Abstract
Objective
Coronavirus disease 2019 (COVID‐19) has progressed rapidly around the world, reaching a lethality of up to 20% due to acute respiratory distress syndrome (ARDS). This latter condition is a relevant concern for systemic lupus erythematosus (SLE); however, data on this topic are limited to few case series. Our objective was to evaluate in hospitalized patients with SLE and with COVID‐19–associated ARDS (confirmed by reverse transcription‐polymerase chain reaction) the risk of mortality and combined poor outcomes (death, intensive care unit [ICU] admission, and/or mechanical ventilation [MV] use) and to compare with that of patients without SLE.
Methods
This is a nationwide cross‐sectional study of patients with severe acute respiratory syndrome coronavirus 2 nested in the national Influenza Epidemiological Surveillance Information System (Sistema de Informação de Vigilância Epidemiológica da Gripe [SIVEP‐gripe]). Mortality rates, frequencies of ICU admissions, and MV use for 319 patients with SLE and 251 800 patients without SLE were calculated as well as relative risks (RRs). A fully adjusted multiple logistic regression was performed to adjust factors, such as age and well‐known comorbidities, that might impact worse outcomes.
Results
Patients with SLE had an increased risk of death and combined poor outcome compared with patients without SLE (RR = 1.738, 95% confidence interval [CI]: 1.557‐1.914, and RR = 1.391, 95% CI: 1.282‐1.492, respectively). Among all investigated comorbidities, SLE yielded the higher risk of death and combined poor outcomes (RR = 2.205, 95% CI: 1.780‐2.633, and RR = 1.654, 95% CI: 1.410‐1.88, respectively).
Conclusions
This study provides novel evidence that patients with SLE hospitalized because of COVID‐19 have significantly higher risks of death and poor outcomes compared with patients without comorbidities and patients with other comorbidities.
Significance & Innovations.
This is the first study to evaluate the prognosis of coronavirus disease 2019 (COVID‐19) in a large population with systemic lupus erythematosus (SLE) hospitalized because of acute respiratory distress syndrome.
Patients with SLE had a significant increase in the risk of death and combined poor outcomes when compared with patients without SLE.
Among all investigated comorbidities, SLE yielded the highest risk for death and combined poor outcomes (relative risk [RR] = 2.205, 95% confidence interval [CI]: 1.780‐2.633; RR = 1.654, 95% CI: 1.410‐1.88).
This is an important alert to those caring for patients with SLE, and it is an additional reinforcement of the importance of preventive measures during the pandemic for this population.
INTRODUCTION
The pandemic of coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, has progressed rapidly around the world, reaching a lethality of up to 20% in different regions due to acute respiratory distress syndrome (ARDS) (1). Brazil has had more than 8 million cases of COVID‐19 since its first confirmed case on February 27, 2020, with more than 215 000 deaths until January 2021, a lethality rate of 2.5% (2, 3). More severe cases and high mortality rates have been reported in elderly and comorbid patients, particularly in those with cardiovascular or chronic respiratory diseases, diabetes mellitus, and arterial hypertension (4).
COVID‐19 raised particular concern for people with autoimmune rheumatic diseases (5, 6), particularly those with systemic lupus erythematosus (SLE), because of the known increased susceptibility to infections (6, 7). Underlying this susceptibility is the chronic inflammatory autoimmune dysregulation of the disease and the use of immunosuppressive medications (8). These factors may be associated with a high risk for SARS‐CoV‐2 and worse prognosis (8, 9). Reinforcing this possibility, Mathian et al (9) observed a high hospitalization rate (82%) in a small case series with 17 patients with SLE, including seven (41%) patients who were admitted to an intensive care unit (ICU), five (29%) who needed mechanical ventilation (MV), and two (14%) who died . A meta‐analysis including SLE data (10), showed lower figures, with hospitalization and mortality rates of 33% and 6.9%, respectively, in patients with lupus.
With regard to the incidence of COVID‐19 in patients with SLE’s, data are still controversial, with some reports suggesting comparable incidences (7, 11, 12, 13) and others suggesting higher frequencies than the general population (14, 15, 16). Recently, a meta‐analysis showed that the risk of COVID‐19 in patients with autoimmune rheumatic diseases was higher compared with control subjects without autoimmune rheumatic diseases (odds ratio: 2.19). An increased risk for lupus was also observed compared with other autoimmune rheumatic diseases (3.4% vs. 0.9%) (10); however, patients with lupus were categorized in the same group of patients with Sjogren syndrome and scleroderma, precluding a definitive conclusion.
Few studies specifically evaluated the prognosis of patients with SLE and with COVID‐19 (8, 9, 10). Fernandez‐Ruiz et al (8), observed that patients with SLE and COVID‐19 had high hospitalization rates and found that comorbidities were independent predictors of hospitalization in these patients. The small representation of patients (n = 41) with positive real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) in their analysis and lack of comparison with the non‐SLE group hamper an accurate interpretation of their findings.
Therefore, the primary aim of this study is to analyze the risk of death, ICU admission, and MV use in a large population of hospitalized patients with SLE who developed ARDS due to SARS‐CoV‐2 infection (confirmed by RT‐PCR) and compare them with patients without SLE.
METHODS
Data sources and study cohorts
We conducted a nationwide cross‐sectional study of SARS‐CoV‐2 infection with patients nested in the national Influenza Epidemiological Surveillance Information System (Sistema de Informação de Vigilância Epidemiológica da Gripe [SIVEP‐gripe]). In Brazil, ARDS is a compulsory reported disease, and a standardized electronic database (SIVEP‐gripe) captures relevant information such as demographic data and clinical and laboratory features of the hospitalized patients from the public and private systems. It is a nationally representative sample of ARDS cases in the country and an important tool to recognize outbreaks and pandemics.
This study was approved by the Local Ethics Committee of our University Hospital (33118820.0.0000.0068).
ARDS diagnosis
The definite diagnosis of ARDS requires the presence of flu‐like symptoms in addition to any of the following criteria: dyspnea/respiratory discomfort, persistent pressure in the chest, desaturation less than 95% in room air, or bluish color of the lips or face (17).
SARS‐CoV‐2 infection diagnosis
For this study, a more restricted definition of SARS‐CoV‐2 infection diagnosis than the one established in the SIVEP‐gripe database was used (17). The definite diagnosis was considered when a patient presented with a flu‐like syndrome in addition to dyspnea/respiratory discomfort and/or desaturation less than 95% in room air and/or bluish color of lips and laboratory SARS‐CoV‐2 confirmation by the RT‐PCR method (17). Additional clinical and epidemiological parameters (such as anosmia, ageusia, tomographic findings, and contact in the last 14 days with a confirmed COVID‐19 case) were also evaluated but were not considered obligatory for definite diagnosis criteria.
Covariates
Baseline information regarding demographics (age, sex, and region of Brazil) and comorbidities (arterial hypertension, diabetes mellitus, heart disease, hepatic disease, lung disease, renal disease, neurological disease, oncological disease, transplants, smoking, alcoholism, obesity/overweight status, and pregnancy) were systematically captured. Potential confounders included the already known risk factors for poor prognosis in COVID‐19, such as age, sex, and comorbidities. The region of Brazil was also considered as a potential confounder.
Outcomes
The outcomes of interest were mortality in hospitalized patients with COVID‐19 (death due to SARS‐Cov‐2 infection or death due to complications caused by SARS‐Cov‐2) and combined poor outcomes (ICU admission and/or MV use and/or death) in COVID‐19 hospitalized patients.
Statistical analysis
Propensity score
We compared the risk for mortality and combined poor outcomes between groups of patients with SLE and patients without SLE using relative risks (RRs) and their corresponding 95% CIs from generalized estimating equations. Models were fully adjusted using the propensity score (PS) stratification method for potential confounders (all demographics and comorbidities were covariates). The fully adjusted PS was estimated using a logistic regression model including all prespecified covariates (all demographic and comorbidities). The matching method performed was the stratification technique.
To control confounding variables, which included demographic data (age group, sex, and region) and comorbidities (heart, liver, lung, hypertension, diabetes, neurological, renal, neoplasms, dyslipidemia and obesity, pregnancy, smoking, and drinking), an adjustment instrument for the PS was used. Thus, a logistic regression model was developed for the SLE prediction using all variables understood as possibly confounding as explanatory. The model was applied for the entire sample, and the stratification was performed in 10 different groups (method stratification). These 10 groups were more homogeneous in relation to the probability of SLE diagnosis, neutralizing the effect of the confounders, than allowing adjusted comparison. The risks of death were weighted according to each group probability, allowing calculation of the final adjusted risk.
To adjust for comorbidities, logistic regression models were developed for each comorbidity as a response variable (having the disease or not) and demographic variables as predictor variables. The resulting probability of the model was used to stratify patients into 10 different groups (method stratification). It is important to mention that each comorbidity was assessed alone (the patient had only one disease), and overlap cases were excluded from this analysis to avoid bias.
Our database presented only 2% of missing data. For all binary covariates, missing data were treated as negative/not present. For the continuous or categoric covariates, patients with missing data were excluded from the analysis. The assessment of normal distribution data was performed with the Kolmogorov‐Smirnov test. Comparisons between categorical variables were performed using Fisher exact test or χ2 test when appropriate. Continuous variables were compared using the Student t test or Mann‐Whitney test when appropriate. The tests were conducted with a significance level of 5%. IBM SPSS (version 20) and Microsoft Excel 365 software were used to perform these analyses.
Confounder summary score
To better control the confounders bias, a second analysis was performed to assess the exposure risk score using the confounder summary score matching method (18). A three‐step methodology was used as follows: 1) a multiple logistic regression was performed to evaluate the outcome score in the entire database excluding the SLE population; 2) a score matching technique was performed to ensure proper adjustment of important confounders (such as age and comorbidities) and to ensure that both populations with and without SLE would enter the score with the same a priori risk; 3) a second multiple logistic regression, now including the SLE population, was performed to allow the assessment of the risk increment that SLE caused.
RESULTS
From January 1 to August 17, 2020, 607 326 ARDS cases had been reported in Brazil, of which312 419 (51.4%) were due to COVID‐19. Among these patients, 252 119 (80.7%) had RT‐PCR SARS‐CoV‐2 infection confirmation. In the overall cohort, we found 382 patients with SLE, and in the group with positive RT‐PCR results, there were 319 patients with SLE. Table 1 shows the distribution of demographic data, clinical characteristics, and outcomes of both cohorts comparing patients with SLE and patients without SLE.
Table 1.
Demographic and clinical features of patients hospitalized with SARS‐CoV‐2 infection in 2020
| Demographic | SARS‐CoV‐2 Cohort | Positive RT‐PCR SARS‐CoV‐2 Cohort | ||||
|---|---|---|---|---|---|---|
| SLE (n = 382) | Non‐SLE (n = 312 047) | P Value | SLE (n = 319) | Non‐SLE (n = 251 800) | P Value | |
| Age, mean (SD), yr | 45.1 (16.6) | 58.6 (19.1) | <0.0001 | 45.5 (16.5) | 58.7 (18.9) | <0.0001 |
| Female sex, n (%) | 327 (85.6) | 136 593 (43.8) | <0.001 | 274 (85.9) | 110 309 (43.8) | <0.001 |
| Comorbidities, n (%) | ||||||
| Any comorbidity | 236 (61.8) | 179 205 (57.4) | 0.086 | 198 (62.1) | 147 815 (58.7) | 0.245 |
| Arterial hypertension | 60 (15.7) | 37 799 (12.1) | 0.032 | 50 (15.7) | 30 846 (12.3) | 0.075 |
| Diabetes mellitus | 74 (19.4) | 76 689 (24.6) | 0.018 | 59 (18.5) | 63 010 (25.0) | 0.009 |
| Heart disease | 110 (28.8) | 99 909 (32.0) | 0.177 | 92 (28.8) | 82 987 (33.0) | 0.133 |
| Hepatic disease | 10 (2.6) | 2900 (0.9) | 0.001 | 10 (3.1) | 2425 (1.0) | <0.001 |
| Lung disease | 24 (6.3) | 21 184 (6.8) | 0.694 | 20 (6.3) | 17 919 (7.1) | 0.632 |
| Renal disease | 66 (17.3) | 15 658 (5.0) | <0.001 | 50 (15.7) | 13 291(5.3) | <0.001 |
| Neurological disease | 16 (4.2) | 12 854 (4.1) | 0.946 | 10 (3.1) | 11 028 (4.4) | 0.343 |
| Oncological disease | 8 (2.1) | 7608 (2.4) | 0.663 | 6 (1.9) | 6631 (2.6) | 0.507 |
| Transplant patients | 3 (0.8) | 548 (0.2) | 0.031 | 3 (0.9) | 510 (0.2) | 0.021 |
| Smoking | 3 (0.8) | 19 053 (6.1) | <0.001 | 3 (0.9) | 16 363 (6.5) | <0.001 |
| Alcoholism | 1 (0.3) | 3224 (1.0) | 0.1982 | 1 (0.3) | 2577 (1.0) | 0.327 |
| Obesity/overweight | 27 (7.1) | 1374 (0.4) | <0.001 | 23 (7.2) | 1065 (0.4) | <0.001 |
| COVID‐19 | ||||||
| Symptoms, n (%) | ||||||
| Fever | 245 (64.1) | 207 738 (66.6) | 0.313 | 203 (63.6) | 169 270 (67.2) | 0.192 |
| Cough | 259 (67.8) | 225 449 (72.2) | 0.052 | 218 (68.3) | 184 013 (73.1) | 0.065 |
| Sore throat | 68 (17.8) | 61 970 (19.9) | 0.314 | 49 (15.4) | 48 698 (19.3) | 0.084 |
| Dyspnea | 287 (75.1) | 212 359 (68.1) | 0.003 | 240 (75.2) | 172 859 (68.6) | 0.013 |
| Respiratory discomfort | 232 (60.7) | 169 922 (54.5) | 0.014 | 185 (58.0) | 138 125 (54.9) | 0.285 |
| Desaturation | 229 (59.9) | 168 426 (54.0) | 0.019 | 188 (58.9) | 139 003 (55.2) | 0.016 |
| Diarrhea | 69 (18.1) | 42 077 (13.5) | 0.009 | 55 (17.2) | 34 329 (13.6) | 0.073 |
| Vomiting | 50 (13.1) | 24 217 (7.8) | <0.001 | 39 (12.2) | 19 653 (7.8) | 0.005 |
| Abdominal pain | 8 (2.1) | 4694 (1.5) | 0.344 | 7 (2.2) | 3485 (1.4) | 0.318 |
| Fatigue | 31 (8.1) | 26 610 (8.5) | 0.773 | 23 (7.2) | 21 087 (8.4) | 0.516 |
| Anosmia | 26 (6.8) | 17 099 (5.5) | 0.255 | 24 (7.5) | 13 164 (5.2) | 0.086 |
| Ageusia | 10 (2.6) | 12 416 (4.0) | 0.174 | 9 (2.8) | 9317 (3.7) | 0.495 |
| ICU admission, n (%) | 159 (41.6) | 90 999 (29.2) | <0.001 | 132 (41.4) | 77 572 (30.8) | <0.001 |
| Ventilatory support, n (%) | 251 (65.7) | 172 318 (55.2) | <0.001 | 206 (64.6) | 141 512 (56.2) | 0.003 |
| Invasive ventilation | 105 (27.5) | 53 014 (17.0) | <0.001 | 83 (26.0) | 44 028 (17.5) | <0.001 |
| Noninvasive ventilation | 146 (38.2) | 119 304 (38.2) | 0.996 | 123 (38.6) | 97 484 (38.7) | 1.000 |
| ICU length of stay, mean (SD), d | 10.1 (9.9) | 10.3 (10.5) | 0.734 | 10.7 (10.3) | 10.5 (10.6) | 0.734 |
| Hospital length of stay, mean (SD), d | 11.7 (13.7) | 11.1 (12.1) | 0.376 | 12.0 (14.3) | 11.4 (12.1) | 0.376 |
| Outcome, n (%) | ||||||
| Deaths | 154 (40.3) | 103 039 (33.0) | 0.003 | 126 (39.5) | 82 895 (32.9) | 0.015 |
| Combined poor outcomes (ICU admission + MV need + death) | 210 (54.9) | 148 459 (47.5) | <0.001 | 173 (54.2) | 122 341 (48.5) | <0.001 |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; MV, mechanical ventilation; RT‐PCR, reverse transcription‐polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SLE, systemic lupus erythematosus.
The positive RT‐PCR cohort analysis revealed that patients with SLE were younger patients (45.5 ± 16.5 years vs. 58.7 ± 18.9 years, P < 0.0001) and had a greater predominance of women (85.9% vs. 43.8%; P < 0.001) than patients without SLE. ICU admission and MV use was more frequent in the SLE group than in the non‐SLE group (132 [41.4%] vs. 77 572 [30.8%], P < 0.001, and 83 [26%] vs. 44 028 [17.5%], P < 0.001, respectively). The death rate was also significantly higher in the former group (126 [39.5%] vs. 82 895 [32.9%], P = 0.015) (Table 1). Regarding symptoms presentation, dyspnea (240 [75.2%] vs. 172 859 [68.6%], P = 0.013), oxygen desaturation (188 [58.9%] vs. 139 003 [55.2%], P = 0.016), and vomiting (39 [12.2%] vs. 19 653 [7.8%], P = 0.016) were more often observed in patients with SLE than in patients without SLE (Table 1). For both groups, SLE and non‐SLE, the three more frequent symptoms were dyspnea, cough, and fever (Table 1).
Table 2 compares the RR of death and combined poor outcomes among different groups with SARS‐CoV‐2 infection confirmed by positive RT‐PCR results. After adjustment for additional potential confounders through PS analysis, patients with SLE had an increased risk of death and combined poor outcomes compared with patients without SLE (RR = 1.738 [95% CI: 1.557‐1.914] and RR = 1.391 [95% CI: 1.282‐1.492], respectively). Compared with patients without SLE without comorbidities, patients with SLE also had an increased risk of death and combined poor outcomes (RR = 2.249 [95% CI: 1.987‐2.511] and RR = 1.697 [95% CI: 1.549‐1.837], respectively). Patients with SLE also had an increased risk of death and combined poor outcomes compared with patients without SLE with any other comorbidity (RR = 1.397 [95% CI: 1.254‐1.536] and RR =1.174 [95% CI: 1.084‐1.258], respectively).
Table 2.
RRs and 95% CIs comparing the risk of death and combined poor outcomes in patients with SLE with positive COVID‐19‐19 RT‐PCR
| RR (95% CI) | ||||
|---|---|---|---|---|
| Numerator | Denominator | Crude Analysis | Fully Adjusted PS Analysis | |
| Death | ||||
| SLE versus non‐SLE | 0.395 | 0.329 | 1.200 (1.047‐1.374) | 1.738 (1.557‐1.914) |
| SLE versus non‐SLE without comorbidities | 0.395 | 0.226 | 1.749 (1.527‐2.005) | 2.249 (1.987‐2.511) |
| SLE versus non‐SLE with comorbidities | 0.395 | 0.396 | 0.998 (0.871‐1.143) | 1.397 (1.254 ‐1.536) |
| Combined poor outcome | ||||
| SLE versus non‐SLE | 0.542 | 0.486 | 1.116 (1.009‐1.235) | 1.391 (1.282‐1.492) |
| SLE versus non‐SLE without comorbidities | 0.542 | 0.365 | 1.486 (1.343‐1.644) | 1.697 (1.549‐1.837) |
| SLE versus non‐SLE with comorbidities | 0.542 | 0.564 | 0.962 (0.870‐1.064) | 1.174 (1.084‐1.258) |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; PS, propensity score; RR, relative risk; RT‐PCR, reverse transcription‐polymerase chain reaction; SLE, systemic lupus erythematosus. Combined poor outcome: death, intensive care unit admission and/or mechanical ventilation use.
Comparisons of the risks of death and combined poor outcomes of all comorbidities with patients without SLE without comorbidities are presented in Table 3. After adjustment for additional potential confounders through PS analysis, SLE diagnosis had a 2.21‐fold (95% CI: 1.178‐2.633) increased risk for mortality, followed by oncological disease (RR = 1.926 [95% CI: 1.846‐2.008]), alcoholism (RR = 1.897 [95% CI: 1.314‐2.531]), hepatic disease (RR = 1.865 [95% CI: 1.659‐2.077]), transplant (RR = 1.783 [95% CI: 1.065‐2.612]), renal disease (RR = 1.750 [95% CI: 1.621‐1.882]), obesity/overweight (RR = 1.704 [95% CI: 1.618‐1.792]), neurological disease (RR = 1.470 [95% CI: 1.396‐1.547]), lung disease (RR = 1.220 [95% CI: 1.152‐1.290]), and diabetes mellitus (RR = 1.192 [95% CI: 1.151‐1.234]) (Table 3 and Figure 1). SLE diagnosis was also associated with the highest risk for overall combined poor outcomes, with a RR of 1.654 (95% CI: 1.410‐1.881) (Table 3). Alcoholism yielded a 1.61‐fold (95% CI: 1.214‐1.976) risk increase, followed by obesity/overweight (RR = 1.613 [95% CI: 1.567‐1.659]), oncological disease (RR = 1.558 [95% CI: 1.507‐1.609]), transplant (RR = 1.519 [95% CI: 1.011‐1.990]), renal disease (RR = 1.482 [95% CI: 1.400‐1.564]), neurological disease (RR = 1.468 [95% CI: 1.414‐1.522]), smoking (RR = 1.442 [95% CI: 1.156‐1.722]), and hepatic disease (RR = 1.438 [95% CI: 1.307‐1.568]). Lung disease and diabetes mellitus resulted in less significant increase in the risk of poorer outcomes, with RRs of 1.218 (95% CI: 1.170‐1.266) and 1.205 (95% CI: 1.175‐1.235), respectively (Table 3 and Figure 2).
Table 3.
RRs and 95% CIs of death and combined poor outcome of each comorbidity compared with patients without systemic lupus erythematosus and without comorbidities
| Fully Adjusted PS Analysis, RR (95% CI) | ||
|---|---|---|
| Death | Combined Poor Outcome | |
| SLE | 2.205 (1.780‐2.633) | 1.654 (1.410‐1.881) |
| Heart disease | 0.982 (0.955‐1.010) | 1.079 (1.057‐1.101) |
| Hepatic disease | 1.865 (1.659‐2.077) | 1.438 (1.307 ‐1.568) |
| Lung disease | 1.220 (1.152‐1.290) | 1.218 (1.170‐1.266) |
| Arterial hypertension | 0.976 (0.940‐1.013) | 1.082 (1.053‐1.111) |
| Diabetes mellitus | 1.192 (1.151‐1.234) | 1.205 (1.175‐1.235) |
| Neurological disease | 1.470 (1.396‐1.547) | 1.468 (1.414‐1.522) |
| Renal disease | 1.750 (1.621‐1.882) | 1.482 (1.400‐1.564) |
| Oncological disease | 1.926 (1.846‐2.008) | 1.558 (1.507‐1.609) |
| Transplant | 1.783 (1.065‐2.612) | 1.519 (1.011‐1.990) |
| Obesity/overweight | 1.704 (1.618‐1.792) | 1.613 (1.567‐1.659) |
| Smoking | 1.294 (0.949‐1.704) | 1.442 (1.156‐1.722) |
| Alcoholism | 1.897 (1.314‐2.531) | 1.614 (1.214‐1.976) |
Abbreviations: CI, confidence interval; PS, propensity score; RR, relative risk; SLE, systemic lupus erythematosus Combined poor outcome: death, intensive care unit admission, and/or mechanical ventilation use.
Figure 1.
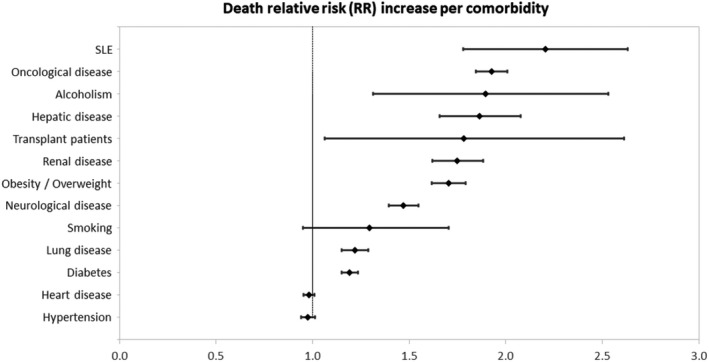
Relative risks (RRs) 95% confidence intervals (CIs) comparing the risk of death of patients with all comorbidities compared with patients without systemic lupus erythematosus (SLE) and comorbidities.
Figure 2.

Relative risks (RRs) 95% confidence intervals (CIs) comparing the risk of combined poor outcomes of each comorbidity compared with patients without lupus and comorbidities SLE, systemic lupus erythematosus.
The confounder summary score analysis confirmed the finding in the PS analysis that SLE significantly increases the risk of death (RR = 1.893, 95% CI: 1.652‐2.169) and combined poor outcomes (RR = 1.425, 95% CI: 1.289‐1.577) in patients hospitalized with ARDS due to COVID‐19 (Appendix 1). In the matched model, having an SLE diagnosis accounts for a risk comparable to a 20‐year increase in patient age.
DISCUSSION
To our knowledge, this is the first study to evaluate the prognosis of COVID‐19 due to ARDS in a large hospitalized SLE population and clearly show a high risk of death and combined poor outcomes. Among the investigated comorbidities, SLE remained as one of the diseases with a worse prognosis.
The great advantage of this study is the large sample size, provided by the nationwide database, with an evaluation of 382 hospitalized patients with SLE and 312 047 hospitalized patients without SLE, all with SARS‐CoV‐2 infection. Previous studies had exceedingly small sample sizes (n < 25) of hospitalized patients with SLE with SARS‐CoV‐2 infection (11, 13, 14, 19, 20). Another advantage of the present study was the restricted requirement of positive RT‐PCR for SARS‐CoV‐2 infection, which ensured a homogenous population with the correct etiologic diagnosis. This criterion was not observed in several previous reports, precluding a definitive conclusion about their findings (7, 13, 14, 20, 21, 22). The fully adjusted PS analysis was also relevant and minimized the impact of confounding variables, such as age, sex, and comorbidities. These are known risk factors for severe COVID‐19 infection (4). Importantly, regions of Brazil were also considered as a potential confounder due to discrepancies in socioeconomic status and quality of the health care system in some regions and therefore adjusted in PS analysis (23).
This study provides novel data demonstrating that SLE diagnosis doubled the risk of death and combined poor outcomes of SARS‐CoV‐2 infection in hospitalized patients compared with patients without SLE. This finding is in line with previous observations of higher severity of COVID‐19 infection in patients with autoimmune rheumatic disease (16) and of more hospitalization (8) in patients with lupus than the general population. In contrast, Hausmann et al (24) observed that only 5.5% of patients with autoimmune rheumatic diseases (a total of 9393 patients) developed COVID‐19, and the majority did not require hospitalization.
Of note, the majority of previous studies performed an overall analysis of autoimmune rheumatic diseases (pooling data) (2, 4, 10, 16, 19, 21, 24). This methodology provides the overall risk of COVID‐19 in the immunosuppressed population, but the heterogeneity of the pathogenesis of the distinct diseases and their variable prevalence (eg, 0.1% SLE vs. 1% rheumatoid arthritis) precludes a more stringent evaluation of the real impact per disease.
This increased mortality and worse prognosis observed in our study could be explained by a recent report that suggested that the angiotensin‐converting enzyme 2 (ACE2) gene is demethylated and overexpressed in patients with SLE, which could lead to enhanced susceptibility to SARS‐CoV‐2 infection (25). Alternatively, patients with lupus could be at increased risk for cytokine storm during SARS‐CoV‐2 infection (25).
Another contributing factor for COVID‐19 severity in lupus is the reported thromboembolic complications associated with this infection (26). The mechanism seems to be multifactorial and can be explained by previous cardiovascular risk, immobilization, direct endothelial damage by the virus via activation of ACE2, complement overactivation, disseminated intravascular coagulation, thrombotic microangiopathy, coagulopathy secondary to cytokine storm, formation of extracellular neutrophil traps and, more recently, the presence of antiphospholipid antibodies (26, 27, 28). Lupus also has a higher predisposition for thromboembolic events due to accelerated atherosclerosis (arterial hypertension, hyperlipidemia, aberrant innate and adaptive immune responses); positive antiphospholipid antibodies; increase activation of the coagulation cascade; abnormal interactions between platelets, leucocytes/SLE macrophages, and the endothelium; endothelial dysfunction and disease activity markers (such as high anti–double‐stranded DNA antibody and low C3 levels) (29, 30, 31). We, therefore, hypothesized that these preexisting conditions might have increased the chances of thrombotic complications and influenced the poor outcome of patients with lupus with a severe form of the disease (SARS‐CoV‐2) observed herein. Further studies are necessary to confirm this possibility because the SIVEP‐gripe data set (17) does not provide information regarding thrombotic complications.
The main limitation of our epidemiologic database cohort is related to the study design, which narrows down the analysis to the predefined covariates available in the SIVEP‐gripe data set. No data were provided regarding SLE classification criteria, autoantibody profile, disease activity and damage indexes, or immunosuppressive treatments. Missing data were not a relevant problem because they represented only 2% of the data, but heterogeneity on the accuracy of the information is expected with Brazilian regional differences, and this parameter was included in our analysis as a confounding variable. The 70% sensitivity of RT‐PCR for SARS‐CoV‐2 infection from the nasopharyngeal swab is also a concern (32, 33) because it might have decreased the number of reported cases of SARS‐CoV‐2 infection and consequently reduced the number of patients analyzed in the present study.
In conclusion, this is the first study to evaluate the prognosis of COVID‐19 due to ARDS in a large hospitalized SLE population and clearly show a significantly higher risk of death and combined poor outcomes compared with patients without lupus and without comorbidities and with other comorbidities. This is an important alert to those caring for patients with SLE, and it is an additional reinforcement of the importance of preventive measures, such as vaccines, during a pandemic for this population.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Bertoglio had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Bertoglio, Valim, Daffre, Aikawa, Silva, Bonfá, Ugolini‐Lopes.
Acquisition of data
Bertoglio, Valim, Daffre, Aikawa, Silva, Bonfá, Ugolini‐Lopes.
Analysis and interpretation of data
Bertoglio, Valim, Daffre, Aikawa, Silva, Bonfá, Ugolini‐Lopes.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors thank Alex Cardoso Lopes, BSc, for helping with the risk modeling and statistical analysis; his expertise allowed a more thorough work.
This was an epidemiological nationwide cross‐sectional study that used a public disidentified data set. Data are available in a public, open access repository at covid.saude.gov.br and opendatasus.saude.gov.br/dataset/bd‐srag‐2020.
Drs. Aikawa, Silva, and Bonfá’s work was supported by a grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (2015/03756‐4). Drs Silva and Bonfá’s work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (304984/2020‐5 and 305242/2019‐9, respectively).
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Ye C, Cai S, Shen G, Guan H, Zhou L, Hu Y, et al. Clinical features of rheumatic patients infected with COVID‐19 in Wuhan, China. Ann Rheum Dis 2020;79:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marques C, Pinheiro MM, Neto ETR, Dantas AT, Ribeiro FM, Melo AKG. COVID‐19 in patients with rheumatic diseases: what is the real mortality risk? Ann Rheum Dis 2020. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3. Coronavírus Brasil . Painel Coronavírus. 2020. URL: https://covid.saude.gov.br.
- 4. Emmi G, Bettiol A, Mattioli I, Silvestri E, Di Scala G, Urban ML, et al. SARS‐CoV‐2 infection among patients with systemic autoimmune diseases. Autoimmun Rev 2020;19:102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramirez GA, Gerosa M, Beretta L, Bellocchi C, Argolini LM, Moroni L, et al. COVID‐19 in systemic lupus erythematosus: data from a survey on 417 patients. Semin Arthritis Rheum 2020;50:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Favalli EG, Gerosa M, Murgo A, Caporali R. Are patients with systemic lupus erythematosus at increased risk for COVID‐19? Ann Rheum Dis 2021;80:e25. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez‐Ruiz R, Masson M, Kim MY, Myers B, Haberman RH, Castillo R, et al. Leveraging the United States epicenter to provide insights on COVID‐19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020;72:1971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathian A, Mahevas M, Rohmer J, Roumier M, Cohen‐Aubart F, Amador‐Borrero B, et al. Clinical course of coronavirus disease 2019 (COVID‐19) in a series of 17 patients with systemic lupus erythematosus under long‐term treatment with hydroxychloroquine. Ann Rheum Dis 2020;79:837–9. [DOI] [PubMed] [Google Scholar]
- 10. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID‐19 in patients with autoimmune diseases: a systematic review and meta‐analysis. Ann Rheum Dis 2020. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11. Chen C. The plight of patients with lupus nephritis during the outbreak of COVID‐19 in Wuhan, China. J Rheumatol 2020;47:1452. [DOI] [PubMed] [Google Scholar]
- 12. Briones‐Figueroa A, García‐Villanueva M, Andreu Suárez, Tortosa‐Cabañas M, Corral‐Bote A, Garrote‐Corral S, et al. Clinical characteristics and impact of the COVID‐19 pandemic in systemic lupus erythematosus patients in a Spanish tertiary hospital [abstract]. Arthritis Rheumatol 2020;72 Suppl 10. URL: https://acrabstracts.org/abstract/clinical‐characteristics‐and‐impact‐of‐the‐covid‐19‐pandemic‐in‐systemic‐lupus‐erythematosus‐patients‐in‐a‐spanish‐tertiary‐hospital/. [Google Scholar]
- 13. Gendebien Z, von Frenckell C, Ribbens C, André B, Thys M, Gangolf M, et al. Systematic analysis of COVID‐19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments. Ann Rheum Dis 2020. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14. Bozzalla Cassione E, Zanframundo G, Biglia A, Codullo V, Montecucco C, Cavagna L. COVID‐19 infection in a northern‐Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis 2020;79:1382–3. [DOI] [PubMed] [Google Scholar]
- 15. Gartshteyn Y, Askanase AD, Schmidt NM, Bernstein EJ, Khalili L, Drolet R, et al. COVID‐19 and systemic lupus erythematosus: a case series. Lancet Rheumatol 2020;2:e452–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Silva K, Jorge A, Lu N, Zhang Y, Wallace Z, Choi H. Outcomes of coronavirus disease 2019 infection among patients living with rheumatic diseases: a matched cohort study from a US multi‐ center research network [abstract]. Arthritis Rheumatol 2020;72 Suppl 10. URL: https://acrabstracts.org/abstract/outcomes‐of‐coronavirus‐disease‐2019‐infection‐among‐patients‐living‐with‐rheumatic‐diseases‐a‐matched‐cohort‐study‐from‐a‐us‐multi‐center‐research‐network/. [Google Scholar]
- 17. Ministério da Saúde . Government of Brazil. 2020. URL: https://coronavirus.saude.gov.br/definicao‐de‐caso‐e‐notificacao.
- 18. Greenland S. Confounder summary score. Wiley StatsRef: Statistics Reference Online 2015:1–3.
- 19. Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, et al. SARS‐CoV‐2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross‐sectional study on 916 patients. J Autoimmun 2020;112:102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goyal M, Patil P, Pathak H, Santhanam S, Goel A, Sharma V, et al. Impact of COVID‐19 pandemic on patients with SLE: results of a large multicentric survey from India. Ann Rheum Dis 2020. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21. Favalli EG, Agape E, Caporali R. Incidence and clinical course of COVID‐19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol 2020;47:8. [DOI] [PubMed] [Google Scholar]
- 22. Holubar J, Le Quintrec M, Letaief H, Faillie JL, Pers YM, Jorgensen C. Monitoring of patients with systemic lupus erythematosus during the COVID‐19 outbreak. Ann Rheum Dis 2020. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23. IBGE, Coordenação de Trabalho e Rendimento . Pesquisa nacional de saúde: 2019. Rio de Janeiro: IBGE; 2020. [Google Scholar]
- 24. Hausmann J, Kennedy K, Surangiwala S, Larche M, Levine M, Liew J, et al. Characteristics of adult patients with rheumatic diseases during the COVID‐19 pandemic: data from an international patient survey [abstract]. Arthritis Rheumatol 2020;72 Suppl 10. URL: https://acrabstracts.org/abstract/characteristics‐of‐adult‐patients‐with‐rheumatic‐diseases‐during‐the‐covid‐19‐pandemic‐data‐from‐an‐international‐patient‐survey/. [Google Scholar]
- 25. Sawalha AH, Manzi S. Coronavirus disease‐2019: implication for the care and management of patients with systemic lupus erythematosus. Eur J Rheumatol 2020;7 Suppl 2:S117–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonjorno lP, Lopes F, Louzada Junior P, Oliveira RDR. Imunopatologia induzida por COVID‐19: avaliação da resposta imune inata e adaptativa. Rev Paul Reumatol 2020;19:6–11. [Google Scholar]
- 27. Assad RL. Mecanismos envolvidos na trombogênese em pacientes com COVID‐19. Parte 1. Trombogênese em SARS‐CoV2: mecanismo da doença. Rev Paul Reumatol 2020;19:19–21. [Google Scholar]
- 28. Andrade D. Mecanismos envolvidos na trombogênese em pacientes com COVID‐19. Parte 2. Síndrome antifosfolípide (SAF) e infecção por SARS‐CoV2 (COVID). Rev Paul Reumatol 2020;19:22–6. [Google Scholar]
- 29. Ramirez GA, Efthymiou M, Isenberg DA, Cohen H. Under crossfire: thromboembolic risk in systemic lupus erythematosus. Rheumatology (Oxford) 2019;58:940–52. [DOI] [PubMed] [Google Scholar]
- 30. de Groot PG, de Laat B. Mechanisms of thrombosis in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract Res Clin Rheumatol 2017;31:334–341. [DOI] [PubMed] [Google Scholar]
- 31. Petri M. Thrombosis and systemic lupus erythematosus: the Hopkins Lupus Cohort perspective. Scand J Rheumatol 1996;25:191–3. [DOI] [PubMed] [Google Scholar]
- 32. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID‐19: comparison to RT‐PCR. Radiology 2020;296:E115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT‐PCR testing for coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2020;296:E32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


