Abstract
RAF kinase is a family of isoforms including A-RAF, B-RAF, and C-RAF. Despite the important role of RAF in cell growth and proliferation, little evidence exists for isoform-specific function of RAF family members. Using Western analysis and immunogold labeling, A-RAF was selectively localized in highly purified rat liver mitochondria. Two novel human proteins, which interact specifically with A-RAF, were identified, and the full-length sequences are reported. These proteins, referred to as hTOM and hTIM, are similar to components of mitochondrial outer and inner membrane protein-import receptors from lower organisms, implicating their involvement in the mitochondrial transport of A-RAF. hTOM contains multiple tetratricopeptide repeat (TPR) domains, which function in protein-protein interactions. TPR domains are frequently present in proteins involved in cellular transport systems. In contrast, protein 14-3-3, an abundant cytosolic protein that participates in many facets of signal transduction, was found to interact with C-RAF but not with A-RAF N-terminal domain. This information is discussed in view of the important role of mitochondria in cellular functions involving energy balance, proliferation, and apoptosis and the potential role of A-RAF in regulating these systems.
Numerous protein isoform families exist in the mammalian genome, yet unique roles for such isoforms are largely unknown. Protein isoforms are particularly abundant in intracellular signal transduction systems, where complex networks (37) are frequently found. The potential complexity of these transduction systems is exemplified by the activation of RAF, the downstream effector of RAS in the growth factor–mitogen-activated protein (MAP) kinase signal transduction pathway. The RAS-MAP kinase pathway is a major system coordinating growth factor receptor activity with nuclear events involved in the control of cell division (16). We have sought to understand potential isoform-specific function of the RAF isoforms by exploiting subtle differences in the ability to form protein-protein interactions among family members.
RAF is centrally located in the MAP kinase signal transduction pathway, where it is uniquely situated to integrate collateral, regulatory influences (recently reviewed in reference 39). Integration is accomplished both by protein-protein interactions and by extensive phosphorylation of RAF (20). Various kinases impinge on the MAP kinase system including protein kinase A, protein kinase C, and tyrosine kinases (21). Phosphorylation of RAF at various sites either inhibits or activates the enzyme, thereby modulating the activity of the pathway.
Protein interactions at the level of RAF have been extensively studied. Multiple low-molecular-weight G proteins, heat shock proteins, 14-3-3 proteins, and various other proteins are capable of binding RAF (3, 22, 38). The interaction of the RAS family of low-molecular-weight G protein with RAF's RAS binding domain (RBD) has been investigated by biophysical and biochemical techniques (reviewed by Avruch et al. [3]). A cysteine-rich domain (CRD) also has been implicated in important secondary RAS interactions (22), but little is known about the binding of other proteins to the CRD domain. Current information suggests this list of RAF binding proteins may be incomplete (39) and has compelled us to search for isoform-specific protein interactions as clues to unique functions for RAF isoforms. This search led us to the discovery of two novel and isoform-specific protein interactions of A-RAF, interactions with mitochondrial protein transport system proteins hTOM and hTIM and with the localization of A-RAF inside mitochondria. The results serve as the basis for a model of A-RAF involvement in the coordinated cellular control over mitochondrial function.
Mammalian cells dedicate elaborate machinery to transfer protein between different cellular organelles, attesting to the vital physiological importance of protein compartmentalization. Mitochondria, for example, rely in large part on gene products from the cell nucleus. Protein transport systems which facilitate selective cytoplasmic protein uptake into mitochondria have been characterized extensively in yeast and lower organisms (26) but very little in mammalian systems. The finding of a mitochondrial transport system that distinguishes between protein isoforms is likely to have implications concerning mitochondrial function. This likelihood is increased since the process by which mammalian cells balance energy production at the level of the mitochondria with cell growth and replication is largely unknown. Furthermore, the mechanisms whereby cells coordinate mitochondrial replication with cell division are largely unexplored.
MATERIALS AND METHODS
DNA manipulations and plasmid construction.
Cloning was done using standard molecular biological techniques (25). The cDNA regions encoding the N-terminal halves of RAF isoforms (see Table 1 for vector details) were cloned by PCR using Pfu polymerase (Stratagene catalog no. 600153) from Quick-Clone cDNA (Clontech catalog no. 7169-1) into a GAL4 DNA binding domain vector. This vector contains a GAL4 DNA binding domain coding region (amino acids 1 to 147), a Saccharomyces cerevisiae CYH2 gene used for counterselection of the plasmid by cycloheximide, and a tryptophan (TRP1)-selectable marker gene. The correct fusion of baits was checked by Western analysis of yeast protein extracts using an anti-GAL4 DNA binding domain monoclonal antibody (Santa Cruz Biotechnology catalog no. sc-510) and by a two-hybrid assay with a hemagglutinin-RAS fusion using a GAL4 activation domain in a pGAD10 vector (Clontech catalog no. 5398-1).
TABLE 1.
Interactions observed in the two-hybrid assays
| GAL4 DNA binding domain fusion (bait) | GAL4 activation domain fusion (prey)
|
||||||
|---|---|---|---|---|---|---|---|
| A-RAF | B-RAF | C-RAF | R-RAS | hTIM44 | hTOM | 14-3-3β | |
| A-RAF | ++ | ++ | ++ | − | |||
| C-RAF | ++ | − | ++ | ++ | |||
| hTOM | ++ | − | − | − | +/− | ++ | +/− |
| mCTD | − | − | − | − | − | − | − |
The two-hybrid method used was similar to that described by Chevray and Nathans (4). The relative strength of interaction is depicted as ++, +/−, and − to indicate the intensity of yeast growth resulting from the protein-protein interaction between RAF bait and prey fusion proteins. Lack of an entry indicates that the experiment was not performed. Human A-RAF bait contained amino acids 2 to 314, rat A-RAF 2 to 311, human C-RAF 2 to 348, and human B-RAF 2 to 460. B-RAF–GAL4 DNA binding domain is self-active in the two-hybrid assay, and so this construct was not used extensively. mCTD is the mouse C-terminal domain of RNA polymerase II.
Yeast growth, transformation, and two-hybrid screen.
Yeast cells were grown at 30°C in a minimal dropout medium containing appropriate amino acids (Bio 101 catalog no. 4025-012 and 4530-912). Transformation of yeast was done by the alkali-cation method using a Bio 101 kit (catalog no. 2 200-200) as described in the kit protocol except that the heat shock was done for 25 min in the presence of 5% dimethyl sulfoxide, using herring sperm DNA (Bethesda Research Laboratories catalog no. 14430-011) as a carrier. Before transformation with the library DNA (Clontech catalog no. HL4000AA), the transformation efficiency of each strain was tested with a pGAD10 vector DNA. Appropriate amounts of yeast cells and library DNA were taken to achieve the desired number of transformants.
Two-hybrid screens were performed in S. cerevisiae Y190 (MATa ura3-52 his3 ade 2-101 trp1-901 leu2-3,112) (12). Positive clones were selected in the presence of 30 mM 3-aminotriazole (3-AT) on synthetic complete medium (SC) plates lacking uracil, leucine, tryptophan, and histidine (3-AT/SC-Leu-Trp-Ura-His). For a typical screen, selection was done for 5 days, and then positive clones were picked and grown on 3-AT/SC-Leu-Trp-Ura-His plates. Subsequently positives were grown on SC-Leu-Ura plates, and β-galactosidase (β-Gal) assays were performed as described by Chevray and Nathans (4). On average, only 60 to 70% of 3-AT-resistant colonies had β-Gal activity. All β-Gal-positive clones were grown on SC-Leu-Ura plates containing 4 μg of cycloheximide per ml to counterselect the bait plasmid; then the β-Gal analysis was repeated to detect self-activating clones. All non-self-active clones were regrown in 5 ml of liquid SC-Leu-Ura medium in the presence of 4 μg of cycloheximide per ml. Subsequently, the library plasmids were isolated from yeast in the presence of phenol by the glass bead method (2) and transformed into Escherichia coli by electroporation. One colony from each transformation plate was grown in 2 ml of Luria-Bertani medium; the plasmids were purified by the standard alkaline lysis procedure (25) and retransformed into the PCY2 strain (4) together with the bait plasmid or vector plasmid alone. The β-Gal assay was performed, and positive plasmids were reselected. All positive clones were analyzed by restriction enzyme digestion using EcoRI and XhoI endonucleases. Identical plasmids were determined by a combination of DNA hybridization using the Rapid-Hyb system (Amersham catalog no. RPN1635), restriction analysis, and sequencing of flanking regions.
DNA sequencing and analysis.
All sequencing was done at the Novartis sequencing facility at Research Triangle Park, N.C., using an ABI sequencer with primers complementing the GAL4 activation domain and ADH1 terminator sequences. To obtain more internal sequences, appropriate deletions of plasmids were made. Some sequences were obtained from the GenBank expressed sequence tag database using the BLASTN program (1).
Western analysis.
Antibodies used are listed in figure legends. Anti-A-RAF polyclonal antibody was from Santa Cruz Biotechnology (catalog no. sc-408P). Results with this antibody were confirmed with two other antibodies: Transduction Laboratories mouse monoclonal antibody (catalog no. R14320) and Upstate Biotechnology Inc. rabbit polyclonal antibody (catalog no. 06-251). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting was performed using standard techniques described by Ausubel et al. (2). An anti-human B-RAF monoclonal antibody was obtained from Ulf Rapp, University of Wuerzburg, Wuerzburg, Germany.
Electron microscopy.
Purified rat liver mitochondria was fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetroxide followed by 1% uranyl acetate, dehydrated through a graded series of ethanol washes, and embedded in LX112 resin (LADD Research Industries, Burlington, Vt.). Ultrathin sections were cut on a Reichert Ultracut E, stained with uranyl acetate followed by lead citrate, and viewed on a JEOL 100CX transmission electron microscope at 80 kV.
Immunogold labeling.
Samples were fixed with 4% paraformaldehyde–0.1% glutaraldehyde in 0.1 M sodium cacodylate buffer, dehydrated through a graded series of ethanol washes, and embedded in LR White resin (London Resin Company). Sections of embedded mitochondria were blocked first with 1× Tris-buffered saline (TBS) containing 50 mM glycine followed by 1× TBS containing 5% bovine serum albumin (BSA) and 5% goat serum. After overnight incubation in 1× TBS with 1% BSA containing different primary antibody dilutions, sections were washed six times with 1× TBS–1% BSA and incubated for 2 h with a 1:10 dilution of the secondary (10-nm) immunogold-labeled goat anti-rabbit antibody conjugated to 10-mm colloidal gold from Goldmark Biologicals (catalog no. EMGAR).
Nucleotide sequence accession numbers.
The amino acid sequences of hTIM44 and hTOM have been deposited as GenBank accession no. AF026030 and AF026031, respectively.
RESULTS AND DISCUSSION
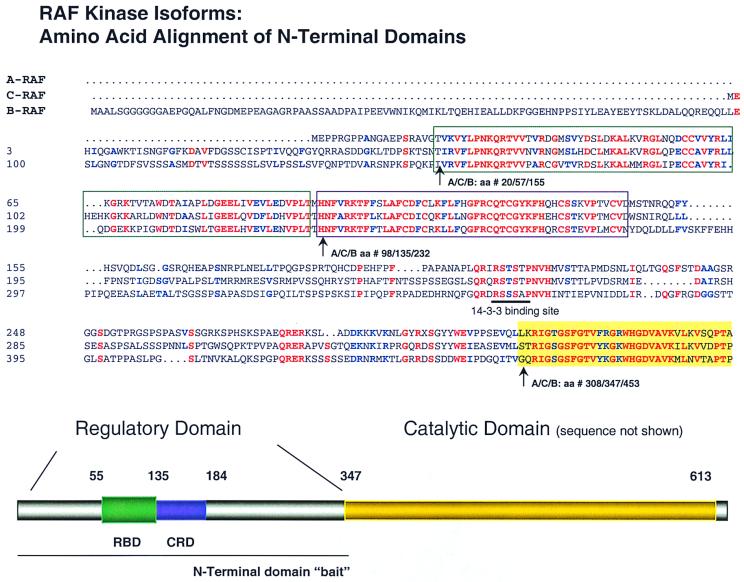
The two-hybrid screening method was used to search for protein-protein interactions with the three RAF isoforms, A-, B-, and C-RAF. Assuming that isoform-specific functions are mediated by protein-protein interactions, they are likely to involve the most diverged regions. Sequence alignment of RAF isoforms reveals that the greatest divergence resides in the N-terminal regulatory domain (Fig. 1) of approximately 350 amino acids. In this domain, excluding the 100-amino-acid N-terminal extension of B-RAF, human A-, B-, and C-RAF are only 62% homologous (39). When this comparison is made in more detail, however, the N-terminal regulatory half of RAF kinases contain highly conserved subdomains, namely, the CRD and RBD domains, interspaced by more highly divergent interdomain regions. As shown in Fig. 1, the RBD is identical between A-, B-, and C-RAF at 40 of 87 residues and homologous at 54 of 87 residues. Likewise, the CRD is identical between the three isoforms at 33 of 47 residues and homologous at 39 of 47 residues. Moreover, as detailed below, of the eight divergent residues in the CRD, five replacements result in charge changes. Furthermore, these residues are situated on one face of the protein, implying a particular role for this interface to dictate specificity of protein interactions.
FIG. 1.
Alignment of the N-terminal regulatory domain of the three RAF isoforms. Amino acid sequences of human A-, C-, and B-isoforms are aligned. Identical residues are colored red, homologous residues are blue, and nonconserved residues are black. The RBD and CRD are indicated (top and bottom). While both domains are relatively conserved across isoforms, important differences exist which may account for isoform-specific protein interactions (see text). The amino acids for each isoform are numbered to the right. The end of the N-terminal domain is indicated by the gold block and corresponds to A/C/B-RAF as number 308/347/453, respectively. Similarly, the RBD and CRD begin at amino acids 14/55/148 and 98/135/232, respectively, for A/C/B-RAF. The N-terminal domain baits used for subsequent experiments end at amino acids (A/C/B) 314/348/460, as detailed in Table 1.
The human A-RAF N-terminal domain (amino acids 2 to 353 in the A-RAF sequence) was fused as bait to the GAL4 DNA binding domain and different clones identified as prey from a HeLa cell cDNA fusion library (4, 12). From a library containing 14.4 million transformants, 12 different cDNAs were identified. Two of the independent cDNA pools were found to be related to mitochondrial protein import systems and are detailed here.
One cDNA (isolated in 10 independent clones) was sequenced, and the corresponding protein sequence revealed a significant similarity to a yeast mitochondrial inner membrane protein yTIM44 or ISP45 (GenBank accession no. Q01852) (Fig. 2). This protein is referred to as hTIM44, using the nomenclature of Schatz (26). The homology between the predicted hTIM44 and yTIM44 proteins is 74%, which extends uniformly across the polypeptide chain. yTIM44 is known to be a mitochondrial inner membrane protein which functions as part of a multisubunit complex serving in the ATP-dependent import of cytosolic proteins (14).
FIG. 2.
Similarity of A-RAF-specific binding protein to TIM mitochondrial transport proteins from lower organisms. The amino acid sequence of hTIM44 as predicted from the gene sequence is presented with its alignment to yeast TIM44. Identical amino acids are shown in red, and conserved substitutions are in blue. The alignment was done using the BESTFIT program with a gap penalty of 0.25, which resulted in a 74% homology score. The 5′-end 77 residues of the hTIM44 gene were cloned by RACE (rapid amplification of cDNA ends) PCR using a human hypocampus Marathon-Ready cDNA (Clontech catalog no. 7419-1). The original two-hybrid clone, which interacted with A-RAF, begins from amino acid 77 and is highlighted.
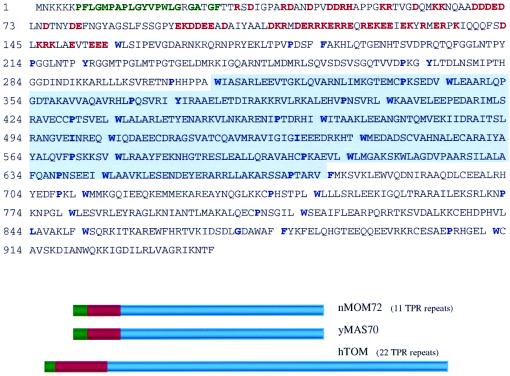
A second set of 10 overlapping cDNA clones was found whose structure resembled that of the outer mitochondrial membrane import receptors of S. cerevisiae (yMAS70; GenBank accession no. PO7213) and Neurospora crassa (nMOM72; GenBank accession no. P23231). This protein is referred to as hTOM (Fig. 3). yMAS70 and nMOM72 are known to reside on the cytoplasmic face of the mitochondrial outer membrane, where they also function as subunits of import receptors for cytosolic proteins (13, 28). yMAS70 and nMOM72 consist of multiple tetratricopeptide repeats (TPRs) (17). TPR motifs have been detected (typically as multiple repeats) in a family of cellular proteins involved in mitochondrial and peroxisomal protein import (17). Such TPR repeat-containing proteins frequently mediate protein-protein interactions found in multiprotein complexes (5). These repeats occupy 75% of yMAS70 and nMOM72 and are located at the C terminus. In addition, both nMOM72 and yMAS70 contain a stretch of hydrophobic amino acids at the N terminus that is implicated as a membrane-anchoring region (13, 28). This membrane-anchoring region is immediately followed by a 60-amino-acid sequence with multiple charges. hTOM (Fig. 3) has a similar domain structure containing 22 TPR repeats, roughly twice as many TPRs as the mitochondrial import receptors from lower eukaryotes. Like both yeast and Neurospora receptors, hTOM has a putative anchoring region followed by a highly charged membrane-proximal domain. Recently, the novel yeast protein yTom71, which is homologous to yMAS70, was also shown to be associated with the yeast mitochondrial protein import complex (27).
FIG. 3.
Similarity of A-RAF-specific binding protein to TOM mitochondrial transport proteins from lower organisms. The amino acid sequence of hTOM protein is presented as predicted from the gene sequence. The 5′-end sequence was obtained by RACE PCR as in Fig. 2. TPRs, also found in yeast (yMAS70; also called yTOM70) and N. crassa (nMOM72; also called nTOM72) mitochondrial transport proteins, are separated by breaks. Key repetitive hydrophobic and proline residues (17) are indicated in blue. The predicted transmembrane domain is shown in green. Charged amino acids in the membrane-proximal region are in red. Below the sequence is a schematic comparison of hTOM, yeast MAS70, and N. crassa MOM72 proteins. hTOM resembles nMOM72 and yMAS70 by overall protein domain organization and structure. Among these three proteins, the charged region was 26% and the transmembrane region was 20% homologous. For the TPRs, the best homology was with hTOM amino acids 216 to 904, the 11 C-terminal TPRs. This region was 25% homologous with the TPR regions of yMAS70 and nMOM72. Analysis of overlapping clones from the two-hybrid screen showed that the minimal region required for hTOM interaction with A-RAF contained TPR5 through TPR15; this region is highlighted.
Control experiments confirmed the specific nature of the interactions identified in the two-hybrid screen. hTIM44 was found to interact with A-RAF but not with C-RAF (Table 1). None of the identified clones interacted with an irrelevant partner, the C-terminal domain of RNA polymerase II. hTOM protein also interacted with A-RAF in swap experiments in which the fusion partners were switched. hTIM44 protein was not tested in swap experiments. Curiously, hTOM-GAL4 activation domain fusions interacted with both A- and C-RAF–GAL4 DNA binding domain fusions. In the swap experiment, hTOM-GAL4 DNA binding domain interacted with A-RAF but not C-RAF. We attribute this change to a subtle difference in affinity that falls below detection for C-RAF in the swap orientation but is sufficient to be detected in the normal orientation.
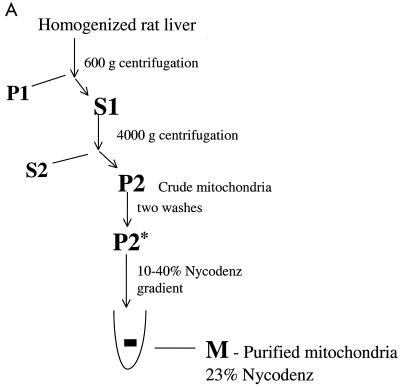
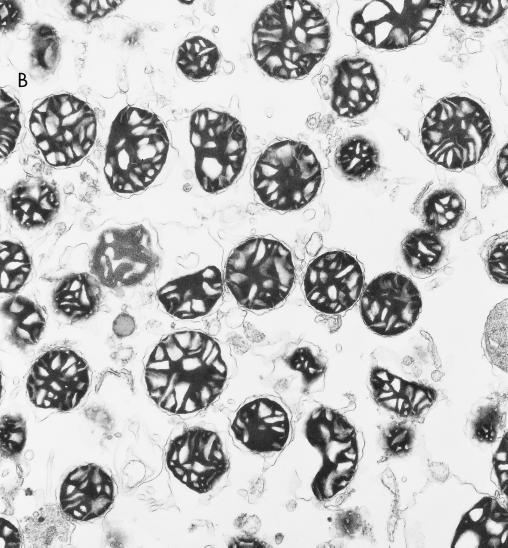
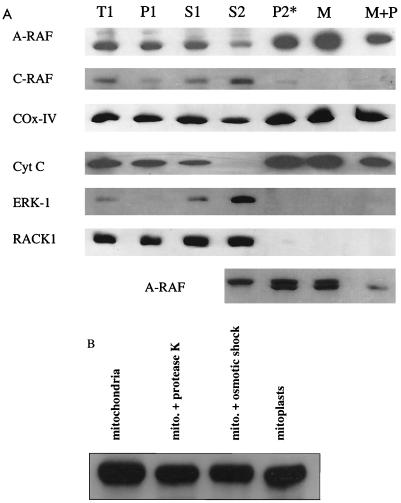
The identification of putative mitochondrial import proteins which interact with A-RAF suggested that A-RAF might be localized in mitochondria. Mitochondria were isolated from rat liver as depicted in Fig. 4, using differential sedimentation followed by a gradient fractionation using Nycodenz (10). The purity of the mitochondrial fractions was judged by Western analysis by monitoring the mitochondrial marker proteins cytochrome c and cytochrome oxidase subunit IV. Simultaneously, nonmitochondrial cytosolic proteins RACK-1 and ERK-1 (Fig. 5) were evaluated. The purified mitochondrial fractions were highly enriched with cytochrome oxidase IV and cytochrome c (Table 2), while ERK-1 and RACK-1 were absent. In addition, electron microscopy of purified mitochondrial fractions (Fig. 4) revealed a high degree of enrichment of intact mitochondria. Through these methods, purity of mitochondria in the Nycodenz fraction was estimated to be greater than 90% (Table 2).
FIG. 4.
Purification of rat liver mitochondria. (A) Outline of the mitochondrial fractionation scheme. Mitochondria from rat liver were isolated as described by Mihara and Omura (19) with subsequent purification by a linear Nycodenz gradient (10) in 0.22 M mannitol–0.075 M sucrose–10 mM HEPES-KOH (pH 7.4)–1 mM EDTA. (B) Electron micrograph of the purified mitochondria, demonstrating a high level of enrichment, estimated at >90% purity.
FIG. 5.
Western analysis of fractions from rat liver mitochondrial preparation. (A) Western analysis of fractions performed with 50 μg of protein per lane (except the lowest lane, where 100 μg was used) using SDS-PAGE with 10% polyacrylamide gels. Standard procedures were used to blot gels to nitrocellulose membranes and to perform Western analysis with enhanced chemiluminescence detection using appropriate alkaline phosphatase-conjugated secondary antibodies (except with the lowest panel). Purified mitochondria were treated with protease K (10) to judge if A-RAF would be protected. Anti-A-RAF polyclonal antibodies were from Santa Cruz Biotechnologies (catalog no. sc-408). Monoclonal antibodies to various proteins were as follows: C-RAF (Transduction Laboratories catalog no. R19120), ERK-1 (Transduction Laboratories catalog no. M37520), cytochrome c (Cyt C) (PharMingen catalog no. 65981A), cytochrome oxidase subunit IV (COX-IV) (Molecular Probes catalog no. A-6403), and RACK-1 (Transduction Laboratories catalog no. R20620). For the lowest panel, Western analysis was performed as described above except that an iodinated secondary antibody (ICN catalog no. 68086) was used to increase the resolution. This method gave sharper bands than a horseradish peroxidase-conjugated second antibody–chemiluminescence method, where broad bands were detected and the doublet could not be resolved. (B) Experiment to judge the protection of A-RAF immunoreactivity upon preparation of mitoplasts using Western analysis with the technique described for panel A. The mitoplast preparation was performed as described by Glick (9). Note that (low-resolution) chemiluminescence was used, and therefore only a single band of A-RAF was detected.
TABLE 2.
Analysis of marker proteins during purification of mitochondriaa
| Fraction | Total protein (mg) | A-RAF
|
Cytochrome c
|
C-RAF
|
ERK-1
|
||||
|---|---|---|---|---|---|---|---|---|---|
| % Recovery | Enrichment | % Recovery | Enrichment | % Recovery | Enrichment | % Recovery | Enrichment | ||
| S1 | 2,240 | 100 | 1 | 100 | 1 | 100 | 1 | 100 | 1 |
| S2 | 1,197 | 57 | 1.06 | 0.01 | 0.014 | 99 | 1.89 | 152 | 2.8 |
| P2 | 315 | 28 | 2 | 119 | 8.6 | 4 | 0.27 | 2.3 | 0.17 |
| Mitochondrial | 58.5 | 4.6 | 1.7 | 24 | 9 | 0.3 | 0.09 | ND | |
Western analysis was performed with 125I-labeled secondary antibodies. The intensity of bands on the gel was quantitated on a Molecular Dynamics Storm PhosphorImager, and percent recovery is expressed as relative units. The results are from a typical experiment performed on four occasions.
ND, not detectable in purified mitochondrial fractions.
A-RAF but not C-RAF was detected in these highly purified mitochondrial fractions (Fig. 5). As shown in Fig. 5B, A-RAF remained present in mitochondria treated with either protease K or osmotic shock or in mitochondrion-mitoplast preparations. Mitoplasts are mitochondria stripped of outer membranes and intermembrane components, prepared from intact mitochondria by gentle osmotic shock in the presence of protease K (Fig. 5B). The results suggest that a proportion of A-RAF is present in the inner matrix compartment of mitochondria. The A-RAF band was quantitatively displaced when the peptide derived from the C terminus of A-RAF—the peptide antigen used to produce the antibody—was included (at 10 μg/ml) in the Western analysis. The same band was detected in purified mitochondria with two other A-RAF-specific antibodies (Materials and Methods). B-RAF was not detected in any rat liver fraction using an anti-human B-RAF monoclonal antibody.
In separate experiments, Western analysis of purified mitochondria was performed using a 125I-labeled second-antibody detection method, in order to increase the resolution of the protein separation. Using this system, A-RAF was detected as a doublet of ∼67 kDa in the pure mitochondrial fraction (Fig. 5A, lower panel). The nature of the distinction between doublet bands of A-RAF has not been resolved. The second band could arise upon alternative splicing of A-RAF or protein phosphorylation. However, proteins imported into mitochondria typically are cleaved at their N-termini to remove a transport peptide (26), and such processed imported proteins are usually protected from proteolysis. Consistent with this perspective, the bottom band of the doublet was found to be protected from digestion by protease K in intact mitochondria much more than the upper band. This suggests that the second band may correspond to A-RAF lacking its transient peptide after being imported into the mitochondrial matrix. Regardless of its origin, this band is highly enriched in mitochondrial fractions and therefore is mitochondrion specific. The doublet of A-RAF is not seen in the cytosolic fraction (S2) but arises only in the mitochondrial compartment after import into mitochondria. For this reason, alternate mRNA processing leading to splice variants of A-RAF was not considered likely.
The amount of A-RAF imported into mitochondria was estimated by Western analysis as shown in Table 2. Although Western analysis is typically only a semiquantitative method, it is clear from the quantitation of cytochrome c (enriched by a factor of 9-fold during purification) and A-RAF (enriched by ∼2-fold) that cellular A-RAF is not completely localized in mitochondria. The results imply, but do not prove, that A-RAF may be partitioned both in mitochondria and in the cytoplasm. One can speculate that a dynamic equilibrium may exist between A-RAF levels of these compartments, where the amount of A-RAF in the mitochondria may be changed in response to growth or apoptosis signals, which in turn may regulate mitochondrial response to these signals. C-RAF, in contrast, was absent from (negatively [0.27-fold] enriched) purified mitochondria. To estimate the degree of C-RAF present in the mitochondrial fraction, overexposure of the autoradiograms was required. This overexposure would be expected to detect proteins contaminating the highly enriched mitochondrial preparations.
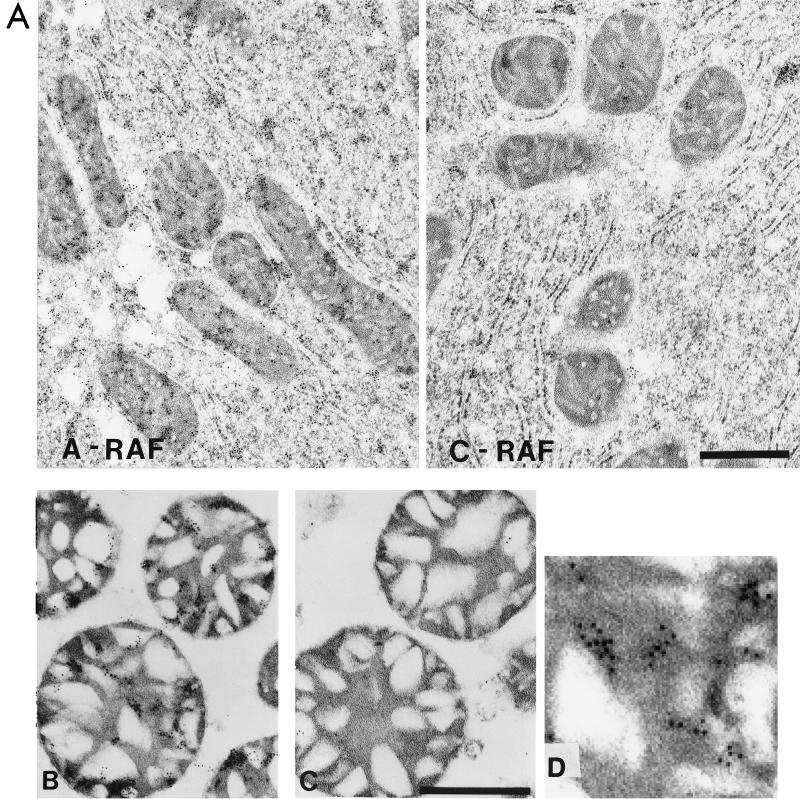
Immunogold labeling with anti-RAF antibodies confirmed the presence of A-RAF and the absence of C-RAF in mitochondria (Fig. 6A). For these studies, we performed preliminary experiments to demonstrate that the amount of gold label was proportional to the concentration of primary anti-A-RAF antibody, thus excluding the possibility of labeling due to secondary antibody binding. The identical labeling of mitochondria with anti-C-RAF antibodies did not yield significant staining. Labeling of rat liver thin sections with C-RAF and A-RAF antibodies is shown in Fig. 6B. Whereas A-RAF is seen clearly both in the mitochondrial fraction and cytoplasm, C-RAF is seen primarily in the cytosol. For both isoforms, homogeneous labeling of the cytosol was seen, indicating that the antibodies did not preferentially label membranous structures. It is of interest to estimate the suborganelle localization of A-RAF in mitochondria using this method. There were gold particles evident over mitochondrial regions corresponding to both intermembrane space and the matrix compartment. When gold particles, corresponding to A-RAF, were counted in the two mitochondrial compartments of these micrographs, approximately 77% of particles were found in the darkly stained matrix compartment (see reference 10 for identification of the mitochondrial compartments in these preparations). Taking this evidence along with the protease protection studies (Fig. 5), it can be concluded that A-RAF is transported into mitochondria intermembranous space and may additionally exist in the matrix compartment.
FIG. 6.
Transmission electron micrographs of thin sections of mitochondrial preparations. (A) Rat liver was treated with either A-RAF or C-RAF antibody and stained with gold as described below for mitochondrial sections. The bar equals 1 μm. (B and C) Purified mitochondrial samples were fixed for immunogold labeling with 4% paraformaldehyde-0.1% glutaraldehyde in 0.1 M sodium cacodylate buffer, dehydrated through a graded series of ethanol, and embedded in LR White resin (London Resin Company). Sections of embedded mitochondria were blocked with TBS containing first 50 mM glycine and then containing 5% BSA and 5% goat serum. After overnight incubation in TBS with 1% BSA containing different primary antibody dilutions of anti-A-RAF polyclonal antibody (Santa Cruz Biotechnologies catalog no. sc-408) (B) or anti-C-RAF polyclonal antibody (Santa Cruz Biotechnologies catalog no. sc-227) (C), sections were washed six times with 1× TBS–1% BSA and incubated for 2 h with a 1:10 dilution of goat anti-rabbit antibody conjugated to 10-nm colloidal gold (Goldmark Biologicals). The bar equals 0.5 μm. (D) Enlargement of a section of panel B showing the gold particles.
The domain of A-RAF that is sufficient for interaction with hTOM and hTIM44 was mapped by testing various fragments of the A-RAF N-terminal domain. A-RAF amino acids 2 to 72 (corresponding to amino acids 2 to 112 in C-RAF), 66 to 155 (106 to 195 in C-RAF), and 148 to 314 (190 to 353 in C-RAF) were independently fused to the GAL4 DNA binding domain and tested against hTOM- and hTIM44-GAL4 activation domain fusion proteins in the two-hybrid assay. In both cases, only the 66–155 fusion gave a positive signal (data not shown). This region of A-RAF contains the CRD which has a zinc finger structure (22). It is evident from this and other information (A. Yuryev and L. P. Wennogle, unpublished data) that the RAF CRD may be specialized to serve as a general sensor for proteins contacts to the RAF system. This result was contrary to our original assumption that the least conserved among RAF isoforms regions are responsible for the isoform-specific functions. We have investigated this further and found that, indeed, both hTIM44 and hTOM did not interact with C-RAF CRD–GAL4 fusion (amino acids 106 to 195 of the C-RAF sequence). A-RAF and C-RAF CRD differ only in five nonconserved and five conserved amino acid substitutions. Nevertheless, it appears to be enough to achieve isoform specificity of binding.
Protein 14-3-3 previously has been shown to interact with B- and C-RAF (7, 8, 23, 33, 34). We have confirmed these observations for C-RAF and extended them to show that protein 14-3-3 does not interact with A-RAF N-terminal domain by yeast two-hybrid analysis (Table 1). Unfortunately, the B-RAF N-terminal half fusion with the GAL4 DNA binding domain was self-active in the two-hybrid assay and thus was difficult to test for binding to 14-3-3 or any other protein. Since 14-3-3 is an abundant cytoplasmic protein, by virtue of binding to C- and B-RAF, it provides a mechanism for retention of these isoforms in the cytoplasm by mass action. Lacking a strong affinity for cytosolic 14-3-3 protein, A-RAF presumably would readily be available for transport into mitochondria. The difference between A- and C-RAF binding to 14-3-3 was surprising because the consensus sequence known to bind 14-3-3 is a phosphoserine-containing sequence (RSXS*XP [S* = phosphoserine]), and such a consensus sequence is conserved in the N-terminal domains of both A- and C-RAF (C-RAF amino acids 256 to 261, RSTS*TP). There are several possible explanations for this result. The 14-3-3 binding affinity to A-RAF may be lower than the detectable limit for this two-hybrid method, and the respective protein interaction domains may be more extensive than the minimal peptide sequence suggested by this consensus. Alternatively, 14-3-3 binding is known to be dependent on the phosphorylation state of the sequence, and the state of phosphorylation of the RAF isoforms in yeast is not known. Since a second binding site for protein 14-3-3 exists in the C-terminal domain of RAF, we cannot conclude that A-RAF does not bind to 14-3-3 as a full-length construct. It would be of interest to explore this aspect further and to evaluate other 14-3-3 isoforms in this regard.
We have presented evidence that A-RAF is imported into mitochondria via a system for isoform-specific uptake into this organelle. There is little information about localization of other MAP kinase pathway components in mitochondria (see also references 29 and 32). Using Western analysis techniques, R-RAS was localized in purified mitochondrial fractions (data not shown), suggesting the possibility that other members of the MAP kinase cascade may be localized in mitochondria.
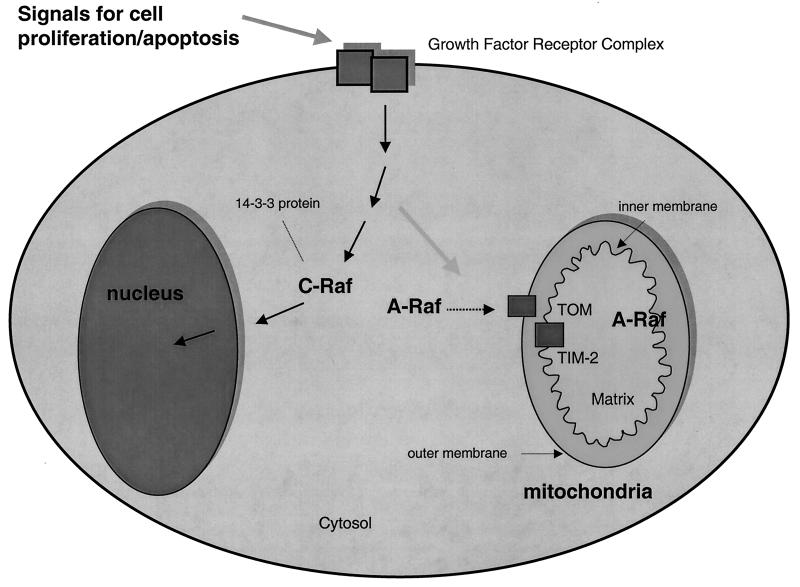
The detection of A-RAF inside mitochondria implies the existence of a novel mode for cell regulation of mitochondrial activities by phosphorylation of mitochondrial proteins. It seems logical to speculate that the cytoplasmic signal for cell proliferation carried through the RAS/RAF/MAP kinase pathway is coordinately transmitted not only to the nuclei for regulation of cell division but also to the major compartment for chemical energy production, the mitochondria (Fig. 7). Parallel regulation of mitochondrial activity would be beneficial to the cell both with respect to ATP production to fuel cell division and for the regulation of mitochondrial replication. Consistent with these ideas, ATP biosynthesis is known to be increased after stimulation with growth factors (11, 30, 31) in concert with DNA synthesis. Na+/H+ exchangers in Xenopus oocytes are up-regulated by RAF (15).
FIG. 7.
Model for the localization of RAF isoforms relative to mitochondria. The model summarizes the information from this study showing A-RAF localization inside and outside mitochondria and C-RAF localization uniquely outside mitochondria. The model implies a role of mitochondria A-RAF influencing the proliferation and activity of this organelle (see text). Protein 14-3-3 is an abundant cytosolic protein shown to bind the C- but not A-RAF N-terminal domain.
Much attention has been paid to the role of mitochondria in apoptosis, and the role of C-RAF in this process has been implicated (6, 35, 40). Recent evidence (18, 24, 36) has supported the initial observations and C-RAF was shown capable of binding the mitochondrion-associated protein Bcl-2, a protein known to inhibit apoptosis, and to phosphorylate BAD in correlation with inhibition of apoptotic events. Presumably these events occur at the outer membrane (cytoplasmic facing) surface of the mitochondria, accessible to Bcl-2 and BAD. The failure in the present study to find C-RAF associated with the exterior face of mitochondria may be due to a low-affinity interaction that does not survive the washing conditions required for isolation of purified mitochondria. Our findings, therefore, do not contradict the current hypothesis that C-RAF is involved in apoptotic signaling through its association with Bcl-2 and mitochondrial outer membrane. In contrast, they suggest that more than one RAF isoform may be implicated in these processes. In view of the results presented here, the role of A-RAF in apoptotic processes should be further investigated.
Experiments to assess the potential dynamic role of A-RAF in mitochondria are in progress. These experiments will attempt to document changes in the level of mitochondrial A-RAF in cell-cultured hepatocytes subject to different conditions of growth factor stimulation and apoptosis. Likewise, experiments are designed to document mitochondrial protein phosphorylated by A-RAF and to evaluate the impact of these modifications.
ACKNOWLEDGMENT
We thank Sreekala Mandiyan for her excellent technical help and for the help in preparing the manuscript.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1992. [Google Scholar]
- 3.Avruch J, Zhang X, Kyriakis J M. Raf meets Ras: completing the framework of signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 4.Chevray P M, Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci USA. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das A K, Cohen P W, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Sarabia M J, Bischoff J R. Bcl-2 associates with the ras-related protein R-ras p23. Nature. 1993;366:274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- 7.Freed E, Symons M, Macdonald S G, McCormick F, Ruggieri R. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science. 1994;265:1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- 8.Fu H, Xia K, Pallas D C, Cui C, Conroy K, Narsiman R P, Mamon H, Collier R J, Roberts T M. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science. 1994;266:126–128. doi: 10.1126/science.7939632. [DOI] [PubMed] [Google Scholar]
- 9.Glick B S. Pathways and energetics of mitochondrial protein import in Saccharomyces cerevisiae. Methods Enzymol. 1995;260A:225–226. doi: 10.1016/0076-6879(95)60140-6. [DOI] [PubMed] [Google Scholar]
- 10.Glick B S, Pon L A. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 1995;260A:215–217. doi: 10.1016/0076-6879(95)60139-2. [DOI] [PubMed] [Google Scholar]
- 11.Grummt F, Paul D, Grummt I. Regulation of ATP pools, rRNA and DNA synthesis in 3T3 cells in response to serum or hypoxanthine. Eur J Biochem. 1977;76:7–12. doi: 10.1111/j.1432-1033.1977.tb11564.x. [DOI] [PubMed] [Google Scholar]
- 12.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 13.Hase T, Riezman H, Suda K, Schatz G. Import of proteins into mitochondria: nucleotide sequence of the gene for a 70-kd protein of the yeast mitochondrial outer membrane. EMBO J. 1983;2:2169–2172. doi: 10.1002/j.1460-2075.1983.tb01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horst M, Jeno P, Kronidou N G, Bolliger L, Oppliger W, Scherer P, Manning-Krieg U, Jascur T, Schatz G. Protein import into yeast mitochondria: the inner membrane import site protein ISP45 is the MPI1 gene product. EMBO J. 1993;12:3035–3041. doi: 10.1002/j.1460-2075.1993.tb05972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang M G, Kulisz A, Wasserman W J. Raf-1 kinase, a potential regulator of intracellular pH in Xenopus oocytes. Biol Cell. 1998;90:477–485. [PubMed] [Google Scholar]
- 16.Kerkhoff E, Rapp U R. Cell cycle targets of Ras/Raf signalling. Oncogene. 1998;17:1457–1462. doi: 10.1038/sj.onc.1202185. [DOI] [PubMed] [Google Scholar]
- 17.Lamb J R, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- 18.Majewski M, Nieborowska-Skorska M, Salomoni P, Slupianek A, Reiss K, Trotta R, Calabretta B, Skorski T. Activation of mitochondrial Raf-1 is involved in the antiapoptotic effects of Akt. Cancer Res. 1999;59:2815–2819. [PubMed] [Google Scholar]
- 19.Mihara K, Omura T. Protein import into mammalian mitochondria. Methods Enzymol. 1995;260:302–310. doi: 10.1016/0076-6879(95)60147-3. [DOI] [PubMed] [Google Scholar]
- 20.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 21.Morrison D K, Heidecker G, Rapp U R, Copeland T D. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268:17309–17316. [PubMed] [Google Scholar]
- 22.Mott H R, Carpenter J W, Zhong S, Ghosh S, Bell R M, Campbell S L. The solution structure of the Raf-1 cysteine-rich domain: a novel ras and phospholipid binding site. Proc Natl Acad Sci USA. 1996;93:8312–8317. doi: 10.1073/pnas.93.16.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papin C, Denouel A, Calothy G, Eychene A. Identification of signalling proteins interacting with B-Raf in the yeast two-hybrid system. Oncogene. 1996;12:2213–2221. [PubMed] [Google Scholar]
- 24.Salomoni P, Wasik M A, Riedel R F, Reiss K, Choi J K, Skorski T, Calabretta B. Expression of constitutively active Raf-1 in the mitochondria restores antiapoptotic and leukemogenic potential of a transformation-deficient BCR/ABL mutant. J Exp Med. 1998;187:1995–2007. doi: 10.1084/jem.187.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schatz G. The protein import system of mitochondria. J Biol Chem. 1996;271:31763–31766. doi: 10.1074/jbc.271.50.31763. [DOI] [PubMed] [Google Scholar]
- 27.Schlossmann J, Lill R, Neupert W, Court D A. Tom 71, a novel homologue of the mitochondrial preprotein receptor Tom 70. J Biol Chem. 1996;271:17890–17895. doi: 10.1074/jbc.271.30.17890. [DOI] [PubMed] [Google Scholar]
- 28.Steger H F, Sollner T, Kiebler M, Dietmeier K A, Pfaller R, Trulzsch K S, Tropschug M, Neupert W, Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990;111:2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda S, Kita K, Miyazaki H, Natori S, Sekimizu K. Purification of low-molecular-weight GTP-binding proteins structurally related to MTG33, a mitochondrial GTP-binding protein of Ehrlich ascites tumor cells. J Biochem (Tokyo) 1995;118:791–795. doi: 10.1093/oxfordjournals.jbchem.a124981. [DOI] [PubMed] [Google Scholar]
- 30.Talha S, Harel L. Anisomycin and cycloheximide, like growth factors, stimulate rapidly ATP turnover in 3T3 cells. Cell Biol Int Rep. 1986;10:249–255. doi: 10.1016/0309-1651(86)90071-8. [DOI] [PubMed] [Google Scholar]
- 31.Talha S, Harel L. Early effect of growth factors (EGF + insulin) upon ATP turnover in 3T3 cells. Inhibition by cytochalasin B and D. Exp Cell Res. 1986;163:261–265. doi: 10.1016/0014-4827(86)90579-3. [DOI] [PubMed] [Google Scholar]
- 32.Thomson M, Korn M, Hall P F. GTP-binding proteins in adrenocortical mitochondria. Biochim Biophys Acta. 1995;1248:159–169. doi: 10.1016/0167-4838(94)00234-8. [DOI] [PubMed] [Google Scholar]
- 33.Thorson J A, Yu L W, Hsu A L, Shih N Y, Graves P R, Tanner J W, Allen P M, Piwnica-Worms H, Shaw A S. 14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity. Mol Cell Biol. 1998;18:5229–5238. doi: 10.1128/mcb.18.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzivion G, Luo Z, Avruch J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 1998;394:88–92. doi: 10.1038/27938. [DOI] [PubMed] [Google Scholar]
- 35.Wang H G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 36.Wang H G, Reed J C. Bcl-2, Raf-1 and mitochondrial regulation of apoptosis. Biofactors. 1998;8:13–16. doi: 10.1002/biof.5520080103. [DOI] [PubMed] [Google Scholar]
- 37.Weng G, Bhalla U S, Iyengar R. Complexity in biological signaling systems. Science. 1999;284:92–96. doi: 10.1126/science.284.5411.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittinghofer A, Herrmann C. Ras-effector interactions, the problem of specificity. FEBS Lett. 1995;369:52–56. doi: 10.1016/0014-5793(95)00667-x. [DOI] [PubMed] [Google Scholar]
- 39.Yuryev A, Wennogle L P. The RAF family: an expanding network of post-translational controls and protein-protein interactions. Cell Res. 1998;8:81–98. doi: 10.1038/cr.1998.9. [DOI] [PubMed] [Google Scholar]
- 40.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]