Abstract
Objectives:
Describe outcomes among preterm infants diagnosed with single-vessel primary pulmonary vein stenosis (PPVS) initially treated using conservative management (active surveillance with deferral of treatment).
Study Design:
Retrospective cohort study at a single, tertiary-center (2009-2019) among infants <37 weeks’ gestation with single-vessel PPVS. Infants were classified into 2 categories: disease progression and disease stabilization. Cardiopulmonary outcomes were examined, and a Kaplan-Meier survival analysis performed.
Results:
Twenty infants were included. Compared to infants in the stable group (0/10, 0%), all infants in the progressive group had development of at least severe stenosis or atresia (10/10, 100%; P<0.01). Severe pulmonary hypertension at diagnosis was increased in the progressive (5/10, 50%) than the stable group (0/10, 0%; P=0.03). Survival was lower among infants in the progressive than the stable group (Log-Rank Test, P<0.01).
Conclusion:
Among preterm infants with single-vessel PPVS, risk stratification may be possible, wherein more targeted, individualized therapies could be applied.
Introduction:
Pulmonary vein stenosis (PVS) is a rare, often lethal, cardiac disease. PVS obstructs venous return to the left atrium, raising pulmonary venous pressure.1 Consequent clinical manifestations include pulmonary edema, right heart failure, pulmonary hypertension (PH), frequent re-hospitalizations, and death.1, 2, 3, 4 In contrast to secondary (acquired) PVS following surgery for total anomalous pulmonary venous connection (TAPVC) or other cardiac lesions, primary PVS (PPVS) occurs in the absence of preceding intervention.4 In view of the growing awareness of PPVS among preterm infants, the American Heart Association (AHA) and Pediatric Pulmonary Hypertension Network (PPHN) have issued statements identifying the need for a better understanding of optimal evaluation, management, and treatment strategies.5, 6, 7, 8
Treatments with catheter-based interventions or surgery are widely accepted for infants with PPVS, including use among infants with single vessel (isolated) disease.3, 9 However, in the absence of evidence-based guidance, treatments have been applied broadly, largely irrespective of clinical presentation and disease severity (e.g. number of stenosed veins).9 Rather than an “all-or none” strategy, accurate characterization of potential clinical phenotypes may inform prognosis and provide opportunities for more individualized, targeted approaches.8
The present study describes short and longer-term outcomes among infants born <37 weeks’ gestation, diagnosed with single vessel PPVS, and treated initially using conservative management (active surveillance with deferral of treatment until evidence of disease progression). Since this approach to management has not been adopted widely across centers, factors associated with disease progression to multivessel disease (or death) are described. We hypothesized that single-vessel PPVS is a diverse disease, including a subset of infants who can be managed conservatively.
Methods:
All study activities were approved by the Nationwide Children’s Hospital (NCH) Institutional Review Board (IRB #IRB 00000957). Two authors (E.Z., C.B.) independently searched institutional databases in pediatric cardiology and neonatology at NCH for preterm infants with a diagnosis of PPVS from 2009-2019.
Eligibility Criteria:
Because PVS among older, more mature patients may represent a separate disease process with unique causes and outcomes, we included premature infants born at <37 weeks gestation and <1 year of age with an initial diagnosis of single-vessel pulmonary vein stenosis.10, 11 Infants with an initial diagnosis of multivessel disease were excluded; stenosis of a right or left common vein was considered multivessel disease.
To minimize confounding effects, we excluded infants with life-threatening congenital or chromosomal anomalies; infants with Trisomy 21 were included. We excluded infants with cardiac anomalies (e.g. TAPVC, partial anomalous pulmonary venous connection), other than atrial septal defects (ASD), patent ductus arteriosus (PDA), or muscular ventricular septal defects (VSD). Infants transferred to our facility were included if medical records, with Doppler transthoracic echocardiograms (ECHOs), were available. We used a pragmatic definition of bronchopulmonary dysplasia (BPD), as defined by Jensen et al.12 Severity of pulmonary hypertension (PH), as defined by Carlton et. al, as determined by cardiologist blinded to the infant’s clinical status.13 Right ventricular dysfunction was assessed qualitatively.
PVS Score:
Consistent with previous studies3, the degree of stenosis at the veno-atrial junction was determined based on mean ECHO gradients: mild (2 to 4 mmHg), moderate (5 to 7 mmHg), or severe (>7 mmHg). No stenosis was defined by the absence of PV narrowing, biphasic blood flow, and a mean ECHO gradient <2 mmHg. The stenosis was described as focal when affecting a very short length of the PV with associated upstream dilatation of the PV, and as diffuse when affecting a significant length of the PV with no upstream dilation of the PV. To better define the level of stenosis and the status of downstream pulmonary veins, assessments in 75% of infants (15/20) were supplemented by additional imaging, including cardiac catheterization and/or computed topography (CT).
Each vein was given a score of 0 (no stenosis), 1.0 (mild focal), 1.5 (mild diffuse), 2.0 (moderate focal), 2.5 (moderate diffuse), 3.0 (severe focal), 3.5 (severe diffuse), or 4.0 (atresia). The overall severity of stenosis (PVS score) reflects the sum of four individual PV scores, with an additional 2.0 added in cases of progression to bilateral disease (score range, 0-18).3 Pulse-width Doppler interrogations were attempted for all veins on each study; however, in settings where PV anatomy was not clearly delineated (limited acoustic windows, patient agitation), the PVS score from the previous study was used. To verify the diagnosis, 3 pediatric cardiologists (C.C., C.S., C.B.) independently reviewed cardiac imaging. Disagreements in the determination of disease severity were discussed with a 4th cardiologist (B.B.), and consensus reached.
Disease stabilization or progression:
Based on comparisons to imaging at initial diagnosis, infants were classified into one of 2 categories: disease progression or stabilization.14 Disease progression was defined as 1) new stenosis (or atresia) in a previously unaffected vein (e.g. single-vessel disease progressing to multivessel disease); 2) worsening stenosis in a single vein, defined a-priori as an increase in the PVS score of ≥2 (e.g. progression from mild to severe stenosis). Disease stabilization was defined as ≥12 months without evidence of disease progression.
Treatment approach:
For infants with single-vessel PPVS, NCH had adopted conservative approaches, with active surveillance and deferral of treatment until evidence of disease progression. The timing and nature of interventions were at the discretion of the attending health care providers. Diagnostic cardiac catheterizations in the absence of directed therapies (e.g. balloon angioplasty) were not considered interventions.
Disease surveillance and follow-up:
Surveillance of PPVS disease was conducted by serial ECHO. In most cases, surveillance was performed monthly to bimonthly for the first 12 months following initial diagnosis. All cardiac testing was recommended, rather than required. Longer-term outcomes beyond initial hospitalization were reviewed. Surviving infants had follow-up for at least 1 year following initial PPVS diagnosis.
Statistical analysis:
All statistical analyses were performed using SPSS V.19 statistical software or GraphPad Prism. Characteristics of infants with disease progression versus disease stabilization are presented as means ± standard deviations or medians with range. Group comparisons for normally-distributed continuous variables were made using the Student’s t-test; for nonparametric analyses, the Mann-Whitney test was used. Chi-squared or Fisher’s exact tests were used for categorical variables. A Kaplan-Meier survival analysis was performed among infants with disease progression versus stabilization using the log-rank test. A P< 0.05 was considered statistically significant.
Results:
A total of 20 infants met inclusion criteria and were initially diagnosed with single-vessel PPVS (Figure 1, on-line only). Clinical characteristics are provided in Table 1. All infants had ECHOs in their first month of life documenting normal cardiac anatomy, including pulmonary venous structures. Four infants (20%) were transferred from other institutions, with documented evidence of normal ECHOs at the referring institution. Overall, 214 ECHOs (median 11 per patient, range 3-24) were reviewed. The median number of ECHOs prior to a diagnosis of PPVS was 3 (range 1-17). We observed no differences in the median number of ECHOs over the first 12 months following initial diagnosis among infants with disease progression (5, range 3-10) versus disease stabilization (4, range 1-9; p=0.14). Associated cardiac lesions at diagnosis included ASD (8/20, 40%), PDA (5/20, 25%), and muscular VSD (1/20, 5%). We observed no differences between the two groups in the distribution of associated cardiac lesions. No infants in either group were exposed to postnatal corticosteroids (e.g. dexamethasone). Compared to infants in the progressive group (10/10, 100%), less than half (4/10, 40%) of infants in stable group had evidence of mild or greater PH at 36 weeks corrected gestational age (p=0.01).
Figure 1:
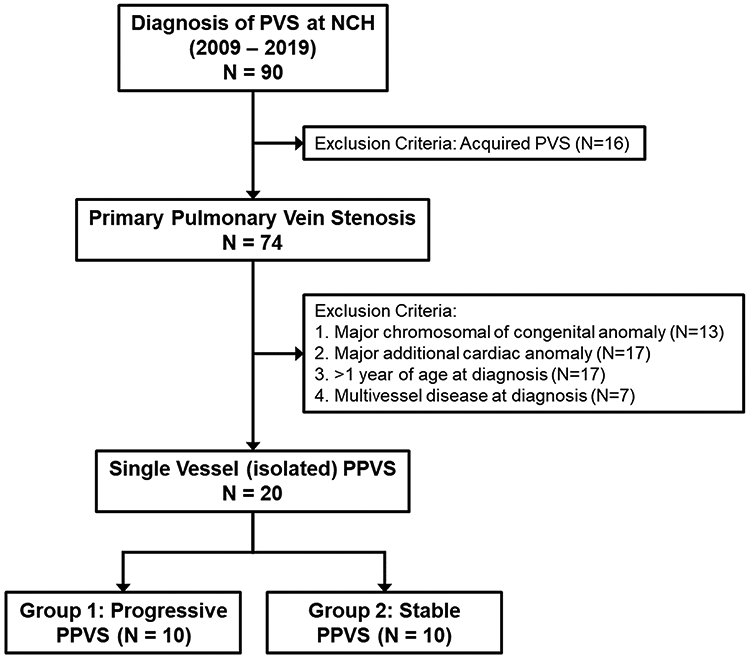
Patient selection flowchart.
Table:
Clinical characteristics
| Characteristics | Stable Disease (N=10) |
Progressive Disease (N=10) |
P- Value |
|---|---|---|---|
| GA (weeks) | 30.4 ± 4.5 | 26.2 ± 1.7 | 0.01 |
| Birth weight (grams) | 1146.7 ± 919.0 | 640.8 ± 182.0 | 0.12 |
| Diagnosis of PPVS (day) | 110.7 ± 82.0 | 144.1 ± 82.4 | 0.38 |
| Female gender | 6 (60%) | 7 (70%) | >0.99 |
| Trisomy 21 | 2 (20%) | 0 (0%) | 0.47 |
| IUGR | 7 (70%) | 6 (60%) | >0.99 |
| Multiple gestation | 0 (0%) | 2 (20%) | 0.47 |
| PDA closure1 | 4 (40%) | 4 (40%) | >0.99 |
| BPD (grade 3)2 | 5 (50%) | 8 (80%) | 0.35 |
| NEC3 | 1 (10%) | 1 (10%) | >0.99 |
| Spontaneous Intestinal Perforation | 1 (10%) | 1 (10%) | >0.99 |
| Severe PH4 | 0 (0%) | 5 (50%) | 0.03 |
PPVS: primary pulmonary vein stenosis; GA: Gestational age; IUGR: intrauterine growth restriction; PDA: patent ductus arteriosus; BPD: bronchopulmonary dysplasia; PH: Pulmonary hypertension; NEC: necrotizing enterocolitis
Percutaneous closure (n=7); surgical ligation (n=1) performed prior to transfer to NCH.
BPD criteria as defined by Jensen et. al.12
As defined by Bell et al.15
PH at time of PPVS diagnosis.13
PPVS characterization:
Initial diagnosis was made by ECHO in all infants. We observed no differences in the median corrected gestational age (weeks) at time of diagnosis among infants with disease progression (45.4 weeks ± 14.1 weeks) versus disease stabilization (45.9 weeks ± 8.1 weeks; p=0.92). At the time of PPVS diagnosis, infants in the progressive group were more likely to have a diagnosis of severe PH (5/10, 50%) than infants in the stable group (0/10, 0%; P=0.03). Among the progressive group, all infants underwent additional diagnostic testing, including cardiac catheterization (n=5) or chest CT (n=5); among the stable group, 5 infants underwent additional testing, including cardiac catheterization (n=4) and chest CT (n=1). At the time of diagnosis, stenosis was observed in the following pulmonary veins: right upper (n=7, 35%), left lower (n=6, 30%), left upper (n=6, 30%), and right lower (1, 5%). We observed no differences between the two groups in the location of stenosis. We observed no association between the location of stenosis and the likelihood of disease progression.
Agreement on the severity of PVS Score among reviewers was good (K= 0.78). Due to limited acoustic windows and patient agitation, the PVS score from the previous ECHO was used in 21 of 214 (9.8%) occurrences. We observed no differences in the median PVS score at diagnosis among infants in the progressive (2.0, range 1.0-4.0) versus stable groups (1.25, range 1.0-2.5; p=0.09). Following initial diagnosis, compared to infants in the stable group (0/10, 0%), all infants (10/10, 100%) in the progressive group had subsequent evidence of at least severe stenosis or atresia (P <0.01). No infants in the stable group had a PVS score ≥3 throughout hospitalization. Reasons for characterization of disease progression included: 1) evidence of new stenosis (or atresia) in a previously unaffected vein (n=6) and progression from mild to severe stenosis (n=3) or progression from mild to atresia (n=1). Among infants in the progressive group, the median time from initial diagnosis to worsening disease was 1.5 months (range 0.1 – 7.1 months). Not surprisingly, we observed differences in the maximum PVS score throughout hospitalization among infants in the progressive group (median 7.0, range 3.0-12.0) versus stable group (median 2.0, range 1.0-2.5; P<0.01).
Treatment modalities:
No infants in the stable group underwent intervention (catheter-based or surgery) for PPVS. In contrast, 2 (20%) of the infants in the progressive group underwent cutting balloon angioplasty (n=2). None of the infants underwent surgery or lung transplantation. We observed no differences between the progressive group versus stable group in the use of furosemide (9/10 vs 8/10; p>0.99), spironolactone (7/10 vs 2/10; p=0.07), sildenafil (3/10 vs 4/10, p >0.99) or inhaled nitric oxide (7/10 vs 3/10; p=0.18); however, limited numbers preclude meaningful comparisons. Evidence of moderate-to-severe RV dysfunction was observed among 3 (30%) infants in the progressive group, compared to 1 (10%) infant in the stable group (P=0.58). None of the patients developed pulmonary edema after anti-PH therapies. All infants who died were on anti-PH therapy at the time of their death.
Mortality and longer-term follow:
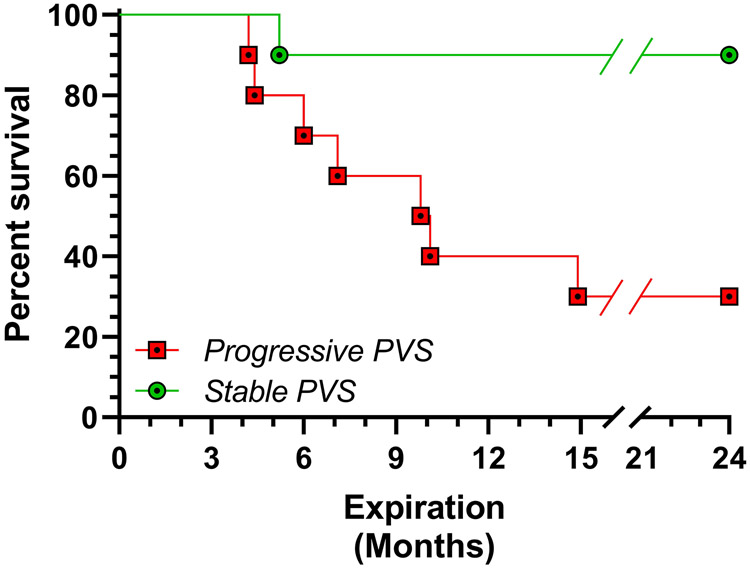
Survival was lower among infants in the progressive group than the stable group (Log Rank Test, P<0.01; Figure 2). The primary cause of death in the PPVS progressive group included PH crisis (n=5) and cardiac decompensation of unclear etiology prompting withdrawal of support at the request of caregivers (n=2). At the time of death, the PVS score in the progressive group was 7.1 (± 3.4). In contrast, we observed one death in the stable group in a Trisomy 21 infant due to an acute respiratory deterioration of unclear etiology and subsequent caregiver withdrawal of care; PVS score at time of death was 1.5. Median follow-up duration was 4 years (IQR, 1-8.5 years). All infants discharged home with PPVS continue to have evidence of PPVS disease at longest available follow-up. No infants died after 15 months corrected gestational age. Follow-up was 100% complete.
Figure 2:
Kaplan-Meier survival plot among infants with PPVS disease progression or disease stabilization. Survival is shown on the y-axis. Follow-up time in months is shown on the X-axis. Difference in survival was determined by log-rank test. P-value <0.01.
Discussion:
Although the present study is a single-center experience, the number of premature infants with single-vessel PPVS exceeds those in multicenter registries and benefits from consistently applied entry criteria and disease definitions.3 Similar to previous investigators, we observed that the natural history of PPVS can vary considerably from asymptomatic to rapidly progressive.4, 8 Observed differences between infants with progressive disease versus stable disease (more premature, PVS score >2.5, severe PH) suggest that risk stratification may be possible, wherein more targeted, individualized therapies could be applied. Without direct comparisons, conclusions on the optimal treatment among premature infants with single-vessel PPVS remain unanswered.3, 8, 16 Thus, our results do not support the widespread acceptance of conservative management for PPVS, but rather provide a foundation for selection of potential subgroups of infants for enrollment in comparative trials necessary to inform the practice of evidence-based medicine.
While evidence on the value of aggressive surveillance, intervention, and reintervention for multivessel PPVS is growing, optimal treatments for infants with single-vessel disease remains highly controversial.8, 17, 18 In a multicenter cohort study, Mahgoub et. al described similar outcomes among infants with single-vessel PPVS who were treated conservatively (non-intervention) versus those who underwent surgical intervention.16 While data are mixed, preterm infants may be at higher risk for procedure-related complications than more mature counterparts, wherein a period of conservative management is attractive.19, 20, 21 In the present cohort, outcomes of infants with stable disease treated conservatively are encouraging, but evidence of disease progression in half of infants initially diagnosed with single-vessel PPVS is noteworthy. In fact, delayed intervention among infants who developed progressive disease may have contributed to worse outcomes, wherein earlier, more aggressive intervention would be warranted.18
Evidence on the pathobiology, pathophysiology, and natural history of premature infants with single-vessel PPVS is limited by the small numbers of infants available for study at any one institution. To date, accurate characterization of clinical phenotypes and best practices have been obscured by marked inter- and intra-center variability in disease surveillance and management strategies.22 To define subgroups of infants with distinct physiologic phenotypes, characterize common patterns of care, and identify best practices, multidisciplinary collaborations are needed.23, 24 We eagerly await findings from the PVS Network, an ongoing collaborative among centers in the US and abroad, to provide evidence-based approaches to the care of infants with PPVS.25
Limitations:
The present study has several limitations. Unmeasured confounders may have influenced the observed findings and cannot be accounted for in a retrospective study design. Despite a comprehensive search, we acknowledge the inherent risk of selection bias, including the potential for subclinical cases (“silent” PPVS disease) to have been missed. To mitigate the risk of potential misdiagnosis, images were reviewed independently and consensus reached; however, definitions for PPVS are highly variable and inconsistently applied.3, 22 Previous investigators have described the validity of the PVS Score, an ECHO-based assessment of disease severity.3, 9 However, redistribution of blood flow to non-affected segments of the lung may result in misclassification of disease severity using ECHO alone.26 Consistent with growing evidence on the value of advanced cardiac imaging modalities to more clearly determine the level of stenosis and the status of downstream pulmonary veins is acknowledged, most infants in the present study underwent additional cardiac imaging.6, 7, 24 Given complex cardiopulmonary interactions in the setting of PPVS and associated PH, robust characterization of RV performance may provide opportunities for more tailored treatment strategies (afterload reduction, inotropy enhancement).27 Multicenter, interdisciplinary studies are needed to better characterize inflammatory profiles among infants with PPVS, and to determine if those profiles are mediated by prenatal (IUGR) and postnatal (NEC) influences.28, 29 The results of the present study do not pertain to infants with multivessel PPVS.5, 17
Conclusion:
Outcomes among a subgroup of infants with stable disease are encouraging, but conclusions on the optimal treatment among premature infants with single-vessel PPVS remain unanswered. Observed patient and diagnostic-level differences among infants with progressive disease versus stable disease provide opportunities for risk stratification. These findings may help inform patient selection for infants with PPVS and direct targeted research efforts to support the practice of evidence-based medicine.
Acknowledgments
The authors (C.B., J.S. and B.C.) are funded by the National Heart, Lung, and Blood Institute of the US National Institutes of Health (R01HL145032, R01HL13693).
Footnotes
Conflict of Interest Statement: The authors have no commercial or financial conflicts of interest to disclose.
REFERENCES
- 1.Holt DB, Moller JH, Larson S, Johnson MC. Primary pulmonary vein stenosis. Am J Cardiol 2007, 99(4): 568–572. [DOI] [PubMed] [Google Scholar]
- 2.Bini RM, Cleveland DC, Ceballos R, Bargeron LM Jr., Pacifico AD, Kirklin JW. Congenital pulmonary vein stenosis. Am J Cardiol 1984, 54(3): 369–375. [DOI] [PubMed] [Google Scholar]
- 3.Kalfa D, Belli E, Bacha E, Lambert V, di Carlo D, Kostolny M, et al. Primary Pulmonary Vein Stenosis: Outcomes, Risk Factors, and Severity Score in a Multicentric Study. Ann Thorac Surg 2017, 104(1): 182–189. [DOI] [PubMed] [Google Scholar]
- 4.Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation 2007, 115(1): 103–108. [DOI] [PubMed] [Google Scholar]
- 5.del Cerro MJ, Sabate Rotes A, Carton A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol 2014, 49(1): 49–59. [DOI] [PubMed] [Google Scholar]
- 6.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation 2015, 132(21): 2037–2099. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan U, Feinstein JA, Adatia I, Austin ED, Mullen MP, Hopper RK, et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J Pediatr 2017, 188: 24–34 e21. [DOI] [PubMed] [Google Scholar]
- 8.Kanaan UB, Mahle WT. New Paradigms for Pulmonary Vein Stenosis Treatment: When Surgery and Transcatheter Therapy Aren't Good Enough. J Pediatr 2018, 198: 12–13. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblum JM, Altin HF, Gillespie SE, Bauser-Heaton H, Kanter KA, Sinha R, et al. Management outcomes of primary pulmonary vein stenosis. J Thorac Cardiovasc Surg 2020, 159(3): 1029–1036 e1021. [DOI] [PubMed] [Google Scholar]
- 10.Drossner DM, Kim D, Maher KO, Mahle WT. Prematurity is a strong risk factor for developing pulmonary vein stenosis. J Am Coll Cardiol 2008, 51(10): A378–A379. [Google Scholar]
- 11.Drossner DM, Kim DW, Maher KO, Mahle WT. Pulmonary vein stenosis: Prematurity and associated conditions. Pediatrics 2008, 122(3): E656–E661. [DOI] [PubMed] [Google Scholar]
- 12.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med 2019, 200(6): 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlton EF, Sontag MK, Younoszai A, DiMaria MV, Miller JI, Poindexter BB, et al. Reliability of Echocardiographic Indicators of Pulmonary Vascular Disease in Preterm Infants at Risk for Bronchopulmonary Dysplasia. J Pediatr 2017, 186: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan R, Kieran MW, Baird CW, Colan SD, Gauvreau K, Ireland CM, et al. Adjunct Targeted Biologic Inhibition Agents to Treat Aggressive Multivessel Intraluminal Pediatric Pulmonary Vein Stenosis. J Pediatr 2018, 198: 29–35 e25. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978, 187(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahgoub L, Kaddoura T, Kameny AR, Lopez Ortego P, Vanderlaan RD, Kakadekar A, et al. Pulmonary vein stenosis of ex-premature infants with pulmonary hypertension and bronchopulmonary dysplasia, epidemiology, and survival from a multicenter cohort. Pediatr Pulmonol 2017, 52(8): 1063–1070. [DOI] [PubMed] [Google Scholar]
- 17.Hong H Commentary: Management outcomes of primary pulmonary vein stenosis: What we know now and what we can expect in the future. J Thorac Cardiovasc Surg 2020, 159(3): 1039. [DOI] [PubMed] [Google Scholar]
- 18.Cory MJ, Ooi YK, Kelleman MS, Vincent RN, Kim DW, Petit CJ. Reintervention Is Associated With Improved Survival in Pediatric Patients With Pulmonary Vein Stenosis. JACC Cardiovasc Interv 2017, 10(17): 1788–1798. [DOI] [PubMed] [Google Scholar]
- 19.Bergersen L, Gauvreau K, Foerster SR, Marshall AC, McElhinney DB, Beekman RH 3rd, et al. Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM). JACC Cardiovasc Interv 2011, 4(9): 1037–1046. [DOI] [PubMed] [Google Scholar]
- 20.Bergersen L, Gauvreau K, Marshall A, Kreutzer J, Beekman R, Hirsch R, et al. Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circ Cardiovasc Interv 2011, 4(2): 188–194. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002, 123(1): 110–118. [DOI] [PubMed] [Google Scholar]
- 22.Backes CH, Nealon E, Armstrong AK, Cua CL, Mitchell C, Krishnan U, et al. Pulmonary Vein Stenosis in Infants: A Systematic Review, Meta-Analysis, and Meta-Regression. J Pediatr 2018, 198: 36–45 e33. [DOI] [PubMed] [Google Scholar]
- 23.Lo Rito M, Gazzaz T, Wilder TJ, Vanderlaan RD, Van Arsdell GS, Honjo O, et al. Pulmonary vein stenosis: Severity and location predict survival after surgical repair. J Thorac Cardiovasc Surg 2016, 151(3): 657–666 e652. [DOI] [PubMed] [Google Scholar]
- 24.Levy PT, Jain A, Nawaytou H, Teitel D, Keller R, Fineman J, et al. Risk Assessment and Monitoring of Chronic Pulmonary Hypertension in Premature Infants. J Pediatr 2020, 217: 199–209 e194. [DOI] [PubMed] [Google Scholar]
- 25.Vanderlaan RD, Honjo O. Commentary: It takes a village: Changing the trajectory of pulmonary vein stenosis outcomes. J Thorac Cardiovasc Surg 2020, 159(3): 1037–1038. [DOI] [PubMed] [Google Scholar]
- 26.Morales-Nebreda L, Chung CS, Agrawal R, Yeldandi AV, Singer BD, Bharat A, et al. Headed in the Wrong Direction: Chronic and Acute Derangements in Pulmonary Blood Flow Distribution in a Patient with Severe Pulmonary Vein Stenosis. Ann Am Thorac Soc 2019, 16(10): 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy PT, Jain A, Nawaytou H, Teitel D, Keller R, Fineman J, et al. Risk assessment and monitoring of chronic pulmonary hypertension in premature infants. J Pediatr 2020, 217:199–209.e4. [DOI] [PubMed] [Google Scholar]
- 28.Heching HJ, Turner M, Farkouh-Christiana, Krishnan U. Pulmonary vein stenosis and necrotising enterocolitis: is there a possible link with necrotising enterocolitis? Arch Dis Child Fetal Neonatal Ed 2014, 99(4): F282–F285. [DOI] [PubMed] [Google Scholar]
- 29.Swier NL, Richards B, Cua CL, Lynch SK, Yin H, Nelin LD, et al. Pulmonary vein stenosis in neonates with severe bronchopulmonary dysplasia. Am J Perinatol 2016, 33(7): 671–677. [DOI] [PubMed] [Google Scholar]