Abstract
Millions of cancer survivors suffer from a persistent neurological syndrome that includes deficits in memory, attention, information processing, and mental health. Cancer therapy-related cognitive impairment can cause mild to severe disruptions to quality of life for these cancer survivors. Understanding the cellular and molecular underpinnings of this disorder will facilitate new therapeutic strategies aimed at ameliorating these long-lasting impairments. Accumulating evidence suggests that a range of cancer therapies induce persistent activation of the brain’s resident immune cells, microglia. Cancer therapy-induced microglial activation disrupts numerous mechanisms of neuroplasticity, and emerging findings suggest that this impairment in plasticity is central to cancer therapy-related cognitive impairment. This review explores reactive microglial dysregulation of neural circuit structure and function following cancer therapy.
Keywords: Chemotherapy, radiation, immunotherapy, synapse, myelin
Cancer Therapy-Related Cognitive Impairment
Millions of people are living with the long-term consequences of cancer therapies. For example, in the United States nearly 17 million children and adults are cancer survivors. The number of cancer survivors is only projected to grow, as technological advancements in cancer management, such as radiation, chemotherapy, and immunotherapy, continue to extend the survival of patients. This is especially true for children, in which the 5-year survival rate for all childhood cancers in the US has increased over the past three decades from 58% to 84%, resulting in approximately 400,000 survivors of childhood cancer [1]. While this is a remarkable testament to the persistence and innovation of the cancer scientific and medical communities, the long-term neurological effects of cancer therapies require increased attention, mechanistic understanding and therapeutic strategies for mitigation.
Up to approximately 70% of cancer survivors report persistent deficits in memory, attention, speed of information processing, multi-tasking, and mental health functioning. While the severity and duration of CRCI can vary depending on age, cancer type, and treatment regimens, compromised cognition is generally more severe in children who were younger at the time of treatment [2], following cranial radiation [3] or with certain chemotherapies [4]. Broadly, the cognitive symptoms that follow exposure to cancer therapy implicate white matter and hippocampal dysfunction. This syndrome of cognitive impairment is associated with both central nervous system (CNS) and non-CNS cancers. While overt structural damage to the brain is typically absent in standard clinical imaging studies, changes in hippocampal structure and in white matter integrity have been demonstrated using advancing neuroimaging techniques[5, 6]. Studies in both experimental animal models and patient populations suggest long-term dysregulation of intercellular interactions involving multiple neural cell types [7–9], with consequent dysregulation of neural circuit dynamics.
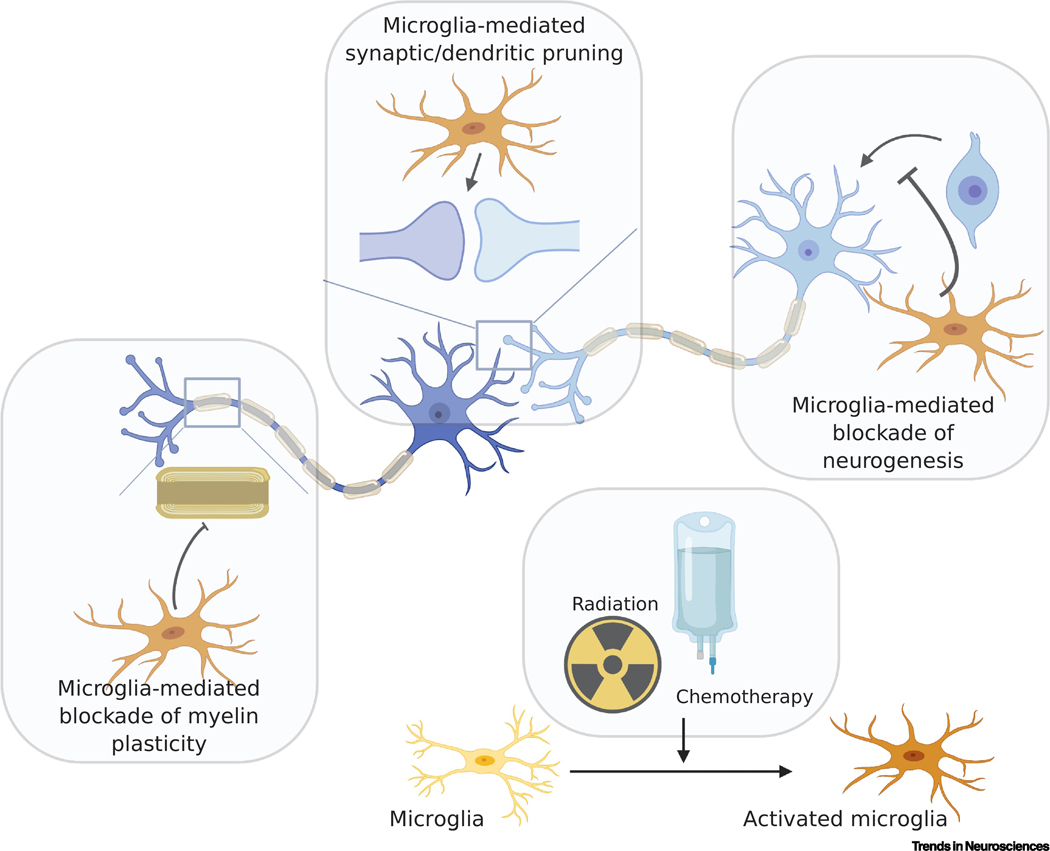
For neural circuits to function properly, neural signals must propagate in the appropriate temporal and spatial pattern. The dynamic circuit changes that underlie adaptive brain functions like learning occur in myriad ways, but two of the most prevalent mediators of dynamical circuit adaptation are modulation of synapses (see glossary) and myelin. Synapses, the junctions between neurons that are sculpted throughout life by glial cells, pass neural impulses from one neuron to the next via vesicular release of neurotransmitters. Neural networks function through the convergence and integration of numerous excitatory and inhibitory signals. The timing of these signals can be modified by changes to myelination of the various neuronal components of the circuit. Myelin is the multilaminar structure that ensheaths axons to decrease transverse capacitance and enable efficient, saltatory neural conduction. Experience-dependent fine tuning of myelin, known as myelin plasticity, allows for the adaptive temporal cadence of these signals, as even subtle changes in myelination can promote neural circuit coordination [10]. Recent work discerning the mechanisms mediating cancer therapy-related cognitive impairment indicates disruption to neural circuit structure and function, from the synapses between neurons to myelin structure and plasticity (Figure 1). This review will center on how cancer therapies disrupt components of neural network architecture to cause long-term cognitive impairment, with particular focus on microglia, the resident macrophages of the CNS, which have emerged as central to the mechanisms causing neural dysfunction after cancer therapies (Figure 1). Additional mechanisms that may contribute to long-term neurotoxicity, such as microvascular disease and axonal injury, represent important topics that are outside the scope of this review. We will largely focus on preclinical studies in which mechanistic testing is possible, with clinical observations discussed where available to ground and validate the relevance of the preclinical studies.
Figure 1:
Cancer therapies such as radiation and chemotherapy dysregulate neural signaling, leading to persistent cognitive dysfunction. Much of this process is modulated by cancer therapy-induced activation of microglia (yellow to orange cells, bottom/right panel) and consequent alterations in neural circuit architecture. Activated microglia may block neurogenesis (light blue cells, top/right) through inhibition of precursor cell proliferation and their differentiation into new neurons. Microglia are also vital to the establishment and maintenance of synapses through pruning of dendrites (dark blue) and axonal terminals (central panel). Changes in microglial activation state can lead to aberrant pruning which can alter subsequent synaptic transmission. Cancer therapy-induced microglial reactivity also disrupts myelin dynamics (left panel) through the blockade of neuronal activity-mediated myelin plasticity, potentially resulting in changes to neural impulse conduction time. Cumulatively, these alterations in neural circuit structure can lead to profound changes in function, laying the foundation for the persistent neurological dysregulation suffered by millions of cancer survivors.
Microglia: Central modulators of neural circuit form and function in health and disease
Microglia derive from the yolk sac during embryonic development and populate the brain at this early developmental timepoint, eventually establishing 10–15% of brain parenchymal cells. These cells develop intricate branching morphology and exhibit rapid motility in response to injury or pathology, moving to the site of injury to phagocytose debris. In developmental and homeostatic states, these cells help to sculpt neural circuit refinement through dendritic and synaptic pruning [11, 12]. However, in disease states such as Alzheimer’s disease [13] and Parkinson’s disease [14], microglial activation may contribute to aberrantly increased synaptic pruning. Furthermore, microglia can transition from a homeostatic, neurotrophic state to a neurotoxic state. As discussed in more detail in later sections, this transition appears to be instigated by many cancer therapies. While whole brain radiotherapy and chemotherapy treatments aim to target malignant cells, they may also cause both acute and chronic changes in the brain including activation of microglia. The role of these activated microglia in the etiology of cancer therapy-related cognitive impairment is highlighted by numerous studies showing that blockade of microglia through colony-stimulating factor 1 receptor (CSF1R) inhibition can prevent fractionated whole brain irradiation-induced memory deficits [15–17] and chemotherapy-induced cognitive deficits [18–20] in preclinical models. It is important to note that the physical presence of the tumor itself, especially related to CNS malignancies, can affect cognitive function [21], and the relative contribution of a tumor to cognitive dysfunction will be dependent on precise tumor location, molecular subtype-specific effects of the tumor on neural circuit remodeling [22] and on effects of the surgical resection. Emphasizing the importance of non-tumor factors, one recent study using a glioma mouse model suggests that in the context of that mouse glioma model, the resultant memory dysfunction is related more to cranial irradiation-induced microglial activation than tumor growth [17], although the relevance of the findings to humans remains to be evaluated, and the specifics may vary amongst glioma subtypes.
Microglial influence on neurogenesis after cancer therapy
New neuron generation occurs in the hippocampi of rodents throughout life (as reviewed in [23, 24]) and is thought to contribute to some forms of hippocampal-dependent memory function. Conversely, disruption of hippocampal neurogenesis is thought to contribute to memory impairment in many disease contexts (as reviewed in [25]). While most hippocampal neurogenesis studies have been conducted in rodents, several studies have investigated hippocampal neurogenesis in humans. The majority of studies find evidence of hippocampal neurogenesis in the human brain during childhood and adolescence [8, 26]. It is more controversial whether human hippocampal neurogenesis continues at a lower level in older adulthood [27, 28, 29]. Some studies have found evidence of human hippocampal neurogenesis throughout adulthood [8, 26–28, 30] while others have not [29].
Radiation:
Studies in rats and mice showed that decreases in hippocampal neurogenesis following ionizing irradiation occur in a dose-dependent fashion, with increasing dosages associated with greater impairment in both proliferating precursor cells and newly-formed neurons [31, 32]. The deficit in neurogenesis is chiefly due to radiation-induced perturbations in the neurogenic niche, rather than cell-intrinsic effects on the precursor cells [33]. Radiation-induced microglial activation and consequent interleukin-6-mediated blockade of neuronal differentiation was found to be at the core of this microenvironmental disruption [7, 33]. In preclinical models, the direct role of this radiation-induced microglial inflammatory state as a central modulator of cranial irradiation-mediated memory deficits is supported by the findings that anti-inflammatory drugs targeting microglia [7] or depletion of microglia by CSF1R inhibitors [15] restore hippocampal neurogenesis and improve cognitive function following irradiation.
Chemotherapy:
Chemotherapeutics vary in mechanism of action and in blood-brain-barrier (BBB) penetration. Most longitudinal studies have found that 10–70% of patients exhibit cognitive impairment associated with chemotherapy exposure [34–37]. This wide range of incidence reflects the heterogeneity in chemotherapeutic regimens and patient populations. Rodent models of chemotherapy-related cognitive impairment using cytotoxic agents like cyclophosphamide [38, 39], thioTEPA [40, 41], fluorouracil (5-FU; [42, 43]), doxorubicin [44], methotrexate (MTX; [45–47]) or combinations of these agents [48, 49] and others have consistently found acute and chronic depletion of hippocampal precursor cell proliferation, immature neurons or mature neurons following chemotherapy exposure. In rodents, methotrexate activates microglia [18, 19, 50] which can persist for 6 months post-exposure [18] or longer; however the mechanisms of this sustained microglial activation remain incompletely understood. While most studies support a role for microglia-induced influences on neural stem/precursor cell populations and subsequent neurogenesis following chemotherapy exposure, one study found that decreased neurogenesis following chemotherapy in mice is not associated with changes in overall numbers of microglia, although activation state was not assessed in this study [51]. Also, it is not yet known how various targeted therapies, such as tyrosine kinase inhibitors - which have similarly been shown to impair memory function in mice influence microglial reactivity [52]. Microglia exhibit a wide range of cellular states [53], and the various reactive states of microglia in the context of exposure to each cancer therapeutic may differentially influence precursor cell proliferation and neurogenesis. Studies dedicated to understanding the microglial response to each agent will be vital to determining the role of neuroinflammation in cancer therapy-related cognitive impairment associated with each cancer therapeutic or combination of cancer therapeutics.
Microglial influence on synaptic structure and function after cancer therapy
Radiation:
Microglia are increased in both number and activation state following therapeutic doses of cranial radiation in rodent models [7, 33] and in humans [8]. Microglia are well-established modulators of synaptic structure through pruning of both axonal terminals and dendritic spines, as well as secretion of immune-related factors that can alter neural transmission [54]. Microglia-associated cytokines, a robust and diverse group of small signaling proteins such as colony stimulating factors, tumor necrosis factors, and interleukins, are especially important in the development and plasticity associated with synapses. The diversity of their roles in the synaptic connectivity of circuits include synaptogenesis, synapse maturation, synaptic strength, pruning, and plasticity, with alterations in both presynaptic and postsynaptic processes occurring (for review see [55]). Their crucial role in mediating synaptic form has implications in both physiological and pathological states, placing microglia as central players in synaptic function and dysfunction. This is particularly true following cranial radiation therapy. Dose-dependent thinning of the cerebral cortex has been demonstrated in neuroimaging studies following cranial radiation in cancer patients [56]. This cortical thinning may reflect comprise of synaptic structures following cranial irradiation, as rodent models of cranial irradiation demonstrate decreases in the number of hippocampal dendritic branches, dendritic length and area, spine density, number of overall spines and filopodia spines, and expression of synaptic proteins [57]. These changes in synaptic structures are found concomitant with alterations to expression of genes associated with neuronal activity and plasticity, including Arc, cFos, and CREB within the hippocampus and cortex [58]. Insights about the possible effects of irradiation, and microglial roles in this context, can be gleaned also from rodent studies aimed to assess the effects of astronauts’ exposure to radiation in space. Specifically, studies of cranial radiation commensurate with the levels to which astronauts may be exposed in space showed synapse loss and persistent cognitive and behavioral deficits, including changes in anxiety-like behaviors, sociability, social memory, and attention [59, 60]. Depletion of microglia via CSF1R inhibition prevents radiation-induced cognitive deficits in rodent models [15–17]. When microglia repopulate following cessation of microglia-depleting CSFR1 inhibitor therapy, microglia in male mice exhibit reduced expression of scavenger receptors, lysosome membrane protein and complement receptors, resulting in lower phagocytic activity, while microglia in female mice do not [59, 60], highlighting a potential mechanism underlying the sex difference often identified in cognitive impairment following radiation therapy [61, 62].
Chemotherapy:
Numerous chemotherapeutic agents alter cortical thickness, synaptic structure and signaling similar to radiation exposure. In breast cancer survivors treated with chemotherapy, longitudinal neuroimaging studies demonstrate decreased cortical thickness for up to one-year post-treatment that correlates with impaired learning and memory [63]. Loss of hippocampal dendritic spines and synapses is dose-dependent, with higher doses of cisplatin resulting in more rapid loss and simplification of dendritic branching [64]. Mice exposed to adriamycin and cyclophosphamide also exhibit reductions in total dendritic length, ramifications, and branch complexity [51]. These chemotherapy-induced changes in spine complexity results in deficits in cognition [65] and are associated with a neuroinflammatory response as anti-inflammatory compounds such as ginsenoside (Rg1) reverse the loss of dendritic spines and deficits in neuronal activity in the prefrontal cortex (PFC) and hippocampal circuitry [66]. Changes in circuit signaling are further supported by findings that chemotherapeutic agents like 5-FU [67] and carboplatin [68] alter dopamine levels and reuptake. Glutamatergic signaling is impaired in mice following doxorubicin exposure via both reuptake in the frontal cortex and clearance in the hippocampus [69]. Cognitive deficits in mice following exposure to cyclophosphamide are associated with microglial activation together with decreased dendritic length, volume, and complexity [70]. While these associations of chemotherapy with structural changes are intriguing, the role of microglial activation in upregulated synaptic and dendritic pruning following chemotherapy remains to be directly tested and represents an area for future investigation. Moreover, microglial reactivity induces neurotoxic astrocyte reactivity [71]; as astrocytes promote synaptogenesis [72, 73] (for review see [74]), and regulate synaptic function (for review see [75]), it remains to be clarified how cancer therapy-induced microglial reactivity alters the astrocytic influence on synaptogenesis and synaptic function.
Microglial influence on myelin plasticity after cancer therapy
Myelin plasticity has been receiving growing appreciation as a mechanism by which neuronal activity can modulate circuit function through adaptive changes in myelin structure. Neuronal activity can induce oligodendrocyte proliferation, oligodendrogenesis and myelination [9]. This does not appear to occur in all CNS circuits [9], but has been well demonstrated in neocortex, cortico-callosal projections and hippocampal projections to frontal cortex [9, 10, 19, 76, 77]. Neuronal brain-derived neurotrophic factor (BDNF) signaling to the TrkB receptor on oligodendrocyte precursor cells (OPCs) is a required mechanistic component in plasticity of cortical projection neuron myelination [19]. BDNF signaling is a well-documented mediator of neurogenesis and synaptic plasticity [78], and its recently identified role in myelin plasticity emphasizes the unique role BDNF plays in modulating numerous forms of neural plasticity. While BDNF-TrkB signaling is required, it is likely only one component of a more complex mechanism that may include neuregulin [79] and endothelin signaling [80]. Activity-regulated oligodendrogenesis and myelin changes include both de novo formation of new internodes on previously unmyelinated axons or axon segments [10, 76, 77, 81] and remodeling of existing myelin [80]. Such activity-regulated myelin changes can modulate conduction velocity and consequently circuit dynamics to promote coordinated circuit activity [10]. Concordantly, adaptive changes in myelin contribute to a range of neurological functions, including motor function [9], motor learning [82, 83], attention and short-term memory [19], hippocampal-dependent learning and memory consolidation [10, 84]. Recent work outlined below demonstrates that in a rodent model, myelin plasticity is lost following exposure to methotrexate chemotherapy and this loss contributes to cognitive impairment in the model [19].
Chemotherapy:
Myelin changes have been identified in the brains of cancer survivors and in animal models following chemotherapy treatment. Both human and animal studies reveal changes in conduction parameters such as latency that are associated with disrupted or dysregulated myelination following chemotherapy exposure. Mice exposed to MTX and 5-FU exhibit a reduction in the ability to gate incoming auditory signals as assessed using whole brain event-related potentials [85]. A study in mice described effects on the latency of impulse conduction in the auditory system that can last up to 6 months post-exposure to 5-FU. This latency deficit, which is functionally related to atypical white matter, is associated with a decrease in progenitor cells and oligodendrocytes, altered transcriptional regulation of oligodendrogenesis, and dysmyelination [86]. In human breast cancer survivors treated with varying chemotherapeutic regimens, patients with chemotherapy had lower auditory P3 amplitude and latency than controls when assessed using an auditory oddball task [87]. This finding suggests changes in neural signaling that could be mediated by axonal injury or alterations in myelin structure. These findings are concordant with previous work indicating that glial progenitor cells are exquisitely sensitive to chemotherapeutic agents [88, 89]. Chemotherapy agents cause loss of OPCs and earlier precursors [89], but these cellular populations are expected rapidly replenish [90] after the last exposure to chemotherapy if the factors regulating oligodendroglial lineage dynamics are intact. However, a persistent depletion of oligodendroglial lineage precursors was demonstrated in both humans and in mouse models following chemotherapy [18]. This persistent OPC depletion represents disruption of the gliogenic microenvironment for which microglial activation is central [18]. It is important to note that while microglia inhibit myelination after methotrexate chemotherapy, microglial influence on myelination varies depending on microglial cell state, with pre-regenerative microglia promoting myelination after injury in other disease contexts [91].
A mouse model of high-dose MTX exposure demonstrated persistent microglial activation for at least 6 months following chemotherapy exposure, with consequent astrocyte reactivity, oligodendroglial lineage dysregulation, dysmyelination, and motor and cognitive dysfunction. Depletion of microglia with a CSF1R inhibitor (PLX5622) following MTX exposure normalized astrocyte reactivity, oligodendroglial lineage populations, myelination, and cognitive function. This study emphasizes that microglial dysregulation is a central mediator of chemotherapy-induced white matter toxicity, at least for this animal model [18]. The same model of high-dose MTX exhibits impaired neuronal activity-mediated myelin plasticity and depletion of neuronal BDNF attributable to microglial reactivity [19]. Pharmacological replacement of BDNF-TrkB signaling using a specific partial agonist of TrkB rescues cognition in this mouse model even without microglial depletion. Importantly, cognitive rescue depended on intact OPC expression of TrkB [19]. This indicates that, while BDNF exerts numerous effects on mechanisms of neural plasticity, the key effect is on oligodendroglial cells in this context.
It remains to be determined how reactive microglia alter neuronal BDNF expression. BDNF-TrkB signaling also may be altered in the hippocampus and frontal cortex following exposure to multiple chemotherapeutic agents [19, 92, 93]. In humans, BDNF Val66Met polymorphisms predict clinical outcomes in chemotherapy-induced cognitive dysfunction [94]. Collectively, these data suggest that BDNF-mediated myelin plasticity is dysregulated following chemotherapy exposure and places it as a possible target of therapeutic strategies to abrogate chemotherapy-related cognitive impairment. Mitigating the persistent deficits in precursor cells following chemotherapy exposure is another a crucial therapeutic goal required to abrogate chemotherapy-induced cognitive impairment. Additional potential therapeutic avenues include metformin and nasal administration of mesenchymal stem cells, each of which have been shown to moderate cisplatin-induced changes in white matter structure and cognitive deficits [95, 96].
Radiation:
White matter deficits following cranial irradiation are dose-dependent, similar to radiation effects on neurogenesis, [97]. Using diffusion tensor imaging (DTI), magnetoencephalography (MEG), and computational modeling, pediatric patients with cranial radiation were found to exhibit white matter microstructural differences and subsequent decreased phase synchrony during visual attention tasks, as well as slower reaction times when compared to healthy controls [98]. These findings suggest that changes in white matter function led to changes in neural synchrony and signal propagation following cranial irradiation. Whole brain or focal radiation administered to infant mice results in decreased brain volume in young adulthood, with off-target white matter regions like the corpus callosum most impacted following focal irradiation [5]. Cranial radiation-induced changes in white matter are further supported by findings from non-human primate models, in which the most robust changes in gene expression 1 year following whole brain irradiation were found in white matter and were associated with numerous changes in transcription of neuroinflammatory genes [99].
Since the phenotype of activated microglia shares some similarities with brain-infiltrating activated macrophages, it is possible that the inflammatory cells within the brain parenchyma following cancer treatment originate from peripheral cells penetrating the BBB and are not resident immune cells of the CNS. Bone-marrow derived cells are recruited from the periphery to the site of cranial irradiation in a dose-dependent fashion and then differentiate predominately into MAC3+ and CD11b+ macrophages similar to microglia [100]. A specific monocyte population has been identified as the precursor of adult-generated microglia in the peripheral blood of mice. These monocytes are recruited to the brain and differentiate into microglia-like cells only if the brain is primed by irradiation [101]. Interestingly, in mice, repopulation of bone marrow-derived cells into the brain parenchyma was only observed following myeloablation caused by total body irradiation, not chemotherapy [102]. Cranial irradiation can result in acute decreased brain-residing microglia with increased penetration of peripheral macrophages. Cranial irradiation was also associated with increased secretion of chemoattractants associated with migration and recruitment of monocytes [103]. Recently, these bone marrow-derived macrophages and monocytes have been directly shown to aid in the recovery of myelin and cognition after cranial radiation [104]. Coalescing the above, the brain inflammatory response associated with cancer therapy can occur through direct activation of resident immune cells or through the invasion of the brain by peripheral bone-marrow derived cells that then differentiate into macrophages or microglia, implicating a complex interaction between central and peripheral responses to cancer therapy.
Immuno-oncology and implications for cancer therapy-related cognitive impairment
Immunotherapies are revolutionizing cancer care. Two major classes of immunotherapy include checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell. Given the central role of microglial activation in the pathophysiology of circuit dysfunction and cognitive impairment following cancer therapy, immuno-oncology strategies have the potential to exacerbate or induce cognitive impairment. While immunotherapy is a relatively young field, early indications of cognitive toxicities demand further investigation and the development of strategies to ameliorate putative cognitive sequelae.
Checkpoint inhibitors:
Checkpoint inhibitors have transformed the outcomes for certain cancers such as metastatic melanoma. The goal of checkpoint inhibitors is to produce a proinflammatory microenvironment to enable immune cell clearance of cancer cells [105, 106]. Two of the most clinically successful checkpoint inhibitors target CTLA4, a receptor expressed on the surface of helper and regulatory T cells, and PD-1, a receptor expressed on T cells and pro-B cells [107]. Less than 4% of patients treated with anti-CTLA4 immunotherapy, ~6% of patient treated with anti-PD-1, and 12% treated with a combination of the inhibitors exhibit acute adverse neurological events. The majority of the neurological adverse effects that occur during treatment include headaches, encephalopathies and meningitis which can typically be circumvented with steroids or drug interruption strategies [108]. The impact of checkpoint inhibitors on longer-term cognitive function is just beginning to be evaluated [109]. In non-CNS tumor-bearing mice, treatment with radiation therapy and anti-CTLA4 immunotherapy resulted in increased anxiety and decreased performance in the novel object recognition task, in conjunction with changes in microglial activation and proinflammatory cytokine levels [110]. The long-term effects of checkpoint inhibitors alone and in combination with other cancer therapies remain to be elucidated in cancer survivors.
CAR T cell therapy:
Chimeric antigen receptor (CAR) T-cell therapy has provided a major therapeutic advance for relapsed/refractory lymphoid malignancies and holds promise for solid malignancies. CAR T-cell immunotherapy is often associated with acute neurotoxicity, such as confusion, aphasia, and seizures [111]. One study in patients investigating neurocognitive abilities immediately (1 month) following CD22 CAR T-cell therapy reported stable cognitive functioning in terms of cognitive flexibility, attention/inhibitory control, working memory and speed of information processing [112]. However, the long-term effects of CAR T cell therapy on neurological function are largely unknown. In patients with relapsed/refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, and acute lymphoblastic leukemia, over 47% of patients reported at least one cognitive difficulty, depression or anxiety 1–5 years following CAR T-cell treatment, with younger ages of treatment and pre-treatment anxiety levels associated with worse long-term prognosis in terms of mental health [113]. Further studies are required to define the long-term neurocognitive effects of CAR T cell immunotherapy and any putative relationship to the initial systemic inflammatory response and severity of cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS).
Concluding Remarks
By the close of this decade, over 20 million cancer survivors will be living in the United States [1], with the majority experiencing neurological dysfunction that ranges from mild to life-altering. Advances in cancer therapy have contributed to this remarkable increase in cancer survivors. However, these successes in cancer survival mean that more people will be living with the long-term cognitive dysfunction that afflicts many survivors. Recent evidence that broad neuroregenerative strategies such as aerobic exercise training [114] can promote structural and functional brain recovery after combined chemoradiation exposure in childhood offers an exciting proof-of-principle demonstration that cancer therapy-associated cognitive impairment can be mitigated. Future molecular imaging studies providing details about the microglial activation state in human patients following specific cancer therapies, the kinetics of microglial reactivity and evolution of the states of various microglial subtypes over time after therapy will contribute to the development of therapeutic strategies to improve cognitive outcomes for cancer patients. Thoroughly understanding the biological underpinnings causing this persistent neurological dysfunction (see Outstanding Questions) is imperative to preventing or remediating neurotoxicity and improving the quality of life for millions of cancer survivors.
Outstanding questions box.
Are the reactive states of microglia following cancer therapy identical across therapies, or are there important differences induced by different therapies?
Are there sex differences in human microglia in physiological and pathological states and if so, how do sex differences in microglia affect their response to cancer therapy?
How do activated microglia following chemotherapy exposure alter synaptic and dendritic pruning?
Through what mechanism do reactive microglia alter neuronal BDNF expression following chemotherapy exposure?
What are the long-term neurological effects of immunotherapeutic strategies, such as checkpoint inhibitor therapy and chimeric antigen receptor T-cell therapy, and do microglia play a role in the context of these therapies as well?
Highlights:
Cancer survivors frequently exhibit persistent disruptions in cognitive function, including deficits in memory, attention, speed of information processing, and mental health.
Studies from both human cancer survivors and pre-clinical rodent models of chemotherapy and radiation exposure indicate dysregulation of neural plasticity mechanisms underlying cancer therapy-related cognitive impairment.
Both cranial irradiation and systemic chemotherapy treatment have been shown to induce a reactive state in microglia.
Activated microglia are associated with deficits in neural precursor cell population maintenance and neurogenesis, in synaptic structure and function, and myelin plasticity.
Acknowledgements
The authors gratefully acknowledge support from the National Institute of Neurological Disorders and Stroke (R01NS092597 to M.M), NIH Director’s Pioneer Award (DP1NS111132 to M.M.), Kleberg Foundation (M.M.), Unravel Pediatric Cancer (M.M.), McKenna Claire Foundation (M.M.), the National MS Society (E.M.G.), Departent of Psychiatry Innovator Award (E.M.G.)
Glossary:
- Blood-Brain-Barrier
semi-permeable barrier surrounding the CNS that is formed by endothelial cells and tightly moderates the movements of biological agents between the blood and CNS
- Checkpoint inhibitor therapy
immunotherapy strategy in oncology which targets immune checkpoints, resulting in enhancement of the immune system attack on cancer cells
- Chimeric Antigen Receptor T-cell Therapy
immunotherapy strategy in oncology in which a patient’s T cells are genetically engineered to target a specific protein on cancer cells
- Complement System
pro-inflammatory component of the immune system that increases the efficacy of antibodies and phagocytic cells to clear debris, damaged, or excess cells or cellular processes
- Cytokines
small signaling proteins that are secreted by immune cells
- Immune effector cell-associated neurotoxicity syndrome (ICANS)
neurotoxic syndrome associated with immunotherapy that is characterized by global encephalopathy, seizures, aphasia, tremors, and hallucinations
- Microglia
resident immune cells of the central nervous system (CNS), a specialized macrophage type with key roles in central nervous system development and plasticity
- Myelin
lipid-rich structure produced by oligodendrocytes that ensheaths axons, reduces transverse capacitance, and allows for saltatory neural transmission
- Myelin Plasticity
the dynamic nature of myelin through which neuronal activity can modulate adaptive changes in myelin structure and function
- Neurogenesis
production of new neurons from neural stem cells
- Synapse
junction between neurons that consists of a presynaptic axonal bouton and a postsynaptic compartment, and in which neurotransmitters are passed
Footnotes
Declaration of Interests
M.M. declares being a member of the editorial advisory board for Neuron, Cancer Cell, Cell Stem Cell, Cell Reports Medicine and Neuro-Oncology. M.M. is an inventor on a patent application, coordinated through Stanford University, regarding use of LM22A-4 to promote myelination in disease.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.American Cancer Society, Cancer Treatment & Survivorship Facts & Figures 2019–2021. 2019: Atlanta, Georgia. [Google Scholar]
- 2.Askins MA and Moore BD 3rd, Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol, 2008. 23(10): p. 1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene-Schloesser D. and Robbins ME, Radiation-induced cognitive impairment--from bench to bedside. Neuro Oncol, 2012. 14 Suppl 4: p. iv37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesler SR and Blayney DW, Neurotoxic Effects of Anthracycline- vs Nonanthracycline-Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol, 2016. 2(2): p. 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beera KG, et al. , Altered brain morphology after focal radiation reveals impact of off-target effects: implications for white matter development and neurogenesis. Neuro Oncol, 2018. 20(6): p. 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menning S, et al. , Changes in brain white matter integrity after systemic treatment for breast cancer: a prospective longitudinal study. Brain Imaging Behav, 2018. 12(2): p. 324–334. [DOI] [PubMed] [Google Scholar]
- 7.Monje ML, Toda H, and Palmer TD, Inflammatory blockade restores adult hippocampal neurogenesis. Science, 2003. 302(5651): p. 1760–5. [DOI] [PubMed] [Google Scholar]
- 8.Monje ML, et al. , Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol, 2007. 62(5): p. 515–20. [DOI] [PubMed] [Google Scholar]
- 9.Gibson EM, et al. , Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science, 2014. 344(6183): p. 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steadman PE, et al. , Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron, 2020. 105(1): p. 150–164 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens B, et al. , The classical complement cascade mediates CNS synapse elimination. Cell, 2007. 131(6): p. 1164–78. [DOI] [PubMed] [Google Scholar]
- 12.Schafer DP, et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S, et al. , Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 2016. 352(6286): p. 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecours C, et al. , Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions? Front Cell Neurosci, 2018. 12: p. 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acharya MM, et al. , Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep, 2016. 6: p. 31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng X, et al. , Colony-stimulating factor 1 receptor blockade prevents fractionated whole-brain irradiation-induced memory deficits. J Neuroinflammation, 2016. 13(1): p. 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng X, et al. , Rescue of cognitive function following fractionated brain irradiation in a novel preclinical glioma model. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson EM, et al. , Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell, 2019. 176(1–2): p. 43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geraghty AC, et al. , Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron, 2019. 103(2): p. 250–265 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen BD, et al. , Attenuation of neuroinflammation reverses Adriamycin-induced cognitive impairments. Acta Neuropathol Commun, 2019. 7(1): p. 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheibel RS, Meyers CA, and Levin VA, Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol, 1996. 30(1): p. 61–9. [DOI] [PubMed] [Google Scholar]
- 22.Yu K, et al. , PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature, 2020. 578(7793): p. 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jessberger S. and Gage FH, Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol, 2014. 24(10): p. 558–63. [DOI] [PubMed] [Google Scholar]
- 24.Kempermann G, et al. , Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell, 2018. 23(1): p. 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian KM, Song H, and Ming GL, Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci, 2014. 37: p. 243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spalding KL, et al. , Dynamics of hippocampal neurogenesis in adult humans. Cell, 2013. 153(6): p. 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno-Jimenez EP, et al. , Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med, 2019. 25(4): p. 554–560. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson PS, et al. , Neurogenesis in the adult human hippocampus. Nat Med, 1998. 4(11): p. 131–37. [DOI] [PubMed] [Google Scholar]
- 29.Sorrells SF, et al. , Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature, 2018. 555(7696): p. 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flor-Garcia M, et al. , Unraveling human adult hippocampal neurogenesis. Nat Protoc, 2020. 15(2): p. 668–693. [DOI] [PubMed] [Google Scholar]
- 31.Tada E, et al. , Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol, 1999. 160(1): p. 66–77. [DOI] [PubMed] [Google Scholar]
- 32.Mizumatsu S, et al. , Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res, 2003. 63(14): p. 4021–7. [PubMed] [Google Scholar]
- 33.Monje ML, et al. , Irradiation induces neural precursor-cell dysfunction. Nat Med, 2002. 8(9): p. 955–62. [DOI] [PubMed] [Google Scholar]
- 34.Wefel JS, et al. , Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer, 2010. 116(14): p. 3348–56. [DOI] [PubMed] [Google Scholar]
- 35.Deprez S, et al. , Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp, 2011. 32(3): p. 480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahles TA, Root JC, and Ryan EL, Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol, 2012. 30(30): p. 3675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wefel JS and Schagen SB, Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep, 2012. 12(3): p. 267–75. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, et al. , Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem, 2010. 93(4): p. 487–94. [DOI] [PubMed] [Google Scholar]
- 39.Lyons L, et al. , The effects of cyclophosphamide on hippocampal cell proliferation and spatial working memory in rat. PLoS One, 2011. 6(6): p. e21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondie CM, et al. , The chemotherapy agent, thioTEPA, yields long-term impairment of hippocampal cell proliferation and memory deficits but not depression-related behaviors in mice. Behav Brain Res, 2010. 209(1): p. 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson CL and Weber ET, Chemotherapy drug thioTEPA exacerbates stress-induced anhedonia and corticosteroid responses but not impairment of hippocampal cell proliferation in adult mice. Behav Brain Res, 2013. 236(1): p. 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ElBeltagy M, et al. , Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav Brain Res, 2010. 208(1): p. 112–7. [DOI] [PubMed] [Google Scholar]
- 43.Lyons L, et al. , Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS One, 2012. 7(1): p. e30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Agamy SE, et al. , Astaxanthin Ameliorates Doxorubicin-Induced Cognitive Impairment (Chemobrain) in Experimental Rat Model: Impact on Oxidative, Inflammatory, and Apoptotic Machineries. Mol Neurobiol, 2018. 55(7): p. 5727–5740. [DOI] [PubMed] [Google Scholar]
- 45.Lyons L, et al. , Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology (Berl), 2011. 215(1): p. 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seigers R, et al. , Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res, 2009. 201(2): p. 279–84. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, et al. , Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem Pharmacol, 2011. 82(1): p. 72–80. [DOI] [PubMed] [Google Scholar]
- 48.Briones TL and Woods J, Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci, 2011. 12: p. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christie LA, et al. , Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res, 2012. 18(7): p. 1954–65. [DOI] [PubMed] [Google Scholar]
- 50.Seigers R, et al. , Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res, 2010. 207(2): p. 265–72. [DOI] [PubMed] [Google Scholar]
- 51.Kang S, et al. , Chronic Treatment with Combined Chemotherapeutic Agents Affects Hippocampal Micromorphometry and Function in Mice, Independently of Neuroinflammation. Exp Neurobiol, 2018. 27(5): p. 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Aziz AK, et al. , The tyrosine kinase inhibitor, sunitinib malate, induces cognitive impairment in vivo via dysregulating VEGFR signaling, apoptotic and autophagic machineries. Exp Neurol, 2016. 283(Pt A): p. 129–41. [DOI] [PubMed] [Google Scholar]
- 53.Hammond T, et al. , Complex cell-state changes revealed by single cell RNA sequencing of 76,149 microglia throughout the mouse lifespan and in the injured brain. bioRxiv, 2018. 10.1101/406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, et al. , Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol, 2015. 36(10): p. 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werneburg S, et al. , A microglia-cytokine axis to modulate synaptic connectivity and function. Curr Opin Neurobiol, 2017. 47: p. 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagtegaal SHJ, et al. , Effect of radiation therapy on cerebral cortical thickness in glioma patients: Treatment-induced thinning of the healthy cortex. Neurooncol Adv, 2020. 2(1): p. vdaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parihar VK and Limoli CL, Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A, 2013. 110(31): p. 12822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kempf SJ, et al. , The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol Neurodegener, 2014. 9: p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krukowski K, et al. , Temporary microglia-depletion after cosmic radiation modifies phagocytic activity and prevents cognitive deficits. Sci Rep, 2018. 8(1): p. 7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krukowski K, et al. , Female mice are protected from space radiation-induced maladaptive responses. Brain Behav Immun, 2018. 74: p. 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armstrong GT, et al. , Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol, 2007. 25(28): p. 4477–89. [DOI] [PubMed] [Google Scholar]
- 62.Hinkle JJ, et al. , Cranial irradiation mediated spine loss is sex-specific and complement receptor-3 dependent in male mice. Sci Rep, 2019. 9(1): p. 18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henneghan A, et al. , Cortical Brain Age from Pre-treatment to Post-chemotherapy in Patients with Breast Cancer. Neurotox Res, 2020. 37(4): p. 788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andres AL, et al. , Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp Neurol, 2014. 255: p. 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi DD, et al. , Chemotherapy-Induced Cognitive Impairment Is Associated with Cytokine Dysregulation and Disruptions in Neuroplasticity. Mol Neurobiol, 2019. 56(3): p. 2234–2243. [DOI] [PubMed] [Google Scholar]
- 66.Shi DD, et al. , Ginsenoside Rg1 Prevents Chemotherapy-Induced Cognitive Impairment: Associations with Microglia-Mediated Cytokines, Neuroinflammation, and Neuroplasticity. Mol Neurobiol, 2019. [DOI] [PubMed] [Google Scholar]
- 67.Jarmolowicz DP, et al. , 5-Fluorouracil impairs attention and dopamine release in rats. Behav Brain Res, 2019. 362: p. 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaplan SV, et al. , Impaired Brain Dopamine and Serotonin Release and Uptake in Wistar Rats Following Treatment with Carboplatin. ACS Chem Neurosci, 2016. 7(6): p. 689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas TC, et al. , Acute treatment with doxorubicin affects glutamate neurotransmission in the mouse frontal cortex and hippocampus. Brain Res, 2017. 1672: p. 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Acharya MM, et al. , Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res, 2015. 75(4): p. 676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liddelow SA, et al. , Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 2017. 541(7638): p. 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christopherson KS, et al. , Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell, 2005. 120(3): p. 421–33. [DOI] [PubMed] [Google Scholar]
- 73.Ullian EM, et al. , Control of synapse number by glia. Science, 2001. 291(5504): p. 657–61. [DOI] [PubMed] [Google Scholar]
- 74.Bolton MM and Eroglu C, Look who is weaving the neural web: glial control of synapse formation. Curr Opin Neurobiol, 2009. 19(5): p. 491–7. [DOI] [PubMed] [Google Scholar]
- 75.Allen NJ and Eroglu C, Cell Biology of Astrocyte-Synapse Interactions. Neuron, 2017. 96(3): p. 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitew S, et al. , Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun, 2018. 9(1): p. 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes EG, et al. , Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Vincenti AP, et al. , Mechanisms That Modulate and Diversify BDNF Functions: Implications for Hippocampal Synaptic Plasticity. Front Cell Neurosci, 2019. 13: p. 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundgaard I, et al. , Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol, 2013. 11(12): p. e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swire M, et al. , Endothelin signalling mediates experience-dependent myelination in the CNS. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomassy GS, et al. , Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science, 2014. 344(6181): p. 319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McKenzie IA, et al. , Motor skill learning requires active central myelination. Science, 2014. 346(6207): p. 318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao L, et al. , Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci, 2016. 19(9): p. 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pan S, et al. , Preservation of a remote fear memory requires new myelin formation. Nat Neurosci, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gandal MJ, et al. , A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience, 2008. 157(1): p. 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han R, et al. , Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol, 2008. 7(4): p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kreukels BPC, et al. , ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin Neurophysiol, 2008. 119(3): p. 533–541. [DOI] [PubMed] [Google Scholar]
- 88.Morris GM, Hopewell JW, and Morris AD, A comparison of the effects of methotrexate and misonidazole on the germinal cells of the subependymal plate of the rat. Br J Radiol, 1995. 68(808): p. 406–12. [DOI] [PubMed] [Google Scholar]
- 89.Dietrich J, et al. , CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol, 2006. 5(7): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes EG, et al. , Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci, 2013. 16(6): p. 668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miron VE, et al. , M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci, 2013. 16(9): p. 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi DD, et al. , Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiol Dis, 2018. 114: p. 164–173. [DOI] [PubMed] [Google Scholar]
- 93.Park HS, et al. , Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology, 2018. 133: p. 451–461. [DOI] [PubMed] [Google Scholar]
- 94.Tan CJ, et al. , Replication and Meta-analysis of the Association between BDNF Val66Met Polymorphism and Cognitive Impairment in Patients Receiving Chemotherapy. Mol Neurobiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou W, Kavelaars A, and Heijnen CJ, Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLoS One, 2016. 11(3): p. e0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiu GS, et al. , Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice. Oncotarget, 2018. 9(85): p. 35581–35597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connor M, et al. , Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol, 2016. 121(2): p. 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bells S, et al. , Changes in White Matter Microstructure Impact Cognition by Disrupting the Ability of Neural Assemblies to Synchronize. J Neurosci, 2017. 37(34): p. 8227–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrews RN, et al. , White Matter is the Predilection Site of Late-Delayed Radiation-Induced Brain Injury in Non-Human Primates. Radiat Res, 2019. 191(3): p. 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burrell K, Hill RP, and Zadeh G, High-resolution in-vivo analysis of normal brain response to cranial irradiation. PLoS One, 2012. 7(6): p. e38366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mildner A, et al. , Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci, 2007. 10(12): p. 1544–53. [DOI] [PubMed] [Google Scholar]
- 102.Lampron A, Lessard M, and Rivest S, Effects of myeloablation, peripheral chimerism, and whole-body irradiation on the entry of bone marrow-derived cells into the brain. Cell Transplant, 2012. 21(6): p. 1149–59. [DOI] [PubMed] [Google Scholar]
- 103.Morganti JM, et al. , Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS One, 2014. 9(4): p. e93650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dietrich J, et al. , Bone marrow drives central nervous system regeneration after radiation injury. J Clin Invest, 2018. 128(1): p. 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galon J, et al. , Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science, 2006. 313(5795): p. 1960–4. [DOI] [PubMed] [Google Scholar]
- 106.DeNardo DG, et al. , Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov, 2011. 1(1): p. 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGinnis GJ and Raber J, CNS side effects of immune checkpoint inhibitors: preclinical models, genetics and multimodality therapy. Immunotherapy, 2017. 9(11): p. 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cuzzubbo S, et al. , Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer, 2017. 73: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 109.Cuzzubbo S, et al. , Assessing cognitive function in patients treated with immune checkpoint inhibitors: A feasibility study. Psychooncology, 2018. 27(7): p. 1861–1864. [DOI] [PubMed] [Google Scholar]
- 110.McGinnis GJ, et al. , Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget, 2017. 8(6): p. 9155–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karschnia P, et al. , Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood, 2019. 133(20): p. 2212–2221. [DOI] [PubMed] [Google Scholar]
- 112.Shalabi H, et al. , A Prospective Evaluation of Neurocognitive Function and Neurologic Symptoms in Pediatric and Young Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) Undergoing Anti-CD22 Chimeric Antigen Receptor Therapy. Blood, 2016. 128(22): p. 1625.27354722 [Google Scholar]
- 113.Ruark J, et al. , Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant, 2020. 26(1): p. 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riggs L, et al. , Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol, 2017. 19(3): p. 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]