Abstract
Cardiopulmonary exercise testing (CPET) has become an invaluable tool in healthcare, improving the diagnosis of disease and the quality, efficacy, assessment and safety of treatment across a range of pathologies. CPET’s superior ability to measure the global exercise response of the respiratory, cardiovascular and skeletal muscle systems simultaneously in a time and cost-efficient manner has led to the application of CPET in a range of settings from diagnosis of disease to preoperative assessment. The Association for Respiratory Technology and Physiology Statement on Cardiopulmonary Exercise Testing 2021 provides the practitioner and scientist with an outstanding resource to support and enhance practice, from equipment to testing to leadership, helping them deliver a quality assured service for the benefit of all patient groups.
Keywords: exercise, respiratory measurement
Key messages.
This statement outlines the latest best practice guidance for the performance of cardiopulmonary exercise testing (CPET) within a healthcare environment.
Recommendations are provided on how to undertake CPET safely, to quality assured standards and using the most suitable reference values available for the interpretation of results.
Introduction
Initially used in sports and exercise science to determine aerobic and anaerobic fitness thresholds in athletes, cardiopulmonary exercise testing (CPET) is increasingly being used in the healthcare setting and has been used in preoperative medicine for over 20 years. CPET is used for diagnostic assessment of patients presenting with dyspnoea of unknown origin, assessment of respiratory or cardiovascular disease or for presurgical assessment prior to major elective surgery.
Patients are often poor at estimating their own cardiopulmonary fitness. This can lead to difficulty in identifying the cause of exercise intolerance during clinician assessment. Pulmonary function testing can be considered as a weak predictor of disability and quality of life in patients with chronic respiratory conditions,1 while traditional static tests of cardiac function have been shown to correlate poorly with physical fitness.2
Field exercise tests, such as 6 min walk tests, are repeatable, reproducible and sensitive to therapeutic intervention3 but crucially lack the information required to precisely determine aerobic capacity. Dynamic assessments, such as dobutamine stress echocardiography, do not allow for objective measurement of functional capacity.4 Treadmill-based stress electrocardiography provides an indirect assessment of functional capacity but has been shown to be poorly tolerated by elderly patients and have a negative predictive value for postoperative outcomes in elective surgical patients.5
The benefit of performing CPET is that it is a relatively non-invasive objective test which provides a direct objective measurement of the global exercise responses of the respiratory, cardiovascular and skeletal muscle systems during incremental exercise. Analysis of the integrative exercise responses of these body systems can offer a wealth of information when compared with traditional individual diagnostic tests performed at rest.
Since 2008, the number of departments in the NHS performing CPET has increased by 40%, and over 15 000 CPETs are now performed annually in the UK.6 Within UK respiratory departments, the number of CPET increased by 81% between 2005 and 2015 with an estimated annual increase of 8% per year.7 CPET is routinely performed on both adult and paediatric patients, in respiratory or cardiology laboratories, and is increasingly being performed in anaesthetic departments or presurgical assessment units.
The interpretation and clinical value of CPET relies on a series of interventions before, during and after the act of measurement. In recent years, this has become increasingly important. CPET is no longer just a diagnostic tool to assess patients with suspected cardiovascular and respiratory disease. The quantification of risk prior to major surgery can now be determined more confidently. A decision to proceed to major surgery or the level of care required postoperatively can be supported by gas exchange data recorded during CPET. Modern, commercially available exercise testing systems require less and less awareness from the end user on potential sources of error. These systems are often more complex, and the risk of introducing systematic bias into measurement is higher. CPET is complex; the integrative nature of the information allows scrutinising the onset of unexplained symptoms on exertion that no other investigation is capable of.
The aim of this document was to provide comprehensive guidance for performing CPET in the clinical setting, offering a standardised approach to CPET based on current scientific knowledge and best practice. The document offers recommendations around specific indications for CPET, test protocol selection, testing equipment, appropriate personnel, and patient and test safety. The intended audience for this document is those healthcare professionals who plan to, or currently, perform CPET in the clinical setting.
This document is a consensus statement from experts in the field and is supported by previously published literature. The Association for Respiratory Technology and Physiology (ARTP) CPET working party group consists of respiratory physiologists and clinical scientists with a wide range of clinical experience in cardiopulmonary exercise testing (CPET) in adult and paediatric patients, clinical research in the field of exercise physiology, CPET service development, preoperative evaluation of surgical patients and exercise prescription.
General procedures
Indications
CPET has a wide range of indications in a clinical setting (box 1), evaluating exercise tolerance, assessment of surgical risk, identifying specific pathophysiology and monitoring their progression and/or response to treatment. Although it can provide large amounts of detailed data, it should be used as a supplementary assessment when questions remain after baseline clinical examinations, including routine pulmonary function testing, to fine-tune a diagnosis or reveal abnormalities that are not distinguishable on resting measurements.
Box 1. Indications for performing cardiopulmonary exercise testing.
Indications
Investigation of unexplained dyspnoea.
Evaluation of cardiovascular disease.
Evaluation of respiratory disease.
Preoperative assessment for major surgery.
Exercise prescription.
Evaluation of impairment/disability.
Evaluation of exercise tolerance.
Risk stratification
CPET is generally a safe procedure (box 2), more so in patients with no pre-existing comorbidity. Various studies place complication rates at 2–5 per 100 000 tests.8 9 Recent UK data have reported no serious adverse events from 4983 tests during pulmonary assessment and an occurrence of a patient safety incident in 2 per 1000 tests.10 It is important to remember that CPET is a symptom limited and often maximal effort test; hence, there is some risk of inducing syncope, exercise-induced hypoxaemia and malignant cardiac dysrhythmias, and exacerbating previously latent conditions. Thorough pretest preparation and adherence to safety recommendations will help to mitigate these risks significantly.
Box 2. Known risk of adverse events associated with performing cardiopulmonary exercise testing.
Risk of adverse events
Incidence of a complication requiring hospitalisation of ≤2 in 1000.
Incidence of a major cardiac event of 1.2 per 10 000.
Incidence of mortality of 2–5 per 100 000.
Previously published guidance8 11 12 have described certain clinical conditions in which CPET is contraindicated, either as an absolute, and so the test is not performed, or relative, where the test is recommended to be conducted under medical supervision. In line with more recent publications,13 14 we recommend an approach that considers and balances risk versus benefit of undertaking a CPET. As with any test, clinical CPET should only be undertaken if the results will alter patient management. Accordingly, if the risk of performing a CPET is felt to be high, then it may be appropriate to ensure there are adequate measures in place to mitigate the risk; however, if not sufficient, then it may be advisable to consider an alternative diagnostic modality, in place of CPET.
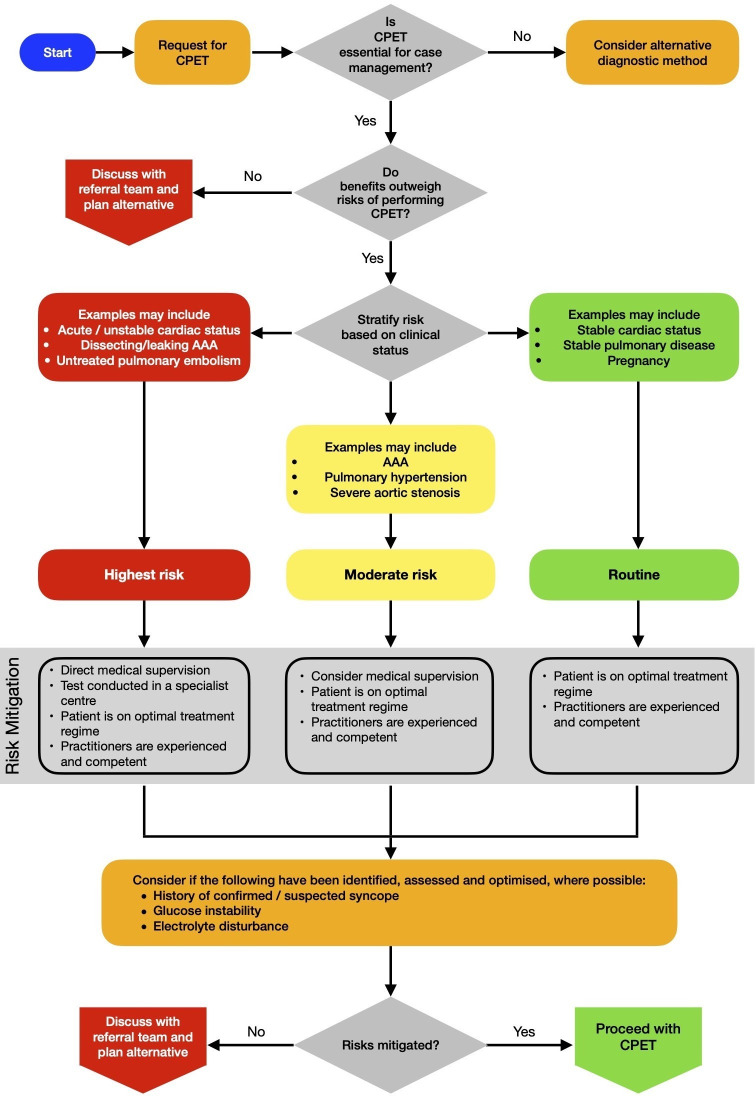
We have outlined in figure 1 a tool for assessing the risk associated with performing CPET in various clinical scenarios. The healthcare environment in which the procedure is being performed, the specialist support that is available etc. should also be taken into consideration. Risk assessment is likely to be different if a CPET is conducted in a specialist heart and lung centre where specialist assistance is available for complex cardiorespiratory conditions or whether the assessment is performed in a non-specialist setting.
Figure 1.
Tool for assessing the risk associated with performing CPET in various clinical scenarios. AAA, abdominal aortic aneurysm; CPET, cardiopulmonary exercise testing.
As an example, clarity has often been sought by those involved in CPET testing regarding the safety of performing maximal effort exercise testing in patients with abdominal aortic aneurysm (AAA). The combination of exercise and aneurysms raises theoretical concerns regarding the risk of expansion and rupture; however, the published evidence available suggests that performing exercise stress testing in a hospital setting is safe and presents a low incidence of acute adverse events.15–19 There is one published case study of an abdominal aortic dissection following cardiopulmonary testing.20
A conservative approach should be taken for those undergoing CPET for preoperative assessment of AAA. Cardiovascular exercise is generally regarded as safe in the absence of hypertension,21 but there are currently no published data on what would constitute an excessively hypertensive response during cardiovascular exercise in the presence of aneurysms. The consensus opinion of the authors of this document is that CPET should be discontinued if blood pressure exceeds 200 mm Hg systolic or 110 mm Hg diastolic. Although maximal effort symptom limited exercise testing provides a full assessment of functional capacity, in the presurgical assessment of patients with AAA, consideration should be given to performing a submaximal test, terminating the test as soon as there is confirmation that anaerobic threshold has been achieved.
Consent
Consent is the principle that a person must give permission before they receive any type of medical treatment, test or examination. Consent is an essential component of both medical ethics and human rights law. Consent is only valid if it is voluntary and informed. Importantly, the person giving consent must have the capacity to make the decision.
Any patient attending for CPET should therefore be provided with all relevant information about the test process, the risks and benefits of completing the investigation, and implications of not performing the test. This information should be presented in lay terms (box 3) and provided in plenty of time ahead of the appointment date to allow the patient sufficient time to formulate and raise any queries.
Box 3. Lay terminology for describing the risk of adverse events to patients attending for cardiopulmonary exercise testing testing.
Lay terminology
The risk of side effects is the same as performing mild exercise.
The number of patients who develop complications during the test is low.
Complications may include abnormal blood pressure, fainting or a change in heart rhythm.
Extremely rare, serious complications such as heart attack or stroke may occur
Patients may also give non-verbal consent, as long as they understand the treatment or examination about to take place, for example, holding out an arm for a blood test, allowing the CPET mask to be fitted or taking up position on the cycle ergometer.
Our recommendation would be that formal written consent is obtained, that is, completion and signing of a consent form, prior to the investigation taking place if the CPET test will include invasive aspects of physiological measurement, for example, measurement of arterial or capillary blood gas or laryngoscopy.
Consent should be given to the healthcare professional directly responsible for the person’s current tests or treatment. If the subjects change their mind at any point before the test commences or even during the test, the test must be stopped.
Children under the age of 16 years can provide consent if they are believed to have enough intelligence, competence and understanding to fully appreciate what is involved in their treatment. This is known as being ‘Gillick competent’. If the child is not Gillick competent, then someone with ‘parental responsibility’ can provide consent for them. This could be the following:
The child’s mother or father.
The child’s legally appointed guardian.
A person with a residence order concerning the child.
A local authority designated to care for the child.
A local authority or person with an emergency protection order for the child.
The person with parental responsibility must have the capacity to give consent.
Preparation
Before, during and after CPET, the practitioner must consider all aspects involved with the patient’s test. Some of these may have a direct or indirect impact on the test accuracy and patient experience. All clinical staff working in the exercise laboratory setting will have the responsibility to consider the standards set out further. This should ensure a good patient experience, reduce risk and eliminate any potential sources of bias.
General patient considerations
Practitioners should remain professional at all times, answer any patient questions honestly and avoid asking leading questions. Patients may have very specific needs that should be accommodated by the attending member of staff (where possible). This may be due to previous experiences and ethnic, religious or cultural background. Also consider disability: wheelchair users, deafness, blindness and patients with learning disabilities. Interpreters should also be organised as necessary. If the patient appears anxious or uncomfortable, anticipate their needs, be cordial and communicate clearly. Patients attending for presurgical assessment are likely to be extremely anxious ahead of potentially life-changing major surgery; recognition and anticipation of this is essential to ensure the patient is reassured as much as possible to promote successful test completion and to ensure the patient can perform optimally. Interpreters, chaperones, friends or relatives should accompany the patient to the testing room (space permitting), with the patient’s consent. However, consideration for the patient’s dignity when undressing and during ECG preparation should be paramount.
Pretest instructions
Patients attending for a CPET should receive clear information ahead of the appointment, which clearly outlines the test procedure and provides a rationale for why the test is required. Clear guidance on any special measures the patient is expected to take before attending should also be included (box 4).
Box 4. Pretest patient instructions for issue to patients ahead of attending for cardiopulmonary exercise testing appointment.
Refrain from exercise on the day of the test and be well rested.
Eat a light meal or breakfast no less than 2 hours hours previously.
Maintain hydration by drinking water.
Avoid caffeine and alcohol prior to the test.
Take all routine/normal medication and bring along a medication list.
Bring along all rescue medications, for example, inhalers or nitrolingual sprays.
Wear light comfortable clothing and shoes suitable to exercise in.
Avoid use of body lotion on the upper body, as this may affect ECG electrode placement.
Abstain from smoking for at least 8 hours hours prior to the test.
Refrain from wearing any nail varnish or false nails.
Adapted from the American Thoracic Society and American College of Chest Physicians.8
Are there any permanent or temporary conditions that could affect their ability to move, bear weight, balance or walk? Is the patient pregnant? Obtaining this information will ensure that valuable appointment slots are not wasted. The use of a wheelchair or walking aid does/will not necessarily render CPET unfeasible. The patients will also need to be aware that if they feel unwell (eg, suffering from a chest infection, cold, etc), it will be a requirement to reschedule the appointment to ensure optimal effort can be invested into the test.
It is also important to ascertain information regarding the patient’s mobility prior to the test. Good practice is therefore to trial the patient on the cycle ergometer before preparing the patient for the ECG electrode placement. Patients with severe end-stage arthritis undergoing preassessment for total hip replacement or knee replacement are capable of maximal symptom limited exercise using ergometry methods.22 In practice, patients awaiting lower limb joint replacement often tolerate cycle ergometry, with a slightly higher saddle position, reducing the required flexion at the knee.
Patients should be as relaxed as possible before the test and should be seated for 5–10 min prior to testing. The purpose and nature of the test should be clearly and fully explained to the patient,14 allowing opportunities for the patient to ask questions or to clarify any concerns.
Infection control
There is often a diverse range of patients attending for a CPET. Some patients may pose an infection risk, whereas others may themselves be vulnerable to a range of infections, creating potential risk of cross-infection. However, the degree of risk remains unknown with limited evidence available.
Cross-infection may occur either by direct patient contact or indirect contact, which is most likely when the patient comes into contact with contaminated surfaces, equipment or healthcare personnel, and inhalation of aerosol particles or droplets via airborne route through tubing, mouthpieces or masks.23
CPET has a raised potential for cross-infection and should be classed as a high-risk procedure due to the nature of increased levels of patient ventilation24 and greater capacity to generate aerosols. To combat this, consideration should be given to environmental ventilation levels in the testing area. Negative pressure testing rooms, use of high efficiency particulate air (HEPA) filtration or rooms with higher (>12) air changes per hour (ACH) provide the lowest-risk environment. Rooms with ACH of >6 but <12 provide a moderate risk environment, require regular cleaning and ‘fallow periods’ between patients. Testing environments with ACH of <6 should be classed as the highest-risk testing environment.24
It is important for laboratories to practise appropriate routine cleaning and decontamination of all non-disposable consumables, equipment, work surfaces and personnel with local policies in place, including cleaning protocols, cleaning logs, practising good hand hygiene and appropriate use of personal protective equipment (PPE).
Historically, the use of PPE in respiratory laboratories has not been enforced, even though there are numerous respiratory infections that are transmitted via aerosol-generating procedures (AGPs), such as CPET. Therefore, PPE should be used in all AGP environments in accordance with the type of infection and level of risk posed (table 1).
Table 1.
Recommended levels of PPE in relation to levels of infection risk
| Level of risk | Known infection | Community prevalence | Recommended PPE |
| Level 1 | No known infection risk | No pandemic | Three-ply surgical mask, disposable apron, surgical gloves |
| Level 2 | Upper respiratory tract infection, lower respiratory tract infection, influenza | Pandemic with low community prevalence | Face shield, three-ply surgical mask, disposable apron, surgical gloves |
| Level 3 | Tuberculosis, family Coronaviridae, SARS, pandemic influenza | Global pandemic with high community prevalence | FFP3 mask with face shield or respirator hood, isolation gown, surgical gloves |
PPE, personal protective equipment.
Cleaning and decontamination of all non-disposable consumables and equipment can be performed using multiple methods. An appropriate area for cleaning of non-disposable consumables should be identified; all cleaning and decontamination should only take place in this specific area. The use of chemicals should be in accordance with local control of substances hazardous to health (COSHH) policies, local infection control teams and manufacturer guidelines; some chemicals may cause unnecessary degrading of equipment. Clean items should be clearly labelled and stored in such a way as to avoid contamination.
With known infectious patients, additional measures may be considered such as employing the use of a bacterial viral filter to prevent aerosolisation of micro-organisms.25 The filters used in pulmonary function testing are not recommended for clinical exercise testing due to increased production of water vapour in exhaled breath at high ventilatory frequencies during vigorous exercise, saturating the filter. Increased water vapour is likely to impact on time to volitional fatigue, especially in those who are ventilatory capacity limited,26 and to reduce the effectiveness of the filter once excessively saturated, as well as impair the quality of the gas exchange data.
There is very limited evidence supporting the use of a filter during CPET. A recent study performed by University Hospital of Wales on a small cohort of healthy subjects suggested CPET can be performed effectively while employing a filter and reducing risk of transmitting aerosolised particles,27 but further research in the use of a bacterial viral filter during CPET is warranted.
No research has been conducted in elderly or respiratory compromised population; therefore, the impact of potential increased resistance posed to the respiratory effort in these populations is unknown and the use of filters cannot be recommended.
Quality assurance (QA)
QA describes the systematic processes used to ensure a clinical service meets quality standards. Good QA systems will use a robust series of checks before, during and after the patient’s visit to ensure that the results generated will be as accurate and precise as possible. The role of QA and quality control (QC) within the testing environment is of immense value to ensure that those who perform the test can resolve arising issues in a proactive manner, providing accurate, meaningful information to the referring clinician, and ensure that the patients get the desired outcome as a result of performing the test, and the reported results are the best possible representation of the clinical status of the patient. In clinical and research settings, QA and QC have different purposes. QA can be defined as a group of routines and interventions to ensure the data recorded are of high quality, while QC is the process of measuring and monitoring the quality of the data.28
Our recommendation is that any department performing CPET should subscribe to an appropriate and recognised accreditation scheme as a quality marker, enabling demonstration of compliance with governance standards. In the UK, physiological services should align with the Improving Quality in Physiological Services (IQIPS) programme.29
IQIPS is a systematic accreditation process delivered by United Kingdom Accreditation Service in the UK for physiological services, including cardiac, respiratory and sleep services. This involves provision of evidence that demonstrates services are compliant with the domains of the accreditation process. Accredited services are required to constantly monitor and review their delivery of patient care. IQIPS is recognised by the Care Quality Commission. IQIPS can also act as a service improvement tool to highlight areas where services are below the required standard to facilitate service improvement.
QA and QC
Physiologists/scientists must give consideration to all ‘sources of variation other than disease’.30 Any form of clinical measurement is highly complex, as there are numerous sources of variability inherent to the different processes required to record information. This tends to start with a referral and terminates with a set of numerical values and graphs. All test and non-test specific factors within these two landmarks need to be taken into account. This includes the laboratory environment, the patient, the test operator, the equipment and test protocols. Common sources of error in CPET testing are documented in table 2. The next sections will detail considerations, routines and interventions within each of these factors that are expected to be part of the QA programme, including QC methods to measure and monitor data accuracy and precision.
Table 2.
Common sources of error in CPET testing
| Source of error | Description | Impact on data | |
| Patient | Failure to follow information disclosed on patient information leaflet | All patients should be given a patient information leaflet and/or advice on what to avoid prior to performing an exercise test. | Various implications, specifically limiting exercise tolerance and impairing gas exchange data |
| Poor effort/cooperation/motivation to perform exercise test | Patients need to understand the reason why the test is being performed. Failure to do so may result in suboptimal effort. | Underestimation of all indices, including workload, AT, VO2 and VCO2. | |
| Test operator | Failure to give standardised instruction and encouragement during exercise | Throughout the different phases of exercise, there should be clear and standardised instructions to patients. | Various implications, specifically lack of consistency in data across different test operators |
| Failure to select correct load (watts) in view of patient’s activity level/fitness | Incremental workloads that result in exercise duration of <8 or >12 min do not accurately reflect aerobic status. | Various implications, although more commonly underestimation of gas exchange indices | |
| Lack awareness/guidance on the use of well-defined end of test criteria | Exercise may be stopped too early or too late in what should be a symptom limited exercise test. | If exercise is stopped early (eg, pulse rate), gas exchange indices can be underestimated. | |
| Incorrect determination/identification of the AT | There should be a clear definition of what AT is and processes in place to promote discussion and review agreement. | Inappropriate estimation of level of fitness or degree of impairment in O2 delivery/use | |
| Incorrect determination/measurement of slopes (ΔVE/ΔVCO2, OUES and ΔVO2/ΔWR) | The determination of slopes based on linear regression models require correct identification of the start and end points. | Incorrect inferences from data (VE/VCO2 mismatch, cardiovascular impairment, among others) | |
| Equipment | Inaccurate output of power by treadmill/ergometer | Treadmill (speed/grade) and ergometer (resistance) power outputs require yearly servicing (more often if regularly moved). | Various implications, particularly overestimation or underestimation of gas exchange indices |
| Non-calibrated weighing scales and stadiometer | Weighing scales and stadiometers require regular servicing and calibration if there is a suspicion of erroneous measurements. | Incorrect estimation of predicted data and consequent inaccurate inferences from recorded data | |
| Excessive condensation at the point of gas analysis | Gas analysis should meet BTPS conditions, particularly humidity/water vapour pressure. | Various implications, although more commonly underestimation of gas exchange indices | |
| Volume drift | Thermal or offset volume drift may occur as a result of large fluctuations in temperature or incorrect calibration | Various implications, particularly inaccurate ventilatory and gas exchange indices | |
| Delayed response time and transit time in gas exchange parameters | Under certain testing conditions, there may be delay from the point of sampling to the point of gas analysis. | Normally, underestimation of gas exchange data due to dispersion of expired gases | |
| High/low sampling rates/delta time/data averaging of gas exchange data | Data averaging below 30 or above 60 s will affect validity of gas exchange measurements. | Either high fluctuations or excessive attenuation in gas exchange data |
AT, anaerobic threshold; BTPS, body temperature and pressure saturated; CPET, cardiopulmonary exercise testing; OUES, oxygen uptake efficiency slope; VE/VCO2, ventilatory equivalent for carbon dioxide production; ΔVE/ΔVCO2, slope of the ventilatory response; ΔVO2/ΔWR, slope of the metabolic response.
Test operator
A well-constructed and well-adhered to QA programme is founded on enthusiastic and motivated staff. The role of the healthcare professional involved in the preparation, monitoring, evaluation and interpretation of CPET is vital to ensure the data recorded are a good representation of the patient’s exercise capacity and that meaningful information is given to the referring clinician. There is a clear degree of crossover between cardiac and respiratory physiology that needs to be well understood by test operators to take advantage of, fully comprehend and translate into a clear description of the integrative nature of CPET.
Staff involved in equipment maintenance and calibration must be familiar with the advantages and caveats of different methods of measurement, as well as technical concepts as this allows higher standards in QA and monitoring.
Audit and feedback
There are a series of aspects before, during and after CPET that altogether may contribute to bias in measurement, almost like a ‘snowball effect’. The process by which some of these aspects are controlled is normally the responsibility of the members of staff conducting the test. For the purpose of learning, QA and service improvement, simple, short-term audits are an effective way of measuring practice against local and national standards. In order to complete a process of audit, there has to be feedback at the end to those involved in the process, that being referring clinicians or test operators. Plan, do, check and act cycle is an easy and practical method to use, with each cycle lasting no longer than 1 or 2 months. Our recommended regular short-term audit processes are detailed in table 3.
Table 3.
Recommended regular short-term audit processes
| Audit processes | ||
| Audit | Purpose | Actions |
| Quality of referral information | Do referral forms provide sufficient information? |
|
| Adherence to pretest information | Are patients being given patient information? |
|
| Consistency in instructions issued on the day of testing | Are different test operators giving set instructions? |
|
| Correct power determination | Are patients exercising between 8 and 12 min? |
|
| Dead space correction | Is dead space being updated when using different sized masks? |
|
| Leak at peak exercise | How often does it occur and what are the implications? |
|
| Post-test review | Are different members of staff reviewing the information consistently? |
|
| Patient satisfaction | Are patients satisfied with the service? |
|
CPET, cardiopulmonary exercise testing.
All the recommended audit processes detailed as follows should be complemented with simple descriptive statistics and performed at regular time intervals (eg, every 6 months). Over time, assuming that, at the end of each audit cycle, clear and constructive feedback is given to the stakeholders, one would only expect an increase in standards and ultimately quality and assurance in service provision.
Equipment
Standard CPET equipment consists of the following components (figure 2).
Figure 2.
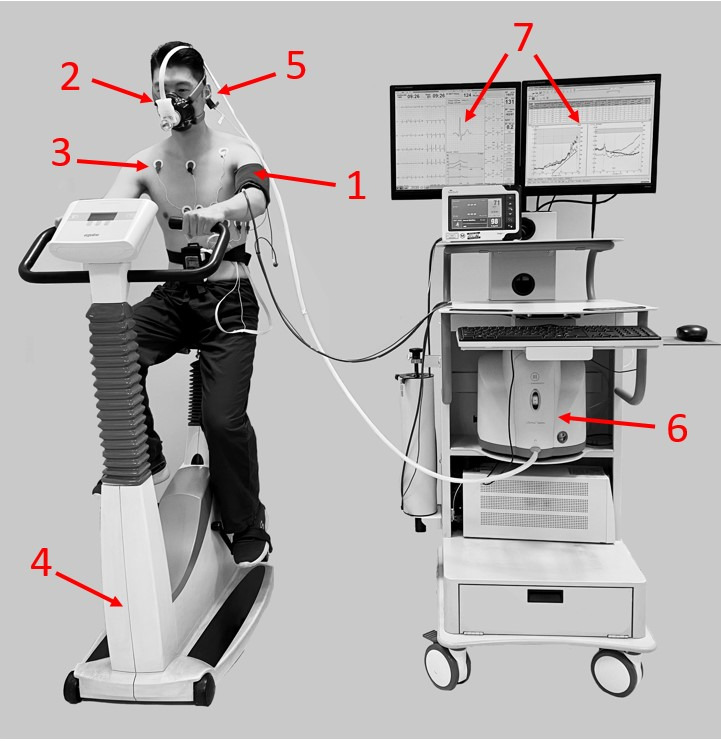
Standard CPET equipment. CPET, cardiopulmonary exercise testing. 1, blood pressure monitor; 2, mask, volume sensor and gas analyser tubing; 3, 12-lead ECG; 4, ergometer (cycle, treadmill, arm crank, etc); 5, pulse oximeter (finger, earlobe and forehead placement); 6, gas analyser; 7, display of breath by breath data and exercise ECG. CPET, cardiopulmonary exercise testing.
QC must not be regarded as a tick box exercise. A well-designed QC routine is ingrained within day-to-day activities, provides factual evidence of reliability, and allows a proactive stance at detecting, correcting and reducing equipment-related sources of error. Serial QC data are the excellence hallmark of CPET service provision.
All equipment directly or indirectly used for CPET must be regularly serviced, calibrated and quality controlled.8 9 31 An easily accessible, up-to-date log of all interventions (repairs, troubleshooting, service history, calibrations, biological controls and respective coefficient of variance for the different output measures) must be kept within the exercise service. All logs must be kept for a period of at least 2 years.32 Older logs should be scanned and saved electronically should a review of this information be required in the foreseeable future.
Planned preventative maintenance (PPM)
Routine day-to-day maintenance is essential to keep equipment in prime condition. A PPM schedule specifies what intervention is needed at what interval. The system used to track PPM (commonly a logbook) should identify when an intervention is due and then when it was performed and by whom. Items recorded will depend on the actual equipment in use but must include the manufacturer’s service visits and replacement of parts, including software updates.
Servicing of equipment should be carried out at the frequency recommended by the manufacturer but should not exceed a period of 12 months, using appropriately qualified and authorised personnel. Following service visits, the authorised agent should certify that the equipment is working to specification before it is returned to clinical use.
Servicing and maintenance
During laboratory downtime, all equipment should be subject to a series of checks.31 We recommend this should occur on a weekly basis and/or additionally when concerns arise around equipment performance. Manufacturer-specific recommendations should be followed in terms of equipment components that need to be checked. This will involve
Evaluation of integrity of reusable pieces of equipment (flow sensors and sample lines).
Review of serial data recorded during physical/biological controls.
Checking of gas cylinders (expiry date, pressure and remaining capacity).
Wired connections and system leaks.
On a yearly basis, equipment must be subject to servicing by the manufacturer. We recommend service contracts be in place to ensure technical support is readily available when an equipment fault is suspected. Servicing of CPET equipment should include
Ambient conditions (accuracy).
Flow/volume calibration (accuracy and linearity).
Gas analyser calibration (response time and delay time, accuracy and linearity).
Treadmill (speed and grade).
Cycle ergometer (calibration and validation of power).
Pulse oximetry (accuracy).
Electrocardiography (deflection and rate).
Blood pressure (leaks and accuracy of pressure transducer).
The engineer on site must be able to provide at the end of the servicing visit a detailed description of corrective actions. This document should be kept in a folder as evidence of regular service history.
Calibration and verification
The terms calibration and verification are sometimes used interchangeably but refer to different procedures in the QC process.
Calibration: changing the relationship between the input (measured parameters such as FeO2) and the output as measured and displayed on the equipment and applying this change to future measurements.
Verification: checking the relationship between the input (measured parameters such as FeO2) and the output as measured and displayed on the equipment, but not making intrinsic changes to how that measurement is being made.
Prior to purchasing a piece of equipment, the manufacturer should
Explain measurement principles of each sensor, how they are combined and any calculation or corrections that are used. This should include which procedures act as calibrations and which are only verifications.
Provide evidence for accuracy and repeatability of each sensor and the overall measurement. That may be a white paper, an internal audit or even independently published articles.
Demonstrate how to correctly calibrate or verify each sensor and explain what constitutes a pass or fail. Be aware there are often arbitrary values used to define ‘out of range’, and most devices will not detect some of the subtler problems that may be detected by using the procedures described in this document.
It is the responsibility of the laboratory personnel to monitor and maintain the accuracy and repeatability of their measurements by performing calibration and verification prior to every patient test. It is imperative that calibration data are recorded, trended and interpreted in the same way as biological control data. The data should be interrogated regularly on the day of testing and on a weekly basis. Although not always practical, best practice would see this performed after every verification to ensure there is no ‘out of control’ or ‘warning’ to identify the expected calibration values for each piece of equipment and to identify subtle trends. Westgard’s rules for QC (see Physical QC section for more details) should be used to quantitatively analyse these trends.
A good example of where this is essential is when examining a common misconception about calibrations. Specifically, some manufacturers use the percentage change from the previous calibration to flag ‘faulty’ calibrations. In the following example, the CO2 analyser gain is highlighted as out of range because the percentage change from the previous calibration is large (arbitrary values are often chosen, in this case greater than 5%). In a continuation of this misapprehension, users are encouraged to repeat the same calibration and this appears to ‘fix’ the problem. However, when seen in the context of a trend (figure 3), it becomes clear that the aforementioned interpretation is misleading. This outcome can result from two opposite situations with contrasting consequences.
Figure 3.
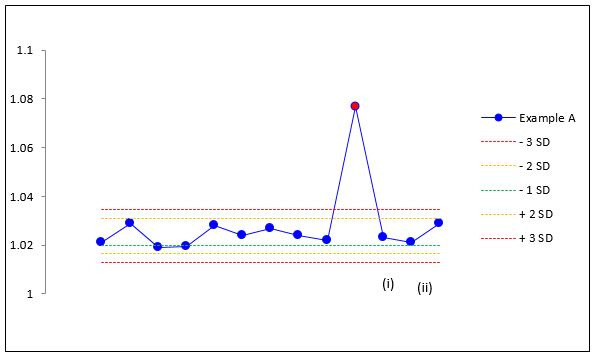
Calibration example situation A trend data.
In one situation A, a user performs an incorrect calibration (eg, with a damaged sample line) and does not check the gain value before saving the new calibration, thus saving a value out of the expected range. When a subsequent calibration (using an undamaged sample line) is performed, the user gets values as described in table 4i, flagging this calibration as an error when in fact the value has dropped back down into the expected range. When another calibration is performed (table 4ii) in an attempt to fix the problem, there is no change in the gain and the calibration is a ‘pass’ according to the device. In fact, both calibrations were a pass and the second one was not required. There was nothing to fix but a faulty calibration has gone unnoticed.
Table 4.
Calibration example situation A
| Situation A | ||
| (i) Incorrect ‘fail’ | ||
| Previous | Current | % Diff |
| 1.077 | 1.023 | −5.0% |
| (ii) Correct ‘pass’ but unnecessary | ||
| Previous | Current | % Diff |
| 1.023 | 1.021 | −0.2% |
In alternative situation B, the device is performing normally until the user performs an incorrect calibration, for example, with a damaged flow sensor. The device flags the calibration as having deviated from the previous one by an unacceptable percentage, giving the values in table 5i. Again, in an attempt to fix the problem, the user repeats the calibration (table 5ii). However, because the faulty sensor is still in place, the gain value does not change from the previous incorrect value and the device ‘passes’ this calibration. Rather than being fixed, this only signifies that this calibration is equally as bad as the previous one, but this fault will now go unnoticed.
Table 5.
Calibration example situation B
| Situation B | ||
| (i) Correct ‘fail’ | ||
| Previous | Current | % Diff |
| 1.022 | 1.077 | 5.4% |
| (ii) Incorrect ‘pass’ | ||
| Previous | Current | % Diff |
| 1.077 | 1.073 | −0.4% |
Looking at the device data in isolation (tables 4 and 5) does not allow the user to discriminate between these situations. Looking at trends A and B (figures 3 and 4) allows the user to identify what has happened from the first calibration attempt. In A, no second calibration is required, but the fact that a previous calibration error went undetected should be flagged and any test performed on that date should be re-examined. In B, the out of range gain should prompt a fault-finding procedure that should detect the faulty sensor. Once this is replaced, the gain value will drop back within range and clinical testing can be performed.
Figure 4.
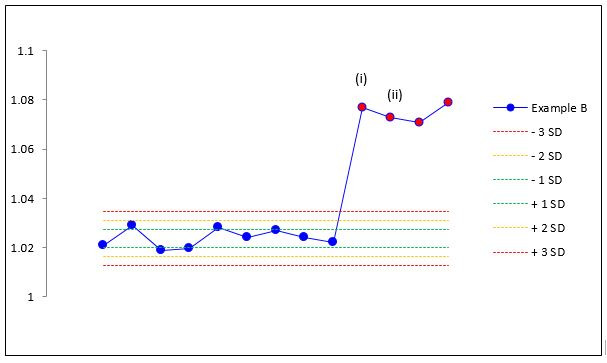
Calibration example situation B trend data.
Physical QC
QC using physical (mechanical) or non-biological methods is often incorrectly denominated calibration. It is no more than a one-point or two-point verification between the measurement sensor and a volume or gas of a known composition. ‘True’ calibration is normally performed at factory level or on site by service engineers. The equipment used to perform calibrations is expensive and cumbersome and usually not readily available in a clinical environment. The use of simulators and mass flow sensors is described in literature, but its applicability seems to be more of interest for research purposes.8 9
The number of manufacturers with commercially available exercise testing equipment integrated with gas exchange has risen greatly over the last few years. It is therefore difficult to stereotype the type of flow, volumes and gas analysis methods used in current practice. In case of doubt, manufacturer recommendations must be followed to ensure the testing system is in good working condition.
Ambient conditions are normally measured by built-in sensors in the testing system. Ideally, these sensors should be open to room air but as close as possible to the gas analysers. It is good practice to verify measurements of humidity, barometric pressure and temperature on a daily basis against a validated weather station (table 6). Ambient conditions should be measured and entered into the software immediately prior to a test.
Table 6.
Calibration and verification methods and frequencies for various ambient condition sensors
| Ambient conditions | ||
| Method/sensor | Verification/calibration | Frequency |
| Hygrometer, thermometer, barometer, pressure/barometric altimeter | Validated weather station (check for agreement ±3%) | Daily/sessional or before each test if unable to control ambient temperature |
There are well-known advantages and caveats of a series of volume and flow measuring devices. These differences are well described elsewhere.33 Regardless of the type of volume/flow sensor, verification of volumes and flows should be performed before each test using the same breathing circuit used during testing. Calibration should be performed in a manner consistent with the manufacturer’s guidance, but a follow-up verification should be performed with a 3 L syringe to check flow dependence. This should use a wide variety of flow rates, and the resulting volume should be within the recommended repeatability (table 7). Ultimately, these sensors should comply with European Respiratory Society (ERS)/American Thoracic Society (ATS) standards for spirometers.34
Table 7.
Calibration and verification methods and frequencies for various volume and flow sensors
| Volume (L) and flow (L/s) | ||
| Method/sensor | Verification/calibration | Frequency |
| Pneumotachograph | Manual Validated 3 L syringe (±3%) using low, mid and high flows34 Automatic (equipment) Low and high flows (±3%) |
Before each test |
| Pitot tube | ||
| Turbine | ||
| Hot-wire anemometers | ||
Douglas bags or systems which use mixing chambers are rarely used in clinical practice, so this description pertains to breath-by-breath systems which most clinical laboratories use. These systems employ ‘fast’ gas analysers with rapid analyser response times and specified delay times.
All analysers are affected by water vapour concentration and consequent condensation at the point of analysis. The use of a sulfuric acid lined Teflon tubing allows the addition (during verification) or removal (during testing) of moisture from gas samples. This means that by the point the expired gas reaches the analysers, the water vapour should be equivalent to ambient conditions, which is neither wet nor dry.
It is paramount, however, that every verification is performed with a clean and properly dried breathing circuit, including the sampling line. With regular usage, the latter will need to be replaced, particularly when the transparent appearance becomes yellow/orange in colour (saturated). It is also good practice to ensure the dead space input on the testing system is actually correct (this can normally be found in the equipment manual). The process of verification should consist of a two-point verification, normally to room air and to the gas cylinder mixture (table 8). The use of a second gas cylinder with different composition to test the accuracy and precision of the analysers at the extreme of measurable ranges is considered good practice (eg, 26% oxygen cylinder, nitrogen balance).
Table 8.
Calibration and verification methods and frequencies for various O2 and CO2 gas analysers
| O2 and CO2 gas analysers | ||
| Method/sensor | Verification/calibration | Frequency |
| Mass spectrometers/infrared | 2-point calibration Known FiO2 and FiCO2 (±1%)8 9 Mass flow sensor/simulator 21% O2 in N2 (balance) at (x) flow (±1%) |
Before each test |
| Electrochemical | Not common in clinical setting | |
Although unlikely, it is possible, for calibration gas to be supplied with an unacceptable variance from the labelled concentrations. In this situation the device may pass calibration but may result in significant variance from biological controls. For this reason, it is best practice to store an additional gas bottle for use for fault finding or in the event of the routine gas bottle running out.
Procedures vary from one manufacturer to the next, but there are generally at least four parameters to be determined by a gas calibration:
Slope: often referred to as ‘gain’, it is the ratio of change in the input parameter versus change in output parameter. The slope is one of two parts to the equation used to linearise the relationship between the upper and lower gas calibration concentrations.
Offset: the ‘offset’ is where the calibration line crosses the Y-axis.
Delay time: the time from when the gas is introduced into the system to when it is detected by the gas analysers. The delay time is dictated by the length of the sampling tube.
Response time: the inherent ‘response time’ of the system which has a predictable time constant.
It is worth noting that the processes described previously are a simplification of what actually happens in commercially available metabolic carts, and even different models from the same manufacturer can differ in their principles of operation. It is important to be aware of the aforementioned general principles, but equally, it is unfair to expect that all systems will operate identically. As a result, operators and clinicians should take a keen interest in the particulars of their own specific equipment and, as previously mentioned, ask their supplier detailed questions about its internal workings.
Gas calibration should be performed prior to testing. Calibration should be repeated if any part of the gas sampling apparatus is changed between tests, for example, the gas sample line. This is particularly pertinent for systems which use oxygen analysers that react with O2 to produce a voltage and, in the process, consume themselves. These types of ‘cell’ change their voltage to gas concentration relationship over time. As such, care should be taken to ascertain over what period of time this relationship changes. It should not result in a measurable difference over the length of time that a normal test takes. To do this, calibrate the system as normal, then perform a biological control test. After the test is complete, run a verification of the calibration and check that the variance is within the expected range. If the gain has increased over that short period of time, it may indicate that the ‘O2 cell’ is coming to the end of its life and needs to be replaced.
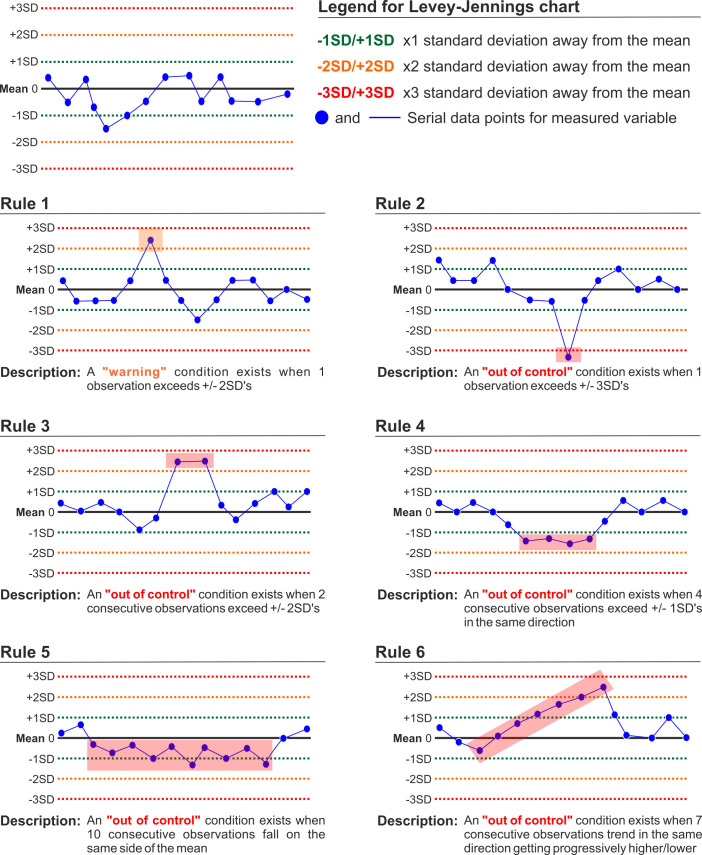
As a result of performing regular physical QC procedures, there is often a considerable amount of data that can be used to further provide evidence on the stability of the measuring equipment. This often does not take place in clinical practice and may mask potential trends in equipment function that are not readily noticeable when comparing single verifications. In most testing systems, the calibration data is easily transferable to an Excel spreadsheet, where basic descriptive statistics can be performed to explore the data. The work of Westgard and colleagues35 details a series of steps to understand variation within the data as well as a group of criteria to identify ‘out of control’ conditions. Once processed this information can be easily plotted in a Levey-Jennings control chart (mean, 2 SDs and 3 SDs from mean) to visually and very intuitively check whether there are data trends that could meet an ‘out of control” condition. Figure 5 describes and depicts ‘out of control” conditions according to Westgard and Barry.36
Figure 5.
Example of Westgard criteria plotted in a Levey-Jennings chart which can be used to visually and intuitively identify data trends that could meet an ‘out of control’ condition.35 36
Treadmill and cycle ergometers are commonly used to generate a measurable workload during exercise testing. Inaccuracies in power output, particularly in cycle ergometers, have been reported in literature and can vary as much as 18%.37 This has significant implications on the measurable gas exchange parameters. There are fewer reports implicating inaccuracies in the use of treadmill ergometry; however, the process of verification should be regarded as equally important (tables 9 and 10).
Table 9.
Calibration and verification methods and frequencies for various cycle ergometers
| Cycle ergometry (power output) | ||
| Method/sensor | Verification/calibration | Frequency |
| Mechanically braked | ±2% or ±3 W (above 25 W)8 9 Dynamic torque Metre, specialist, not readily available equipment (often performed by manufacturer) |
Yearly as part of servicing, when moved or on suspicion of malfunction |
| Electromagnetically braked | ||
Table 10.
Calibration and verification methods and frequencies for treadmill
| Treadmill (speed and grade) | ||
| Method/sensor | Verification/calibration | Frequency |
| Manual/digital control | ±0.2 mph and ±0.5% grade8 9 Time revolution of belt and carpenter’s rule. Specialist, not readily available equipment (often performed by manufacturer) |
Yearly as part of servicing, when move, or on suspicion of malfunction |
Both methods of testing should be subject to verification and calibration (if required) when moved. The equipment required is often not readily available in respiratory laboratories as it is expensive and bulky. Alternative, supposedly simpler, verification methods have been described,38 39 but their practical application is difficult and likely to generate confusion rather than assurance. In view of this, constant workload biological QC testing is perhaps the best way of ensuring adequate working conditions on a short-term basis. In case of doubt, service engineers should be called onsite to investigate the problem or carry out required maintenance or servicing, as required.
Other equipment, not directly related with CPET testing systems, should be regularly verified. This includes stadiometers, weighing scales, blood gas analysers, manual blood pressure cuffs and automatic defibrillators. The clinical engineering department will often be responsible to ensure the equipment listed previously is regularly serviced, is in good working condition and is compliant with electrical safety requirements.
Biological QC
Regular biological QC (also referred to as physiological control) using one or more healthy testing subjects at regular intervals, similar time of day and under controlled conditions, is important to monitor the precision of the testing equipment as a whole.40 Healthy testing subjects could be drawn from volunteering staff members. They should be free from test contraindications and consent for their data to be used for QC purposes. Using healthy subjects who are practised and familiar with the equipment and QC protocol is recommended and has been shown to return very little biological variability.40 Biological QC allows a holistic evaluation of the testing system and can uncover systematic errors that physical methods alone are not able to.41 Testing should be carried out during protected laboratory downtime. There are currently three different protocol designs which can be used as part of a biological QC programme. We recommend that one or more of these protocols are deployed on a weekly basis:
Incremental ramp (to monitor precision of flow and gas measuring devices at the extremes of working ranges).
Constant work protocol (to monitor serial precision and stability of flow and gas measuring devices as well as rule out indirect sources of bias).
Isotime protocol (allows for direct measurement of the dynamic slopes used in CPET interpretation).
Services should produce and maintain standard operating procedures which detail the QC protocol or protocols in use currently within that service.
Incremental ramp protocol
During incremental ramp the test can be conducted if used on the exact same terms as it is performed in patients. Initially, at a shorter time interval, serial tests should be performed to determine baseline mean values to then allow ongoing comparison with less frequent testing to calculate the coefficient of variation (CoV). Previous data has been published on what is considered to be acceptable CoV.32 41 This is shown in table 11.
Table 11.
CoV standards for various physiological variables measured during a biological quality control programme
| Biological QC standards for CPET | |||||||||
| Variable | HR | BF | VE | VO2 | VCO2 | RER | VTex | ΔVO2/ΔWR | ΔVE/ΔVCO2 |
| CoV | 5% | 8% | 8% | 6% | 6% | 8% | 8% | 10% | 10% |
BF, breathing frequency; CoV, coefficient of variation; CPET, cardiopulmonary exercise testing; RER, respiratory exchange ratio; VTex, expiratory tidal volume.
Constant work protocol
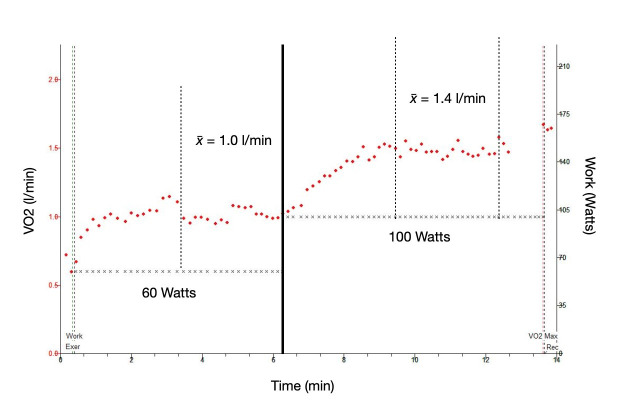
The constant work protocol has been presented by other authors.11 It involves the use of at least two power outputs, low from around 30 to 60 W and high, from 70 to 100 W. It is imperative that both work rates (WRs) are subanaerobic threshold. The testing subject should be able to tolerate both loads for at least 6 min. Once again, testing should be performed in similar conditions to patient testing. The protocol should start with at least 3 min of baseline measurements, followed by 3 min of unloaded cycling, 6 min at a low and constant load and finally a further 6 min at a high and constant loads (figure 6). It is expected that within 3 min of pedalling at a constant load, gas exchange parameters reach a steady state. Mean values for parameters such as VO2, VCO2 and VE should be derived from the steady-state portion of the test. These data should be recorded in a database for serial analysis and identification of differences in expected variables. The Westgard rules, described previously, should be applied to these data, however, it is important to note that the use of Westgard rules to monitor biological QC data may not inform that an error is about to happen and may miss actual errors due to subject variability.42
Figure 6.
Graphical representation of the constant work protocol used during a biological quality control session. Steady-state VO2 at 60 and 100 W is collected in a normal healthy control subject.
The relationship between VO2 and WR is a useful measure to record as it provides an overall ‘sense check’ of the testing equipment. In health, one would expect an increase in VO2 of approximately 10 mL/min/W increase in WR; therefore, the measured change in VO2 between two differing constant WR protocols would be directly proportional to the difference in WR by a factor of 10. ΔVO2/ΔWR values which deviate above or below the 95% CI (~8.5 to 12.5 mL/min/W)43 44 should raise suspicion of a significant deviation in the measurement.11
Isotime protocol
The steady-state method of QC is the reference method for biological control with considerable supporting evidence; however, it can be time consuming. Between set-up and waiting for gas kinetics to stabilise, there is a large portion of the procedure which does not contribute to the outcomes. This method also suffers from a lack of specificity because it uses steady-state measurements to infer the accuracy of an incremental test. Using a submaximal incremental protocol and measuring values at isotime points during the test are an alternative that has the convenience of being more practical and more activity specific. It also allows for direct measurement of the dynamic slopes used in CPET interpretation.
The protocol starts with a ‘check’ phase to ensure the resting data are stable before proceeding. Resting data are captured for 3 min before starting an unloaded phase where the subject pedals at approximately 60–80 revs/min with no applied WR (0 Watts) for a further 3 min. Providing the respiratory exchange ratio (RER) is stable the ramp phase is started at an increment of between 15 and 30 W/min. The increment used can be determined from the subject’s previous maximal ramp tests and adjusted with the aim of obtaining repeatable submaximal data over a clinically relevant time course that is equivalent to a normal patient test, specifically 8 min. It is important that subsequent tests use the same cadence and ramp increment. After 8 min, pedalling is stopped. If recovery data are of interest, the subject can remain on the ergometer for an additional 3 min.
Measurement of all relevant parameters can be sampled at 2.5, 5.0 and 7.5 min. Providing the same averaging that is used during clinical tests is applied, each isotime should only use a single data point. The slopes should use a statistical ‘best fit’ average between 2.5 and 7.5 min to avoid interoperator variability. The first 2.5 min is omitted in order to exclude gas kinetic curves at the onset of the WR ramp. An example of data taken at an isotime of 5.0 min during this type of protocol is shown in table 12. This is averaged over the last 10 tests and shows the type of variability that can be expected.
Table 12.
Example of data taken at an isotime of 5 min during an isotime quality control protocol; this is averaged over the last 10 tests and shows the type of variability that can be expected
| Parameter | Units | Mean | Variance (%) |
| Power | W | 190 | 0.5 |
| HR | beats/min | 117 | 3.1 |
| VO2 | mL/min | 2608 | 4.1 |
| VCO2 | mL/min | 2091 | 3.7 |
| SPO2 | % | 99 | 1.0 |
| RPM | revs/min | 79 | 2.0 |
| BF | breaths/min | 27 | 14.1 |
| VE | L/min | 63 | 5.3 |
| VTEX | L | 2 | 11.0 |
| PETCO2 | kPa | 5 | 3.5 |
| PETO2 | kPa | 14 | 2.1 |
| TI/TTOT | 44 | 2.9 | |
| VE/VO2 | 23 | 6.5 | |
| VE/VCO2 | 29 | 4.8 | |
| BPSYS | mm Hg | 150 | 13.1 |
| BPDIA | mm Hg | 69 | 11.9 |
| ΔHR/ΔVO2 | beats/mL | 23 | 10.3 |
| ΔVE/ΔVCO2 | 27 | 7.1 | |
| ΔVO2/ΔWR | mL/min/W | 10 | 5.7 |
| ΔVO2/ΔLOG10VE | 4902 | 10.0 |
Source: P. Jamieson Respiratory Laboratory, Hairmyres Hospital, NHS Lanarkshire, UK.
Where multiple exercise testing systems are available, efforts to exercise the same biological control in different pieces of equipment are highly recommended. Ideally, a difference in measured gas exchange variables of no more than ±5% should be witnessed. In real terms, however, this can normally be expected to increase to ±10% and still be considered acceptable. Patients who are expected to perform serial exercise tests should always be set up on the same testing system to decrease sources of non-physiological bias.
Regardless of the type of protocol used for biological QC, there are important technical aspects inherent to the method of gas exchange analysis that need to be taken into account. Most systems in use have adopted the breath-by-breath method, although there are mixed reviews on whether mixing chamber systems may be superior.45 The latter requires ponderous consideration between tidal volume and size of the mixing bag.31 Inappropriate equipment selection in this method would result in either large or subtle fluctuations in gas concentration affecting the validity of measurements. A similar argument is raised as an issue in breath-by-breath systems with regard to lack of standardisation in sampling rates and consequent effect on measured VO2 and VCO2.46 Signal averaging between 30 and 60 s, as long as unchanged across serial measurements and is consistently adopted within the laboratory protocol, is considered normal practice in a clinical setting.8
Facilities
The exercise laboratory should be free of clutter and welcoming to patients. CPET equipment often varies in size and may consist of any combination of bicycle ergometer, arm crank ergometer, treadmill, ECG, blood pressure monitoring equipment and ventilatory gas exchange analyser. It is therefore important that the exercise laboratory is not only large enough to accommodate such equipment but also provides enough space to enable staff and patients to walk around freely, allowing adequate access to the patient in case of an emergency.47
Patients may be easily distracted during the test protocol. Where possible, monitoring screens should not be visible to the patient. Dialogue between test operators must be kept to a minimum as this can distract the patient and potentially alter peak values. The position of the ergometer in relation to the system cart must allow clear communication. Communication between the patient (hand gestures) and the test operator (verbal feedback and encouragement) is key and predisposes to maximal effort.
Resuscitation facilities must be available in the event of a cardiac/respiratory arrest and must include an automatic external defibrillator (AED), arrest trolley, oxygen and suction in line with current UK Resuscitation Council guidelines.48 This equipment should be checked and deemed fully functional before the start of each testing session. There must also be a system in place for rapidly summoning a hospital arrest team in case of an emergency, for example, an alarm switch or telephone bleep system. All staff working in the area must be fully briefed on the protocol for summoning the hospital arrest team before participating in CPET.
The laboratory should be positioned, within the hospital, in such a way as to allow fast access from the arrest team. There should be a private area within the exercise laboratory which patients can use for changing clothes before and after the test, and efforts to maintain privacy throughout the test should be made; this includes minimising interruptions throughout the test49 and ensuring that only required members of staff are present during the CPET.
The testing area should be clean, well lit and well ventilated in line with health and safety guidelines50 and ARTP guidance.47 Furthermore, the laboratory should be temperature and humidity controlled, where possible, as heart rate and rating of perceived exertion increase as ambient temperature increases.49 The American Heart Association has made recommendations that a temperature of between 20°C and 22°C is comfortable for the patient to exercise and suggest that a cooler dryer exercise environment enhances cutaneous heat exchange, helping to dissipate heat generated by exercise.49 The laboratory should contain a thermometer, barometer and hygrometer to allow gas exchange measurements to be adjusted for ambient conditions.
Although CPET testing has been historically performed under physician supervision due to the additional risk of cardiac arrest during or soon after vigorous exercise,51 it has been concluded that the risk of medical complications is related to the underlying disease, rather than the test itself, and the mortality rate for patients during exercise testing is 2–5 per 100 000 clinical exercise tests.8 A comparison of CPET performed by physicians and by trained physiologists/nurses suggests that the incidence of adverse events during CPET is no different.51 It is therefore now widely accepted that CPET can be safely performed by adequately trained and experienced healthcare professionals.
Personnel
A minimum of two qualified healthcare professionals are required to safely perform CPET and should be present during the exercise and recovery stage of the test.52 A physician should be present for the CPET if deemed necessary by the referring physician or if the healthcare professionals performing the test believe the patient is at sufficient risk of developing complications during testing.11 Ultimately, a physician should be immediately available during all exercise tests52 53 if required. Healthcare professionals from several disciplines may possess the training and experience required to competently perform CPET.49 These include physiologists working within the field of respiratory or cardiology, exercise physiologists, nursing staff and physician assistants.
If tests are performed within a secondary care centre or acute NHS Trust, where there is rapid access to an arrest trolley and emergency resuscitation (crash) team, we recommend that healthcare professionals performing CPET be trained in basic life support with AED. If CPET is performed in any other location, we recommend that healthcare professionals performing CPET are trained to the level of immediate life support (ILS).
Leadership
The test must be led by a competent CPET practitioner. It is the role of the lead practitioner to accurately review the medical history,49 supervise the test and ensure that all protocols are followed correctly regarding test procedure. It is the role of the lead practitioner to interpret the test data and produce an accurate report while ensuring that all absolute contraindications have been excluded before commencing the test and ensuring that staff involved with the test are suitably qualified.52
The second practitioner required for CPET should work in an assisting role52 and should be assessed as competent to assist in line with local departmental policies. The role of the assistant may include duties such as performing accurate calibration of the test equipment before each CPET, preparing the patient pretest by accurately positioning the 12-lead ECG electrodes, performing any pretest procedures such as pulmonary function testing or capillary blood gas testing, encouraging the patient throughout the CPET as instructed by the lead practitioner and assisting in the safe recovery of the patient following the exercise test. It is vital that both the supervisor and assistant are fully aware of the indications for test termination and that either member of staff may end the test when required.
Competency
All competent CPET practitioners must be able to identify and manage adverse events in relation to CPET by discriminating between normal and abnormal responses to exercise.11 The lead practitioner will have extensive exercise test experience and practical or academic knowledge attained through a formal training programme, for example, a CPET-specific course which provides the underpinning knowledge required to safely perform and interpret the CPET. The lead practitioner should have an advanced knowledge of ECG interpretation.
Levels of competency will reflect the specific requirements of the service and may differ between services. However, in line with other organisations,11 CPET practitioners who will be performing and reporting CPET tests should have completed a suitable course, provided by an accredited organisation, and covering key learning outcomes (box 5).
Box 5. Suggested key learning outcomes which should be covered during any suitable cardiopulmonary exercise testing course provided by an accredited organisation.
Key learning outcomes
Normal cardiac and respiratory physiological responses to exercise.
-
Safe practice, contraindications to test, when to terminate test.
Preparing the patient for the test.
Performing the test.
Protocol selection based on reason for referral.
Patient set-up.
Exercise ECG interpretation.
Test termination.
Appropriate data collection for analysis.
Interpretative strategies based on reason for referral.
Identifying an abnormal response to exercise.
Generating an effective report and answering the clinical question.
Delivering a quality assured service.
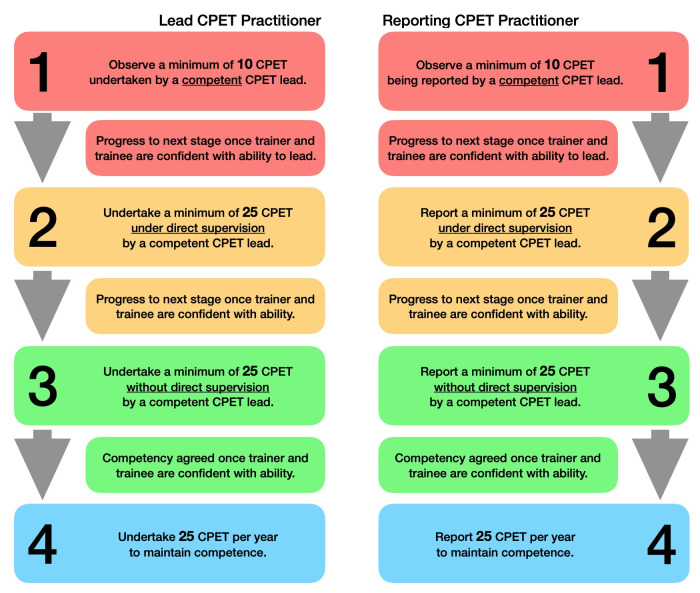
Additionally, the competency pathways detailed in figure 7 should be followed to ensure competency to perform CPET in a lead practitioner role and/or competency to report CPET. Where possible, new practitioners working towards competency should expose themselves to a mixed cohort of patient testing; this should include patients referred for diagnostic or preoperative assessment and patients referred from differing specialities, for example, respiratory or cardiology.
Figure 7.
Recommended competence pathways for lead CPET practitioners and reporting CPET practitioners. CPET, cardiopulmonary exercise testing.
Once a practitioner is deemed to be competent to perform or report CPET, ongoing maintenance of competency is essential to minimise clinical risk and potential adverse events. Primarily, competence is maintained with frequent performance of CPET. We would suggest a minimum of 25 tests per year. Continued professional development is essential and should reflect the skills and knowledge base presented in current literature and clinical guidelines. Additionally, routine monitoring of interpretive skills and clinical reporting should be adhered to. This can be achieved with periodic review and over reading by a competent colleague. Peer review could be completed within the practitioner’s own service or by a competent colleague working within a neighbouring service. Gaps in knowledge identified during this process should be resolved with further training.
As a minimum, the assistant should be competent in the use of the CPET equipment52 and trained in a field related to the test itself while having at least a basic knowledge of normal and abnormal exercise responses, and able to recognise an abnormal ECG,8 oxygen desaturation, hypotension and hypertension.
Test performance
Protocol selection
The aim of the clinical CPET is to make measurements of cardiorespiratory function while the patient performs work. The work should incrementally progress from a period of unloaded exercise to maximal exertion within approximately 8–12 min.54 When deciding which protocol to use during CPET, consideration should be given to the individual performing the test and the desired outcome measures: is the patient attending for preoperative fitness assessment or for diagnostic purposes? This question is important for deciding the appropriate protocol. It is possible to estimate how rapidly WR should be progressed based on the patient’s predicted peak work load and subtracting the work of unloaded exercise. The following equation can be used to calculate the optimum cycle ergometry work increment for individual patients55:
Work rate increment=predicted VO2Max–VO2unloaded)/103
However, consideration of pulmonary function impairment, patient activity or fitness levels should also influence the practitioner’s decision as to which exercise protocol will be best suited to achieve the desired outcome measures. In line with other authors,12 we recommend adjusting the protocol by ±5 W/min.
During treadmill-based CPET, belt speed and/or incline are adjusted over time in order to elicit an increase in exercise intensity. Several established protocols exist,56–58 which are based on these principles; however, none of these protocols allow for a linear increase in WR as a ramp or minute by minute increment.12 Algorithm-based protocols have been developed59 more recently which aim to produce linear increases in WR which more closely replicate exercise responses seen in cycle ergometry.
Measurement principles
Pretest spirometry
Quality assured spirometry should be performed prior to CPET by a practitioner who is qualified and competent to do so. If investigating the presence of exercise induced dynamic hyperinflation, baseline measurement of inspiratory capacity (IC) should be completed. Forced expiratory volume in the first second (FEV1) and forced vital capacity are used to determine the presence of airway obstruction or a restrictive defect. For details on the performance of spirometry, please see the ARTP Statement on Lung Function 2020.14
The measurement of maximum voluntary ventilation (MVV) can be indirectly estimated from the spirometry manoeuvre. This approach has now superseded the direct measurement of MVV60 and is not recommended here. There are many methods suggested in the literature for indirect estimation of MVV using various multiplication factors of FEV1; however, most laboratories use the measured FEV1×40 method for estimating MVV. The consensus opinion of the authors of this document is that MVV should be estimated indirectly by multiplying the measured FEV1×40.
Basic components
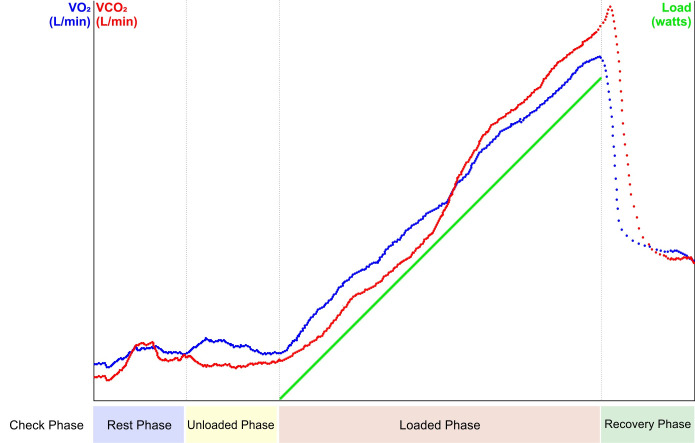
There should be five basic components to any cardiopulmonary exercise protocol (figure 8).
Figure 8.
Five basic components of a standard CPET test. CPET, cardiopulmonary exercise testing.
Check phase: during the check phase, the patient is attached to all equipment and visualisation of the outputs is made possible. This is an opportunity to ensure there are no software or hardware issues before any measurements are made and that all parameters appear to be reasonable and physiologically plausible.
Rest phase: during rest, all parameters are being recorded without any exercise being performed. This is an opportunity to determine whether there are any resting physiological variables that may be influencing exercise performance (eg, resting hyperventilation). This phase typically lasts a minimum of 2 min and until a stable baseline has been achieved.
Unloaded phase: this is the point in the exercise test where the patient performs unloaded exercise, that is, the metabolic demand on the system to move the limbs used for exercise only. It allows measurements of loaded exercise to be made from a true zero point. It is not possible to perform unloaded exercise on a treadmill as load is required as soon as the treadmill begins to move and hence is a potential drawback in using treadmill exercise. Unloaded exercise can only be truly performed using an electromagnetically braked cycle ergometer which will assist patients to turn their legs at low levels of exercise. This phase usually lasts no longer than 3 min.
Loaded phase: at this point in the exercise test, a load will be introduced to the patient. For the treadmill, this will result in an increase in speed and/or incline. For the cycle ergometer, an increase in resistance required to turn the flywheel is applied. Depending on the chosen protocol, this load may be introduced at low levels to begin and increase at specific time intervals (eg, step or ramp protocols) or commence at higher-intensity levels and remain constant for the duration that the patient is able to continue exercising (eg, endurance protocols). For cycle ergometry, the duration of this phase should be no less than 8 min to ensure the patient does not stop due to an excessive workload and/or that important phases during the exercise test are not missed. The test duration should also be no greater than 12 min to reduce the likelihood of the patient stopping due to something other than symptom limitation (eg, boredom or saddle soreness).
Recovery phase: during the recovery phase, load is reduced and variables are continually monitored to measure the patient’s ability to recover from the exercise protocol. The recovery phase can be terminated, for instance, when VO2 has returned to 50% of peak values or heart rate returns to within 20 beats/min of pretest resting values. Some sites choose to use the period as a warm-down for the patient, during which time the patient continues to cycle at reduced load in the hope of maintaining blood flow to the muscles and removing by-products of exercise (eg, lactate build-up). We would suggest that recovery variables are collected, and once targets have been achieved, a warm down is allowed to ensure venous return and appropriate muscle recovery.
Blood gas analysis
The performance of arterial blood gas (ABG) or capillary blood gas (CBG) during the rest phase and immediately postexercise adds additional benefit when investigating the cause of unexplained exertional breathlessness. Hyperventilation and gas exchange abnormalities can present with similar response patterns, that is, raised ventilatory slope, raised ventilatory equivalents and reduced end-tidal carbon dioxide levels. However, to accurately distinguish between these response patterns, it is essential to understand whether there is a wide alveolar to arterial oxygen partial pressure difference (P(A-a)O2) and/or a wide end-tidal to arterial difference in carbon dioxide (P(a-ET)CO2) and hence the need for blood gas sampling. Oxygen saturation levels recorded by pulse oximetry are not accurate enough in all subjects to allow accurate distinction. If one or both of these are wider than the expected normal, then this is highly suggestive of a gas exchange and/or dead space problem.
Additionally, clinically relevant information on arterial carbon dioxide tension, pH, bicarbonate and blood lactate levels can be obtained from arterialised blood gas sampling at rest and immediately postexercise. The ratio of physiological dead space ventilation (VD) to tidal ventilation (VT) can be calculated by the metabolic system using the measurement of arterial carbon dioxide tension. VD/VT provides further assessment of the efficiency of gas exchange. Although most metabolic systems will provide a calculated VD/VT without measurement of arterial carbon dioxide tension, this is based on end tidal gas tensions and should be considered inaccurate.
It is important that the blood is collected while the patient is connected to the mask/mouthpiece and breath samples are being recorded. The metabolic system must be notified at the point at which the sample is collected so the breath values can be directly compared with the blood sample values. This ensures an accurate comparison between arterial gas values and gases being inspired/expired. For details on the performance of ABGs/CBGs, please see the ARTP Statement on Lung Function 2020.14
Ergometer selection
The measurement of cardiac and ventilatory responses to linear increases to WR enables accurate, non-invasive identification of exercise thresholds. A plethora of exercise ergometer devices is available for measuring incremental physical work during exercise testing, including specific machines for swimming, kayaking, Nordic skiing and rowing. These more specialised devices are often specific to exercise physiology and sports science laboratories, and used predominantly in the assessment of athletes. In the clinical environment, cycle ergometers and treadmills are most commonly used as these are more widely available and replicate the type of activity which most patients can easily relate to (table 13).
Table 13.
Expected characteristic differences between cycle ergometry and treadmill modalities for CPET testing
| Cycle | Treadmill | |
| Peak oxygen uptake | Lower | Higher |
| Ability to implement ramp protocol | Easier | Harder |
| Quantifiable external work | Yes | Based on algorithm |
| Blood gas collection | Easier | Harder |
| ECG quality | Higher | Lower |
| Noise and artefacts | Less | More |
| Safety | Safer | Increased risk of falls |
| Weight bearing in obese | Less | More |
| Degree of leg muscle training | Less | More |
| Possible use in recumbent position | Yes | No |
| More appropriate for | Patients | Active normal subjects |
| Cost | Lower | Higher |
| Size | Smaller | Larger |
| Ease of movement | Easier | Harder |
Adapted from the American Thoracic Society and American College of Chest Physicians and Mezzani.8 90
CPET, cardiopulmonary exercise testing.
Treadmills are more often used in cardiology laboratories and offer the advantage of allowing exercise, which is more representative of daily activities for most people, for example, walking, jogging and running, and for this reason are often well tolerated by most patients. Exercising on a treadmill does involve upper body muscle mass due to the global nature of ambulatory movement; the effect of this is that peak VO2 can often be 5%–10% higher than on a cycle ergometer.31 This is an obvious advantage in the assessment of athletes or those patients where determination of VO2peak is desirable. The disadvantage of increased upper body muscular involvement is the possibility of introducing movement artefact to measurement of blood pressure, oximetry, ventilatory and gas exchange indices. A further important factor for consideration when using a motorised treadmill is that oxygen use may be reduced if the patient requires the assistance of the hand rails61 due to the weight-bearing benefit of leaning against the equipment.
There are many established treadmill protocols used in clinical practice. These are often employed in cardiac exercise tests in which increases in ventilatory or metabolic indices are not considered. Traditional treadmill WR protocols which use ‘stepped’ or non-uniform increases in belt speed and gradient are not always suitable for CPET as they are likely to result in non-linear increases in metabolic rate,59 rendering non-invasive detection of the anaerobic threshold difficult. Additionally, protocols which call for high constant belt speed may not be tolerated by some clinical patients and may result in failure to perform the test or early termination of exercise. For CPET, treadmill protocols should consist of a low initial WR which is increased continuously by adjustment of belt speed and angle to produce a linear increase in work intensity56 and, therefore, metabolic rate, over an adequate period of time to allow sufficient physiological response to exercise.
Cycle ergometers are a popular alternative to the treadmill in the clinical setting. These devices are physically smaller and usually less expensive62 than most treadmills. Exercising on a stationary bicycle is often well tolerated by most individuals but may be difficult for those not experienced in using a cycle or those with lower limb orthopaedic or vascular limitations. The cycle ergometer is often considered a safer option than the treadmill in patients who have balance or gait problems,61 for obvious reasons. On a cycle ergometer, the patient can stop cycling as soon as they develop any mobility issues or are unable to continue the test, whereas those exercising on a motorised treadmill must first signal to the operator that they wish to stop before the belt is slowed to a stop.31
In the obese patient, use of the cycle ergometer over a treadmill may provide an advantage. As the patient is supported at all times and the nature of the exercise modality is less weight bearing, obese individuals who are unable to mobilise on a treadmill may be able to exercise for a sufficient length of time to generate enough data for analysis. The weight restrictions on commercial cycle ergometers are the obvious limiting factor in this instance. As an alternative to the traditional upright cycle ergometer, semirecumbent cycle ergometers are now commercially available and offer a higher ceiling on weight restriction. This particular cycle type is often more comfortable for obese individuals who may be physically limited by physical build in a traditional cycling position.
The advantage of using a cycle ergometer is that WR can be directly quantified8 and incremental WR protocols can be tightly controlled to allow for smaller increments as and when required. This is particularly useful when elderly or frail patients are tested and require much more gentle increases in exercise intensity than a younger or fitter individual. Compared with the treadmill, the action of exercising on a cycle ergometer limits the muscular involvement of the arms and upper body. The obvious disadvantage of this is that peak VO2 will be lower compared with treadmill testing; however, this often reduces movement and noise artefact, which can otherwise make ECG, blood pressure, blood gas sampling and oxygen saturation analysis difficult.
Amputees and patients with sufficient disability or lower limb mobility issues may not be able to perform leg exercise on either a cycle or treadmill. In this instance, these individuals can perform CPET on an arm crank ergometer and generate physiological response data which is similar to leg ergometry.63 Standalone arm crank ergometers are now commercially available; however, it is not uncommon for modified cycle ergometers to be used. There are, however, limitations with this exercise test modality. Primarily, the action required to turn an arm crank ergometer involves and interferes with respiratory accessory muscles.8 This may result in increased dyspnoea and/or the early termination of exercise in those patients with respiratory disease. The rhythmic nature of turning the arm crank is also not an action which many individuals are experienced in; this has also been suggested as a possible cause of early onset of fatigue during this exercise modality.64
Additionally, the metabolic stress induced during arm crank exercise is significantly lower than leg ergometry due to a much smaller active muscle mass61 resulting in lower reported peak VO2 values and earlier onset of metabolic acidosis.8 65 To date, arm crank ergometry has gained little traction in clinical assessment of patients, and this is largely due to lack of research in this area. It is widely accepted that comparing the measured physiological exercise response patterns to age-matched normal predicted values is common practice. The predicted values used should be matched to the appropriate exercise modality,31 an area that is still lacking in arm crank ergometry.
In the field of presurgical assessment via CPET, there is an established wealth of literature focusing on the use of cycle or treadmill exercise, but to date, very little exists to support the use of arm crank ergometry in the surgical cohort. This area of research is now beginning to develop and, recently, it has been suggested that the relationship between arm crank and leg ergometry is not a simple one and likely does not share a linear relationship based on muscle mass alone. While some clinical value may be obtained from CPET via arm crank (eg, identification of cardiac/ventilatory limitation or ECG changes), values obtained through arm crank ergometry are poor predictors of those values obtained through cycle ergometry.66 As a result of this, using arm crank ergometry for risk stratification of surgery is an insufficient substitute for traditional leg exercise, until further research has been conducted in this area.
Test end criteria
The criteria for determining when the CPET operator should consider terminating the exercise test prematurely are presented in box 6. The patient and their symptoms should be the priority when determining if it is safe to continue with an exercise test.
Box 6. Premature test termination criteria Adapted from Levett et al and The Society for Cardiological Science and Technology.11 52.
Premature test termination criteria
-
Angina:
Significant arrhythmias causing symptoms or haemodynamic compromise
Fall in systolic blood pressure >20 mm Hg from the highest value during the test
-
Hypertension >250 mm Hg systolic and >120 mm Hg diastolic
Patients with abdominal aortic aneurysms undergoing preoperative assessment >200 mm Hg systolic or >110 mm Hg diastolic.
Severe desaturation: SpO2 <80% (lower may be accepted in patients with known underlying lung disease or congenital heart disease if agreed with referring clinician).
Loss of coordination.
Mental confusion.
Dizziness or faintness.
Post-test instructions
On completion of the exercise test, the following precautionary advice should be relayed to the patient before leaving the exercise laboratory:
To not take a hot shower for at least 1 hour following the test. Blood vessels expand following exercise; a hot shower may attenuate this further causing a possible drop in blood pressure which may lead to dizziness and the risk of a fall or feeling faint.
To rest further in the waiting area if they wish to do so before leaving the department.
The patient can eat and drink as usual following the test.
The patient should also be advised that if on leaving the hospital they begin to experience chest pain that lasts more than 10 min, or if patient’s own, prescribed, glyceryl trinitrate (GTN) medication does not relieve the symptoms, they should call 111/999 or report to the nearest accident and emergency (A&E) department.
Technical comments
It is important that for all exercise tests technical information about the test is documented. The following should be considered on all reports to assist with both data interpretation and to confirm test quality:
Document the type of exercise ergometer used during the test.
The incremental WR should be stated and also how long the patient exercised for.
The reason for stopping the test: was the test terminated by the patient (see box 7) or the clinician? The test will be terminated either due to maximum patient effort or clinical deterioration. Ascertain the patient’s symptoms and what they felt limited their ability to exercise.
Box 7. Common reasons patients will stop a cardiopulmonary exercise testing.
Test termination by patient
Dyspnoea.
Leg fatigue.
Chest pain.
Pain/physical discomfort.
Dizziness.
Saddle discomfort.
Palpitations
Determination of maximal test
The level of effort achieved by the patient is important for the interpretation of an exercise test. Patients must reach their maximal capacity for exertion to extrapolate the findings at peak to their surgical risk or functional capacity. Box 8 lists the criteria for determining if a patient’s effort during CPET could be considered maximal.
Box 8. Criteria for determining if patient effort is maximal during cardiopulmonary exercise testing.
Criteria for determining if effort is maximal
Achieves a plateau in VO2 (indicating the patient has reached their VO2max).
Heart rate reaches 90% of predicted or heart rate reserve is ≤15 beats/min
There is evidence of ventilation limitation (breathing reserve <15%, expiratory flow limitation, also consider significant increase in end-expiratory lung volume).
mBorg for leg fatigue or breathlessness is ≥9/10.
Peak exercise blood lactate concentration ≥8 mmol/L (if measured).
The RER is not a reliable indicator of maximal exertion due to its variability with hyperventilation and erratic breathing patterns. However, if the graphical data presents itself normally (ie, it is not >1 during rest or reference exercise, it is not oscillatory as a result of an erratic breathing pattern), the RER may be supportive of maximal exertion when >1.15. We strongly recommend against using a value of 1.05 to determine maximal exertion.67
Reference values
Reference values are a core component of a clinical exercise test. Reference values provide context to measured values and allow for data interpretation. Compared with previous standard documents,9 the availability of relevant references values has increased considerably. However, it is not always clear which reference sets are appropriate. Takken and colleagues68 69 have published an admirable review of the available literature which encapsulates most relevant studies and sets out 14 methodological criteria with which to assess them. It is important to point out that none of the reference papers they examined achieved acceptability in all criteria. As such, choosing reference values is still a compromise and a laboratory should look to find the reference set which best reflects their priorities from the following characteristics.
Contemporary—Many metabolic carts are still sold with some of the ‘classic’ reference values set as the default. These studies are generally decades old, published in the previous century. Modern sets of reference values are continuously being published. In the last 5 years; more data have been published than in the preceding 35 years.68 Methodologies, equipment and technology are continuously improving. It is therefore imperative that current CPET practitioners move on to reference values published more recently (see table 14).
Table 14.
Recommended reference values
Geographically specific: the sample tested should be drawn from a similar background and geographical area as the laboratory’s testing population and include a mix of similar ethnic origins.
Adequate sample size: a larger sample size is favourable; studies with small sample sizes should be avoided.
Equality of sex distribution: the closer to 50/50 female/male, the better for most normal populations. However, there may be instances such as perioperative assessment for a sex-specific cancer that may be best served by single-sex reference values.
Equality of age distribution: optimally, studies should cover paediatrics through to geriatrics, but as minimum reference values should cover the upper and lower ages of the population being tested in the intended laboratory.
Relevant testing parameters: having reference values for multiple parameters (O2pulse, AT, ΔVE/ΔVCO2, end test PETCO2, etc) included in the same study makes these values more internally consistent and is preferred to having values taken from a mix of different studies.
Statistical relevance and utility: even modern studies often only publish mean or median equations for the parameters studied. This is at odds with the trend within modern medicine of using lower and upper limits of normal and centiles or z-scores to estimate the level of impairment. Precedence should be given to studies that provide more detailed descriptions of the values measured.
Comparable methodologies—The test protocols used should be consistent with that used in the intended laboratory and should include averaging time, apparatus, mode of exercise and choice of ergometer.
At the time of publishing, there is no one set of reference values which ticks all of these boxes, and so the onus is on individual laboratories to choose the most appropriate for them. In the future, the aim must be to match efforts such as the Global Lung Initiative70 and produce a set of regularly updated multicentre reference values that use sophisticated statistical modelling, include longitudinal data, and cover children and the elderly.
We recommend the following reference values (table 14) for use in adults. These studies represent a good compromise in the areas described previously for clinical laboratories in the UK. It may also act as a consensus to provide short-term continuity between centres across the country if these are adopted as a default in the absence of better reference values.
Paediatric considerations
There is high clinical value in performing CPET in paediatrics in various circumstances. The non-invasive nature of the test makes it suitable for young children through to adolescents. Most children 10 years and above will be able to perform a technically acceptable test. However, this should not be a strict rule as often children as young as 6 will manage the test. The fundamental principles of CPET are the same in paediatrics as in adults. There are some slight modifications in the way the test is performed, the equipment used and the interpretation of the data. We detail these differences as follows based on published evidence and experience. Using this guidance, along with the recommendations already detailed, will give a comprehensive guide to CPET in paediatrics.
Indications
Exercise testing plays a vital role in the diagnosis and monitoring of paediatric cardiorespiratory disease. Physical activity is an important part in the daily life of children and adolescents both in health and disease. CPET is a useful tool which can assist in excluding pathology in children and adolescents who are experiencing shortness of breath on exertion (SOBOE). Asthma is the most common chronic disease in childhood,71 and this can often be brought on or exacerbated by exercise. It is important to note that CPET is not a sensitive test to diagnose exercise-induced bronchoconstriction (EIB). If EIB is suspected, a constant load 6 min treadmill challenge test preferably in a cold, dry environment which provokes a ventilation of 40%–60% of MVV or 80% of predicted peak heart rate should be the test of choice.72 If this test is negative, then CPET can be used as a second-line test to exclude any other pathology causing the symptoms.
CPET is the gold standard for measurement of aerobic fitness.8 In children with cystic fibrosis (CF), aerobic fitness has been shown to be an independent predictor of mortality.73 Current European guidelines recommend that CPET be used routinely in children aged ≥10 years.74 The UK CF Trust also recommends the use of exercise testing at annual review when clinically indicated.75 CPET has been shown to be a clinically useful test as part of an annual review procedure in children with CF.76
Children with congenital heart disease will often experience SOBOE as they go from childhood to adolescence. Often, they may require intervention and CPET plays an important role in deciding the timing of such interventions. It will also help the clinician elucidate whether the symptoms are due to a progression of the cardiac disease, coexistent respiratory disease, deconditioning or a combination of these.77
Set-up and equipment
The setup of the CPET in children will be the same as that in adults described in the previous section. Equipment must be available in a range of sizes to accommodate young children through to adolescents. Smaller child specific mask sizes should be used for younger children to ensure an adequate seal. SpO2 sensors, ECG electrodes and blood pressure cuffs should be available in paediatric sizes.
The test can be performed on a treadmill or cycle ergometer. The cycle ergometer is preferred for safety reasons as there is less chance of children injuring themselves. However, if they are not tall enough to reach the pedals adequately or if there is a specific symptom that occurs during running, then a treadmill protocol can be used. There are paediatric specific cycle ergometers available. However, the main difference between these and a standard cycle ergometer is the child friendly colours and not the size. If using a standard adult ergometer, there should be adjustable cranks to allow you to shorten them for smaller children and the option of a paediatric saddle, which will again help fit smaller children onto the cycle ergometer.
Performance of the test and protocol selection
The performance of the CPET in children is very similar to that in adults. The five basic components described previously are the same in children—check, rest, unloaded, loaded and recovery phases. In terms of protocol selection, the ramp protocol is the most common and most suitable. The gradual increment in workload will minimise effort perception and provide the gradational stimulus to span the intensity of the work domains. It is recommended that if your equipment allows it, the watts/min increment should be broken down into increments of 10–12 s increments. For example, a 15 W/min protocol will actually be increased by 2.5 W every 10 s. As described in the previous chapter, the ideal test duration is 8–12 min. The choice of ramp should be based on the child’s weight. The following equation requires calculation of peak VO278 and unloaded VO279 and can be used to calculate the optimum work increment for individual patients:
Male=((52.78×weight (kg)–303.4) – (5.8×weight (kg)+151))/103
Female=((28.5×weight (kg)+288.1) – (5.8×weight (kg)+151))/103
If the child has significant pulmonary or cardiac impairment, then the ramp calculated from the aforementioned equation may be too difficult and choosing the ramp as folllows may be more appropriate.
Treadmill protocols are more difficult to individualise. However, in our experience, using a Standard Exponential Exercise Protocol (STEEP) often provides a ‘one size fits all’ in most children. This protocol was originally developed to accommodate a wide range of adult patients with a single protocol.80 The STEEP protocol begins at a low workload with 15% increments in workload each minute (table 15).
Table 15.
Steep protocol
| Stage (min) | |||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Speed (mph) |
1.5 | 2.0 | 2.0 | 2.0 | 2.5 | 2.5 | 2.5 | 3.0 | 3.0 | 3.0 | 3.5 | 3.5 | 3.5 | 4.2 | 5.0 |
| Speed (kph) |
2.4 | 3.2 | 3.2 | 3.2 | 4.0 | 4.0 | 4.0 | 4.8 | 4.8 | 4.8 | 5.6 | 5.6 | 5.6 | 6.7 | 8.0 |
| Incline (degrees) |
0 | 0 | 1.5 | 3 | 3 | 5 | 7 | 7 | 9 | 11 | 11 | 13 | 16 | 16 | 16 |
Some children/adolescents that are referred for CPET may be involved in competitive sports and this is where their symptoms are occurring. If the sport mainly involves running, then specific incremental athletic treadmill protocols can be used. These generally involve a small constant incline with increments in speed at each stage.
Staffing/supervision
Ischaemic heart disease is very rare in a young population; therefore, exercise testing in children poses less of a risk to that in adults. There are no published data on the incidence of mortality in paediatric exercise testing, but this will undoubtedly be less than what is stated in the previous chapter for adults. In a recent American Heart Association statement,81 it was recommended that clinical exercise tests be safely supervised by appropriately trained non-physician healthcare professionals. We echo this statement and recommend that the test be supervised by at least two properly trained healthcare professionals who have the experience and qualifications that have been detailed in the previous section. It should also be noted that the physician’s role as final authority for safety, quality and interpretation in the delivery of the CPET service is vital.
End test criteria
CPET is a test to a volitional maximum. Children can sometimes be more difficult than adults to motivate. However, with the correct coaching and encouragement, it is entirely possible. A gold standard for a maximal test is a plateau in the VO2. However, this is rarely seen in children. There are several other criteria to indicate a maximal effort in children during an exercise test. For children with normal cardiovascular function, peak heart rate should be within 15 beats of the predicted value.
The traditional 220 minus age equation has been shown to overestimate peak heart rate in children and adolescents.82 Recent normal values of peak heart rate in children and adolescents during cycle ergometry CPET show that boys aged 8 years have a mean of 184 beats/min with a small linear increase to 191 beats/min at 14 years, which then remains stable until 18 years old. Females aged 8 years had a mean of 187 beats/min which had a small linear increase to 191 beats/min at 11 years. It then stabilised at this until 14 years after which it showed a small linear decline to 189 at 18 years old.83 An RER, >1.1 can be used in most paediatric patients. However, some small children will not have big enough lungs to generate RERs as high as this during exercise; therefore, this should be interpreted with caution in these patients. Finally, the presence of respiratory compensation will indicate a near maximal test.
Reference values
As with any reference values, the ones which are chosen should best reflect the characteristics of the population tested and the equipment and methodology used. In a recent review of paediatric CPET reference values,84 it was concluded that current reference values are based on heterogeneous protocols with variable normalisation strategies and that high-quality reference values are still needed. The important work of Cooper and colleagues85–87 looking at normal values in children is still commonly used today. These authors looked at North American children and developed reference equations for VO2peak, peak O2pulse, anaerobic threshold and the ΔVE/ΔVCO2. They used a ramp protocol on a cycle ergometer in 109 children aged 6–17 years. Despite the widespread use of these values, they are outdated, use simple linear regression and only contain a small number of children in each age bracket.
More recently, Bongers et al83 have published reference values in 214 Dutch children aged 8–18 years old. They used the least median of squares (LMS) regression statistical method, which is superior to simple regression.88 The major disadvantage of this reference set is that they were in relation to sex and age only. Height and weight were not taken into account. Bearing in mind that the function of the cardiopulmonary system is heavily dependent on anthropometric variables, we do not find these useful for most parameters.
In 2018, Blanchard et al89 published reference equations for CPET using cycle ergometry in 12–17 year olds. They looked at six maximal parameters including VO2, O2pulse, workload, ventilation, heart rate and RER. They also looked at a range of submaximal parameters including OUES, ΔVE/ΔVCO2, VO2 at the ventilatory threshold, ΔVO2/ΔWR and heart rate recovery after 1 and 2 min. Polynomial regression equations were used, taking into account sex, age, height and weight. Another major benefit is that they allow for the generation of Z scores. They found that CPET parameters had a strong correlation with fat-free mass but a weak correlation with fat mass. This led to development of prediction equations that use ideal body weight if the subject has a high body mass index.
Currently, we would recommend using the Blanchard predicted set when the patient is aged 12–17. If the patient is <12 years old, then the Cooper values are recommended. However, care should be taken when interpreting these values if the patient is overweight. If testing is performed on a treadmill, then the predicted VO2 from cycle ergometry should be increased by 11% to take into account the different exercise modality.31
Footnotes
Contributors: PA, PJ, TH, MP, BP, CJP, JP, TM and SKP were involved in writing, editing and review of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; internally peer reviewed.
Author note: The group was originally convened in 2016 and a review of the current literature undertaken by each chapter contributor for their relevant section. Literature was further reviewed throughout the development process up until the point of submission, in light of newer best practice publications. All of the authors have approved the contents of this paper and the manuscript was then sent for consultation to the Association for Respiratory Technology and Physiology (ARTP) Editorial Board prior to submission. The manuscript has also been reviewed by an ARTP CPET specialist panel, which consisted of expert practitioners working within in the field of cardiopulmonary exercise testing. These panel members were independent of the authors.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Jones P, Miravitlles M, van der Molen T, et al. Beyond FEV1 in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis 2012;7:697–709. 10.2147/COPD.S32675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodyear SJ, Yow H, Saedon M, et al. Risk stratification by pre-operative cardiopulmonary exercise testing improves outcomes following elective abdominal aortic aneurysm surgery: a cohort study. Perioper Med 2013;2:10. 10.1186/2047-0525-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fotheringham I, Meakin G, Punekar Y, et al. Comparison of laboratory- and field-based exercise tests for COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 2015;10:625–43. 10.2147/COPD.S70518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Older P, Hall A. Clinical review: how to identify high-risk surgical patients. Crit Care 2004;8:369. 10.1186/cc2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Older P, Smith R, Hall A, et al. Preoperative cardiopulmonary risk assessment by cardiopulmonary exercise testing. Crit Care Resusc 2000;2:198–208. [PubMed] [Google Scholar]
- 6.Huddart S, Young EL, Smith R-L, et al. Preoperative cardiopulmonary exercise testing in England – a national survey. Perioper Med 2013;2:4. 10.1186/2047-0525-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucknall M, Butterfield K. ARTP Workforce & Workload Survey, 2018. Available: https://www.artp.org.uk/Reports/a5056393-cb7b-401a-bd4a-c114b383fc55 [Accessed 08 Jul 2021].
- 8.American Thoracic Society, American College of Chest Physicians . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–77. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 9.Roca J, Whipp BJ, Agusti AGN. Clinical exercise testing with reference to lung diseases: indications standardization and interpretation strategies, 1997. Available: https://moh-it.pure.elsevier.com/en/publications/clinical-exercise-testing-with-reference-to-lung-diseases-indicat [DOI] [PubMed]
- 10.Roberts C, Ward S, Walsted E, et al. Safety of pulmonary function testing: data from 20 years. Thorax 2018;73:385–7. 10.1136/thoraxjnl-2017-210246 [DOI] [PubMed] [Google Scholar]
- 11.Levett DZH, Jack S, Swart M, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth 2018;120:484–500. 10.1016/j.bja.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 12.Radtke T, Crook S, Kaltsakas G, et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019;28. 10.1183/16000617.0101-2018. [Epub ahead of print: 31 Dec 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper BG. An update on contraindications for lung function testing. Thorax 2011;66:714–23. 10.1136/thx.2010.139881 [DOI] [PubMed] [Google Scholar]
- 14.Sylvester KP, Clayton N, Cliff I, et al. ARTP statement on pulmonary function testing 2020. BMJ Open Resp Res 2020;7:e000575. 10.1136/bmjresp-2020-000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Best PJ, Tajik AJ, Gibbons RJ. The safety of treadmill exercise stress testing in patients with abdominal aortic aneurysms. Ann Intern Med 1998;129:628–31. 10.7326/0003-4819-129-8-199810150-00008 [DOI] [PubMed] [Google Scholar]
- 16.McArthur S, Robson A, Mitchard N. An audit of adverse events during pulmonary function testing (PFT) and cardiopulmonary exercise testing (CPEX) in pre-operative abdominal aortic anuerysm (AAA) patients. Eur Respir J 2013;42 https://erj.ersjournals.com/content/42/Suppl_57/1794 [Google Scholar]
- 17.Tew GA, Moss J, Crank H, et al. Endurance exercise training in patients with small abdominal aortic aneurysm: a randomized controlled pilot study. Arch Phys Med Rehabil 2012;93:2148–53. 10.1016/j.apmr.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Myers JN, White JJ, Narasimhan B, et al. Effects of exercise training in patients with abdominal aortic aneurysm: preliminary results from a randomized trial. J Cardiopulm Rehabil Prev 2010;30:374–83. 10.1097/HCR.0b013e3181ebf2db [DOI] [PubMed] [Google Scholar]
- 19.Myers J, McElrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc 2014;46:2–9. 10.1249/MSS.0b013e3182a088b8 [DOI] [PubMed] [Google Scholar]
- 20.Robertson V, Rayt H, Kirkbride D, et al. Abdominal aortic aneurysm rupture following cardiopulmonary exercise testing. Anaesth Cases 2017;5:38–43. 10.21466/ac.ACOAAAR.2017 [DOI] [Google Scholar]
- 21.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation 2005;111:816–28. 10.1161/01.CIR.0000154569.08857.7A [DOI] [PubMed] [Google Scholar]
- 22.Philbin EF, Ries MD, French TS. Feasibility of maximal cardiopulmonary exercise testing in patients with end-stage arthritis of the hip and knee prior to total joint arthroplasty. Chest 1995;108:174–81. 10.1378/chest.108.1.174 [DOI] [PubMed] [Google Scholar]
- 23.Goodman RA, Solomon SL. Transmission of infectious diseases in outpatient health care settings. JAMA 1991;265:2377–81. 10.1001/jama.1991.03460180083038 [DOI] [PubMed] [Google Scholar]
- 24.ARTP COVID-19 Group . Guidelines for recommencing physiological services during the coronavirus disease 2019 (COVID-19) endemic phase, 2020. Available: https://www.artp.org.uk/write/MediaUploads/Standards/COVID19/ARTP_COVID-19_endemic__guidance_Vers_5.6_final.pdf [Accessed 08 Jul 2021].
- 25.Kendrick AH, Johns DP, Leeming JP. Infection control of lung function equipment: a practical approach. Respir Med 2003;97:1163–79. 10.1016/S0954-6111(03)00223-3 [DOI] [PubMed] [Google Scholar]
- 26.Faghy MA, Sylvester KP, Cooper BG, et al. Cardiopulmonary exercise testing in the COVID-19 endemic phase. Br J Anaesth 2020;125:447–9. 10.1016/j.bja.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacey BS, Rose GA, Davies RA, et al. Effect of a novel viral filter on cardiopulmonary exercise testing during the COVID-19 pandemic. Anaesthesia 2021;76:1003–4. 10.1111/anae.15451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagnon J, Province MA, Bouchard C, et al. The heritage family study: quality assurance and quality control. Ann Epidemiol 1996;6:520–9. 10.1016/S1047-2797(96)00068-3 [DOI] [PubMed] [Google Scholar]
- 29.United Kingdom Accreditation Society . Improving quality in physiological services (IQIPS). UKAS. Available: https://www.ukas.com/accreditation/standards/iqips/ [Accessed 12 Jul 2021].
- 30.Becklake MR. Concepts of normality applied to the measurement of lung function. Am J Med 1986;80:1158–64. 10.1016/0002-9343(86)90678-9 [DOI] [PubMed] [Google Scholar]
- 31.Wasserman K. Principles of exercise testing and interpretation : including pathophysiology and clinical applications. 5 edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 32.Cooper B, Butterfield A. Quality control in lung function testing. In: ERS Buyers’ Guide Respir Care Prod, 2009: 24–38. [Google Scholar]
- 33.Cooper BG, Evans AE, Kendrick AH. Practical handbook of respiratory function testing: part two. Association of respiratory technology and physiology, 2005. [Google Scholar]
- 34.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. an official American thoracic society and european respiratory society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westgard JO, Groth T, Aronsson T, et al. Performance characteristics of rules for internal quality control: probabilities for false rejection and error detection. Clin Chem 1977;23:1857–67. 10.1093/clinchem/23.10.1857 [DOI] [PubMed] [Google Scholar]
- 36.Westgard JO, Barry PL. Chapter 4 in Cost-effective quality control: managing the quality and productivity of analytical processes. In: Improving quality control by use of Multirule control procedures, 1986. [Google Scholar]
- 37.Guiraud T, Léger L, Long A, et al. Vo2 requirement at different displayed power outputs on five cycle ergometer models: a preliminary study. Br J Sports Med 2010;44:449–54. 10.1136/bjsm.2007.044826 [DOI] [PubMed] [Google Scholar]
- 38.Clark JH, Greenleaf JE. Electronic bicycle ergometer: a simple calibration procedure. J Appl Physiol 1971;30:440–2. 10.1152/jappl.1971.30.3.440 [DOI] [PubMed] [Google Scholar]
- 39.Van Praagh E, Bedu M, Roddier P, et al. A simple calibration method for mechanically braked cycle ergometers. Int J Sports Med 1992;13:27–30. 10.1055/s-2007-1021229 [DOI] [PubMed] [Google Scholar]
- 40.Revill SM, Morgan MD. Biological quality control for exercise testing. Thorax 2000;55:63–6. 10.1136/thorax.55.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cramer D, Ward S, Unstead M. Biological quality control data relating to lung function and cardioplumonary exercise. Inspire 2011;11:15–26. [Google Scholar]
- 42.Swanney MP, Stanton JD. Spirometry biological controls: the scientist who cried wolf. Eur Respir J 2016;48. 10.1183/13993003.congress-2016.OA286 [DOI] [Google Scholar]
- 43.Hansen JE, Sue DY, Oren A, et al. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol 1987;59:669–74. 10.1016/0002-9149(87)91190-8 [DOI] [PubMed] [Google Scholar]
- 44.Hansen JE, Casaburi R, Cooper DM, et al. Oxygen uptake as related to work rate increment during cycle ergometer exercise. Eur J Appl Physiol Occup Physiol 1988;57:140–5. 10.1007/BF00640653 [DOI] [PubMed] [Google Scholar]
- 45.Beijst C, Schep G, Breda Evan, van BE, et al. Accuracy and precision of CPET equipment: a comparison of breath-by-breath and mixing chamber systems. J Med Eng Technol 2013;37:35–42. 10.3109/03091902.2012.733057 [DOI] [PubMed] [Google Scholar]
- 46.Hill DW, Stephens LP, Blumoff-Ross SA, et al. Effect of sampling strategy on measures of VO2peak obtained using commercial breath-by-breath systems. Eur J Appl Physiol 2003;89:564–9. 10.1007/s00421-003-0843-1 [DOI] [PubMed] [Google Scholar]
- 47.ARTP Department Size Working Group . Lung function department size and space recommendations, 2006. Available: https://www.artp.org.uk/Reports/86896265-92a0-454d-9aad-80e40af8b12392
- 48.Resuscitation Council UK . Quality standards for cardiopulmonary resuscitation practice and training: acute care - equipment and drug lists, 2020. Available: https://www.resus.org.uk/quality-standards/acute-care-equipment-and-drug-lists/ [Accessed 13 Jul 2021].
- 49.Myers J, Arena R, Franklin B, et al. Recommendations for clinical exercise laboratories: a scientific statement from the American heart association. Circulation 2009;119:3144–61. 10.1161/CIRCULATIONAHA.109.192520 [DOI] [PubMed] [Google Scholar]
- 50.Health and Safety Executive . Workplace health, safety and welfare: a short guide for managers, 2017. Available: http://www.hse.gov.uk/pubns/indg244.pdf [Accessed 13 Jul 2021].
- 51.Franklin BA, Gordon S, Timmis GC, et al. Is direct physician supervision of exercise stress testing routinely necessary? Chest 1997;111:262–5. 10.1378/chest.111.2.262 [DOI] [PubMed] [Google Scholar]
- 52.The Society for Cardiological Science & Technology . Clinical guidance by consensus: recommendations for clinical exercise tolerance testing, 2008. Available: https://fd.org.ua/wp-content/uploads/2019/03/Clinical-Guidance-by-Consensus-Recommendations-for-Clinical-Exercise-Tolerance-Testing.pdf [Accessed 13 Jul 2021].
- 53.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American heart association. Circulation 2001;104:1694–740. 10.1161/hc3901.095960 [DOI] [PubMed] [Google Scholar]
- 54.Arena R, Myers J, Williams MA, et al. Assessment of functional capacity in clinical and research settings: a scientific statement from the American heart association Committee on exercise, rehabilitation, and prevention of the Council on clinical cardiology and the Council on cardiovascular nursing. Circulation 2007;116:329–43. 10.1161/CIRCULATIONAHA.106.184461 [DOI] [PubMed] [Google Scholar]
- 55.Cooper C, Storer TW. Exercise testing and interpretation: a practical approach. Cambride University Press, 2001: 79–82. [Google Scholar]
- 56.Naughton J, Sevelius G, Balke B. Physiological responses of normal and pathological subjects to a modified work capacity test. J Sports Med Phys Fitness 1963;3:201–7. [PubMed] [Google Scholar]
- 57.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 1973;85:546–62. 10.1016/0002-8703(73)90502-4 [DOI] [PubMed] [Google Scholar]
- 58.Nagle FJ, Balke B, Naughton JP. Gradational step tests for assessing work capacity. J Appl Physiol 1965;20:745–8. 10.1152/jappl.1965.20.4.745 [DOI] [PubMed] [Google Scholar]
- 59.Porszasz J, Casaburi R, Somfay A, et al. A treadmill ramp protocol using simultaneous changes in speed and grade. Med Sci Sports Exerc 2003;35:1596–603. 10.1249/01.MSS.0000084593.56786.DA [DOI] [PubMed] [Google Scholar]
- 60.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 61.Forman DE, Myers J, Lavie CJ, et al. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med 2010;122:68–86. 10.3810/pgm.2010.11.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albouaini K, Egred M, Alahmar A, et al. Cardiopulmonary exercise testing and its application. Postgrad Med J 2007;83:675–82. 10.1136/hrt.2007.121558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schrieks IC, Barnes MJ, Hodges LD. Comparison study of treadmill versus arm ergometry. Clin Physiol Funct Imaging 2011;31:326–31. 10.1111/j.1475-097X.2011.01014.x [DOI] [PubMed] [Google Scholar]
- 64.Davis JA, Vodak P, Wilmore JH, et al. Anaerobic threshold and maximal aerobic power for three modes of exercise. J Appl Physiol 1976;41:544–50. 10.1152/jappl.1976.41.4.544 [DOI] [PubMed] [Google Scholar]
- 65.Reybrouck T, Heigenhauser GF, Faulkner JA. Limitations to maximum oxygen uptake in arms, leg, and combined arm-leg ergometry. J Appl Physiol 1975;38:774–9. 10.1152/jappl.1975.38.5.774 [DOI] [PubMed] [Google Scholar]
- 66.Loughney L, West M, Pintus S, et al. Comparison of oxygen uptake during arm or leg cardiopulmonary exercise testing in vascular surgery patients and control subjects. Br J Anaesth 2014;112:57–65. 10.1093/bja/aet370 [DOI] [PubMed] [Google Scholar]
- 67.Thomas M, Sylvester K. An rER of 1.05 should not be used to determine maximal effort during CPET, 2020. Available: https://www.ers-education.org/lr/show-details/?idP=246195
- 68.Takken T, Mylius CF, Paap D, et al. Reference values for cardiopulmonary exercise testing in healthy subjects - an updated systematic review. Expert Rev Cardiovasc Ther 2019;17:413–26. 10.1080/14779072.2019.1627874 [DOI] [PubMed] [Google Scholar]
- 69.Paap D, Takken T. Reference values for cardiopulmonary exercise testing in healthy adults: a systematic review. Expert Rev Cardiovasc Ther 2014;12:1439–53. 10.1586/14779072.2014.985657 [DOI] [PubMed] [Google Scholar]
- 70.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017;50:1700010. 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 71.Asher I, Pearce N. Global burden of asthma among children. int j tuberc lung dis 2014;18:1269–78. 10.5588/ijtld.14.0170 [DOI] [PubMed] [Google Scholar]
- 72.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. this official statement of the American thoracic Society was adopted by the ats board of directors, July 1999. Am J Respir Crit Care Med 2000;161:309–29. 10.1164/ajrccm.161.1.ats11-99 [DOI] [PubMed] [Google Scholar]
- 73.Pianosi P, Leblanc J, Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax 2005;60:50–4. 10.1136/thx.2003.008102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hebestreit H, Arets HGM, Aurora P, et al. Statement on exercise testing in cystic fibrosis. Respiration 2015;90:332–51. 10.1159/000439057 [DOI] [PubMed] [Google Scholar]
- 75.Cystic Fibrosis Trust . Standards for the clinical care of children and adults with cystic fibrosis in the UK, 2011. Available: https://www.cysticfibrosis.org.uk/sites/default/files/2020-12/Cystic%20Fibrosis%20Trust%20Standards%20of%20care.pdf
- 76.Weir E, Burns PD, Devenny A, et al. Cardiopulmonary exercise testing in children with cystic fibrosis: one centre's experience. Arch Dis Child 2017;102:440–4. 10.1136/archdischild-2016-310651 [DOI] [PubMed] [Google Scholar]
- 77.Massin MM. The role of exercise testing in pediatric cardiology. Arch Cardiovasc Dis 2014;107:319–27. 10.1016/j.acvd.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 78.Wasserman K, Hansen JE, Sue DY. Principles of exercise testing and interpretation : including pathophysiology and clinical applications. In: Principles of exercise testing and interpretation. 166. Philadelphia: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- 79.Wasserman K, Hansen JE, Sue DY. Principles of exercise testing and interpretation : including pathophysiology and clinical applications. In: Principles of exercise testing and interpretation. 19. Philadelphia: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- 80.Northridge DB, Grant S, Ford I, et al. Novel exercise protocol suitable for use on a treadmill or a bicycle ergometer. Heart 1990;64:313–6. 10.1136/hrt.64.5.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Myers J, Forman DE, Balady GJ, et al. Supervision of exercise testing by nonphysicians: a scientific statement from the American heart association. Circulation 2014;130:1014–27. 10.1161/CIR.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahon AD, Marjerrison AD, Lee JD, et al. Evaluating the prediction of maximal heart rate in children and adolescents. Res Q Exerc Sport 2010;81:466–71. 10.1080/02701367.2010.10599707 [DOI] [PubMed] [Google Scholar]
- 83.Bongers BC, van Brussel M, Hulzebos EHJ. Pediatric norms for cardiopulmonary exercise testing: in relation to sex and age, 2014. [Google Scholar]
- 84.Blais S, Berbari J, Counil F-P, et al. A systematic review of reference values in pediatric cardiopulmonary exercise testing. Pediatr Cardiol 2015;36:1553–64. 10.1007/s00246-015-1205-6 [DOI] [PubMed] [Google Scholar]
- 85.Cooper DM, Weiler-Ravell D, Whipp BJ, et al. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol Respir Environ Exerc Physiol 1984;56:628–34. 10.1152/jappl.1984.56.3.628 [DOI] [PubMed] [Google Scholar]
- 86.Cooper DM, Weiler-Ravell D, Whipp BJ, et al. Growth-Related changes in oxygen uptake and heart rate during progressive exercise in children. Pediatr Res 1984;18:845–51. 10.1203/00006450-198409000-00008 [DOI] [PubMed] [Google Scholar]
- 87.Cooper DM, Kaplan MR, Baumgarten L, et al. Coupling of ventilation and CO2 production during exercise in children. Pediatr Res 1987;21:568–72. 10.1203/00006450-198706000-00012 [DOI] [PubMed] [Google Scholar]
- 88.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60. [PubMed] [Google Scholar]
- 89.Blanchard J, Blais S, Chetaille P, et al. New reference values for cardiopulmonary exercise testing in children. Med Sci Sports Exerc 2018;50:1125–33. 10.1249/MSS.0000000000001559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mezzani A. Cardiopulmonary exercise testing: basics of methodology and measurements. Ann Am Thorac Soc 2017;14:S3–11. 10.1513/AnnalsATS.201612-997FR [DOI] [PubMed] [Google Scholar]
- 91.Gläser S, Ittermann T, Schäper C, et al. [The study of health in pomerania (SHIP) reference values for cardiopulmonary exercise testing]. Pneumologie 2013;67:58–63. 10.1055/s-0032-1325951 [DOI] [PubMed] [Google Scholar]
- 92.Edvardsen E, Hansen BH, Holme IM, et al. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest 2013;144:241–8. 10.1378/chest.12-1458 [DOI] [PubMed] [Google Scholar]