lower chlorinated congeners with 2–4 chlorines increased gradually as the irradiation time elapsed (data not shown). Baron et al. have tested photolytic removal of toxic dioxin-like PCBs including # 77, #81, #126, #169, #105, #114, #118, #123, #158, #157, #167, and #189. They have revealed an increase in the toxic equivalency concentration induced by the dechlorination of PCBs with 5 – 7 chlorine atoms. The concentrations of PCBs (#105, #118, #123, #158, #157, #167, and #189) with more chlorines and lower toxic equivalency factor (TEF) values decreased, while the concentration of PCBs (#77, #81, #126, and #169) with less chlorine and higher TEF value increased after 5 days of UV irradiation [60]. In this study, no dioxin-like PCBs were detected in the photolytic process using Aroclor 1260. The concentration of dioxin-like PCBs was measured using a high-resolution gas chromatography/high-resolution mass spectrometer. Therefore, the photolytic process was suggested as a post-washing leachate treatment process.
Fig. 5.
Photolytic removal of PCBs (Aroclor 1260) in methanol under various UVC (λ = 254 nm) intensities. The inset represents the average pseudo-first-order reaction rate constants for various UVC intensities.
4. Conclusion
The effect of ultrasound on soil washing processes using organic solvents, was investigated in a 28 kHz double bath-type sonoreactor for the development of novel remediation technology for PCBs-contaminated soils. Sonophysical damage in the aluminum foil was found to be more severe in methanol than n-hexane. PCBs removal from soils was found to be significantly higher with ultrasonic soil washing than conventional soil washing, owing to macro- and micro-scale ultrasonic and cavitational actions. The highest washing efficiencies of 90% for freshly contaminated soil (C0 = 2.5 mg/L) and approximately 70% for weathered contaminated soil (C0 = 0.5 mg/L) were obtained with the ultrasonic washing processes that used methanol. It was found that ultrasonic soil washing was more effective than conventional soil washing in terms of washing efficiency, consumption of washing agents, treatment of washing leachate, and operation time. Photolytic and sonolytic processes, such as the post-treatment of washing leachate consisting of organic solvent and desorbed PCBs from soils, were investigated. A high removal efficiency of 80 % was obtained for 60 min in photolytic processes via photolytic dechlorination, while no significant removal was observed in the sonolytic processes due to the presence of excess organic solvent molecules. Therefore, it was concluded that ultrasonic soil washing could be considered a promising alternative to conventional thermal treatment technologies for the remediation of POPs-contaminated soils and sediments. Further investigation of large-scale ultrasonic soil washing using real POPs-contaminated soils under various soil, environmental and operating conditions is therefore necessary.
CRediT authorship contribution statement
Dukyoung Lee: Methodology, Investigation, Validation, Writing – original draft. Younggyu Son: Conceptualization, Methodology, Writing – review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Research Foundation of Korea [NRF-2021R1A2C1005470], and by the Korea Ministry of Environment (MOE) as “Subsurface Environment Management (SEM)” Program [project No. 2021002470001].
References
- 1.Naile J.E., Khim J.S., Wang T., Wan Y.i., Luo W., Hu W., Jiao W., Park J., Ryu J., Hong S., Jones P.D., Lu Y., Giesy J.P. Sources and distribution of polychlorinated-dibenzo-p-dioxins and -dibenzofurans in soil and sediment from the Yellow Sea region of China and Korea. Environ. Pollut. 2011;159(4):907–917. doi: 10.1016/j.envpol.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Kim E.-K., Barghi M., Choi M., Moon H.-B. Spatial and temporal trends of PCDD/Fs in sediment and bivalves along the Korean coasts during 2001–2012. Mar. Pollut. Bull. 2019;146:183–189. doi: 10.1016/j.marpolbul.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Jing R., Fusi S., Kjellerup B.V. Remediation of Polychlorinated Biphenyls (PCBs) in Contaminated Soils and Sediment: State of Knowledge and Perspectives. Front. Environ. Sci. 2018;6 [Google Scholar]
- 4.Guemiza K., Coudert L., Metahni S., Mercier G., Besner S., Blais J.-F. Treatment technologies used for the removal of As, Cr, Cu, PCP and/or PCDD/F from contaminated soil: A review. J. Hazard. Mater. 2017;333:194–214. doi: 10.1016/j.jhazmat.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Gomes H.I., Dias-Ferreira C., Ribeiro A.B. Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci. Total Environ. 2013;445-446:237–260. doi: 10.1016/j.scitotenv.2012.11.098. [DOI] [PubMed] [Google Scholar]
- 6.Tran H.T., Lin C., Hoang H.G., Bui X.T., Le V.G., Vu C.T. Soil washing for the remediation of dioxin-contaminated soil: A review. J. Hazard. Mater. 2022;421:126767. doi: 10.1016/j.jhazmat.2021.126767. [DOI] [PubMed] [Google Scholar]
- 7.Sau T.K., Truong N.X., Hanh T.T.T., Le Hung B., Thang N.D., T. Le Lan Anh, Ambient air monitoring around the dioxin remediation site in Da Nang, Vietnam, using passive air samplers. Environ. Monit. Assess. 2021;193:434. doi: 10.1007/s10661-021-09223-7. [DOI] [PubMed] [Google Scholar]
- 8.Rathna R., Varjani S., Nakkeeran E. Recent developments and prospects of dioxins and furans remediation. J. Environ. Manage. 2018;223:797–806. doi: 10.1016/j.jenvman.2018.06.095. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z., Ni M., Li X., Buekens A., Yan J. Combined mechanochemical and thermal treatment of PCBs contaminated soil. RSC Adv. 2017;7(34):21180–21186. [Google Scholar]
- 10.Qi Z., Chen T., Bai S., Yan M., Lu S., Buekens A., Yan J., Bulmău C., Li X. Effect of temperature and particle size on the thermal desorption of PCBs from contaminated soil. Environ. Sci. Pollut. Res. 2014;21(6):4697–4704. doi: 10.1007/s11356-013-2392-4. [DOI] [PubMed] [Google Scholar]
- 11.Trellu C., Mousset E., Pechaud Y., Huguenot D., van Hullebusch E.D., Esposito G., Oturan M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016;306:149–174. doi: 10.1016/j.jhazmat.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Cagnetta G., Huang J., Yu G. A mini-review on mechanochemical treatment of contaminated soil: From laboratory to large-scale. Crit. Rev. Environ. Sci. Tec. 2018;48(7-9):723–771. [Google Scholar]
- 13.Jonsson S., Lind H., Lundstedt S., Haglund P., Tysklind M. Dioxin removal from contaminated soils by ethanol washing. J. Hazard. Mater. 2010;179(1-3):393–399. doi: 10.1016/j.jhazmat.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Nakamiya K., Furuichi T., Ishii K. Evaluation of the optimal washing conditions for dioxin-contaminated soils from the circumference of an incinerator. J. Mater. Cycles Waste Manage. 2003;5(1):63–68. [Google Scholar]
- 15.Meguro H., Lee B.‐D., Nakai S., Hosomi M. Evaluation of ethanol washing on dioxins polluted soil and sediment based on adsorption relationships. Environ. Technol. 2008;29(3):325–332. doi: 10.1080/09593330802102132. [DOI] [PubMed] [Google Scholar]
- 16.Majid A., Argue S., Sparks B.D. Removal of Aroclor 1016 from contaminated soil by Solvent Extraction Soil Agglomeration Process. J. Environ. Eng. Sci. 2002;1(1):59–64. [Google Scholar]
- 17.Takigami H., Etoh T., Nishio T., Sakai S.-I. Chemical and bioassay monitoring of PCB-contaminated soil remediation using solvent extraction technology. J. Environ. Eng. Sci. 2008;10:198–205. doi: 10.1039/b715474g. [DOI] [PubMed] [Google Scholar]
- 18.Vu C.T., Lin C., Hung W., Huang W.-Y., Kaewlaoyoong A., Yotapukdee S., Chen J.R., Shen Y.-H. Ultrasonic Soil Washing with Fish Oil Extract to Remove Polychlorinated Dibenzo-p-dioxins (PCDDs), Dibenzofurans (PCDFs) from Highly Contaminated Field Soils. Water Air Soil Pollut. 2017;228:343. [Google Scholar]
- 19.Vu C.T., Tran H.T., Kaewlaoyoong A., Huang W.Y., Lin C. Efficacy of Indigenously Prepared Sugarcane and Pineapple Wine Solvents for Washing Highly Dioxin-Contaminated Field Soils. Appl. Sci. 2019;9:61. [Google Scholar]
- 20.Isosaari P., Tuhkanen T., Vartiainen T. Use of Olive Oil for Soil Extraction and Ultraviolet Degradation of Polychlorinated Dibenzo-p-dioxins and Dibenzofurans. Environ. Sci. Technol. 2001;35(6):1259–1265. doi: 10.1021/es000190d. [DOI] [PubMed] [Google Scholar]
- 21.Son Y., Lee D., Lee W., Park J., Hyoung Lee W., Ashokkumar M. Cavitational activity in heterogeneous systems containing fine particles. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Park B., Son Y. Ultrasonic and mechanical soil washing processes for the removal of heavy metals from soils. Ultrason. Sonochem. 2017;35:640–645. doi: 10.1016/j.ultsonch.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Son Y., Nam S., Ashokkumar M., Khim J. Comparison of energy consumptions between ultrasonic, mechanical, and combined soil washing processes. Ultrason. Sonochem. 2012;19(3):395–398. doi: 10.1016/j.ultsonch.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Son Y., Cha J., Lim M., Ashokkumar M., Khim J. Comparison of Ultrasonic and Conventional Mechanical Soil-Washing Processes for Diesel-Contaminated Sand. Ind. Eng. Chem. Res. 2011;50(4):2400–2407. [Google Scholar]
- 25.Choi J., Lee D., Son Y. Ultrasound-assisted soil washing processes for the remediation of heavy metals contaminated soils: The mechanism of the ultrasonic desorption. Ultrason. Sonochem. 2021;74:105574. doi: 10.1016/j.ultsonch.2021.105574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Effendi A.J., Wulandari M., Setiadi T. Ultrasonic application in contaminated soil remediation. Curr. Opin. Environ. Sci. Health. 2019;12:66–71. [Google Scholar]
- 27.Tao Y., Wu Y., Han Y., Chemat F., Li D., Show P.L. Insight into mass transfer during ultrasound-enhanced adsorption/desorption of blueberry anthocyanins on macroporous resins by numerical simulation considering ultrasonic influence on resin properties. Chem. Eng. J. 2020;380 [Google Scholar]
- 28.Yusof N.S.M., Babgi B., Alghamdi Y., Aksu M., Madhavan J., Ashokkumar M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016;29:568–576. doi: 10.1016/j.ultsonch.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Tran K.V.B., Kimura T., Kondo T., Koda S. Quantification of frequency dependence of mechanical effects induced by ultrasound. Ultrason. Sonochem. 2014;21(2):716–721. doi: 10.1016/j.ultsonch.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Hung W., Huang W.-Y., Lin C., Vu C.T., Yotapukdee S., Kaewlaoyoong A., Chen J.-R., Shen Y.-H. The use of ultrasound-assisted anaerobic compost tea washing to remove poly-chlorinated dibenzo-p-dioxins (PCDDs), dibenzo-furans (PCDFs) from highly contaminated field soils. Environ. Sci. Pollut. Res. 2017;24(23):18936–18945. doi: 10.1007/s11356-017-9517-0. [DOI] [PubMed] [Google Scholar]
- 31.Son Y. Simple design strategy for bath-type high-frequency sonoreactors. Chem. Eng. J. 2017;328:654–664. [Google Scholar]
- 32.Thanh Nguyen T., Asakura Y., Koda S., Yasuda K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrason. Sonochem. 2017;39:301–306. doi: 10.1016/j.ultsonch.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Khuyen Viet Bao T., Yoshiyuki A., Shinobu K. Influence of Liquid Height on Mechanical and Chemical Effects in 20 kHz Sonication. Jpn. J. Appl. Phys. 2013;52:07HE07. [Google Scholar]
- 34.Polychlorinated biphenyls and polybrominated biphenyls: IARC monographs on the evaluation of carcinogenic risks to humans, International Agency for Research on Cancer, Lyon, France, 2016. [PMC free article] [PubMed]
- 35.Ayris S., Harrad S. The fate and persistence of polychlorinated biphenyls in soil. J. Environ. Monit. 1999;1(4):395–401. doi: 10.1039/a903017d. [DOI] [PubMed] [Google Scholar]
- 36.Tam N.F.Y., Yao M.W.Y. Concentrations of PCBs in coastal mangrove sediments of Hong Kong. Mar. Pollut. Bull. 2002;44(7):642–651. doi: 10.1016/s0025-326x(01)00306-x. [DOI] [PubMed] [Google Scholar]
- 37.Toma M., Fukutomi S., Asakura Y., Koda S. A calorimetric study of energy conversion efficiency of a sonochemical reactor at 500 kHz for organic solvents. Ultrason. Sonochem. 2011;18:197–208. doi: 10.1016/j.ultsonch.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Dular M., Osterman A. Pit clustering in cavitation erosion. Wear. 2008;265:811–820. [Google Scholar]
- 39.Niemczewski B. A comparison of ultrasonic cavitation intensity in liquids. Ultrasonics. 1980;18:107–110. [Google Scholar]
- 40.Mason T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016;29:519–523. doi: 10.1016/j.ultsonch.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 42.Mason T.J., Lorimer J.P. Wiley-VCH Verlag GmbH; Weinheim: 2002. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing. [Google Scholar]
- 43.Löning J.-M., Horst C., Hoffmann U. Investigations on the energy conversion in sonochemical processes. Ultrason. Sonochem. 2002;9(3):169–179. doi: 10.1016/s1350-4177(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 44.Brezhneva N., Dezhkunov N.V., Ulasevich S.A., Skorb E.V. Characterization of transient cavitation activity during sonochemical modification of magnesium particles. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enomoto N., Okitsu K. In: Sonochemistry and the Acoustic Bubble. Grieser F., Choi P.K., Enomoto N., Harada H., Okitsu K., Yasui K., editors. Elsevier; Amsterdam: 2015. Chapter 8 - Application of Ultrasound in Inorganic Synthesis; pp. 187–206. [Google Scholar]
- 46.Haynes W.M., Lide D.R., Bruno T.J. 97th ed., CRC Press; Florida: 2016. CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. 2016–2017. [Google Scholar]
- 47.René P.M.G., Schwarzenbach P., Imboden D.M. third ed. John Wiley & Sons Inc; New Jersey: 2016. Environmental Organic Chemistry. [Google Scholar]
- 48.Ballschmiter K., Zell M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography, Fresenius' Zeitschrift für analytische. Chemie. 1980;302:20–31. [Google Scholar]
- 49.Hawker D.W., Connell D.W. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environ. Sci. Technol. 1988;22(4):382–387. [Google Scholar]
- 50.Nam P., Kapila S., Liu Q., Tumiatti W., Porciani A., Flanigan V. Solvent extraction and tandem dechlorination for decontamination of soil. Chemosphere. 2001;43:485–491. doi: 10.1016/s0045-6535(00)00398-2. [DOI] [PubMed] [Google Scholar]
- 51.Kimbrough D.E., Chin R., Wakakuwa J. Wide-spread and systematic errors in the analysis of soils for polychlorinated biphenyls. Part 2. Comparison of extraction systems. Analyst. 1994;119:1283–1292. [Google Scholar]
- 52.Nardella A., Massetti F., Sisto R., Tomaciello R. Clean-up of polluted wet soils by solvent extraction. Environ. Prog. 1999;18(4):243–249. [Google Scholar]
- 53.Brady N.C., Weil R.R. 14th ed., Prentice Hall; New Jersey: 2008. The Nature and Properties of Soils. [Google Scholar]
- 54.Wu G., Li X., Coulon F., Li H., Lian J., Sui H. Recycling of solvent used in a solvent extraction of petroleum hydrocarbons contaminated soil. J. Hazard. Mater. 2011;186(1):533–539. doi: 10.1016/j.jhazmat.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 55.Buettner J., Gutierrez M., Henglein A. Sonolysis of water-methanol mixtures. J. Phys. Chem. 1991;95(4):1528–1530. [Google Scholar]
- 56.Zhang G., Hua I. Cavitation Chemistry of Polychlorinated Biphenyls: Decomposition Mechanisms and Rates. Environ. Sci. Technol. 2000;34:1529–1534. [Google Scholar]
- 57.Miao X.-S., Chu S.-G., Xu X.-B. Degradation pathways of PCBs upon UV irradiation in hexane. Chemosphere. 1999;39:1639–1650. doi: 10.1016/s0045-6535(99)00062-4. [DOI] [PubMed] [Google Scholar]
- 58.Chang F.-C., Chiu T.-C., Yen J.-H., Wang Y.-S. Dechlorination pathways of ortho-substituted PCBs by UV irradiation in n-hexane and their correlation to the charge distribution on carbon atom. Chemosphere. 2003;51:775–784. doi: 10.1016/S0045-6535(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 59.Tang T., Zheng Z., Wang R., Huang K., Li H., Tao X., Dang Z., Yin H., Lu G. Photodegradation behaviors of polychlorinated biphenyls in methanol by UV-irradiation: Solvent adducts and sigmatropic arrangement. Chemosphere. 2018;193:861–868. doi: 10.1016/j.chemosphere.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 60.Baron C.P., Børresen T., Jacobsen C. UV Treatment of Fishmeal: A Method To Remove Dioxins? J. Agri. Food Chem. 2005;53:7091–7097. doi: 10.1021/jf0509963. [DOI] [PubMed] [Google Scholar]