Abstract
Acute oesophageal necrosis, black oesophagus (BE) or Gurvits syndrome (GS) is a rare form of severe oesophagitis appearing as a striking circumferential discolouration of distal mucosa with various proximal extensions abruptly terminating at the gastro-oesophageal junction. It is most commonly associated with acute exacerbations of medical comorbidities, while associations with altered gut anatomy are rare. We present a unique constellation of BE, Cameron ulcers (CU), and gastric volvulus from a large paraesophageal hiatal hernia. Our patient recently recovered from COVID-19 and was malnourished and frail, while the expanding paraesophageal hiatal hernia turned into an acute organoaxial gastric volvulus with accompanying outlet obstruction. In low-flow post-COVID coagulopathic states, compensatory mechanisms may lack against gastric stunning and sudden massive reflux on the oesophagus. We additionally performed a systematic review and discovered additional cases with coexistent volvulus and paraesophageal hernia, although there are no previous reports of BE with CU, which makes this study the first.
Keywords: gastroenterology, oesophagus, gastro-oesophageal reflux, gastrointestinal surgery
Background
Acute oesophageal necrosis (AEN), black oesophagus (BE) or Gurvits syndrome (GS) is a rare but emerging cause of gastrointestinal (GI) bleeding first described in autopsy studies in the pre-endoscopic era.1 However, its incidence has increased over the past two decades as case recognition, reporting and understanding have grown. It presents on endoscopy as striking black circumferential distal oesophageal mucosal discolouration with various proximal extensions and abrupt cessation at the gastro-oesophageal junction (GEJ), often seen in acutely ill patients.2 The aetiology of BE is multifactorial—a combination of tissue hypoperfusion, massive reflux of gastric contents, and compromised local mucosal barriers. Diagnosis is endoscopic and does not require tissue sampling. Associated medical conditions may include sepsis, cardiovascular disease, diabetes mellitus with ketoacidosis, thromboembolic phenomena, alcohol abuse, oncologic disorders and malnourishment. Alterations in foregut anatomy seen in the setting of significant hiatal hernia may play a role in the pathogenesis of the syndrome. Mucosal recovery is common when underlying derangement is identified and corrected, while post-endoscopy care and surveillance schedules are individualised when strictures or stenosis complicate oesophageal recovery. All-cause mortality nears 32% due to comorbid medical conditions, but fatality specific to AEN is much lower at around 6%.3 Our case represents an unusual case of AEN in post-COVID coagulopathy, altered local circulation, and outlet obstruction in the setting of a large volvulating paraesophageal hernia in a malnourished patient. We additionally performed a systematic search to parse out unique features of our case and define the common pathogenesis of this syndrome in such a setting.
Case presentation
A 77-year-old woman was presented to our emergency department with abdominal pain, multiple emesis episodes and transient loss of consciousness. Her medical history included chronic obstructive pulmonary disease, gastro-oesophageal reflux disease, peptic ulcer disease, hiatal hernia, malnutrition and recent COVID-19 infection. She was haemodynamically stable but ill-appearing with diffuse tenderness on abdominal palpation. Laboratory analysis showed a remarkable count for white blood cell count at 18.5 k/μL, haemoglobin at 9.8 g/dL, serum creatinine at 1.4 mg/dL and serum albumin at 3.3 g/dL. A CT scan of the abdomen revealed large paraesophageal hiatal with organoaxial volvulation and distal oesophageal distention (figures 1 and 2). She was made nil-per-os, resuscitated with intravenous ringers lactate solution and started on continuous intravenous pantoprazole infusion. An oesophagogastroduodenoscopy revealed a large hiatal hernia, CU and necrotic discolouration of the mid-to-lower third of the oesophagus with an abrupt transition at the gastro-oesophageal junction (figures 3 and 4). The patient declined surgical intervention to correct the hernia and volvulus. She was eventually discharged home in stable condition tolerating oral intake with hospice services.
Figure 1.
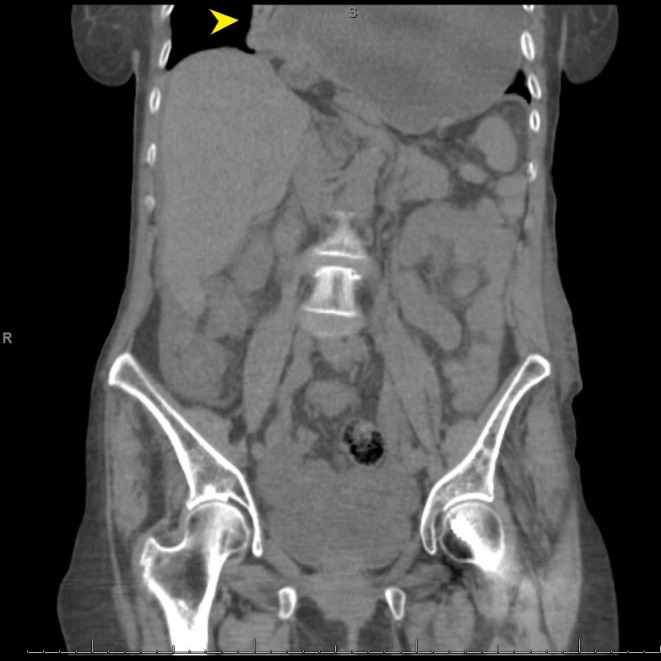
CT abdomen reveals complete herniation of the stomach into the posterior mediastinum (arrows).
Figure 2.
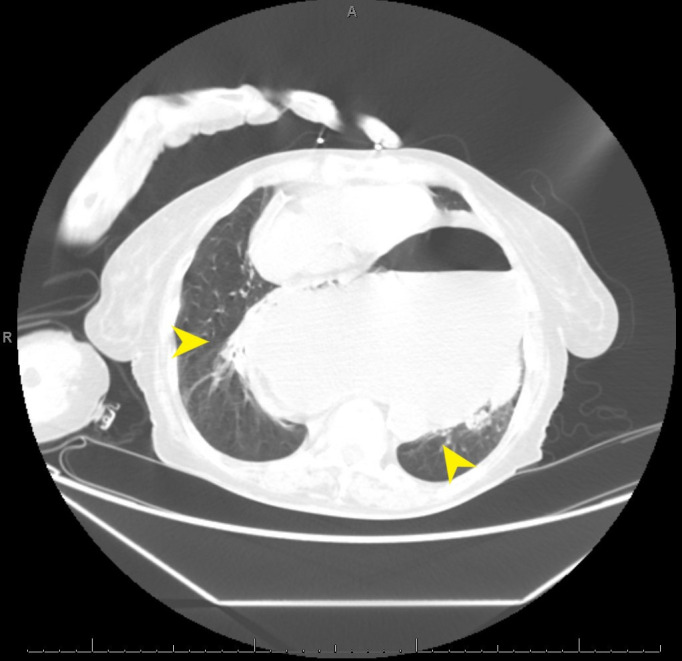
CT abdomen reveals herniation of the stomach into the posterior mediastinum (arrows), oesophageal dilatation, and gastric distension.
Figure 3.
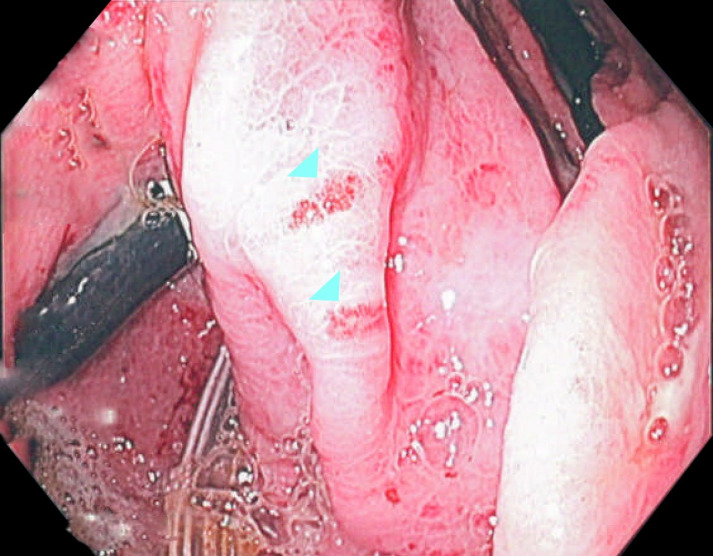
Esophagogastroduodenoscopy (EGD) demonstrating necrotic lesions along the oesophageal lumen (arrowheads).
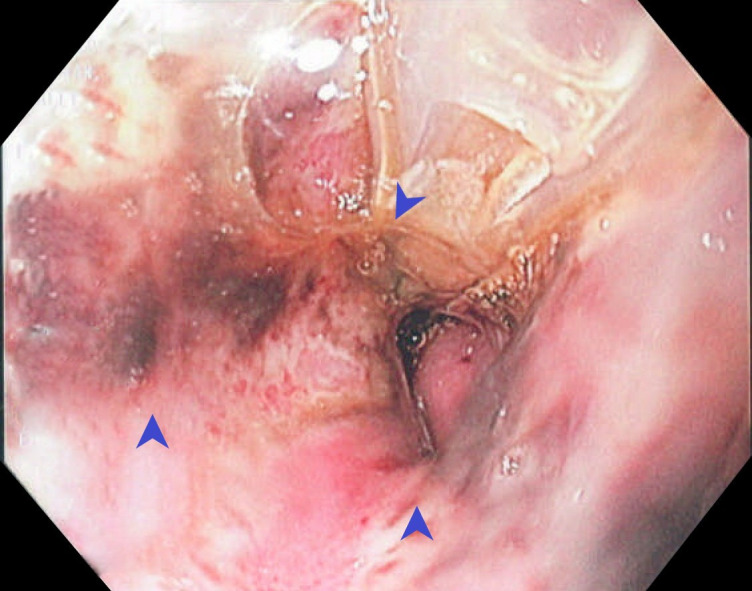
Figure 4.
Esophagogastroduodenoscopy (EGD) demonstrating cameron ulcers (arrowheads).
Outcome and follow-up
Despite eventual weaning off mechanical ventilation and advance on an oral diet, the patient declined surgical intervention for correction of hiatal hernia and was eventually discharged home in stable condition tolerating oral intake. She was found to be well after her 6 month follow-up but refused repeat endoscopy and surgical intervention.
Discussion
The incidence of AEN or GS ranges between 0.01% and 0.30%, although these estimates are under-reported as autopsy studies were the primary method of diagnosis in the pre-endoscopy era. At the same time, sicker patients at the highest risk for AEN would often forgo or delay endoscopy due to their tenuous states.2 3 Gurvits staging system was introduced in 2007 to describe the natural history of AEN from the endoscopic and histopathologic analyses2, subdividing it into black appearing mucosa with tissue necrosis in Stage 1, transition changes in Stage 2 and normal mucosal appearance with microscopically present granulation tissue in Stage 3. Rapid recovery from Stage 1 may be seen as early as the first week of diagnosis3 and effectively underestimate AEN incidence. The diagnosis of GS requires the exclusion of pseudomelanosis, malignant melanoma and caustic injuries.4 On performing a systematic search of other similar presentations, only a handful of AEN cases with a concurrent hernia and/or gastric volvulus have been reported.5–10 Our study is the first to report the case of AEN associated with severe mechanical features of a large hiatal hernia, including CU. These constellations of findings are unique and may imply a common underlying pathophysiological mechanism that stems from large hiatal hernias and their effect on the surrounding structures.11 12
The occurence of necrosis seen in AEN is thought to be a consequence of multiple derangements in the setting of an acute illness and long-standing comorbidities. 2 3An important factor is a sudden state of hypoperfusion or vascular compromise that preferentially affects watershed areas of the distal oesophagus with various proximal extension with colloquial hindering mucosal regeneration and the inability to protect against massive reflux of gastric contents. In our patient, various mechanisms can account for AEN, including post-COVID hypercoagulable state, compromise to the blood flow to the lower oesophagus traversing through the lesser gastric curvature during gastric rotation and increase in esophageal intraluminal pressure on the mucosal and submucosal vessels with effective compression.13 Gastric outlet obstruction, partial or complete, as seen in the gastric volvulation, leads to massive reflux of corrosive secretions to the distal esophagus overwhelming local mucosal barriers and local buffering systems that were already relatively compromised in this chronically malnourished elderly patient. Interestingly, under normal circumstances, the oesophagus may compensate for this sudden reflux by increasing blood flow, but this compensatory mechanism is lost in a coexistent low-flow state.4
A laparoscopic transabdominal repair was proposed to our patient; however, this was declined by her. Traditionally, surgery can be approached from the thorax or abdomen, and although no head-tohead studies exist—laparoscopic transabdominal is increasingly used in practice as thoracic approaches can be challenging. Inpatient sample studies have demonstrated lower readmission, reoperation and complication rates in laparoscopic approaches.14 15
Increased recognition of AEN in the 20th century has led to its ascent as the fourth leading cause of acute haemorrhage from the upper intestinal tract and a valid differential for GI bleeding.1 Among known cases of GS, a direct effect from alteration of upper GI anatomy is rare; its management and prognosis are less defined, placing importance on case reports and nonrandomised studies to increase awareness (table 1). Future explorations may provide additional insight on pathogenesis and management with a clear goal of improving morbidity and mortality of such patients with AEN.
Table 1.
Summary of previous cases of acute oesophageal necrosis (AEN) associated with gastric volvulus and hiatal hernias medical literature
| Author/Year | Article type | Presenting symptoms | Gastric volvulus | Hiatal hernia | Segment affected | Management | Outcomes |
| Kram et al5 2000 | Full manuscript | Coffee-ground emesis | ✓ | ✓ | Distal | Laparotomy and oesophagectomy | Full recovery and alive |
| Hwang16 2007 | Full manuscript | Coffee-ground emesis | X | ✓ | Pan-oesophageal | Laparoscopy, hernia repair and percutaneous endoscopic gastrostomy (PEG) | Alive and removal of PEG and resolution of acute oesophageal necrosis (AEN) on repeat endoscopy |
| Matsumoto et al6 2009 | Full manuscript | Vomiting and epigastric pain | ✓ | ✓ | Distal | Laparotomy, oesophagectomy and total gastrectomy | Alive and oesophageal reconstruction |
| Garas et al17 2011 | Full manuscript | epigastric pain | ✓ | ✓ | Distal | Esophagectomy with gastric pull-up | Multiorgan failure and death |
| Mclaughlin18 2011 | Full manuscript | Coffee-ground emesis, nausea, and chest pain | X | ✓ | Middle-distal | Nissen fundoplication | Alive with AEN resolution on repeat endoscopy |
| Nunes et al19 2017 | Case Image | Coffee-ground emesis and epigastric pain | ✓ | ✓ | Middle-distal | Laparotomy and Nissen fundoplication | Alive and discharged without any complications |
| Moore et al9 2018 | Full manuscript | Nausea and abdominal pain | ✓ | X* | Middle-distal | Laparotomy with volvulating laparoscopic band removal | Discharge and AEN resolution on repeat endoscopy |
| Chowdhury7 2019 | Abstract | Dysphagia | ✓ | ✓ | Middle-distal | Laparoscopy and PEG | Alive and discharge |
*volvulus secondary to slipped laparoscopic gastric band
Learning points.
Acute esophageal necrosis (AEN) is gaining recognition as an emergent cause of upper gastrointestinal hemorrhage in patients presenting for hospital care.
Alteration in gastrointestinal anatomy, including paraesophageal hiatal hernias, are rare but essential causes of AEN.
AEN in the setting of a large paraesophageal hiatal hernia may represent regional compression of distal esophageal vasculature.
Gastric volvulation may lead to massive reflux of corrosive secretions to the distal esophagus, contributing to AEN development.
Future studies can explore AEN incidence within cases of altered gut anatomy.
Acknowledgments
We would like to thank Dr. Madhu Vennikandam - Gastroenterology fellow at Michigan State University for her input on this case.
Footnotes
Twitter: @smitdeliwalaMD
Contributors: SD - conception, draft, and acquisitionMurtaza Hussain - Data collection, draft AP - Data collection, draft GB - Draft and review GEG - Interpretation and review
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Yasuda H, Yamada M, Endo Y, et al. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol 2006;41:193–7. 10.1007/s00535-005-1741-6 [DOI] [PubMed] [Google Scholar]
- 2.Gurvits GE, Shapsis A, Lau N, et al. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007;42:29–38. 10.1007/s00535-006-1974-z [DOI] [PubMed] [Google Scholar]
- 3.Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010;16:3219–25. 10.3748/wjg.v16.i26.3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben Soussan E, Savoye G, Hochain P, et al. Acute esophageal necrosis: a 1-year prospective study. Gastrointest Endosc 2002;56:213–7. 10.1016/S0016-5107(02)70180-6 [DOI] [PubMed] [Google Scholar]
- 5.Kram M, Gorenstein L, Eisen D, et al. Acute esophageal necrosis associated with gastric volvulus. Gastrointest Endosc 2000;51:610–2. 10.1016/S0016-5107(00)70304-X [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto N, Oki E, Morita M, et al. Successful treatment of acute esophageal necrosis caused by intrathoracic gastric volvulus: report of a case. Surg Today 2009;39:1068–72. 10.1007/s00595-008-3983-4 [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury N, Alom MS, Saleem A. 1824 Acute Esophageal Necrosis Secondary to Intrathoracic Gastric Volvulus Without Deadly Outcome. Am J Gastroenterol 2019;114:S1024. 10.14309/01.ajg.0000596828.44656.2b [DOI] [Google Scholar]
- 8.Nunes G, Patita M, Fernandes V. Paraesophageal hernia and gastric volvulus: an uncommon etiology of vomiting and upper gastrointestinal bleeding. Rev Esp Enferm Dig 2017;109:294–5. [PubMed] [Google Scholar]
- 9.Moore C, Matthews LR, Danner O, et al. "Black Esophagus" and Gastric Volvulus Following Slipped Laparoscopic Adjustable Gastric Band. Obes Surg 2018;28:2941–8. 10.1007/s11695-018-3354-1 [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin CW, Person TD, Denlinger CE. Management of acute esophageal necrosis syndrome. J Thorac Cardiovasc Surg 2011;141:e23–4. 10.1016/j.jtcvs.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Tang S-J, Daram SR, Wu R, et al. Pathogenesis, diagnosis, and management of gastric ischemia. Clin Gastroenterol Hepatol 2014;12:246–52. 10.1016/j.cgh.2013.07.025 [DOI] [PubMed] [Google Scholar]
- 12.Manno V, Lentini N, Chirico A, et al. Acute esophageal necrosis (black esophagus): a case report and literature review. Acta Diabetol 2017;54:1061–3. 10.1007/s00592-017-1028-4 [DOI] [PubMed] [Google Scholar]
- 13.Zullo A, Manta R, De Francesco V, et al. Cameron lesions: a still overlooked diagnosis. Case report and systematic review of literature. Clin Res Hepatol Gastroenterol 2018;42:604–9. 10.1016/j.clinre.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Schlosser KA, Maloney SR, Prasad T, et al. Mesh reinforcement of paraesophageal hernia repair: trends and outcomes from a national database. Surgery 2019;166:879–85. 10.1016/j.surg.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 15.Migliore M, Arcerito M, Vagliasindi A, et al. The place of Belsey Mark IV fundoplication in the era of laparoscopic surgery. Eur J Cardiothorac Surg 2003;24:625–30. 10.1016/S1010-7940(03)00445-7 [DOI] [PubMed] [Google Scholar]
- 16.Hwang J, Weigel TL. Acute esophageal necrosis: "black esophagus". JSLS 2007;11:165–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Garas G, Wou C, Sawyer J, et al. Acute oesophageal necrosis syndrome. BMJ Case Rep 2011;2011. 10.1136/bcr.10.2010.3423. [Epub ahead of print: 03 Mar 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin CW, Person TD, Denlinger CE. Management of acute esophageal necrosis syndrome. J Thorac Cardiovasc Surg 2011;141:e23–4. 10.1016/j.jtcvs.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Nunes G, Patita M, Fernandes V, et al. Paraesophageal hernia and gastric volvulus: an uncommon etiology of vomiting and upper gastrointestinal bleeding. Rev Esp Enferm Dig 2017;109:294–5. [PubMed] [Google Scholar]