Abstract
The pathophysiology and risk factors of nonalcoholic fatty liver disease (NAFLD) among lean patients is poorly understood and therefore investigated. We performed a meta‐analysis of observational studies. Of 1175 articles found through searching from Medline/PubMed, Banglajol, and Google Scholar by two independent investigators, 22 were selected. Data from lean (n = 6768) and obese (n = 9253) patients with NAFLD were analyzed; lean (n = 43 398) and obese (n = 9619) subjects without NAFLD served as controls. Age, body mass index, waist circumference, systolic blood pressure, and diastolic blood pressure (DBP) had significantly higher estimates in lean NAFLD patients than in lean non‐NAFLD controls. Fasting blood sugar [MD(mean difference) 5.17 mg/dl, 95% CI(confidence interval) 4.14–6.16], HbA1c [MD 0.29%, 95% CI 0.11–0.48], and insulin resistance [HOMA‐IR] [MD 0.49 U, 95% CI 0.29–0.68]) were higher in lean NAFLD patients than in lean non‐NAFLD controls. All components of the lipid profile were raised significantly in the former group except high‐density lipoprotein. An increased uric acid (UA) level was found to be associated with the presence of NAFLD among lean. Cardio‐metabolic profiles of nonlean NAFLD patients significantly differs from the counter group. However, the magnitude of the difference of lipid and glycemic profile barely reached statistical significance when subjects were grouped according to lean and nonlean NAFLD. But DBP (slope: 0.19, P < 0.037), HOMA‐IR (slope: 0.58, P < 0.001), and UA (slope: 0.36, P = 0.022) were significantly higher if NAFLD was present compared to that of non‐NAFLD group. Lean and nonlean NAFLD patients are metabolically similar and share common risk factors.
Keywords: lean, meta‐analysis, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, nonlean, nonobese, risk factors, systematic review
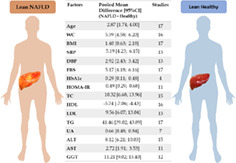
A meta‐analysis of 22 articles comprising data from lean (n = 6768) and obese (n = 9253) patients with nonalcoholic fatty liver disease (NAFLD), and lean (n = 43398) and obese (n = 9619) subjects without NAFLD (healthy) revealed that both lean and obese patients shared similar risk factors associated with NAFLD including age, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, HbA1c, insulin resistance (HOMA‐IR), total cholesterol, low‐density lipoprotein, high‐density lipoprotein, triglycerides, and uric acid.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an emerging public health problem. 1 , 2 It encompasses a spectrum of diseases from nonalcoholic fatty liver to nonalcoholic steatohepatitis (NASH) and fibrosis. 3 It is the most common cause of chronic liver disease 4 and has been identified as the leading etiology of liver transplantation worldwide. 5
The pathogenesis of the NAFLD is multifactorial and the underlying mechanism yet to be fully understood. Most mechanisms for developing NAFLD are linked with changes in lipid metabolism and development of insulin resistance. 6 , 7 , 8 Former thought about underlying pathophysiology was based on excess body fat or obesity but recent trends of NAFLD in lean patients with a body mass index (BMI) <25 kg/m2 has changed that thought. 9 , 10 , 11 The role of factors like diet, ethnicity, derangement of gut liver axis, and gut microbiota came into light. 12 , 13 , 14 , 15 While this phenomenon was initially observed in Asian population, 16 it has now been recognized as a global health issue. 17 In addition, due to increased incidence of NAFLD in lean (nonobese) patients, research focus has recently been emphasized to this population. 16 However, obesity, age, advanced insulin resistance, or type 2 diabetes mellitus have repeatedly been reported as risk factors for progression from NAFLD to NASH. 6 , 11 , 12 , 14 , 18
Overall prevalence of lean NAFLD among general population was 10.2%, and among the NAFLD population the prevalence of lean NAFLD was 19.2% with a broad countrywide variation. 19 , 20 In an average around 40% of people with NAFLD are not obese but they have severe histological phenotype like that of obese people. Mortality rate is higher and almost 40% of nonobese people with NAFLD have NASH and almost 30% have significant fibrosis. 13 , 18 , 19 , 20 In addition, the management plan of both group of patients has a distinct difference. For example: successful weight reduction strategy has potential benefits in nonlean patients but there are limited benefits in lean NAFLD patients. 21 Based on the previous literature and considering the differences in the underlying mechanisms of lean and nonlean NAFLD, we hypothesized that risks factors of NAFLD might differ between those groups and needs to be assessed. Therefore, the aim of this study was to systematically evaluate the risk factors of lean NAFLD and to aid in management strategies for the prevention and control of NAFLD and related diseases.
Methods
This work was delineated on the basis of the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis Protocols (PRISMA‐P) guidelines 22 for evaluating the risk factor responsible for development of NAFLD in lean individuals. A PRISMA‐P checklist for this work is attached (Table S1, Supporting information). The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42019136129).
Ethical clearance was waivered as there was no involvement of live subjects. However, formal notification and permission was taken from Institutional Review Board (IRB) of Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh. IRB registration no: BSMMU/2020/1012.
Search strategy and selection criteria
This meta‐analysis was based on systematic searches in several electronic databases comprising Medline/PubMed, BanglaJOL, and Google Scholar from inception to 30 March 2020. The keywords used during searching were made before final searching in collaboration with an experienced medical librarian so that as many relevant articles as could be retrieved. To enhance the sensitivity, bibliographic search of the selected article was also performed for additional article. Our search term for PubMed (from inception to 30 March 2020) with details of our search strategy and data collection, including terms for other databases, are described in the Table S2.
We included original research articles that defined their population as nonobese or lean individuals aged 18‐years or older and defined NAFLD with stratification according to weight status (nonobese or lean). In this review, lean was defined as patients with a BMI <25 kg/m2 and nonlean was defined when BMI ≥25 kg/m2. Hence only studies using this cut‐off point for differentiating nonlean (obese) from lean (nonobese) were included. Studies that described possible risk factors of NAFLD or lean NAFLD/NASH patients were considered for initial inclusion and there was no restriction in accordance with study setting either hospital or community. We included prospective comparative cohort studies of which baseline data is available, case–control studies, and cross‐sectional studies which detailed the risk factors of lean NAFLD.
The exclusion criteria for studies were: reporting population <18 years of age including children, or people with mental disorders or studies concerning patients with other liver diseases or causes for steatosis, and alcoholic fatty liver disease or articles including patients who have a daily alcohol consumption ≥30 g for men and ≥20 g for women. 23 Studies unable to ascertain how NAFLD was diagnosed were also removed. Randomized controlled trials (RCTs), systematic reviews, review articles, trial protocols, ongoing trials, editorials, letters, and conference papers were excluded from the analysis. Articles which do not have full texts available or lack of information regarding age, sex, country of origin, study design, method of assessment of fatty liver infiltration, anthropometric variables (waist circumference [WC]) or exclusive to one gender and duplicate publication were excluded. Gray literatures were also excluded. Inclusion of the studies were restricted to those published in English.
In order to exclude articles irrelevant to the systematic review, two investigators (Mohammad Jahid Hasan and Md Abdullah Saeed Khan) initially independently reviewed the title and abstract of each the references using rigorous inclusion criteria. Any dispute between two investigators were resolved through discussion with the principal investigator (Shahinul Alam). Then search results passed on to Mendeley (reference management software) which excluded the duplicates. In the second stage, the two investigators independently read the full texts of the articles that were included in the initial stage, and then selected the articles that met the inclusion criteria. Differences of ideas regarding the selection of articles were resolved through group discussion. In cases where details were missing on study design, population, intervention, or outcomes, the authors of included studies were contacted by email. After the first contact attempt, if no response received, the study authors were contacted two more times approximately 3 to 4 weeks apart. The searches were re‐run just before the final analyses and further studies retrieved for inclusion were checked. The literature search, data review, and data extraction were done with a case report form to provide consistency throughout the data collection process. Data were extracted independently by two investigators (any of two Mohammad Jahid Hasan, Md Abdullah Saeed Khan, Kamrul Anam, and S K M Nazmul Hasan). Discordance and disagreements were resolved by consensus between the two investigators or by consultation with the principal investigator (Shahinul Alam).
Assessment of study quality
We used a quality assessment scale based on the Newcastle‐Ottawa Scale (NOS) for this study, ranging from 0 to 9, with 7–9 representing high quality scores, 4–6 representing medium scores, and 1–3 representing low scores. 24 For cross‐sectional study, adapted version of the NOS was used. More details are depicted in Table S3.
Data management
The data extraction was done by two researchers (Mohammad Jahid Hasan, Md Abdullah Saeed Khan) using a standardized and pretested format. Data extraction included: title, first author, publication year, study design, settings (hospital‐based or population‐based), sample size, ethnicity, participant groups (lean vs nonlean, NAFLD vs non‐NAFLD), effect estimates for age, WC, BMI, systolic blood pressure, diastolic blood pressure, fasting blood sugar (FBS), HbA1c, insulin resistance (HOMA‐IR), total cholesterol (TC), high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), triglycerides (TG), and uric acid (UA). Besides this, the search results and necessary notes were uploaded and managed using Microsoft Excel 2016.
Statistical analysis
To summarize effects sizes a random effect model was adopted to allow for the variability of measurements across the studies included in the analysis. To specifically provide measures of the absolute difference between the mean values of each variable of interest calculated for any two groups (e.g. lean‐NAFLD vs lean non‐NAFLD patients, or lean‐NAFLD vs nonlean NAFLD, or lean non‐NAFLD vs nonlean non‐NAFLD), we used the difference in means. All the data presented as median (range), and median (interquartile range [IQR]) were converted to mean (SD) using an online calculator 25 particularly using equations by Luo et al. 26 and Wan et al. 27 We measured the variables of interest on the same scale/unit. Results from studies that report laboratory data on SI units were converted to conventional units using appropriate conversion factors. For each analysis, a forest plot was generated to display results. Subgroup analysis across ethnicity, and study population were conducted to assess sources of heterogeneity. Corresponding forest plots with estimates of effects sizes across groups are presented in Supporting Information. Funnel plots were drawn to assess publication bias for risk factors which was reported in 10 or more studies. Details regarding subgroup analyses, meta‐regression, heterogeneity, and publication bias are fully disclosed in Supporting Information. The meta‐analysis was conducted using the statistical software “R” version 3.6.0 for Windows version 10 (Microsoft corporation, Redmond, WA, USA).
Results
Study identification and selection
The PRISMA flow chart visualizes the overall study screening process (Fig. 1). Initially 1175 articles were identified through search strategy which was narrowed down to 47 articles that matched the purpose of the study. After further scrutiny, 22 articles were finally selected for inclusion (Table S5 enlists the 25 articles which were excluded with main reasons for exclusion). Among the included articles, there were 19 cross‐sectional studies, 11 , 16 , 17 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 1 case–control study, 44 and 2 cohort studies. 12 , 45
Figure 1.
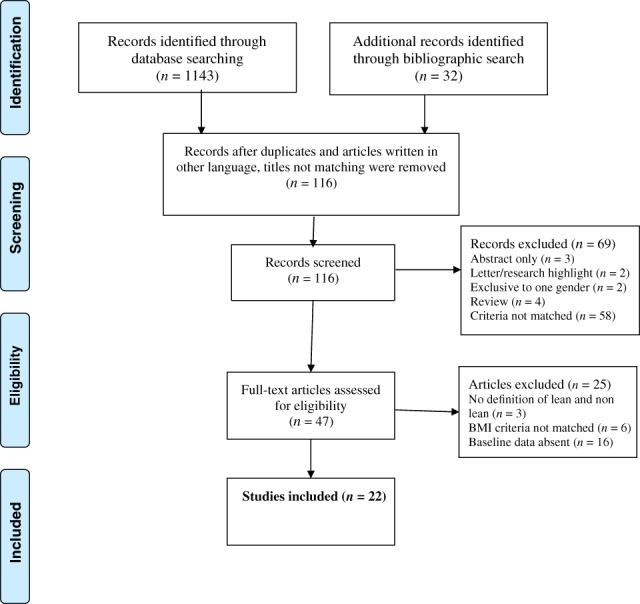
PRISMA 2009 flow diagram. BMI, body mass index.
Study characteristics
Detailed study characteristics for the 22 included studies are outlined in Table S4. Total sample size was 69 038 (lean NAFLD = 6768, lean non‐NAFLD = 43 398, nonlean NAFLD = 9253, and nonlean non‐NAFLD = 9619). All the studies included adults of both sexes.
Fifteen studies were conducted in hospital 11 , 12 , 29 , 30 , 31 , 33 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 45 and seven studies were conducted in the community setting. 16 , 17 , 28 , 32 , 34 , 35 , 38 , 44
Fatty liver was assessed by ultrasonography (USG) of liver in 15 studies 11 , 17 , 28 , 29 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 by computed tomography scan in 1 study, 44 by magnetic resonance spectroscopy in 1 study, 16 and by percutaneous liver biopsy in 5 studies. 13 , 30 , 31 , 40 , 45
Eight studies were of Caucasian origin 11 , 17 , 30 , 31 , 38 , 39 , 44 , 45 and 14 studies were of East‐Asian origin. 11 , 12 , 16 , 17 , 28 , 29 , 32 , 33 , 34 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44
Two studies 4 , 6 presented values as median (range), five studies 14 , 15 , 16 , 19 , 21 presented as median (IQR), and rest of the studies 1 , 2 , 3 , 5 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 17 , 18 , 20 , 22 presented as mean (SD). Median values were converted to mean using procedures described in Methods section.
Meta‐analysis results
Age and risk of NAFLD in lean people
For risk of NAFLD in lean individual, 15 studies 17 , 28 , 29 , 30 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 compared age of lean NAFLD and non‐NAFLD participants. Among these, two studies 33 , 35 presented comparison separately for male and female. Only one study was case–control study, 44 and rest of the studies were cross‐sectional studies. 11 , 12 , 16 , 17 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 45 Figure 2 shows that lean NAFLD patients were significantly older than lean non‐NAFLD participants (mean difference [MD] 2.87 years, 95% confidence interval [CI] 1.74–4.00). The studies were considerably heterogenous (I 2 = 95%). Hence, a random effect model was used. Subgroup analysis across ethnicity showed that studies of Korean, Japanese, and Chinese origin (I 2 = 94, 98 and 85% respectively) were more heterogenous than that of Caucasian origin (I 2 = 68%) (Figure S2A). Studies stratified by study population (population‐based vs hospital‐based) did not show any significant change in heterogeneity (Figure S2B). However, a stratification across geographic location (Eastern vs other) showed less heterogeneity in other studies (I 2 = 93 vs 68% respectively) (Figure S2C).
Figure 2.
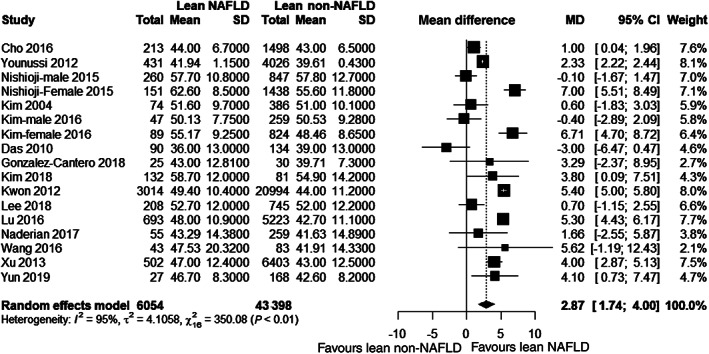
Forest plot for age. CI, confidence interval; MD, mean difference; NAFLD, nonalcoholic fatty liver disease.
BMI and risk of NAFLD in lean people
For risk of NAFLD in lean people, same number of studies as that of age compared BMI between lean NAFLD patients and lean non‐NAFLD controls. Figure 3a shows that lean NAFLD patients had significantly higher BMI than lean non‐NAFLD participants (MD 1.40 kg/m2, 95% CI 0.63–2.18). The studies were considerably heterogenous (I 2 = 100%). Subgroup analysis across ethnicity did not show any change in heterogeneity (Figure S3A). Studies stratified by population (population‐based vs hospital‐based) and by geographic location (Eastern vs other) showed similar heterogeneity (Figure S3B and S3C).
Figure 3.
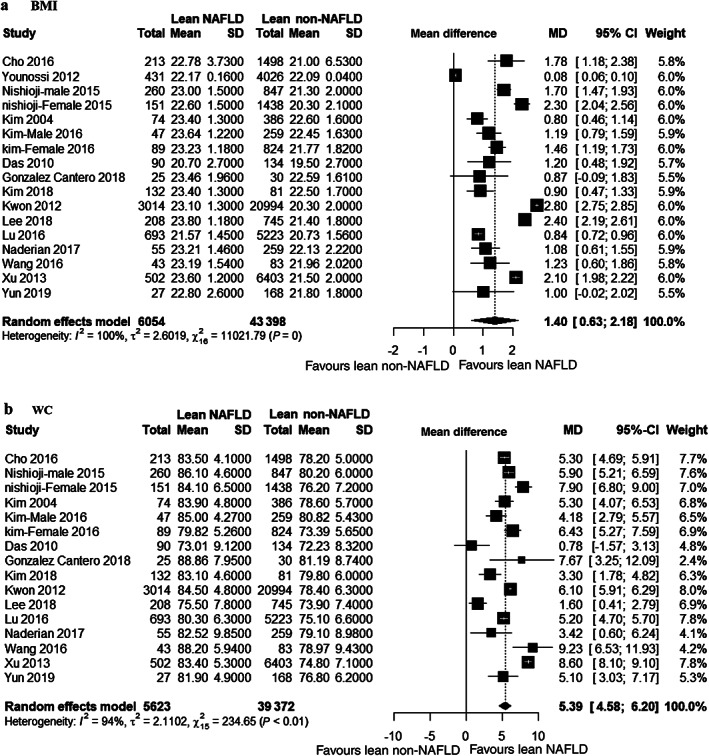
Forest plot for body mass index (BMI) (a) and waist circumference (WC) (b). CI, confidence interval; MD, mean difference; NAFLD, nonalcoholic fatty liver disease.
WC and risk of NAFLD in lean people
For risk of NAFLD in lean people, same number of studies as that of age compared WC between lean NAFLD patients and lean non‐NAFLD controls except one cross‐sectional study. 17 Figure 3b shows that lean NAFLD patients had significantly higher WC than lean non‐NAFLD participants (MD 5.39 cm, 95% CI 4.58–6.20). These studies were considerably heterogenous (I 2 = 94%). Subgroup analysis across ethnicity showed that Chinese studies explained the most heterogeneity (I 2 = 98%). Korean (I 2 = 78%) and Caucasian (I 2 = 74%) studies were less heterogenous than that of Japanese (I 2 = 89%) and Chinese studies (Figure S4A). Studies stratified by population (population‐based vs hospital‐based) had similar high heterogeneity (Figure S4B). But, a stratification across geographic location (Eastern vs other) showed less heterogeneity in other studies (I 2 = 94 vs 74%, respectively) (Figure S4C).
Blood pressure and risk of NAFLD in lean people
Ten studies 28 , 29 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 43 compared blood pressure (BP) (both systolic blood pressure [SBP] and diastolic blood pressure [DBP]) of lean NAFLD and lean non‐NAFLD participants. Among those, three studies 29 , 33 , 35 presented comparison separately for male and female. All the studies were cross‐sectional. Figure 4a,b shows that lean NAFLD patients had significantly higher BP than lean non‐NAFLD participants (SBP: MD 5.39 mmHg, 95% CI 4.58–6.20; DBP: MD 2.92 mmHg, 95% CI 2.43–3.42) with considerable heterogeneity (I 2 = 94% for SBP and 98% for DBP). Ethnicity showed that Chinese studies were relatively homogenous for SBP (I 2 = 39%, P = 0.19) than other. Heterogeneity was mostly explained by Korean studies (I 2 = 100%) (Figure S5A). Heterogeneity of DBP was explained by both Korean (I 2 = 99%) and Chinese (I 2 = 96%) studies (Figure S6A). Studies stratified by settings showed population‐based studies were less heterogenous than hospital‐based (I 2 = 77 vs 100% respectively for SBP and 63 vs 99% respectively for DBP) (Figure S5B and S6B). However, stratification across geographic location (Eastern vs others) did not reveal any difference as only one study in the latter group reported BP (Figure S5C and S6C).
Figure 4.
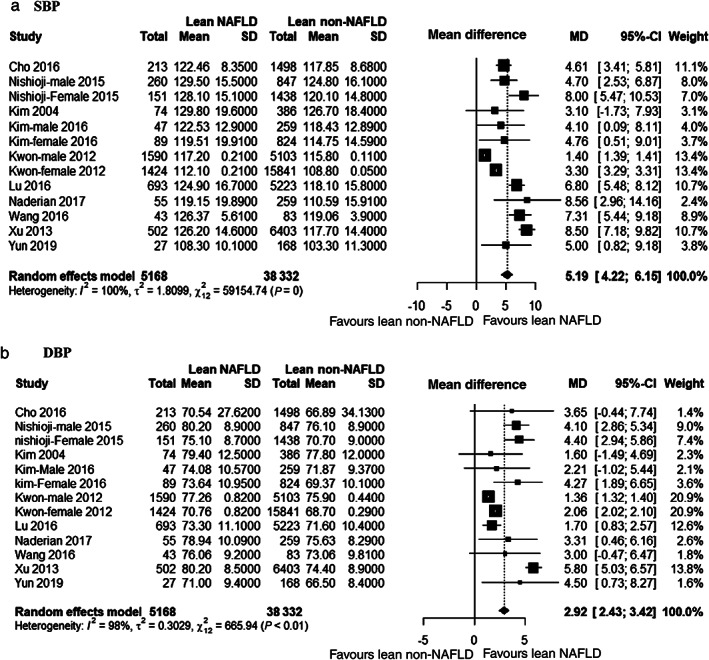
Forest plot for systolic blood pressure (SBP) (a) and diastolic blood pressure (DBP) (b).
FBS and risk of NAFLD in lean people
Fifteen studies compared FBS between lean NAFLD patients and lean non‐NAFLD controls. Figure 5a shows that lean NAFLD patients had significantly higher FBS than lean non‐NAFLD controls (MD 5.17 mg/dL, 95% CI 4.17–6.16). These studies were considerably heterogenous (I 2 = 99%). Subgroup analysis across ethnicity showed that heterogeneity was mostly explained by Korean (I 2 = 100%), Japanese (I 2 = 87%), and Chinese (I 2 = 87%) studies (Figure S7A). Heterogeneity of the studies can be explained by hospital‐based studies and Eastern studies (Figure S7B and S7C).
Figure 5.
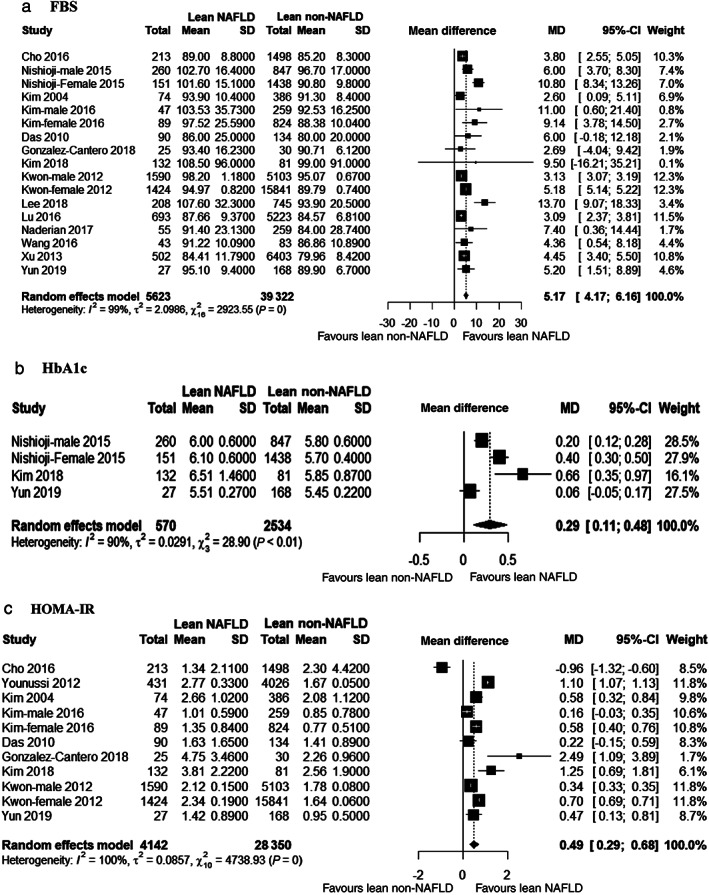
Forest plot for fasting blood sugar (FBS) (a), HbA1c (b), and (c) insulin resistance (HOMA‐IR).
HbA1c and risk of NAFLD in lean people
Only three studies 33 , 40 , 43 compared HbA1c between lean NAFLD and lean non‐NAFLD controls. One study 33 compared HbA1c by sex. Figure 5b shows that lean NAFLD patients had significantly higher HbA1c than lean non‐NAFLD controls (MD 0.29%, 95% CI 0.11–0.48). These studies were considerably heterogenous (I 2 = 90%). Subgroup analysis by ethnicity showed similar heterogeneity (Figure S8A).
HOMA‐IR and risk of NAFLD in lean people
Nine studies 17 , 28 , 29 , 34 , 35 , 39 , 40 , 43 , 44 compared HOMA‐IR of lean NAFLD and non‐NAFLD participants. One study 35 presented gender difference, and one 44 was case–control study. Figure 5c shows that lean NAFLD patients had significantly higher HOMA‐IR than lean non‐NAFLD controls (MD 0.49 U, 95% CI 0.29–0.68). These studies were considerably heterogenous (I 2 = 100%). Subgroup analysis did not show any difference in heterogeneity across any stratification (Figure S9A–C).
Serum lipid profile and risk of NAFLD in lean people
For risk of NAFLD in lean people, 13 studies 28 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 43 , 44 compared TC of lean NAFLD and non‐NAFLD participants. Same studies except two, 41 , 44 except one, 41 and plus one 29 study also compared LDL, HDL, and TG between those groups, respectively. Only two studies 33 , 35 presented comparison separately for male and female and one study was case–control. 44 Figure 6a–d show that lean NAFLD patients had significantly higher TC, higher LDL, lower HDL, and higher TG than lean non‐NAFLD controls (TC: MD 10.32 mg/dL, 95% CI 6.68–13.96; LDL: MD 9.56 mg/dL, 95% CI 6.07–13.04; HDL: MD −5.74 mg/dL, 95% CI −7.06 to 4.43; and TG: MD 41.46 mg/dL, 95% CI 39.02–43.89). These studies were substantially heterogenous (I 2 = 76%, 76, 100 and 97% respectively for TC, LDL, HDL, and TG). Subgroup analysis by ethnicity is shown in Figures S10A, S11A, S12A, and S13A. Studies stratified by population (population‐based vs hospital‐based) showed that heterogeneity in TC is completely explained by population‐based studies (I 2 = 83%), but heterogeneity in LDL and HDL is explained by hospital‐based studies (I 2 = 74% each) (Figure S10B, S11B, and S12B). However, it was same across study population in case of TG (Figure S13B). Stratification across geographic location (Eastern vs other) showed that Eastern studies were overall more heterogenous than other studies for LDL, HDL, and TG and other way around for TC (Figures S10C, S11C, S12C, and S13C).
Figure 6.
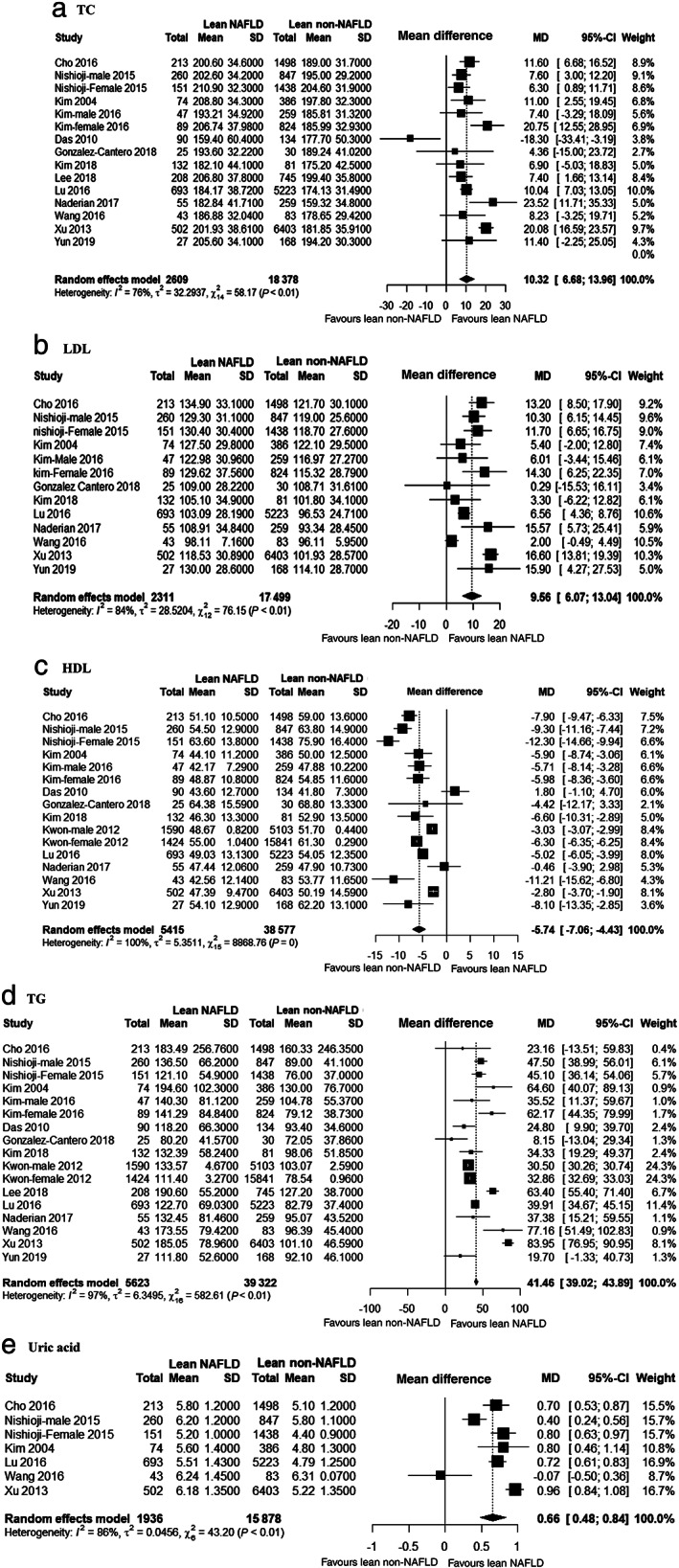
Forest plot for total cholesterol (TC) (a), low‐density lipoprotein (LDL) (b), high‐density lipoprotein (HDL) (c), triglyceride (TG) (d), and uric acid (e).
Serum UA and risk of NAFLD in lean people
For risk of NAFLD in lean people, only six studies 28 , 32 , 33 , 34 , 36 , 38 compared UA between lean NAFLD patients and lean non‐NAFLD controls. One study 33 compared UA by sex. All were cross‐sectional studies. Figure 6e showed that lean NAFLD patients had significantly higher UA than lean non‐NAFLD controls (MD 0.66 mg/dL, 95% CI 0.48–0.84). These studies were considerably heterogenous (I 2 = 86%). Subgroup analysis by ethnicity and study settings are shown in Figures S14A and S14B.
Liver function tests in lean NAFLD versus non‐NAFLD
Alanine aminotransferase (ALT) was reported in 13 studies. 17 , 28 , 29 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 43 Aspartate aminotransferase (AST) was reported in 10 studies 17 , 28 , 29 , 33 , 34 , 37 , 38 , 39 , 40 , 41 , 43 and gamma glutamyl transferase (GGT) was reported in 10 studies. 28 , 29 , 32 , 33 , 34 , 35 , 36 , 37 , 39 , 40 , 41 , 43 Compared to non‐NAFLD controls lean NAFLD patients had significantly higher mean values of ALT (MD 8.12 U/L, 95% CI 6.21–10.02, Figure S15A), AST (MD 2.72 U/L, 95% 1.91–3.63, Figure S16A), and GGT (MD 11.21 U/L, 95% CI 9.02–13.40, Figure S17A). Analysis for all three variables were considerably heterogenous. Subgroup analysis showed that heterogeneity is mostly explained by Korean studies for ALT, Caucasian studies for AST, and Chinese studies for GGT (Figures S15B, S16B, and S17B). Whereas subgroup analysis by population (population‐based vs hospital‐based) showed that heterogeneity was mainly due to hospital‐based studies (Figures S15C, S16C, and S17C). Subgroup analysis by Eastern versus other studies showed that heterogeneity was higher among Eastern studies for ALT and higher among other studies for AST (Figures S15D, S16D, and S17D).
Summary of the findings
A summary of the meta‐analysis results along with subgroup analysis for individual demographic, anthropometric, and cardiometabolic risk factors for NAFLD in lean people are given in Tables S6‐S8. All the studies were considerably heterogenous and heterogeneity varied by ethnicity, study population, and risk factor under consideration. Random effect model analysis showed that all of the factors had significantly different effects estimates between lean NAFLD patients and non‐NAFLD controls.
Publication bias
For risk factors with 10 or more studies, funnel plot analysis was conducted (Figure S18). Almost all the plots showed asymmetry which could be explained mostly by heterogeneity of the studies and partly by biased reporting of studies with significant effect sizes. A visual inspection of the funnel plots shows that studies reporting SBP, DBP, GGT, TC, and LDL were moderately asymmetric. While other risk factors showed a high asymmetry.
Comparison of risk factors of nonlean and lean NAFLD
A summary of differences in effect size between lean and nonlean NAFLD patients, and lean and nonlean controls are given in Table 1. Nonlean NAFLD patients had significantly different effect estimates than lean NAFLD patients in cardio metabolic profiles: BMI (mean difference, P‐value: 5.62 kg/m2, P < 0.001), WC (16.44 cm, P < 0.001), SBP (4.97 mmHg, P < 0.001), DBP (3.45 mmHg, P < 0.001), FBS (3.37 mg/dL, P < 0.001), HbA1c (0.14%, P = 0.02), HOMA‐IR (1.07, P < 0.001), HDL (−3.12 mg/dL, P < 0.001), TG (13.07 mg/dL, P = 0.001), UA (0.37 mg/dL, P = 0.003), ALT (5.66 U/L, P < 0.001), AST (3.31 U/L, P < 0.001), and GGT (4.58 U/L, P = 0.027). Similarly nonlean controls had significantly different values in cardiometabolic risk than lean control: BMI (5.25 kg/m2, P < 0.001), WC (11.36 cm, P < 0.001), SBP (6.71 mmHg, P < 0.001), DBP (3.64 mmHg, P < 0.001), FBS (2.91 mmol/L, P = 0.003), HbA1c (0.06%, P = 0.044), HOMA‐IR (0.53, P = 0.009), TC (5.13 mg/dL, P = 0.001), LDL (6.25 mg/dL, P < 0.001), HDL (−5.67, P < 0.001), TG (22.36, P < 0.001), UA (0.36 mg/dL, P = 0.007), ALT (2.71 U/L, P < 0.001), AST (3.31, P < 0.001), and GGT (4.58, P = 0.027). In addition, nonlean controls were significantly older than lean controls (2.63 years, P < 0.001).
Table 1.
Meta‐regression analysis to examine the impact of nonalcoholic fatty liver disease (NAFLD) on the risk factors
| Outcome | NAFLD lean versus nonlean | Non‐NAFLD lean versus nonlean | Meta‐regression analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) | P‐value | Sample size | No of sub studies | Mean difference (95% CI) | P‐value | Sample size | No of sub studies | Slope | P‐value | |
| Age | 0.12 [−2.72; 2.96] | 0.934 | 9212/5385 | 19 | 2.63 [1.71; 3.55] | <0.001 | 9576/29717 | 10 | −0.06 | 0.839 |
| BMI | 5.62 [3.66; 7.58] | <0.001 | 8565/5231 | 17 | 5.25 [4.58; 5.93] | <0.001 | 9576/29717 | 10 | 1.12 | <0.001 |
| WC | 16.44 [13.87; 19.00] | <0.001 | 7192/5044 | 20 | 11.36 [9.72; 13.00] | <0.001 | 1470/4697 | 8 | 1.15 | <0.001 |
| SBP | 4.97 [3.25; 6.69] | <0.001 | 6142/4543 | 14 | 6.71 [3.68; 9.73] | <0.001 | 3683/24688 | 8 | 0.09 | 0.459 |
| DBP | 3.45 [2.76; 4.13] | <0.001 | 6142/4543 | 14 | 3.64 [2.21; 5.07] | <0.001 | 3683/24866 | 8 | 0.19 | 0.037 |
| FBS | 3.37 [2.44; 4.30] | <0.001 | 7151/4954 | 19 | 2.91 [1.34; 4.47] | 0.003 | 4481/25641 | 10 | 0.19 | 0.064 |
| HbA1c | 0.14 [0.02; 0.25] | 0.021 | 1661/814 | 7 | 0.06 [0.00; 0.13] | 0.044 | 205/2453 | 3 | 0.00 | 0.993 |
| HOMA‐IR | 1.07 [0.57; 1.57] | <0.001 | 8155/4675 | 15 | 0.53 [0.13; 0.92] | 0.009 | 8625/26637 | 8 | 0.58 | <0.001 |
| TC | 0.49 [−1.71; 2.70] | 0.660 | 4126/1940 | 17 | 5.13 [2.08; 8.18] | 0.001 | 1470/4697 | 8 | 0.13 | 0.226 |
| LDL | 0.58 [−0.99; 2.14] | 0.470 | 3841/1732 | 16 | 6.25 [2.96; 9.54] | <0.001 | 700/3952 | 7 | 0.08 | 0.244 |
| HDL | −3.12 [−3.74; −2.49] | <0.001 | 6866/4746 | 18 | −5.67 [−6.94; −4.40] | <0.001 | 3711/24896 | 9 | −0.01 | 0.964 |
| TG | 13.07 [5.36; 20.77] | 0.001 | 7151/4954 | 19 | 22.36 [18.99; 25.73] | <0.001 | 4481/25641 | 10 | 0.02 | 0.910 |
| UA | 0.37 [0.13; 0.61] | 0.003 | 1846/1022 | 6 | 0.36 [0.10; 0.63] | 0.007 | 383/2671 | 3 | 0.36 | 0.022 |
| ALT | 5.66 [3.59; 7.72] | <0.001 | 9212/5385 | 19 | 2.71 [1.03; 4.40] | 0.002 | 9576/29717 | 10 | 0.11 | 0.233 |
| AST | 3.31 [2.32; 4.30] | <0.001 | 8716/5041 | 16 | 0.33 [−0.81; 1.48] | 0.569 | 8541/27889 | 7 | 0.27 | 0.003 |
| GGT | 4.58 [0.52; 8.65] | 0.027 | 5096/4123 | 12 | 6.75 [2.30; 11.21] | 0.003 | 3509/24560 | 7 | 0.32 | 0.019 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; FBS, fasting blood sugar; GGT, gamma glutamyl transferase; HDL, high‐density lipoprotein; HOMA‐IR, insulin resistance; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UA, uric acid; WC, waist circumference.
The differences in values of individual risk factors between lean and nonlean patients and controls could be explained by the significant differences in BMI and WC. However, the meta‐regression analysis showed that the magnitude of the difference in the value of the lipid profile and glycemic profile barely reached statistical significance when subjects were grouped according to the presence or absence of NAFLD (Table 1). But for cardio metabolic variables, the magnitude of effect was significantly higher if NAFLD was present compared to that of non‐NAFLD group. These are: DBP (slope: 0.19, P < 0.037), HOMA‐IR (slope: 0.58, P < 0.001), UA (slope: 0.36, P = 0.022). Also, the presence of NAFLD is associated with significantly higher values of AST (slope: 0.27, P = 0.003) and GGT (slope: 0.32, P = 0.019) in blood.
Discussion
NAFLD in lean persons is now a widely recognized problem. Initially, described in Asian populations and considered as a “third world phenotype,” this subset of NAFLD has since been described in other populations, including in Europe and the United States. 20 , 46 Therefore, risk factors associated with lean NAFLD are being studied worldwide. A previous metanalysis, which synthesized evidence based on research studies published up to 2016, found that risk factors of NAFLD are shared between lean and obese individuals. 47 Since then more data have been generated, especially the importance of uric acid, which was found to have a significant positive nonlinear association with risk of cardiovascular disease (CVD) mortality (Hazard Ratio [HR] 1.45, 95% CI 1.33–1.58, I 2 = 79%) 48 as a risk factor of NAFLD was being studied. Hence, an updated metanalysis was warranted.
Our study found that lean NAFLD patients were significantly older than lean non‐NAFLD controls. This is different from that found by Sookoian et al. 47 The difference in age varied across the studies. Usually, studies with small sample sizes 28 , 35 , 37 , 38 , 39 , 40 , 41 , 44 reported small nonsignificant difference in age while studies with large sample sizes 17 , 32 , 33 , 44 found a significant difference. Finally, a pooled estimate produced a significant effect size. Hamaguchi et al. studied the effect of aging on NAFLD in detail and found that aging is a significant nonmodifiable risk factor for NAFLD in premenopausal women independent of weight gain and metabolic syndrome. 49 We found that lean NAFLD patients were 2.87 years (95% CI 1.74–4.00, P < 0.01) older than lean non‐NAFLD individuals, while this difference was reported to be 3.79 (95% CI 2.38–5.20) years in the previous study. 47 In addition, we found that the differences were more in hospital‐based studies (3.86; 95% CI 2.29–5.43) in comparison to population‐based studies (1.99; 95% CI 0.74–3.25). Interestingly, the difference was mainly due to Eastern studies, because other non‐Eastern studies did not find a significant difference of age between these two groups. These suggest that lean individuals are more likely to develop NAFLD with an increasing age. Although age cannot be modified or treated, lean individuals who have other concomitant risk factors of NAFLD should be actively monitored and sought for presence of fatty liver with an increasing age, so that they can be included in management strategies as early as possible.
All components of metabolic syndrome (high BP, hyperglycemia, insulin resistance, visceral adiposity, and lipid profile) along with UA were present in both lean and nonlean NAFLD patients. Even in lean patients, NAFLD was characterized by the presence of significantly higher BMI and WC than non‐NAFLD controls. WC is a surrogate of visceral obesity. 50 Excess free fatty acid (FFA) released from visceral adipose tissue leads to over‐exposure of FFA to hepatic and extra‐hepatic tissue promoting aberrations in insulin action and dynamics. 51 Moreover, FFA was found to be significantly higher in NAFLD patients than non‐NAFLD controls irrespective of BMI. 52 This also explains the benefit of weight reduction in lean NAFLD patients. 21
FBS showed less heterogeneity among Caucasian people and in population‐based studies. Most of the heterogeneity in FBS was explained by Asian, and hospital‐based studies. HbA1c, BP (SBP and DBP), lipid profiles (TC, LDL, HDL, and TG), and UA showed considerable heterogeneity across different ethnicity and study design. The random effect model analysis showed that all these variables were significantly higher in lean NAFLD patients than non‐NAFLD controls (except HDL which was significantly lower). These findings are consistent with that of Sookoian and Pirola 14 reported nearly similar differences in risk factors between lean NAFLD and non‐NAFLD individuals. Unlike them, we did a subgroup analysis based on the population and geographic region (Eastern vs others) where some interesting findings were noted. A significantly higher mean difference was noted in BMI, WC, HOMA‐IR, ALT, and AST for hospital‐based studies and in BP, lipid profiles, uric acid, and GGT for population‐based studies. Several possible reasons could explain this difference. As some of the participants in hospital were more likely to have some sorts of illness or comorbidities, they might have been taking medications for BP, lipid profile, or even uric acid. This may explain the lower values of these pooled parameters in the hospital‐based population. While the anthropometric parameters, which do not change immediately on medication and which are expected to be higher in symptomatic NAFLD individuals coming to take health care service, were found high in the hospital‐based population. On the other hand, nearly all the parameters showed a higher mean difference in Eastern studies rather the non‐Eastern ones. Although overall population of the Eastern studies were highly heterogenous, the ethnic characteristics shared by Japanese, Chinese, and Korean population might explain the difference than other studies comprising Indian, Iranian, and American population of mixed ethnicity. Overall, our findings suggest that if lean people have any suspicious rise of these risk factors' parameters, they should be screened for NAFLD. Also, lean‐NAFLD patients with symptoms and/or comorbidities need to be treated aggressively and monitored closely for compliance, and lean people of Eastern origin if screened positive for NAFLD should be given proper attention as required.
Uric acid was found to be an independent risk factor for NAFLD in lean person by several other studies conducted in Iran 53 and China. 54 , 55 It is reportedly responsible for lipid metabolism impairment and inflammation. 56 , 57 , 58 Thus, there may be interaction between high serum UA and increased weight in the pathogenesis of NAFLD. Hence, all suspected or diagnosed lean NAFLD patients should undergo UA assay and specified management for UA. Further studies on lean NAFLD patients must include UA as a part of laboratory tests.
The meta‐regression analysis showed that the presence of higher DBP, HOMA‐IR, and UA in both lean and nonlean patients were independently associated with NAFLD irrespective of BMI and WC. Therefore, NAFLD could be considered an important but separate component within the spectrum of metabolic syndrome which interacts with other components and influences each other. This hypothesis is strengthened by several other studies where the authors suggest that NAFLD works as a precursor of metabolic syndrome 59 independent of central obesity and insulin resistance. 60 Besides our findings suggest that treatment of NAFLD, particularly in lean patients, should involve uncompromising management of diastolic blood pressure, insulin resistance, and uric acid.
Taken together, patients with lean NAFLD, who do not have any infectious‐inflammatory and drug cause 46 may have additional pathogenetic mechanisms other than metabolic causes of NAFLD. Some of these mechanisms are as follows. At least four genetic variants have shown robust association with development and progression of NAFLD. 61 These are: PNPLA3 (rs738409 C > G), TM6SF2 (rs58542926 C > T), MBOAT7 (rs641738 C > T), and GCKR (rs1260326 C > T). However, there is a paucity of literature regarding specific genetic markers among lean NAFLD patients. Mixed findings are reported regarding frequency of PNPLA3 and TM6SF2 variants when compared between lean and nonlean NAFLD patients. 62 , 63 Polymorphism in IFNL3, CETP, and PEMT has been shown to be associated with the progression of NAFLD among lean or nonobese individuals by several studies. 46 Further studies are needed to find any strong association of genetic variants with NAFLD among lean population.
Fecal, gut, and blood microbiome has recently been brought into focus as probable risk factors for NAFLD in lean patients. 37 , 43 , 62 , 64 , 65 It was considered because dysbiosis between beneficial and pathogenic bacteria may lead to obesity, insulin resistance, and NAFLD 65 particularly in nonobese individual. 37 Yun et al. found that NAFLD patients showed a distinct bacterial community with a lower biodiversity and a far distant phylotype compared to control group, and fecal and blood microbiota profiles showed different patterns between subjects with obese and lean NAFLD 43 However, relationship between microbiota, and metabolic and inflammatory response is complex which warrants extensive investigation.
Several studies also found significant associations of serum sialic acid, 36 bile acid, 62 and apolipoproteins (apoB1 level and apoB/A1 ratio), 66 with lean NAFLD which need further research for confirmation.
The meta‐regression results also indicated that serum levels of AST and GGT, but not ALT were markedly modulated by the presence of NAFLD. Sookoian and Pirola 14 previously noted a similar finding except that it was only for AST. Accumulation of fat in liver causes increased energy demand and in response to that synthesis of transaminase, particularly the mitochondrial isoform AST, increases. 47 On the other hand, Hossain et al. 67 proposed that GGT is an independent determinant of association of insulin resistance with nonalcoholic fatty liver disease in adults. Our result endorses their findings.
Finally, the comparison of risk factors between lean versus nonlean NAFLD in our study found that almost all the components were present in significantly higher amount among nonlean subjects. A higher BMI and visceral obesity may explain this difference. It is obvious that nonlean (overweight/obese) NAFLD patients are in need of stringent management of weight and other metabolic parameters. However, our study results emphasize the importance of this strategy for both lean and nonlean NAFLD patients.
To summarize, lean and nonlean NAFLD patients share similar cardiovascular and metabolic risk factors. Genetic, microbiological, and other metabolic factors warrant further investigation to identify unique determinants of NAFLD in lean individuals. Regardless of the underlying mechanisms, lean NAFLD needs to be given appropriate clinical attention similar to that of nonlean NAFLD.
Limitations and strengths
The current study was limited in several aspects. Selected articles were mostly heterogenous and mostly it was due to the inclusion of the studies from different ethnic origin as well as inclusion of both hospital‐ and population‐based studies. Even within a unique ethnic setting, separate studies were considerably heterogenous with respect to different variables under consideration. This indicates remarkable individual differences in risk factors within same ethnic population. Also, a separate analysis for different BMI cut‐off points used in definition of lean and nonlean was not possible. Therefore, implication of the risk factors of NAFLD with respect to Asian population, where a different BMI cut‐off point for overweight and obesity often used could not be determined. Many authors did not report separate analysis for male and female. Therefore, a sex‐based subgroup analysis was not possible. Multivariate analysis to determine independent associations between risk factors and lean‐NAFLD could not be done, because of unavailability of data, leading to an inference of only general associations among different risk factors and NAFLD.
The strength of the study was its large sample size of 69 038 individuals, comprising 6768 lean NAFLD patients, 9253 nonlean NAFLD patients, 43 398 lean controls, and 9619 nonlean controls. Also, consistent findings across different cardiovascular and metabolic risk factors strengthened our results. Hope that the study could be used as an update of previous metanalysis in this topic and will be evident for future clinicians as well as policy makers.
Recommendations for future studies
NAFLD could be considered a part of an interactome of metabolic syndrome components working in a coordinated fashion to develop chronic diseases and complications involving different systems of the body. Therefore, to incorporate NAFLD within the definition of metabolic syndrome extensive research is necessary. Well‐designed case–control and cohort studies for risk factor analysis of both lean and non‐NAFLD are lacking. Multivariate analysis of risk factors is also warranted. Further explorative research on unique determinants of lean‐NAFLD are recommended.
In conclusion, Age, BMI, WC, BP, FBS, HbA1c, insulin resistance, uric acid, TC, LDL, HDL, and TG are the important risk factors of NAFLD shared equally among lean and nonlean. In other words, lean and nonlean NAFLD are anthropometrically different but metabolically similar entities. The findings provide a scientific basis for a further understanding of the nature of NAFLD in lean individual, and might provide a guide to determine the management strategies for lean‐NAFLD.
Supporting information
Appendix S1. Supporting information.
Acknowledgments
The investigator team duly acknowledge “Pi Research Consultancy Center (www.pircc.org)” Bangladesh for their support during development of protocol, data extraction, data analysis, manuscript formatting, and submission. The authors acknowledge the role of Farha Musharrat Noor for her assistance during data analysis. The authors also acknowledge all of the faculties of the Department of Hepatology BSSMU and the IRB team members.
Declaration of conflict of interest: None.
Author contribution: Shahinul Alam contributed to the conceptualization, methodology, writing‐original draft, writing‐review and editing, and supervision. Mohammad Jahid Hasan contributed to the methodology, investigation; data curation, writing‐original draft, writing‐review and editing, and project administration. Md Abdullah Saeed Khan contributed to the investigation, data curation, formal analysis, writing‐original draft, and writing‐review and editing. Mohammad Abdul Baker Chowdhury contributed to the formal analysis. Rosmawati Mohamed contributed to the writing‐review and editing. Mohammad Eslam contributed to the writing‐review and editing. Kamrul Anam contributed to the investigation and writing‐review and editing. Nazmul S K M Hasan contributed to the investigation and writing‐review and editing.
References
- 1. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020; 158: 1851–64. [DOI] [PubMed] [Google Scholar]
- 2. Eslam M, Newsome PN, Sarin SK et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J. Hepatol. 2020; 73: 202–9. [DOI] [PubMed] [Google Scholar]
- 3. Huang T, Behary J, Zekry A. Non‐alcoholic fatty liver disease (NAFLD): a review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 2019; 50:1038–47. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 5. Pais R, Barritt AS 4th, Calmus Y et al. NAFLD and liver transplantation: current burden and expected challenges. J. Hepatol. 2016; 65: 1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maximos M, Bril F, Portillo Sanchez P et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology. 2015; 61: 153–60. [DOI] [PubMed] [Google Scholar]
- 7. Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol. Commun. 2020; 4: 478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J. Gastrointest. Pathophysiol. 2016; 7: 211–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldman A, Eder SK, Felder TK et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non‐alcoholic Fatty Liver. Am. J. Gastroenterol. 2017; 112: 102–10. [DOI] [PubMed] [Google Scholar]
- 10. Wattacheril J, Sanyal AJ, Hospital P. Lean NAFLD: an underrecognized outlier. Curr. Hepatol. Rep. 2016; 15: 134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alam S, Gupta UD, Alam M, Kabir J, Chowdhury ZR, Alam AK. Clinical, anthropometric, biochemical, and histological characteristics of nonobese nonalcoholic fatty liver disease patients of Bangladesh. Indian J. Gastroenterol. 2014; 33: 452–7. [DOI] [PubMed] [Google Scholar]
- 12. Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016; 13: 412–25. [DOI] [PubMed] [Google Scholar]
- 13. Leung JC, Loong TC, Wei JL et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017; 65: 54–64. [DOI] [PubMed] [Google Scholar]
- 14. Sookoian S, Pirola CJ. Systematic review with meta‐analysis: risk factors for non‐alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment. Pharmacol. Ther. 2017; 46: 85–95. [DOI] [PubMed] [Google Scholar]
- 15. Tripathi A, Debelius J, Brenner DA et al. The gut‐liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018; 15: 157–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei JL, Leung JC, Loong TC et al. Prevalence and severity of nonalcoholic fatty liver disease in non‐obese patients: a population study using proton‐magnetic resonance spectroscopy. Am. J. Gastroenterol. 2015; 110: 11006–315. [DOI] [PubMed] [Google Scholar]
- 17. Younossi ZM, Stepanova M, Negro F et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine. 2012; 9: 319–27. [DOI] [PubMed] [Google Scholar]
- 18. Denkmayr L, Feldman A, Stechemesser L et al. Lean patients with non‐alcoholic fatty liver disease have a severe histological phenotype similar to obese patients. J. Clin. Med. 2018; 7: 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ye Q, Zou B, Yeo YH et al. Global prevalence, incidence, and outcomes of non‐obese or lean non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Lancet Gastroenterol. Hepatol. 2020; 5: 12531–14. [DOI] [PubMed] [Google Scholar]
- 20. Shi Y, Wang Q, Sun Y et al. The prevalence of lean/nonobese nonalcoholic fatty liver disease: a systematic review and meta‐analysis. J. Clin. Gastroenterol. 2020; 54: 378–87. [DOI] [PubMed] [Google Scholar]
- 21. Alam S, Jahid Hasan M, Khan MAS, Alam M, Hasan N. Effect of weight reduction on histological activity and fibrosis of lean nonalcoholic steatohepatitis patient. J. Transl. Intern. Med. 2019; 7: 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J. Clin. Epidemiol. 2009; 6: e100009. [PMC free article] [PubMed] [Google Scholar]
- 23. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J. Hepatol. 2016; 59: 1121–40. [DOI] [PubMed] [Google Scholar]
- 24. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25. Online‐calculator. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range Available from URL: http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html [DOI] [PMC free article] [PubMed]
- 26. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat. Methods Med. Res. 2018; 27: 1785–1805. [DOI] [PubMed] [Google Scholar]
- 27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014; 14: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim HJ, Kim HJ, Lee KE et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Intern. Med. 2004; 164: 2169–75. [DOI] [PubMed] [Google Scholar]
- 29. Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am. J. Gastroenterol. 2012; 107: 1852–8. [DOI] [PubMed] [Google Scholar]
- 30. Margariti E, Deutsch M, Manolakopoulos S, Papatheodoridis GV. Non‐alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann. Gastroenterol. 2012; 25: 45–51. [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar R, Rastogi A, Sharma M et al. Clinicopathological characteristics and metabolic profiles of non‐alcoholic fatty liver disease in Indian patients with normal body mass index: do they differ from obese or overweight non‐alcoholic fatty liver disease? Indian J. Endocrinol. Metab. 2013; 17: 665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai study. Am. J. Gastroenterol. 2013; 108: 1299–304. [DOI] [PubMed] [Google Scholar]
- 33. Nishioji K, Sumida Y, Kamaguchi M et al. Prevalence of and risk factors for non‐alcoholic fatty liver disease in a non‐obese Japanese population, 2011‐2012. J. Gastroenterol. 2015; 50: 95–108. [DOI] [PubMed] [Google Scholar]
- 34. Cho HC. Prevalence and factors associated with nonalcoholic fatty liver disease in a nonobese Korean population. Gut Liver. 2016; 10: 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JY, Lee C, Oh M et al. Relationship between non‐alcoholic fatty liver disease, metabolic syndrome and insulin resistance in Korean adults: a cross‐sectional study. Clin. Chim. Acta. 2016; 458: 12–17. [DOI] [PubMed] [Google Scholar]
- 36. Lu Z, Ma H, Xu C, Shao Z, Cen C, Li Y. Serum sialic acid level is significantly associated with nonalcoholic fatty liver disease in a nonobese Chinese population: a cross‐sectional study. Biomed. Res. Int. 2016; 2016: 5921589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang B, Jiang X, Cao M et al. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non‐alcoholic fatty liver disease. Sci. Rep. 2016; 6: 32002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naderian M, Kolahdoozan S, Garmaroudi H et al. Assessment of lean patients with non‐alcoholic fatty liver disease in a middle income country; prevalence and its association with metabolic disorders: a cross‐sectional study. Arch. Iran. Med. 2017; 20: 211–17. [PubMed] [Google Scholar]
- 39. Gonzalez‐Cantero J, Martin‐Rodriguez JL, Gonzalez‐Cantero A, Arrebola JP, Gonzalez‐Calvin JL. Insulin resistance in lean and overweight nondiabetic Caucasian adults: study of its relationship with liver triglyceride content, waist circumference and BMI. PLoS One. 2018; 13: e0192663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim D, Kim W, Joo SK et al. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non‐obese nonalcoholic fatty liver disease. Liver Int. 2019; 39: 392–41. [DOI] [PubMed] [Google Scholar]
- 41. Lee SW, Lee TY, Yang SS, Tung CF, Yeh HZ, Chang C. Risk factors and metabolic abnormality of patients with non‐alcoholic fatty liver disease: either non‐obese or obese Chinese population. Hepatobiliary Pancreat. Dis. Int. 2018; 17: 45–48. [DOI] [PubMed] [Google Scholar]
- 42. Shao C, Ye J, Li F, Feng S, Wang W, Zhong B. Different predictors of steatosis and fibrosis severity among lean, overweight and obese patients with nonalcoholic fatty liver disease. Dig. Liver Dis. 2019; 51: 1392–9. [DOI] [PubMed] [Google Scholar]
- 43. Yun Y, Kim HN, Lee EJ et al. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS One. 2019; 14: e0213692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Das K, Das K, Mukherjee PS et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010; 51: 1593–602. [DOI] [PubMed] [Google Scholar]
- 45. Akyuz U, Yesil A, Yilmaz Y. Characterization of lean patients with nonalcoholic fatty liver disease: potential role of high hemoglobin levels. Scand. J. Gastroenterol. 2014; 50: 501–346. [DOI] [PubMed] [Google Scholar]
- 46. Albhaisi S, Chowdhury A, Sanyal AJ. Non‐alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019; 1: 19–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sookoian S, Castaño GO, Scian R et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016: 103: 422–34. [DOI] [PubMed] [Google Scholar]
- 48. Rahimi‐Sakak F, Maroofi M, Rahmani J, Bellissimo N, Hekmatdoost A. Serum uric acid and risk of cardiovascular mortality: a systematic review and dose‐response meta‐analysis of cohort studies of over a million participants. BMC Cardiovasc. Disord. 2019; 19: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J. Gastroenterol. 2012; 18: 187–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fracanzani AL, Petta S, Lombardi R et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin. Gastroenterol. Hepatol. 2017; 15: 1604–1611.e1. [DOI] [PubMed] [Google Scholar]
- 51. Bosello O, Zamboni M. Visceral obesity and metabolic syndrome. Obes. Rev. 2000; 1: 47–56. [DOI] [PubMed] [Google Scholar]
- 52. Feng R, Luo C, Li C et al. Free fatty acids profile among lean, overweight and obese non‐alcoholic fatty liver disease patients: a case ‐ control study. Lipids Health Dis. 2017; 16: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eshraghian A, Nikeghbalian S, Geramizadeh B, Kazemi K, Shamsaeefar A, Malek‐Hosseini SA. Characterization of biopsy proven non‐alcoholic fatty liver disease in healthy non‐obese and lean population of living liver donors: the impact of uric acid. Clin. Res. Hepatol. Gastroenterol. 2020; 44: 572–8. [DOI] [PubMed] [Google Scholar]
- 54. Yang C, Yang S, Xu W, Zhang J, Fu W, Feng C. Association between the hyperuricemia and nonalcoholic fatty liver disease risk in a Chinese population: a retrospective cohort study. PLoS One. 2017; 12: e0177249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng X, Gong L, Luo R et al. Serum uric acid and non‐alcoholic fatty liver disease in non‐obesity Chinese adults. Lipids Health Dis. 2017; 16: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choi YJ, Shin HS, Choi HS et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP‐1c activation in hepatocytes. Lab. Invest. 2014; 94: 1114–25. [DOI] [PubMed] [Google Scholar]
- 57. Lanaspa MA, Sanchez‐Lozada LG, Cicerchi C et al. Uric Acid Stimulates Fructokinase and Accelerates Fructose Metabolism in the Development of Fatty Liver. PLoS One. 2012; 7: e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wan X, Xu C, Lin Y et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome‐dependent mechanism. J. Hepatol. 2016; 64: 925–32. [DOI] [PubMed] [Google Scholar]
- 59. Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig. Liver Dis. 2015; 47: 181–90. [DOI] [PubMed] [Google Scholar]
- 60. Yang KC, Hung HF, Lu CW, Chang HH, Lee LT, Huang KC. Association of Non‐alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Sci. Rep. 2016; 6: 27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J. Hepatol. 2018; 68: 268–79. [DOI] [PubMed] [Google Scholar]
- 62. Chen F, Esmaili S, Rogers GB et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020; 71: 1213–27. [DOI] [PubMed] [Google Scholar]
- 63. Honda Y, Yoneda M, Kessoku T et al. Characteristics of non‐obese non‐alcoholic fatty liver disease: effect of genetic and environmental factors. Hepatol. Res. 2016; 46: 1011–18. [DOI] [PubMed] [Google Scholar]
- 64. Duarte SMB, Stefano JT, Miele L et al. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: a prospective pilot study. Nutr. Metab. Cardiovasc. Dis. 2018; 28: 369–84. [DOI] [PubMed] [Google Scholar]
- 65. Machado MV, Cortez‐Pinto H. Diet, microbiota, obesity, and NAFLD: a dangerous quartet. Int. J. Mol. Sci. 2016; 17: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang MH, Sung J, Gwak GY. The associations between apolipoprotein B, A1, and the B/A1 ratio and nonalcoholic fatty liver disease in both normal‐weight and overweight Korean population. J. Clin. Lipidol. 2016; 10: 289–98. [DOI] [PubMed] [Google Scholar]
- 67. Hossain IA, Rahman Shah MM, Rahman MK, Ali L. Gamma glutamyl transferase is an independent determinant for the association of insulin resistance with nonalcoholic fatty liver disease in Bangladeshi adults: association of GGT and HOMA‐IR with NAFLD. Diabetes Metab. Syndr. Clin. Res. Rev. 2016; 10: S25–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


