Abstract
The present study aimed to characterize the effect of microRNA (miR)-367-3p on sevoflurane anesthesia and elucidate the underlying mechanism. A total of 36 4-month-old adult Sprague-Dawley rats were divided into six groups. Sevoflurane was inhaled at concentrations of 0, 1, 2, 4, 8 and 16% for a total of 6 h; the hippocampus of the brain was subsequently minced and digested, and astrocytes were isolated. Various methods, including reverse transcription-quantitative (RT-q)PCR, western blotting and TUNEL staining, were used to determine the expression levels of Bax, BCL-2 and BCL-2-like protein 11 (BCL2L11), as well as the level of apoptosis. The rats were treated with 8% sevoflurane and the astrocytes from the rats were transfected with miR-367-3p or anti-miR-367-3p. The present study demonstrated that sevoflurane promoted astrocytes apoptosis. Western blotting revealed that with an increase of sevoflurane concentration, the expression levels of the apoptotic proteins Bax and BCL2L11 were significantly increased, whereas the protein expression levels of BCL-2 were significantly decreased. However, overexpression of miR-367-3p reversed these effects. TUNEL staining revealed that sevoflurane promoted the apoptosis of astrocytes, while apoptosis was reversed by miR-367-3p overexpression. RT-qPCR demonstrated that sevoflurane inhibited the expression of miR-367-3p. Notably, miR-367-3p reduced the expression of BCL2L11, thereby inhibiting the apoptosis of astrocytes originating from the hippocampal area of adult rats induced by sevoflurane. Therefore, miR-367-3p and BCL2L11 may act as effective targets for the study of anesthesia.
Keywords: microRNA-367-3p, sevoflurane, BCL-2-like protein 11, hippocampal astrocytes
Introduction
Sevoflurane is a general inhalation anesthetic commonly used in the clinic, which exhibits rapid induction and recovery, and is widely used in cesarean sections and pediatric surgery (1,2). However, the use of sevoflurane may result in the decline of cognitive function during the recovery period, which may be associated with the apoptosis of nerve cells during anesthesia (3). The hippocampus is an important area of consciousness and memory in mammals (4), and sevoflurane may damage neurons in this particular area, causing cognitive impairment in anesthetized individuals (5). In the present study, the cause of cognitive impairment induced by sevoflurane was investigated.
It has been reported that sevoflurane inhibits the activity of neurons and even causes neuronal apoptosis (6-8). In the present study, the effect of sevoflurane on neurons and its association with Bax/BCL-2 was investigated. Bax is a well-known apoptosis-promoting factor, which activates the caspase cascade reaction to induce apoptosis (9). BCL-2 is an anti-apoptotic molecule and BCL-2-like protein 11 (BCL2L11) is a member of the BCL-2 family (10). The increase of BCL-2 levels and the decrease of Bax levels indicate that the resistance of cells to apoptosis is enhanced (11). It has been revealed that inhibition of BCL-2 or BCL2L11 gene expression promotes apoptosis (12).
MicroRNA (miRNA/miR)-367-3p is a type of non-coding endogenous miRNA consisting of 21 nucleotides, which widely exists in eukaryotic cells (13). miR-367-3p specifically acts on target mRNAs, and regulates gene expression via incomplete complementation, inhibition of translation and complete complementation (14). It has been confirmed that miR-367-3p is involved in the process of hippocampal neuronal damage, although the specific underlying mechanism remains unclear, and miR-367-3p target gene regulation is still unknown (15). In the present study, TargetScan software (16) was used to screen targets for miR-367-3p and a luciferase reporter assay was employed to explore the interaction between miR-367-3p and the BCL2L11 gene. The potential mechanisms underlying the apoptosis of astrocytes in the hippocampus induced by sevoflurane were also studied.
Materials and methods
Animal treatment and primary astrocytes culture
All animal experiments were approved by the Committee on Animal Experimentation of The Affiliated Hospital of Putian University (Putian, China; approval no. 2018-0023) and performed in compliance with the Guidelines for the Care and Use of Laboratory Animals (17). A total of 36 4-month-old adult male Sprague-Dawley rats (weight, 180-250 g) purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. were divided into six groups (n=3). The rats were kept in an animal breeding room for 2 weeks under the same conditions of temperature (22±2˚C), humidity (50±10%) and controlled habitat with a 12-h light/dark cycle (lights were on between 8:00 a.m. and 8:00 p.m.). Rats were administered food and water ab libitum.
Primary astrocytes were used in the present study, which were isolated as follows: Rats were anesthetized with different concentrations of sevoflurane (cat. no. 1612540; Millipore Sigma) as follows: 0, 1, 2, 4, 8 or 16%, from 9:00 a.m. to 3:00 p.m. Specifically, rats were housed in a chamber (length, 50 cm; width, 30 cm; height, 30 cm) containing 30% O2, 68% N2 and the corresponding concentrations of sevoflurane for 6 h. Immediately after anesthesia, the animals were euthanized via cervical dislocation and the heads were removed. The isolated brains of rats were sterilized with PBS containing 10% penicillin and streptomycin (cat. no. 10378016; Gibco; Thermo Fisher Scientific, Inc.) and placed in precooled sterile calcium- and magnesium-free (CMF) Hank's Balanced Salt Solution (HBSS) (cat. no. H9394; Sigma-Aldrich; Merck KGaA). All the cerebral hemispheres were collected in a petri dish on ice containing 5 ml CMF-HBSS, and the collected cortical tissue was minced as finely as possible. The tissue was transferred into a 15 ml tube with 12 ml CMF-HBSS. A total of 1.5 ml 2.5% trypsin and 10 mg/ml DNase solution were added, and the mixture was incubated at 37˚C for 15 min. The tissue was triturated 10-15 times using a glass Pasteur pipette and the mixture was further incubated at 37˚C for 5 min. The supernatant was filtered using a 100-µm cell strainer. The suspension was centrifuged at 130 x g for 6 min at 4˚C and the supernatant was carefully discarded. It was ensured that the pelleted cells at the bottom were not disturbed. Every group of samples was mixed together with up to 20 ml of astrocyte medium (cat. no. A1261301; Thermo Fisher Scientific, Inc.) to resuspend the combined dissociated glial cells, which were subsequently cultured in a flask in a humidified incubator at 37˚C with 5% CO2. The astrocyte medium was changed on the next day. A total of 4 days after seeding the cells, the flask was vigorously shaken 5-10 times to remove the unwanted cell types, and the supernatant was discarded. A total of 10 ml fresh CMF-HBSS was added in each flask, which was vigorously shaken 5-10 times and the supernatant was again discarded. A total of 20 ml fresh astrocyte medium was added into each flask. The confluent cells were the desired astrocytes. The astrocytes were maintained in high glucose DMEM (cat. no. 11965084) containing 10% FBS (cat. no. 16141061), and 1% penicillin and streptomycin (cat. no. 10378016; all from Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator at 37˚C with 5% CO2. The astrocytes were then used for the following experiments.
Transfection
Astrocytes were grouped into four groups: Anti-miR-negative control (NC) [infected with lentivirus (LV)-miR-367-3p inhibitor negative control], anti-miR-367-3p (infected with LV-miR-367-3p inhibitor), miR-NC (infected with LV-miR-367-3p mimic negative control), miR-367-3p (infected with LV-miR-367-3p mimic). The lentiviral miRNA inhibitor vector was pCLenti-U6-shRNA-CMV-EGFP-WPRE, and the lentiviral miRNA overexpression vector was pCLenti-U6-miR30 (miRNA)-CMV-EGFP-F2A-Puro-WPRE, which were purchased from OBiO Technology (Shanghai) Corp., Ltd. miR-367-3p inhibitor (5'-AGTAATGGCCATCACCATTGCT-3'), inhibitor-NC (5'-CCATTACTAGCAATGGTGATGG-3'), miR-367-3p mimic (5'-CCATTACATGCAATGGTGATGGAU-3') and mimic-NC (5'-AUCCATCACCATTGCTAGTAATGG-3') were constructed by Sangon Biotech Co., Ltd. Lentiviral particles were packaged by co-transfection with lentivirus and pHelper 1.0 and 2.0 vectors carrying the miR-367-3p inhibitor, inhibitor-NC, miR-367-3p mimic or mimic-NC into 293T cells (Procell Life Science & Technology Co., Ltd.). In brief, a total of 20 µg lentiviral plasmids, including pHelper 1.0: pHelper 2.0: pLVX (LV-miR-367-3p inhibitor or NC; LV-miR-367-3p mimic or NC)=7.5 µg: 2.5 µg: 10 µg, were added into 500 µl serum free DMEM (Solution A). A total of 60 µl Lipofectamine® 3000 (cat. no. L3000015; Invitrogen; Thermo Fisher Scientific, Inc.) was added into 500 µl serum free DMEM (Solution B). Solution B was added drop by drop into solution A, mixed gently and incubated for 20 min at room temperature to prepare solution C. Solution C was added into 293T cells drop by drop and mixed gently, and 293T cells were cultured in a 37˚C incubator for 16 h. Subsequently, serum free DMEM was changed into complete DMEM medium with 10% FBS. A total of 48 h after transfection at 37˚C, lentivirus particles were harvested from the cell culture supernatant and concentrated to 108 transduction units/ml.
A total of 24 h before lentivirus transduction, astrocytes were seeded in 6-well plates with ~1x105 cells to obtain ~30% confluency on the 2nd day. On day 2, 6.8 µl lentiviral particles (MOI=80) were added to each well. A total of 16 h later, the medium was replaced with lentivirus-free complete DMEM medium, and the astrocytes were cultured for another 48 h for transduction. Then, the lentiviral transduction efficiency was evaluated under a fluorescence microscope (Eclipse Ts2; Nikon Corporation).
For overexpression of BCL2L11, BCL2L11 open reading frame was inserted into pGV358-GFP vector (TsingKe Biological Technology) to construct pGV358-GFP-BCL2L11. pGV358-GFP was used as NC. The following primers (forward, 5'-TCAGTGCCTTCTCCAGACCAGACG-3'; and reverse, 5'-CATCAGAAGGTTGCTTGGCCAT-3') were designed to amplify the cDNA of BCL2L11. The lentiviral particles of pGV358-GFP-BCL2L11 were packaged following the procedures aforementioned. In brief, a total of 20 µg lentiviral plasmids including pHelper 1.0 and 2.0 vectors and pGV358-GFP-BCL2L11 were incubated with 60 µl Lipofectamine 3000 in 500 µl serum free DMEM for 20 min at room temperature and then added to 293T cells for co-transfection for 48 h in a 37˚C incubator. Subsequently, lentiviral particles were obtained from the cell supernatant and concentrated to 108 transduction units/ml. Astrocytes were then infected with the packaged pGV358-GFP-BCL2L11 lentiviral particles for 48 h, followed by adding G418 to the astrocytes for 4 weeks to obtain stable BCL2L11-overexpressing astrocytes.
For co-transfection with pGV358-GFP-BCL2L11 and LV-miR-367-3p mimic, stable BCL2L11-overexpressing astrocytes were used, and 6.8 µl LV-miR-367-3p mimic lentiviral particles (MOI=80) were added to each well of 6-well plate. A total of 16 h later, the medium was replaced with lentivirus-free complete DMEM, and the astrocytes were cultured for another 48 h.
Cell Counting Kit-8 (CCK-8) assay for cell viability
As aforementioned, the hippocampal regions of the brain were minced and digested into cell suspensions. These cell suspensions were then added to a 96-well plate at 3x103 cells per well in 100 µl complete DMEM medium, and cultured at 37˚C in a 5% CO2 incubator for 6 h. A total of 10 µl CCK-8 reagent (cat. no. 96992; Sigma-Aldrich; Merck KGaA) was added to each well and cultured for 4 h. The absorbance was measured at 450 nm. Each sample was assayed in triplicate (18). The absorbance of the astrocytes sample from rats anesthetized with 0% sevoflurane was used as control group and calculated as 100% cell viability. The cell viability of the other groups was calculated as the absorbance of 1, 2, 4, 8 or 16% sevoflurane group/the absorbance of 0% sevoflurane group x100%.
Western blotting
Total protein was extracted from the astrocytes that had been previously digested from hippocampal tissue using trypsin. RIPA buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.) with PMSF (ratio, 1 ml:10 µl) was used to lyse the cells. Following homogenization, the supernatant was prepared by centrifugation at 1,000 x g at 4˚C for 20 min, and the transparent colloidal mucus was discarded. The protein was quantified using the BCA assay. A total of 20 µg of the total protein per lane was mixed with SDS loading buffer and proteins were denatured at 95-100˚C for 5 min. The proteins were separated by 10% SDS-PAGE, and the separated proteins were transferred to a PVDF membrane. The membrane was blocked using 5% skimmed milk containing 0.1% Tween-20 (TBST) at room temperature for 1 h, prior to incubation with the corresponding primary antibody at 4˚C for 12 h. The membranes were subsequently washed in TBST and incubated with the goat anti-rabbit IgG H&L (HRP) secondary antibody (1:10,000; cat. no. ab6721; Abcam) at room temperature for 2 h. The primary antibodies were as follows: i) Bax (1:5,000; anti-rabbit; cat. no. ab32503; Abcam); ii) BCL-2 (1:1,000; anti-rabbit; cat. no. ab32124; Abcam); iii) BCL2L11 (1:2,000; anti-rabbit; cat. no. ab32158; Abcam); and iv) GAPDH as a loading control (1:10,000; anti rabbit; cat. no. ab181602; Abcam). Bands were developed using Immobilon ECL Ultra Western HRP Substrate (cat. no. WBULS0100; MilliporeSigma) and the image were analyzed using ImageJ v1.8.0 (National Institutes of Health) (19).
TUNEL staining
TUNEL staining was carried out using Click-iT™ TUNEL Alexa Fluor™ 488 Imaging Assay kit (cat. no. C10245; Invitrogen; Thermo Fisher Scientific, Inc.). The experiment was conducted following the manufacturer's protocol. In brief, a total of 2.5x105 astrocytes were seeded on coverslips and cultured in 6-well plates (Corning, Inc.) for 24 h before TUNEL staining. The astrocytes were rinsed with PBS three times, and then fixed with 4% paraformaldehyde for 15 min at room temperature. A total of 100 µl 0.25% Triton X-100 was added to completely cover the coverslips and incubated for 20 min at room temperature. A total of 100 µl terminal deoxynucleotidyl transferase (TdT) reaction buffer was added on each coverslip and the solution was allowed to spread completely over the surface. The coverslips were incubated for 10 min at room temperature. Another 100 µl of the TdT reaction cocktail containing TdT reaction buffer, EdUTP (dUTP modified with an alkyne) and TdT, was added to each coverslip and incubated them for 60 min at 37˚C. The coverslips were washed twice with 3% BSA (cat. no. B2064; MilliporeSigma) in PBS. A total of 100 µl Click-iT reaction cocktail containing Click-iT reaction buffer and Click-iT reaction buffer additive was added to each coverslip and incubated them for 30 min at room temperature in the dark. The coverslips were washed twice with 3% BSA in PBS. The primary antibody solution was added as supplemented by the manufacturer and incubated for 6 h at 4˚C, protected from light. The coverslips were washed with 3% BSA in PBS twice, and subsequently incubated with the secondary antibody for 0.5 h at room temperature, protected from light, followed by washing with 3% BSA in PBS twice. A total of 100 µl of 1X Hoechst 33342 solution per coverslip was added for 15 min at room temperature in the dark. The coverslips were washed twice with PBS, and subsequently imaged coverslips under a fluorescence microscope (Nikon Corporation) with fluorescence excitation at 495 nm and emission at 519 nm. At least 5 fields were captured per coverslip and 3 coverslips per group were analyzed to calculate the TUNEL-positive cells (%).
Dual luciferase reporter assay
TargetScan v7.2 software (http://www.targetscan.org/vert_72/) was used to predict the potential miRNAs interacting with the BCL2L11 3'-untranslated region (3'-UTR). Dual luciferase reporter assay was carried out using Pierce™ Renilla-Firefly Luciferase Dual Assay Kit (cat. no. 16185; Thermo Fisher Scientific, Inc.). In brief, the reporter plasmids (80 ng) containing wild type (WT) or mutant (Mut) 3'-UTR of BCL2L11were co-transfected with miR-367-3p mimic (50 nM) or miR-NC (50 nM) into 293T cells using Lipofectamine 3000. miR-367-3p mimic (5'-CCATTACATGCAATGGTGATGGAU-3') and mimic-NC (5'-AUCCATCACCATTGCTAGTAATGG-3') were constructed by Sangon Biotech Co., Ltd.
A total of 48 h after transfection, the luciferase assay reagent was added and luminescence signals were read at 640 nm for firefly luciferase and at 525 nm for green Renilla luciferase. Firefly luciferase was an experimental reporter and Renilla luciferase was used as a normalization control.
Reverse transcription-quantitative (RT-q)PCR
Hippocampal tissue that had been previously digested into individual cells using trypsin was used for PCR. RNA was extracted from the cells using 1 ml TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and 200 µl chloroform according to the manufacturer's protocol, incubated on a shaker, and centrifuged at 10,000 x g at 4˚C for 15 min. The upper solution was transferred to a clean RNase-free centrifuge tube and mixed with an equal volume of isopropanol, incubated for 15 min on ice, and centrifuged at 10,000 x g at 4˚C for 15 min. The RNA quality was evaluated as the absorbance at 260/280 nm ratio being 1.8-2.1. The samples were reverse transcribed to cDNA with PrimeScript RT reagent kit (cat. no. RR036Q; Takara Bio, Inc.). The RT reaction was performed at 37˚C for 15 min followed by 85˚C for 5 sec and 4˚C on hold. The TB Green® Premix Ex Taq™ II kit (cat. no. RR820A; Takara Bio, Inc. Takara) with an ABI 7500 real-time qPCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to conduct qPCR. The thermocycling conditions used were as follows: 95˚C for 30 sec; followed by 40 cycles of 95˚C for 5 sec and 60˚C for 10 sec. The 2-IICq method was applied for calculating the relative quantification (20). U6 was used as reference control for miR-367-3p.
The following primers were used: miR-367-3p forward, 5'-TTCTCCGAACTTTGCACGTTT-3' and reverse, 5'-ACGTGACACGTTCGGAGAATT-3'; U6 forward, 5'-GCTTCGGCAGCACATATACTAA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'.
Statistical analysis
All experimental data are presented as the mean ± standard deviation. The data were analyzed using GraphPad Prism 6 statistical software (GraphPad Software, Inc.). Unpaired Student's t-test was used for comparisons between two groups (21) and one-way ANOVA followed by Bonferroni's correction was used for comparisons between multiple groups. All experiments were repeated three times (n=3). P<0.05 was considered to indicate a statistically significant difference.
Results
Sevoflurane inhibits the viability of astrocytes in the hippocampus of adult rats and promotes astrocytes apoptosis
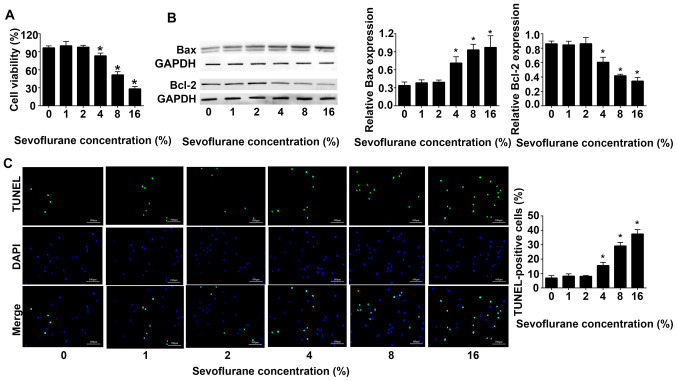
Sevoflurane inhibited the viability of astrocytes in a dose-dependent manner. The results of the CCK-8 cell viability assay revealed that at ≥4% sevoflurane the cell viability decreased in a dose-dependent manner compared with 0% sevoflurane (Fig. 1A). Western blot analysis indicated that compared with 0% sevoflurane, the expression levels of Bax were gradually increased, whereas the expression levels of Bcl-2 were gradually decreased. When the concentration of sevoflurane was 16%, the expression levels of Bax reached a maximum, and those of Bcl-2 were the lowest (Fig. 1B). The results of TUNEL assays demonstrated that with the increase of sevoflurane concentration, the apoptosis of astrocytes increased. When the concentration of sevoflurane reached 16%, the apoptosis of astrocytes was the highest (Fig. 1C).
Figure 1.
Sevoflurane inhibits the viability and promotes apoptosis of adult rat astrocytes. (A) Astrocyte viability was detected under the condition of different concentrations of sevoflurane inhaled by rats. (B) Bax and Bcl-2 expression was detected using western blotting. (C) Astrocyte apoptosis was detected using TUNEL staining (n=3). Scale bars, 100 µm. *P<0.05 vs. 0% sevoflurane group.
Sevoflurane inhibits the expression of miR-367-3p in the hippocampal astrocytes
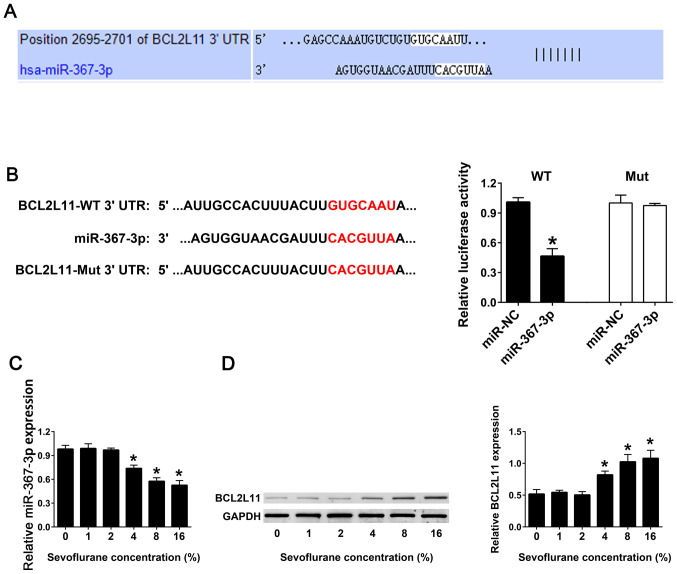
TargetScan software was used to predict the potential miRNAs interacting with the BCL2L11 3'-UTR, and miR-367-3p was identified (Fig. 2A). Luciferase reporter assay was used to analyze the binding ability between the WT or Mut 3'-UTRs of BCL2L11 and miR-367-3p. The results demonstrated that co-transfection of miR-367-3p and BCL2L11-WT 3'-UTR exhibited a lower luciferase activity compared with co-transfection of miR-NC and BCL2L11-WT 3'-UTR. Co-transfection of the miR-367-3p or miR-NC and BCL2L11-Mut 3'-UTR demonstrated no difference in the luciferase activity (Fig. 2B). RT-qPCR results revealed that with an increase in the concentration of sevoflurane, the expression levels of miR-367-3p in astrocytes gradually decreased, compared with 0% sevoflurane. When the concentration of sevoflurane reached 16%, the expression levels of miR-367-3p were the lowest, compared with 0% sevoflurane (Fig. 2C). Western blot analysis was used to detect the protein expression levels of BCL2L11 in astrocytes. The results demonstrated that with the increase of sevoflurane concentration, the expression levels of the BCL2L11 protein in hippocampal astrocytes were significantly increased. When the concentration of sevoflurane reached 16%, the expression of BCL2L11 protein was the highest, compared with 0% sevoflurane (Fig. 2D).
Figure 2.
Sevoflurane inhibits the expression of miR-367-3p and promotes the expression of BCL2L11 in hippocampal astrocytes. (A) TargetScan v7.2 was used to analyze the interaction between miR-367-3p and BCL2L11 gene. (B) Schematic diagram of the sequences co-transfected in dual luciferase activity assay, and bar graph of the dual luciferase activity assay results. (C) miR-367-3p expression was detected using reverse transcription-quantitative PCR. (D) BCL2L11 expression was detected using western blot assay. (n=3). *P<0.05 vs. 0% sevoflurane group. miR, microRNA; BCL2L11, BCL-2-like protein 11.
miR-367-3p inhibits the expression of BCL2L11 in astrocytes
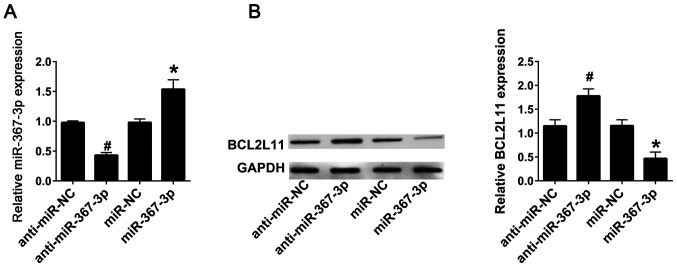
In order to detect the interaction between miR-367-3p and BCL2L11, lentiviral infection with anti-miR-367-3p or miR-367-3p into the astrocytes from rats not treated with sevoflurane was performed. RT-qPCR results indicated that transfection with anti-miR-367-3p significantly decreased the expression levels of miR-367-3p compared with the anti-miR-NC group, whereas transfection with miR-367-3p significantly increased the expression levels of miR-367-3p compared with miR-NC group (Fig. 3A). Western blot analysis revealed that the protein expression levels of BCL2L11 were significantly increased following transfection with anti-miR-367-3p, and were significantly decreased after transfection with miR-367-3p, compared with anti-miR-NC or miR-NC, respectively (Fig. 3B).
Figure 3.
miR-367-3p inhibits BCL2L11 in astrocytes. (A) The expression of miR-367-3p was detected using reverse transcription-quantitative PCR following transfection with anti-miR-NC, anti-miR-367-3p, miR-NC or miR-367-3p. (B) The expression of BCL2L11 was detected using western blotting following transfection with anti-miR-NC, anti-miR-367-3p, miR-NC or miR-367-3p. (n=3). #P<0.05 vs. anti-miR-NC; *P<0.05 vs. miR-NC. miR, microRNA; BCL2L11, BCL-2-like protein 11; NC, negative control; WT, wild-type; Mut, mutant; UTR, untranslated region.
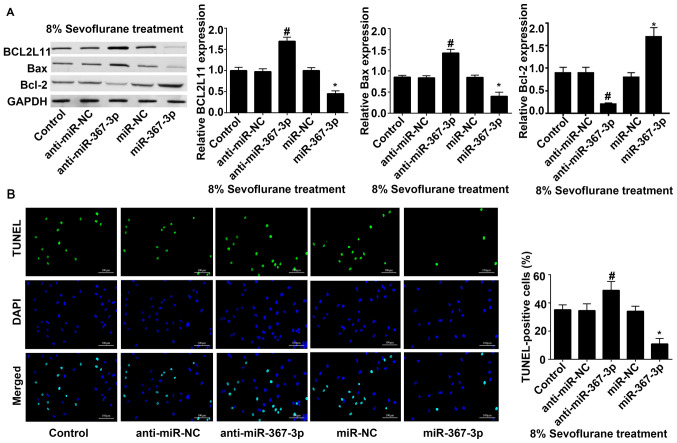
Transfection with miR-367-3p inhibits the expression of BCL2L11 and prevents the sevoflurane-induced apoptosis of rat hippocampal astrocytes
Western blot analysis revealed that under 8% sevoflurane treatment, the protein expression of BCL2L11 and Bax in the astrocytes transfected with anti-miR-367-3p was significantly increased, while the protein expression of Bcl-2 was decreased, compared with astrocytes transfected with anti-miR-NC. The protein expression of BCL2L11 and Bax in astrocytes transfected with miR-367-3p were significantly decreased, and the protein expression of BcL-2 were significantly increased, compared with astrocytes transfected with miR-NC (Fig. 4A). The results of TUNEL staining revealed that apoptosis of the anti-miR-367-3p-transfected astrocytes were significantly increased, and that of the miR-367-3p-transfected astrocytes were significantly decreased (Fig. 4B), compared with anti-miR-NC or miR-NC, respectively.
Figure 4.
Transfection with miR-367-3p inhibits the expression of BCL2L11 and prevents sevoflurane-induced apoptosis of rat hippocampal astrocytes. (A) The expression of BCL2L11, Bax and Bcl-2 was detected using western blot assay following transfection with anti-miR-NC, anti-miR-367-3p, miR-NC, miR-367-3p under the condition of 8% sevoflurane treatment. (B) TUNEL staining was conducted following transfection with anti-miR-NC, anti-miR-367-3p, miR-NC, miR-367-3p under the condition of 8% sevoflurane treatment. Scale bars, 100 µm. (n=3). #P<0.05 vs. anti-miR-NC; *P<0.05 vs. miR-NC. miR, microRNA; BCL2L11, BCL-2-like protein 11; NC, negative control.
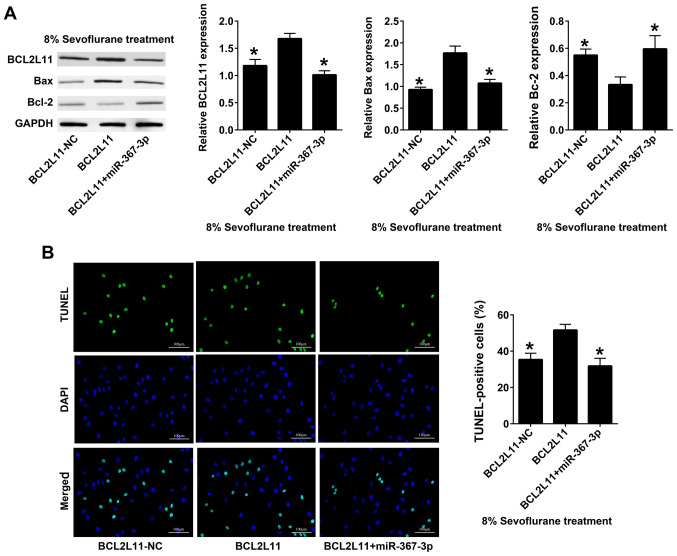
miR-367-3p decreases the effect of recombinant BCL2L11 on astrocytes apoptosis in sevoflurane-treated rats
BCL2L11 and miR-367-3p were co-transfected into rat hippocampal astrocytes; BCL2L11-NC was used as the control group. Western blot analysis revealed that the expression levels of BCL2L11 and Bax in co-transfected BCL2L11 + miR-367-3p astrocytes were significantly lower compared with those transfected with BCL2L11 alone, whereas the expression levels of BcL-2 in co-transfected BCL2L11 + miR-367-3p astrocytes were significantly higher than those transfected with BCL2L11 alone (Fig. 5A). The results of TUNEL staining demonstrated that the apoptosis of co-transfected BCL2L11 + miR-367-3p astrocytes was lower than that of cells transfected with BCL2L11 alone (Fig. 5B).
Figure 5.
miR-367-3p decreases the effect of recombinant BCL2L11 on astrocyte apoptosis in sevoflurane-treated rats. (A) The expression of BCL2L11, Bax and Bcl-2 was detected using western blotting following transfection of BCL2L11-NC, BCL2L11, BCL2L11 + miR-367-3p under the condition of 8% sevoflurane treatment. (B) TUNEL staining was conducted following transfection of BCL2L11-NC, BCL2L11, BCL2L11 + miR-367-3p under the condition of 8% sevoflurane treatment. Scale bars, 100 µm. (n=3). *P<0.05 vs. BCL2L11. miR, microRNA; BCL2L11, BCL-2-like protein 11; NC, negative control.
Discussion
Sevoflurane was initially discovered by Terrell (22) and was approved by the Japanese Drug Administration as a clinically inhaled anesthetic in 1990(23). Sevoflurane is a common drug in inhalation anesthesia (24). However, in recent years, studies have demonstrated that sevoflurane is neurotoxic. For example, Beekoo et al (25) demonstrated that sevoflurane may cause behavioral and developmental cognitive disorders in children <3-years-old. Zhou et al (26) treated 7-day-old rats with 2.3% sevoflurane for 6 h, in order to demonstrate that exposure of the developing brain to sevoflurane may lead to hippocampal synaptic plasticity damage, hippocampal area-dependent learning and memory impairment. Yang et al (27) used the Morris water maze test to detect the cognitive function of rats (P30) who had inhaled 3% sevoflurane for 4 h. The results revealed that sevoflurane caused cognitive impairment in rats, which may have been due to the promotion of apoptosis of neurons in the hippocampus. The results of the present study confirmed the aforementioned hypothesis. Sevoflurane caused apoptosis of nerve cells in the hippocampus, promoted the expression of the apoptotic protein Bax and BCL2L11, and inhibited the expression of the anti-apoptotic proteins BCL-2. BCL-2 has been indicated to serve an important role in sevoflurane-mediated cell death; sevoflurane was previously shown to facilitate cell apoptosis in glioma progression by inhibiting BCL-2(28). miR-34c has previously been shown to participate in apoptosis mediated by sevoflurane in rat brains via the mitochondrial pathway (29). Zhu et al (30) exposed 14-day-old mice to 3% sevoflurane for 2 h for 3 consecutive days. The results revealed that sevoflurane increased the expression of Bax, caspase-3 and other apoptosis-related genes in the hippocampus, and inhibited the expression of BCL-2.
miR-367-3p is an endogenous small molecule non-coding RNA. In the present study, miR-367-3p reversed the induction of apoptosis by sevoflurane, potentially rendering neurons in the hippocampus resistant to sevoflurane. A study by Kaid et al (31) demonstrated that miR-367-3p exhibited certain stem cell-enhancing characteristics, and improved the pluripotency of embryonic stem cells and the invasiveness of cancer-like stem cells. Wang et al (32) revealed that treatment with adriamycin induced apoptosis of osteosarcoma cells with upregulation of miR-367-3p. Additionally, miR-367-3p has been demonstrated to be downregulated in brain homogenates with sustained ischemia (15). However, the association between miR-367-3p and neuronal cell apoptosis remains unclear. The results of the present study revealed that miR-367-3p inhibited the expression of Bax and BCL2L11, and promoted the expression of BCL-2. The target gene of miR-367-3p was also verified as BCL2L11 using TargetScan, a target gene database of mammalian miRNA. Therefore, these findings indicated that miR-367-3p inhibited apoptosis and protected neuronal cells.
In conclusion, miR-367-3p reversed the apoptosis-promoting effect of sevoflurane on neurons. miR-367-3p suppressed sevoflurane-induced neuronal apoptosis by targeting BCL2L11. Sevoflurane promoted the expression of Bax and BCL2L11, and inhibited the expression of BCL-2, whereas miR-367-3p reversed these expression changes. miR-367-3p may therefore be a target in the field of anesthesia recovery and also an important target in protection from anesthesia toxicity/adverse effects.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DMX and CBZ conceived and supervised the study; JYL designed the experiments; WHC and WL performed the experiments and analyzed the data; DMX and CBZ confirm the authenticity of all the raw data; DMX wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Committee on Animal Experimentation of The Affiliated Hospital of Putian University (Putian, China). All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Edgington TL, Muco E, Maani CV (eds) Sevoflurane. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL, 2021. [PubMed] [Google Scholar]
- 2.Brioni JD, Varughese S, Ahmed R, Bein B. A clinical review of inhalation anesthesia with sevoflurane: From early research to emerging topics. J Anesth. 2017;31:764–778. doi: 10.1007/s00540-017-2375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Zhang W, Wu H, Fu S, Yang J, Liu S, Zhao Y, Zhang X, Liu J. Downregulation of CDK5 restores sevoflurane-induced cognitive dysfunction by promoting SIRT1-mediated autophagy. Cell Mol Neurobiol. 2020;40:955–965. doi: 10.1007/s10571-020-00786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo S, Liu L, Wang C, Jiang Q, Dong Y, Tian Y. Repeated exposure to sevoflurane impairs the learning and memory of older male rats. Life Sci. 2018;192:75–83. doi: 10.1016/j.lfs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Satomoto M, Sun Z, Adachi YU, Kinoshita H, Makita K. Sevoflurane preconditioning ameliorates lipopolysaccharide-induced cognitive impairment in mice. Exp Anim. 2018;67:193–200. doi: 10.1538/expanim.17-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andropoulos DB. Effect of anesthesia on the developing brain: Infant and fetus. Fetal Diagn Ther. 2018;43:1–11. doi: 10.1159/000475928. [DOI] [PubMed] [Google Scholar]
- 7.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22:368–373. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 8.Zuo Y, Chang Y, Thirupathi A, Zhou C, Shi Z. Prenatal sevoflurane exposure: Effects of iron metabolic dysfunction on offspring cognition and potential mechanism. Int J Dev Neurosci. 2021;81:1–9. doi: 10.1002/jdn.10080. [DOI] [PubMed] [Google Scholar]
- 9.Qi Z, Li S, Su Y, Zhang J, Kang Y, Huang Y, Jin F, Xing Q. doi: 10.1002/jcp.28323. Role of microRNA-145 in protection against myocardial ischemia/reperfusion injury in mice by regulating expression of GZMK with the treatment of sevoflurane. J Cell Physiol: Mar 14, 2019 (Epub ahead of print). doi: 10.1002/jcp.28323. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Zhou X, Lu D, Yang X, Zhou Z, Chen X, Chen Y, He W, Feng X. Aberrantly expressed long noncoding RNAs are involved in sevoflurane-induced developing hippocampal neuronal apoptosis: A microarray related study. Metab Brain Dis. 2016;31:1031–1040. doi: 10.1007/s11011-016-9838-6. [DOI] [PubMed] [Google Scholar]
- 11.Bedirli N, Bagriacik EU, Yilmaz G, Ozkose Z, Kavutçu M, Cavunt Bayraktar A, Bedirli A. Sevoflurane exerts brain-protective effects against sepsis-associated encephalopathy and memory impairment through caspase 3/9 and Bax/Bcl signaling pathway in a rat model of sepsis. J Int Med Res. 2018;46:2828–2842. doi: 10.1177/0300060518773265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang LM, Zhao XC, Sun WB, Li R, Jiang XJ. Sevoflurane post-conditioning protects primary rat cortical neurons against oxygen-glucose deprivation/resuscitation via down-regulation in mitochondrial apoptosis axis of Bid, Bim, Puma-Bax and Bak mediated by Erk1/2. J Neurol Sci. 2015;357:80–87. doi: 10.1016/j.jns.2015.06.070. [DOI] [PubMed] [Google Scholar]
- 13.Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaid C, Silva PB, Cortez BA, Rodini CO, Semedo-Kuriki P, Okamoto OK. miR-367-3p promotes proliferation and stem-like traits in medulloblastoma cells. Cancer Sci. 2015;106:1188–1195. doi: 10.1111/cas.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabet F, Lee S, Zhu W, Levin MG, Toth CL, Cuesta Torres LF, Vinh A, Kim HA, Chu HX, Evans MA, et al. MicroRNA-367-3p regulation of GPRC5A is suppressed in ischemic stroke. J Cereb Blood Flow Metab. 2020;40:1300–1315. doi: 10.1177/0271678X19858637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, Dong Y, Zhou H, Liu J, Zhao W. miR-143-3p directly targets GLUT9 to reduce uric acid reabsorption and inflammatory response of renal tubular epithelial cells. Biochem Biophys Res Commun. 2019;517:413–420. doi: 10.1016/j.bbrc.2019.07.114. [DOI] [PubMed] [Google Scholar]
- 17.Jones-Bolin S. Guidelines for the care and use of laboratory animals in biomedical research. Curr Protoc Pharmacol. 2012;59:A.4B.1–A.4B.9. doi: 10.1002/0471141755.pha04bs59. doi: 10.1002/0471141755.pha04bs59. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Wang H, Li F, Heindl LM, He X, Yu J, Yang J, Ge S, Ruan J, Jia R, Fan X. Long non-coding RNA LINC-PINT suppresses cell proliferation and migration of melanoma via recruiting EZH2. Front Cell Dev Biol. 2019;7(350) doi: 10.3389/fcell.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abd-Elrahim Batran S. Toxicity of environmental ozone exposure on mice olfactory bulbs, using western blot technique. Toxicol Rep. 2020;7:453–459. doi: 10.1016/j.toxrep.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Geng C, Qiao Y, Guo Y, Han W, Wu B, Wang C, Zhang J, Chen D, Yang M, Jiang P. Integrated metabolomics and lipidomics profiling of hippocampus reveal metabolite biomarkers in a rat model of chronic unpredictable mild stress-induced depression. Ann Transl Med. 2019;7(781) doi: 10.21037/atm.2019.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrell RC. The invention and development of enflurane, isoflurane, sevoflurane, and desflurane. Anesthesiology. 2008;108:531–533. doi: 10.1097/ALN.0b013e31816499cc. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara M, Ichinohe T, Okamoto S, Okada R, Kanbe H, Matsuura N. Concomitant administration of nitrous oxide and remifentanil reduces oral tissue blood flow without decreasing blood pressure during sevoflurane anesthesia in rabbits. J Anesth. 2015;29:421–425. doi: 10.1007/s00540-014-1944-1. [DOI] [PubMed] [Google Scholar]
- 24.Olutoye OA, Baker BW, Belfort MA, Olutoye OO. Food and drug administration warning on anesthesia and brain development: Implications for obstetric and fetal surgery. Am J Obstet Gynecol. 2018;218:98–102. doi: 10.1016/j.ajog.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 25.Beekoo D, Yuan K, Dai S, Chen L, Di M, Wang S, Liu H, ShangGuan W. Analyzing electroencephalography (EEG) waves provides a reliable tool to assess the depth of sevoflurane anesthesia in pediatric patients. Med Sci Monit. 2019;25:4035–4040. doi: 10.12659/MSM.915640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Li W, Chen X, Yang X, Zhou Z, Lu D, Feng X. Dose-dependent effects of sevoflurane exposure during early lifetime on apoptosis in hippocampus and neurocognitive outcomes in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol. 2016;8:111–119. [PMC free article] [PubMed] [Google Scholar]
- 27.Yang F, Shan Y, Tang Z, Wu X, Bi C, Zhang Y, Gao Y, Liu H. The neuroprotective effect of hemin and the related mechanism in sevoflurane exposed neonatal rats. Front Neurosci. 2019;13(537) doi: 10.3389/fnins.2019.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Xia T, Guan Y, Yu Y. Sevoflurane regulates glioma progression by Circ_0002755/miR-628-5p/MAGT1 axis. Cancer Manag Res. 2020;12:5085–5098. doi: 10.2147/CMAR.S242135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Xian D, Xia J, Tang Y, Li W, Chen X, Zhou Z, Lu D, Feng X. MicroRNA-34c is regulated by p53 and is involved in sevoflurane-induced apoptosis in the developing rat brain potentially via the mitochondrial pathway. Mol Med Rep. 2017;15:2204–2212. doi: 10.3892/mmr.2017.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Zhang Z, Jia J, Wang L, Yang Q, Wang Y, Chen C. Sevoflurane induces learning and memory impairment in young mice through a reduction in neuronal glucose transporter 3. Cell Mol Neurobiol. 2020;40:879–895. doi: 10.1007/s10571-019-00779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaid C, Jordan D, Bueno HMS, Araujo BHS, Assoni A, Okamoto OK. miR-367 as a therapeutic target in stem-like cells from embryonal central nervous system tumors. Mol Oncol. 2019;13:2574–2587. doi: 10.1002/1878-0261.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GC, He QY, Tong DK, Wang CF, Liu K, Ding C, Ji F, Zhang H. miR-367 negatively regulates apoptosis induced by adriamycin in osteosarcoma cells by targeting KLF4. J Bone Oncol. 2016;5:51–56. doi: 10.1016/j.jbo.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.